Temporally controlled modulation of FGF/ERK signaling directs midbrain dopaminergic neural progenitor fate in mouse and human pluripotent stem cells (original) (raw)
Abstract
Effective induction of midbrain-specific dopamine (mDA) neurons from stem cells is fundamental for realizing their potential in biomedical applications relevant to Parkinson’s disease. During early development, the Otx2-positive neural tissues are patterned anterior-posteriorly to form the forebrain and midbrain under the influence of extracellular signaling such as FGF and Wnt. In the mesencephalon, sonic hedgehog (Shh) specifies a ventral progenitor fate in the floor plate region that later gives rise to mDA neurons. In this study, we systematically investigated the temporal actions of FGF signaling in mDA neuron fate specification of mouse and human pluripotent stem cells and mouse induced pluripotent stem cells. We show that a brief blockade of FGF signaling on exit of the lineage-primed epiblast pluripotent state initiates an early induction of Lmx1a and Foxa2 in nascent neural progenitors. In addition to inducing ventral midbrain characteristics, the FGF signaling blockade during neural induction also directs a midbrain fate in the anterior-posterior axis by suppressing caudalization as well as forebrain induction, leading to the maintenance of midbrain Otx2. Following a period of endogenous FGF signaling, subsequent enhancement of FGF signaling by Fgf8, in combination with Shh, promotes mDA neurogenesis and restricts alternative fates. Thus, a stepwise control of FGF signaling during distinct stages of stem cell neural fate conversion is crucial for reliable and highly efficient production of functional, authentic midbrain-specific dopaminergic neurons. Importantly, we provide evidence that this novel, small-molecule-based strategy applies to both mouse and human pluripotent stem cells.
Keywords: Pluripotent stem cell, Dopamine neuron, Cell fate, Mouse, Human
INTRODUCTION
The midbrain dopaminergic (mDA) neuron has been a prime target in stem cell research and developmental neurobiology owing to its association with Parkinson's disease. Recent years have witnessed a rapid advancement in understanding the regulatory cascade that governs ventral midbrain neuroepithelial cell fate specification and the differentiation and maintenance of mDA neurons (Andersson et al., 2006; Ferri et al., 2007; Nakatani et al., 2009). Overexpression of mDA transcription factors offers a valid strategy for generating DA neurons from embryonic stem cells (ESCs) (Andersson et al., 2006; Chung et al., 2005; Kittappa et al., 2007; Konstantoulas et al., 2010; Maxwell et al., 2005). However, mDA specification via genetic manipulation is limited and context dependent (Andersson et al., 2006; Friling et al., 2009; Parmar and Li, 2007; Roybon et al., 2008). This could be attributed to the fact that, during development, the transcription factors employed act only on regionally specified mDA-competent ventral midbrain progenitors, but the extent to which such progenitor populations can be generated from pluripotent stem cells using currently available paradigms remains unclear. Several studies indicate that ESCs give rise to forebrain-like identity under defined conditions or to heterogeneous progenitor phenotypes, which exhibit a broad range of anterior-posterior domain-specific expression profiles, when differentiated under the influence of stromal feeders and/or patterning cues (Bouhon et al., 2005; Gaspard et al., 2008; Kawasaki et al., 2000; Watanabe et al., 2005).
During development, mDA neurons are generated from the ventral midbrain floor plate cells under the cooperative action of the homeobox transcription factor Lmx1a and the forkhead transcription factor Foxa2 (Andersson et al., 2006; Chung et al., 2009; Lin et al., 2009; Nakatani et al., 2009). A recent gain-of-function study in ESCs reported that the early expression of an Lmx1a transgene in ESC-derived neural progenitors is crucial for its mDA neuron-inducing activity (Friling et al., 2009), suggesting that temporally restricted characteristics essential for the mDA-competent state become limited in ESC-derived neural cultures soon after neural induction. This change in progenitor competency might explain why sonic hedgehog (Shh), which plays a pivotal role in ventral midbrain patterning and mDA neuron fate specification during development (Ye et al., 1998), is reported to have a widely varying ability to promote mDA neuron production from mouse or human ESCs (Andersson et al., 2006; Friling et al., 2009; Kim et al., 2002; Parmar and Li, 2007; Perrier et al., 2004; Yan et al., 2005). Thus, there is a pressing need to elucidate the essential attributes of mDA-competent neural progenitors and the regulators that are capable of inducing this state.
Molecular signaling is essential for shaping cell fate choice during development. FGF signaling plays multiple roles during the induction and maintenance of the telencephalon (Paek et al., 2009) and, together with Wnt, participates in the formation of the isthmus organizer (IsO) at the midbrain and hindbrain boundary (Olander et al., 2006). However, prior to IsO induction, phosphorylated ERK1/2, which marks regions of FGF signaling, is scarcely detectable in the epiblast of pre-streak vertebrate embryos (Lunn et al., 2007) and in the prospective ventral midbrain of early gastrulating embryos (Corson et al., 2003; Lunn et al., 2007). This temporospatial restriction of FGF/ERK signaling might be necessary for initiating the regulatory cascade that induces competency and cell fate potentials of prospective midbrain neural progenitors.
Using mouse and human pluripotent stem cells and mouse induced pluripotent stem cells (iPSCs), we report here for the first time that a pharmacological blockade of FGF/ERK signaling upon neural induction induces midbrain-specific characteristics, whereas a subsequent activation of FGF signaling consolidates and maintains dopaminergic traits. Combinatorial stimulation with Shh in this experimental paradigm leads to robust production of `authentic' mDA neurons from mouse and human pluripotent stem cells.
MATERIALS AND METHODS
Cell culture and neural differentiation
Mouse ESCs, mouse iPSCs and mouse epiblast stem cells (EpiSCs) were maintained feeder-free as previously described (Guo et al., 2009; Parmar and Li, 2007; Ying et al., 2003). Mouse EpiSCs were established from E14tg2a, Sox1-GFP (also referred to as 46C), Pitx3-GFP and Lmx1a-GFP mouse ESCs as described (Guo et al., 2009). Human ESCs (hESCs; H1 and H7) were cultured on mitomycin C-inactivated feeder cells in knockout DMEM supplemented with 20% knockout serum replacement (KSR) and 8 ng/ml FGF2. Neural differentiation of hESCs was induced with a 3-day treatment with Smad inhibitor, but otherwise similar to that described by Chambers et al. (Chambers et al., 2009). Monolayer differentiation of EpiSCs was developed based on the method by Ying et al. (Ying et al., 2003). Briefly, EpiSCs were plated on fibronectin-coated plastics and cultured in EpiSC media until 50% confluency. Cells were then rinsed twice with PBS and cultured in retinol-free N2B27. Medium was refreshed every other day, as with ESC differentiation, where the day cells are switched to N2B27 is designated as d0 MD. Where indicated, PD0325901 (1 μM, Axon), PD173074 (50 ng/ml, Sigma), FGF8b (100 ng/ml, Peprotech), Shh (200 ng/ml, C25 II-N, R&D) or cyclopamine (2 μM, Sigma) were added to the cultures.
Immunocytochemistry
Cultures were washed twice in PBS then fixed in 4% paraformaldehyde for 20 minutes. Fixed cultures were washed three times in PBS containing 0.3% Triton X-100 followed by incubation in blocking solution containing the above plus 1% BSA and 10% serum compatible with the secondary antibodies. Cells were then incubated overnight at 4°C with the appropriate primary antibody diluted in blocking solution. Cells were washed three times in PBS followed by incubation for 1-2 hours with fluorescently labeled secondary antibodies and DAPI (Molecular Probes). Primary antibodies used were: TH (rabbit, Pel-Freez), TH (sheep, Pel-Freez), β3-tubulin (mouse, Babco), Pitx3 (rabbit, gift of M. Smidt, University of Amsterdam), Foxa2 (goat, Santa Cruz), Lmx1a (rabbit, gift of M. German, University of California, San Francisco), nestin (DSHB), GFP (mouse, Roche), Otx2 (rabbit, Millipore) and Nurr1 (rabbit, Santa Cruz).
Images were captured using a Leica TCS SP5 confocal microscope. The number of Otx2+ cells was determined using ImageJ macro (NIH), based on the total number of pixels of Otx2-labeled and DAPI-labeled nuclei. Quantification of other markers was carried out manually by examining randomly selected fields from at least three independent experiments and data are presented as mean ± s.e.m. Statistical significance was determined using a two-tailed Student's _t_-test.
Quantitative (q) PCR
Total RNA was extracted using TRI Reagent (Sigma) and processed for RT-PCR on a Chromo4 real-time PCR detection system according to the manufacturer's protocols (Bio-Rad). All PCR data were normalized to the average of two reference genes: Hmbs and cyclophilin (peptidylprolyl isomerase A). For PCR primers, see Table S1 in the supplementary material. All qPCR data are presented as mean ± s.e.m. of at least three biological replicates.
Electrophysiology
Day 14-16 MD cultures derived from Pitx3-GFP EpiSCs were placed in a recording chamber and viewed using an Olympus BX51WI microscope with a 40× water-immersion lens and DIC optics. Cells were bathed in 140 mM NaCl, 3.5 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, 10 mM HEPES pH 7.4. For whole-cell recordings, low-resistance recording pipettes (9-12 MΩ) were pulled from capillary glass (Harvard Apparatus) and coated with ski wax to reduce pipette capacitance. Recording pipettes were filled with 140 mM potassium gluconate, 5 mM NaCl, 2 mM MgATP, 0.5 mM LiGTP, 0.1 mM CaCl2, 1 mM MgCl2, 1 mM EGTA, 10 mM HEPES pH 7.4. The osmolarity and pH of both solutions were adjusted before experiments. Prior to recording, the GFP+ neurons were identified and targeted for recording using fluorescence via a GFP-selective filter (X-Cite series 120, EXFO). For analysis of action potential frequency and the coefficient of variation of the interspike interval (CV-ISI), the first 30 seconds of recording time were used to avoid potential effects of washout. Data were acquired at room temperature (20-22°C) using an Axon Multiclamp 700B amplifier and a Digidata 1440a acquisition system, with pClamp 10 software (Molecular Devices). Data analysis was carried out using Clampfit 10.2 (Axon), OriginPro 8.1 (OriginLab) and Spike2v5 (Cambridge Electronic Design) software. Data are presented as mean ± s.e.m.
RESULTS
EpiSCs offer an alternative model for efficient neural induction in vitro
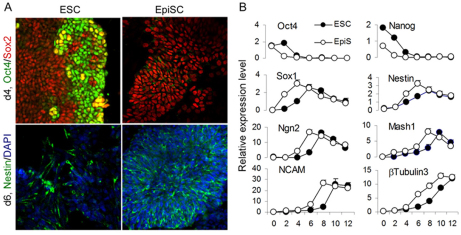
In vitro differentiation of ESCs is asynchronous, generating scenarios in which intermediate progenitors of distinct developmental stages and/or positional identity co-exist and elicit heterogeneous responses to a given inductive signal. Mouse ESCs differentiate into somatic cells via the primitive ectoderm/epiblast stage, when lines of EpiSCs can be established (Brons et al., 2007; Guo et al., 2009; Tesar et al., 2007). Thus, EpiSCs are developmentally primed pluripotent stem cells, which might offer a better in vitro differentiation model than mouse ESCs. We therefore subjected EpiSCs to a monolayer differentiation (MD) protocol previously developed in ESCs (Ying et al., 2003). We found that EpiSCs convert to neuroectoderm cells more quickly than ESCs (Fig. 1). At day 4 (d4) MD, when Oct4+ cells were still abundant in ESC MD cultures, few were detected in EpiSC cultures. However, Sox2, which is expressed in both pluripotent stem cells and neuroepithelial stem cells, was detected in the Oct4– d4 EpiSC derivatives (Fig. 1A). At d6, EpiSC cultures contained numerous nestin+ neural progenitors, whereas significantly fewer were found in ESC progeny.
Fig. 1.
EpiSCs enter the neuroectoderm lineage faster than ESCs. (A) Mouse ESC and EpiSC MD cultures immunostained for Oct4 and Sox2 at day 4 (top) and nestin at day 6 (bottom). DAPI labeling is in blue. (B) qPCR analysis for the expression of pluripotency and neural lineage genes over 12-day MD cultures of ESCs and EpiSCs. Data represent mean±s.e.m. from triplicate cultures of a single experiment.
Consistent with the above observation, qPCR analysis showed a more rapid reduction of Oct4 (Pou5f1 – Mouse Genome Informatics) and Nanog transcripts in EpiSC than in ESC MD cultures. By contrast, we observed a greater rate of upregulation of the neuroepithelial genes Sox1 and nestin in EpiSC cultures. This was followed by an earlier upregulation of the pro-neural genes Ngn2 and Mash1 (Neurog2 and Ascl1 – Mouse Genome Informatics) and, subsequently, of the neuronal marker genes Ncam1 and β3-tubulin (Tubb3) in EpiSC cultures (Fig. 1B).
Taken together, this survey of marker expression indicates that EpiSCs offer a more rapid neural differentiation system than ESCs. Furthermore, the pattern of stage-specific gene expression observed indicates that neural induction occurs during the first 2-3 days of MD using the EpiSC system.
FGF/ERK signaling blockade accelerates the conversion from the epiblast pluripotent state toward neuroectoderm
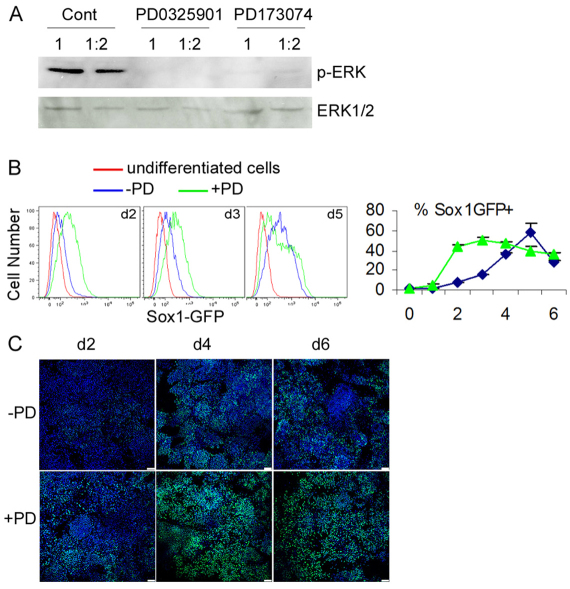
FGF/ERK signaling promotes the conversion of ESCs toward neural progenitors and this signal may be required at multiple points during neural differentiation (Kunath et al., 2007; Stavridis et al., 2007). Kunath et al. (Kunath et al., 2007) suggest that ERK-dependent FGF signaling is required for the exit from an inner cell mass-like state toward epiblast ectoderm, whereas Stavridis et al. (Stavridis et al., 2007) argue that this signaling acts in the transition of epiblast-like cells to neural progenitors. To distinguish between these possibilities, we performed EpiSC MD in the presence of the specific FGF receptor inhibitor PD173074 or the potent MEK blocker PD0325901 (Stavridis et al., 2007; Ying et al., 2008). Both compounds prevented the phosphorylation of ERK1/2 (Mapk3/1 – Mouse Genome Informatics) (Fig. 2A). Using EpiSCs derived from the 46C ESCs, which harbor a knock-in GFP reporter in the Sox1 locus (Ying et al., 2003), we observed an early appearance of Sox1-GFP+ neural progenitors (Fig. 2B). This was supported by immunocytochemical analysis for another neuroepithelial marker, PLZF (Zbtb16 – Mouse Genome Informatics) (Fig. 2C). This finding indicates that FGF/ERK signaling blockade accelerates neural induction of EpiSCs.
Fig. 2.
FGF/ERK inhibition accelerates neural fate conversion of EpiSCs. (A) Western blot analysis for phospho-ERK in day-2 mouse EpiSC differentiation cultures treated with PD0325901 (PD) or PD173074. Samples were loaded undiluted (1) or diluted 1:2 in loading buffer. (B) MD cultures of Sox1-GFP EpiSCs in the presence or absence of PD. Sox1-GFP reporter expression was analyzed by flow cytometry and the percentage of Sox1-GFP+ cells was determined from three independent cultures. Error bars indicate s.e.m. (C) Antibody staining for a neural rosette marker PLZF (green) in d2, d4 and d6 MD cultures with or without PD. DAPI labeling is in blue. Scale bars: 100 μm.
Blockade of FGF/ERK signaling at the onset of EpiSC differentiation induces mDA neural progenitor characteristics
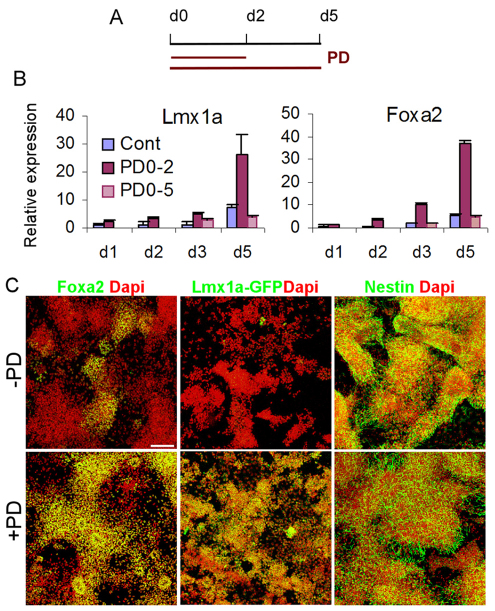
Isthmus-derived Fgf8 plays an important role in midbrain-rostral hindbrain patterning and mDA development and has been used as a standard inductive cue in all ESC differentiation protocols targeting dopamine neurons (Barberi et al., 2003; Kawasaki et al., 2000; Kim et al., 2002; Lee et al., 2000; Perrier et al., 2004; Ye et al., 1998). Surprisingly, we found that treatment with either PD173074 or PD0325901 at the onset of MD led to early induction of Lmx1a and Foxa2 (Fig. 3A,B, see Fig. S1 in the supplementary material). Moreover, cultures that experienced FGF/ERK blockade for the first 2 days continued to show elevated levels of Lmx1a and Foxa2 between d3 and d5, as compared with no-PD controls. By contrast, MD cultures exposed to PD0325901 continuously for 5 days showed a similar level of Foxa2 and a reduced level of Lmx1a transcript compared with the no-PD culture at d3 and d5 (Fig. 3A,B). Consistent with the RNA analysis, at d5 MD, we observed a pronounced increase of neural progenitors expressing Foxa2 protein or a knock-in Lmx1a-GFP reporter in cultures exposed to 2 days of PD173074 or PD0325901 (Fig. 3C). These results demonstrate that blocking FGF/ERK activity on exit of the epiblast pluripotent state induces appropriate gene markers for the presumptive ventral midbrain in newly converted neural progenitors, which then require FGF/ERK signaling to maintain and consolidate this progenitor trait.
Fig. 3.
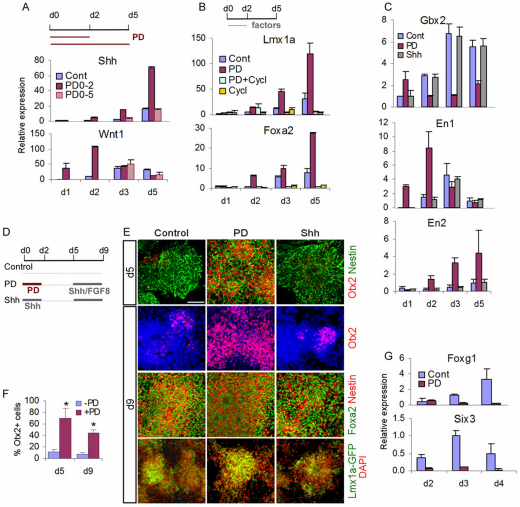
FGF/ERK blockade at the onset of neural fate conversion induces mDA regulatory genes. (A) Experimental schemes. E14tg2a mouse EpiSC monolayer cultures were exposed to PD0325901 (PD) from d0 MD for either 2 days (PD0-2) or 5 days (PD0-5). Cultures were harvested at d1, d2, d3 and d5 MD. (B) qPCR analysis of Lmx1a and Foxa2. The value of the d1 MD control (Cont) was set as 1. Error bars indicate mean±s.e.m. of two sets of experiments performed in duplicate. (C) d5 MD of Lmx1a-GFP EpiSCs exposed to PD from d0 MD for 2 days were immunostained for Foxa2 and GFP (both green) and counterstained with DAPI (red). Scale bar: 100 μm.
We obtained similar results with PD0325901 and PD173074 for the gene markers described above (Fig. 3) and the additional markers presented in Fig. S1A in the supplementary material. The studies described below were therefore performed with PD0325901 (hereafter referred to as PD) unless stated otherwise.
Temporally controlled modulation of FGF/ERK signaling leads to highly reliable and efficient production of mDA neurons
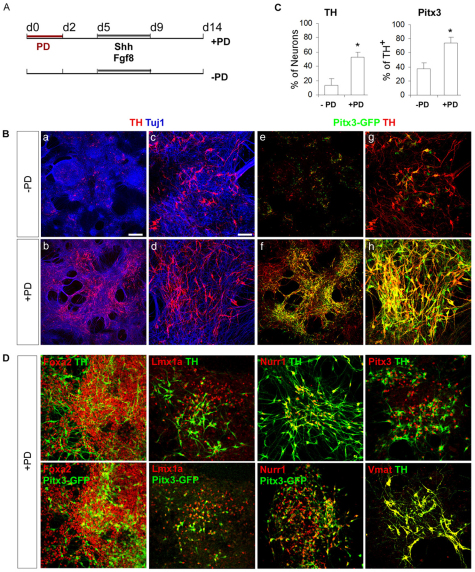
To investigate whether the observed induction of the mDA progenitor phenotype translates to mDA neuron production either quantitatively [the number of tyrosine hydroxylase-positive (Th+) neurons] or qualitatively (the midbrain-specific identity of Th+ neurons), we assessed a panel of markers by immunocytochemistry at d14 MD. We employed a scheme that is analogous to most DA differentiation protocols in which Shh is applied to nestin+ cells (Fig. 4A, see Fig. S2 in the supplementary material) (Barberi et al., 2003; Kawasaki et al., 2000; Kim et al., 2002; Lee et al., 2000; Parmar and Li, 2007; Perrier et al., 2004). We found that, despite the robust induction of Foxa2+ Lmx1a+ neural progenitors at d5, cultures treated with PD alone did not produce more Th+ neurons than the standard control culture treated with Shh and FGF8 (SF control, see Fig. S2A,B in the supplementary material). This might indicate that the d5 Foxa2+ Lmx1a+ neural progenitors were not sufficiently specified toward an mDA fate. However, when further stimulated with Shh and FGF8 for 4 days, PD-treated cultures had an almost 4-fold increase in Th+ neurons as compared with the SF control (PD, 52.3±8%; control, 13.5±9%) (Fig. 4B,C). Consistent with the increase of Th+ neurons, we detected a near 3-fold increase in extracellular dopamine levels in PD-treated culture supernatant compared with the SF controls (see Fig. S2D in the supplementary material). Using another line of EpiSCs, which carrys a GFP reporter knocked into the Pitx3 locus and drives mDA-specific GFP expression (Maxwell et al., 2005; Zhao et al., 2004), we found a substantial increase of Pitx3-GFP+ cells in PD-treated cultures as compared with the SF controls (40±6.6% versus 5±3.2%; P<0.01) (Fig. 4Be-h). Furthermore, the majority of Th+ neurons in PD-treated cultures were Pitx3-GFP+ (73.8±10.2%). This was also the case for other mDA and pan-DA neuronal markers such as Lmx1a (88.5±3.9%), Foxa2 (99.5±1.2%), Nurr1 (Nr4a2 – Mouse Genome Informatics) (85.4±4%) and VMAT (Slc18a1 – Mouse Genome Informatics) (85.6±12%) (Fig. 4D, see Fig. S2C in the supplementary material).
Fig. 4.
Stepwise inactivation and activation of FGF/ERK signaling leads to the highly efficient production of dopamine neurons exhibiting mDA components. (A) Outline of the experiment using the Pitx3-GFP EpiSCs. (B) Immunostaining of mouse d14 MD cultures for (a-d) Th (red) and Tuj1 (blue) as well as (e-h) Th (red) and Pitx3-GFP (green). (C) Quantification of the immunostaining in B, illustrating the percentage of Th+ neurons and Th+ neurons co-expressing Pitx3. Mean±s.e.m. *, P<0.01 relative to no-PD control (Student's _t_-test). (D) Immunostaining of d14 MD cultures for various midbrain and pan-DA markers. Scale bars: 200 μm for Ba,b,e,f; 50 μm for Bc,d,g,h,D.
We next tested the efficacy of this experimental scheme on mouse iPSCs. Consistent with the report that FGF/ERK signaling is required for the progression from the naïve to the primed pluripotent state (Kunath et al., 2007), we found that PD treatment from d0 MD inhibited neural induction (data not shown). However, PD treatment for 2 days either at d1-3 or d2-4 MD significantly enhanced the production of Th+ neurons compared with controls without PD (see Fig. S3 in the supplementary material). Similar to EpiSCs, iPSC-derived Th+ neurons co-expressed Foxa2. Together, these data demonstrate that FGF signaling blockade during neural induction primes a midbrain regional fate in derived neural progenitors that is necessary for their terminal differentiation into DA neurons exhibiting midbrain characteristics.
Blockade of FGF signaling upon exit from the epiblast pluripotent state induces Shh and Wnt1
We next investigated how FGF inhibition confers mDA competence. Wnt1 and Shh are two essential signaling molecules that govern mDA progenitor fate specification and neurogenesis by forming regulatory loops with Lmx1a and Foxa2 (Andersson et al., 2006; Chung et al., 2009; Echelard et al., 1993). We examined Wnt1 and Shh expression by qPCR in MD cultures treated with PD at d0-2. PD treatment resulted in a sharp increase in Wnt1 transcript at d1, with a further increase at d2 (Fig. 5A, see Fig. S2B in the supplementary material). This effect was also observed with PD173074 (see Fig. S1A in the supplementary material). Intriguingly, Wnt1 transcript returned to, or dropped below, control levels at d3-5 irrespective of the continued presence or absence of PD, indicating that early FGF/ERK blockade in EpiSCs initiates downstream molecular events that negatively regulate Wnt1 expression. For Shh, similar (low) levels of transcript were detected at d1 in PD173074- or PD0325901-treated culture and in non-treated cultures. However, 1.5-fold and 4-fold increases of Shh transcript were observed at d2 in PD173074 and PD0325901, respectively. The level of Shh continued to increase (up to 8-fold) post-PD exposure between d3 and d5 (Fig. 5A, see Fig. S1A in the supplementary material). The kinetics of PD-mediated Wnt1 induction at d1 and d2 MD is similar to that of Lmx1a, whereas the pattern of Shh upregulation is similar to that of Foxa2 (Fig. 3, see Fig. S1B in the supplementary material), indicating a regulatory relationship.
Fig. 5.
FGF inhibition directs a midbrain neural progenitor fate. (A) Mouse E14tg2a EpiSC monolayer cultures were exposed to PD0325901 (PD) from d0 MD for 2 (PD0-2) or 5 (PD0-5) days. Cultures were harvested at day 1, 2, 3 or 5 for qPCR analysis of Shh and Wnt1. (B) E14tg2a EpiSC MD cultures were treated with PD or cyclopamine (Cycl) or a combination of the two as indicated. Cultures were harvested at day 1, 2, 3 or 5 for qPCR analysis of Lmx1a and Foxa2. (C) EpiSCs were differentiated in the presence of PD or Shh for 2 days from d0 MD, as in B, and analyzed for Gbx2, En1 and En2 by qPCR at d1-3 and d5 MD. (D) Experimental schemes for E-G. (E) Immunostaining of d5 and d9 MD E14tg2a (top two rows) and Lmx1a-GFP (bottom two rows) EpiSCs. Antibodies against Otx2 (red) and nestin (green), or Otx2 alone (red), were used in E14tg2a EpiSCs. Foxa2 (green), nestin (red) and GFP (green) were used in the Lmx1a-GFP panels. Scale bar: 100 μm. (F) Quantification of immunostaining in E illustrating the percentage of cells expressing Otx2. Mean±s.e.m. of ten fields from two independent experiments. *, P<0.01 relative to no-PD control (Student's _t_-test). (G) qPCR analysis of the telencephalic markers Six3 and Foxg1 in d2-4 MD cultures with or without PD. Data in A-C,G are mean±s.e.m. of three replicate cultures.
We then investigated the contribution of Shh signaling to the upregulation of Lmx1a and Foxa2, as these genes are known to be induced by Shh (Andersson et al., 2006; Chung et al., 2009). We performed additional MD in the presence of the Shh inhibitor cyclopamine. This treatment abolished the induction of Foxa2 by PD, suggesting that PD-mediated Foxa2 regulation is strictly dependent on Shh signaling (Fig. 5B). Interestingly, cyclopamine did not affect PD-induced Lmx1a expression at d1-2, but did exhibit an effect at d3 and d5 (Fig. 5B). Because the Lmx1a transcript was already induced at d1 MD after 24 hours of FGF/ERK inhibition, and because Shh was not induced until d2 (Fig. 5A,B, see Fig. S1B in the supplementary material), our data suggest that the early induction of Lmx1a by PD might be elicited by Wnt1 in an Shh-independent feed-forward fashion. However, sustained expression of Lmx1a between d3 and d5 MD requires Shh signaling.
FGF/ERK inhibition promotes mDA characteristics via modulation of anterior-posteriorization and the maintenance of Otx2
Although PD induces Shh, we found that replacing PD with Shh at d0-2 did not recapitulate the highly efficient production of mDA neurons at d14 MD (see Fig. S2A-C in the supplementary material). This could be due to the relatively late and weaker stimulation of Lmx1a and Foxa2 by Shh compared with PD (see Fig. S4A in the supplementary material). However, it is also possible that FGF/ERK inhibition induces other properties that are necessary for mDA fate specification.
An attractive hypothesis is that blocking FGF/ERK maintains anterior neural character, as FGF is known to promote caudal attributes (for a review, see Wilson and Rubenstein, 2000). Consistent with this, we found using qPCR analysis that cultures exposed to PD for 2 days consistently exhibited reduced levels of Gbx2 (Fig. 5C), which encodes a homeobox protein that is expressed in the anterior hindbrain and prospective spinal cord. By contrast, 2 days of stimulation by Shh had no effect on Gbx2 expression. Also consistent with this hypothesis, neural progenitors co-expressing Otx2, an anteriorly expressed homeobox protein that forms a mutually repressive circuit with Gbx2, were significantly more abundant in PD-treated cultures than in control cultures (Fig. 5E, see Fig. S4B in the supplementary material) (Millet et al., 1999). Again, Shh in place of PD at d0-2 MD did not increase the number of Otx2+ neural progenitors at d5 MD (Fig. 5E,F). Furthermore, PD-treated and non-treated cultures exposed to Shh and FGF8 for a further 4 days at d5-9 maintained the differential expression of Otx2 at d9 MD (Fig. 5D-F). These findings suggest that FGF/ERK blockade promotes the generation of anterior neural progenitors by suppressing posteriorization.
FGF signaling from the anterior neural ridge plays an important role in the formation of forebrain (Paek et al., 2009). We therefore determined the effect of FGF/ERK inhibition on the expression of the telencephalic gene markers Six3 and Foxg1. As expected, increases of Six3 and Foxg1 transcripts were readily detected from d2 and d3 MD, respectively, in the control cultures without PD (Fig. 5G). By contrast, PD-treated cultures did not upregulate either of the telencephalic marker genes. Furthermore, we detected a sharp increase in the mesencephalic transcription factor genes engrailed 1 (En1) at d1 and En2 from d2 MD in PD-treated, but not Shh-treated, cultures (Fig. 5C). Taken together with the finding that PD induces Wnt1, these data suggest that FGF/ERK inhibition confers a midbrain bias within the Otx2+ anterior neural progenitor population. Otx2 has been shown to play a regulatory role in Lmx1a transcription and in the proliferation and differentiation of mDA progenitors (Brodski et al., 2003; Chung et al., 2009; Omodei et al., 2008; Ono et al., 2007). Thus, the retention of Otx2 in neural progenitors, and the early induction of isthmus-patterning molecules by FGF/ERK inhibition, are likely to serve as additional attributes essential for the observed efficient production of mDA neurons.
It is worth noting that, at d9 MD following 4 days exposure to Shh/FGF8, PD-treated and untreated control cultures had similar proportions of Foxa2+/Lmx1a+ neural progenitors (Fig. 5E), indicating that either Foxa2/Lmx1a neural progenitor expression at later stage of MD, or Foxa2/Lmx1a expression alone, is not sufficient for mDA commitment, at least in pluripotent stem cell cultures in vitro.
Shh and Fgf8 participate in distinct regulatory pathways governing mDA fate specification and differentiation
Our study shows that both Shh and Fgf8 are necessary during the period d5-9 for achieving the highest number of mDA neurons: those co-expressing Th, Pitx3, Lmx1a and Foxa2. Cultures treated with PD followed by Shh alone contained a large number of faintly stained Th+ cells with immature neuronal morphology. In addition, few of these Th+ neurons co-expressed Pitx3-GFP (see Fig. S2 in the supplementary material). By contrast, Th+ neurons present in cultures treated with PD, followed by FGF8 alone, were mature in appearance and mostly Pitx3-GFP+. However, the number of Th+ neurons was similar to that found in the no-PD control cultures. These observations suggest that PD-generated d5 neural progenitors require sustained Shh signaling to specify a dopaminergic phenotype, whereas FGF8 facilitates progenitor differentiation involving Pitx3 expression.
Day 5-9 MD spans the peak of neurogenesis during which the expression of neuronal subtype determination factors rapidly increases (Fig. 1; data not shown). To further elucidate how Shh and FGF affect neural progenitor behavior, we examined their effect on the expression of a number of genes that encode mDA and non-dopaminergic neural progenitor domains by RT-PCR (Fig. 6). We found that Shh added either at d3-6 MD or d5-9 MD induced a further increase in Foxa2 and Msx1 expression, although it had no effect on either Lmx1a or Shh (Fig. 6B). FGF8 stimulation, by contrast, led to significant upregulation of Wnt1, En1 and En2, which are all known to participate in mDA differentiation and survival (Alberi et al., 2004; Joksimovic et al., 2009) (Fig. 6B,C).
Fig. 6.
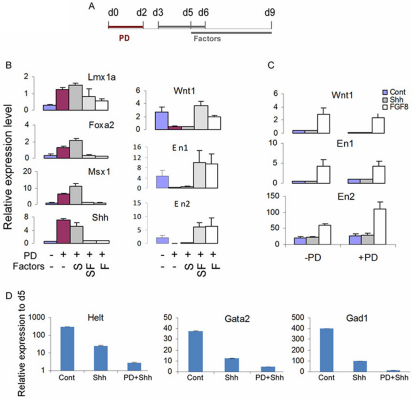
Shh and FGF8 act on distinct aspects of mDA neuron differentiation. (A) Experimental scheme for data in B and C. (B) qPCR analysis of mouse d6 MD cultures for the ventral mesencephalic regulator genes Lmx1a, Foxa2, Msx1 and Shh and the isthmus-expressed midbrain patterning genes Wnt1, En1 and En2. (C) qPCR analysis at d9 MD for Wnt1, En1 and En2. PD0325901 (PD) was added for 2 days from d0-2, whereas Shh and/or FGF8 were added from d5. (D) qPCR analysis of Helt, Gata2 and Gad1 in d5 and d9 MD cultures. Expression levels were examined in control MD cultures with no added factors, control cultures treated with Shh between d5 and d9 alone, as well as in cultures treated with PD at d0-2 followed by Shh between d5 and d9 MD. Data in B and C are mean±s.e.m. of two experiments performed in duplicate, whereas data in D are mean± s.e.m. of triplicate cultures from one representative experiment.
We also found that cultures without any exogenous stimuli experienced a sharp increase of gene transcripts controlling GABAergic fate, such as Gata2 (38-fold), Helt (293-fold) and Gad1 (401-fold) (Fig. 6D), consistent with the suggestion that in vitro-derived neurons are mostly GABAergic (Kala et al., 2009; Nakatani et al., 2007). However, Shh stimulation at d5-9 suppressed the expression of these genes, and progenitors exposed to sequential PD and Shh treatment showed a further reduction in these non-DA markers. Thus, the fold increases in Helt and Gad1 transcripts in the PD-Shh cultures were only 10% of the level of Shh alone cultures and were less than 1% of the level of no factor controls. Together, our data demonstrate that Shh and Fgf8 participate in distinct regulatory pathways that contribute to efficient mDA fate specification and differentiation.
Interestingly, FGF8 treatment, with or without Shh, from d3 MD immediately after PD treatment eliminated the PD-mediated upregulation of Lmx1a, Foxa2, Shh and Msx1 (Fig. 6B). Consequently, we observed little increase in the number of Th+ neurons in these cultures at d14 MD compared with the untreated control (data not shown). Together with the finding that persistent FGF/ERK blockade after neural induction abolishes PD-mediated induction of mDA regulators (Fig. 3B, Fig. 5B), our data indicate that a period of autocrine FGF/ERK signaling is crucial for newly converted neural progenitors to process the patterning information that leads to the eventual commitment of the dopaminergic neuron fate.
In vitro-generated mDA neurons exhibit functional neuronal characteristics
We examined whether dopamine neurons generated using these protocols had functional neuron-like properties. We took advantage of the Pitx3-GFP reporter system (Zhao et al., 2004), in which we could target dopamine neurons by their GFP signal (Fig. 7A). We conducted whole-cell recordings at d14-16 MD, from GFP+ cells differentiated with or without PD. First we examined the passive membrane properties of the differentiated cells. All cells exhibited a negative resting membrane potential typical of neurons (control, –67±1.3 mV, _n_=6; PD, –37±4.6, _n_=13; P<0.05). Interestingly, cells treated with PD had a resting membrane potential that was significantly more depolarized than that of control cells. Both groups had similar input resistance (control, 422±198 MΩ, _n_=8; PD, 558±79 MΩ, _n_=15; _P_>0.05) and whole-cell capacitance (control, 9.6±1 pF, _n_=8; PD, 12±5 pF, _n_=15; _P_>0.05) values, suggesting that PD treatment did not affect cell size.
Fig. 7.
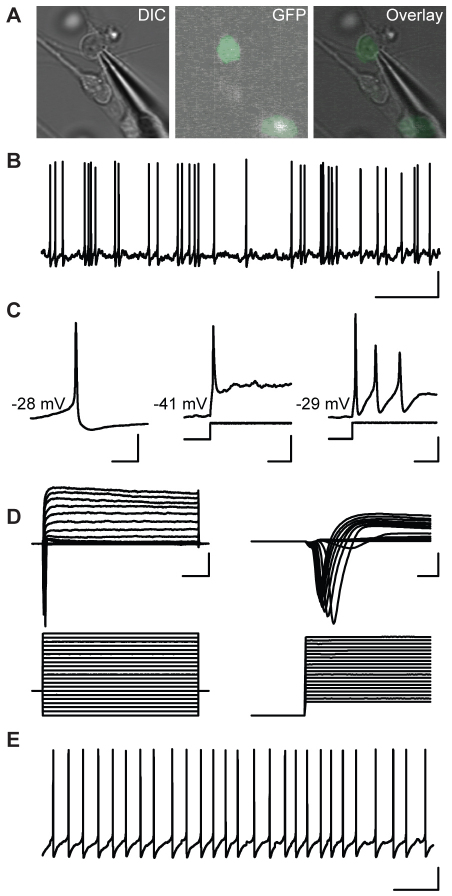
Pitx3-GFP+ cells exhibit functional neuron-like electrophysiological properties. (A) DIC and GFP image showing a recording pipette patched onto a mouse Pitx3-GFP+ cell. (B) Spontaneous action potentials fired by a day-14 neuron. Vertical scale bar, 20 mV; horizontal scale bar, 5 mseconds. (C) A single spontaneous action potential (left). A single action potential fired in response to a 50 pA depolarizing current pulse (middle). Multiple action potentials fired in response to a 50 pA depolarizing current pulse (right). Vertical bars, 20 mV; horizontal bars, 50, 100 and 100 mseconds, respectively. (D) Outward current evoked by a serious of voltage steps (left). Fast inward current evoked by a series of voltage steps (right). Vertical bars, 1 nA; horizontal bars, 50 mseconds. (E) Pacemaker-like spontaneous firing a day-41 neuron. Vertical bar, 20 mV; horizontal bar, 1 second.
At d14-16 MD, 14% of these cells (control, 1/7 cells; PD, 2/14 cells) exhibited multiple spontaneous action potentials (Fig. 7B). In all cells, application of a depolarizing current pulse evoked an overshooting action potential (_n_=21), and 19% of cells (control, 1/7 cells; PD, 3/14 cells) fired multiple action potentials in response to the stimulus (Fig. 7C). The cells that were spontaneously active fired at a low frequency (0.95±0.14 Hz; _n_=3) and in an irregular pattern (CV-ISI, 1.19±0.24; _n_=3). However, 67% (6/9) of cells in MD cultures at d40 or later fired spontaneous action potentials. Importantly, these older cells exhibited the pacemaker-like spontaneous activity (Fig. 7E) that is characteristic of mDA neurons (3.54±0.8 Hz; CV-ISI, 0.49±0.08; _n_=6). In voltage-clamp mode, all differentiated cells exhibited a large outward current, typical of a delayed-rectifier K+ current (control, 2.91±0.39 nA, _n_=7; PD, 2.19±0.28 nA, _n_=15; _P_>0.05) (Fig. 7D, left panel). Fast-activating, fast-inactivating inward currents were also observed that were typical of Na+ currents (control, 1.38±0.14 nA, _n_=7; PD, 1.69±0.25 nA, _n_=15; _P_>0.05) (Fig. 7D, right panel). Taken together, these recordings show that dopaminergic cells generated using our differentiation protocol exhibit functional, neuron-like properties.
A conserved role for FGF/ERK signaling in mDA fate specification of hESCs
Finally, we asked whether the modulation of FGF/ERK signaling could also promote mDA neuron differentiation of hESCs. H1 and H7 hESCs were induced to differentiate by a 3-day exposure to Smad inhibitors. Under this condition, the primitive ectoderm marker FGF5 peaked at d3 MD and the majority of the cells became NES+ neural precursors at d11 for H1 and d20 for H7. To mimic the condition experienced by the mouse cells, we applied PD for 4 days from d3 MD (Fig. 8A). Similar to the finding in mouse cells, PD treatment in hESCs also resulted in an increase in OTX2, WNT1, EN1/2 and in the suppression of SIX3, FOXG1 and GBX2 (Fig. 8B), indicating the induction of a midbrain fate and the suppression of caudalization as well as forebrain induction. We also observed similar induction of ventral fate regulators such as SHH, LMX1A, FOXA2 and MSX1. Interestingly, PD treatment led to robust induction of DMRT5, a newly identified midbrain floor plate marker that confers mDA progenitor identity following forced expression in ESC-derived neural progenitors (Fig. 8B) (Gennet et al., 2011).
Fig. 8.
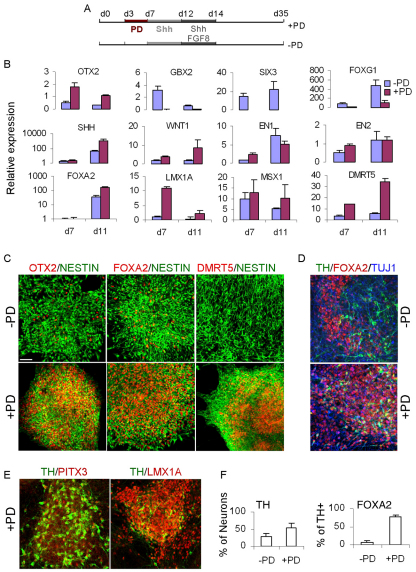
Generation of mDA neurons from human ESCs. (A) Experimental schemes. (B) qPCR analysis of regional markers in d7 and d11 H7 MD cultures with or without PD0325901 (PD). Data are mean±s.e.m. of three replicate cultures. (C) Double immunostaining of d13 H1 MD cultures for nestin (green) plus OTX2, FOXA2 or DMRT5 (all in red). (D) Triple antibody staining of d35 H7 cultures for FOXA2 (red), TH (green) and β3-tubulin (TUJ1, blue). (E) Immunostaining of PD-treated d32 H7 MD cultures for PITX3 (red) and TH (green), and for LMX1Aa (red) and TH (green). (F) Quantification of the immunostaining in E, illustrating the percentage of TH+ neurons and TH+ neurons co-expressing FOXA2. Error bars indicate s.e.m. Scale bars: 75 μm.
Consistent with the transcript analysis, the major population of NES+ progenitors in PD-treated cultures co-expressed OTX2, FOXA2 and DMRT5 (Fig. 8C). Of the PD-treated cells, 47.5±7.1% were FOXA2+ OTX2+, a molecular profile characteristic of mDA neural progenitors (see Fig. S5 in the supplementary material). By contrast, this cellular phenotype only constituted 6.8±1.5% of the control population. As cultures progressed toward neuronal differentiation at d35 MD, we detected numerous TH+ neurons co-expressing FOXA2, LMX1A and PITX3 in PD-treated cultures (Fig. 8D,E), which was in dramatic contrast to the no-PD controls, in which LMX1A+ and PITX3+ cells were rarely detected (data not shown). Furthermore, we found that, although FOXA2+ cells were present in the no-PD cultures, they were mostly mutually exclusive with TH+ neurons (Fig. 8D). Thus, PD treatment resulted in a near 7-fold increase of FOXA2+ TH+ neurons when compared with no-PD controls (Fig. 8D-F). Together, the above data demonstrate that FGF/ERK signaling plays a conserved role in mouse and human pluripotent stem cells.
DISCUSSION
Dopamine neurons derived from pluripotent stem cells constitute a powerful resource in neurobiological research. In the context of patient-specific iPS cells, in vitro-derived dopamine neurons also provide a valuable tool for drug discovery, modeling of Parkinson's disease and as a potential alternative cell source for transplantation-based therapy.
Here, we demonstrate a functional impact of the FGF/ERK signaling level on the course of mDA neuron differentiation of mouse and human pluripotent stem cells. Pharmacological inactivation of FGF/ERK activity upon exit of the lineage-primed epiblast pluripotent state initiates transcription activities that govern early mesencephalic patterning of both the anterior-posterior and dorsal-ventral axes, leading to the induction of mDA neural progenitor characteristics and maintenance of dopaminergic competence. The consolidation of these characteristics, however, requires a period of autocrine/paracrine FGF/ERK signaling immediately after neural induction. Either continued FGF/ERK blockade in newly derived neural progenitors, or enhancing FGF signaling activity by exogenous FGF8 in these cells, abolishes the effects of PD. These findings demonstrate a previously unrecognized inhibitory role of FGF/ERK in the induction of ventral midbrain neural progenitors and offer a novel strategy for mDA neuron production from mouse and human pluripotent stem cells and iPSCs. Furthermore, the current method represents a simple, small-molecule-based paradigm for significantly improved efficiency and high reproducibility compared with previously reported transgene-free protocols. Importantly, our strategy directs a midbrain regional identity in the derived dopamine neurons, a property that is essential for functional integration of transplanted dopamine neurons in the Parkinsonian brain (Hudson et al., 1994).
Stimulation of ESC-derived neural progenitors with Shh and FGF8 is used by almost all dopamine differentiation protocols (Barberi et al., 2003; Kim et al., 2002; Lee et al., 2000; Perrier et al., 2004; Yan et al., 2005). However, unless combined with genetic manipulation of mDA transcription factors, such as Pitx3 or Lmx1a (Andersson et al., 2006; Chung et al., 2002; Konstantoulas et al., 2010; Maxwell et al., 2005), the midbrain regional identity of the dopamine neurons generated has remained uncertain. Furthermore, the yield of Th+ neurons has often proved unreliable between experiments and even highly variable between different microscopic fields within a single culture. A major limiting factor is the temporal and spatial heterogeneity of ESC-derived neural progenitors. Our findings demonstrate that the above issues can be addressed using EpiSCs. We found that, in the absence of FGF/ERK signaling manipulation, nearly 40% of Th+ neurons generated by EpiSCs already co-expressed Pitx3. This represents a significant improvement over ESC-derived monolayer cultures, where Pitx3+ neurons are rarely observed (Andersson et al., 2006; Friling et al., 2009; Parmar and Li, 2007). This improvement is likely to be due to the more synchronous conversion of EpiSCs to the neuroepithelial fate, which would allow for the effective capture of mDA-competent progenitors.
However, without additional FGF/ERK inhibitor treatment at the neural induction phase, the total numbers of Th+ Pitx3+ cells remained low due to the overall poor efficiency in producing Th+ cells. The early induction of both Lmx1a and Foxa2 by inhibiting FGF receptor or ERK is likely to be a key factor in the observed high efficiency in our experiments. This hypothesis is based on the following observations: (1) d5 PD-treated (EpiSC) MD cultures are highly enriched for Foxa2+ Lmx1a+ neural progenitors compared with untreated controls; (2) although Shh treatment in d5-9 MD results in comparable numbers of Foxa2+ Lmx1a+ cells in PD-primed and no-PD cultures, mDA neuron production was not enhanced in the manner observed with PD treatment; (3) replacing PD with Shh, which turned out to be a slower and less effective inducer of Lmx1a and Foxa2, also led to poor mDA production; and (4) previous reports have credited the dopaminergic-promoting activity of Lmx1a to its early transgene expression in ESC-derived neural progenitors (Friling et al., 2009) and indicated that Lmx1a functions by cooperating with Foxa2 in specifying mDA fate during midbrain development (Lin et al., 2009; Nakatani et al., 2009).
The robust induction of Wnt1 and its targets in naïve neural progenitors is likely to be a key downstream mediator that confers the observed early induction of Lmx1a, in light of the recent finding that it can be directly regulated by Wnt1/β-catenin signaling (Chung et al., 2009). The same study also showed that, although Otx2 itself had no effect in promoting the expression of terminal mDA neuronal marker genes such as Th, Pitx3 and Nurr1, it significantly enhanced the regulatory effect of Lmx1a and Foxa2 on the expression of these genes. Thus, Otx2 plays a permissive role in Lmx1a/Foxa2-mediated mDA neuronal production. It is worth noting that a significant effect of FGF/ERK blockade is the maintenance of Otx2 in derived neural progenitors.
Our study also shows that, in addition to inducing a regulatory cascade for ventralizing nascent neural progenitors, FGF/ERK inhibition suppresses forebrain specification while promoting anterior neural induction, as demonstrated by the strong and consistent repression of the forebrain regulator genes Six3 and Foxg1 and the hindbrain marker Gbx2. Thus, blocking FGF/ERK at the onset of neural induction leads to a direct and early induction of the midbrain fate at the expense of forebrain and caudal neural fates. Our finding is consistent with the developmental role of FGF signaling in regionalization of the forebrain (Corson et al., 2003; Shimamura and Rubenstein, 1997).
Furthermore, we demonstrate the importance of precise temporal control of cell signaling and its cross-regulation with other signaling pathways in mDA neuronal fate specification. During development, Fgf8-mediated signaling can induce the patterned expression of many midbrain/rostral hindbrain genes and is required for normal development of the midbrain and cerebellum (Chi et al., 2003; Liu et al., 1999; Meyers et al., 1998). Fgf8-induced Wnt1 and engrailed are key regulators of midbrain and cerebellum patterning, as well as of the differentiation and survival of dopamine neurons (Prakash et al., 2006; Simon et al., 2001; Tang et al., 2009). In EpiSC-derived neural cultures, after an initial burst of upregulation induced by PD exposure, Wnt1 expression was subsequently reduced to a level below the no-PD control by unknown factors in the newly generated neural progenitors in d3-5 MD. This is the period when Shh, Lmx1a and Foxa2 expression levels continued to rise. Given that Shh and Wnt1 play opposing roles with regard to mDA neurogenesis (Joksimovic et al., 2009), our findings suggest that the delay in FGF reactivation, which suppresses Wnt1 levels, might be crucial for achieving high numbers of Th+ neurons by consolidating Lmx1a and Foxa2 expression via Shh signaling.
From a technological standpoint, we describe a novel method of mDA neuron differentiation that employs temporally controlled exposure of human and mouse pluripotent stem cells to an FGF/ERK-deficient environment. The highly reliable nature of this method was demonstrated using five independent mouse EpiSC lines, a mouse iPS cell line and two human ESC lines. This protocol offers several advantages over current methods of generating midbrain-specific DA neurons in that it is adherent culture-based and free from genetic manipulation and thus could be readily applied to other cell lines of interest. Furthermore, because it is fully chemically defined, this paradigm could be readily adapted for use in a clinical setting or scaled up for toxicity and drug screening relevant to developing new therapeutics for Parkinson's disease.
Supplementary Material
Supplementary Material
Acknowledgments
We thank Dr Michael German for Lmx1a antibody, Dr Marten Smidt for Pitx3 antibody, Drs Johan Ericson and Thomas Perlmann for the Lmx1a-GFP ESCs, Dr Dirk Dormann for expert advice on confocal microscopy and Drs Austin Smith, Tilo Kunath and Tristan Rodriguez for discussion and critical reading of the manuscript.
Footnotes
Funding
This work was supported by the UK Medical Research Council (MRC, G117/560, U120005004) and EU framework program 7 NeuroStemcell, no. 222943 to M.L.; and by the MRC (U120085816) and a Royal Society University Research Fellowship to M.A.U. M.L. was an MRC Senior Non-Clinical Research Fellow. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
References
- Alberi L., Sgado P., Simon H. H. (2004). Engrailed genes are cell-autonomously required to prevent apoptosis in mesencephalic dopaminergic neurons. Development 131, 3229-3236 [DOI] [PubMed] [Google Scholar]
- Andersson E., Tryggvason U., Deng Q., Friling S., Alekseenko Z., Robert B., Perlmann T., Ericson J. (2006). Identification of intrinsic determinants of midbrain dopamine neurons. Cell 124, 393-405 [DOI] [PubMed] [Google Scholar]
- Barberi T., Klivenyi P., Calingasan N. Y., Lee H., Kawamata H., Loonam K., Perrier A. L., Bruses J., Rubio M. E., Topf N., et al. (2003). Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nat. Biotechnol. 21, 1200-1207 [DOI] [PubMed] [Google Scholar]
- Bouhon I. A., Kato H., Chandran S., Allen N. D. (2005). Neural differentiation of mouse embryonic stem cells in chemically defined medium. Brain Res. Bull. 68, 62-75 [DOI] [PubMed] [Google Scholar]
- Brodski C., Weisenhorn D. M., Signore M., Sillaber I., Oesterheld M., Broccoli V., Acampora D., Simeone A., Wurst W. (2003). Location and size of dopaminergic and serotonergic cell populations are controlled by the position of the midbrain-hindbrain organizer. J. Neurosci. 23, 4199-4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brons I. G., Smithers L. E., Trotter M. W., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S. M., Howlett S. K., Clarkson A., Ahrlund-Richter L., Pedersen R. A., et al. (2007). Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191-195 [DOI] [PubMed] [Google Scholar]
- Chambers S. M., Fasano C. A., Papapetrou E. P., Tomishima M., Sadelain M., Studer L. (2009). Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi C. L., Martinez S., Wurst W., Martin G. R. (2003). The isthmic organizer signal FGF8 is required for cell survival in the prospective midbrain and cerebellum. Development 130, 2633-2644 [DOI] [PubMed] [Google Scholar]
- Chung S., Sonntag K. C., Andersson T., Bjorklund L. M., Park J. J., Kim D. W., Kang U. J., Isacson O., Kim K. S. (2002). Genetic engineering of mouse embryonic stem cells by Nurr1 enhances differentiation and maturation into dopaminergic neurons. Eur. J. Neurosci. 16, 1829-1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Hedlund E., Hwang M., Kim D. W., Shin B. S., Hwang D. Y., Jung Kang U., Isacson O., Kim K. S. (2005). The homeodomain transcription factor Pitx3 facilitates differentiation of mouse embryonic stem cells into AHD2-expressing dopaminergic neurons. Mol. Cell. Neurosci. 28, 241-252 [DOI] [PubMed] [Google Scholar]
- Chung S., Leung A., Han B. S., Chang M. Y., Moon J. I., Kim C. H., Hong S., Pruszak J., Isacson O., Kim K. S. (2009). Wnt1-lmx1a forms a novel autoregulatory loop and controls midbrain dopaminergic differentiation synergistically with the SHH-FoxA2 pathway. Cell Stem Cell 5, 646-658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corson L. B., Yamanaka Y., Lai K. M., Rossant J. (2003). Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development 130, 4527-4537 [DOI] [PubMed] [Google Scholar]
- Echelard Y., Epstein D. J., St-Jacques B., Shen L., Mohler J., McMahon J. A., McMahon A. P. (1993). Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417-1430 [DOI] [PubMed] [Google Scholar]
- Ferri A. L., Lin W., Mavromatakis Y. E., Wang J. C., Sasaki H., Whitsett J. A., Ang S. L. (2007). Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development 134, 2761-2769 [DOI] [PubMed] [Google Scholar]
- Friling S., Andersson E., Thompson L. H., Jonsson M. E., Hebsgaard J. B., Nanou E., Alekseenko Z., Marklund U., Kjellander S., Volakakis N., et al. (2009). Efficient production of mesencephalic dopamine neurons by Lmx1a expression in embryonic stem cells. Proc. Natl. Acad. Sci. USA 106, 7613-7618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspard N., Bouschet T., Hourez R., Dimidschstein J., Naeije G., van den Ameele J., Espuny-Camacho I., Herpoel A., Passante L., Schiffmann S. N., et al. (2008). An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature 455, 351-357 [DOI] [PubMed] [Google Scholar]
- Gennet N., Gale E., Nan X., Farley E., Takacs K., Oberwallner B., Chambers D., Li M. (2011). Doublesex and mab-3-related transcription factor 5 promotes midbrain dopaminergic identity in pluripotent stem cells by enforcing a ventral-medial progenitor fate. Proc. Natl. Acad. Sci. USA 108, 9131-9136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G., Yang J., Nichols J., Hall J. S., Eyres I., Mansfield W., Smith A. (2009). Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136, 1063-1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. L., Bickford P., Johansson M., Hoffer B. J., Stromberg I. (1994). Target and neurotransmitter specificity of fetal central nervous system transplants: importance for functional reinnervation. J. Neurosci. 14, 283-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksimovic M., Yun B. A., Kittappa R., Anderegg A. M., Chang W. W., Taketo M. M., McKay R. D., Awatramani R. B. (2009). Wnt antagonism of Shh facilitates midbrain floor plate neurogenesis. Nat. Neurosci. 12, 125-131 [DOI] [PubMed] [Google Scholar]
- Kala K., Haugas M., Lillevali K., Guimera J., Wurst W., Salminen M., Partanen J. (2009). Gata2 is a tissue-specific post-mitotic selector gene for midbrain GABAergic neurons. Development 136, 253-262 [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Mizuseki K., Nishikawa S., Kaneko S., Kuwana Y., Nakanishi S., Nishikawa S. I., Sasai Y. (2000). Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 28, 31-40 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Auerbach J. M., Rodriguez-Gomez J. A., Velasco I., Gavin D., Lumelsky N., Lee S. H., Nguyen J., Sanchez-Pernaute R., Bankiewicz K., et al. (2002). Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature 418, 50-56 [DOI] [PubMed] [Google Scholar]
- Kittappa R., Chang W. W., Awatramani R. B., McKay R. D. (2007). The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol. 5, e325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantoulas C. J., Parmar M., Li M. (2010). FoxP1 promotes midbrain identity in embryonic stem cell-derived dopamine neurons by regulating Pitx3. J. Neurochem. 113, 836-847 [DOI] [PubMed] [Google Scholar]
- Kunath T., Saba-El-Leil M. K., Almousailleakh M., Wray J., Meloche S., Smith A. (2007). FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 134, 2895-2902 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Lumelsky N., Studer L., Auerbach J. M., McKay R. D. (2000). Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat. Biotechnol. 18, 675-679 [DOI] [PubMed] [Google Scholar]
- Lin W., Metzakopian E., Mavromatakis Y. E., Gao N., Balaskas N., Sasaki H., Briscoe J., Whitsett J. A., Goulding M., Kaestner K. H., et al. (2009). Foxa1 and Foxa2 function both upstream of and cooperatively with Lmx1a and Lmx1b in a feedforward loop promoting mesodiencephalic dopaminergic neuron development. Dev. Biol. 333, 386-396 [DOI] [PubMed] [Google Scholar]
- Liu A., Losos K., Joyner A. L. (1999). FGF8 can activate Gbx2 and transform regions of the rostral mouse brain into a hindbrain fate. Development 126, 4827-4838 [DOI] [PubMed] [Google Scholar]
- Lunn J. S., Fishwick K. J., Halley P. A., Storey K. G. (2007). A spatial and temporal map of FGF/Erk1/2 activity and response repertoires in the early chick embryo. Dev. Biol. 302, 536-552 [DOI] [PubMed] [Google Scholar]
- Maxwell S. L., Ho H. Y., Kuehner E., Zhao S., Li M. (2005). Pitx3 regulates tyrosine hydroxylase expression in the substantia nigra and identifies a subgroup of mesencephalic dopaminergic progenitor neurons during mouse development. Dev. Biol. 282, 467-479 [DOI] [PubMed] [Google Scholar]
- Meyers E. N., Lewandoski M., Martin G. R. (1998). An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat. Genet. 18, 136-141 [DOI] [PubMed] [Google Scholar]
- Millet S., Campbell K., Epstein D. J., Losos K., Harris E., Joyner A. L. (1999). A role for Gbx2 in repression of Otx2 and positioning the mid/hindbrain organizer. Nature 401, 161-164 [DOI] [PubMed] [Google Scholar]
- Nakatani T., Minaki Y., Kumai M., Ono Y. (2007). Helt determines GABAergic over glutamatergic neuronal fate by repressing Ngn genes in the developing mesencephalon. Development 134, 2783-2793 [DOI] [PubMed] [Google Scholar]
- Nakatani T., Kumai M., Mizuhara E., Minaki Y., Ono Y. (2009). Lmx1a and Lmx1b cooperate with Foxa2 to coordinate the specification of dopaminergic neurons and control of floor plate cell differentiation in the developing mesencephalon. Dev. Biol. 339, 101-113 [DOI] [PubMed] [Google Scholar]
- Olander S., Nordstrom U., Patthey C., Edlund T. (2006). Convergent Wnt and FGF signaling at the gastrula stage induce the formation of the isthmic organizer. Mech. Dev. 123, 166-176 [DOI] [PubMed] [Google Scholar]
- Omodei D., Acampora D., Mancuso P., Prakash N., Di Giovannantonio L. G., Wurst W., Simeone A. (2008). Anterior-posterior graded response to Otx2 controls proliferation and differentiation of dopaminergic progenitors in the ventral mesencephalon. Development 135, 3459-3470 [DOI] [PubMed] [Google Scholar]
- Ono Y., Nakatani T., Sakamoto Y., Mizuhara E., Minaki Y., Kumai M., Hamaguchi A., Nishimura M., Inoue Y., Hayashi H., et al. (2007). Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development 134, 3213-3225 [DOI] [PubMed] [Google Scholar]
- Paek H., Gutin G., Hebert J. M. (2009). FGF signaling is strictly required to maintain early telencephalic precursor cell survival. Development 136, 2457-2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar M., Li M. (2007). Early specification of dopaminergic phenotype during ES cell differentiation. BMC Dev. Biol. 7, 86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier A. L., Tabar V., Barberi T., Rubio M. E., Bruses J., Topf N., Harrison N. L., Studer L. (2004). Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 101, 12543-12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash N., Brodski C., Naserke T., Puelles E., Gogoi R., Hall A., Panhuysen M., Echevarria D., Sussel L., Weisenhorn D. M., et al. (2006). A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo. Development 133, 89-98 [DOI] [PubMed] [Google Scholar]
- Roybon L., Hjalt T., Christophersen N. S., Li J. Y., Brundin P. (2008). Effects on differentiation of embryonic ventral midbrain progenitors by Lmx1a, MSX1, Ngn2, and Pitx3. J. Neurosci. 28, 3644-3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura K., Rubenstein J. L. (1997). Inductive interactions direct early regionalization of the mouse forebrain. Development 124, 2709-2718 [DOI] [PubMed] [Google Scholar]
- Simon H. H., Saueressig H., Wurst W., Goulding M. D., O’Leary D. D. (2001). Fate of midbrain dopaminergic neurons controlled by the engrailed genes. J. Neurosci. 21, 3126-3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavridis M. P., Lunn J. S., Collins B. J., Storey K. G. (2007). A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development 134, 2889-2894 [DOI] [PubMed] [Google Scholar]
- Tang M., Villaescusa J. C., Luo S. X., Guitarte C., Lei S., Miyamoto Y., Taketo M. M., Arenas E., Huang E. J. (2009). Interactions of Wnt/beta-catenin signaling and sonic hedgehog regulate the neurogenesis of ventral midbrain dopamine neurons. J. Neurosci. 30, 9280-9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar P. J., Chenoweth J. G., Brook F. A., Davies T. J., Evans E. P., Mack D. L., Gardner R. L., McKay R. D. (2007). New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448, 196-199 [DOI] [PubMed] [Google Scholar]
- Watanabe K., Kamiya D., Nishiyama A., Katayama T., Nozaki S., Kawasaki H., Watanabe Y., Mizuseki K., Sasai Y. (2005). Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 8, 288-296 [DOI] [PubMed] [Google Scholar]
- Wilson S. W., Rubenstein J. L. (2000). Induction and dorsoventral patterning of the telencephalon. Neuron 28, 641-651 [DOI] [PubMed] [Google Scholar]
- Yan Y., Yang D., Zarnowska E. D., Du Z., Werbel B., Valliere C., Pearce R. A., Thomson J. A., Zhang S. C. (2005). Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 23, 781-790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W., Shimamura K., Rubenstein J. L., Hynes M. A., Rosenthal A. (1998). FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell 93, 755-766 [DOI] [PubMed] [Google Scholar]
- Ying Q. L., Stavridis M., Griffiths D., Li M., Smith A. (2003). Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183-186 [DOI] [PubMed] [Google Scholar]
- Ying Q. L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. (2008). The ground state of embryonic stem cell self-renewal. Nature 453, 519-523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Maxwell S., Jimenez-Beristain A., Vives J., Kuehner E., Zhao J., O’Brien C., de Felipe C., Semina E., Li M. (2004). Generation of embryonic stem cells and transgenic mice expressing green fluorescence protein in midbrain dopaminergic neurons. Eur. J. Neurosci. 19, 1133-1140 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material