Functional Properties and Differential Neuromodulation of Nav1.6 Channels (original) (raw)
. Author manuscript; available in PMC: 2012 Sep 4.
Published in final edited form as: Mol Cell Neurosci. 2008 May 20;38(4):607–615. doi: 10.1016/j.mcn.2008.05.009
Abstract
The voltage-gated sodium channel Nav1.6 plays unique roles in the nervous system, but its functional properties and neuromodulation are not as well established as for NaV1.2 channels. We found no significant differences in voltage-dependent activation or fast inactivation between NaV1.6 and NaV1.2 channels expressed in non-excitable cells. In contrast, the voltage dependence of slow inactivation was more positive for Nav1.6 channels, they conducted substantially larger persistent sodium currents than Nav1.2 channels, and they were much less sensitive to inhibtion by phosphorylation by cAMP-dependent protein kinase and protein kinase C. Resurgent sodium current, a hallmark of Nav1.6 channels in neurons, was not observed for NaV1.6 expressed alone or with the auxiliary β4 subunit. The unique properties of NaV1.6 channels, together with the resurgent currents that they conduct in neurons, make these channels well-suited to provide the driving force for sustained repetitive firing, a crucial property of neurons.
Introduction
Voltage-gated sodium channels are widely distributed in neurons of the central nervous system to maintain normal patterns of neuronal electrical activity. Through their role in initiation and propagation of action potentials, sodium channels are essential in defining the input-output relationships of neurons, and they influence integration of dendritic responses, action potential threshold, burst duration, and pattern of firing (Colbert et al., 1997; Jung et al., 1997; Mickus et al., 1999; Johnston et al., 1999; Stuart, 1999; Stuart and Haussner, 1999; Gonzalez-Burgos and Barrionuevo, 2001). Different central neurons have distinct firing properties, and differential expression, function, and regulation of distinct sodium channel isoforms may contribute to these neuron-specific functions.
Voltage-gated sodium channels are encoded by a family of 10 genes in mammals (Catterall, 2000; Catterall et al., 2005). NaV1.3 channels are primarily expressed in embryonic and neonatal rodent brain, whereas Nav1.1, Nav1.2, and Nav1.6 are highly expressed in adult brain (Catterall et al., 2005). In contrast to rodents, NaV1.3 expression remains high in adult human brain (Chen et al., 2000; Whitaker et al., 2001). Deletion of each of the genes encoding the Nav1.1, Nav1.2, and Nav1.6 channel isoforms expressed in adult rodent brain leads to lethality (Burgess et al., 1995; Planell-Cases et al., 2000; Yu et al., 2006), suggesting that these sodium channels are essential for life and have unique functional roles. Electrophysiological studies have revealed only subtle differences in the properties of these sodium channel subtypes; however, slight changes in sodium channel function can alter action potential firing, as in inherited forms of periodic paralysis, cardiac arrhythmia, epilepsy, chronic pain, and congenital indifference to pain (Balser, 2002; Heron et al., 2007; Keating and Sanguinetti, 2001; Lossin et al., 2002; Meisler et al., 2001; Sugawara et al., 2001; Venance et al., 2006; Dib-Hajj et al., 2007) Although the brain sodium channel isoforms have similar functional properties, distinct subcellular localization and/or regulation of these isoforms may give them unique functional roles. Nav1.1 and Nav1.3 are localized primarily in the soma of CNS neurons, whereas Nav1.2 is primarily in unmyelinated axons (Westenbroek et al., 1989, 1992; Gong et al., 1999). In contrast, Nav1.6 channels are localized in high density in nodes of Ranvier and axon initial segments (Boiko et al., 2001; Caldwell et al., 2000) and in lower density in dendrites and cell bodies of some neurons. They are selectively expressed at high levels in cerebellar Purkinje neurons (Raman et al., 1997).
cAMP-dependent protein kinase (PKA) and protein kinase C (PKC) phosphorylate brain sodium channels in vitro and in intact neurons (Costa et al., 1982; Costa and Catterall, 1984a, b; Rossie and Catterall, 1987; 1989; Rossie et al., 1987) and reduce peak sodium currents in heterologous expression systems (Dascal and Lotan, 1991; Numann et al., 1991) and in neurons (Numann et al., 1991; Surmeier et al., 1992; Li et al., 1992; Cantrell et al., 1996; 1997; 1999a; Surmeier and Kitai, 1997; Carr et al., 2002). By reducing sodium currents, protein phosphorylation can regulate burst duration and pattern of action potential firing in neurons (Carr et al., 2003). The inhibition of sodium channel activity by PKA and PKC is voltage-dependent and involves enhancement of the intrinsic slow inactivation gating process (Li et al., 1993; Cantrell et al., 1999a, 2002; Carr et al., 2003; Chen et al., 2006). Regulation by PKC requires anchored PKCε (Chen et al., 2005). Regulation by PKA requires interaction with A Kinase Anchoring Protein 15 (AKAP15), which binds to the intracellular loop between domains I and II (LI–II; Cantrell et al., 1999b, 2002; Few et al., 2007). The key sites of phosphorylation by PKA and PKC in NaV1.1 and NaV1.2 channels are located in the inactivation gate (West et al., 1991) and in LI–II (Murphy et al., 1993; Smith and Goldin, 1996, 1997; Cantrell et al., 1997, 2002).
The Nav1.6 channel has been extensively studied in Purkinje neurons, where it conducts substantial peak, persistent, and resurgent sodium currents (Raman and Bean, 1997; Raman et al., 1997). The NaV1.6 channel contributes to peak sodium currents and repetitive firing of neurons in cortical pyramidal cells (Maurice et al., 2001), subthalamic neurons (Do and Bean, 2004), dorsal root ganglion neurons (Cummins et al., 2005), retinal ganglion cells (Van Wat and Matthews, 2005), globus pallidus neurons (Mercer et al., 2007), and trigeminal neurons (Enomoto et al., 2007). Nav1.6 has also been expressed in Xenopus oocytes and in dorsal root ganglia neurons, and its functional properties have been compared with Nav1.1 and Nav1.2 channels in those cell types (Smith et al., 1998; Rush et al., 2005; Rush et al., 2007). In this study, we transfected Nav1.6 channels into tsA-201 cells in order to analyze this channel expressed alone in mammalian non-neuronal cells, compare its functional properties and neuromodulation with NaV1.2 channels, and identify functional and regulatory properties that may be important for its role in action potential generation and repetitive firing.
Results
Activation and fast inactivation of Nav1.6 channels
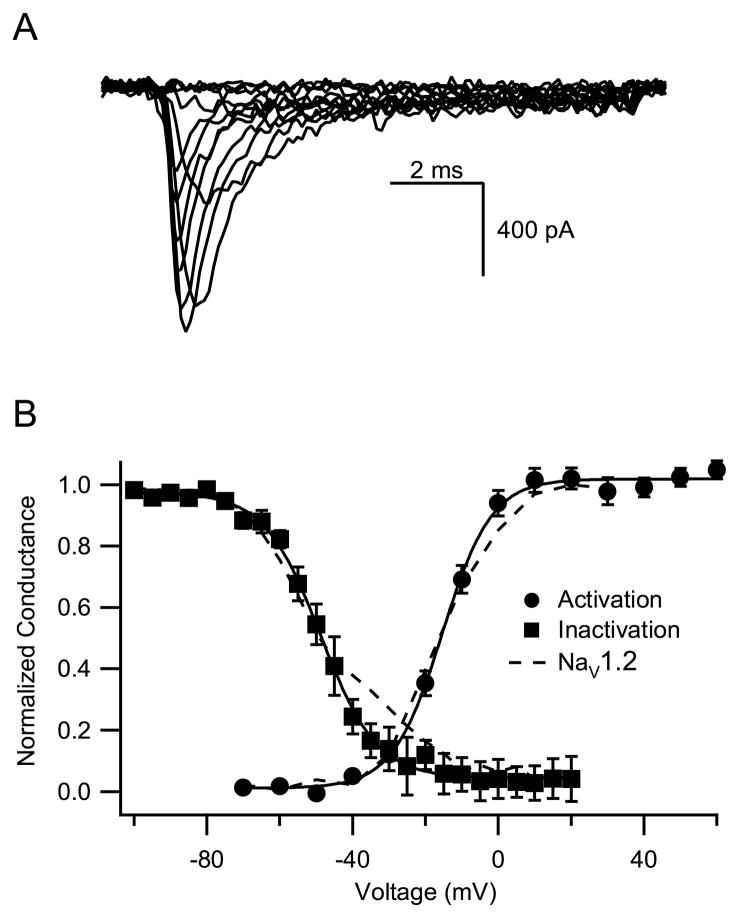
The properties of voltage-gated sodium channels vary depending on the cell background in which they are expressed. This is reflected in the variable findings in previous reports of the properties of NaV1.6 channels (Burbridge et al., 2002; Rush et al., 2005; Smith et al., 1998). To allow comparison with previous reports on the functional properties and neuromodulation of Nav1.2a channels from our laboratory, we examined the functional properties of Nav1.6 α subunits with clearly defined primary amino acid sequence expressed alone in tsA-201 cells. Sodium currents were evoked by 20-ms test pulses from a holding potential of −100 mV to potentials from −70 mV to +70 mV. The kinetics and voltage dependence of activation of the sodium current were similar for NaV1.2a and NaV1.6 channels in this cell background (Fig. 1A). The conductance-voltage relationship calculated from these results (Fig. 1B) yielded a value for the voltage of half-maximal activation, Va, for Nav1.6 of −13.6 ± 0.6 mV (n=9), not significantly different from Nav1.2 channels (Va= −14.9 ± 3.7 mV; Chen et al., 2006). The voltage dependence of fast inactivation was also similar for NaV1.6 and NaV1.2 channels (Fig. 1B). From a holding potential of −100 mV, prepulses of 100-ms duration to potentials between −100 mV and +20 mV were applied followed by test pulses to +10 mV. The half-maximal value for fast inactivation of Nav1.6 during these prepulses was −47.4 ± 2.4 mV (n=4), not significantly different from −48.8 ± 0.8 mV for Nav1.2a (Chen et al., 2006). Thus, there is little difference between these voltage-dependent properties of Nav1.2 and NaV1.6 when expressed under identical conditions in mammalian cells.
Fig. 1.
Voltage dependence of activation and fast inactivation of NaV1.6 channels. (A) Example sodium current records. (B) Activation. From a holding potential of −100 mV, a test pulse to the indicated potentials was applied for 10 ms and the sodium current was measured. Conductance was calculated from the peak sodium current and the reversal potential. Nav1.6, V1/2 = −13.6±0.6 mV, k = 6.7± 0.3, n=9; Nav1.2a, V1/2 = −14.9±3.7 mV, k = 6.8 ± 0.6. Fast inactivation. From a holding potential of −100 mV, a prepulse to the indicated potentials was applied for 100 ms, followed by a test pulse to 10 mV for 10 ms. Peak sodium currents during the test pulses were recorded and plotted versus the potential of the prepulse. Nav1.6, V1/2 = −47.4 ± 2.4, k = 8.4 ± 1.2, n=4; Nav1.2a = −48.8 ± 0.8 mV, k = 9.5 ± 0.7
Slow inactivation of NaV1.6 channels
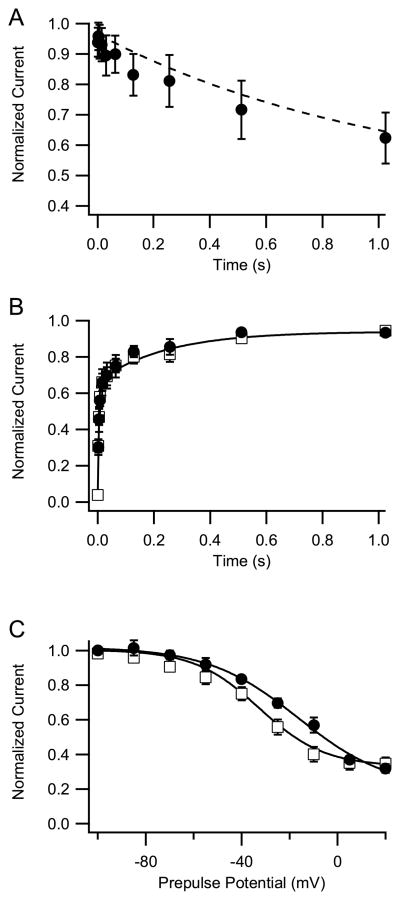
In addition to fast inactivation, sodium channels undergo a separate slow inactivation process on the time scale of seconds (Rudy, 1978). This slow inactivation process is an important factor governing sodium channel availability during periods of high activity (Carr et al., 2003). We measured entry into the slow inactivated state during prepulses to +10 mV of variable duration up to 1 s followed by a 20-ms repolarization to −100 mV to allow recovery from fast inactivation, and a final test pulse to +10 mV (Fig. 2_A_). The ratio of sodium current remaining in the test pulse following prepulses was plotted as a function of prepulse duration. Little difference in the rate of onset of slow inactivation between NaV1.6 and Nav1.2 channels was observed at this potential (Fig. 2_A_). We also examined recovery from fast and slow inactivation. Cells were depolarized to +10 mV for 1s. The membrane potential was returned to −100 mV to allow recovery from inactivation for times from 1 ms to 1000 ms, followed by a test pulse to +10 mV. The test pulse current was plotted as a function of the duration of repolarization to assess the rate of recovery from inactivation. Recovery from fast and slow inactivation of NaV1.6 was similar to that of Nav1.2 channels (Fig. 2_B_). We examined the voltage dependence of slow inactivation of NaV1.6 using 5-s prepulses. With this protocol, NaV1.6 inactivated less completely during prepulses ranging from −50 mV to −10 mV compared to NaV1.2a channels (Fig. 2_C_). The resulting changes in sodium channel availability may contribute to reduced use-dependent inactivation of NaV1.6 sodium channels, as previously reported (Rush et al., 2005), and could influence modulation by protein phosphorylation, which inhibits sodium channels by enhancing slow inactivation.
Fig. 2.
Slow inactivation of NaV1.6 channels. (A) Onset of slow inactivation. From a holding potential of −100 mV, a test pulse to 10 mV for 10 ms was applied and the sodium current was measured. A prepulse to 10 mV for the indicated times was then applied, the cells were repolarized to −100 mV for 20 ms to allow recovery from fast inactivation, and a second test pulse to 10 mV for 10 ms was applied. The mean (±SEM) ratio of peak sodium currents measured during the second test pulse to those measured during the first test pulse is plotted against prepulse duration (n=5). The dashed line is the fit to the NaV1.2 data from Chen et al. (2006) recorded under identical conditions. The data at different prepulse durations were fit with a single exponential. Circles, NaV1.6; dashed line, NaV1.2. (B) Recovery from slow inactivation. From a holding potential of −100 mV, a test pulse to 10 mV for 10 ms was applied and the sodium current was measured. A prepulse to 10 mV for 1s was then applied, the cells were repolarized to −100 mV for the indicated times to allow recovery from inactivation, a second test pulse to 10 mV for 2 ms was applied, and sodium currents were measured for WT and the indicated mutants. The mean (±SEM) ratio of peak amplitude of sodium current evoked in the second test pulse to that evoked in the first test pulse is plotted against the duration of the interpulse and fit to a single exponential (n=6). NaV1.6, circles; NaV1.2a, squares. (C) Voltage dependence of slow inactivation. From a holding potential of −100 mV, a test pulse to 10 mV for 10 ms was applied and the sodium current was measured. A conditioning prepulse to the indicated potentials was then applied for 5s, the cells were repolarized to −100 mV for 20 ms to allow recovery of fast inactivation, a second test pulse to 10 mV for 10 ms was applied, and sodium currents were measured. The mean (±SEM) ratios of the peak sodium current recorded during the test pulse to the sodium current recorded before the conditioning prepulse were plotted against the voltage of the conditioning pulse, and the data were fit to the Boltzmann equation. Nav1.6, V1/2 =−16.4 ± 3.5, k = 18.9 ± 2.9, n= 6; Nav1.2a, V1/2 =−33.1, k = 13.0 ± 1.5. NaV1.6, circles; NaV1.2a, squares. All NaV1.2a data are from Chen et. al. (2006).
Persistent sodium currents conducted by Nav1.6 channels
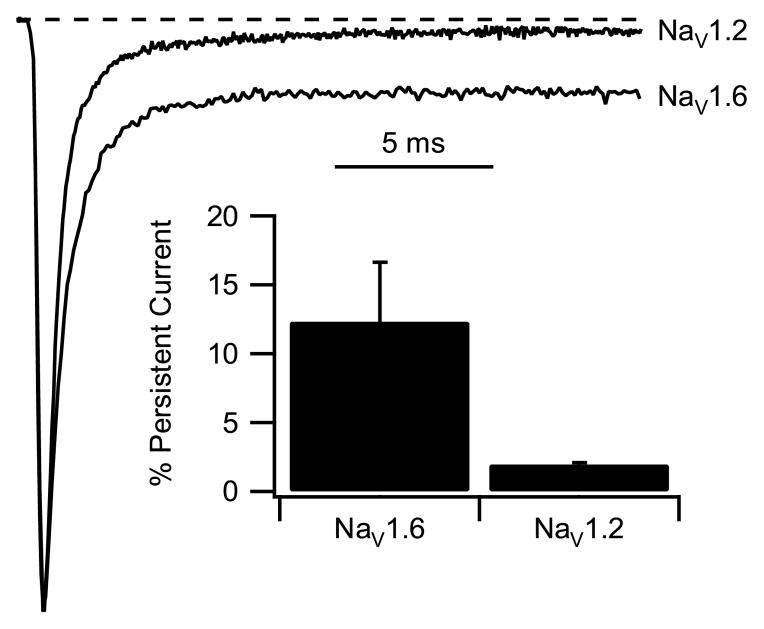
Persistent sodium current fails to inactivate after fast inactivation has reached steady state (Crill, 1996; Segal and Douglas, 1997). Sodium currents were measured in response to test pulses to +30 mV from a holding potential at −100 mV. This positive test potential was chosen to avoid contamination of the measurements of persistent sodium current by “window current” resulting from the overlap of activation and inactivation curves in the negative potential range. Persistent sodium current was measured as the mean current between 16 and 18 ms after the beginning of depolarization, when fast inactivation is complete at +30 mV, and the measured values for persistent sodium current were normalized to the peak inward transient sodium current. Mean persistent sodium current for NaV1.6 channels was significantly larger than for NaV1.2 channels (Fig. 3). For Nav1.6 channels, the persistent component of sodium current was 12.3 ± 4.3% of peak current (n=6) compared to 2.0 ± 0.8% (n=6) for Nav1.2 (p<0.01) (Fig. 3). Increased persistent sodium current would enhance dendritic integration of synaptic responses and increase repetitive firing of neurons expressing NaV1.6 channels.
Fig. 3.
Persistent sodium current of Nav1.6 channels. Average current traces depolarization to +30 mV from a holding potential of −100 mV. Lower trace, NaV1.6; upper trace, Nav1.2a. Inset. Bar graph representing the percentage of persistent current. Persistent currents were averaged between 16 to 18 ms after the beginning of the depolarization and divided by their peak amplitude. NaV1.6, 12.3 ± 4.3%, n=6; NaV1.2a, 2.0 ± 0.8%.
Resurgent sodium current conducted by Nav1.6 channels
Resurgent current is a unique form of sodium current, first described in Purkinje neurons (Raman and Bean, 1997; Raman et al., 1997). Resurgent sodium currents activate with a slow time course upon repolarization and then deactivate. It has been proposed that resurgent sodium currents arise from inactivated sodium channels that recover from inactivation via the open state (Raman and Bean, 2001) and that a blocking particle may serve to hold the pore open and reveal resurgent current as it dissociates (Raman and Bean, 2001; Grieco et al., 2005). As analyzed in knock-out mice, Nav1.6 channels contribute substantially to the resurgent sodium current in Purkinje neurons (Raman et al., 1997), although persistent sodium current is also generated by other sodium channel isoforms (Do and Bean, 2004), including NaV1.1 channels in Purkinje neurons (Kalume et al., 2007).
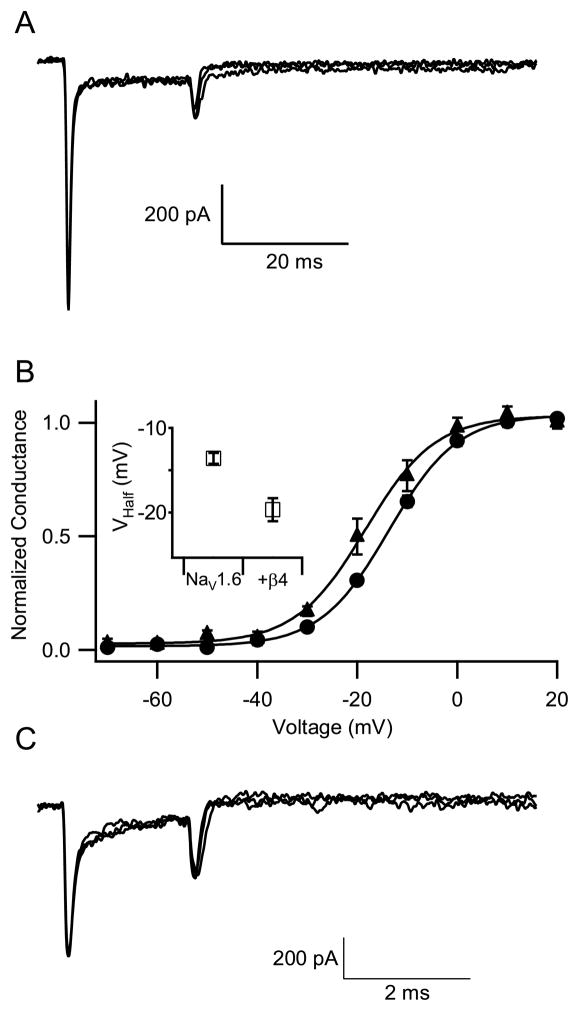
To investigate resurgent sodium current conducted by Nav1.6 channels, we activated sodium current with a 20-ms pulse to +30 mV from a holding potential of −100 mV (Fig. 4_A_). The sodium current during this test pulse activated and then inactivated. To elicit resurgent sodium current, the cell was repolarized to membrane potentials that ranged from −60 mV to +10 mV for 40 ms. An analogous protocol elicits prominent resurgent currents in Purkinje neurons, where it has a slow time course with a prominent peak 5 to 10 ms after repolarization (Raman and Bean, 1997; Raman et al., 1997). In contrast, in cells transfected with Nav1.6, no resurgent sodium current was observed (Fig. 4_A_, n=13).
Fig. 4.
Resurgent sodium current of NaV1.6 channels. (A) From a holding potential of −100 mV, a cell expressing NaV1.6 channels was depolarized by a prepulse to +30 mV for 40 ms to activate and inactivate sodium channels. Each prepulse was followed immediately by a single 20-ms test pulse to potentials from −60 mV to +20 mV in 10 mV increments. Representative current traces for test pulses to −60, −50, and −40 mV are shown. (B) Effect of β4 subunits on the voltage dependence of activation. The voltage dependence of activation was measured as in Fig. 1. NaV1.6, circles; NaV1.6 + β4, triangles. Inset. The graph shows the voltage of half-maximal activation for NaV1.6 alone (−13.6±0.6 mV, n=9) and Nav1.6 co-expressed with β4 subunits (−19.7±1.4 mV, n=6, p<0.05). (C) An experiment similar to panel A with NaV1.6 channels co-expressed with β4 subunits.
Resurgent current may arise when sodium channels conducting persistent current become blocked by an endogenous particle that prevents inactivation (Raman and Bean, 2001). According to this model, resurgent current is observed upon repolarization because the block is reversed when the cell is repolarized while the channels are still open. This allows current to flow transiently, before the sodium channel deactivates. Grieco et al. (2005) proposed that the cytoplasmic tail of the β4 subunit of the sodium channel is the endogenous particle responsible for reversible block of open sodium channels and production of resurgent current, based on experiments in which a peptide with the amino acid sequence of the cytoplasmic tail of this subunit was perfused at high concentration inside hippocampal pyramidal cells under whole-cell voltage clamp. To examine this idea further, we transfected NaV1.6 α subunits without and with β4 subunits in tsA-201 cells and looked for resurgent sodium currents (Fig. 4_B_, C). Co-transfection of the β4 subunit resulted in a −6 mV shift in the voltage-dependence of NaV1.6 activation (Fig. 4_B_; NaV1.6 alone, Va= −13.6 ± 0.6, n=6; NaV1.6 + β4, Va= −19.7 ± 1.4 mV, n=6, p<0.01). These results agree with the previously observed effect of β4 expression on the voltage dependence of activation of NaV1.2 and NaV1.4 channels (Yu et al., 2003) and confirm that the β4 subunits are effectively expressed and associate with NaV1.6 channels in tsA-201 cells. As illustrated in Fig. 4_C_, we were unable to detect resurgent sodium currents in NaV1.6 cells co-expressing full-length wild-type β4 subunit. This result indicates that co-expression of full-length β4 subunit with NaV1.6 channels is not sufficient by itself to reconstitute resurgent sodium currents in tsA-201 cells and suggests that other protein(s), which may act in concert with β4 subunits in neurons, may be required for induction of resurgent sodium currents.
Modulation of Nav1.6 channels by PKA and PKC
Serine/threonine protein kinases directly modulate sodium channels via phosphorylation of a family of sites in the inactivation gate and LI–II of NaV1.2 channels (Fig. 6A; Cantrell et al., 2001, 2002). Although the PKC phosphorylation site in the inactivation gate is identical in NaV1.2 and NaV1.6 channels, alignment of LI–II from NaV1.6 and NaV1.2 channels revealed significant alterations in the other known phosphorylation sites. Table 1 illustrates the amino acid sequences of the PKA and PKC phosphorylation sites in NaV1.2 channels, the corresponding amino acid sequences of NaV1.1 and NaV1.6, and the propensity of those sites for phosphorylation as determined with the Scansite 2.0 algorithm (www.scansite.mit.edu) (Obenauer et al., 2003). This program provides a percentile rank of a phosphorylation site of interest against all other serine/threonine sites, so a low percentile rank indicates a high propensity for phosphorylation. For example, S573 in NaV1.2 channels has a percentile rank of 0.85%, indicating that it is more likely to be phosphorylated by PKA than 99.15% of serine/threonine residues, and the corresponding residues in NaV1.1 channels has an even higher percentile rank of 0.47%. S573 is required for regulation of NaV1.2 channels by PKA and PKC (Smith and Goldin, 1997; Cantrell et al., 1997, 2002), but its sequence context is altered NaV1.6 channels making it much less likely to be phosphorylated (Table 1). Of the five additional phosphorylation sites (S554, S576, S610, S623, and S687) that are also necessary for optimal modulation of the sodium current by PKA and PKC in transfected tsA-201 cells (Cantrell et al., 2002), Ser687 is also much less likely to be phosphorylated in NaV1.6 channels (Table 1). These amino acid sequence comparisons suggest that NaV1.2 and NaV1.6 channels may be differentially regulated by PKA and/or PKC.
Fig. 6.
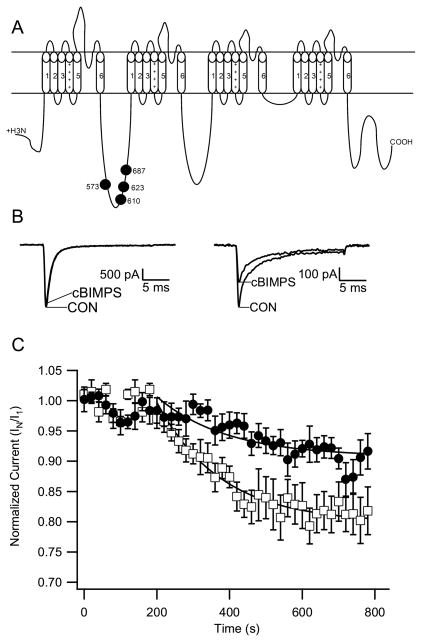
Modulation of Nav1.6 channels by PKA. (A) PKA phosphorylation sites on NaV1.2 channels. (B) From a holding potential of −70 mV, sodium currents were evoked every 20 s by a 20-ms test pulse to 10 mV. Perfusion with cBIMPS (50 μM) began at 60 s. Representative current traces for Nav1.6 before and after cBIMPS (left) and Nav1.2a before and after cBIMPS (right) are shown. (C) Time course of mean (±SEM) normalized peak sodium currents following addition of cBIMPS. The peak sodium currents were reduced by the following amounts at 200 ms: NaV1.6, 8.4 ± 2.9% (circles, n = 6); NaV1.2a, 21.7 ± 3.1% (squares, n = 5).
Table 1.
Phosphorylation Sites in LI–II of NaV1.1, NaV1.2, and NaV1.6 channels
| Nav1.2 Site | Channel | Sequence | PKA %ile Score | PKC %ile Score |
|---|---|---|---|---|
| Nav1.1 | KRYSSPHQS | 0.57, >5 | >5, >5 | |
| S554 | Nav1.2 | KRFSSPHQS | 1.98, 4.79 | >5, >5 |
| Nav1.6 | RKFSIMNQSLL | 0.28, NP | 1.98;NP | |
| Nav1.1 | PRRNSRTSL | 0.47, 3.69 | >5, 0.13 | |
| S573, S576 | Nav1.2 | PRRNSRASL | 0.85, 2.57 | >5. 0.16 |
| Nav1.6 | SRHNSKSSIFSFRGP | 4.9;>5 | >5;0.24 | |
| Nav1.1 | SRRDSLFVP | 0.19 | >5 | |
| S610 | Nav1.2 | SRRDSLFVP | 0.30 | >5 |
| Nav1.6 | GRRDSLFIPIRA | 0.06 | >5 | |
| Nav1.1 | ERRNSNLSQ | 0.44 | >5 | |
| S623 | Nav1.2 | ERRPSNVSQ | 0.59 | >5 |
| Nav1.6 | ERRSSYSGYSGY | >5;0.09 | >5;2.25 | |
| Nav1.1 | RKRRSSSFHVS | 0.39, 0.43, >5 | >5, 0.57, 1.86 | |
| S687 | Nav1.2 | RKRRSSSYHVS | 0.38, 0.53, 2.92 | >5, 0.48, 4.58 |
| Nav1.6 | KKKGPGSLLVS | NP,NP,>5 | NP,NP,>5 |
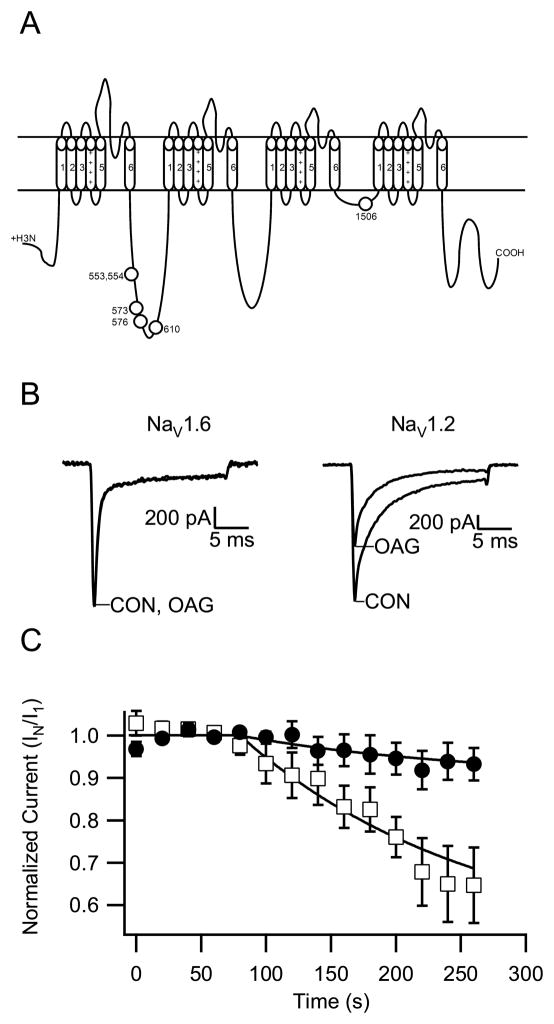
To examine regulation of NaV1.6 channels by PKC phosphorylation, we used 1-oleoyl-2-acetyl-sn-glycerol (OAG), an analog of the endogenous PKC activator diacylglycerol, to stimulate the activity of endogenous PKC in tsA-201 cells. OAG mimics the regulation of sodium channels by acetylcholine acting through muscarinic acetylcholine receptors and serotonin acting through 5-HT2a/c receptors and has been used extensively to study PKC effects on sodium channels in neurons and transfected cells (Cantrell et al., 2002; Cantrell et al., 1996; Carr et al., 2003; Chen et al., 2006; Dascal and Lotan, 1991; Numann et al., 1991; Sigel and Baur, 1988; West et al., 1991). To make a well-controlled, quantitative comparison, Nav1.6 and Nav1.2 channels were transfected into tsA-201 cells contemporaneously, and their sodium currents examined on the same day under similar conditions and using the same batches of solutions. In order to measure the voltage-dependent modulation by PKC, the cells were maintained at a holding potential of −70 mV, and sodium currents were recorded every 20 s in response to a 20-ms test pulse to +10 mV. Following acquisition of 3 to 5 identical records of peak sodium currents, we began perfusion of the cells with 50 μM OAG in the extracellular solution. We normalized peak amplitude of sodium currents to the average currents recorded prior to OAG and plotted against time. As presented in Figure 5, the peak sodium currents in cells expressing Nav1.2a channels declined by 35.3 ± 8.9% (n=7) during application of OAG for 5 min, which is a similar level of inhibition as in our previous experiments on NaV1.2 channels in neurons and transfected cells (Cantrell et al., 1996, 2002; Chen et al., 2005, 2006). In contrast, we found that the peak current of Nav1.6 channels decreased only slightly in the same period OAG perfusion (6.8 ± 3.9%, n=6, Fig. 5). This result suggests that Nav1.6 is much less sensitive to modulation by PKC than Nav1.2.
Fig. 5.
Modulation of Nav1.6 channels by PKC. (A) PKC phosphorylation sites of NaV1.2 channels. (B) From a holding potential of −70 mV, sodium currents were evoked every 20 s by a 20-ms test pulse to 10 mV. Perfusion with OAG (50 μM) began at 60 s. Representative current traces for Nav1.6 before and after OAG (left) and Nav1.2a before and after OAG (right) are shown. (C) Time course of mean (± SEM) normalized peak sodium currents following addition of OAG. At 200 s, the mean peak sodium currents were reduced as follows: NaV1.6, 6.8 ± 3.9% (n=6); NaV1.2a, 35.3 ± 8.9% (n=7).
We also studied the effect of activation of PKA on Nav1.6 sodium currents. We used the membrane-permeable cAMP analog DCl-cBIMPS (cBIMPS) as PKA activator in a similar manner to our previous experiments in neurons and transfected cells (Li et al., 1992; Cantrell et al., 1997, 1999a; Carr et al., 2003; Chen et al., 2006). Using the same protocol as described in the PKC experiment above, we found that peak sodium currents through Nav1.2 sodium channels declined by 21.7 ± 3.1% (n=5) after 11 min of external perfusion with cBIMPS (Figure 6). However, sodium currents conducted by Nav1.6 channels were inhibited only to 9.4 ± 2.9% (n=6) in the same time. These results indicate that neuromodulation by PKA is substantially reduced in Nav1.6 channels compared to Nav1.2a channels.
Discussion
Similar functional properties of NaV1.2 and NaV1.6 channels
Voltage-gated sodium channels are critical for neuronal excitation because they initiate and propagate action potentials. Three isoforms of sodium channels, Nav1.1, Nav1.2 and Nav1.6, are highly expressed in adult central nervous system (Westenbroek et al., 1989; Trimmer and Rhodes, 2004). The kinetics and voltage-dependence of activation and fast inactivation of Nav1.1 and Nav1.2 channels are essentially identical when expressed in tsA-201 cells (Chen et al., unpublished results). Sodium currents from Purkinje neurons that express high levels of Nav1.6 and hippocampal CA3 neurons that express high levels of Nav1.2 channels were found to be largely similar in their steady-state activation and fast inactivation properties, suggesting similar functional properties between Nav1.6 and Nav1.2 in neurons (Raman and Bean, 1997). Purkinje neurons express only NaV1.1 and NaV1.6 channels (Kalume et al., 2007). No differences in sodium current properties were found in cerebellar Purkinje neurons between Nav1.1 null mice and wild type mice, suggesting that there are no significant differences between activation and fast inactivation Nav1.1 and Nav1.6 in neurons (Kalume et al., 2007). However, a small shift in steady-state fast inactivation was reported in Purkinje neurons of Nav1.6 null mice compared to the wild type (Raman et al., 1997). Taken together, these data suggest that these three sodium channel subtypes share similar or identical steady-state activation and fast inactivation properties in transfected cells and neurons. Consistent with these previous results, we found that there are no significant differences in activation and fast inactivation between Nav1.6 and Nav1.2a expressed alone in the tsA-201 cell line.
In contrast, Nav1.6 properties are significantly different from Nav1.2 when expressed in other heterologous cells types. When Nav1.6 channels were transfected into dorsal root ganglion neurons (Rush et al., 2005), the voltage dependence of activation and fast inactivation were negatively shifted compared to those of Nav1.2 channels. On the other hand, Nav1.6 channels expressed in Xenopus oocytes (Smith et al., 1998) had more positive voltage dependence of activation and more negative voltage dependence of fast inactivation, although these differences were reduced by co-expression of β1 and β2 auxiliary sodium channel subunits. Evidently, different cellular contexts and auxiliary subunits may contribute to these apparent differences in sodium channel function. Our experiments show that NaV1.2 and NaV1.6 channels expressed alone in mammalian non-neuronal cells, without auxiliary subunits or other modulatory influences from excitable cells, have essentially identical activation and fast inactivation. These results are consistent with the nearly exact conservation of amino acid sequence of the voltage sensors and inactivation gates in these channels.
Slow inactivation
Although we did not find differences between Nav1.6 and Nav1.2a in activation or fast inactivation, we observed that Nav1.6 was significantly more resistant to slow inactivation in the voltage range from −60 mV to 0 mV. Exogenous Nav1.6 channels expressed in DRG neurons were better able to sustain high frequency repetitive firing than Nav1.2 channels, perhaps owing to this resistance to slow inactivation (Rush et al 2005). This more positive voltage dependence of slow inactivation would also be consistent with resistance to basal modulation by PKA and PKC in tsA-201 cells, since phosphorylation by these protein kinases enhances slow inactivation (Chen et al., 2006). However, as the level of slow inactivation was largely unchanged at −70 mV and −80 mV (Fig. 4), where the effects of phosphorylation by PKA and PKC are prominent (Chen et al., 2006), it is more likely that our results reflect an intrinsic difference in the voltage dependence of slow inactivation of NaV1.6 channels.
Persistent sodium current
Comparatively large persistent sodium currents are a common feature of Nav1.6 channels in many cell types (Smith et al., 1998; Rush et al., 2005; Burbidge, et al., 2002). Our results also show increased persistent sodium current by Nav1.6 compared to Nav1.2 channels when expressed side-by-side in tsA-201 cells. While the magnitude of persistent sodium current is typically a small fraction of the peak transient current in neurons, it can profoundly affect excitability by influencing net current at subthreshold membrane potentials where few voltage-gated channels are activated and input resistance is high, and persistent sodium currents can play a critical role in setting pacemaker activity of neurons that fire rhythmically. Persistent sodium current has been measured in many central neurons (Taddese and Bean, 2002; Do and Bean, 2004; Yue et al., 2005), and many disease mutations in sodium channels cause a gain of function to conduct more persistent inward current (Lossin et al., 2002; Sugawara et al., 2001, 2003; Vanoye et al., 2006). Our results indicate that NaV1.6 channels contribute more to persistent sodium currents than NaV1.2 channels and are therefore likely to have a more substantial influence on repetitive firing in central neurons where both are expressed.
Resurgent sodium current
Nav1.6 channels conduct resurgent sodium currents in cerebellar Purkinje neurons (Raman et al., 1997), although other channel isoforms do contribute a substantial proportion of total resurgent sodium currents (Do and Bean, 2004; Kalume et al., 2007). The mechanism underlying this resurgent current remains an active area of investigation. Raman and colleagues have recapitulated resurgent current by perfusing a peptide corresponding to the intracellular domain of the β4 subunit into hippocampal neurons that show no native resurgent currents (Grieco et al., 2005). They proposed that the positively charged amino acid residues in the intracellular domain of β4 subunit cause open channel block that can be relieved upon repolarization and reveal resurgent sodium current (Grieco et al., 2005). Our present results do not support this hypothesis for the full-length β4 subunit expressed at similar concentration to the subunit. No resurgent currents were recorded from cells co-transfected with Nav1.6 and full-length β4 subunit, even though the co-expressed β4 subunit was able to modify the voltage dependence of activation. Although typical sodium currents recorded in Nav1.6-transfected cells were usually 10-fold smaller than those in Purkinje neurons, we would easily have observed resurgent sodium currents comparable to those recorded in Purkinje neurons. Evidently, full-length β4 auxiliary subunits are not sufficient by themselves to induce resurgent sodium currents in tsA-201 cells. Either additional protein(s) are required for this effect of β4 subunits in neurons or the effect of the intracellular domain of the β4 subunit observed in previous experiments (Grieco et al., 2005) requires a high concentration of peptide that is not achieved by expression of full-length β4 in intact cells.
Differential regulation of NaV1.2 and NaV1.6 channels by protein phosphorylation
Nav1.2 channels are very sensitive to regulation by protein phosphorylation, resulting in a reduction of peak sodium currents (Cantrell et al., 1996, 1997; Carr et al., 2002, 2003; Chen et al., 2005, 2006; Li et al., 1992; Numann et al., 1991). In contrast, we find here that Nav1.6 channels are largely refractory to modulation by PKA or PKC. This striking difference in sodium channel regulation is likely to have important physiological significance.
In hippocampal pyramidal neurons, peak sodium currents are substantially reduced by dopamine acting at D1-like receptors and acetylcholine acting at muscarinic acetylcholine receptors through phosphorylation by PKA and PKC (Cantrell et al., 1996, 1997). This voltage-dependent reduction in peak sodium current reflects enhanced slow inactivation (Chen et al., 2006). In prefrontal cortex neurons, stimulation of 5-HT2A/C receptors activates PKC, reduces Na+ current, increases spike threshold, and reduces spike train duration (Carr 2002, 2003). These effects are also caused by enhancement of slow inactivation by PKC phosphorylation (Carr et al., 2003; Chen et al., 2006). The lack of modulation of NaV1.6 channels by these protein phosphorylation pathways indicates that these channels do not contribute to neurotransmitter modulation of sodium channel function via the PKA and PKC pathways, and therefore do not contribute substantially to modulation of threshold and firing pattern via this mechanism. These results are consistent with the findings of Maurice et al. (2004) that deletion of NaV1.6 channels in knockout mice does not alter the regulation of persistent sodium currents by dopamine activation of the PKA pathway.
Inspection of the amino acid sequences of the phosphorylation sites in NaV1.2 channels and their potential counterparts in Nav1.6 channels indicates a clear molecular basis for the difference in regulation of these two channel types (Table 1). The protein kinase modulation sites in LI–II of the NaV1.2 channel are nearly exactly conserved in NaV1.1 channels, which have comparable modulation by PKA and PKC (Chen et al., unpublished results), but are not well-conserved in the NaV1.6 channel. In particular, the amino acid sequence contexts of the phosphorylation sites at Ser573 and Ser687 in NaV1.2 channels are altered such that phosphorylation of the corresponding sites by PKA and PKC is much less likely in NaV1.6 channels. Evidently, the molecular properties of the NaV1.6 channel prevent its effective modulation by the PKA and PKC pathways.
NaV1.6 channels and sustained repetitive firing
Overall, there are four characteristic differences in properties of Nav1.6 channels compared to the NaV1.2 channels: larger persistent current, resistance to slow inactivation, unique resurgent current (Raman and Bean, 1997), and the resistance to inhibition by protein phosphorylation. All of these properties would support sustained high-frequency neuronal excitation (Rush et al., 2005). In agreement with this notion, Nav1.6 channels are abundant in Purkinje neurons whose repetitive firing is important for cerebellar function in control and fine-tuning of motor activities. The larger persistent sodium currents of Nav1.6 channels shown here would maintain depolarization of membrane potential near threshold and thereby permit firing of additional action potentials. The resurgent currents of Nav1.6 channels generate inward current after each action potential in Purkinje neurons and permit rapid recovery from inactivation, thereby facilitating high-frequency firing (Raman and Bean, 1997). Finally, the relative insensitivity of Nav1.6 sodium channels to slow inactivation and to enhancement of slow inactivation by protein phosphorylation shown in our results provides an additional margin of safety for repetitive firing. Consistent with a key role for NaV1.6 channels in sustained repetitive firing, recent studies with knockout mice show that haploinsufficiency of NaV1.6 channels is anti-epileptic and can compensate for the pro-epileptic effect of haploinsufficiency of the NaV1.1 channel (Martin et al., 2007). Our results elucidate the functional basis for this important role of NaV1.6 channels in the central nervous system.
Experimental Procedures
Molecular biology, transfection, and cell culture
Plasmid pCDM8-rIIA containing the cDNA encoding the full-length rat Nav1.2a α subunit has been described previously (Auld et al., 1990; Linford et al., 1998). Wild-type mouse Nav1.6 cDNA was a kind gift of Dr. Alan Goldin (UC Irvine). It was expanded and subcloned it into pCDM8 for expression in mammalian cells. Following subcloning into pCDM8, we fully sequenced the cDNA and corrected its sequence to correspond with the RefSeq database (NM_011323). The full-length human β4 cDNA was subcloned into pCDNA3.1 (Invitrogen, San Diego, CA).
TsA-201 cells, a subclone of human embryonic kidney HEK293 cells, were maintained as described (Herlitze et al., 1996). Sodium channel expression plasmids were transiently transfected using the calcium phosphate co-precipitation method. The cDNA encoding the CD8 receptor in pCD8-NEO was used as a marker of the transfected cells (Margolskee et al., 1993). Twelve hours later, transfected cells were replated at low density for electrophysiological recordings. The transfected cells were identified by labeling with CD8-specific antibody-coated microspheres (Dynal, Oslo, Norway). The mean sodium current among 63 transfected cells was approximately 1 nA.
Electrophysiology
Whole-cell patch-clamp recordings were performed at room temperature using an Axopatch 200 amplifier (Axon Instruments, Union City, CA). The voltage clamp data waere filtered at 10 kHz then digitized at 20 μs per point. 80–90% series resistance compensation was routinely used. Leak and capacitive transients were subtracted using a P/−4 protocol. Whole-cell voltage clamp experiments were conducted using two intracellular solutions. Most of the recordings were with cesium aspartate-based intracellular solution: 100 mM CsAspartate, 1 mM NaCl, 4 mM MgCl2, 10 mM EGTA, 0.5 mM CaCl2, 25 mM phosphocreatine (Tris salt), 2 mM ATP (Na salt), 0.2 mM GTP (Na salt) and 40 mM HEPES with pH 7.3 by CsOH. Measurements of persistent sodium currents were made with an intracellular solution consisting of: 179 mM NMG, 1 mM NaCl, 4 mM MgCl2, 10 mM EGTA, 0.5 mM CaCl2, 25 mM phosphocreatine (Tris salt), 2 mM ATP (Na salt), 0.2 mM GTP (Na salt) and 40 mM HEPES with pH 7.3 by H2SO4. The common extracellular solution contained 140 mM NaCl, 10 mM CsCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 50 mM glucose at pH 7.3.
Data were analyzed using IgorPro (Wave Metrics, Lake Oswego, OR) or SigmaPlot (Jandel Scientific Co., San Rafael, CA). Conductance-voltage (g-V) relationships (activation curves) were calculated from the current-voltage (I-V) relationships according to g = INa/(V-ENa), where INa was the peak Na+ current measured at potential, V, and ENa, the calculated equilibrium potential. Normalized activation and inactivation curves were fit to Boltzmann relationships of the form: y = 1/{1 + exp [(V - V1/2)/k]} + A, where y is normalized gNa or INa, A, the baseline, V, the membrane potential, V1/2, the voltage of half-maximal activation or inactivation, and k is a slope factor. Slow inactivation-voltage curves were fit with a modified Boltzmann equation (Carr et al., 2003) of the form: I/Imax = (1 - Iresid)/((1 + exp((Vm - V1/2)/k)) + Iresid, where Iresid is the residual fraction of current at the end of the test pulse and k is the slope factor. All averaged data are the mean ± s.e.m. We determined the statistical significance of differences between groups by Student’s t-test or paired t-test. The threshold P value for statistical significance was 0.05.
Acknowledgments
This work was supported by National Institutes of Health Research Grant NS15751 to W. A. C. and NIH NRSA NS43065 to Y. C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auld VJ, Goldin AL, Krafte DS, Catterall WA, Lester HA, Davidson N, Dunn RJ. A neutral amino acid change in segment IIS4 dramatically alters the gating properties of the voltage-dependent sodium channel. Proc Natl Acad Sci U S A. 1990;87:323–327. doi: 10.1073/pnas.87.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balser JR. Inherited sodium channelopathies: novel therapeutic and proarrhythmic molecular mechanisms. Trends Cardiovasc Med. 2002;11:229–237. doi: 10.1016/s1050-1738(01)00116-5. [DOI] [PubMed] [Google Scholar]
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. doi: 10.1016/s0896-6273(01)00265-3. [DOI] [PubMed] [Google Scholar]
- Burbidge SA, Dale TJ, Powell AJ, Whitaker WR, Xie XM, Romanos MA, Clare JJ. Molecular cloning, distribution and functional analysis of the NA(V)1.6. Voltage-gated sodium channel from human brain. Mol Brain Res. 2002;103:80–90. doi: 10.1016/s0169-328x(02)00188-2. [DOI] [PubMed] [Google Scholar]
- Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, Meisler MH. Mutation of a new sodium channel gene, Scn8a, in the mouse mutant ‘motor endplate disease’. Nat Genet. 1995;10:461–465. doi: 10.1038/ng0895-461. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Nav1.6 is localized at nodes of Ranvier, dendrites, and synapses. Proc Natl Acad Sci U S A. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nature Reviews/Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Tibbs VC, Westenbroek RE, Scheuer T, Catterall WA. Dopaminergic modulation of voltage-gated Na+ current in rat hippocampal neurons requires anchoring of cAMP-dependent protein kinase. J Neurosci. 1999b;19:RC21. doi: 10.1523/JNEUROSCI.19-17-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Ma JY, Scheuer T, Catterall WA. Muscarinic modulation of sodium current by activation of protein kinase C in rat hippocampal neurons. Neuron. 1996;16:1019–1026. doi: 10.1016/s0896-6273(00)80125-7. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Scheuer T, Catterall WA. Voltage-dependent neuromodulation of Na+ channels by D1-like dopamine receptors in rat hippocampal neurons. J Neurosci. 1999a;19:5301–5310. doi: 10.1523/JNEUROSCI.19-13-05301.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Smith RD, Goldin AL, Scheuer T, Catterall WA. Dopaminergic modulation of sodium current in hippocampal neurons via cAMP-dependent phosphorylation of specific sites in the sodium channel α subunit. J Neurosci. 1997;17:7330–7338. doi: 10.1523/JNEUROSCI.17-19-07330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Yu FH, Murphy BJ, Sharp EM, Qu Y, Catterall WA, Scheuer T. Molecular mechanisms of convergent regulation of brain Na+ channels by protein kinase A and protein kinase C. Mol. Cell Neurosci. 2002;21:63–80. doi: 10.1006/mcne.2002.1162. [DOI] [PubMed] [Google Scholar]
- Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci. 2002;22:6846–55. doi: 10.1523/JNEUROSCI.22-16-06846.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron. 2003;39:793–806. doi: 10.1016/s0896-6273(03)00531-2. [DOI] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cantrell AR, Messing RO, Scheuer T, Catterall WA. Specific modulation of Na+ channels in hippocampal neurons by protein kinase C-ε. J Neurosci. 2005;25:507–513. doi: 10.1523/JNEUROSCI.4089-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Dale TJ, Romanos MA, Whitaker WR, Xie XM, Clare JJ. Cloning, distribution and functional analysis of the type III sodium channel from human brain. Eur J Neurosci. 2000;12:4281–4289. [PubMed] [Google Scholar]
- Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase. Neuron. 2006;49:409–420. doi: 10.1016/j.neuron.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Magee JC, Hoffman DA, Johnston D. Slow recovery from inactivation of sodium channels underlies the activity dependent attenuation of dendritic action potentials in hippocampal CA1 pyramidal neurons. J Neurosci. 1997;17:6512–6521. doi: 10.1523/JNEUROSCI.17-17-06512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MRC, Catterall WA. Cyclic-AMP-dependent phosphorylation of the α subunit of the sodium channel in synaptic nerve ending particles. J Biol Chem. l984a;259:8210–8218. [PubMed] [Google Scholar]
- Costa MRC, Catterall WA. Phosphorylation of the α subunit of the sodium channel by protein kinase C. Cell Mol Neurobiol. 1984b;4:29l–297. doi: 10.1007/BF00733592. [DOI] [PubMed] [Google Scholar]
- Costa MRC, Casnellie JE, Catterall WA. Selective phosphorylation of the α subunit of the sodium channel by cAMP dependent protein kinase. J Biol Chem. 1982;257:7918–7921. [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Herzog RI, Waxman SG. NaV1.6 channels generate resurgent sodium currents in spinal sensory neurons. FEBS Lett. 2005;579:2166–2170. doi: 10.1016/j.febslet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Dascal N, Lotan I. Activation of protein kinase C alters the voltage-dependence of sodium channel. Neuron. 1991;6:165–175. doi: 10.1016/0896-6273(91)90131-i. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. From genes to pain: Na(v)1.7 and human pain disorders. Trends Neurosci. 2007;30:555–563. doi: 10.1016/j.tins.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Do MT, Bean BP. Sodium currents in subthalamic nucleus neurons from Nav1.6-null mice. J Neurophysiol. 2004;92:726–733. doi: 10.1152/jn.00186.2004. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Han JM, Hsiao CF, Chandler SH. Sodium currents in mesencephalic trigeminal neurons from NaV1.6 null mice. J Neurophysiol. 2007;98:710–719. doi: 10.1152/jn.00292.2007. [DOI] [PubMed] [Google Scholar]
- Few WP, Scheuer T, Catterall WA. Dopamine modulation of neuronal Na+ channels requires binding of A kinase-anchoring protein 15 and PKA by a modified leucine zipper motif. Proc Natl Acad Sci U S A. 2007;104:5187–5192. doi: 10.1073/pnas.0611619104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Rhodes KJ, Bekele-Arcuri Z, Trimmer JS. Type I and type II Na+ channel alpha-subunit polypeptides exhibit distinct spatial and temporal patterning, and association with auxiliary subunits in rat brain. J Comp Neurol. 1999;412:342–352. [PubMed] [Google Scholar]
- Gonzalez-Burgos GR, Barrioneuvo G. Voltage-gated sodium channels shape subthreshold EPSPs in layer 5 pyramidal neurons from rat prefrontal cortex. J Neurophysiol. 2001;86:1671–1684. doi: 10.1152/jn.2001.86.4.1671. [DOI] [PubMed] [Google Scholar]
- Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM. Open-channel block by the cytoplasmic tail of sodium channel beta4 as a mechanism for resurgent sodium current. Neuron. 2005;45:233–244. doi: 10.1016/j.neuron.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Heron SE, Scheffer IE, Berkovic SF, Dibbens LM, Mulley JC. Channelopathies in idiopathic epilepsy. Neurotherapeutics. 2007;4:295–304. doi: 10.1016/j.nurt.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Colbert CM, Magee JC. Regulation of back-propagating action potentials in hippocampal neurons. Curr Opin Neurobiol. 1999;9:288–292. doi: 10.1016/s0959-4388(99)80042-7. [DOI] [PubMed] [Google Scholar]
- Jung HY, Mickus T, Spruston N. Prolonged sodium channel inactivation contributes to dendritic action potential attenuation in hippocampal pyramidal neurons. J Neurosci. 1997;17:6639–6646. doi: 10.1523/JNEUROSCI.17-17-06639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J Neurosci. 2007;27:11065–11074. doi: 10.1523/JNEUROSCI.2162-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Levin SI, Khaliq ZM, Aman TK, Grieco TM, Kearney JA, Raman IM, Meisler MH. Impaired motor function in mice with cell-specific knockout of sodium channelScn8a (NaV1.6) in cerebellar purkinje neurons and granule cells. J Neurophysiol. 2006 Aug;96(2):785–93. doi: 10.1152/jn.01193.2005. [DOI] [PubMed] [Google Scholar]
- Li M, West JW, Numann R, Murphy BJ, Scheuer T, Catterall WA. Convergent regulation of Na+ channels by protein kinase C and cAMP-dependent protein kinase. Science. 1993;261:1439–1442. doi: 10.1126/science.8396273. [DOI] [PubMed] [Google Scholar]
- Li M, West JW, Lai Y, Scheuer T, Catterall WA. Functional modulation of brain sodium channels by cAMP-dependent phosphorylation. Neuron. 1992;8:1151–1159. doi: 10.1016/0896-6273(92)90135-z. [DOI] [PubMed] [Google Scholar]
- Linford NJ, Cantrell AR, Qu Y, Scheuer T, Catterall WA. Interaction of batrachotoxin with the local anesthetic receptor site in transmembrane segment IVS6 of the voltage-gated sodium channel. Proc Natl Acad Sci U S A. 1998;95:13947–13952. doi: 10.1073/pnas.95.23.13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossin C, Wang DW, Rhodes TH, Vanoye CG, George AL., Jr Molecular basis of an inherited epilepsy. Neuron. 2002;34:877–884. doi: 10.1016/s0896-6273(02)00714-6. [DOI] [PubMed] [Google Scholar]
- Margolskee RF, McHendry-Rinde B, Horn R. Panning transfected cells for electrophysiological studies. Biotechniques. 1993;5:906–911. [PubMed] [Google Scholar]
- Martin MS, Tang B, Papale LA, Yu FH, Catterall WA, Escayg A. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy. Hum Mol Genet. 2007;16:2892–2899. doi: 10.1093/hmg/ddm248. [DOI] [PubMed] [Google Scholar]
- Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci. 2001;21:2268–2277. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Mercer J, Chan CS, Hernandez-Lopez S, Held J, Tkatch T, Surmeier DJ. D2 dopamine receptor-mediated modulation of voltage-dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci. 2004;24:10289–10301. doi: 10.1523/JNEUROSCI.2155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler MH, Kearney J, Ottman R, Escayg A. Identification of epilepsy genes in human and mouse. Annu Rev Genet. 2001;35:567–588. doi: 10.1146/annurev.genet.35.102401.091142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer JN, Chan CS, Tkatch T, Held J, Surmeier DJ. Nav1.6 sodium channels are critical to pacemaking and fast spiking in globus pallidus neurons. J Neurosci. 2007;27:13552–13566. doi: 10.1523/JNEUROSCI.3430-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickus T, Jung H, Spruston N. Properties of slow, cumulative sodium channel inactivation in rat hippocampal CA1 pyramidal neurons. Biophys J. 1999;76:846–860. doi: 10.1016/S0006-3495(99)77248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BJ, Rossie S, DeJongh KS, Catterall WA. Identification of the sites of selective phosphorylation and dephosphorylation of the rat brain sodium channel alpha subunit by cAMP-dependent protein kinase and phosphoprotein phosphatases. J Biol Chem. 1993;268:27355–27362. [PubMed] [Google Scholar]
- Numann R, Catterall WA, Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991;254:115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- Obenauer JC, Cantley LC, Yaffe MB. Scansite 2.0: Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planells-Cases R, Caprini M, Zhang J, Rockenstein EM, Rivera RR, Murre C, Masliah E, Montal M. Neuronal death and perinatal lethality in voltage-gated sodium channel alpha(II)-deficient mice. Biophys J. 2000;78:2878–2891. doi: 10.1016/S0006-3495(00)76829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Inactivation and recovery of sodium currents in cerebellar Purkinje neurons: evidence for two mechanisms. Biophys J. 2001;80:729–737. doi: 10.1016/S0006-3495(01)76052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17:4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 1997;19:881–891. doi: 10.1016/s0896-6273(00)80969-1. [DOI] [PubMed] [Google Scholar]
- Rossie S, Catterall WA. Cyclic AMP-dependent phosphorylation of voltage-sensitive sodium channels in primary cultures of rat brain neurons. J Biol Chem. 1987;262:12735–12744. [PubMed] [Google Scholar]
- Rossie S, Catterall WA. Phosphorylation of the α subunit of rat brain sodium channels by cAMP-dependent protein kinase at a new site containing Ser686 and Ser687. J Biol Chem. 1989;264:14220–14224. [PubMed] [Google Scholar]
- Rossie S, Gordon D, Catterall WA. Identification of an intracellular domain of the sodium channel having multiple cyclic AMP-dependent phosphorylation sites. J Biol Chem. 1987;262:17530–17535. [PubMed] [Google Scholar]
- Rudy B. Slow inactivation of the sodium conductance in squid giant axons. Pronase resistance. J Physiol. 1978;283:1–21. doi: 10.1113/jphysiol.1978.sp012485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J Physiol. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AM, Dib-Hajj SD, Waxman SG. Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones. J Physiol. 2005;564:803–815. doi: 10.1113/jphysiol.2005.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal MM, Douglas AF. Late Sodium Channel Openings Underlying Epileptiform Activity Are Preferentially Diminished by the Anticonvulsant Phenytoin. The Journal of Neurophysiology. 1997;77:3021–3034. doi: 10.1152/jn.1997.77.6.3021. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R. Activation of protein kinase C differentially modulates neuronal Na+, Ca2+, and gamma-aminobutyrate type A channels. Proc Natl Acad Sci U S A. 1988;85:6192–6196. doi: 10.1073/pnas.85.16.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Smith RD, Plummer NW, Meisler MH, Goldin AL. Functional analysis of the mouse Scn8a sodium channel. J Neurosci. 1998;18:6093–6102. doi: 10.1523/JNEUROSCI.18-16-06093.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RD, Goldin AL. Phosphorylation of brain sodium channels in the I-II linker modulates channel function in Xenopus oocytes. J Neurosci. 1996;16:1965–1974. doi: 10.1523/JNEUROSCI.16-06-01965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RD, Goldin AL. Phosphorylation at a single site in the brain sodium channel is necessary and sufficient for current reduction by protein kinase A. J Neurosci. 1997;17:6086–6093. doi: 10.1523/JNEUROSCI.17-16-06086.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G. Voltage-activated sodium channels amplify inhibition in neocortical pyramidal neurons. Nature Neurosci. 1999;2:144–150. doi: 10.1038/5698. [DOI] [PubMed] [Google Scholar]
- Stuart G, Haussner M. Dendritic coincidence detection of EPSPs and action potentials. Nature Neurosci. 2001;4:63–71. doi: 10.1038/82910. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Tsurubuchi Y, Agarwala KL, Ito M, Fukuma G, Mazaki-Miyazaki E, Nagafuji H, Noda M, Imoto K, Wada K, Mitsudome A, Kaneko S, Montal M, Nagata K, Hirose S, Yamakawa K. A missense mutation of the Na+ channel alpha II subunit gene NaV1.2 in a patient with febrile and afebrile seizures causes channel dysfunction. Proc Natl Acad Sci U S A. 2001;98:6384–6389. doi: 10.1073/pnas.111065098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Tsurubuchi Y, Fujiwara T, Mazaki-Miyazaki E, Nagata K, Montal M, Inoue Y, Yamakawa K. Nav1.1 channels with mutations of severe myoclonic epilepsy in infancy display attenuated currents. Epilepsy Res. 2003;54:201–207. doi: 10.1016/s0920-1211(03)00084-6. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Kitai ST. State-dependent regulation of neuronal excitability by dopamine. Nihon Shinkei Seishin Yakurigaku Zasshi. 1997;17:105–110. [PubMed] [Google Scholar]
- Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci USA. 1992;89:10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddese A, Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron. 2002;33:587–600. doi: 10.1016/s0896-6273(02)00574-3. [DOI] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- Van Wart A, Matthews G. Impaired firing and cell-specific compensation in neurons lacking NaV1.6 sodium channels. J Neurosci. 2006;26:7172–7180. doi: 10.1523/JNEUROSCI.1101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanoye CG, Lossin C, Rhodes TH, George AL., Jr Single-channel properties of human NaV1.1 and mechanism of channel dysfunction in SCN1A-associated epilepsy. J Gen Physiol. 2006;127:1–14. doi: 10.1085/jgp.200509373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venance SL, Cannon SC, Fialho D, Fontaine B, Hanna MG, Ptacek LJ, Tristani-Firouzi M, Tawil R, Griggs RC. The primary periodic paralyses: diagnosis, pathogenesis and treatment. Brain. 2006;129:8–17. doi: 10.1093/brain/awh639. [DOI] [PubMed] [Google Scholar]
- West JW, Numann R, Murphy BJ, Scheuer T, Catterall WA. A phosphorylation site in the Na+ channel required for modulation by protein kinase C. Science. 1991;254:866–868. doi: 10.1126/science.1658937. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Merrick DK, Catterall WA. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989;3:695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Noebels JL, Catterall WA. Elevated expression of type II Na+ channels in hypomyelinated axons of shiverer mouse brain. J Neurosci. 1992;12:2259–2267. doi: 10.1523/JNEUROSCI.12-06-02259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker WR, Faull RL, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ. Comparative distribution of voltage-gated sodium channel proteins in human brain. Brain Res Mol Brain Res. 2001;88:37–53. doi: 10.1016/s0169-328x(00)00289-8. [DOI] [PubMed] [Google Scholar]
- Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R. Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity tobeta2. J Neurosci. 2003;23:7577–7585. doi: 10.1523/JNEUROSCI.23-20-07577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- Yue C, Remy S, Su H, Beck H, Yaari Y. Proximal persistent Na+ channels drive spike afterdepolarizations and associated bursting in adult CA1 pyramidal cells. J Neurosci. 2005;25:9704–9720. doi: 10.1523/JNEUROSCI.1621-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]