Sex Disparities in Cancer Mortality and Survival (original) (raw)
. Author manuscript; available in PMC: 2012 Aug 1.
Published in final edited form as: Cancer Epidemiol Biomarkers Prev. 2011 Jul 12;20(8):1629–1637. doi: 10.1158/1055-9965.EPI-11-0246
Abstract
BACKGROUND
Previous research has noted higher cancer mortality rates and lower survival among males than females. However, systematic comparisons of these two metrics by sex has been limited.
METHODS
We extracted U.S. vital rates and survival data from the Surveillance, Epidemiology and End Results Database for 36 cancers by sex and age for the period 1977–2006. We compared sex-specific mortality rates and male-to-female mortality rate ratios (MRRs). We also extracted case data which included age and date of diagnosis, sex, primary cancer site, tumor stage and grade, survival time, vital status, and cause of death. Relative cancer-specific hazard ratios (HRs) for death in the 5-year period following diagnosis were estimated with Cox proportional hazards models, adjusted for covariates.
RESULTS
For the vast majority of cancers, age-adjusted mortality rates were higher among males than females with the highest male-to-female MRR for lip (5.51), larynx (5.37), hypopharynx (4.47), esophagus (4.08) and urinary bladder (3.36). Cancer-specific survival was, for most cancers, worse for males than females, but such disparities were drastically less than corresponding MRRs; e.g., lip (HR = 0.93), larynx (1.09), hypopharynx (0.98), esophagus (1.05), and urinary bladder (0.83).
CONCLUSIONS
Male-to-female MRRs differed markedly while cancer survival disparities were much less pronounced. This suggests that sex-related cancer disparities are more strongly related to etiology than prognosis.
IMPACT
Future analytical studies should attempt to understand causes of observed sex disparities in cancer.
Keywords: Sex, Male, Female, SEER program, Neoplasms, Mortality, Epidemiology
Introduction
Sex is known to be an important factor in the pathogenesis, diagnosis, and prognosis of many diseases (1). Cancer is a stark example of such—the risk of malignancy is much higher in males, relative to females, for a majority of cancers at most ages (2). Exposure factors implicated in these sex disparities include hormones (3), body mass index (kg/m2) (4), viral infections (5, 6), carcinogenic susceptibility (7), and health-care access and utilization (8).
Less information on potential sex differences in cancer mortality and cancer survival is available. Prior reports suggest that certain cancers are disproportionate by sex in these metrics (9–14), but no study has explicitly and systematically tested for sex differences in cancer mortality and cancer survival. These are important questions, because disparities in cancer mortality result from the combined effects of incidence and survival; if sex differences exist in cancer mortality and are the result of sex differences in cancer incidence, and not cancer survival, then such evidence may suggest etiologic clues for future analytical studies. If cancer survival is also highly disproportionate between men and women, then reasoning becomes more complex because, in addition to etiologic factors, this metric may suggest sex differences in the natural history of disease, access to medical care, response to treatment, or some combination of these.
In a previous report, we described sex disparities in cancer incidence rates in the United States using Surveillance Epidemiology and End Results (SEER) in an attempt to bring attention to sex as an important consideration in studies of cancer (2). In this complementary manuscript, we now utilize SEER and National Center for Health Statistics (NCHS) data to assess sex differences in cancer mortality and cancer survival.
Methodology
The April 2009 submission of the Surveillance Epidemiology and End Results (SEER) Program (15) mortality database in SEER*Stat v6.6.2 was used to calculate cancer mortality counts and rates per 100,000 person-years (age-adjusted in single years to the 2000 US standard population) for each cancer, stratified by sex for the periods 1977–1986, 1987–1996, 1997–2006, and 1977–2006. The National Vital Statistics System makes this data available through NCHS via SEER*Stat. Investigated cancers were: all malignant cancers, all malignant cancers excluding sex-specific (cervix uteri, corpus uteri, uterus NOS, ovary, vagina, vulva, other female genital organs, prostate, testis, penis, other male genital organs), all malignant cancers excluding sex-specific and breast, and individual cancers according to the SEER Cause of Death Recode (16). Male-to-female mortality rate ratios (MRRs) were calculated for cancers which had at least 10 deaths in each sex by using the male age-adjusted mortality rate as the numerator and the female age-adjusted mortality rate as the denominator. Confidence intervals for the male-to-female MRRs were generated in SEER*Stat (17). Graphs of male-to-female MRRs and sex-specific mortality rates plotted by age at death (ten-year age groups) were produced for each cancer. Each data point (age group) of these plots was required to have at least 25 cases for each sex. Cancers that had the most extreme (>10%) and consistent changes in male-to-female MRR over the three decades studied and a mortality rate of at least 3 per 100,000 in one of the sexes are illustrated and discussed herein (graphs for other cancers can be accessed online as Supplementary Material).
SEER 17 (18) incidence data were extracted for survival analyses. The geographic area and calendar period covered by each of these registries is available online (19). For each cancer, we extracted the variables: patient id, sex, primary site, histologic type, tumor stage, tumor grade, age at diagnosis, year of diagnosis, month of diagnosis, survival time, vital status, and cause of death from a case-listing session. Data were restricted to individuals with a single primary diagnosis of malignant cancer diagnosed during 1973–2006. Cox proportional hazards models were used to estimate the male, relative to female, hazard of cause-specific mortality, defined here as the cause of death being the specific-cancer originally diagnosed and death being within five years of cancer diagnosis. (All-cause mortality analyses for the five years following cancer diagnosis were also conducted, the results of which are provided as supplementary materials.) All analyses were adjusted for age at diagnosis (ten-year age groups to 80+) and stratified (equal coefficients across strata but with baseline hazard unique to each stratum) by year of cancer diagnosis (1973–1979, 1980–1986, 1987–1993, 1994–2000, 2001–2006).
Subsequent analyses adjusted for additional variables of cancer stage and grade were restricted to cancers where such information was available for at least 60% of cases that were included in the age-adjusted model. When models adjusted for age and year of diagnosis were restricted to those with stage and/or stage and grade information, similar estimates were attained, thus the maximal number of participants were retained in each of the models conducted. Log-log plots of survival against analysis time indicated that the proportional hazards assumption was upheld for each of the cancers assessed (data not shown). Data for the cancer peritoneum, omentum and mesentery were not amenable to Cox proportional hazards models due to small numbers.
Data were analyzed using STATA version 10.1 (StataCorp LP, College Station, TX). Graphs were generated using SigmaPlot version 11.0 (Systat Software, Inc., San Jose, CA).
Results
Sex-specific, age-adjusted cancer mortality rates per 100,000 person years and male-to-female MRRs are shown in Table 1 for the periods 1977–1986, 1987–1996, 1997–2006, and 1977–2006. For the vast majority of cancers studied, age-adjusted mortality rates were higher for males than females; over the entire period, the ten cancers with the highest male-to-female MRR were lip (5.51, 95% CI: 5.05–6.03), larynx (5.37, 95% CI: 5.29–5.45), hypopharynx (4.47, 95% CI: 4.30–4.65), esophagus (4.08, 95% CI: 4.05–4.11), urinary bladder (3.36, 95% CI: 3.34–3.39), tonsil (3.28, 95% CI: 3.17–3.39), oropharynx (3.05, 95% CI: 2.95–3.16), floor of mouth (2.89, 95% CI: 2.77–3.03), tongue (2.53, 95% CI: 2.49–2.58), and nasopharynx (2.47, 95% CI: 2.40–2.55). Only three cancers had a higher mortality rate in women than men: peritoneum, omentum and mesentery (MRR=0.39, 95% CI: 0.38–0.41), gallbladder (0.56, 95% CI: 0.55–0.57), and anus, anal canal and anorectum (0.78, 95% CI: 0.75–0.81). Male-to-female MRRs have changed over time for certain cancers; for example, lung and bronchus, larynx, and pancreas cancers had relatively high male-to-female MRRs in the 1977–1986 period which consistently decreased with time over the 30-year period. In contrast, the male-to-female MRRs consistently increased for several sites between 1977 and 2006, including the esophagus, skin excluding basal and squamous, and liver and intrahepatic bile duct.
Table 1.
Sex-Specific Mortality Rates and Male-to-Female Mortality Rate Ratios by Cancer, SEER 9, 1977–2006
| Site | Cancer mortality per 100,000 man/woman years | Male-to-female MRR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1977–1986 | 1987–1996 | 1997–2006 | 1977–1986 | 1987–1996 | 1997–2006 | 1977–2006 | ||||
| male | female | male | female | male | female | |||||
| All Malignant Cancers | 271.93 | 167.12 | 275.03 | 173.52 | 240.33 | 162.06 | 1.63 | 1.58 | 1.48 | 1.56 (1.56–1.56) |
| All Malignant Cancers excluding Sex-specific | 237.63 | 147.72 | 236.63 | 155.67 | 211.38 | 145.57 | 1.61 | 1.52 | 1.45 | 1.52 (1.52–1.52) |
| All Malignant Cancers excluding Sex-specific and Breast | 237.31 | 115.48 | 236.30 | 123.81 | 211.06 | 119.89 | 2.05 | 1.91 | 1.76 | 1.89 (1.89–1.89) |
| Lip | 0.15 | 0.02 | 0.08 | 0.01 | 0.04 | 0.01 | 7.54 | 5.11 | 3.88 | 5.51 (5.05–6.03) |
| Tongue | 1.43 | 0.52 | 1.14 | 0.45 | 0.94 | 0.39 | 2.76 | 2.51 | 2.39 | 2.53 (2.49–2.58) |
| Salivary Gland | 0.45 | 0.21 | 0.42 | 0.18 | 0.37 | 0.15 | 2.20 | 2.34 | 2.43 | 2.32 (2.25–2.39) |
| Floor of Mouth | 0.36 | 0.11 | 0.19 | 0.07 | 0.08 | 0.03 | 3.31 | 2.62 | 2.60 | 2.89 (2.77–3.03) |
| Gum and Other Mouth | 1.04 | 0.47 | 0.79 | 0.41 | 0.54 | 0.32 | 2.20 | 1.93 | 1.70 | 1.92 (1.88–1.96) |
| Nasopharynx | 0.47 | 0.19 | 0.42 | 0.17 | 0.33 | 0.13 | 2.52 | 2.48 | 2.44 | 2.47 (2.40–2.55) |
| Tonsil | 0.52 | 0.16 | 0.38 | 0.12 | 0.34 | 0.09 | 3.19 | 3.17 | 3.59 | 3.28 (3.17–3.39) |
| Oropharynx | 0.34 | 0.11 | 0.35 | 0.12 | 0.33 | 0.11 | 3.20 | 2.96 | 3.06 | 3.05 (2.95–3.16) |
| Hypopharynx | 0.54 | 0.11 | 0.36 | 0.08 | 0.21 | 0.05 | 4.69 | 4.44 | 4.44 | 4.47 (4.30–4.65) |
| Esophagus | 6.56 | 1.80 | 7.29 | 1.80 | 7.76 | 1.75 | 3.64 | 4.04 | 4.43 | 4.08 (4.05–4.11) |
| Stomach | 10.34 | 4.90 | 8.39 | 3.93 | 6.02 | 3.04 | 2.11 | 2.14 | 1.98 | 2.07 (2.06–2.08) |
| Small Intestine | 0.48 | 0.32 | 0.51 | 0.33 | 0.47 | 0.31 | 1.49 | 1.54 | 1.50 | 1.51 (1.47–1.55) |
| Colon and Rectum | 33.13 | 23.83 | 29.44 | 20.15 | 23.56 | 16.47 | 1.39 | 1.46 | 1.43 | 1.42 (1.41–1.42) |
| Anus, Anal Canal and Anorectum | 0.10 | 0.13 | 0.14 | 0.18 | 0.15 | 0.20 | 0.75 | 0.81 | 0.77 | 0.78 (0.75–0.81) |
| Liver and Intrahepatic Bile Duct | 4.14 | 2.04 | 5.58 | 2.56 | 7.14 | 3.06 | 2.03 | 2.18 | 2.33 | 2.23 (2.22–2.25) |
| Gallbladder | 0.78 | 1.44 | 0.60 | 1.07 | 0.50 | 0.84 | 0.54 | 0.56 | 0.59 | 0.56 (0.55–0.57) |
| Other Biliary | 1.18 | 0.88 | 0.91 | 0.65 | 0.63 | 0.47 | 1.33 | 1.38 | 1.34 | 1.34 (1.32–1.37) |
| Pancreas | 13.12 | 8.96 | 12.45 | 9.22 | 12.26 | 9.26 | 1.46 | 1.35 | 1.32 | 1.37 (1.36–1.38) |
| Retroperitoneum | 0.24 | 0.16 | 0.14 | 0.10 | 0.10 | 0.07 | 1.53 | 1.35 | 1.38 | 1.42 (1.37–1.48) |
| Peritoneum, Omentum and Mesentery | 0.10 | 0.10 | 0.09 | 0.14 | 0.05 | 0.31 | 1.00 | 0.64 | 0.16 | 0.39 (0.38–0.41) |
| Nose, Nasal Cavity and Middle Ear | 0.35 | 0.17 | 0.27 | 0.15 | 0.22 | 0.12 | 2.06 | 1.82 | 1.87 | 1.91 (1.84–1.97) |
| Larynx | 3.21 | 0.51 | 2.97 | 0.57 | 2.41 | 0.50 | 6.34 | 5.25 | 4.82 | 5.37 (5.29–5.45) |
| Lung and Bronchus | 85.59 | 25.94 | 87.77 | 37.59 | 74.08 | 40.81 | 3.30 | 2.33 | 1.82 | 2.31 (2.31–2.32) |
| Trachea, Mediastinum and Other Respiratory Organs | 0.30 | 0.13 | 0.19 | 0.10 | 0.12 | 0.06 | 2.36 | 2.03 | 1.95 | 2.13 (2.04–2.22) |
| Bones and Joints | 0.73 | 0.43 | 0.58 | 0.37 | 0.55 | 0.35 | 1.68 | 1.59 | 1.55 | 1.60 (1.57–1.63) |
| Soft Tissue including Heart | 1.35 | 1.10 | 1.57 | 1.32 | 1.49 | 1.20 | 1.22 | 1.20 | 1.24 | 1.22 (1.21–1.24) |
| Skin excluding Basal and Squamous | 4.70 | 2.31 | 5.39 | 2.32 | 5.32 | 2.17 | 2.04 | 2.32 | 2.45 | 2.30 (2.28–2.32) |
| Urinary Bladder | 8.95 | 2.62 | 7.92 | 2.37 | 7.59 | 2.27 | 3.41 | 3.35 | 3.34 | 3.36 (3.34–3.39) |
| Kidney and Renal Pelvis | 5.57 | 2.54 | 6.13 | 2.84 | 6.05 | 2.75 | 2.19 | 2.16 | 2.20 | 2.19 (2.17–2.20) |
| Ureter | 0.20 | 0.10 | 0.18 | 0.09 | 0.15 | 0.09 | 1.89 | 1.97 | 1.74 | 1.86 (1.78–1.94) |
| Eye and Orbit | 0.16 | 0.12 | 0.13 | 0.09 | 0.10 | 0.07 | 1.33 | 1.39 | 1.41 | 1.36 (1.30–1.42) |
| Brain and Other Nervous System | 5.45 | 3.64 | 5.84 | 3.94 | 5.45 | 3.64 | 1.50 | 1.48 | 1.50 | 1.49 (1.48–1.50) |
| Endocrine System | 0.78 | 0.83 | 0.77 | 0.76 | 0.80 | 0.76 | 0.94 | 1.01 | 1.05 | 1.01 (0.99–1.02) |
| Lymphoma | 9.07 | 6.12 | 10.95 | 7.19 | 10.28 | 6.64 | 1.48 | 1.52 | 1.55 | 1.52 (1.52–1.53) |
| Myeloma | 4.21 | 2.82 | 4.82 | 3.19 | 4.63 | 3.10 | 1.49 | 1.51 | 1.50 | 1.50 (1.49–1.51) |
| Leukemia | 10.92 | 6.30 | 10.63 | 6.10 | 10.03 | 5.69 | 1.73 | 1.74 | 1.76 | 1.75 (1.74–1.76) |
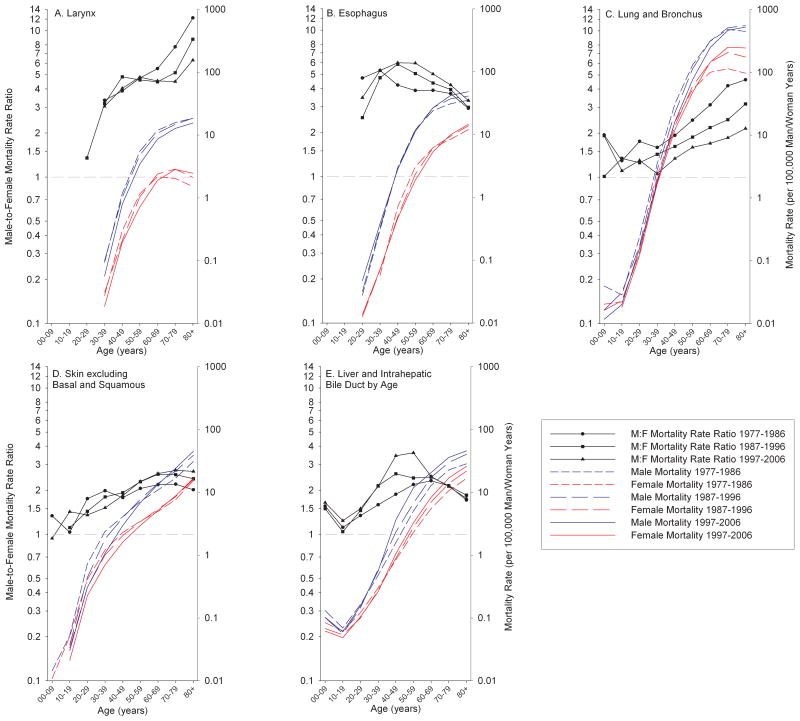
Next, we examined whether sex-specific mortality rates and male-to-female MRRs changed with age from 1977 to 2006 (Figure 1a–e; Supplementary Figures 1–35, can be accessed online). Several patterns emerged. For example, laryngeal cancer mortality (Figure 1a) has been decreasing in both sexes for the majority of ages, but this trend has been more rapid in males. In addition, female laryngeal cancer mortality has been increasing in older age groups (70+ years). Both of these factors have contributed to the decline in male-to-female MRR of laryngeal cancer over the 30-year time period of analysis among those 60+ years of age. Esophageal cancer (Figure 1b) has been trending in the opposite direction with an increasing MRR being observed, particularly between the ages of 40–69 years. This has been caused by a decrease in the female mortality rate, while the equivalent rates for males have remained fairly stable. In the oldest age groups (70+ years), esophageal cancer mortality rates have been increasing in both sexes, but at a faster rate in males, resulting in increased MRRs for these age groups. Lung and bronchus cancer (Figure 1c) has been decreasing in males but has remained fairly stable, and has even increased at older ages, in females during 1977–2006. This has caused the male-to-female MRR to dramatically decrease for all adult age groups. Mortality from skin cancer (Figure 1d) has also been more incident in males than females. Moreover, the MRR for these cancers has been increasing in the age groups 50+ years due to increases in male mortality yet stable female mortality. Mortality rates of liver and intrahepatic bile duct (Figure 1e) follow a similar trend, although the observed increases have been substantially greater for males, relative to females, between the ages 30–59, which has resulted in a large increase in the MRR during the observed 30-year period.
Figure 1.
Male-To-Female Mortality Rate Ratios and Sex-Specific Mortality Rates by Age for Selected Cancers, 1977–2006.
a. Larynx
b. Esophagus
c. Lung and bronchus
d. Skin excluding basal and squamous
e. Liver and intrahepatic bile duct
Next, we examined cancer survival. Cox proportional hazards models, adjusted for age, stage, and grade, were used to test for sex differences in survival in the five years following cancer diagnosis (Table 2). A large number of cancers had higher hazards of death for men (i.e., worse survival) than women including, but not limited to, skin excluding basal and squamous (HR=1.58, 95% CI: 1.52–1.64), endocrine system (HR=1.32, 95% CI: 1.24–1.42), floor of mouth (HR=1.32, 95% CI: 1.07–1.63), anus, anal canal and anorectum (HR=1.21, 95% CI: 1.02–1.43), lymphoma (HR=1.20, 95% CI: 1.18–1.22), and lung and bronchus (HR=1.17, 95% CI: 1.16–1.18). In contrast, two sites were notable for their decreased risk of cause-specific mortality in men relative to women: urinary bladder (HR=0.83, 95% CI: 0.81–0.86) and tongue (HR=0.89, 95% CI: 0.83–0.95). Adjustment for stage and grade, when available, had moderate effects for some cancers, e.g. the HR for tongue cancer went from 1.07 to 0.89. The excess male hazards for the cancers salivary gland and skin excluding basal and squamous were attenuated when adjusted for stage. In contrast, the HRs for the two cancers ureter and anus, anal canal and anorectum were strengthened after adjustment for both stage and grade. Other cancers, such as urinary bladder had a more complicated pattern: higher risks in woman were attenuated yet persisted after adjustment for stage and grade.
Table 2.
Cox Proportional Hazards Regression Models Estimating Male-to-Female Hazard Ratios for Cancer-Specific Death in the Five-Year Period Following Cancer Diagnosis, SEER 17, 1973–2006
| Site | Cox Proportional Hazards Models | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted for Age | Adjusted for Age and Stage | Adjusted for Age, Stage and Grade | |||||||||||||
| N | Deaths | HR | 95% CI | P value | N | Deaths | HR | 95% CI | P value | N | Deaths | HR | 95% CI | P value | |
| Lip | 9,280 | 154 | 0.91 | 0.61 – 1.34 | 0.618 | 8,460 | 131 | 0.89 | 0.58 – 1.36 | 0.593 | 5,538 | 97 | 0.93 | 0.55 – 1.56 | 0.778 |
| Tongue | 20,585 | 4,690 | 1.07 | 1.00 – 1.14 | 0.036 | 19,332 | 4,307 | 0.92 | 0.86 – 0.98 | 0.010 | 15,577 | 3,476 | 0.89 | 0.83 – 0.95 | 0.001 |
| Salivary Gland | 9,661 | 1,419 | 1.52 | 1.37 – 1.70 | <0.001 | 8,960 | 1,281 | 1.27 | 1.13 – 1.42 | <0.001 | - | - | - | - - | - |
| Floor of Mouth | 6,879 | 608 | 1.35 | 1.13 – 1.62 | 0.001 | 6,386 | 553 | 1.29 | 1.07 – 1.57 | 0.008 | 5,069 | 453 | 1.32 | 1.07 – 1.63 | 0.010 |
| Gum and Other Mouth | 12,788 | 1,725 | 1.19 | 1.08 – 1.31 | 0.000 | 11,488 | 1,486 | 1.14 | 1.03 – 1.27 | 0.014 | 8,740 | 1,154 | 1.05 | 0.93 – 1.18 | 0.437 |
| Nasopharynx | 6,671 | 1,814 | 1.17 | 1.05 – 1.29 | 0.003 | 6,170 | 1,664 | 1.11 | 0.99 – 1.23 | 0.068 | 4,509 | 1,251 | 1.10 | 0.97 – 1.25 | 0.125 |
| Tonsil | 11,164 | 1,394 | 1.13 | 1.00 – 1.27 | 0.060 | 10,592 | 1,286 | 1.07 | 0.94 – 1.22 | 0.293 | 8,684 | 1,014 | 1.06 | 0.91 – 1.22 | 0.468 |
| Oropharynx | 2,501 | 246 | 0.96 | 0.73 – 1.26 | 0.754 | 2,291 | 205 | 1.03 | 0.76 – 1.40 | 0.841 | 1,763 | 150 | 1.02 | 0.71 – 1.45 | 0.934 |
| Hypopharynx | 6,924 | 1,081 | 1.00 | 0.87 – 1.15 | 0.982 | 6,621 | 1,024 | 1.01 | 0.87 – 1.17 | 0.894 | 5,325 | 824 | 0.98 | 0.84 – 1.16 | 0.832 |
| Esophagus | 36,962 | 25,493 | 1.08 | 1.05 – 1.11 | <0.001 | 30,217 | 20,644 | 1.05 | 1.01 – 1.08 | 0.006 | 24,065 | 16,527 | 1.05 | 1.01 – 1.09 | 0.011 |
| Stomach | 76,687 | 43,115 | 1.04 | 1.02 – 1.06 | <0.001 | 67,514 | 37,645 | 1.00 | 0.98 – 1.02 | 0.964 | 51,754 | 28,889 | 1.01 | 0.98 – 1.03 | 0.513 |
| Small Intestine | 11,539 | 2,207 | 1.22 | 1.12 – 1.33 | <0.001 | 10,751 | 2,029 | 1.25 | 1.14 – 1.36 | <0.001 | - | - | - | - - | - |
| Colon and Rectum | 414,964 | 138,305 | 1.08 | 1.07 – 1.09 | <0.001 | 391,548 | 127,303 | 1.08 | 1.06 – 1.09 | <0.001 | 320,549 | 100,990 | 1.08 | 1.07 – 1.10 | <0.001 |
| Anus, Anal Canal and Anorectum | 10,297 | 995 | 1.07 | 0.94 – 1.22 | 0.318 | 9,215 | 856 | 1.10 | 0.95 – 1.27 | 0.194 | 6,203 | 598 | 1.21 | 1.02 – 1.43 | 0.031 |
| Liver and Intrahepatic Bile Duct | 41,809 | 25,735 | 1.16 | 1.13 – 1.19 | <0.001 | 33,085 | 19,555 | 1.17 | 1.13 – 1.20 | <0.001 | - | - | - | - - | - |
| Gallbladder | 11,305 | 6,168 | 0.99 | 0.93 – 1.04 | 0.639 | 10,802 | 5,882 | 0.97 | 0.91 – 1.03 | 0.281 | 7,430 | 4,005 | 1.01 | 0.94 – 1.09 | 0.806 |
| Other Biliary | 13,017 | 3,355 | 0.98 | 0.91 – 1.05 | 0.531 | 10,541 | 2,589 | 1.03 | 0.95 – 1.11 | 0.453 | - | - | - | - - | - |
| Pancreas | 92,133 | 74,979 | 1.07 | 1.05 – 1.08 | <0.001 | 78,100 | 63,331 | 1.05 | 1.03 – 1.07 | <0.001 | - | - | - | - - | - |
| Retroperitoneum | 3,916 | 437 | 1.34 | 1.11 – 1.62 | 0.003 | 3,511 | 354 | 1.29 | 1.04 – 1.59 | 0.020 | - | - | - | - - | - |
| Nose, Nasal Cavity and Middle Ear | 5,949 | 1,094 | 1.19 | 1.05 – 1.34 | 0.006 | 4,533 | 756 | 1.20 | 1.03 – 1.39 | 0.016 | - | - | - | - - | - |
| Larynx | 31,755 | 6,197 | 1.00 | 0.94 – 1.07 | 0.999 | 25,041 | 5,263 | 1.04 | 0.97 – 1.12 | 0.217 | 19,611 | 4,275 | 1.09 | 1.00 – 1.17 | 0.037 |
| Lung and Bronchus | 506,173 | 365,361 | 1.19 | 1.19 – 1.20 | <0.001 | 346,725 | 242,451 | 1.17 | 1.16 – 1.18 | <0.001 | - | - | - | - - | - |
| Trachea, Mediastinum and Other Respiratory Organs | 2,105 | 277 | 1.34 | 1.02 – 1.77 | 0.036 | 1,445 | 211 | 1.24 | 0.90 – 1.70 | 0.182 | - | - | - | - - | - |
| Bones and Joints | 8,977 | 2,018 | 1.26 | 1.15 – 1.38 | <0.001 | 8,069 | 1,810 | 1.13 | 1.03 – 1.24 | 0.012 | - | - | - | - - | - |
| Soft Tissue including Heart | 24,690 | 4,934 | 1.08 | 1.02 – 1.15 | 0.005 | 22,605 | 4,426 | 1.10 | 1.03 – 1.16 | 0.003 | - | - | - | - - | - |
| Skin excluding Basal and Squamous | 140,700 | 13,309 | 1.81 | 1.75 – 1.88 | <0.001 | 132,910 | 11,881 | 1.58 | 1.52 – 1.64 | <0.001 | - | - | - | - - | - |
| Urinary Bladder | 143,334 | 23,826 | 0.73 | 0.71 – 0.75 | <0.001 | 137,323 | 22,112 | 0.85 | 0.82 – 0.87 | <0.001 | 124,971 | 19,928 | 0.83 | 0.81 – 0.86 | <0.001 |
| Kidney and Renal Pelvis | 86,116 | 25,294 | 1.12 | 1.09 – 1.15 | <0.001 | 81,740 | 23,326 | 0.96 | 0.93 – 0.99 | 0.003 | - | - | - | - - | - |
| Ureter | 2,588 | 489 | 0.90 | 0.75 – 1.07 | 0.230 | 2,356 | 439 | 1.06 | 0.87 – 1.28 | 0.557 | 2,002 | 344 | 1.17 | 0.94 – 1.45 | 0.168 |
| Eye and Orbit | 7,422 | 482 | 1.09 | 0.91 – 1.31 | 0.331 | 6,494 | 411 | 1.06 | 0.87 – 1.29 | 0.573 | - | - | - | - - | - |
| Brain and Other Nervous System | 63,256 | 34,104 | 1.13 | 1.10 – 1.15 | <0.001 | - | - | - | - - | - | - | - | - | - - | - |
| Endocrine System | 74,540 | 4,043 | 1.86 | 1.75 – 1.99 | <0.001 | 72,112 | 3,704 | 1.32 | 1.24 – 1.42 | <0.001 | - | - | - | - - | - |
| Lymphoma | 178,638 | 48,517 | 1.20 | 1.18 – 1.22 | <0.001 | - | - | - | - - | - | - | - | - | - - | - |
| Myeloma | 47,306 | 23,136 | 1.03 | 1.01 – 1.06 | 0.015 | - | - | - | - - | - | - | - | - | - - | - |
| Leukemia | 104,012 | 41,695 | 1.04 | 1.02 – 1.06 | <0.001 | - | - | - | - - | - | - | - | - | - - | - |
Discussion
In this study we show that cancer mortality was much higher in males relative to females for a majority of cancer types (Table 1 and Figure). Conversely, cancer survival was generally similar between the sexes; even when differences were observed, these sex disparities were relatively modest.
Disparities of cancer mortality have largely paralleled those of cancer incidence, which we have described previously (2). For example, the incidence and mortality rate ratios were largely similar, differing by more than 20% for only four cancer sites (lip: MRR=5.51, IRR=7.16; salivary gland: MRR=2.32, IRR=1.59; skin excluding basal and squamous: MRR=2.30, IRR=1.43; and ureter: MRR=1.86, IRR=2.45). Qualitatively, the patterns of sex-specific mortality rates and male-to-female MRRs by age and stratified by decade (Figure and Supplementary Figures), appeared to be nearly identical to the patterns observed in cancer incidence rates (2).
This supports the idea that sex disparities in cancer mortality arise from the sex differences in cancer incidence. Examples of risk factors that have been implicated in cancer sex disparities include tobacco smoking in lung and bronchus, oral HPV infection in tongue and oropharyngeal (20–22), cosmetic and occupational UV radiation exposure in skin excluding basal and squamous (23, 24), and anal HPV infection in anus, anal canal and anorectum (6, 25). Universal mechanisms that may be causal in observed sex differences in cancer incidence and, thus, mortality include anti-oxidative capacity (26, 27), sex chromosome complement/aneuploidy/aberrations (28, 29), gene expression (30–32), hormones (33, 34), and immunocompetence (35). These issues relating to sex disparities in cancer incidence are discussed in further detail I our previous manuscript (2).
For cancer survival, the largest sex differences occurred for the cancers: skin excluding basal and squamous; endocrine system; floor of mouth; retroperitoneum; salivary gland; small intestine; trachea, mediastinum and other respiratory organs; anus, anal canal and anorectum; lymphoma; nose, nasal cavity and middle ear; lung and bronchus; urinary bladder; and tongue. For all but the last two of these cancers, males had a higher risk of death from cancer. It is feasible that differential environmental exposures and/or physiological processes, such as sex hormones, could explain the observed sex disparities in survival (2, 36, 37).
Alternatively, observed sex differences in survival may be artifactual. In analyses unadjusted for extent of disease, lead time bias could give the false impression of sex disparities in five-year survival rates. For cancers with the largest sex differences in survival, the sex with the poorer survival almost always presented with later stage and higher grade tumors. Additional adjustment for stage and grade substantially attenuated most observed sex differences. However, it is unlikely that categorical metrics of stage and grade fully account for variation in extent of disease, thus residual confounding remains a distinct possibility.
Over-diagnosis through screening could also disproportionately affect sex-specific cancer survival. For example, a large proportion of cancer is thought to be asymptomatic and undiagnosed (38). If asymptomatic cancers tended to be diagnosed more frequently in females, relative to males, females could appear to have better cancer survival (39, 40). In support of this hypothesis, females typically present with earlier-stage, lower-grade, less-aggressive and unifocal cancers, compared with males (39), perhaps because women more readily and more often utilize health resources available to them (37, 41–44). More research on this subject is required to accurately ascertain to what degree this may play a role in sex discrepant cancer survival.
Sex differences in co-morbidity at cancer diagnosis could also skew cancer survival in favor of one sex over the other. Some (45–49), but not all (50–52), studies have suggested that males have more co-morbid conditions at the point of cancer diagnosis than do females. As co-morbidities are independent prognostic indicators, pre-existing chronic conditions may contribute to poorer cancer survival.
Of our results for specific cancers, better survival for lung and bronchus among women is noteworthy. This concurs with previous studies which have addressed this question (40, 53), the results of which have piqued the idea that estrogen receptor β, expressed in lung cancer cells, may be causal to this observation (54, 55), though various other hypotheses have also been suggested (56).
Urinary bladder cancer was unusual in that females had lower five-year survival, compared with males, an observation also made by others (8, 14). Given that US male urinary bladder cancer rates are much higher than female rates (2) and that females often present with later stage and higher grade lesions (8, 15, 57–59), the observed disparity in survival may partly be due to diagnostic misclassification. For example, when presenting with similar symptoms, males may be more readily referred for cystoscopy than women (60).
Cancers of the anus, anal canal and anorectum show the opposite pattern to urinary bladder; these cancers are more common in females, yet males have lower rates of five-year survival. One hypothesis is that men may be more likely than women to have anus, anal canal and anorectum tumors caused by HIV infection (61), and that such tumors may be more aggressive (25).
Cancer of the tongue was unique, in that adjustment for stage and grade changed the HR estimate from indicating a higher risk of death in males to indicating a higher risk of death in females, in the five-years following diagnosis. These unusual observations are difficult to explain, mainly because the etiopathogenesis of this cancer is poorly understood (62, 63).
Strengths of this study include the use of a large, population-based cancer registry database. In addition, SEER has extensive quality control procedures (64, 65). Limitations of this analysis include use of cause of death extracted from death certificates which is known to have problems and imperfections (66). However, inaccuracies are likely to be non-differential by sex. Other limitations include lack of information on co-morbidities and only having adjusted for stage and grade, which may be suboptimal for certain cancers. Lastly, although we utilized the largest US dataset currently available for cancer survival analyses, our results are not perfectly generalizable to the total US population due to the fact that the data are restricted to the 17 cancer registries currently in SEER (18).
In conclusion, this analysis shows that male cancer mortality rates were higher than equivalent female rates for a majority of cancers and these differences largely mirror sex differences in cancer incidence. This analysis also demonstrates modestly, but appreciably, worse survival in men for a number of cancers. Future analytical studies should focus upon the etiological factors responsible for the systematically higher cancer incidence rates among men.
Supplementary Material
1
10
11
12
13
14
15
16
17
18
19
2
20
21
22
23
24
25
26
27
28
29
3
30
31
32
33
34
35
36
37
4
5
6
7
8
9
Acknowledgments
FUNDING
Intramural Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services.
We wish to acknowledge Ms. Sabah Quraishi for her help in figure development.
Footnotes
There are no financial disclosures from any of the authors.
References
- 1.US Institute of Medicine. Exploring the biological contributions to human health: does sex matter? J Womens Health Gend Based Med. 2001;10:433–9. doi: 10.1089/152460901300233902. [DOI] [PubMed] [Google Scholar]
- 2.Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18:1174–82. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shang Y. Hormones and cancer. Cell Res. 2007;17:277–9. doi: 10.1038/cr.2007.26. [DOI] [PubMed] [Google Scholar]
- 4.Whiteman DC, Sadeghi S, Pandeya N, Smithers BM, Gotley DC, Bain CJ, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–80. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 5.D’Souza G, Agrawal Y, Halpern J, Bodison S, Gillison Maura L. Oral Sexual Behaviors Associated with Prevalent Oral Human Papillomavirus Infection. The Journal of Infectious Diseases. 2009;199:1263–9. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewster DH, Bhatti LA. Increasing incidence of squamous cell carcinoma of the anus in Scotland, 1975–2002. Br J Cancer. 2006;95:87–90. doi: 10.1038/sj.bjc.6603175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavitha R, Jyoti DP. Sex Differences in Susceptibility to Carcinogens. Semin Oncol. 2009;36:516–23. doi: 10.1053/j.seminoncol.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 9.Karim-Kos HE, de Vries E, Soerjomataram I, Lemmens V, Siesling S, Coebergh JW. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer. 2008;44:1345–89. doi: 10.1016/j.ejca.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Hebert JR, Daguise VG, Hurley DM, Wilkerson RC, Mosley CM, Adams SA, et al. Mapping cancer mortality-to-incidence ratios to illustrate racial and sex disparities in a high-risk population. Cancer. 2009;115:2539–52. doi: 10.1002/cncr.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levi F, La Vecchia C, Lucchini F, Negri E. Trends in cancer mortality sex ratios in Europe, 1950–1989. World Health Stat Q. 1992;45:117–64. [PubMed] [Google Scholar]
- 12.La Vecchia C, Levi F. Sex differentials in Swiss cancer mortality. Soz Praventivmed. 1988;33:140–3. doi: 10.1007/BF02078420. [DOI] [PubMed] [Google Scholar]
- 13.Foster F. Sex differentials in cancer mortality and morbidity. World Health Stat Q. 1978;31:360–83. [PubMed] [Google Scholar]
- 14.Micheli A, Ciampichini R, Oberaigner W, Ciccolallo L, de Vries E, Izarzugaza I, et al. The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer. 2009;45:1017–27. doi: 10.1016/j.ejca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Surveillance Epidemiology and End Results (SEER) Program. (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Total U.S. (1969–2006) < Single Ages to 85+, Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2006 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2009. Underlying mortality data provided by NCHS (www.cdc.gov/nchs).
- 16.Surveillance Epidemiology and End Results (SEER) SEER Cause of Death Recode 1969+ 2004 Sep 17; [cited 2010 June]; Available from: http://seer.cancer.gov/codrecode/1969+_d09172004/
- 17.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–69. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 18.Surveillance Epidemiology and End Results (SEER) Program. (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 17 Regs Limited-Use + Hurricane Katrina Impacted Louisiana Cases, Nov 2008 Sub (1973–2006 varying) -Linked To County Attributes - Total U.S., 1969–2006 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2009, based on the November 2008 submission.
- 19.Surveillance Epidemiology and End Results (SEER) [cited 2011 April 20th]; Available from: www.seer.cancer.gov.
- 20.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–56. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 21.Smith EM, Ritchie JM, Summersgill KF, Klussmann JP, Lee JH, Wang D, et al. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer. 2004;108:766–72. doi: 10.1002/ijc.11633. [DOI] [PubMed] [Google Scholar]
- 22.Rosenquist K, Wennerberg J, Schildt EB, Bladstrom A, Goran Hansson B, Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125:1327–36. doi: 10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- 23.Miles A, Waller J, Hiom S, Swanston D. SunSmart? Skin cancer knowledge and preventive behaviour in a British population representative sample. Health Educ Res. 2005;20:579–85. doi: 10.1093/her/cyh010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis L, Ritchie J, VanBeek M. Tanning attitudes and beliefs among sorority and fraternity students. Presented at the sixth World Congress on Melanoma; Vancouver, BC. 2005. Abstract BE3–5. [Google Scholar]
- 25.Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer. 2004;101:281–8. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 26.Higdon JV, Frei B. Is there a gender difference in the effect of antioxidants on cancer risk? Br J Nutr. 2005;94:139–40. doi: 10.1079/bjn20051471. [DOI] [PubMed] [Google Scholar]
- 27.Proteggente AR, England TG, Rehman A, Rice-Evans CA, Halliwell B. Gender differences in steady-state levels of oxidative damage to DNA in healthy individuals. Free Radic Res. 2002;36:157–62. doi: 10.1080/10715760290006475. [DOI] [PubMed] [Google Scholar]
- 28.Spatz A, Borg C, Feunteun J. X-chromosome genetics and human cancer. Nat Rev Cancer. 2004;4:617–29. doi: 10.1038/nrc1413. [DOI] [PubMed] [Google Scholar]
- 29.Bottarelli L, Azzoni C, Necchi F, Lagrasta C, Tamburini E, D’Adda T, et al. Sex chromosome alterations associate with tumor progression in sporadic colorectal carcinomas. Clin Cancer Res. 2007;13:4365–70. doi: 10.1158/1078-0432.CCR-06-2736. [DOI] [PubMed] [Google Scholar]
- 30.Ellegren H, Parsch J. The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet. 2007;8:689–98. doi: 10.1038/nrg2167. [DOI] [PubMed] [Google Scholar]
- 31.Rinn JL, Snyder M. Sexual dimorphism in mammalian gene expression. Trends Genet. 2005;21:298–305. doi: 10.1016/j.tig.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9:911–22. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennelly R, Kavanagh DO, Hogan AM, Winter DC. Oestrogen and the colon: potential mechanisms for cancer prevention. Lancet Oncol. 2008;9:385–91. doi: 10.1016/S1470-2045(08)70100-1. [DOI] [PubMed] [Google Scholar]
- 34.Chandanos E, Lagergren J. Oestrogen and the enigmatic male predominance of gastric cancer. Eur J Cancer. 2008;44:2397–403. doi: 10.1016/j.ejca.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 35.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11:411–23. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- 36.Austad SN. Why women live longer than men: sex differences in longevity. Gend Med. 2006;3:79–92. doi: 10.1016/s1550-8579(06)80198-1. [DOI] [PubMed] [Google Scholar]
- 37.Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res. 2008;20:91–102. doi: 10.1007/bf03324754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welch HG, Black WC. Overdiagnosis in Cancer. J Natl Cancer Inst. 2010;102:605–13. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 39.Beisland C, Medby PC, Beisland HO. Renal cell carcinoma: gender difference in incidental detection and cancer-specific survival. Scand J Urol Nephrol. 2002;36:414–8. doi: 10.1080/003655902762467558. [DOI] [PubMed] [Google Scholar]
- 40.Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol. 2002;13:1087–93. doi: 10.1093/annonc/mdf187. [DOI] [PubMed] [Google Scholar]
- 41.National Center for Health Statistics. With Chartbook on Trends in the Health of Americans. Hyattsville, MD: 2009. Health, United States, 2009. [Google Scholar]
- 42.Wingard DL. The sex differential in morbidity, mortality, and lifestyle. Annu Rev Public Health. 1984;5:433–58. doi: 10.1146/annurev.pu.05.050184.002245. [DOI] [PubMed] [Google Scholar]
- 43.Galdas PM, Cheater F, Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs. 2005;49:616–23. doi: 10.1111/j.1365-2648.2004.03331.x. [DOI] [PubMed] [Google Scholar]
- 44.Hamidi M, Moody JS, Kozak KR. Refusal of Radiation Therapy and Its Associated Impact on Survival. Am J Clin Oncol. 2010 doi: 10.1097/COC.0b013e3181d270ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dent OF, Chapuis PH, Renwick AA, Bokey EL. The importance of tumor stage and relative survival analysis for the association between sex and survival after resection of colorectal cancer. Ann Surg. 2009;249:402–8. doi: 10.1097/SLA.0b013e31819a0469. [DOI] [PubMed] [Google Scholar]
- 46.Janssen-Heijnen ML, Houterman S, Lemmens VE, Louwman MW, Maas HA, Coebergh JW. Prognostic impact of increasing age and co-morbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol. 2005;55:231–40. doi: 10.1016/j.critrevonc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Asmis TR, Ding K, Seymour L, Shepherd FA, Leighl NB, Winton TL, et al. Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol. 2008;26:54–9. doi: 10.1200/JCO.2007.12.8322. [DOI] [PubMed] [Google Scholar]
- 48.Kuijpens JL, Janssen-Heijnen ML, Lemmens VE, Haak HR, Heijckmann AC, Coebergh JW. Comorbidity in newly diagnosed thyroid cancer patients: a population-based study on prevalence and the impact on treatment and survival. Clin Endocrinol (Oxf) 2006;64:450–5. doi: 10.1111/j.1365-2265.2006.02492.x. [DOI] [PubMed] [Google Scholar]
- 49.Koppert LB, Janssen-Heijnen ML, Louwman MW, Lemmens VE, Wijnhoven BP, Tilanus HW, et al. Comparison of comorbidity prevalence in oesophageal and gastric carcinoma patients: a population-based study. Eur J Gastroenterol Hepatol. 2004;16:681–8. doi: 10.1097/01.meg.0000108331.52416.f1. [DOI] [PubMed] [Google Scholar]
- 50.Ogle KS, Swanson GM, Woods N, Azzouz F. Cancer and comorbidity: redefining chronic diseases. Cancer. 2000;88:653–63. doi: 10.1002/(sici)1097-0142(20000201)88:3<653::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 51.Yancik R, Wesley MN, Ries LA, Havlik RJ, Long S, Edwards BK, et al. Comorbidity and age as predictors of risk for early mortality of male and female colon carcinoma patients: a population-based study. Cancer. 1998;82:2123–34. [PubMed] [Google Scholar]
- 52.Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. In lung cancer patients, age, race-ethnicity, gender and smoking predict adverse comorbidity, which in turn predicts treatment and survival. J Clin Epidemiol. 2004;57:597–609. doi: 10.1016/j.jclinepi.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 53.Yesner R. Small cell lung cancer: sex and survival. Arch Pathol Lab Med. 2007;131:1631–3. doi: 10.5858/2007-131-1631-SCLCSA. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz AG, Prysak GM, Murphy V, Lonardo F, Pass H, Schwartz J, et al. Nuclear estrogen receptor beta in lung cancer: expression and survival differences by sex. Clin Cancer Res. 2005;11:7280–7. doi: 10.1158/1078-0432.CCR-05-0498. [DOI] [PubMed] [Google Scholar]
- 55.Skov BG, Fischer BM, Pappot H. Oestrogen receptor beta over expression in males with non-small cell lung cancer is associated with better survival. Lung Cancer. 2008;59:88–94. doi: 10.1016/j.lungcan.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 56.Planchard D, Loriot Y, Goubar A, Commo F, Soria JC. Differential expression of biomarkers in men and women. Semin Oncol. 2009;36:553–65. doi: 10.1053/j.seminoncol.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Kiemeney LA, Coebergh JW, Koper NP, van der Heijden LH, Pauwels RP, Schapers RF, et al. Bladder cancer incidence and survival in the south-eastern part of The Netherlands, 1975–1989. Eur J Cancer. 1994;30A:1134–7. doi: 10.1016/0959-8049(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 58.Mungan NA, Kiemeney LA, van Dijck JA, van der Poel HG, Witjes JA. Gender differences in stage distribution of bladder cancer. Urology. 2000;55:368–71. doi: 10.1016/s0090-4295(99)00481-1. [DOI] [PubMed] [Google Scholar]
- 59.Fleshner NE, Herr HW, Stewart AK, Murphy GP, Mettlin C, Menck HR. The National Cancer Data Base report on bladder carcinoma. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1996;78:1505–13. doi: 10.1002/(sici)1097-0142(19961001)78:7<1505::aid-cncr19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 60.Horstmann M, Witthuhn R, Falk M, Stenzl A. Gender-specific differences in bladder cancer: a retrospective analysis. Gend Med. 2008;5:385–94. doi: 10.1016/j.genm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 61.Critchlow CW, Surawicz CM, Holmes KK, Kuypers J, Daling JR, Hawes SE, et al. Prospective study of high grade anal squamous intraepithelial neoplasia in a cohort of homosexual men: influence of HIV infection, immunosuppression and human papillomavirus infection. AIDS. 1995;9:1255–62. doi: 10.1097/00002030-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Papageorge MB. Etiology of oral cancer in the young patient: is tongue cancer becoming the other cancer in women? Oral Maxillofac Surg Clin North Am. 2007;19:163–71. v. doi: 10.1016/j.coms.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20–44 years. Cancer. 2005;103:1843–9. doi: 10.1002/cncr.20998. [DOI] [PubMed] [Google Scholar]
- 64.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76:2343–50. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 65.Nathan H, Pawlik TM. Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol. 2008;15:415–23. doi: 10.1245/s10434-007-9658-3. [DOI] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention. Vital Statistics of the United States: 1999 Mortality Technical Appendix. [cited 2010 July 7th]; Available from: http://www.cdc.gov/nchs/data/statab/techap99.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
10
11
12
13
14
15
16
17
18
19
2
20
21
22
23
24
25
26
27
28
29
3
30
31
32
33
34
35
36
37
4
5
6
7
8
9