Emerging role for ERM proteins in cell adhesion and migration (original) (raw)
Abstract
The highly related ERM (Ezrin, Radixin, Moesin) proteins provide a regulated linkage between the membrane and the underlying actin cytoskeleton. They also provide a platform for the transmission of signals in responses to extracellular cues. Studies in different model organisms and in cultured cells have highlighted the importance of ERM proteins in the generation and maintenance of specific domains of the plasma membrane. A central question is how do ERM proteins coordinate actin filament organization and membrane protein transport/stability with signal transduction pathways to build up complex structures? Through their interaction with numerous partners including membrane proteins, actin cytoskeleton and signaling molecules, ERM proteins have the ability to organize multiprotein complexes in specific cellular compartments. Likewise, ERM proteins participate in diverse functions including cell morphogenesis, endocytosis/exocytosis, adhesion and migration. This review focuses on aspects still poorly understood related to the function of ERM proteins in epithelial cell adhesion and migration.
Key words: epithelial cells, membrane-cytoskeleton interface, morphogenesis, ERM proteins, cell adhesion
Introduction
Epithelial cells are characterized by a high degree of asymmetry critical for their function. Cell-cell and cell-matrix adhesion play a key role in the acquisition of structurally and functionally distinct domains of the plasma membrane and cytoplasm. In particular, junctions between neighboring cells ensure the maintenance of distinct apical and basolateral compartments, whereas adhesion to the extracellular matrix (ECM) defines the axis of polarity. Cell-cell adhesion is initiated by homotypic interactions between the membrane proteins, cadherins, which induce the reorganization of the actin cytoskeleton and membrane proteins on the cytoplasmic surface of the cells. Likewise, cell adhesion to the ECM via the heterodimeric transmembrane receptors, integrins, triggers the assembly of the actin cytoskeleton and regulatory proteins that altogether form a large multiprotein complex named the adhesome.1 Through their association with the actin cytoskeleton and signaling molecules, adhesion complexes create elaborate networks that control a variety of cellular processes in normal and pathological conditions including cell morphogenesis, migration, proliferation, survival and also invasion and metastasis. A family of closely related proteins, the ERM (Ezrin, Radixin, Moesin) proteins, participates in the regulation of these networks through their versatile interaction with membrane proteins, the actin cytoskeleton and signaling molecules. This review highlights the roles of ERM proteins in epithelial cell morphogenesis with particular emphasis on the signaling pathways underlying their functions in cell adhesion and migration.
ERM Proteins Are Conserved through Evolution
The highly homologous ERM proteins2 belong to a superfamily whose founding member is band Four point one (4.1). Within this superfamily the members share a common domain, the FERM domain (Four point one, ERM), which in most cases mediates their association with the membrane. The genes encoding ERM proteins are found in all sequenced metazoans, but are absent from unicellular organisms such as yeast. Therefore their appearance coincides with the apparition of multicellularity and the generation of intercellular junctions. Whereas vertebrate genomes contain three genes encoding the three paralogs ezrin, radixin and moesin, only one ERM gene is found in the genome of non-vertebrate organisms that have been sequenced. ERM proteins from evolutionarily diverse species all share the same structural organization. They are composed of an amino-terminal FERM domain followed by a region rich in α-helices and a carboxy-terminal domain containing the F-actin binding site3 (Fig. 1A). Moreover, ERM proteins are highly conserved in their primary structure throughout evolution with the highest level of identity observed in the FERM domains and the F-actin binding sites. Indeed these domains display ∼76% identity between human ezrin and their homologs Dmoesin from Drosophila melanogaster or ERM-1 from Caenorhabditis elegans.
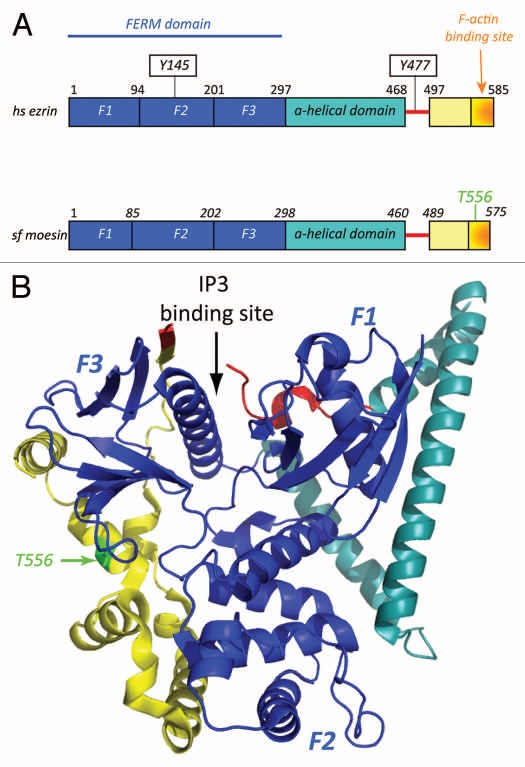
Figure 1.
(A) Domain organization of human ezrin and _Sf_moesin. All ERM proteins display similar domain organization. They are composed of a globular N-terminal domain (FERM domain) followed by a domain rich in α-helices, a linker region and the C-terminal domain. The F-actin binding site has been mapped to the last 30 carboxy-terminal aminoacids.3 Tyrosines 145 and 477 are phosphorylated by Src kinases downstream growth factor stimulation.28,63 These two tyrosines are not conserved in _Sf_moesin. (B) Structure of full-length Spodoptera frugiperda moesin; protein data bank (PDB) identifier 2I1J. The three lobes that composed the FERM domain (F1, F2 and F3) are colored in dark blue, the α-helical domain in turquoise, the linker region in red and the C-terminal domain in yellow. In the closed state, the α-helical region and the C-terminal domain make extensive interactions with the FERM domain that mask the ligand binding sites.10 IP3 binds a basic cleft between domains F1 and F3 of the free FERM domain.7 Phosphorylation of threonine 556, highlighted in green, weakens the interaction between the FERM and the C-terminal domains.
Regulated Linkage of ERM Proteins to the Membrane and to the Actin Cytoskeleton
Biological and biochemical studies have provided important insights into how ERM proteins interact with both membrane components and the actin cytoskeleton. Expressing either full-length ezrin or its N-/C-terminal domains in cells led to the observation that ezrin interacts with membrane components via its N-terminal domain and with the actin cytoskeleton via its C-terminal domain.4 Moreover, whereas both full-length ezrin and its N-terminal domain concentrate at the plasma membrane, the C-terminal domain alone associates with the cortical actin cytoskeleton and actin stress fibers, suggesting that regulatory mechanisms might exist that restrict the localization of the full-length protein to the cell cortex. Biochemical studies demonstrated that ERM proteins are negatively regulated by an intramolecular interaction between the N-terminal domain and the C-terminal ∼100 amino acids called N-/C-ERMAD (ERM Association Domain). In this closed or inactive conformation the actin and membrane binding sites are masked.5
In addition to biochemical studies, considerable insights into how the structure of ERM proteins contributes to their functions have been provided by structural studies. The X-ray structures of the FERM domains,6–8 the FERM domain of moesin complexed with the C-ERMAD (inactive FERM domain conformation),9 and more recently the structure of the full-length moesin from Spodoptera frugiperda (_Sf_moesin),10 have been solved. The FERM domain is composed of three subdomains, F1, F2 and F3, that adopt a clover leaf-like arrangement. The structure of the moesin FERM domain complexed to the C-ERMAD revealed that the C-ERMAD adopts an extended structure that covers a large region of domains F2 and F3 masking the F-actin and membrane binding sites.9 Interestingly the structure of the full-length Sfmoesin uncovered an extensive interaction of the central α-helical region with the FERM domain, indicating that this α-helical region contributes with the C-ERMAD to the masking of a large area of the FERM domain (Fig. 1B). Moreover, it suggests an unexpected role for this α-helical region in ERM protein activation. Indeed, any partners of ERM proteins binding to this α-helical region may contribute to conformational changes and therefore to the activation process.
Activation through the release of the N-/C-ERMAD interaction is therefore required for the unmasking of the membrane and actin binding sites. Numerous studies have indicated that the dissociation of the N-/C-ERMAD interaction is triggered by the binding of the FERM domain to phosphatidyl-inositol-4,5-biphosphate (PIP2) and phosphorylation of a conserved threonine residue present in the F-actin binding site (Thr558, Thr564, Thr567 in human moesin, radixin and ezrin respectively). Initial observations showed that the association of ERM proteins with the cytoplasmic tail of the hyaluronan receptor CD44 requires PIP2 in vitro and in vivo suggesting that PIP2 binding induces a change in the conformation that exposes the membrane protein binding sites in the FERM domain.11,12 Comparison of the crystal structure of the free radixin FERM domain with that in complex with the head group of PIP2, inositol-(1,4,5)-triphosphate (IP3), indicates that structural changes in the FERM domain are associated with IP3 binding. It has been proposed that these changes may contribute to the activation mechanism of ERM proteins.7 The binding of ezrin to PIP2 has also been shown to be a critical step in the activation of ERM proteins in vivo.13 However, the structure of _Sf_moesin suggests that the PIP2 binding site is masked in the full-length proteins by the linker region since IP3 is located in a basic cleft between subdomains F1 and F3 in which three lysines and one arginine contact the phosphate groups of IP3,7,10 (Fig. 1B). Therefore how ERM proteins interact with PIP2 at the membrane remains an open question.
As indicated above, phosphorylation of the conserved threonine residue present in the F-actin binding site has also been implicated in the activation of ERM proteins. Moesin was found phosphorylated at threonine 558 upon platelet activation14 as were ERM proteins following stimulation of Swiss3T3 cells with lysophosphatidic acid.15 Several kinases were shown to phosphorylate this conserved threonine in vertebrates ERM proteins including Protein kinase Cα (PKCα, θ), NIK (NFκB-inducing kinase), LOK (Lymphocyte-Oriented Kinase) and MST4 suggesting that the phosphorylation of ERM proteins may be induced through different pathways.16–20 This threonine is buried in the N-/C-ERMAD interface and its phosphorylation induces steric and electrostatic hindrance preventing the N-/C-ERMAD association.9,15,21 In vivo, the sequence of events consisting of binding to PIP2 followed by phosphorylation at threonine 567 is critical for the proper activation of ezrin, since impairing one of these steps alters its correct localization and functions in epithelial cells.13
Numerous membrane or membrane-associated proteins have been shown to bind the FERM domain when ERM proteins are in an active conformation. ERM proteins interact with the cytoplasmic domain of several membrane proteins including the hyaluronan receptor CD44 which mediates cell adhesion and migration.22 Other membrane proteins such as ion channels, transporters and receptors can bind indirectly through the scaffolding factors NHERF1 (also named EBP50 for ERM-binding phosphoprotein 50) or NHERF2 (Na+-H+ exchanger regulatory factor).23 These proteins contain two PDZ (Postsynaptic Density Protein) domains known to mediate protein interaction followed by an ERM protein binding site.24 Comparison of the crystal structure of the radixin FERM domain complexed with a CD44 cytoplasmic peptide25 and of the moesin FERM domain in complex with a peptide from the C-terminal domain of EBP50 indicates that these two ligands bind to distinct sites of the FERM domain.26
So far few proteins have been shown to interact with the C-terminal domain of ERM proteins. The regulatory subunit RII of protein kinase A (PKA) associates with an α-helical region in ERM proteins (amino acids 417–430 in ezrin). Recently, two subunits of the HOPS complex (Homotypic fusion and Protein Sorting) have been shown to interact with the α-helical domains of ERM proteins.27 Both the regulatory subunit RII of protein kinase A (PKA) and the subunits of the HOPS complex interact with full-length ERM protein suggesting that their binding sites are not masked. The structure of _Sf_moesin also indicates that the linker region in ERM proteins is likely to be accessible when ERM proteins are in a closed conformation. Specific to ezrin is the presence in this region of tyrosine at position 477 which is phosphorylated by Src kinase.28 This linker region likely represents a site for the docking of signaling proteins as discussed below.
ERM Protein Functions in Epithelial Cell Morphogenesis: Insights from Genetic Analyses
Genetic analyses of ERM proteins in different model systems have revealed that they have a crucial role in epithelial cell architecture and in the formation of tubular organ epithelia during development. In all epithelial cell types analyzed a defect in the organization of the actin-rich apical plasma membrane has been observed. In D. melanogaster and C. elegans ERM proteins are essential for viability. In flies, Dmoesin has an important role in epithelial cell integrity and polarity. Imaginal epithelial cells without moesin show reduced cortical actin cytoskeleton, lack junctional markers, detach from and migrate out of the epithelium suggesting a role of Dmoesin in the stabilization of the apical-junctional domain.29 Similarly, ERM-1 in C. elegans functions in apical membrane morphogenesis of epithelial cells.30,31
In adult mammals, the expression of the three ERM proteins is rather tissue-specific with ezrin found primarily in epithelial cells, moesin in endothelial cells and radixin in hepatocytes and hair cells. Inactivation of either ezrin or radixin has provided insights into their function in the particular epithelial cell types where they are expressed. Ezrin knockout mice die soon after birth. Intestinal epithelial cells in these mice present a disorganization of both the terminal web and microvilli and the adhesion complexes appear more elongated and twisted.32 Loss of ezrin leads also to reduction in the apical microvilli of Müller cells and in the microvilli and basal unfoldings of retinal pigment epithelium with, as a consequence, a retardation in the development of photoreceptors.33 In gastric epithelial cells, called parietal cells, ezrin is a key regulator of acid secretion that occurs following the fusion of H+, K+ATPase-rich tubulovesicles with the apical membrane.34 Ezrin knockdown mice suffer from severe achlorhydria due to a defect in the expansion of apical secretory canaliculi leaving the cytoplasm with densely packed tubulovesicles.35 Radixin inactivation causes hyperbilirubinemia likely due to a defect in the localization of Mrp2 (Multidrug resistance protein 2, a protein involved in the secretion of conjugated bilirubin) in the bile canalicular membranes36 and deafness due to progressive degeneration of cochlear stereocilia.37
In addition to these cellular defects in organisms lacking ERM proteins developmental defects are observed in the formation of epithelia that line tubular organs. Loss of the single ortholog erm-1 in Caenorhabditis elegans impairs lumen formation of tubular organ epithelia.30,31 Luminal cysts are observed in the epithelia upon depletion of ERM-1 due to incomplete tubulogenesis. Moreover, intestine-specific phenotypes such as constriction or obstruction are observed.30 It was suggested that these phenotypes are not due to a defect in epithelial cell polarization, but rather arise from premature arrest in the repositioning of the apical junctions to the apico-lateral position during lumen formation resulting in the obstructions observed in the intestine.30 In this latter study, a genetic interaction was found between ERM-1 and the cadherin and catenin homologs HMR-1 and HMP-1, respectively. A possible role for ERM-1 would be to link the cadherin/catenin complex to actin cytoskeleton since the enrichment in F-actin at the apical pole is lost in the intestine of erm-1 (RNAi) embryos.
Similarly, deletion of ezrin results in defects in mouse intestinal villus morphogenesis. Generation of individual villi occurs during embryonic development when the stratified intestinal epithelium is converted into a monolayer of epithelial cells covering the villi. In ezrin-/- mice, villus formation is abnormal. Instead of individual villi observed in wild-type mice, the intestine of ezrin-/- mice displays fused villi. Therefore, the transition from a stratified epithelium to a monolayer, which is initiated by a remodeling of the cell-cell contacts, is altered indicating that ezrin plays a regulatory role in the assembly of the junctions.32
Regulation of Cell-Cell Junction Assembly by ERM Proteins
The role of ERM proteins in cell-cell junction remodeling has been investigated during development. The function of Dmoesin has been addressed in the assembly and positioning of adherens junctions during the formation of primary embryonic epithelium in early D. melanogaster embryo. A microarray-based RNA interference (RNAi) screen devised to characterize regulators of epithelial morphogenesis identified Bitesize (Btsz), a synaptotagmin-like protein recruited to the adherens junctions independently of E-cadherin.38 On loss of Btsz, adherens junctions form properly, but subsequently appear fragmented with a concomitant decrease in E-cadherin expression. This suggests a role for Btsz in the stabilization of the adherens junctions. This stabilization requires Dmoesin, which is recruited to the adherens junctions by Btsz. Interestingly, a dominant negative mutant of ezrin, lacking the C-terminal actin binding sites, when expressed in D. melanogaster embryo during gastrulation shows similar epithelial defects to Btsz mutants.38 These observation indicate that the interaction of Dmoesin with Btsz is required for the stabilization of the membrane-cytoskeleton interface at adherens junctions and thus for the integrity of the adhesion complex.
An interaction of Dmoesin with the Crumbs complex has been reported in Drosophila embryo.39 This complex, localized to the apex of epithelial cells, plays a key role in epithelial cell shape and polarity. The recruitment of the spectrin-based membrane cytoskeleton and Dmoesin by Crumbs to the apical surface would lead to the stabilization of the zonula adherens. Therefore through the formation of diverse molecular complexes, Dmoesin may participate in different steps of junction assembly.
A critical role for ezrin in adherens junction assembly during the formation of the first epithelium, the trophectoderm, in preimplantation mouse development has been demonstrated.40–42 In this model, mutation of ezrin at the conserved threonine 567 inhibits the formation of E-cadherin-mediated cell-cell contacts during blastocyst formation. As a result, the morphology of the blastocyst is impaired, as is the correct differentiation and polarization of the trophectoderm. Moreover, whereas ezrin was found at the cell cortex before the compaction of blastomeres and accumulated at the apical pole during compaction this relocalization was impaired in blastomeres expressing ezrin mutants. Similar relocalization of ERM-1 from junctions to the apical surface of differentiating epithelium has been observed in C. elegans embryos.30 Therefore ERM proteins would be involved in an early step in the formation of cell-cell contacts, before their localization to the apical surface where they have a critical role in the assembly of actin-rich apical structures. Indeed in differentiated epithelial cells, the major pool of ezrin is present at the apical surface although a small fraction can be observed at cell-cell contacts.43
Signaling Pathways Involved in the Regulation of Adherens Junctions by ERM Proteins
A role for ERM proteins in the regulation of cell adhesion was recognized early when it was shown that depletion of ERM proteins by antisense oligonucleotides affects cell-cell and cell-matrix adhesion of epithelial MTD-1A cells.44 Likewise, ERM proteins were shown to be required for the formation of focal adhesion complexes and stress fibers downstream of Rho GTPases activation. 45 Subsequently, ERM proteins were implicated in pathways regulating the dynamic of cell-cell junctions, the Rho pathways and growth factor signaling.
GTPases of the Rho family play important and complex roles in the regulation of cell-cell and cell-matrix adhesion.46 ERM proteins have the potential to act either as effectors of Rho GTPases45 or as regulators of their activity in cell adhesion regulation. Thus, the loss of epithelial cell integrity and junctional markers in dmoesin mutants correlates with excess Rho1 activity, the fly RhoA ortholog29,47 suggesting that Dmoesin negatively regulates RhoA activity. In mammalian cells, the GTPase Rac has been shown to have both positive and negative roles in cell-cell junction dynamics. The production of an ezrin mutant mimicking its constitutive phosphorylation on threonine 567 (ezrin T567D) leads to the formation of lamellipodia in non confluent epithelial cells and to the disruption of cell-cell contacts due to the activation of the small GTPase Rac.48 Not only does the active form of ezrin disrupt the junctions, but it also prevents the proper assembly of cell-cell junctions. Epithelial cells producing this ezrin T567D were neither able to form hollow cysts when grown in a type 1 collagen matrix and remained aggregated nor were they able to organize into tubules, indicating that these cells were not able to establish functional cell-cell contacts.49 Rounds of activation and downregulation of Rac and Rho GTPases have been observed during formation of cell-cell adhesion.50 A constitutively active form of ezrin may prevent the downregulation of Rac therefore perturbing the assembly of the junctions. How do ERM proteins activate Rho GTPases? Rac activation may result from the association of ERM proteins with regulators of small GTPases including Rho GDP-dissociation inhibitor (GDI),51 Rho guanine nucleotide exchange factor (GEF),52,53 and Rho GTPase-activating proteins (GAP).54 In particular, PLEKHG6 which displays an exchange activity towards RhoG and Rac induces extensive lamellipodia when recruited at the lateral surface of the cells.53
Possible mechanisms of adherens junction control by Rac and the ERM proteins include the regulation of local actin cytoskeleton and of endocytosis of E-cadherin. The reorganization of the local actin cytoskeleton at cell-cell contact can be triggered by an array of actin binding proteins including ERM proteins, which would then function both upstream and downstream the Rac GTPase.46,48 Rac GTPase has also been implicated in E-cadherin trafficking as a mean to control adherens junction assembly/disassembly.55–57
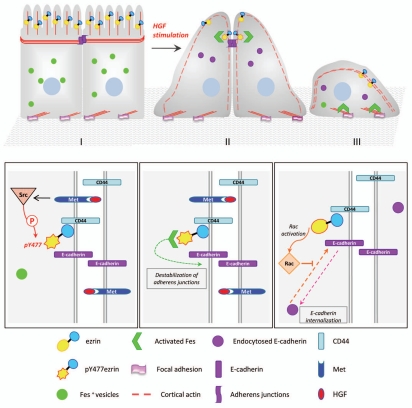
Early studies on ERM proteins indicated that they are the substrates of growth factor receptors. Phosphorylation of ERM proteins on tyrosine residues correlates with changes in the morphology of cells stimulated with growth factor.58–60 The Hepatocyte Growth Factor (HGF) and its receptor c-Met have a prominent role in epithelial cell scattering, motility and morphogenesis, which require extensive adhesion complex remodeling. A role for ezrin in these downstream pathways elicited by HGF stimulation has been demonstrated.61,62 Mutations that abolish phosphorylation of ezrin at specific tyrosine residues have provided insights into the role of ezrin in the transmission of signals elicited by HGF stimulation. Thus, ezrin phosphorylation at tyrosine 145 is required for cell spreading but not for adhesion to the matrix indicating that ezrin is involved in the formation of membrane protrusions.63 Ezrin phosphorylation at tyrosine 477 is important for HGF-induced cell scattering. An effector of phosphorylated ezrin at tyrosine 477 has been identified, the non-receptor tyrosine kinase Fes (Feline Sarcoma) that provides a molecular link between the receptor c-Met and cell-cell adhesion.43 The function of phosphorylated ezrin is to recruit the Fes kinase at cell-cell contacts, where it becomes activated, a prerequisite for HGF-induced cell scattering. If the interaction between ezrin and Fes is abolished, Fes accumulates in focal contacts and cells show defective scattering in response to HGF stimulation (Fig. 2).43 Therefore Fes is likely involved in the phosphorylation of components of the adherens junctions which in turn regulate their dynamics. Moreover, ezrin together with Fes coordinates the cross-talk between cell-cell and cell-matrix adhesion that has been shown to be important for cell scattering.64 Interestingly, the tyrosine 477 in ezrin is not conserved among the other ERM proteins, suggesting a very specific role for ezrin together with Fes in the regulation of the dynamics of adherens junctions.
Figure 2.
Ezrin is an essential component in HGF-induced cell scattering. Upper part: I: HGF stimulation induces morphological changes including breakdown of the microvilli (II), cell flattening (II), cell-cell contact dissociation and increased cell migration (III). Lower part: At the molecular level, stimulation of the Met receptor activates multiple intracellular signaling pathways. (I) Following HGF stimulation, ezrin is recruited to the lateral membrane where it can interact with the adhesion molecule CD44 with concomitant phosphorylation on tyrosine residues. Phosphorylated tyrosine can interact with SH2-domain containing proteins such as the Fes kinase. Fes is recruited and activated to cell-cell contacts by ezrin phosphorylated at tyrosine 477. (II) Phosphorylation of regulatory cell junction proteins by activated Fes can trigger the dissociation of cell-cell contacts. (III) Activated Rac downstream of ezrin is involved in the epithelial to mesenchymal transition by regulating actin cytoskeleton remodeling and E-cadherin trafficking.
The regulation of epithelial cell scattering and signaling induced by HGF also involves the interaction of ERM proteins with the hyaluronan receptor CD44V6, which mediates cell adhesion and migration in a variety of physiological and pathological processes.65 Both biochemical and structural studies have revealed that the cytoplasmic tail of CD44 binds the N-terminal domain of ERM proteins in a regulated manner.22,25,66 Moreover, CD44v6 isoform function as coreceptors for several receptor tyrosine kinases among which is c-Met.67 The binding of ERM proteins to CD44v6 is required to trigger HGF-dependent activation of Ras by the guanine nucleotide exchange factor, Sos.62,65 Therefore through its interaction with CD44, ezrin is part of a signaling network that conveys signals regulating cell proliferation, but also cell adhesion and cell scattering.
ERM Proteins in Cell Migration
ERM proteins are also involved in cell migration following stimulation of membrane receptors by various ligands. Epithelial cell migration is a multi-step process that requires the dissociation of cell-cell contacts, changes in the dynamic of cell adhesion to the matrix and extension of membrane protrusions. As discussed above, ezrin is involved in the dissociation of cell-cell contacts triggered by HGF stimulation through its interaction with the Fes kinase and in the formation of membrane extensions.43 Moreover, ezrin promotes cell motility in response to HGF treatment as measured by the ability of epithelial cells to close a wound.61 Following HGF stimulation of epithelial cells, ezrin is rapidly recruited to the lateral membrane and to the leading edge of migrating cells where it is supposed to play a role in the control of actin polymerization.43,61
Ezrin has also been implicated in cell migration triggered by phorbol-ester stimulation. In this situation, ezrin acts downstream of protein kinase C (PKC) in PKC-induced cell migration and this function requires ezrin phosphorylation at the conserved threonine 567 by PKCα.17 Moreover, activation of PKC leads to the phosphorylation of the transmembrane receptor CD44 and this phosphorylation modulates its interaction with ezrin and CD44-dependent directional cell motility.68
G-protein coupled receptors triggers epithelial cell migration through the activity of the G protein-coupled receptor kinase 2 (GRK2).69 GRK2 was shown to regulate membrane protrusion and cell migration during the wound closure of MDCK cells through the phosphorylation of ERM proteins at the conserved threonine residue present in the F-actin binding sites.70
Altogether these data show that ERM proteins convey signals elicited by membrane receptors to the actin cytoskeleton to regulate cell migration. However, it remains unclear how ERM proteins control the reorganization of the actin cytoskeleton during cell migration. A likely hypothesis is that through their phosphorylation ERM proteins will recruit regulators of actin polymerization.
ERM Proteins in Invasion and Metastasis
There is increasing evidence of a role for ERM proteins in tumor progression. An increase of ezrin expression in metastasic human carcinomas from different origins has been observed by proteomic profiling and immunohistochemical analyses.71–73 These studies and others indicate that not only the expression level of ERM proteins but also their phosphorylation status and subcellular localization should be considered to understand their role in tumor progression. A comparison of the protein profiles of pancreatic cancers without and with lymph node metastasis indicated an increased expression of moesin and radixin in the lymph node metastasis-positive group and a change in ezrin phosphorylation, but not expression.71 Changes in gene expression associated with tumor progression in patients with head and neck squamous cell carcinoma (HNSCC) identified moesin among the genes upregulated in tumors.72 Moesin expression in tissue arrays containing normal squamous cell and the corresponding SCC from the tongue and lymph nodes showed that in normal tissue moesin is present in the basal layer, whereas a strong positive staining for moesin at the membrane and in the cytoplasm is observed in tumor cells in SCC and lymph nodes. Another example of change in ezrin localization associated with a poor prognostic is provided by invasive breast carcinomas.73 A tissue array-based immunohistochemical study showed that an apical ezrin localization is observed in normal breast epithelium, which is associated with a favorable prognostic and node-negative tumor if it is maintained in a tumor. In contrast, the switch of ezrin localization to the cytoplasm or to the membrane in a non-polarized manner is correlated with significant lymph node metastasis and adverse clinical features. This suggests that abnormal cellular localization of ERM proteins may lead to the deregulation of several functions in tumor cells. Among them, the loss of apical localization and the acquisition of an undifferentiated phenotype may impair cell-cell or cell-matrix adhesion and lead to the acquisition of an invasive phenotype. Likewise, the translocation of ERM proteins from the membrane to the cytoplasm may alter the transmission of signals elicited by growth factors or perturb the anchoring of membrane receptors and adhesion molecules during tumor progression.
The function of ezrin in metastasis has been investigated using a breast tumor xenograft model.74 In this model, suppression of ezrin activity inhibits the formation of pulmonary metastasis by the mammary tumor cells. Since deregulation of cell-cell contacts, increased cell motility and invasion are key steps in the metastatic cascade it raises a role for ERM proteins in any of these steps. Using the mouse breast tumor xenograft model described above it has been shown that transplanted tumor cells expressing ezrin non phosphorylable mutants (either Y145F or Y477F ezrin) showed a marked attenuating effect on pulmonary metastasis. Furthermore, transplanted mouse breast carcinoma cells overexpressing Y477F ezrin formed cohesive tumors that were circumscribed by normal stroma with no detectable infiltration into underlying muscle suggesting that the ability of the cells to remodel their intercellular junctions was impaired (Elliott BE, personal communication).43 Increased Src activity and constitutive activation of Met are detected in many invasive tumors. Since a cooperative effect of ezrin and Src kinase deregulates cell-cell contacts75 and that Fes kinase is required for HGF-induced cell scattering,43 the ezrin/Fes interaction appears as a possible mechanism connecting Src activation downstream of Met with changes in the adhesive properties of tumor cells in metastasis progression.
In addition to its role in regulating cell-cell contacts, ezrin may localize adhesion receptors to the front of migrating cells. Indeed an interaction between ezrin and the cell-neural adhesion molecule L1CAM (hereafter referred to as L1) is required for L1-mediated metastasis of colon cancer cells.76 L1, an adhesion molecule expressed in nerve cells where it functions in axonal guidance, outgrowth and pathfinding, is a target of β-catenin-TCF signaling in colorectal cancer cells.77 Because both ezrin and L1 are observed at the front of the tumors it suggests that they may cooperate for the invasion of the cells.
Concluding Remarks
Although a role for ERM proteins in cell adhesion and migration is emerging, little is known on how they regulate changes in cytoskeleton-membrane interaction to promote the assembly/disassembly of adherens junctions or cell motility. ERM proteins have indeed the potential to organize multiprotein complexes in specific compartments in response to external signals. It would be important to understand how ERM proteins regulate in a spatiotemporal manner the assembly of these complexes. Determining how ERM proteins participate in the transmission of signals that control adhesion-dependent processes such as cell proliferation, survival and also actin cytoskeleton remodeling should advance our understanding of epithelial cell function. Another challenge is to understand how the functions of ERM proteins are diverted by tumor cells, which present increased invasive and metastatic properties.
Acknowledgements
We thank Dr. James Sillibourne (Curie Institute) for reading the manuscript. Dafne Chirivino and Ingrid Zwaenepoel were recipients of post-doctoral fellowships from Institut Curie and Fondation pour la Recherche Medicale (FRM) respectively.
References
- 1.Zaidel-Bar R, Geiger B. The switchable integrin adhesome. J Cell Sci. 2010;123:1385–1388. doi: 10.1242/jcs.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992;103:131–143. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- 3.Turunen O, Wahlström T, Vaheri A. Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol. 1994;126:1445–1453. doi: 10.1083/jcb.126.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol. 1993;120:129–139. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gary R, Bretscher A. Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site. Mol Biol Cell. 1995;6:1061–1075. doi: 10.1091/mbc.6.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith WJ, Nassar N, Bretscher A, Cerione RA, Karplus PA. Structure of the active FERM domain of ezrin: conformational and mobility changes identify keystone interactions. J Biol Chem. 2002 doi: 10.1074/jbc.M210601200. [DOI] [PubMed] [Google Scholar]
- 7.Hamada K, Shimizu T, Matsui T, Tsukita S, Tsukita S, Hakoshima T. Structural basis of the membrane-targeting and unmasking mechanisms of the radixin FERM domain. EMBO J. 2000;19:4449–4462. doi: 10.1093/emboj/19.17.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards SD, Keep NH. The 2.7 A crystal structure of the activated FERM domain of moesin: an analysis of structural changes on activation. Biochemistry. 2001;40:7061–7068. doi: 10.1021/bi010419h. [DOI] [PubMed] [Google Scholar]
- 9.Pearson MA, Reczek D, Bretscher A, Karplus PA. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell. 2000;101:259–270. doi: 10.1016/s0092-8674(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Nance MR, Kulikauskas R, Nyberg K, Fehon R, Karplus A, et al. Self-masking in an intact ERM-merlin protein: an active role for the central a-helical domain. J Mol Biol. 2007;365:1446–1459. doi: 10.1016/j.jmb.2006.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, et al. Regulation mechanism of ERM (Ezrin/Radixin/Moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and rho-dependent signaling pathway. J Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yonemura S, Matsui T, Tsukita S, Tsukita S. Rho-dependent and -independent activation mechanisms of ezrin/radixin/moesin proteins: an essential role for polyphosphoinositides in vivo. J Cell Sci. 2002;115:2569–2580. doi: 10.1242/jcs.115.12.2569. [DOI] [PubMed] [Google Scholar]
- 13.Fiévet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, et al. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol. 2004;164:653–659. doi: 10.1083/jcb.200307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura F, Amieva MR, Furthmayr H. Phosphorylation of threonine 558 in the carboxy-terminal actin-binding domain of moesin by thrombin activation of human platelets. J Biol Chem. 1995;270:31377–1385. doi: 10.1074/jbc.270.52.31377. [DOI] [PubMed] [Google Scholar]
- 15.Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, et al. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietromonaco SF, Simons PC, Altman A, Elias L. Protein kinase C-q phosphorylation of moesin in the actin-binding sequence. J Biol Chem. 1998;273:7594–7603. doi: 10.1074/jbc.273.13.7594. [DOI] [PubMed] [Google Scholar]
- 17.Ng T, Parsons M, Hughes W, Monypenny J, Zicha D, Gautreau A, et al. Ezrin is a downstream effector of trafficking PKC/integrin complexes involved in the control of cell motility. EMBO J. 2001;20:2723–2741. doi: 10.1093/emboj/20.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumgartner M, Sillman AL, Blackwood EM, Srivastava J, Madson N, Schilling JW, et al. The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. Proc Natl Acad Sci USA. 2006;103:13391–13396. doi: 10.1073/pnas.0605950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belkina NV, Liu Y, Hao JJ, Karasuyama H, Shaw S. LOK is a major ERM kinase in resting lymphocytes and regulates cytoskeletal rearrangement through ERM phosphorylation. Proc Natl Acad Sci USA. 2009:106. doi: 10.1073/pnas.0805963106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ten Klooster JP, Jansen M, Yuan J, Oorschot V, Begthel H, Di Giacomo V, et al. Mst4 and Ezrin Induce Brush Borders Downstream of the Lkb1/Strad/Mo25. Polarization Complex Dev Cell. 2009;16:551–562. doi: 10.1016/j.devcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura F, Huang L, Pestonjamasp K, Luna EJ, Furthmayr H. Regulation of F-actin binding to platelet moesin in vitro by both phosphorylation of threonine 558 and polyphosphatidylinositides. Mol Biol Cell. 1999;10:2669–2685. doi: 10.1091/mbc.10.8.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yonemura S, Hirao M, Doi Y, Takahashi N, Kondo T, Tsukita S, et al. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43 and ICAM-2. J Cell Biol. 1998;140:885–895. doi: 10.1083/jcb.140.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinman EJ, Hall RA, Friedman PA, Liu-Chen LY, Shenolikar S. The association of NHERF adaptor proteins with g protein-coupled receptors and receptor tyrosine kinases. Annu Rev Physiol. 2006;68:491–505. doi: 10.1146/annurev.physiol.68.040104.131050. [DOI] [PubMed] [Google Scholar]
- 24.Reczek D, Berryman M, Bretscher A. Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139:169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori T, Kitano K, Terawaki SI, Maesaki R, Fukami Y, Hakoshima T. Structural basis for CD44 recognition by ERM proteins. J Biol Chem. 2008;283:29602–29612. doi: 10.1074/jbc.M803606200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finnerty CM, Chambers D, Ingraffea J, Faber HR, Karplus PA, Bretscher A. The EBP50-moesin interaction involves a binding site regulated by direct masking on the FERM domain. J Cell Sci. 2003;117:1547–1552. doi: 10.1242/jcs.01038. [DOI] [PubMed] [Google Scholar]
- 27.Chirivino D, Del Maestro L, Formstecher E, Hupé P, Raposo G, Louvard D, et al. The ERM proteins interact with the class C-Vps/HOPS complex to regulate the maturation of endosomes. Mol Biol Cell. 2011;22:375–385. doi: 10.1091/mbc.E10-09-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heiska L, Carpen O. Src phosphorylates ezrin at tyrosine 477 and induces a phosphospecific association between ezrin and a kelch-repeat protein family member. J Biol Chem. 2005;280:10244–10252. doi: 10.1074/jbc.M411353200. [DOI] [PubMed] [Google Scholar]
- 29.Speck O, Hughes SC, Noren NK, Kulikauskas RM, Fehon RG. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature. 2003;421:83–87. doi: 10.1038/nature01295. [DOI] [PubMed] [Google Scholar]
- 30.Van Fürden D, Johnson K, Segbert C, Bossinger O. The C. elegans ezrin-radixin-moesin protein ERM-1 is necessary for apical junction remodelling and tubulogenesis in the intestine. Dev Biol. 2004;272:262–276. doi: 10.1016/j.ydbio.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Gobel V, Barrett PL, Hall DH, Fleming JT. Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev Cell. 2004;6:865–873. doi: 10.1016/j.devcel.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Bonilha VL, Rayborn ME, Saotome I, McClatchey AI, Hollyfield JG. Microvilli defects in retinas of ezrin knockout mice. Exp Eye Res. 2006;82:720–729. doi: 10.1016/j.exer.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Zhou R, Cao X, Watson C, Miao Y, Guo Z, Forte JG, et al. Characterization of protein kinase A-mediated phosphorylation of ezrin in gastric parietal cell activation. J Biol Chem. 2003;278:35651–35659. doi: 10.1074/jbc.M303416200. [DOI] [PubMed] [Google Scholar]
- 35.Tamura A, Kikuchi S, Hata M, Katsuno T, Matsui T, Hayashi H, et al. Achlorhydria by ezrin knockdown: defects in the formation/expansion of apical canaliculi in gastric parietal cells. J Cell Biol. 2005;169:21–28. doi: 10.1083/jcb.200410083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kikuchi S, Hata M, Fukumoto K, Yamane Y, Matsui T, Tamura A, et al. Radixin deficiency causes conjugated hyperbilirubinemia with loss of Mrp2 from bile canalicular membranes. Nat Genet. 2002;31:320–325. doi: 10.1038/ng905. [DOI] [PubMed] [Google Scholar]
- 37.Kitajiri S, Fukumoto K, Hata M, Sasaki H, Katsuno T, Nakagawa T, et al. Radixin deficiency causes deafness associated with progressive degeneration of cochlear stereocilia. J Cell Biol. 2004;166:559–570. doi: 10.1083/jcb.200402007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pilot F, Philippe JM, Lemmers C, Lecuit T. Spatial control of actin organization at adherens junctions by the synaptotagmin-like protein Btsz. Nature. 2006;442:580–584. doi: 10.1038/nature04935. [DOI] [PubMed] [Google Scholar]
- 39.Médina E, Williams J, Klipfell E, Zarnescu D, Thomas G, Le Bivic A. Crumbs interacts with moesin and beta(Heavy)-spectrin in the apical membrane skeleton of Drosophila. J Cell Biol. 2002;158:941–951. doi: 10.1083/jcb.200203080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dard N, Louvet S, Santa-Maria A, Aghion J, Martin M, Mangeat P, et al. In vivo functional analysis of ezrin during mouse blastocyst formation. Dev Biol. 2001;233:161–173. doi: 10.1006/dbio.2001.0192. [DOI] [PubMed] [Google Scholar]
- 41.Dard N, Louvet-Vallée S, Santa-Maria A, Maro B. Phosphorylation of ezrin on threonine T567 plays a crucial role during compaction in the mouse early embryo. Dev Biol. 2004;271:87–97. doi: 10.1016/j.ydbio.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 42.Louvet S, Aghion J, Santa-Maria A, Mangeat P, Maro B. Ezrin becomes restricted to outer cells following asymmetrical division in the preimplantation mouse embryo. Dev Biol. 1996;177:568–579. doi: 10.1006/dbio.1996.0186. [DOI] [PubMed] [Google Scholar]
- 43.Naba A, Reverdy C, Louvard D, Arpin M. Spatial recruitment and activation of the Fes kinase by ezrin promotes HGF-induced cell scattering. EMBO J. 2008;27:38–50. doi: 10.1038/sj.emboj.7601943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeuchi K, Sato N, Kasahara H, Funayama N, Nagafuchi A, Yonemura S, et al. Perturbation of cell adhesion and microvilli formation by antisense oligonucleotides to ERM family members. J Cell Biol. 1994;125:1371–1384. doi: 10.1083/jcb.125.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackay DJG, Esch F, Furthmayr H, Hall A. Rho- and Rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. J Cell Biol. 1997;138:927–938. doi: 10.1083/jcb.138.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popoff MR, Geny B. Multifaceted role of Rho, Rac, Cdc42 and Ras in intercellular junctions, lessons from toxins. Biochim Biophys Acta. 2009;1788:797–812. doi: 10.1016/j.bbamem.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Hipfner DR, Keller N, Cohen SM. Slik Sterile-20 kinase regulates Moesin activity to promote epithelial integrity during tissue growth. Genes Dev. 2004;18:2243–2248. doi: 10.1101/gad.303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pujuguet P, Del Maestro L, Gautreau A, Louvard D, Arpin M. Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol Biol Cell. 2003;14:2181–2191. doi: 10.1091/mbc.E02-07-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gautreau A, Louvard D, Arpin M. Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. J Cell Biol. 2000;150:193–203. doi: 10.1083/jcb.150.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, et al. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem. 1997;272:23371–23375. doi: 10.1074/jbc.272.37.23371. [DOI] [PubMed] [Google Scholar]
- 52.Prag S, Parsons M, Keppler MD, Ameer-beg SM, Hunt J, Barber P, et al. Ezrin promotes cell migration through recruitment of the GEF Dbl to lipid rafts and downstream activation of Cdc42. Mol Biol Cell. 2007;18:2935–2948. doi: 10.1091/mbc.E06-11-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.D'Angelo R, Aresta S, Blangy A, Del Maestro L, Louvard D, Arpin M. Interaction of ezrin with the novel guanine nucleotide exchange factor PLEKHG6 promotes RhoG-dependent apical cytoskeleton rearrangements in epithelial cells. Mol Biol Cell. 2007;18:4780–4793. doi: 10.1091/mbc.E06-12-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hatzoglou A, Ader I, Splingard A, Flanders J, Saade E, Leroy I, et al. Gem associates with Ezrin and acts via the Rho-GAP protein Gmip to downregulate the Rho pathway. Mol Biol Cell. 2007;18:1242–1252. doi: 10.1091/mbc.E06-06-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akhtar N, Hotchin NA. Rac1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell. 2001;12:847–862. doi: 10.1091/mbc.12.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamei T, Matozaki T, Sakisaka T, Kodama A, Yokoyama S, Peng YF, et al. Coendocytosis of cadherin and c-Met coupled to disruption of cell-cell adhesion in MDCK cells—regulation by Rho, Rac and Rab small G proteins. Oncogene. 1999;18:6776–6784. doi: 10.1038/sj.onc.1203114. [DOI] [PubMed] [Google Scholar]
- 57.Jou TS, Nelson WJ. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol. 1998;142:85–100. doi: 10.1083/jcb.142.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krieg J, Hunter T. Identification of the two major epidermal growth factor-induced tyrosine phosphorylation sites in the microvillar core protein ezrin. J Biol Chem. 1992;267:19258–19265. [PubMed] [Google Scholar]
- 59.Fazioli F, Wong WT, Ullrich SJ, Sakaguchi K, Appella E, Di FP. The ezrin-like family of tyrosine kinase substrates: receptor-specific pattern of tyrosine phosphorylation and relationship to malignant transformation. Oncogene. 1993;8:1335–1345. [PubMed] [Google Scholar]
- 60.Bretscher A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol. 1989;108:921–930. doi: 10.1083/jcb.108.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crepaldi T, Gautreau A, Comoglio PM, Louvard D, Arpin M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J Cell Biol. 1997;138:423–434. doi: 10.1083/jcb.138.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orian-Rousseau V, Morrison H, Matzke A, Kastilan T, Pace G, Herrlich P, et al. Hepatocyte growth factor-induced Ras activation requires ERM proteins linked to both CD44v6 and F-actin. Mol Biol Cell. 2007;18:76–83. doi: 10.1091/mbc.E06-08-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Srivastava J, Elliott BE, Louvard D, Arpin M. Src-dependent ezrin phosphorylation in adhesion-mediated signaling. Mol Biol Cell. 2005;16:1481–1490. doi: 10.1091/mbc.E04-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark P. Modulation of scatter factor/hepatocyte growth factor activity by cell-substratum adhesion. J Cell Sci. 1994;107:1265–1275. doi: 10.1242/jcs.107.5.1265. [DOI] [PubMed] [Google Scholar]
- 65.Orian-Rousseau V, Chen L, Sleeman JP, Herrlich P, Ponta H. CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 2002;16:3074–3086. doi: 10.1101/gad.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Legg JW, Isacke CM. Identification and functional analysis of the ezrin-binding site in the hyaluronan receptor, CD44. Curr Biol. 1998;8:705–708. doi: 10.1016/s0960-9822(98)70277-5. [DOI] [PubMed] [Google Scholar]
- 67.Orian-Rousseau V, Ponta H. Adhesion proteins meet receptors: a common theme? Adv Cancer Res. 2008;101:63–92. doi: 10.1016/S0065-230X(08)00404-1. [DOI] [PubMed] [Google Scholar]
- 68.Legg JW, Lewis CA, Parsons M, Ng T, Isacke CM. A novel PKC-regulated mechanism controls CD44 ezrin association and directional cell motility. Nat Cell Biol. 2002;4:399–407. doi: 10.1038/ncb797. [DOI] [PubMed] [Google Scholar]
- 69.Penela P, Ribas C, Aymerich I, Eijkelkamp N, Barreiro O, Heijnen CJ, et al. G protein-coupled receptor kinase 2 positively regulates epithelial cell migration. EMBO J. 2008;27:1206–1218. doi: 10.1038/emboj.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kahsai AW, Zhu S, Fenteany G. G protein-coupled receptor kinase 2 activates radixin, regulating membrane protrusion and motility in epithelial cells. Biochim Biophys Acta. 2010;1803:300–310. doi: 10.1016/j.bbamcr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui Y, Wu J, Zong M, Song G, Jia Q, Jiang J, et al. Proteomic profiling in pancreatic cancer with and without lymph node metastasis. Int J Cancer. 2009;124:1614–1621. doi: 10.1002/ijc.24163. [DOI] [PubMed] [Google Scholar]
- 72.Belbin TJ, Singh B, Smith RV, Socci ND, Wreesmann VB, Sanchez-Carbayo M, et al. Molecular profiling of tumor progression in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2005;131:10–18. doi: 10.1001/archotol.131.1.10. [DOI] [PubMed] [Google Scholar]
- 73.Sarrio D, Rodriguez-Pinilla SM, Dotor A, Calero F, Hardisson D, Palacios J. Abnormal ezrin localization is associated with clinicopathological features in invasive breast carcinomas. Breast Cancer Res Treatment. 2006;98:71–79. doi: 10.1007/s10549-005-9133-4. [DOI] [PubMed] [Google Scholar]
- 74.Elliott BE, Meens JA, SenGupta SK, Louvard D, Arpin M. The membrane-cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res. 2005;7:365–373. doi: 10.1186/bcr1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Elliott BE, Qiao H, Louvard D, Arpin M. Co-operative effect of c-Src and ezrin in deregulation of cell-cell contacts and scattering of mammary carcinoma cells. J Cell Biochem. 2004;92:16–28. doi: 10.1002/jcb.20033. [DOI] [PubMed] [Google Scholar]
- 76.Gavert N, Ben-Shmuel A, Lemmon V, Brabletz T, Ben-Ze'ev A. Nuclear factorκB signaling and ezrin are essential for L1-mediated metastasis of colon cancer cells. J Cell Sci. 2010;123:2135–2143. doi: 10.1242/jcs.069542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gavert N, Conacci-Sorrell M, Gast D, Schneider A, Altevogt P, Brabletz T, et al. L1, a novel target of β-catenin signaling, transforms cells and is expressed at the invasive front of colon cancers. J Cell Biol. 2005;168:633–642. doi: 10.1083/jcb.200408051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shattuck DL, Miller JK, Laederich M, Funes M, Petersen H, Carraway KL, III, et al. LRIG1 is a novel negative regulator of the Met receptor and opposes Met and Her2 synergy. Mol Cell Biol. 2007;27:1934–1946. doi: 10.1128/MCB.00757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]