A new paramagnetic intermediate formed during the reaction of nitrite with deoxyhemoglobin (original) (raw)
. Author manuscript; available in PMC: 2012 Aug 24.
Published in final edited form as: J Am Chem Soc. 2011 Aug 2;133(33):13010–13022. doi: 10.1021/ja1115088
Abstract
The reduction of nitrite by deoxygenated hemoglobin chains has been implicated in red cell induced vasodilation, although the mechanism for this process has not been established. We have previously demonstrated that the reaction of nitrite with deoxyhemoglobin produces a hybrid intermediate with properties of Hb(II)NO+ and Hb(III)NO that builds up during the reaction retaining potential NO bioactivity. To explain the unexpected stability of this intermediate, which prevents NO release from the Hb(III)NO component, we had implicated the transfer of an electron from the β-93 thiol to NO+ producing •SHb(II)NO. In order to determine if this species is formed and to characterize its properties, we have investigated the electron paramagnetic resonance (EPR) changes taking place during the nitrite reaction. The EPR effects of blocking the thiol group with N-ethyl-maleimide and using carboxypeptidase-A to stabilize the R-quaternary conformation have demonstrated that •SHb(II)NO is formed and that it has the EPR spectrum expected for NO bound to the heme in the β-chain plus that of a thiyl radical. This new NO-related paramagnetic species is in equilibrium with the hybrid intermediate “Hb(II)NO+ ↔ Hb(III)NO” thereby further inhibiting the release of NO from Hb(III)NO. The formation of an NO-related paramagnetic species other than the tightly bound NO in Hb(II)NO was also confirmed by a decrease in the EPR signal by −20°C incubation, which shifts the equilibrium back to the “Hb(II)NO+ ↔ Hb(III)NO” intermediate. This previously unrecognized NO hemoglobin species explains the stability of the intermediates and the buildup of a pool of potentially bioactive NO during nitrite reduction. It also provides a pathway for the formation of β-93 cysteine S-nitrosylated hemoglobin [SNOHb:S-nitrosohemoglobin], which has been shown to induce vasodilation, by a rapid radical-radical reaction of any free NO with the thiyl radical of this new paramagnetic intermediate.
Introduction
Nitric oxide (NO), also known as the endothelium-derived relaxing factor (EDRF), is an important signaling molecule involved in cardiovascular homeostasis1–3. Endogenous NO is derived from both enzymatic and nonenzymatic sources in and near the vasculature. The primary enzymatic source of NO synthesis in the vasculature is from endothelial nitric oxide synthase (eNOS), which catalyzes an oxygen dependent oxidation of L-arginine to generate NO and L-citrulline4. Once formed, NO can diffuse into the vascular smooth muscle cells, activate guanylyl cyclase and result in vasodilation5. However, NO’s bioactivity is readily quenched within the circulation (NO lifetime <0.1 sec in blood)6 in large part due to reactions of NO with RBCs. In RBCs, NO reacts with oxygenated hemoglobin [oxyHb, Hb(II)O2] to form methemoglobin [metHb, Hb(III)] and nitrate, whereas it binds to deoxygenated hemoglobin [deoxyHb, Hb(II)] to form iron-nitrosyl hemoglobin [Hb(II)NO]7,8. These reactions eliminate and trap NO, respectively, thereby limiting the amount of NO available for vasodilation.
A role for bypassing the difficulty associated with the reactions of NO with oxyHb and deoxyHb and providing a mechanism whereby the RBC plays a role in delivering NO to the vasculature was originally proposed by Stamler and collaborators9. They proposed the transfer of NO when hemoglobin is oxygenated from the heme to the β-93 cysteine producing S-nitrosylated hemoglobin [SNOHb]. Once reacted with the thiol the NO is not scavenged by the heme and can be transferred to other thiols by a transnitrosylation reaction. It was subsequently demonstrated10 that during deoxygenation the NO is transferred to a membrane thiol group9 providing a pathway for transport out of the RBC. A number of studies have suggested that this SNOHb hypothesis plays a major role in hypoxic vasodilation that regulates the flow of blood through the microcirculation9–13. S-nitrosothiols, which can originate in part from SNOHb have been linked to many cellular functions11,12. The importance of nitrosothiols has recently been corroborated by studies involving an S-nitrosoglutathione reductase which regulates the level of S-nitrosothiols13.
Although the importance of S-nitrosothiols is established, the significance of Hb S-nitrosylation is controversial. The transfer of NO from the heme to the thiol requires an oxidation process14 and it is not clear how this occurs. Furthermore, the detailed mechanism for the transfer of this pool of SNO’s to the vasculature has not been fully explained. An alternative mechanism proposed for the transport of NO bioactivity by the RBC involves the reduction of nitrite back to NO15,16. In plasma, nanomolar levels of nitrite are present that are thought to at least partially have formed due to oxidation of NO released from the endothelium17–23. The reduction of this nitrite back to NO by deoxyHb has been proposed as a mechanism for maintaining NO bioactivity and a source for hypoxic vasodilation under various physiological and pathological conditions16,24,25. However, the export of NO bioactivity from RBCs requires that the NO formed during nitrite reduction not be immediately scavenged by Hb.
One mechanism to accomplish this involves the formation of SNOHb26,27, which produces a pool of NO that does not react with the heme iron. Basu et al28 recently proposed an alternative mechanism where nitrite binds to the oxidized hemoglobin (metHb) formed during the reduction of nitrite and that the reaction of the NO formed with this complex produces N2O3 that can diffuse out of the cell and/or react with thiols. The difficulty with this mechanism is that it uses the final products formed by the reaction (metHb and NO) and requires that the NO produced react with the low levels of nitrite reacted metHb instead of the readily available oxyHb and deoxyHb. We have recently proposed a mechanism where the nitrite reaction enhances the hypoxic release of ATP, which can stimulate eNOS, thereby producing NO29. The release of ATP provides a potential pathways for RBC nitrite induced vasodilation that does not involve the release of NO from RBCs.
We have also previously proposed the formation of an NO-containing intermediate, characterized as a hybridized species with properties of Hb(II)NO+ and Hb(III)NO, as a potential source of bioactive NO that can accumulate in RBCs30. This intermediate was found to be unexpectedly stable with ~ 25% of the nitrite retained in this form 60 min after the reaction of nitrite with deoxyHb at a 4:1 heme:nitrite molar ratio31. To help understand this stability in the presence of an excess of hemoglobin, the potential involvement of the β-93 thiol group was suggested27. To further investigate the potential role of the β-93 thiol group, we have compared the nitrite reaction with deoxyHb under hypoxic conditions with and without N-ethyl-maleimide (NEM), a thiol group blocking agent. A quantitative comparison of both reactions by EPR indicate that in addition to the hybridized species with properties of Hb(II)NO+ and Hb(III)NO, an additional paramagnetic intermediate with EPR properties of both Hb(II)NO and a thiyl radical (•SHb(II)NO) is detected. Spectral analysis indicates that this additional intermediate is responsible for the overall stability of the hybridized intermediate regulating the dissociation of NO from the Hb(III)NO component.
Combining the current data, which provides a mechanism for the formation of a pool of bioactive NO in the RBC, with recent data showing that nitrite reacted hemoglobin has a high affinity for the membrane29 suggests a potential mechanism, whereby, the release of NO from nitrite reacted hemoglobin on the membrane can be released from the RBC. This hypothesis is supported by a recent study that demonstrated the enhanced release of NO from hemoglobin when nitrite reacted Hb binds to the membrane32. Further studies are required to determine if this actually provides a pathway for the release of NO from RBCs.
Materials and Methods
Reagents
All reagents were obtained from Sigma-Aldrich unless otherwise mentioned.
Solution Preparations
All hemoglobin solutions were prepared in 50mM NaCl and 4mM phosphate buffer (PBS), pH 7.4.
DeoxyHb Preparation
Human hemoglobin was purified from fresh hemolysate by gel filtration using a Sephadex G-100 column equilibrated with PBS, pH 7.4 at 4°C. A 1mM hemoglobin solution was deoxygenated in an anaerobic Coy glove box until the visible spectrum of Hb corresponded to that of deoxygenated Hb. A 0.1 cm path length cuvette was used for spectroscopic measurements of the 1mM Hb solutions.
Semi-Hb preparation
α-CO and β-CO semi-Hbs (dimers of the form α(hemeCO)β(apo) and α(apo)β(hemeCO), respectively) were prepared as previously described33. The CO semi-Hbs were purified by gel filtration through a G-25 Sephadex column equilibrated with PBS, pH 7.4. The CO was removed by strong illumination of the samples and deoxygenation was performed by passing argon gas through septum sealed cuvettes containing the semi-Hb solutions until the visible spectra of each semi-Hb corresponded to that of deoxygenated Hb.
Nitrite Preparation
A 10mM nitrite solution was prepared by dissolving NaNO2− in 10ml of argon-saturated PBS, pH 7.4.
N-ethylmaleimide (NEM) preparation
A 100mM NEM solution was prepared by dissolving NEM in 10ml of PBS, pH 7.4. NEM was reacted with oxygenated Hb at a 10:1, NEM:heme ratio for 30min. NEM treated oxyHb was then deoxygenated in an anaerobic Coy globe box as previously described.
Preparation of Carboxypeptidase A (CPA) reacted Hb
Hemoglobin was incubated with CPA obtained from Sigma Chemical Co., St. Louis, Missouri for 2 hours at 37°C in 0.1M Tris-HCl buffer, pH 8.0 and then passed through a Sephadex G-100 column to remove the enzyme34. This reaction cleaves the terminal histidine and tyrosine on the β-chains stabilizing the R-state, even when hemoglobin is fully deoxygenated.
Azide preparation
A 100mM azide solution was prepared by dissolving azide in 10ml of argon-saturated PBS, pH 7.4. Azide was reacted with deoxyHb or nitrite reacted deoxyHb at a 1:1, azide:heme ratio for 5min.
Hb(II)NO Preparation
NO gas (2.5%) was purified, by passing through concentrated NaOH (5M). Small amounts of this purified gas were added to a septum sealed 100 µM deoxyHb solution to produce Hb(II)NO as confirmed by visible spectroscopy. The Hb(II)NO was then quickly passed down a Sephadex G-25 column anaerobically to remove unreacted NO. The final concentration of Hb(II)NO was determined by visible spectroscopy using the millimolar extinction coefficient for Hb(II)NO of 11.4 at 544 nm. The Hb(II)NO standard solution (~44µM) was flushed with inert gas, and stored in the freezer at −150°C. α-NO and β-NO semi-Hbs were also prepared in the same manner.
MetHb Preparation
Purified human hemoglobin (~ 1.5mM) was oxidized with a 50mM solution of K3Fe(CN)6 (50µl/ml) for five minutes. K3Fe(CN)6 was removed from the metHb solution by gel filtration using a Sephadex G-25 column equilibrated with PBS, pH 7.4. The final concentration of metHb was determined by visible spectroscopy using the millimolar extinction coefficient for metHb of 4.4 at 631 nm. The metHb standard solution (~ 0.75mM) was stored in the freezer at −150°C.
Nitrite Reduction by DeoxyHb
All experiments were carried out inside an anaerobic Coy glove box. 1mM deoxyHb or deoxyHb pretreated with NEM, CPA or CPA plus NEM was reacted with 0.25mM nitrite in PBS, pH 7.4 for up to 60 minutes at room temperature (22°C).
EPR measurements
EPR spectra were measured using a Bruker EMX-61A spectrometer with an Oxford continuous flow ESR-900 cryogenic unit. An Oxford ITC 502 temperature controller was used to maintain the sample temperature in the cavity at 10K with an Oxford VC41 gas flow controller to regulate the liquid Helium flow through the cryostat. EPR spectra were recorded in the region of 500 – 4500 G with a sweep time of 335.54 s, a time constant of 80.42 ms, 2 mW power and 100 kHz modulation frequency. All EPR samples were stored and measured in 4 mm clear fused quartz EPR tubes (707 SQ250M-WILMAD). All EPR measurements were analyzed with Bruker WINEPR software version 2.22 revision 10.
Determination of Hb(II)NO Standards by EPR
The Hb(II)NO standard solution (see above) was diluted accordingly with deoxygenated 3 × diluted PBS, pH 7.4 to make 0.4 ml Hb(II)NO standards of 44, 33, 22, 11 and 5.5 µM. The 0.4ml aliquots were transferred anaerobically into EPR tubes, and frozen by submerging the tubes into liquid nitrogen. The samples were stored in the freezer at −150°C until recorded. The EPR spectrum of each Hb(II)NO standard was recorded and the region from 3150 – 3550 gauss, was integrated. A standard calibration curve for Hb(II)NO was produced by plotting the concentration of Hb(II)NO versus the double integration obtained for each standard.
Determination of metHb Standards by EPR
The Hb(III) standard solution (see above) was diluted accordingly with deoxygenated 3 × diluted PBS, pH 7.4 to make 0.4 ml Hb(III) standards of 260, 130, 65, and 33 µM. The 0.4 ml aliquots were transferred anaerobically into the EPR tubes, and frozen by submerging the tubes into liquid nitrogen. The samples were stored in the freezer at −150°C until recorded. The EPR spectrum of each metHb standard was recorded and the region from 900 – 1500 gauss, was integrated. A standard calibration curve for Hb(III) was produced by plotting the concentration of Hb(III) versus the double integration obtained for each standard.
Determination of metHb and Heme-NO species formed during the reaction of deoxyHb with nitrite by EPR
0.3 ml aliquots of the reaction mixtures of nitrite with one of the deoxyHb samples (see above) was removed at various time intervals and transferred into EPR tubes and immediately frozen by submerging into liquid nitrogen. The samples were stored in the freezer at −150°C until recorded. The EPR spectrum of each reaction mixture was recorded in the region from 900 – 3550 gauss. From the corrected spectra (see data analysis), the concentration of Hb(II)NO and Hb(III) were determined during the nitrite reaction using the Hb(II)NO and Hb(III) standard curves, respectively.
Low temperature incubation of EPR samples
EPR tubes corresponding to the 1min, 15min and 60min reaction mixtures for non-NEM and NEM treated deoxyHb reacted with nitrite, prepared under anaerobic conditions and frozen in liquid nitrogen were incubated at −20°C for 1, 5, and 10 min inside a temperature controlled cryobath (Neslab) set at −20°C. After each incubation, the EPR spectrum was recorded under the experimental conditions described above.
Data analysis
All EPR data was analyzed with the Bruker WINEPR software (version 2.22 revision 10). Mathematical calculations were carried out in Microsoft Office Excel 2007.
Correction of EPR spectra
During the reduction of nitrite by deoxyHb, both metHb and Hb(II)NO species are detected by EPR. MetHb has signals at both, g=6 and the g=2 regions, while the Hb(II)NO spectrum is limited to the g=2 region. In order to quantify the amount of Hb(II)NO formed during the reaction it was necessary to subtract the contribution of metHb to the spectrum. This was carried out by first determining the metHb concentration in the sample from the g=6 region and subtracting the signal in the g=2 region associated with that concentration of metHb from the spectrum. Spectral subtractions were carried out using the algebra/subtraction function of WINEPR.
Calculated spectral contributions to the EPR spectra of each reaction mixture
The spectral contributions to the EPR spectra recorded at various time intervals for the reactions of nitrite with deoxyHb in the absence and presence of NEM, as well as the spectra obtained after −20°C incubations, were calculated by the addition of varying fractions of one or more of five EPR parent spectra. (1) The EPR spectrum of R-state β chain heme-NO (R-state Hb(II)βNO) was obtained using semihemoglobin, which is a dimer with one α globin and a β heme35 where NO is bound to the β heme. (2) The spectrum of fully nitrosylated R-state Hb(II)NO that has NO bound to both α and β heme chains. (3&4) T-state α heme-NO (T-state Hb(II)αNO) and T-state β heme-NO (T-state Hb(II)βNO) spectra obtained from Nihybrids with added IHP to stabilize the T-state36. (5) The spectrum of a thiyl radical species obtained by subtracting the EPR spectra of nitrite reacted CPA-NEM deoxyHb where no radical intermediate can form from nitrite reacted CPA deoxyHb where the intermediates are stabilized.
Calculated DI vs. Experimental DI
As a means to compare whether the spectrum calculated for each reaction mixture represents a good fit for the experimental spectrum observed, the double integration (DI) of the signal in the g=2 region obtained by both methods was compared. The DI value of the signal in the g=2 region for each experimental spectra was obtained using the integration function of WINEPR. For the calculated spectra, the DI values of each of the parent spectra that make up the signal were also calculated using WINEPR and multiplied by the spectral fraction of that component. The summation of the DI values of the individual components that make up each spectrum was compared with its corresponding DI experimental value.
Results and Discussion
At neutral pH the binding of nitrite to Fe(II) hemoglobin is expected to be negligible. However, a number of studies have shown that the nitrite reacts with deoxyHb7,16,30,37,38. This reaction has also been shown to occur in RBCs even at relatively low nitrite concentrations15,29. The final products formed during the reaction of nitrite with deoxygenated hemoglobin subunits are metHb and NO7. In the presence of excess Hb, any NO formed rapidly reacts with deoxyHb and oxyHb37. The high affinity rapid reaction with the Fe(II) of deoxyHb forms Hb(II)NO, which essentially forms an irreversible bond between the Fe(II) and the NO. The reaction with oxyHb oxidizes the heme forming nitrate with no bioactivity.
Our studies indicate that in the presence of an excess of Hb and in the absence of oxygen, within 60 min all of the nitrite was consumed when 250µM nitrite reacted with 1mM deoxyHb. Most of this nitrite that reacts with deoxyHb remains associated with the hemoglobin. However, only about 25% of the total nitrite is released as NO and is subsequently reacted with deoxyHb to form Hb(II)NO. About 50% of the total nitrite is associated with the hemoglobin in this unreduced form, since a strong reducing agent such as ascorbate is required to convert it to NO and it does not react with sulfanilamide, a nitrite complexating agent in acidic media. The slow nitrite reaction observed is consistent with a low affinity of nitrite for the deoxy-heme. However, the initial binding must trigger a protein rearrangement, presumably involving hydrogen bonding of the nitrite in the heme pocket that enhances the affinity. The remaining 25% of the nitrite is retained as a hybridized species with properties of Hb(II)NO+ and Hb(III)NO31. This hybridized complex releases NO when azide binds to the Hb(III)NO component and is stable in the presence of excess hemoglobin. The potential added stability of this hybridized intermediate associated with the transfer of an electron from the NO+ to the β-93 thiol group of Hb was proposed based on studies directed at the formation of SNOHb during the nitrite reaction27,39. By retaining nitrite in the reduced form this complex has potential bioactivity.
Blocking the β-93 thiol of Hb increases the rate for the formation of Hb(II)NO
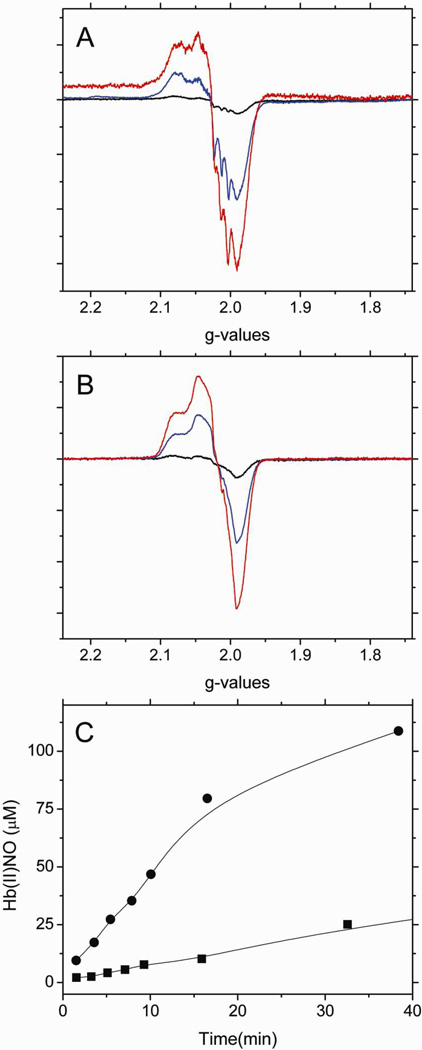
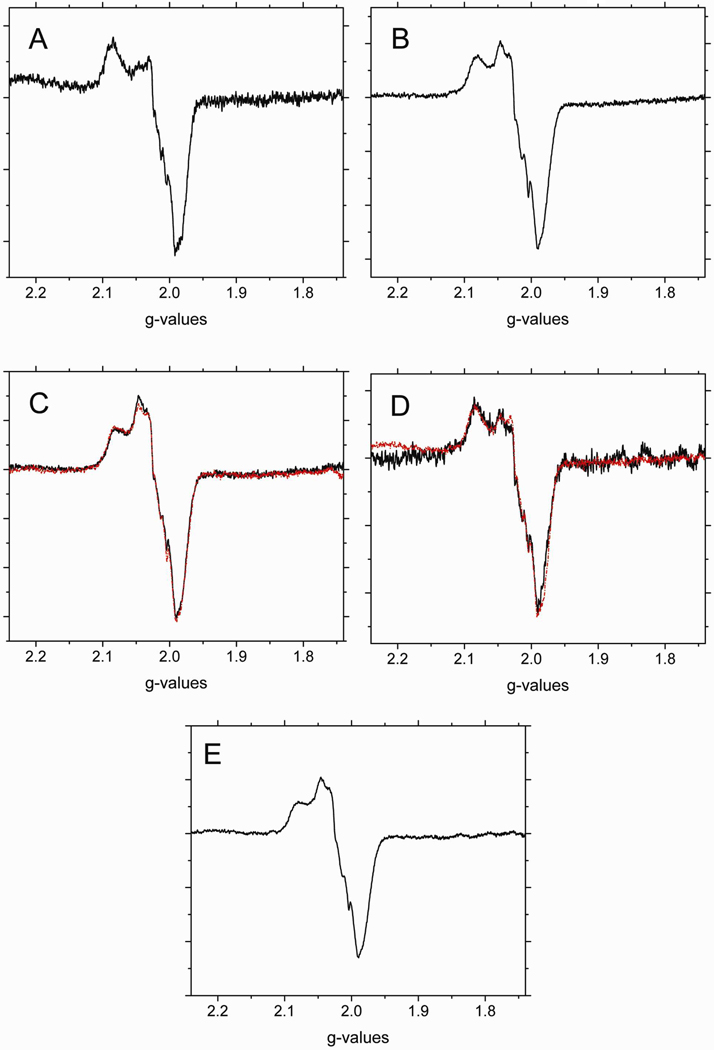
To further investigate the contribution of the β-93 thiol group to the stability of the intermediates, the effect of blocking the thiol group with NEM was investigated by EPR. Figure 1A and 1B respectively show the EPR spectra of the heme-NO complex(es) formed when deoxyHb and NEM treated deoxyHb react with nitrite, at various time intervals. Figure 1C is a comparison of the time dependent formation of Hb(II)NO determined by double integration of its EPR signal in the g=2 region for non-NEM and NEM treated deoxyHb reacted with nitrite. As indicated in Figure 1C, the formation of the Hb(II)NO spectrum is appreciably faster when the thiol group of Hb is blocked by NEM. The dissociation of NO from the hybridized intermediate “Hb(II)NO+ ↔ Hb(III)NO” required to form Hb(II)NO must, therefore be faster when NEM reacts with the thiol group.
Figure 1.
The X-band EPR spectra of Hb(II)NO formed as a function of reaction time when (A) 1mM deoxyHb or (B) 1mM NEM treated deoxyHb are both reacted with 0.25 mM nitrite under anaerobic conditions. (black: 1min; blue: 15min; red: 60min). (C) Total Hb(II)NO concentration as a function of reaction time for non-NEM (■) and NEM treated deoxyHb (●) reacted with nitrite. EPR spectra were recorded at 10 K.
The faster rate for NEM reacted hemoglobin can be attributed to the fact that NEM stabilizes the R-state of Hb40, which has been shown to increase the rate for nitrite reduction15,37. The stabilization of the R-state of Hb is indicated by the elimination of the T-state α-chain hyperfine lines41 of the Hb(II)NO EPR spectra seen in Figure 1A when compared to the EPR spectra of NEM treated deoxyHb reacted with nitrite seen in Figure 1B. Even though the nitrite reduction is taking place predominantly on the β-chains that have the active thiol group, the formation of α-chain Hb(II)NO is attributed to the released NO that reacts with both α and β chains (Eq 1).
| αFe(II)βFe(II)+NO2−→αFe(II)βFe(II)−NO2−→αFe(II)βFe(III)+NO→αFe(II)NOβFe(III) | Eq.1 |
|---|
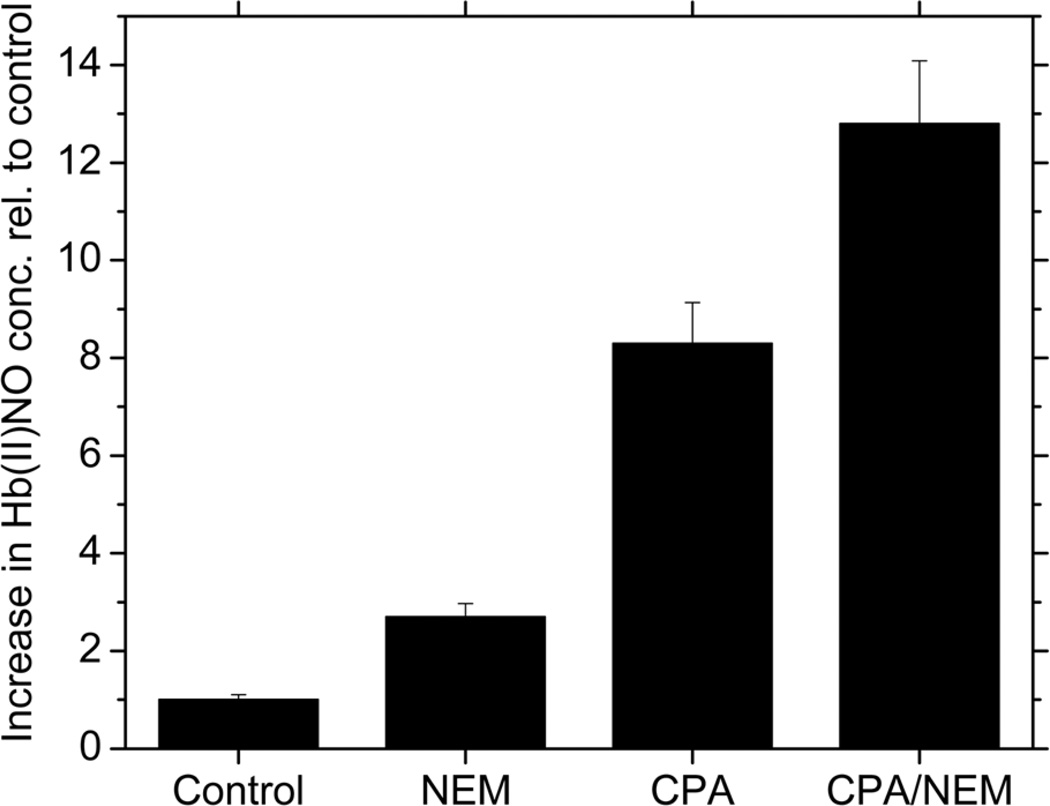
In addition to the conformational change, we wanted to determine if there is a direct effect due to the elimination of the free β-93 thiol group. In order to delineate a potential role of the β-93 thiol group in stabilizing the intermediate(s), it was necessary to convert T-state deoxyHb into an R-state deoxyHb known to have a higher rate for nitrite reduction. For this purpose, Hb was reacted with CPA. CPA cleaves the terminal tyrosine and histidine residues of the β-chain eliminating the crosslinks that stabilize the T-state of Hb and produces R-state Hb even when the hemoglobin is fully deoxygenated34,42. By investigating the effect of NEM blockage of the thiol groups for CPA treated hemoglobin, the effect of the free thiol on the nitrite reaction of R-state hemoglobin was determined. As shown in Figure 2, NEM increases the formation of Hb(II)NO by 2.7 times when compared to the control (untreated deoxyHb). CPA treatment of deoxyHb further increases Hb(II)NO formation to 8.3 times that of the control. However, even for CPA treated Hb which is already in the R-state, NEM further increases the production of Hb(II)NO to 12.8 times higher when compared to the control. Although structures of different R-states have been reported43,44 we have assumed that the increased formation of Hb(II)NO when the β-93 thiol groups on CPA reacted hemoglobin react with NEM is primarily due to blocked thiol groups and not to a shift in the specific R-state of hemoglobin that may further increase the rate for the nitrite reaction. This is consistent with the predicted structural changes associated with CPA and NEM. CPA, removes the terminal tyrosine and histidine of the β-chain, while reactions involving the β-93 cysteine have been reported to displace these residues45–47. This can be contrasted with the structure of R2 hemoglobin where the 2 β-chain terminal histidines stack against one another43.
Figure 2.
Relative increase in Hb(II)NO concentration relative to control for deoxyHb (control), NEM treated, CPA treated and CPA and NEM treated deoxyHb reacted with nitrite for 60min. (Heme:nitrite ratio is 4:1).
A new paramagnetic species that is not Hb(II)NO and requires the β-93 thiol
The stabilization of the hybridized intermediate(s) by the free thiol group and the resultant decrease in the rate for NO release suggests the involvement of the β-93 thiol group. Since this thiol group is ~9 Å from the NO in the R-state and 13Å from the NO in the T-state48, it most likely involves a distinct complex that is in equilibrium with the hybridized intermediate “Hb(II)NO+ ↔ Hb(III)NO”.
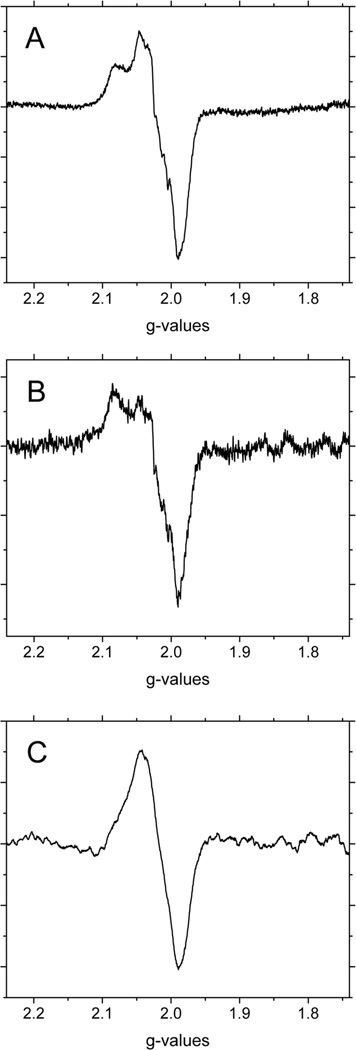
In our previous studies on the autoxidation of hemoglobin we have used sub-zero incubation studies to study the intermediates formed during the reaction49,50. This is based on the concept that in the temperature range from below freezing until about −65°C reactions involving the access of ligands into the heme pocket are expected to be very slow. However, subtle conformational changes necessary for the redistribution of species can take place, which will be driven by the decrease in temperature that favors the more stable species. Based on these earlier studies, we investigated the effect of −20°C incubations on the reaction products present during the nitrite reaction. Figure 3 shows the changes in signal intensity in the g=2 region of the EPR spectra produced after 60 min of the nitrite reaction with A) NEM and B) non-NEM treated deoxyHb incubated at −20°C for 10 min. A small 12% decrease in signal intensity that is considered within experimental error is observed for NEM treated deoxyHb reacted with nitrite after low temperature incubation. However, for non-NEM treated deoxyHb reacted with nitrite, there is a much more significant 32% decrease in the intensity of this signal after low temperature incubation, which is generally attributed to Hb(II)NO. Hb(II)NO is known to be a very stable complex particularly at low temperatures, in the absence of oxygen, and no change in its intensity is expected to be observed. Instead, the observed decrease in signal intensity for Hb(II)NO with a free thiol group suggests the presence of a less stable intermediate species with an EPR signal. But both Hb(II)NO+ and Hb(III)NO, the components of the hybridized intermediate are EPR silent. In our earlier discussion of the contribution of the thiol group to the stability of the hybridized intermediate, the potential transfer of an electron from the thiol to the nitrosonium cation of this intermediate is suggested, resulting in a new intermediate species, •SHb(II)NO (Eq. 2).
| “HSHb(II)NO+↔HSHb(III)NO” ↕¯•SHb(II)NO+H+ | Eq. 2 |
|---|
Figure 3.
The X-band EPR spectra in the g=2 region for (A) 1mM NEM treated deoxyHb and (B) 1mM deoxyHb both reacted with 0.25 mM nitrite for 60min (-·-) and then incubated at −20°C for 10min (—). EPR spectra were recorded at 10 K.
Although we have both the thiyl radical and the NO on the same molecule in •SHb(II)NO, unlike the NO and the Fe(III) in Hb(III)NO, they are not directly linked and are separated by about 9–13 Å and, therefore, its Hb(II)NO EPR signal may still be detected. For this new intermediate (•SHb(II)NO), the thiyl radical can accept an electron from the heme-NO and reform the hybridized intermediate with no EPR signal. This EPR signal is, therefore, much more labile than that of Hb(II)NO and can decrease in intensity during low temperature incubation. Therefore, the observed decrease in EPR signal during the −20°C incubation can be attributed to an electron transfer process which results in a thermodynamically driven shift in equilibrium between the paramagnetic •SHb(II)NO intermediate, which is perhaps less stable at low temperature, and the diamagnetic hybridized “Hb(II)NO+ ↔ Hb(III)NO” intermediate.
The decrease in the EPR signal can also be explained by the transfer of NO from the paramagnetic intermediate •SHb(II)NO to the thiyl radical producing SNOHb(II). This was previously proposed27 to explain the formation of SNOHb during the nitrite reaction.
The EPR spectrum of the Paramagnetic Intermediate
The destabilization of the EPR signal in the g=2 region during low temperature incubation at −20°C (Figure 3) has established the presence of a new paramagnetic intermediate (•SHb(II)NO) formed during the room temperature nitrite reaction that is not formed with NEM treated deoxyHb. The spectrum due to this intermediate could, however, not be determined from the decrease in signal at low temperature (Figure 3B), because of the presence of T-state hemoglobin and a possible shift in Hb’s quaternary conformation during incubation which alters the EPR spectrum of Hb(II)NO. Therefore, in order to study the EPR spectra of this new paramagnetic intermediate, we compared the EPR spectra of CPA and CPA/NEM treated deoxyHb reacted with nitrite, which eliminate any spectral contribution from the T-state quaternary conformation. This comparison is valid even if CPA and NEM reacted CPA exist in different R-states, since the dominant conformational effects on Hb(II)NO involves the T-state, where rupture of the proximal histidine bond in the α-chain produces a spectrum with 3 sharp nitrogen hyperfine lines. In this way we were able to compare the spectral differences between nitrite reacted R-state deoxyHb with a free thiol group and a blocked thiol group, respectively.
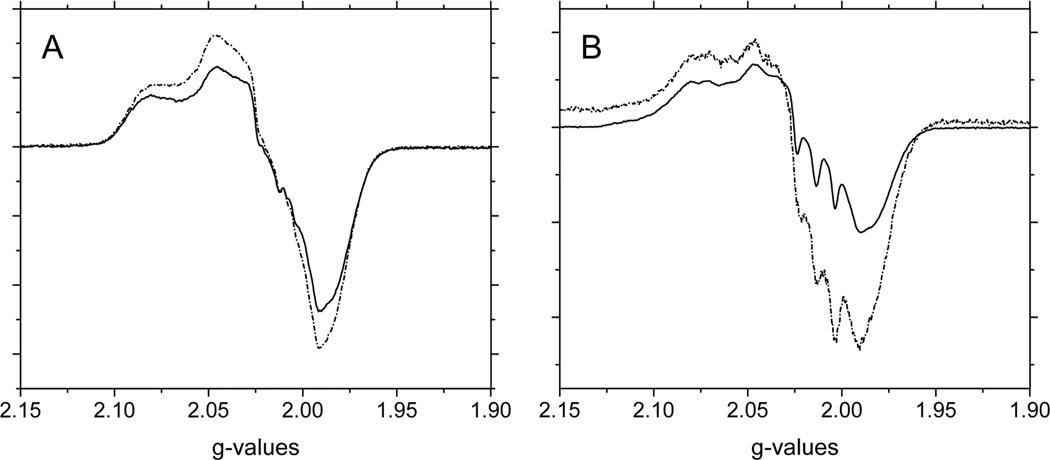
Figure 4 shows the EPR spectra of nitrite reacted A) CPA, and B) CPA/NEM treated deoxyHb. Since both of these treated-hemoglobins are in the R quaternary state, negligible nitrogen hyperfine lines attributed to T-state hemoglobin are observed (compare with Fig. 1A where T-state hyperfine lines are observed). There are, however, subtle differences observed between the two spectra. Since the Hb concentration and the amount of Hb(II)NO formed during the reactions are not the same, the intensities of the signals are not expected to be the same. To compare these spectra the intensities were, therefore, normalized and the CPA/NEM spectrum was subtracted from the CPA spectrum. The spectrum obtained by this subtraction shown in Figure 4C corresponds to an isotropic line in the g=2.015 region with a linewidth of ~ 90 gauss. We attribute this signal to that of a thiyl radical.
Figure 4.
The X-band EPR spectra in the g=2 region for (A) CPA treated deoxyHb and (B) CPA/NEM treated deoxyHb both reacted with nitrite for 60min. (Heme:nitrite ratio is 4:1). (C) The difference EPR spectrum obtained by subtracting the CPA/NEM spectra (4B) from the CPA spectra (4A) after normalization for the intensity differences. EPR spectra were recorded at 10 K.
Other potential protein radicals involving oxygen, nitrogen or carbon would have g-values in the region of 2.003, much closer to the free electron with g=2.0023. For a single crystal of a thiyl radical g=2.03 has been reported51. For sulfur based radicals, increased spin orbital coupling results in higher g-values with a wide range of values51,52 because the g-values are very sensitive to conformations, the environment of the sulfur and the polarity of the region53. For a thiyl radical in a protein environment values in the region of 2.01 have been reported54. The isotropic somewhat broadened signal is expected for a freely rotating thiyl radical within the sulfhydryl group from a cysteine in close proximity to the Fe(II)-heme-NO50. Our g=2.015 value is, therefore, consistent with its designation as a thiyl radical. This analysis indicates that during nitrite reduction by deoxyHb with a free β-thiol group, a new intermediate is formed that involves a thiyl radical.
An alternative designation of the radical species detected could be of a thionitroxide radical that has been reported to be associated with the formation of SNOHb55,56. The formation of the thionitroxide radical would require that the NO/NO+ be transferred from the heme to the thiol. However, the low temperature incubation (Fig. 3) indicates that the intermediate must include in addition to the radical the spectrum of an Hb(II)NO component. This observation is, therefore, consistent with the formation of the putative •SHb(II)NO intermediate, but inconsistent with the formation of the thionitroxide radical.
In order to quantitate the heme-NO contribution to this paramagnetic intermediate, we must differentiate between Hb(II)NO formed by the NO released from the hybridized intermediate and the Hb(II)NO that is part of the radical intermediate. The major difference between the two processes involves the relative contribution of α and β chains. The paramagnetic intermediate is only formed on β chains, while the Hb(II)NO formed by the release of NO from the hybridized intermediate involves both chains (Eq. 1). Since we do not know the relative affinity of this NO with the two types of chains, we used the CPA-NEM reaction to provide a measure for the relative reactivity of α and β chains in the R-quaternary conformation. Although the concentration of Hb(II)NO is different for the CPA and CPA/NEM reaction mixtures, the ratio of β/α chain Hb(II)NO component obtained for the CPA-NEM reaction mixture would indicate the fraction of β-chain Hb(II)NO formed in the CPA sample due to the release of NO from the hybridized intermediate and any excess β-chain Hb(II)NO would be associated with the paramagnetic intermediate (Eq. 3).
| Hb(II)NOrad.−inter.=[Hb(II)βNOCPA]−[Hb(II)αNOCPA]×[Hb(II)βNOCPA−NEM/Hb(II)αNOCPA−NEM] | Eq. 3 |
|---|
Spectral fractions of α-NO semi-Hb (Fig. 5A) and β-NO semi-Hb (Fig.5B) were used to fit the EPR spectra of nitrite reacted CPA and CPA/NEM treated deoxyHb. Although the concentrations of these semihemoglobins were not the same the comparison of the β/α ratio for CPA hemoglobin and NEM reacted CPA hemoglobin is valid. The spectral fits are shown in figures 5C and 5D for the CPA and CPA-NEM reaction mixtures, respectively. For the CPA-NEM sample, the fraction of Hb(II)αNO is 0.35 and the fraction of Hb(II)βNO is 0.1 corresponding to a β/α ratio of 0.29. For the CPA sample the fraction of Hb(II)αNO is 0.25 and for Hb(II)βNO is 0.5. Eventhough the total concentration of Hb(II)NO for the CPA sample is greater than for the CPA-NEM sample (see above), the relative concentrations of Hb(II)αNO and Hb(II)βNO formed by NO released from the hybridized intermediate should be the same for both. Therefore, Eq. 3 can be used to correct the fraction of Hb(II)βNO in the CPA sample for the amount formed by NO released from the hybridized intermediate resulting in a fraction of 0.07 and therefore a 0.43 fraction for Hb(II)βNO associated with the thiyl radical (Hb(II)NOrad.-inter.). By adding a 0.43 fraction of the R-state Hb(II)βNO spectrum (Fig. 5B) plus the thiyl radical spectrum from Fig. 4C, which is present in the CPA sample, we obtain the spectrum of the paramagnetic intermediate shown in Fig. 5E. This figure represents a spectrum of the paramagnetic intermediate that involves both a thiyl radical and an R-state β heme-NO as expected for •SHb(II)NO formed by the transfer of an electron from the β-93 thiol to Hb(II)NO+.
Figure 5.
The X-band EPR spectra in the g=2 region for (A) R-state Hb(II)αNO from semihemoglobin. (B) R-state Hb(II)βNO from semihemoglobin. Spectral fits of nitrite reacted (C) CPA treated deoxyHb and (D) CPA/NEM treated deoxyHb at 60min. (Heme:nitrite ratio is 4:1). (black: observed spectra; red: calculated spectra). [These spectra were fitted using fractions of R-state Hb(II)βNO (Fig. 5A) and R-state Hb(II)αNO (Fig. 5B) and the thiyl radical species (Fig. 4C). (E) The paramagnetic intermediate spectrum calculated from a summation of the thiyl radical spectrum (Fig. 4C) and 0.43 times the R-state Hb(II)βNO spectrum (Fig. 5B) (see text). EPR spectra were recorded at 10 K.
Calculated DI vs. Experimental DI:
CPA calculated DI: 1.19e7, experimental DI: 1.17e7, % error: 2.0%
CPA/NEM calculated DI: 1.81e7, experimental DI: 1.72e7, % error: 5.0%.
Relationship between the hybridized Intermediate and the paramagnetic intermediate
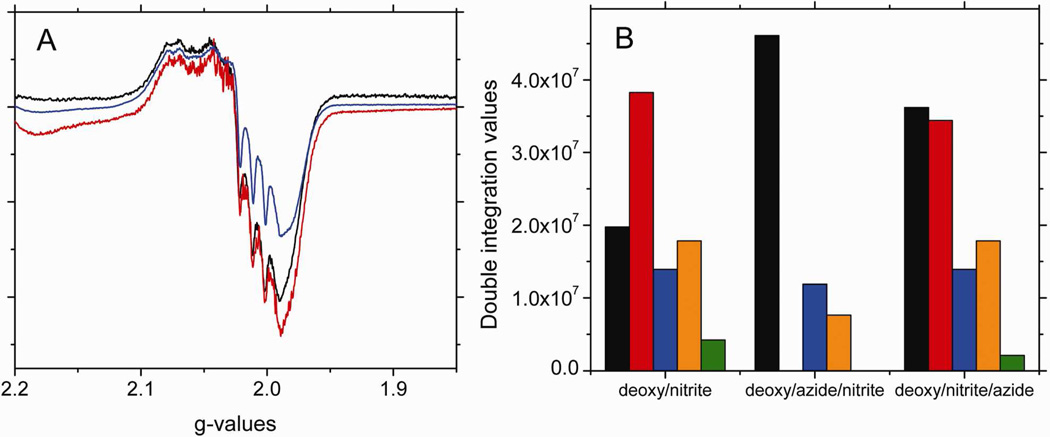
Our analysis of the spectra obtained when nitrite reacts with CPA treated hemoglobin with and without the thiol group blocked by NEM indicates the formation of a thiyl radical. To demonstrate that the thiyl radical containing paramagnetic intermediate •SHb(II)NO is formed from the hybridized intermediate “Hb(II)NO+ ↔ Hb(III)NO” (Eq. 2), we investigated the effect of adding azide to deoxyHb before and after reacting with nitrite. Azide has a high binding affinity to Fe(III)57, and reacts with the Hb(III)NO component of the hybrid, destabilizing the hybridized intermediate and accelerating the release NO to bind to free hemes31. Figure 6A shows the EPR spectra of deoxyHb reacted with nitrite for 60 min, and those of deoxyHb reacted with azide before or after nitrite also for 60min. The EPR intensity in the presence of azide before nitrite addition is significantly decreased. The analysis of the changes in the components of the spectrum for azide added before nitrite (Fig. 6B) show that this decrease is due to the complete elimination of R-state Hb(II)βNO and the thiyl radical, both of which are integral parts of the paramagnetic intermediate. At the same time, there is an increase in R-state Hb(II)NO consistent with the release of NO from the hybridized intermediate. With azide added before the nitrite, it is available to react with the hybridized intermediate as it is formed, and therefore, no paramagnetic intermediate can form. However, when the azide is added after the nitrite has already reacted with the hemoglobin and the paramagnetic intermediate has already formed, the presence of azide for 5 min has only minor effects on the spectrum (Fig. 6A). The analysis of the changes in the components of the spectrum for azide added after nitrite (Fig. 6B), nevertheless indicate a 50% decrease in the thiyl radical and ~50% increase in R-state Hb(II)NO. These changes are consistent with an interaction of azide with the Hb(III)NO component of the hybridized intermediate, which will shift the equilibrium from the radical intermediate to the hybridized intermediate as NO is released from the hybridized intermediate forming Hb(II)NO. The results of adding azide both before and after the nitrite, therefore, support the proposal that the paramagnetic intermediate is formed subsequent to the hybridized intermediate by the transfer of an electron from the thiol to the NO+ (Eq. 4).
| •SHb(II)NO↑↓Hb+NO2−↔Hb−NO2−→“HSHb(II)NO+↔HSHb(III)NO”→Hb(III)+NO | Eq. 4 |
|---|
Figure 6.
The X-band EPR spectra in the g=2 region for (A) 1mM deoxyHb reacted with (black) 0.25 mM nitrite, (blue) 1mM azide before 0.25 mM nitrite addition, and (red) 1mM azide after 0.25 mM nitrite addition for 60 min. (B) The spectral components that make up the calculated EPR spectra of (A). (black: R-state Hb(II)NO, red: R-state Hb(II)βNO, blue: T-state Hb(II)αNO, orange: T-state Hb(II)βNO and green: thiyl radical). [Azide to heme ratio was 1:1. Note: azide has EPR signals at g=2.84, 2.20 and 1.69. These signals do not interact with the heme-NO signal at g=2.0].
Calculated DI vs. Experimental DI:
deoxy/nitrite calculated DI: 9.40e7, experimental DI: 8.38e7, % error: 12.2%
deoxy/azide/nitrite calculated DI: 6.57e7, experimental DI: 5.87e7, % error: 11.8%
deoxy/nitrite/azide calculated DI: 1.04e8, experimental DI:1.07e8, % error: 9.72%
Thus, the release of NO from the Hb(III)NO component, disrupts the hybridized species, which shifts the equilibrium away from the paramagnetic intermediate inhibiting its formation and resulting in the elimination of the paramagnetic intermediate signal from the heme-NO EPR spectrum.
Analysis of spectral changes that take place during the nitrite reaction
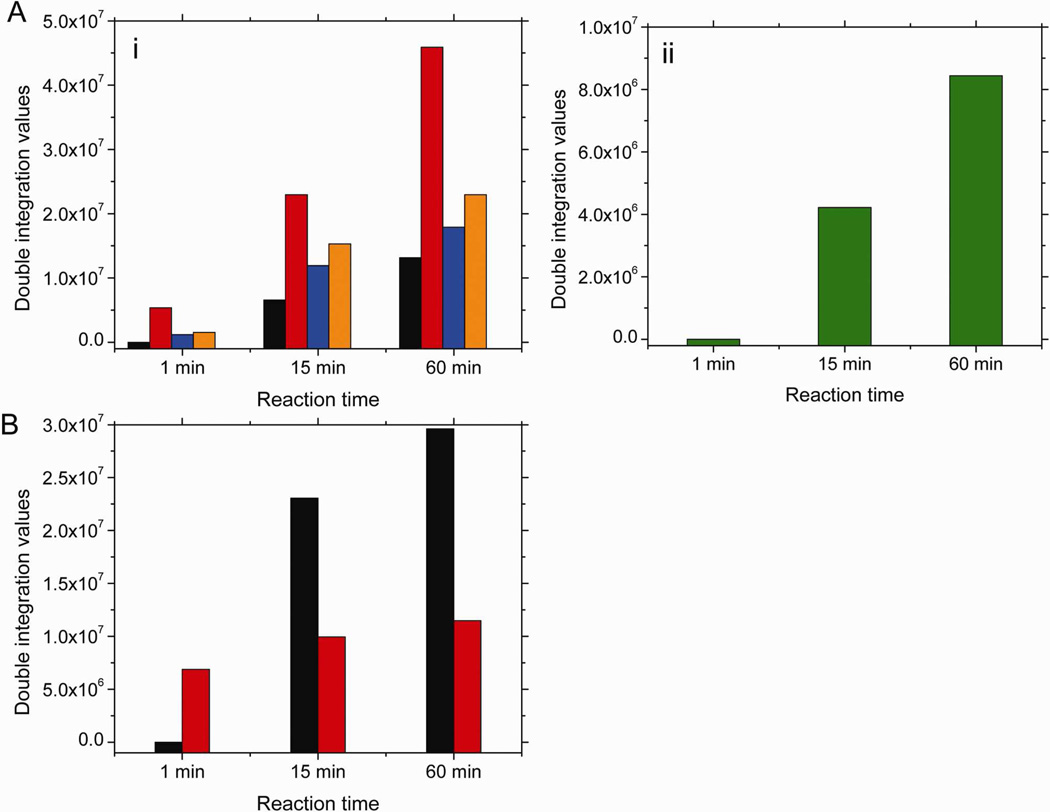
We were able to follow the changes in this new paramagnetic intermediate together with other heme(II)NO species formed during the nitrite reaction at room temperature. For this analysis we included the spectra for T-state Hb(II)αNO and Hb(II)βNO, fully nitrosylated R-state Hb(II)NO, as well as, R-state Hb(II)βNO and the thiyl radical, which are indicative of the new paramagnetic intermediate (•SHb(II)NO). Using these spectra we were able to fit all of the observed spectra shown in Figures 1A and 1B (see supplemental Figure 1). In the absence of NEM, analysis of the concentration of the different components indicates a gradual and significant increase in the paramagnetic intermediate as a function of reaction time as a result of the increase in the components corresponding to R-state Hb(II)βNO (Figure 7Ai) and the thiyl radical signal (Figure 7Aii). Based on the predicted intensity of heme-NO associated with the thiyl radical (Fig. 5C) only 68% of the total heme-NO observed after 60 min of reaction time is due to Hb(II)NO. Based on this analysis, previously reported data38 on the reaction of nitrite with deoxyHb overestimate the formation of Hb(II)NO.
Figure 7.
The spectral components that make up the calculated EPR spectra of the signal in the g=2 region when (A) 1mM deoxyHb and 0.25mM nitrite are reacted for 1, 15 and 60 min; (B) 1mM NEM treated deoxyHb and 0.25mM nitrite are reacted for 1, 15 and 60 min. (black: R-state Hb(II)NO; red: R-state Hb(II)βNO; blue: T-state Hb(II)αNO; orange: T-state Hb(II)βNO; green: thiyl radical). Calculated DI vs. Experimental DI:
(A) 1 min calculated DI: 8.08e6, experimental DI: 1.057e7, % error: 23.3%; 15 min calculated DI: 6.10e7, experimental DI: 7.01e7, % error: 13.0%; 60 min calculated DI: 1.08e8, experimental DI: 9.30e7, % error: 16.6%. (B) 1 min calculated DI: 6.89e6, experimental DI: 7.31e6, % error: 5.83%; 15 min calculated DI: 3.30e7, experimental DI: 3.75e7, % error: 11.9%; 60 min calculated DI: 4.11e7, experimental DI: 4.32e7, % error: 4.85%.
For NEM treated deoxyHb reacted with nitrite (Figure 7B) no T-state heme-NO is observed and no thiyl radical is observed. We do, however, observe a much higher increase in the component corresponding to fully nitrosylated R-state Hb(II)NO (~2.25 times higher than non-NEM deoxyHb), which is consistent with a faster release of NO from Hb(III)NO when the paramagnetic intermediate can not be formed. We also observe a gradual increase in R-state Hb(II)βNO, which is less pronounced (3 times less) then the increase observed for non-NEM deoxyHb. This can be attributed to the preferred β-chain binding of NO released from the hybridized intermediate.
In the presence of NEM, the thiol group necessary for the formation of the paramagnetic intermediate is blocked, and therefore, the thiyl radical is not formed. This data confirms the formation of the thiyl radical intermediate (•SHb(II)NO) during the reaction of nitrite with deoxyHb and its role in slowing down the release of NO from Hb(III)NO to form the reaction product Hb(II)NO.
Analysis of the spectral changes taking place during low temperature incubation
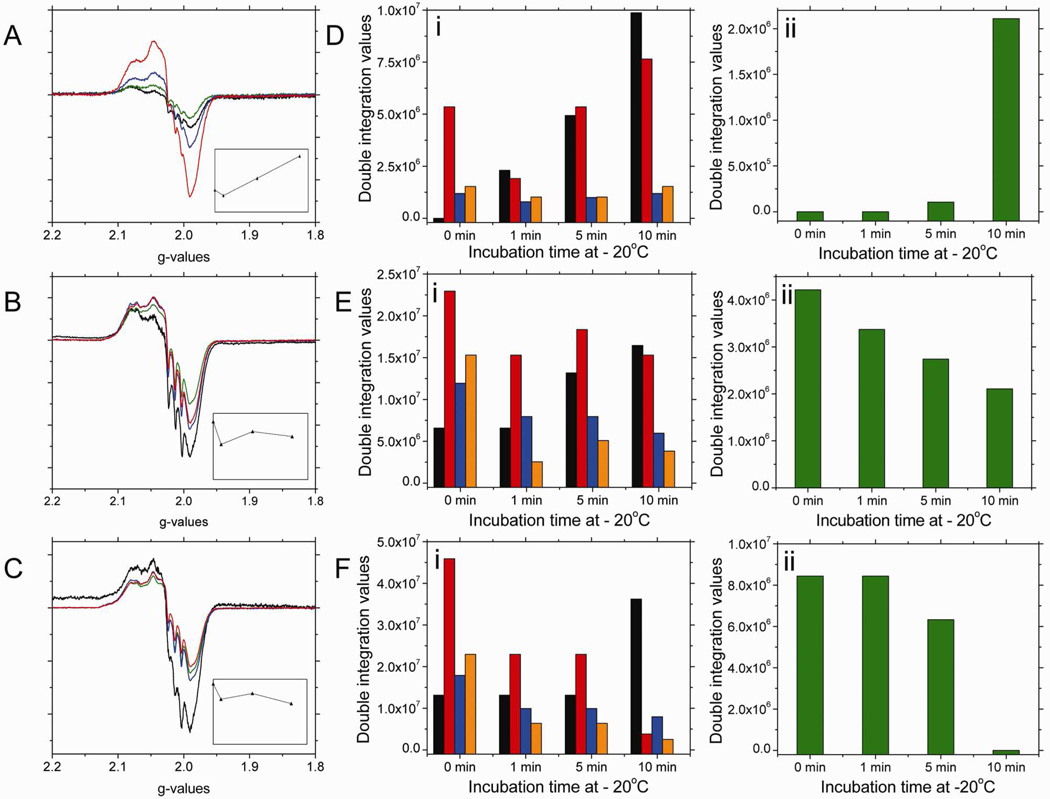
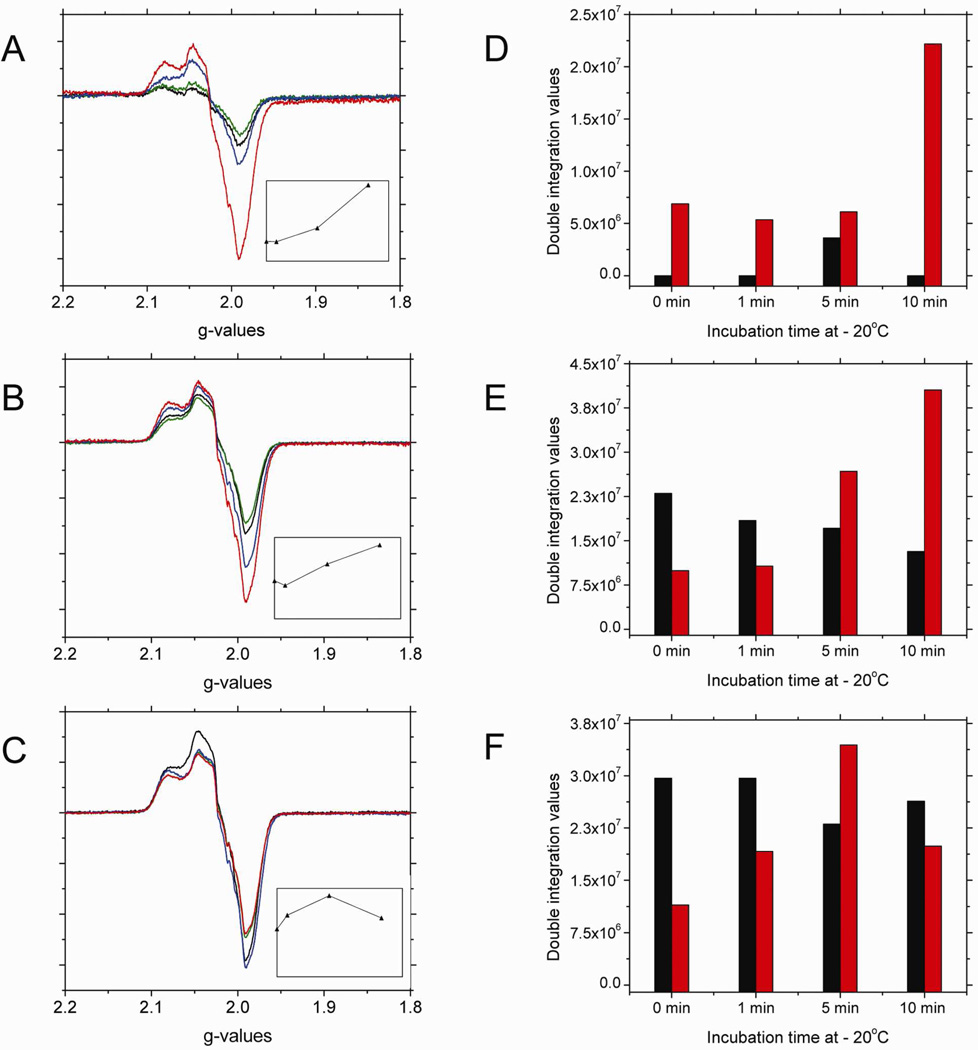
The 10min low temperature incubation results, after a 60min reaction time, shown in Figure 3B indicate that the characteristic signal around g=2 corresponding to the heme-NO species formed during nitrite reduction by deoxyHb decreases. This heme-NO signal is indicative of both R and T-state Hb(II)NO and the paramagnetic intermediate, •SHb(II)NO, as was shown in Figure 7A. To analyze the nature of the spectral changes that take place during low temperature incubations, we have analyzed the spectra of nitrite reacted deoxyHb reacted for 1, 15 and 60 min that have been incubated at low temperature (−20 °C) for 0, 1, 5 and 10 min (Fig. 8).
Figure 8.
The X-band EPR spectra in the g=2 region for a 1mM deoxyHb and 0.25 mM nitrite mixture reacted for (A) 1 min; (B) 15 min; (C) 60 min (black), incubated at −20 °C for 1 (green); 5 (blue); 10 (red) min. [Insets: represent the change in signal intensity in the g=2 region versus incubation time]. The changes in the spectral components as a function of incubation time at −20 °C for the corresponding calculated EPR spectra for the 1 min (D); 15 min (E); and 60 min (F) reaction mixtures. (black: R-state Hb(II)NO; red: R-state Hb(II)βNO; blue: T-state Hb(II)αNO; orange: T-state Hb(II)βNO; green: thiyl radical).
Calculated DI vs. Experimental DI:
(D) 1min reaction time: 1 min incubation calculated DI: 6.03e6, experimental DI: 7.85e6, % error: 23.1%; 5 min incubation calculated DI: 1.24e7, experimental DI: 1.60e7, % error: 22.6%; 10 min incubation calculated DI: 2.24e7, experimental DI: 2.67e7, % error: 16.2%. (E) 15min reaction time: 1 min incubation calculated DI: 3.58e7, experimental DI: 4.25e7, % error: 15.9%; 5 min incubation calculated DI: 4.73e7, experimental DI: 5.80e7, % error: 18.4%; 10 min incubation calculated DI: 4.37e7, experimental DI: 5.20e7, % error: 16.0%. (F) 60min reaction time: 1 min incubation calculated DI: 6.09e7, experimental DI: 6.93e7, % error: 12.2%; 5 min incubation calculated DI: 5.88e7, experimental DI: 7.84e7, % error: 25.0%; 10 min incubation calculated DI: 5.05e7, experimental DI: 6.28e7, % error: 19.5%.
As shown in Figure 8, there are qualitative differences between the results obtained during low temperature incubation for different reaction times. Samples for which the nitrite reaction has only proceeded for 1 min show an increase in the signal intensity in the g=2 region (Fig. 8A). However, for the 15 min and to a greater extent the 60 min samples the signal intensity in the g=2 region decreases (Figs. 8B&C). Based on the analysis of the spectral components at each reaction time (Figs 8D–F), it is clear that the dominant components responsible for both the increased intensity at 1 min and the decrease in intensity at 15 and 60 min correspond to the thiyl radical and the R-state Hb(II)βNO components that are associated with the paramagnetic intermediate, •SHb(II)NO.
After a 1 min reaction, when very little reaction has taken place, a major fraction of the nitrite is associated with the intermediates that have not yet formed paramagnetic species (either the Hb(II)NO formed when NO is released from Hb(III)NO or the paramagnetic intermediate). As the reaction proceeds during low temperature incubation, in addition to the formation of R-state Hb(II)NO associated with the release of NO, we see an increase in the paramagnetic intermediate (Fig. 8D). Therefore, the low temperature incubation after 1min facilitates the formation of the equilibrium distribution between the two intermediates (Eq. 4), such that, the initial formation of the hybridized intermediate is followed by a slower formation of the paramagnetic intermediate. The shift from the EPR silent “Hb(II)NO+ ↔ Hb(III)NO” to the EPR active (•SHb(II)NO) intermediate results in an increase in the EPR signal intensity.
After 15 min of reaction time an appreciable amount of heme-NO species has formed, which include R-state Hb(II)NO, T-state Hb(II)NO, and both the hybridized intermediate, (“Hb(II)NO+ ↔ Hb(III)NO”) and the paramagnetic intermediate (•SHb(II)NO). Under these conditions, a small decrease in signal intensity in the g=2 region is attributed to the low temperature destabilization of the paramagnetic intermediate indicated by the observed decrease in thiyl radical and R-state Hb(II)βNO components (Fig. 8E). This effect is, however, less pronounced than for the 60 min nitrite-reacted sample because after 15min the hybridized intermediate is still being formed at room temperature and the equilibrium distribution between the hybridized and paramagnetic intermediates (Eq. 4) has not been reached.
After a 60 min reaction time when the formation of the hybridized intermediate has leveled off31, the equilibrium distribution of these intermediates is attained. Under these conditions, during the low temperature incubation we observe a significant decrease in both R-state Hb(II)βNO and the thiyl radical components which are attributed to the destabilization of the paramagnetic intermediate relative to the hybridized intermediate. This result shows an apparent decrease in heme-NO species, which does not involve the highly stable Hb(II)NO, but instead involves a transfer of an electron from the NO of •SHb(II)NO to the thiyl resulting in the formation of the HSHb(II)NO+ component of the hybridized intermediate. The transfer of NO from the heme of the paramagnetic intermediate (•SHb(II)NO) to the thiyl radical producing SNOHb(II) can also contribute to the observed decrease in the EPR intensity. However, in the deoxygenated T-state hemoglobin58,59 the formation of SNOHb should be minimal, because of T-state destabilization of SNOHb.
The dominant changes occurring during the −20 °C incubation involves the redistribution between the intermediates. This can be attributed to relatively minor conformational changes, which favor electronic rearrangements that cause a shift between the intermediates. However, at this low temperature appreciable globin rearrangements can also take place, although at a reduced rate. The increase in the R-state Hb(II)NO involving both chains and the decrease on the T-state Hb(II)NO species imply that the low temperature stabilizes the R-quaternary state relative to the T-quaternary state. Since we have previously shown that the release of NO is enhanced in the T-state quaternary conformation60, the low temperature stabilization of the R quaternary conformation would inhibit the release of NO from hemoglobin.
For NEM treated deoxyHb reacted with nitrite (Figure 9), low temperature incubations at −20°C reveal that for the 1 min reaction mixture (Figure 9A) the intensity of the EPR signal in the g=2 region increases as a function of incubation time, such that after 10 min incubation, the signal has tripled in intensity. Spectral fitting of this signal reveal that the intensity increase is correlated to a growth in R-state Hb(II)βNO (Figure 9D). For the 15 min reaction mixture, the total intensity changes for the signal in the g=2 region (Figure 9B) also show an increase in intensity, such that, by the end of 10 min incubation the signal has doubled in intensity. Spectral fitting of this signal reveal that the intensity increase is also correlated to a growth in R-state Hb(II)βNO (Figure 9E). For the reaction mixture at 60 min, low temperature incubations at −20°C decrease the intensity of the signal in the g=2 region by approximately 12% (Figure 9C), this decrease is within experimental error and therefore it is not considered to be significant. Spectral fitting of the signal in the g=2 region reveal that both R-state Hb(II)NO and R-state Hb(II)βNO do not significantly change as a function of incubation time at low temperature (Figure 9F).
Figure 9.
The X-band EPR spectra in the g=2 region for a 1mM NEM treated deoxyHb and 0.25 mM nitrite mixture reacted for (A) 1 min; (B) 15 min; (C) 60 min (black), incubated at −20 °C for 1 (green); 5 (blue); 10 (red) min. [Insets: represent the change in signal intensity in the g=2 region versus incubation time]. The changes in the spectral components as a function of incubation time at −20 °C for the corresponding calculated EPR spectra for the 1 min (D); 15 min (E); and 60 min (F) reaction mixtures. (black: R-state Hb(II)NO; red: R-state Hb(II)βNO).
Calculated DI vs. Experimental DI:
(D) 1min reaction time: 1 min incubation calculated DI: 5.36e6, experimental DI: 7.31e6, % error: 25.7%; 5 min incubation calculated DI: 9.74e6, experimental DI: 1.23e6, % error: 20.7%; 10 min incubation calculated DI: 2.22e7, experimental DI: 2.84e7, % error: 21.7%. (E) 15min reaction time: 1 min incubation calculated DI: 2.91e7, experimental DI: 3.28e7, % error: 11.1%; 5 min incubation calculated DI: 4.39e7, experimental DI: 5.38e7, % error: 18.4%; 10 min incubation calculated DI: 5.37e7, experimental DI: 7.35e7, % error: 25.9%. (F) 60min reaction time: 1 min incubation calculated DI: 4.88e7, experimental DI: 5.56e7, % error: 4.85%; 5 min incubation calculated DI: 5.75e7, experimental DI: 7.34e7, % error: 21.7%; 10 min incubation calculated DI: 4.62e7, experimental DI: 5.31e7, % error: 13.0%.
For NEM treated deoxyHb reacted with nitrite, the only spectral change observed during low temperature incubation at −20 °C is the increase in R-state Hb(II)βNO. This increase in Hb(II)NO must be associated with the relative instability of the hybridized intermediate. Since the paramagnetic intermediate •SHb(II)NO is not formed with NEM treated deoxyHb, the observed increase in intensity in the g=2 region must be due to the binding of NO released from the “Hb(III)NO” component of the hybrid intermediate to reduced Fe(II) subunits. Thus, although diffusion through the globin required for the binding of NO to reduced subunits increasing Hb(II)NO is retarded with the increased levels of released NO, we still observed the formation of Hb(II)NO. The preferred formation of R-state Hb(II)βNO during low temperature incubation (Fig. 9D) suggests that limited globin fluctuations at low temperature inhibits the transfer of NO from a β-chain to an α-chain to a greater extent than between β-chains. The increased release of NO from the hybridized intermediate for NEM treated deoxyHb is also supported by an increase in the formation of metHb (results not shown).
The low temperature incubation studies, thus, indicate that the paramagnetic intermediate •SHb(II)NO is produced subsequent to the hybridized intermediate, by the transfer of an electron from the thiol to the NO+ group of the hybridized intermediate. It also demonstrates that the reduction in temperature stabilizes the hybridized intermediate relative to the paramagnetic intermediate.
Conclusions
The finding that a paramagnetic intermediate is formed during the reaction of nitrite and deoxyHb has important implications. The general assumption that Hb(II)NO measures high affinity binding to an Fe(II) heme that can no longer be released is refuted by the formation of a paramagnetic Hb(II)NO species with a nearby thiyl radical (•SHb(II)NO). This paramagnetic signal can readily be lost by the transfer of an electron back to the thiyl resulting in the re-formation of the hybridized intermediate, which has no EPR signal. This newly characterized paramagnetic intermediate •SHb(II)NO explains the long term stability of our earlier reported hybridized intermediate “Hb(II)NO+ ↔ Hb(III)NO”.
The slow reaction of nitrite with deoxyHb would seem to be incompatible with the physiological requirement of a rapid source of NO. The retention of this pool of bioactive intermediates in the RBC provides a source of nitrite that is already reduced and can potentially be rapidly released from the RBC.
The actual mechanism involved in RBC mediated vasodilation is controversial. However, the accumulation of this pool of potentially bioactive NO in the RBC should have important ramifications for the delivery of NO to the vasculature irrespective of the particular mechanism involved. The involvement of a thiyl radical provides a pathway for the formation of SNOHb. S-nitrosothiols that can be released from RBCs in conjunction with the formation of SNOHb are known to be vasodilators.
There are, however, a number of studies that have addressed a role for the reduction of nitrite to NO by hemoglobin. Whether NO generated by hemoglobin is important physiologically or only when dealing with pharmacological levels of nitrite, a satisfactory mechanism for the release of NO from RBCs needs to be elucidated. The pool of bioactive NO characterized in this paper, which does not spontaneously react with oxyHb or deoxyHb, is a crucial first step required for the release of NO from RBCs. This pool of bioactive NO must release its NO on the membrane in order for it to escape scavenging by hemoglobin. In an earlier publication, we have actually demonstrated that nitrite reacted hemoglobin has an unusually high affinity for the membrane29 and we have further demonstrated the enhanced release of NO from hemoglobin when nitrite reacted Hb binds to the membrane32. NO released from hemoglobin bound to the membrane can potentially diffuse out of the cell without being scavenged by hemoglobin.
S-nitrosothiols released from the RBC are relatively stable in plasma and can interact with thiols on the vasculature. For NO released from the RBC to be transferred to the vasculature we are investigating (1) plasma components that can reversibly bind NO and (2) the direct transfer of NO from the RBC to the vasculature facilitated by transient adherence of RBCs to the vasculature. Further studies are, however, required to determine if, in addition to the transfer of S-nitrosothiols, the transfer of NO from RBCs to the vasculature provides an alternative pathway for the transfer of NO bioactivity.
Supplementary Material
1_si_001
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging. We thank Dr. Antonio Tsuneshige for providing the semihemoglobins samples for this study and Dr. Manoharan for providing the spectra of T-state Hb(II)αNO and Hb(II)βNO. Dr. Manoharan acknowledges the Ramanna Fellowship (SR/S1/RFIC-02-2006) from the DST, Govt. of India and Sir C.V. Raman Chair Professorship from India Gandhi National Open University, New Delhi.
Footnotes
Supporting Information Available: The observed and calculated X-band EPR spectra in the g=2 region for deoxyHb and NEM-treated deoxyHb reacted with nitrite at room temperature and incubated at −20°C for various times. Complete Refs. 24 and 28. This information is available free of charge via the internet at http://pubs.acs.org/.
Reference List
- 1.Palmer RM, Ferrige AG, Moncada S. Nature. 1987;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Higgs EA. Br. J. Pharmacol. 2006;147 Suppl 1:S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ignarro LJ. J. Physiol Pharmacol. 2002;53(4 Pt 1):503–514. [PubMed] [Google Scholar]
- 4.Palmer RM, Ashton DS, Moncada S. Nature. 1988;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 5.Denninger JW, Marletta MA. Biochim. Biophys. Acta. 1999;1411(2–3):334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 6.Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr Proc. Natl. Acad. Sci. U. S. A. 2001;98(1):355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, Pater D. J. Biol. Chem. 1981;256(23):12393–12398. [PubMed] [Google Scholar]
- 8.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr, Olson JS. Biochemistry. 1996;35(22):6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 9.Jia L, Bonaventura C, Bonaventura J, Stamler JS. Nature. 1996;380(6571):221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 10.Pawloski JR, Hess DT, Stamler JS. Nature. 2001;409(6820):622–626. doi: 10.1038/35054560. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, Stamler JS. Cell. 2004;116(4):617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 12.Lima B, Lam GK, Xie L, Diesen DL, Villamizar N, Nienaber J, Messina E, Bowles D, Kontos CD, Hare JM, Stamler JS, Rockman HA. Proc. Natl. Acad. Sci. U. S. A. 2009;106(15):6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanghani PC, Davis WI, Fears SL, Green SL, Zhai L, Tang Y, Martin E, Bryan NS, Sanghani SP. J. Biol. Chem. 2009;284(36):24354–24362. doi: 10.1074/jbc.M109.019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamler JS, Singel DJ, Loscalzo J. Science. 1992;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 15.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. J. Clin. Invest. 2005;115(8):2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. J. Biol. Chem. 2003;278(47):46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 17.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Free Radic. Biol. Med. 2003;35(7):790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 18.Nagababu E, Rifkind JM. Free Radic. Biol. Med. 2007;42(8):1146–1154. doi: 10.1016/j.freeradbiomed.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zweier JL, Samouilov A, Kuppusamy P. Biochim. Biophys. Acta. 1999;1411(2–3):250–262. doi: 10.1016/s0005-2728(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 20.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. FEBS Lett. 1998;427(2):225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 21.Kozlov AV, Staniek K, Nohl H. FEBS Lett. 1999;454(1–2):127–130. doi: 10.1016/s0014-5793(99)00788-7. [DOI] [PubMed] [Google Scholar]
- 22.Castello PR, David PS, McClure T, Crook Z, Poyton RO. Cell Metab. 2006;3(4):277–287. doi: 10.1016/j.cmet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Lundberg JO, Weitzberg E. Arterioscler. Thromb. Vasc. Biol. 2005;25(5):915–922. doi: 10.1161/01.ATV.0000161048.72004.c2. [DOI] [PubMed] [Google Scholar]
- 24.Cosby K, et al. Nat. Med. 2003;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 25.Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, Patel RP. Am. J. Physiol Heart Circ. Physiol. 2006;291(5):H2026–H2035. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 26.Angelo M, Singel DJ, Stamler JS. Proc. Natl. Acad. Sci. U. S. A. 2006;103(22):8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagababu E, Ramasamy S, Rifkind JM. Nitric. Oxide. 2006;15(1):20–29. doi: 10.1016/j.niox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Basu S, et al. Nat. Chem. Biol. 2007;3(12):785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 29.Cao Z, Bell JB, Mohanty JG, Nagababu E, Rifkind JM. Am. J. Physiol Heart Circ. Physiol. 2009;297(4):H1494–H1503. doi: 10.1152/ajpheart.01233.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagababu E, Ramasamy S, Rifkind JM. Biochemistry. 2007;46(41):11650–11659. doi: 10.1021/bi700364e. [DOI] [PubMed] [Google Scholar]
- 31.Salgado MT, Nagababu E, Rifkind JM. J. Biol. Chem. 2009;284(19):12710–12718. doi: 10.1074/jbc.M808647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rifkind JM, Salgado MT, Cao Z. Oxygen Transport to Tissue XXXII. in press. [Google Scholar]
- 33.Tsuneshige A, Kanaori K, Samuni U, Danstker D, Friedman JM, Neya S, Giangiacomo L, Yonetani T. J. Biol. Chem. 2004;279(47):48959–48967. doi: 10.1074/jbc.M405909200. [DOI] [PubMed] [Google Scholar]
- 34.Antonini E, Wyman J, Zito R, Rossi-Fanelli A, Caputo A. J. Biol. Chem. 1961;236:C60–C63. [PubMed] [Google Scholar]
- 35.Cassoly R, Banerjee R. Eur. J. Biochem. 1971;19(4):514–522. doi: 10.1111/j.1432-1033.1971.tb01343.x. [DOI] [PubMed] [Google Scholar]
- 36.Venkateshrao S, Venkatesh B, Manoharan PT. Nitric. Oxide. 2005;13(4):226–231. doi: 10.1016/j.niox.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Grubina R, Huang Z, Shiva S, Joshi MS, Azarov I, Basu S, Ringwood LA, Jiang A, Hogg N, Kim-Shapiro DB, Gladwin MT. J. Biol. Chem. 2007;282(17):12916–12927. doi: 10.1074/jbc.M700546200. [DOI] [PubMed] [Google Scholar]
- 38.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. J. Biol. Chem. 2005;280(35):31126–31131. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 39.Luchsinger BP, Rich EN, Gow AJ, Williams EM, Stamler JS, Singel D. J. Proc. Natl. Acad. Sci. U. S. A. 2003;100(2):461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng Y, Shen TJ, Simplaceanu V, Ho C. Biochemistry. 2002;41(39):11901–11913. doi: 10.1021/bi0202880. [DOI] [PubMed] [Google Scholar]
- 41.Henry Y, Banerjee R. J. Mol. Biol. 1973;73(4):469–482. doi: 10.1016/0022-2836(73)90094-6. [DOI] [PubMed] [Google Scholar]
- 42.Manoharan PT, Alston K, Rifkind JM. Biochemistry. 1989;28(18):7148–7153. doi: 10.1021/bi00444a003. [DOI] [PubMed] [Google Scholar]
- 43.Silva MM, Rogers PH, Arnone A. J. Biol. Chem. 1992;267(24):17248–17256. [PubMed] [Google Scholar]
- 44.Safo MK, Abraham DJ. Biochemistry. 2005;44(23):8347–8359. doi: 10.1021/bi050412q. [DOI] [PubMed] [Google Scholar]
- 45.Chan NL, Rogers PH, Arnone A. Biochemistry. 1998;37(47):16459–16464. doi: 10.1021/bi9816711. [DOI] [PubMed] [Google Scholar]
- 46.Moffat JK. J. Mol. Biol. 1971;55(2):135–146. doi: 10.1016/0022-2836(71)90187-2. [DOI] [PubMed] [Google Scholar]
- 47.Moffat JK. J. Mol. Biol. 1971;58(1):79–88. doi: 10.1016/0022-2836(71)90233-6. [DOI] [PubMed] [Google Scholar]
- 48.Muirhead H, Cox JM, Mazzarella L, Perutz MF. J. Mol. Biol. 1967;28(1):117–156. doi: 10.1016/s0022-2836(67)80082-2. [DOI] [PubMed] [Google Scholar]
- 49.Balagopalakrishna C, Manoharan PT, Abugo OO, Rifkind JM. Biochemistry. 1996;35(20):6393–6398. doi: 10.1021/bi952875+. [DOI] [PubMed] [Google Scholar]
- 50.Balagopalakrishna C, Abugo OO, Horsky J, Manoharan PT, Nagababu E, Rifkind JM. Biochemistry. 1998;37(38):13194–13202. doi: 10.1021/bi980941c. [DOI] [PubMed] [Google Scholar]
- 51.Lassmann G, Kolberg M, Bleifuss G, Gräslund A, Sjöberg BM, Lubitz W. Phys. Chem. Chem. Phys. 2003;5:2442–2453. [Google Scholar]
- 52.Kolberg M, Bleifuss G, Graslund A, Sjoberg BM, Lubitz W, Lendzian F, Lassmann G. Arch. Biochem. Biophys. 2002;403(1):141–144. doi: 10.1016/S0003-9861(02)00264-3. [DOI] [PubMed] [Google Scholar]
- 53.van GM, Lubitz W, Lassmann G, Neese F. J. Am. Chem. Soc. 2004;126(7):2237–2246. doi: 10.1021/ja038813l. [DOI] [PubMed] [Google Scholar]
- 54.Persson AL, Sahlin M, Sjoberg BM. J. Biol. Chem. 1998;273(47):31016–31020. doi: 10.1074/jbc.273.47.31016. [DOI] [PubMed] [Google Scholar]
- 55.Zhao YL, Houk KN. J. Am. Chem. Soc. 2006;128(5):1422–1423. doi: 10.1021/ja057097f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan NL, Kavanaugh JS, Rogers PH, Arnone A. Biochemistry. 2004;43(1):118–132. doi: 10.1021/bi030172j. [DOI] [PubMed] [Google Scholar]
- 57.Brittain T. J. Inorg. Biochem. 2000;81(1–2):99–103. doi: 10.1016/s0162-0134(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 58.Doctor A, Platt R, Sheram ML, Eischeid A, McMahon T, Maxey T, Doherty J, Axelrod M, Kline J, Gurka M, Gow A, Gaston B. Proc. Natl. Acad. Sci. U. S. A. 2005;102(16):5709–5714. doi: 10.1073/pnas.0407490102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palmer LA, Doctor A, Chhabra P, Sheram ML, Laubach VE, Karlinsey MZ, Forbes MS, Macdonald T, Gaston B. J. Clin. Invest. 2007;117(9):2592–2601. doi: 10.1172/JCI29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rifkind JM, Nagababu E, Ramasamy S. Nitric. Oxide. 2011;24(2):102–109. doi: 10.1016/j.niox.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1_si_001