Carbapenemases in Klebsiella pneumoniae and Other Enterobacteriaceae: an Evolving Crisis of Global Dimensions (original) (raw)
Abstract
Summary: The spread of Enterobacteriaceae, primarily Klebsiella pneumoniae, producing KPC, VIM, IMP, and NDM carbapenemases, is causing an unprecedented public health crisis. Carbapenemase-producing enterobacteria (CPE) infect mainly hospitalized patients but also have been spreading in long-term care facilities. Given their multidrug resistance, therapeutic options are limited and, as discussed here, should be reevaluated and optimized. Based on susceptibility data, colistin and tigecycline are commonly used to treat CPE infections. Nevertheless, a review of the literature revealed high failure rates in cases of monotherapy with these drugs, whilst monotherapy with either a carbapenem or an aminoglycoside appeared to be more effective. Combination therapies not including carbapenems were comparable to aminoglycoside and carbapenem monotherapies. Higher success rates have been achieved with carbapenem-containing combinations. Pharmacodynamic simulations and experimental infections indicate that modification of the current patterns of carbapenem use against CPE warrants further attention. Epidemiological data, though fragmentary in many countries, indicate CPE foci and transmission routes, to some extent, whilst also underlining the lack of international collaborative systems that could react promptly and effectively. Fortunately, there are sound studies showing successful containment of CPE by bundles of measures, among which the most important are active surveillance cultures, separation of carriers, and assignment of dedicated nursing staff.
INTRODUCTION
Klebsiella pneumoniae is encountered as a saprophyte in humans and other mammals, colonizing the gastrointestinal tract, skin, and nasopharynx; it is also found in various environmental niches (soil, water, etc.) (11). In the past, it was considered an important causative agent of community-acquired (CA) infections, including a severe form of pneumonia. Recently, while CA pneumonia due to K. pneumoniae has become rare, novel manifestations of CA infections, such as liver abscess complicated by endophthalmitis and other metastatic infections, have been described (140).
In the early 1970s, both the epidemiology and spectrum of infections caused by K. pneumoniae changed dramatically when this bacterium was established in the hospital environment and became a (still) leading cause of nosocomial infections. Not only is it found in the gastrointestinal tracts of patients, at frequencies as high as 80%, but high carriage rates have also been recorded for patient nasopharynges and hands (212). This considerable efficiency of colonization, enhanced by acquired resistance to antibiotics, enables K. pneumoniae to persist and spread rapidly in health care settings (119). Although not inherently resistant to antibiotics, since it produces only moderate amounts of chromosomal penicillinases, K. pneumoniae is a notorious “collector” of multidrug resistance plasmids. During the 1970s to 1980s, these were commonly plasmids encoding resistance to aminoglycosides. Later, however, K. pneumoniae became the index species for plasmids encoding extended-spectrum β-lactamases (ESBLs)—mostly TEMs and SHVs active against newer cephalosporins—along with a variety of genes conferring resistance to drugs other than β-lactams (212). The successive addition of genetic elements encoding resistance to aminoglycosides and extended-spectrum β-lactams, coupled with the rapid accumulation of chromosomal mutations conferring resistance to fluoroquinolones, left carbapenems as the first-choice drugs for the treatment of health care-associated infections caused by K. pneumoniae.
This was true until approximately 2000, when we began witnessing a global crisis of unprecedented dimensions due to the rapid dissemination of multidrug-resistant (MDR) K. pneumoniae strains producing “carbapenemases” encoded by transmissible plasmids. Later, other clinically important enterobacterial species, including Escherichia coli, acquired carbapenemase genes (202). Thus, it appears probable that as in the ESBL “era,” K. pneumoniae again functions as a pool of potent β-lactamases. The clinically most important carbapenemases in Enterobacteriaceae are the class A enzymes of the KPC type and the zinc-dependent class B metallo-β-lactamases (MβLs), represented mainly by the VIM, IMP, and NDM types. The plasmid-expressed class D carbapenemases of the OXA-48 type complete the picture (Table 1) (107, 166, 202).
Table 1.
Types, classification, variants, and species distribution of plasmid-mediated carbapenemases encountered in Enterobacteriaceae
| Type | Molecular class (subclass)a | Functional groupb | Variants | Species |
|---|---|---|---|---|
| KPC | A | 2f | KPC-2 to -13 | K. pneumoniae, E. coli, Klebsiella oxytoca, S. marcescens, Enterobacter spp., C. freundii, Salmonella enterica, Raultella spp. |
| VIM | B (B1) | 3a | VIM-1, -2, -4, -5, -6 | K. pneumoniae, E. coli, K. oxytoca, S. marcescens |
| VIM-11, -12, -13, -19, -23 | Serratia liquefaciens, Enterobacter spp., C. freundii | |||
| VIM-24, -25, -26, -27, -32 | Morganella morganii, Proteus stuartii, P. mirabilis | |||
| IMP | B (B1) | 3a | IMP-1, -3, -4, -6, -8 | K. pneumoniae, E. coli, K. oxytoca, S. marcescens |
| IMP-11, -24, -27 | Enterobacter spp., Citrobacter spp., P. mirabilis, Proteus rettgeri, Shigella flexneri, M. morganii | |||
| NDM | B (B1) | 3a | NDM-1, -4, -5, -6 | K. pneumoniae, E. coli, Enterobacter spp., K. oxytoca, C. freundii, M. morganii, Providencia spp. |
| OXA | D | 2df | OXA-48, -163, -181 | K. pneumoniae, E. coli, C. freundii, P. mirabilis |
Carbapenemase-producing enterobacteria (CPE) cause serious infections in debilitated and immunocompromised patients, in association with prolonged hospital stays and increased mortality rates, ranging from 24% to as high as 70%, depending on the study population (14, 24, 28, 67, 175, 187, 203, 233, 274). Given the critical condition of these patients, treatment should be timely, aggressive, and rapidly efficacious. However, therapeutic options are obviously limited, and unfortunately, the introduction of new antimicrobials such as tigecycline or the “reinvention” of colistin has far from entirely resolved this problem, as discussed in a later section.
In this review, we attempt to (i) describe the microbiological and epidemiological characteristics of carbapenemase-producing Enterobacteriaceae and (ii) present in a critical manner the available data regarding the antimicrobial treatment and infection control practices used to combat infections caused by these bacteria.
GENETIC CONTEXT, SUBSTRATE SPECTRA, AND β-LACTAM RESISTANCE PHENOTYPES
KPC Carbapenemases
KPC β-lactamases (KPC-2 to KPC-13; molecular class A) (www.lahey.org/studies) exhibit activity against a wide spectrum of β-lactams, including penicillins, older and newer cephalosporins, aztreonam, and carbapenems (Table 2) (191). Structural studies and comparisons with the TEM-1 and SHV-1 penicillinases indicated that positioning of the catalytic residues in KPCs may allow accommodation of the bulky α-substituents of carbapenems in a manner facilitating the subsequent acylation and deacylation steps (123).
Table 2.
Hydrolytic efficiencies of representative carbapenemase variants against various β-lactam substrates
| β-Lactamase | Hydrolytic efficiency (_k_cat/Km) (s−1 μM−1)a against: | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Imipenem | Meropenem | Ceftazidime | Cefotaxime | Aztreonam | Cefoxitin | Cephalothin | Penicillin G | ||
| KPC-2 | 0.29 | 0.27 | ND | 0.10 | 0.08 | 0.002 | 0.84 | 1.90 | 271 |
| KPC-3 | 1.90 | 1.40 | 0.03 | 0.50 | ND | 0.50 | 3.50 | ND | 4 |
| VIM-1 | 0.13 | 0.26 | 0.08 | 0.68 | — | 0.20 | 5.10 | 0.04 | 93 |
| VIM-2 | 3.80 | 2.50 | 0.05 | 5.80 | — | 1.20 | 11.8 | 4.0 | 74 |
| VIM-4 | 23.0 | 0.90 | ND | ND | — | ND | 36.0 | 3.10 | 137 |
| VIM-5 | 0.29 | 0.05 | 0.001 | 0.09 | — | ND | ND | 0.26 | 95 |
| VIM-19 | 6.0 | 2.0 | 0.02 | 30.0 | — | 0.50 | ND | 5.0 | 227 |
| VIM-27 | 0.26 | ND | ND | 0.82 | — | 0.03 | 8.30 | ND | 198 |
| IMP-1 | 1.20 | 0.12 | 0.18 | 0.35 | — | 2.0 | 2.40 | 0.62 | 136 |
| IMP-4 | 0.35 | 0.18 | 0.07 | 0.14 | — | ND | 0.43 | 0.08 | 51 |
| NDM-1 | 0.21 | 0.25 | 0.03 | 0.58 | — | 0.02 | 0.40 | 0.68 | 273 |
| NDM-4 | 0.46 | 0.31 | 0.06 | 1.20 | — | — | 0.50 | ND | 189 |
| OXA-48 | 0.14 | <0.001 | 0.001 | 0.05 | — | ND | 0.15 | 6.10 | 217 |
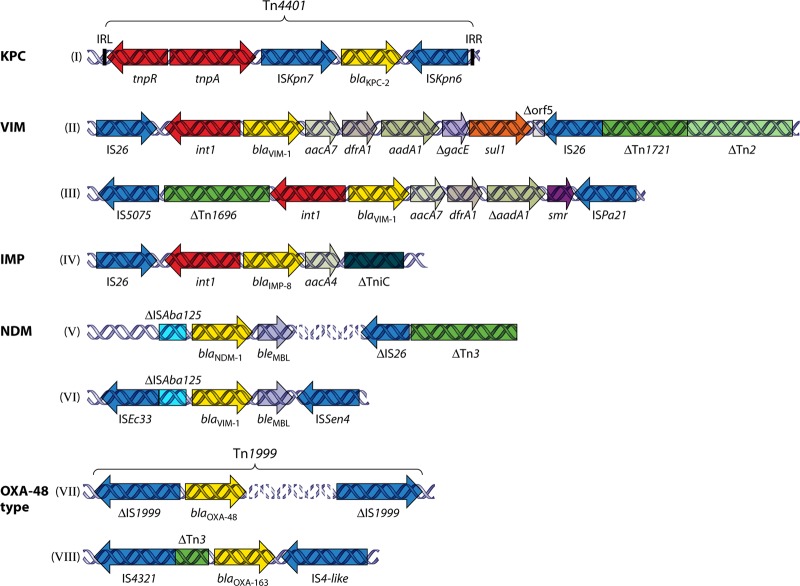
_bla_KPC genes detected so far in K. pneumoniae are all carried on plasmids. Sequences adjacent to bla_KPC genes display rather limited diversity, suggesting a single or at least a limited number of original sources. Segments of the Tn_3_-related Tn_4401 transposon, occurring in four isoforms, are invariably present upstream of bla_KPC (63, 180). Tn_4401 is bracketed by 39-bp imperfect inverted repeats and bounded by different 5-bp target site duplications (Fig. 1, structure I) (180). These structures indicate the operation of a replicative transposition mechanism (typical of Tn_3_-like transposons) that allows spread of KPC-encoding sequences among different genetic units and has resulted in the emergence of distinct KPC-encoding plasmids belonging to various Inc groups, such as FII (probably derivatives of the characteristic FII virulence plasmid of K. pneumoniae), L/M, and N (63). The same genetic structures have been identified in KPC-positive isolates of other enterobacterial species (Table 1).
Fig 1.
Schematic depiction of representative sequences from enterobacterial plasmids, showing the association of carbapenemase-encoding genes with various mobile elements. (I) The bla_KPC-2-containing Tn_4401 transposon from plasmid pNYC (GenBank accession no. EU176011) (180). (II and III) Representative VIM-encoding sequences from plasmids pNL194 (GenBank accession no. GU585907) (167) and pCC416 (GenBank accession no. AJ704863) (59), respectively. (IV) A _bla_IMP-carrying sequence from plasmid pFP10-2 (GenBank accession no. HQ651093) (146). (V and VI) Sequences containing bla_NDM-1 carried by a plasmid from K. pneumoniae 05-506 (GenBank accession no. FN396876) (273) and by plasmid p271A (GenBank accession no. HQ162469) (218), respectively. (VII and VIII) The OXA-48-encoding transposon Tn_1999 from plasmid pA-1 (GenBank accession no. AY236073) (217) and the _bla_OXA-163-containing segment from plasmid p6299 (GenBank accession no. HQ700343) (216), respectively.
In line with their substrate spectra, KPC enzymes confer on enterobacteria decreased susceptibility or resistance to virtually all β-lactam antibiotics. Moreover, there have been studies reporting on the emergence of KPC-positive K. pneumoniae exhibiting a decreased outer membrane permeability that enhances β-lactam resistance levels (134). Although the MICs of carbapenems vary, the latest Clinical and Laboratory Standards Institute (CLSI) and European Committee for Antimicrobial Susceptibility Testing (EUCAST) breakpoints classify most KPC-producing isolates as resistant to these drugs (55; www.eucast.org). It should also be noted that carbapenem and oxyimino-β-lactam MICs are commonly higher for species with derepressed production of their chromosomally encoded AmpCs, such as enterobacters, than for K. pneumoniae (166, 191).
MβLs
MβLs constitute a class of enzymes (molecular class B) that, despite their significant amino acid sequence diversity, share three distinct functional properties: (i) capability of hydrolyzing carbapenems, (ii) resistance to mechanism-based inhibitors, and (iii) susceptibility to chelating agents such as EDTA. The latter property results from their unique mechanism of hydrolysis, in which divalent cations, most commonly Zn2+, are essential for the nucleophilic attack of the β-lactam ring. Phylogenetic analysis suggests the existence of three MβL lineages: B1, B2, and B3 (13). In addition to chromosomally encoded MβLs, subgroup B1 includes the acquired enzymes of the VIM, IMP, GIM, SPM, SIM, AIM, DIM, and NDM types, all of which, surprisingly, are still of unknown origin (61). Of these, several variants of the VIM, IMP, and NDM types have been encountered in K. pneumoniae and other Enterobacteriaceae (Table 1). These β-lactamases are active against penicillins, older and newer cephalosporins, and carbapenems, although significant variations in hydrolytic efficiency exist even between enzymes of the same type (Table 2). Also, they are incapable of inactivating aztreonam. This is due mainly to the fact that B1 MβLs bind monobactams with a very low affinity. Moreover, docking experiments have indicated that positioning of the drug within the active site does not favor hydrolysis (213).
The bla_VIM and bla_IMP variants identified in K. pneumoniae so far occur as gene cassettes incorporated into the variable regions of class 1 integrons (Fig. 1, structures II, III, and IV) (254), with the exception of a class 2 and a class 3 IMP-encoding integron (174, 235). In contrast, bla_NDM genes are not associated with integrons (Fig. 1, structures V and VI) (114, 218, 273). The wide variety of K. pneumoniae plasmids encoding MβLs implies the operation of mobilization mechanisms. Two non-mutually exclusive possibilities are likely to account for this dissemination of MβL genes in distinct plasmids: (i) reshuffling of MβL cassettes among plasmid-borne integrons and (ii) en bloc mobilization of MβL gene-containing structures through transposition and/or recombination events. Indeed, insertion elements (ISs) such as IS_26, IS_Ec33, IS_Sen4, and IS_Aba125_, either alone or as parts of transposons (e.g., Tn_3_ and Tn_1696_), commonly flank MβL-encoding regions (Fig. 1, structures II to VI) (42, 59, 114, 165, 167, 215, 218, 273). At least some of the latter elements apparently participate in the spread of MβL-encoding sequences among different genetic units.
The three MβL types encountered in K. pneumoniae have also been identified on numerous occasions in other enterobacterial species, including E. coli (VIM, IMP, and NDM), Enterobacter cloacae (mainly VIM and IMP), Serratia marcescens (mainly IMP), and Proteus mirabilis (mainly VIM) (Table 1) (61, 166). In these species, most genetic platforms of MβL genes are similar to those found in K. pneumoniae.
The baseline phenotype expected from MβL-producing enterobacteria includes (i) resistance to amino-, carboxy-, and ureido-penicillins, penicillin-clavulanate combinations, and cefoxitin; (ii) decreased susceptibility to piperacillin-tazobactam and oxyimino cephalosporins; and (iii) elevated MICs of carbapenems compared to the epidemiological cutoff values. In actual fact, however, such a minimal resistance phenotype is observed rarely, if at all, among clinical isolates, since MβL producers most often possess additional mechanisms that increase carbapenem resistance levels, such as elevated expression of the MβL itself and, most importantly, impaired outer membrane permeability (40, 152). The latter mechanism apparently plays a significant role in determining carbapenem resistance levels, as also indicated by the wide range of carbapenem MICs among MβL producers belonging to the same species and even to the same lineage (40, 152, 166). Also, ESBLs such as SHVs, often encountered among VIM producers (152, 224), expand the resistance phenotype to include aztreonam resistance.
OXA-48
OXA-type β-lactamases (molecular class D), such as OXA-23, OXA-24/40, and OXA-58, encountered frequently in acinetobacters, exhibit relatively weak carbapenemase activity (256). In 2001, OXA-48, a distinct OXA enzyme (<50% amino acid sequence identity with the other OXA enzymes) with significant carbapenemase activity, was identified in K. pneumoniae (217). Its hydrolytic efficiency against imipenem is approximately 10-fold higher than those of the acinetobacter OXAs (256). Data from the crystal structure of OXA-48 and molecular dynamics studies suggest that the process of carbapenem hydrolysis is different from that for the other OXA carbapenemases. For OXA-48, hydrolysis relies on the rotation of the carbapenem's α-hydroxyethyl group within the active site in a manner that allows movement of the deacylating water toward the acylated serine residue (73). Consequently, OXA-48-producing K. pneumoniae isolates exhibit elevated MICs of carbapenems that are still frequently lower than the respective breakpoints. OXA-48 also hydrolyzes penicillins and early cephalosporins, but its activity against oxyimino cephalosporins is weak (217). Other carbapenem-hydrolyzing variants of OXA-48 (OXA-163 and -181) have also emerged in K. pneumoniae (216, 220, 221). bla_OXA-48 is invariably carried by transmissible plasmids responsible for its spread among K. pneumoniae and other enterobacterial species, such as E. coli and Citrobacter freundii (Table 1) (46, 217). Moreover, the plasmid-borne bla_OXA-48-containing sequences are associated with IS_1999, an IS_4 family element involved in mobilization and expression of β-lactam resistance genes (Fig. 1, structures VII and VIII) (8). This association provides further possibilities for _bla_OXA-48 to be transferred to other genetic units.
GLOBAL SPREAD OF CPE
Producers of KPC Types
A rapid and extensive dissemination of KPC-producing K. pneumoniae was first noticed in the northeastern parts of the United States during the first decade of the 21st century. Surveillance studies suggested that the epicenter of this epidemic was the state of New York (25, 27, 29). Later, isolates producing KPC-2 (270) and KPC-3 (a point mutant of KPC-2) (4) became established in hospitals in neighboring states, apparently due to transfer of colonized patients (83, 84, 126). During the same period, KPC-producing K. pneumoniae also emerged in Latin America (171, 201, 252) and Israel (139). Other countries, such as China (257) and Greece (102), soon followed. The Chinese data originate from a limited number of hospitals; thus, the actual extent of spread of KPC producers in China remains unknown. In Greece, KPC-positive K. pneumoniae became dominant in tertiary care hospitals, reaching epidemic proportions in a matter of approximately 2 years (99). In Northern and Western European countries, KPC prevalence remains low. In these countries (e.g., Switzerland, Ireland, United Kingdom, France, Sweden, Norway, the Netherlands, and Denmark), most reports concern sporadic isolates introduced by patients from high-prevalence areas (181, 230, 266). Nevertheless, a multihospital outbreak has already occurred in France (43). Higher prevalences have been reported from Poland and Italy, where KPC producers appear to be established in various regions (12, 103). The rapid global dissemination of KPC-producing K. pneumoniae implies multiple transmission routes. According to a widely held scenario, an important event was the introduction of KPC-positive K. pneumoniae from the United States to Israel, followed by spread to neighboring countries and via Greece to other European countries (260). However, index cases to confirm this scenario were not identified with certainty (154). KPC enzymes have been detected in a large number of K. pneumoniae sequence types (STs) (99). Nevertheless, the vast majority of isolates with these enzymes worldwide belong to ST258. This ST is strongly associated with KPC production and with isolates exhibiting multidrug resistance, but one could also speculate on additional—though yet unknown—inherent traits responsible for its high rate of transmissibility. Whatever these eventually turn out to be, KPC-producing ST258 K. pneumoniae can undeniably be regarded as one of the most successful multidrug-resistant nosocomial pathogens known to date.
Not unexpectedly, KPC-producing isolates of various other enterobacterial species, including E. coli and E. cloacae, have been reported in settings where the prevalence of KPC-positive K. pneumoniae is high. Outbreaks of KPC-producing E. coli have occurred in health care facilities in various countries, including the United States, Israel, and Greece (29, 105, 160, 249). Also, sporadic KPC-positive isolates of a wide variety of other enterobacterial species have been described worldwide (Table 1) (166, 191).
Producers of MβLs
K. pneumoniae strains producing enzymes belonging to any of the three MβL families (VIM, IMP, and NDM) have already achieved international spread, though significant local differences do exist. VIM-positive K. pneumoniae was first observed around 2001 to 2003 in Southern Europe and was introduced later to Northern Europe (e.g., Germany, France, and the Scandinavian countries) and the United States, mostly through colonized patients transferred from high-prevalence areas (61). Isolation rates of VIM-positive K. pneumoniae in Northern Europe and the United States remain low, though some infection clusters limited to single hospitals have been reported (107). In addition, sporadic cases have been recorded in Tunisia (129), South Korea (272), and Venezuela (155). Until recently, VIM-producing K. pneumoniae and other enterobacteria were frequently isolated in Mediterranean countries, reaching epidemic proportions only in Greece (107, 251). However, up-to-date surveillance data from this country indicate that these organisms have been in decline since 2009 (G. L. Daikos, unpublished data).
Acquisition of IMP MβLs by K. pneumoniae was described during the 1990s, primarily in Japan, as well as in Taiwan and Singapore (225). IMP-positive K. pneumoniae clinical isolates remain frequent in Japan (94). IMP-4-producing K. pneumoniae strains have also caused hospital outbreaks in China (162) and Australia (206). In addition, IMP-positive clinical enterobacteria, such as S. marcescens and E. cloacae, have been reported in the same area, i.e., Japan, South Korea, and Taiwan (225). Dissemination of IMP-producing Enterobacteriaceae in the rest of the world appears to be limited, with single cases identified in Turkey, Lebanon, Brazil, and the United States (3, 72, 148, 174). As usual, limitations and differences in surveillance systems in different countries inevitably affect the reliability and comparability of international epidemiological data on IMP (or indeed VIM)-positive K. pneumoniae.
In stark contrast, the results of the internationally concerted effort and resources allocated for the elucidation of the transmission routes and public health impact of enterobacteria, mainly E. coli and K. pneumoniae strains producing NDM, the most recently identified MβL type, were spectacular. These efforts produced a wealth of data regarding the epidemiology of NDM producers. The epicenter of their epidemic is the Indian subcontinent, where the high isolation frequency of these microorganisms in health care facilities, as well as their extensive spread in various environmental niches, has been documented repeatedly (130, 192). Furthermore, the _bla_NDM genes have spread to various enterobacterial species other than K. pneumoniae and E. coli (Table 1) (255). Also, a second reservoir of NDM-producing K. pneumoniae strains seems to exist in the central Balkans, but its link with the Indian epidemic remains uncertain (108, 150). In contrast, the recent spread of NDM producers in Western Europe, North America, Australia, and the Far East has clearly been attributed to patients who originated mainly from India, Pakistan, and Bangladesh (192). A characteristic of NDM-producing K. pneumoniae isolates has so far been their rapid dissemination; indeed, infected or colonized humans without obvious connection to the Indian epidemic are increasingly being reported in several countries (125, 190, 214).
Producers of OXA-48
OXA-48-producing K. pneumoniae was first detected sporadically in Turkey, in 2001 (217). Hospital outbreaks in the main cities of this country soon followed (45). About the same time, OXA-48-positive K. pneumoniae isolates were also identified in other Middle Eastern and North African countries (46, 62) as well as in Western European countries, including the United Kingdom, Belgium, France, Germany, and the Netherlands. Emergence of OXA-48 producers in the latter countries has been attributed mainly to colonized patients transferred from North Africa (107). Recently, an important outbreak due to an OXA-48-producing K. pneumoniae strain was reported in a Dutch hospital (220). However, there are no indications of an overall significant spread of these microorganisms across Europe. Although the Middle East and North Africa remain the main foci of infection, the recent isolation of K. pneumoniae isolates producing OXA-48-type enzymes in India (47), Senegal (173), and Argentina (216) suggests an expansion that can safely be considered global. Additionally, the recent isolation of OXA-48 producers belonging to species other than K. pneumoniae underlines the spreading potential of _bla_OXA-48 (Table 1) (46).
DETECTION OF CPE
Counterintuitive as it may sound, carbapenemase production by enteric bacilli does not necessarily confer significant resistance to carbapenems. Before the introduction of the new carbapenem breakpoints by CLSI and EUCAST in 2010 (55; www.eucast.org), carbapenemase-positive isolates (as determined by phenotypic tests) with relatively low carbapenem MICs were reported without interpretation of their susceptibility status. This directly implied the possibility of therapeutic failure for carbapenem regimens, passing to clinicians a mixed message of dubious value. Today, after the introduction of the new, lower breakpoints, the situation has been simplified: laboratories report the MICs of carbapenems irrespective of carbapenemase production. On the other hand, various enterobacterial isolates lacking enzymes with appreciable carbapenemase activity may exhibit elevated MICs of carbapenems. Consequently, this may exclude from use a viable group of antibiotics. Application of simple and reliable carbapenemase-detecting tests nevertheless remains useful for monitoring of carbapenemase-producing microorganisms in order to inform appropriate infection control policies in health care settings.
A large-scale and cost-effective approach for deciding which isolates are carbapenemase producers based solely on phenotypic tests should rely on the epidemiological cutoff (ECOFF) values for nonsusceptibility. These values depend on carbapenem MIC distributions of carbapenemase producers as opposed to wild-type strains. Considering the respective distributions for K. pneumoniae and E. coli compiled by EUCAST, MICs of ≥1 μg/ml for imipenem and ≥0.5 μg/ml for meropenem and ertapenem have been proposed (57). According to the CLSI, which has not defined ECOFFs, selection of isolates for testing can rely on clinical breakpoints: isolates that test intermediate or resistant to at least one carbapenem as well as resistant to a “subclass III cephalosporin” (cefotaxime, ceftazidime, ceftriaxone, cefoperazone, or ceftizoxime) should be tested further. Ertapenem is considered the most sensitive indicator (29). It should be noted, however, that use of this drug may cause specificity problems: decreased permeability, combined with either production of CTX-M or overproduction of AmpC β-lactamases, can significantly affect the MIC of ertapenem and therefore lower the detection specificity (57, 264).
Screening criteria, however, may and should be adapted depending on the epidemiological situation in a given ecological setting. Application of the CLSI criteria is expected to be adequate in settings where carbapenemase producers have already been established. On the other hand, occasional adoption of less stringent criteria (i.e., the use of lower cutoffs or reducing the concentrations of the selective agents used for screening) in low-prevalence settings may facilitate the timely detection of CPE emergence or early dissemination and therefore allow the swift implementation of measures preventing their further spread. The potential prevention benefits of such an approach are likely to counterbalance the burden of increased false-positive results.
There have been numerous studies that deal with technical issues of carbapenemase detection methods, comparing their performances mainly for K. pneumoniae and E. coli. We therefore briefly review these methods and their principles.
MHT
The cloverleaf or modified Hodge test (MHT) is based on the inactivation of meropenem or ertapenem by whole cells of carbapenemase-producing organisms. MHT has been used extensively as a phenotypic method for the detection of carbapenemase activity (55), and it is the only carbapenemase detection method recommended by the CLSI for screening purposes. There are, however, various shortcomings with MHT. The assay cannot distinguish the type of carbapenemase involved. Most importantly, false-positive results have been observed with isolates producing CTX-M-type ESBLs or increased amounts of AmpC β-lactamases (cephalosporinases) (166, 200). Moreover, sensitivity problems (false-negative results) may occur, mainly with MβL-producing enterobacterial isolates exhibiting weak carbapenemase activity (166). Also, MHT is probably unreliable in detecting NDM-1-producing K. pneumoniae, though the relevant observations regard a limited number of isolates (49, 169). Replacement of Mueller-Hinton agar by MacConkey agar has been proposed as a means to increase the sensitivity of MHT for detection of isolates producing MβLs or OXA carbapenemases. Improved performance was attributed to the enhanced release of periplasmic enzymes caused by the bile salts included in MacConkey medium (141). This modification, however, has not been evaluated systematically. Overall, MHT, although remaining a convenient assay, cannot be used as the sole method for the detection of carbapenemase-positive Enterobacteriaceae in the clinical laboratory.
Detection of MβLs Based on Chelating Agents
Phenotypic detection of MβL producers in the clinical laboratory is based mainly on the specific inhibition of MβLs by EDTA (193). Additionally, various techniques utilizing other chelating agents, such as dipicolinic acid and 1,10-phenanthroline, as well as thiol compounds such as 2-mercaptopropionic and mercaptoacetic acid, have been developed (166). Use of a combination of chelators, e.g., EDTA plus 2-mercaptopropionic acid, has also been proposed (124). These compounds, by depriving the MβL of hydrolytically essential Zn divalent cations, render it inactive against β-lactams. The most common MβL detection tests employ a disk of a hydrolyzable β-lactam (typically a carbapenem, though ceftazidime has also been used extensively) placed close to a disk with a given amount of an MβL inhibitor (most commonly EDTA), hence the term “double-disk synergy test” (DDST). Formation of a synergy pattern is indicative of MβL production. A drawback of this approach is that interpretation is subjective and cannot be quantified. Alternatively, the β-lactam disk is potentiated with an inhibitor, and the diameter of its inhibition zone is then compared with that of the β-lactam disk alone, hence the term “combined disk test” (CDT). An increase of the inhibition zone diameter above a predefined cutoff value denotes MβL activity. Various gradient diffusion methods (e.g., Etest [bioMérieux, Solna, Sweden]), utilizing strips containing imipenem and EDTA, are based on the same principle. In general, MβL detection methods based on β-lactam–chelator combinations perform well for K. pneumoniae and E. coli, while they have not been tested systematically for other enterobacterial species. Also, the user should always consider the potentially detrimental effects of chelating agents on bacterial growth. Additionally, interpretation difficulties are to be expected with MβL producers exhibiting low carbapenem MICs.
Detection of KPCs Based on Boronates
Phenotypic detection of KPC production is based on the susceptibility of KPCs to boronic acid and its derivatives, i.e., phenylboronic and 3-aminophenylboronic acid. Boronate derivatives, which structurally resemble β-lactams, have long been used in probing the function of β-lactamases, especially class C enzymes. In 2008, Pasteran et al. (201) observed that boronates preferentially inhibit KPC-type β-lactamases. This report was soon followed by studies proposing detection techniques using boronic acids combined with a carbapenem, mostly in the CDT format (75, 104, 248). As with the above-described MβL detection tests, experience with the boronate-based detection of KPC producers is limited mainly to K. pneumoniae. Specificity problems may arise with isolates producing AmpC-type β-lactamases (cephalosporinases), since boronic acid derivatives are potent inhibitors of these enzymes. The problem can be alleviated partly by the simultaneous use of cloxacillin, which preferentially inhibits cephalosporinases (104). It should also be noted that boronate-based assays are ineffective in detecting KPC-positive K. pneumoniae in the case of coproduction of VIM β-lactamase (100).
Detection by Use of Chromogenic Media
At least two selective agar media allowing different carbapenemase-producing microorganisms to be recognized are commercially available: CHROMagar-KPC (CHROMagar; BBL) and Brilliance CRE agar (Thermo Fisher Scientific). Species are distinguished by colony color. The reliability of these media has not yet been evaluated rigorously. Nevertheless, they have been used successfully for surveillance cultures on various occasions (196, 208, 229).
Molecular Detection of Carbapenemase Genes
Many clinical laboratories employ “in-house” PCR-based methods for the detection of carbapenemase genes to get around the problems of phenotypic detection methods and to reduce reporting times. In addition, PCR-based methods allow detection of OXA-type carbapenemases for which specific phenotypic tests have not been developed. Simplex PCR assays targeting a single carbapenemase type have been used successfully in numerous studies, although there is no consensus regarding the oligonucleotide primers that should be used for each bla gene group. Multiplex and real-time PCR methods that allow the identification of multiple carbapenemase gene types and that further shorten the detection time, in the case of real-time PCR, have also been utilized (20, 70, 172, 219, 253). Also, real-time PCR assays can be followed by a melting curve step to allow the accurate identification of carbapenemase gene variants (163). PCR- and hybridization-based kits for detection of the main carbapenemase gene types, for example, Hyplex MBL ID and Hyplex CarbOxa ID kits (BAG Health Care, Lich, Germany), have also been developed by the industry. Although manufacturers claim that these methods have the potential to be used directly on clinical samples (9), their diagnostic usefulness remains to be evaluated systematically in different settings. Microarray technology was recently added to the list of molecular methods aiming at the rapid and reliable identification of multiple resistance determinants. The Check-KPC ESBL microarray and its expanded version, Check-MDR CT102 (Check-Points Health BV, Wageningen, Netherlands), have been used successfully for detection within a single reaction tube of a wide variety of bla genes, including most clinically relevant carbapenemase genes (58, 179). Nevertheless, the term “macroarray” may be more suitable given the relatively small number of genes tested.
An issue with all molecular methods is that the range of resistance genes to be detected is predefined, so these methods may miss novel gene types.
Detection of Carbapenemase Activity by Spectrophotometry
Assessment of carbapenemase activity by spectrophotometry is carried out using crude or partially purified enzyme extracts and a carbapenem, commonly imipenem. It is considered the reference method for the verification of carbapenemase activity. This laborious and technically demanding approach, however, is limited to reference laboratories.
Detection of Carbapenemase Activity by Mass Spectrometry
Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) is the latest advancement in the recognition of carbapenemase activity. The method is based on the ionization in high vacuum of the material under examination and its subsequent acceleration in an electrical field. The sizes of fragments can be inferred from the time of flight within the electrical field. MALDI-TOF MS has been introduced in the clinical laboratory mainly as a means for species identification. However, the method is highly versatile and can be used for the recognition of various compounds, including antibiotic degradation products. Recently, MALDI-TOF MS was used successfully to identify carbapenem hydrolysis products, thus confirming carbapenemase activity in Gram-negative isolates (35, 115). However, experience with this methodology is still limited.
ANTIMICROBIAL AGENTS AGAINST CPE
In Vitro Activity
Susceptibility of the infecting isolate is one of the key factors in deciding on a suitable antimicrobial chemotherapy. In vitro susceptibility data from numerous studies throughout the world indicate that colistin, tigecycline, and fosfomycin are the most effective antibacterials against clinical enterobacteria producing either KPCs or MβLs. Drug effectiveness, however, differs depending on the extent of spread of resistant isolates in each setting. Indeed, a growing number of studies indicate that the activity of these drugs is decreasing rapidly (7, 21, 80, 85, 88, 96, 128, 142, 151, 156, 185, 186, 226, 243, 247, 275, 277). In addition, rates of susceptibility to fluorinated quinolones are generally low, reflecting the multidrug-resistant nature of CPE. Low susceptibility rates are also found for other, clinically less important antimicrobials, such as nitrofurantoin (breakpoints are available only for E. coli) and chloramphenicol, both of which are drugs that are tested less frequently. Among the currently used aminoglycosides, only gentamicin has so far retained good activity against producers of KPCs and acquired MβLs of the VIM type. The majority of NDM producers, however, are resistant to all clinically available aminoglycosides due to coproduction of 16S rRNA methylases (18). Of the β-lactams, the most effective compounds seem to be the carbapenems, which at first sight appears to be paradoxical. However, applications of different carbapenem breakpoints by the CLSI and EUCAST must be taken into account. It has been estimated that in Greek hospitals, some 10 to 15% of CPE (a bacterial population consisting mainly of KPC-2-producing K. pneumoniae strains) appear resistant by the CLSI interpretive criteria but susceptible according to EUCAST (Daikos, unpublished data). Aztreonam, though withstanding hydrolysis by acquired MβLs, exhibits limited activity against the respective CPE due to the frequent coproduction of ESBLs, mainly of the SHV and CTX-M types (60). Finally, temocillin, a 6-α-methoxy derivative of ticarcillin, seems to exhibit moderate activity against KPC-producing K. pneumoniae and E. coli, but the relevant data are limited (1).
The data summarized above, obtained from a wide variety of settings, should nevertheless be treated with caution given the different methodologies used. Additionally, there have been reports that automated systems have “inherent” problems in reliably determining carbapenem MICs for CPE (33, 101, 199, 244, 246). Also, there are difficulties in interpreting carbapenem susceptibility data for KPC producers due to heterogeneous resistance-like phenomena (191).
In Vitro Synergy
Given the limited therapeutic options for the management of infections caused by carbapenemase-producing K. pneumoniae strains, several investigators have evaluated combinations of different antimicrobial agents for potential synergistic effects against these organisms, relying mostly on time-kill methods. It should be noted, however, that in most synergy studies the most frequent compounds used were the polymyxins (polymyxin B and polymyxin E).
In 2005, Bratu and colleagues found that polymyxin B, at 0.5 times its MIC, exhibited synergistic activity with rifampin against 15 of 16 KPC-positive K. pneumoniae isolates. A synergistic effect was also seen in 10 of these isolates when polymyxin B was combined with imipenem (30). In a later study, it was found that a triple-drug combination of polymyxin B with rifampin and doripenem at 1/4 their MICs exhibited high bactericidal activity, defined as a decrease of ≥3 log CFU/ml in 24 h, for five E. coli and two K. pneumoniae clinical isolates, all producing KPC-type enzymes (250). Similarly, the combination of colistin (polymyxin E) and tigecycline has been reported as synergistic against KPC-positive K. pneumoniae isolates in time-kill experiments (222). Interactions of colistin and imipenem were also examined in time-kill experiments with 42 VIM-producing K. pneumoniae isolates from a Greek hospital (241). In general, the combination of imipenem with colistin exhibited improved bactericidal activity against isolates that were susceptible either to both agents or to colistin alone. More specifically, the combination was synergistic against 50% of the colistin-susceptible isolates and indifferent against the remaining 50%, irrespective of the imipenem MIC. In contrast, for isolates that were nonsusceptible to colistin, the combination was antagonistic for 55.6% of the isolates and synergistic for only 11% of the isolates. These data are partly reminiscent of those reported by Elemam et al. (79), who examined the interactions of polymyxin B with several antimicrobials against 12 KPC-producing K. pneumoniae isolates that had elevated polymyxin B MIC values, using a broth microdilution assay in a checkerboard pattern. Synergy was observed with the combinations of polymyxin B plus rifampin and polymyxin B plus doxycycline, at achievable serum drug concentrations for the antimicrobial agents tested. Less pronounced synergy was noted with polymyxin B and tigecycline, whereas no synergy was evident between polymyxin B and the other antimicrobial agents tested, including imipenem and gentamicin.
Given that fosfomycin retains its activity against the majority of CPE, it is reasonable to consider administering this compound against CPE infections, but always in combination with another antimicrobial agent, as the rate of mutation to fosfomycin resistance is worringly high (188). Recently, the interactions of fosfomycin with meropenem, colistin, and gentamicin against KPC-positive K. pneumoniae isolates were studied using time-kill experiments (238). Combinations of fosfomycin with meropenem and colistin were synergistic in 64.7 and 11.8% of KPC-producing K. pneumoniae isolates, respectively, whereas the combination with gentamicin was indifferent. In addition, combinations of fosfomycin with meropenem, colistin, and gentamicin prevented the development of resistance to fosfomycin in 69.2, 53.8, and 81.8% of examined isolates, respectively. Similar results were obtained by another study that demonstrated synergy of fosfomycin with imipenem, meropenem, doripenem, colistin, netilmicin, and tigecycline for 74, 70, 74, 36, 42, and 30% of 50 KPC-producing K. pneumoniae isolates, respectively (228).
Time-kill assays have also been used to comparatively assess the activities of aztreonam and carbapenems. As mentioned previously, aztreonam is not hydrolyzed by MβLs and therefore is a potentially useful agent against MβL producers. A time-kill study assessed the in vitro activity of aztreonam in comparison to carbapenems against VIM-1-producing ESBL-negative K. pneumoniae isolates (197). Aztreonam exhibited slow bactericidal activity that was sustained for 24 h, whereas carbapenems resulted in more rapid bacterial killing for the first 6 h but regrowth to the level of antibiotic-free controls at 24 h.
In Vitro Pharmacodynamic Models
In a chemostat model simulating human pharmacokinetics, it was shown that optimized doses of meropenem (simulation of 2 g every 8 h, infused over 3 h, in humans) can achieve bactericidal activity against KPC-producing K. pneumoniae isolates with low meropenem MICs, despite the presence of an active carbapenemase. In this model, actual meropenem concentrations were significantly lower than intended, presumably due to rapid in vitro hydrolysis of meropenem by the released KPC enzyme. Despite this situation, meropenem achieved a rapid, ≥3-log CFU reduction of all KPC isolates within 6 h, but this effect was maintained for only two of the three KPC-producing isolates (with meropenem MICs of 2 and 8 μg/ml) for which adequate drug exposure had been attained (32).
The effect of tigecycline alone or in combination with meropenem was assessed in an in vitro pharmacodynamic model simulating human epithelial lining fluid drug concentrations against five KPC-producing K. pneumoniae isolates displaying meropenem MICs between 8 and 64 μg/ml and tigecycline MICs between 1 and 2 μg/ml. Tigecycline alone did not produce a reduction in bacterial density in any of the isolates studied, except for one with a tigecycline MIC of 1 μg/ml, in which an initial reduction was nevertheless followed by rapid regrowth. Meropenem alone, on the other hand, produced a rapid bactericidal effect for isolates with meropenem MICs of 8 and 16 μg/ml, but this effect was not maintained and was also followed by regrowth. Unlike monotherapy with tigecycline or meropenem, their combination caused a significant reduction in CFU/ml at 24 and 48 h for isolates with tigecycline and meropenem MICs of ≤2 and ≤16 μg/ml, respectively, compared to the case with either agent alone. None of the studied regimens, however, was able to maintain a significant bactericidal effect for periods over 48 h (262).
Experimental Infection Models
Several investigators have examined the efficacy of different agents, alone or in combination, against CPE isolates by using different experimental infection models. Daikos et al. (66) assessed the activity of two dosing regimens of imipenem (30 and 60 mg/kg of body weight every 2 h [q2h]) against VIM-1-producing K. pneumoniae isolates in the neutropenic murine thigh infection model. Animals were infected with three VIM-1-positive isolates (with imipenem MICs of 2, 4, and 32 μg/ml) and a susceptible clinical isolate (with an imipenem MIC of 0.125 μg/ml) not producing any β-lactamase with broad-spectrum activity. The bactericidal effect was greatest against the susceptible non-VIM-1-producing isolate, intermediate against the “susceptible” VIM-1 producers (imipenem MICs of 2 and 4 μg/ml), and minimal against the resistant VIM-1 isolate (imipenem MIC of 32 μg/ml). However, with administration of a higher dose of imipenem (60 mg/kg q2h) and attainment of a drug exposure (cumulative percentage of a 24-h period that the drug concentration exceeds the MIC under steady-state pharmacokinetic conditions [%_T_MIC]) of approximately 40%, a more pronounced antibacterial effect against all VIM-1-producing isolates, including the highly resistant one, was achieved.
The use of carbapenems in the treatment of CPE infections was pursued further by Bulik and Nicolau (34), who evaluated the efficacy of doripenem against KPC-producing K. pneumoniae isolates with MICs ranging from 4 to 32 μg/ml in both immunocompetent and neutropenic mice. In these experiments, the authors used doripenem doses simulating human pharmacokinetics observed after administration of 1 or 2 g every 8 h as a 4-h infusion. The 1-g dose simulation was able to produce only a bacteriostatic response for the isolates with MICs of 4 and 8 μg/ml, whereas the 2-g dose simulation achieved a similar effect for isolates with MICs of up to 16 μg/ml. Relative to neutropenic mice, a reduction in bacterial density was observed in the immunocompetent animals, with overall decreases of up to 1 log, with either the 1- or 2-g doripenem dose simulation. A critical interpretation of the animal infection model data just summarized suggests that optimized regimens of carbapenems are able to achieve at least a static effect in severely compromised hosts and a modest bactericidal effect in immunocompetent animals infected with KPC-positive isolates with MICs of up to 8 μg/ml.
The efficacy of carbapenems and aztreonam was also evaluated in a rabbit model of peritoneal abscess caused by an ESBL-negative VIM-producing E. coli isolate. MICs of imipenem, meropenem, ertapenem, and aztreonam were 1, ≤0.25, 1.5, and ≤0.25 μg/ml, respectively. Carbapenems and aztreonam were shown to be effective in the treatment of this infection with regard to reductions in bacterial densities and mortality of the animals compared with those of untreated controls. Aztreonam, however, resulted in a more favorable outcome overall than that seen with carbapenems (239).
Comments on Experimental Studies
The number of studies assessing the interaction of antimicrobials with CPE in the laboratory, whether using time-kill assays or experimental animal infections, is remarkably small. In addition, clinically important CPE, such as those producing NDM-type MβLs or OXA-48, have not yet been studied in this manner. It is therefore obvious that additional studies of this kind are required, given the extent and severity of the problem posed by CPE. The synergy data from time-kill studies present some discrepancies. These data should therefore be interpreted with caution, since slight differences in experimental conditions (e.g., a relatively small change in the MIC fraction for one or more drugs) could result in significant changes in the apparent effect of a given combination. Despite these limitations, however, the data from time-kill studies indicate a variety of antibiotic combinations with potential synergistic effects against CPE.
In pharmacodynamic models and, most importantly, experimental infections in animals, the antibiotics preferably evaluated so far have been the carbapenems. This might appear unexpected, since therapy with carbapenems in the majority of human infections caused by CPE would be considered “inappropriate” based on MICs. Yet the relevant data, though limited, may be taken as indicating that approaches such as modification of dosing schemes warrant further attention.
ANTIMICROBIAL THERAPY
In studies examining the outcomes of CPE infections, older age, severity of underlying illness, comorbid conditions of the host, intensive care unit (ICU) stay, resistance to carbapenems, and administration of inappropriate antimicrobial treatment (partly due to CPE multidrug resistance compromising empirical therapy) are the most important independent predictors of treatment failure (14, 67, 175, 205, 233, 274). In the absence of controlled comparative trials, however, an overall critical appraisal of antibiotic treatment schemes inevitably has to be based on a variety of case reports, case series, retrospective studies, and observational studies. Moreover, these studies are focused on K. pneumoniae, as clinical experience with other CPE is quite limited. Therefore, the assessment we attempt here lacks many of the characteristics of a rigorous meta-analysis but may provide some guidance on the treatment of CPE-infected patients.
Review of Clinical Studies
We performed a systematic search of MEDLINE and compiled 34 studies containing the necessary information to estimate the efficacies of different antimicrobials in relation to their MICs for the infecting organisms (Tables 3 and 4). A total of 301 patients were identified, including 161 infected with KPC-producing K. pneumoniae and 140 infected with MβL-producing K. pneumoniae. The vast majority of these patients had serious infections: 244 had bloodstream infections (BSIs), 32 had pneumonia, 8 had urinary tract infections, 4 had tracheobronchitis, 3 had wound infections, and 7 had other infections. Three patients reported as having urinary colonization were excluded. Of the remaining 298 patients, 242 (81.1%) received appropriate therapy (with at least one drug to which the infecting organism was classified as susceptible in vitro), while 56 (18.9%) received inappropriate therapy (no drug to which the infecting organism was classified as susceptible in vitro). To facilitate comparisons, patients were classified into seven groups according to treatment regimen, as follows: regimen A, combination therapy with ≥2 active drugs, one of which was a carbapenem; regimen B, combination therapy with ≥2 active drugs, not including a carbapenem; regimen C, monotherapy with an aminoglycoside; regimen D, monotherapy with a carbapenem; regimen E, monotherapy with tigecycline; regimen F, monotherapy with colistin; and regimen G, inappropriate therapy (Fig. 2). It should be noted that the carbapenem susceptibility status was taken as reported in relevant studies in which the previous CLSI interpretive criteria were applied (54).
Table 3.
Clinical studies, antimicrobial therapies, and outcomes for patients infected with MβL-producing K. pneumoniae
| Reference | Country (yr of publication) | Study design | No. of patients with indicated type of infection | Type of MBL (no. of isolates) | Treatment (no. of patients) | Outcome (no. of successes/no. of failures) |
|---|---|---|---|---|---|---|
| 121 | Greece (2004) | Case reports | 4 (2 BSIs, 1 case of mediastinitis, 1 bone infection) | VIM-1 (4) | Colistin (1) | 1/0 |
| 86 | Greece (2008) | Tigecycline (1) | 1/0 | |||
| 56 | Spain (2008) | Tigecycline-colistin (2) | 1/1 | |||
| 223 | Ireland (2010) | |||||
| 269 | Taiwan (2001) | Case series | 3 BSIs | IMP-8 (3) | Carbapenem (3) | 1/2 |
| 143 | Taiwan (2004) | Case series | 3 (2 pneumonias, 1 BSI) | IMP-type enzyme (3) | Carbapenem (1) | 1/0 |
| Carbapenem-aminoglycoside (2) | 2/0 | |||||
| 240 | Greece (2008) | Case series | 17 (14 BSIs, 3 pneumonias) | VIM-1 (17) | Colistin (6) | 6/0 |
| Tigecycline (1) | 0/1 | |||||
| Colistin-aminoglycoside (2) | 2/0 | |||||
| Colistin doxycycline (1) | 0/1 | |||||
| Carbapenem-colistin (6) | 5/1 | |||||
| Carbapenem-aminoglycoside-doxycycline (1) | 1/0 | |||||
| 175 | Greece (2010) | Case-control study | 18 BSIs | VIM-1 (17) | Colistin (10) | 6/4 |
| VIM-type enzyme (1) | Colistin-aminoglycoside (8) | 4/4 | ||||
| 64 | Greece (2007) | Retrospective study | 28 BSIs | VIM-1 (28) | Carbapenem (8) | 7/1 |
| Colistin (4) | 0/4 | |||||
| Aminoglycoside (3) | 2/1 | |||||
| Carbapenem-aminoglycoside (6) | 6/0 | |||||
| Carbapenem-colistin (1) | 1/0 | |||||
| Aztreonam-aminoglycoside (2) | 1/1 | |||||
| No active drug (4) | 2/2 | |||||
| 67 | Greece (2009) | Prospective observational study | 67 BSIs | VIM-1 (67) | Carbapenem (14) | 11/3 |
| Carbapenem-colistin (8) | 8/0 | |||||
| Carbapenem-aminoglycoside (4) | 3/1 | |||||
| Colistin (15) | 11/4 | |||||
| Aminoglycoside (8) | 5/3 | |||||
| No active drug (18) | 13/5 |
Table 4.
Clinical studies, antimicrobial therapies, and outcomes for patients infected with KPC-producing K. pneumoniae
| Reference | Country (yr of publication) | Study design | No. of patients with indicated infection | Type of β-lactamase (no. of isolates) | Treatment with active drug (no. of patients) | Outcome (no. of successes/no. of failures) |
|---|---|---|---|---|---|---|
| 252 | Colombia (2006) | Case reports | 23 (10 BSIs, 10 pneumonias, 1 endocarditis, 1 liver abscess, 1 empyema) | KPC-2 (19) | Carbapenem (4) | 3/1 |
| 153 | USA (2006) | KPC-3 (2) | Colistin (3) | 2/1 | ||
| 257 | China (2007) | KPC-type enzyme (2) | Tigecycline (1) | 0/1 | ||
| 71 | USA (2007) | Aminoglycoside (2) | 1/1 | |||
| 6 | USA (2008) | Tigecycline-colistin (2) | 1/1 | |||
| 162 | China (2008) | Tigecycline-aminoglycoside (1) | 1/0 | |||
| 81 | USA (2008) | Colistin-aminoglycoside (1) | 1/0 | |||
| 159 | USA (2009) | Aminoglycoside-fluoroquinolone (1) | 1/0 | |||
| 17 | Israel (2009) | Carbapenem-aminoglycoside (1) | 1/0 | |||
| 158 | USA (2009) | No active drug (7) | 1/6 | |||
| 80 | USA (2009) | |||||
| 116 | USA (2010) | |||||
| 138 | Brazil (2011) | |||||
| 52 | Taiwan (2011) | |||||
| 10 | Switzerland (2011) | |||||
| 113 | USA (2011) | |||||
| 25 | USA (2004) | Case series | 4 (1 BSI, 2 urinary tract infections [UTIs], 1 pneumonia) | KPC-2 (4) | Carbapenem (1) | 1/0 |
| Carbapenem-colistin (1) | 1/0 | |||||
| Carbapenem-aminoglycoside (1) | 1/0 | |||||
| Colistin (1) | 0/1 | |||||
| 258 | USA (2009) | Case series | 21 (5 pneumonias, 5 BSIs, 4 cases of tracheobronchitis, 5 UTIs, 1 case of meningitis, 1 surgical site infection [SSI]) | KPC-3 (21) | Carbapenem (4) | 2/2 |
| Tigecycline (5) | 4/1 | |||||
| Aminoglycoside (3) | 3/0 | |||||
| Carbapenem-tigecycline (1) | 0/1 | |||||
| Tigecycline-aminoglycoside (1) | 1/0 | |||||
| No active drug (7) | 3/4 | |||||
| 154 | Greece (2009) | Case series | 13 (9 pneumonias, 4 BSIs) | KPC-2 (13) | Aminoglycoside (2) | 2/0 |
| Tigecycline-colistin (8) | 6/2 | |||||
| Colistin-aminoglycoside (3) | 3/0 | |||||
| 83 | USA (2009) | Case series | 7 (3 BSIs, 1 UTI, 3 urinary colonizations) | KPC-2 (1) | Colistin (1) | 0/1 |
| KPC-3 (6) | Colistin-aminoglycoside (2) | 0/2 | ||||
| No active drug (4) | 0/4 | |||||
| 182 | USA (2009) | Case series | 3 BSIs | KPC-2 (3) | Tetracycline-aminoglycoside (1) | 1/0 |
| Colistin (3) | 1/2 | |||||
| 237 | Greece (2010) | Case series | 17 (11 BSIs, 2 SSIs, 1 UTI, 2 pneumonias, 1 case of cholangitis) | KPC-2 (17) | Colistin (11) | 6/5 |
| Tigecycline (1) | 1/0 | |||||
| Aminoglycoside (1) | 1/0 | |||||
| Colistin-aminoglycoside (2) | 1/1 | |||||
| Tigecycline-colistin-aminoglycoside (1) | 1/0 | |||||
| No active drug (1) | 1/0 | |||||
| 175 | Greece (2010) | Case-control study | 19 BSIs | KPC-2 (19) | Colistin (10) | 2/8 |
| Colistin-aminoglycoside (9) | 4/5 | |||||
| 274 | Greece (2011) | Case-control study | 53 BSIs | KPC-2 (53) | Carbapenem (1) | 0/1 |
| Colistin (7) | 3/4 | |||||
| Tigecycline (5) | 3/2 | |||||
| Aminoglycoside (2) | 2/0 | |||||
| Colistin-aminoglycoside (2) | 2/0 | |||||
| Carbapenem-aminoglycoside (1) | 1/0 | |||||
| Tigecycline-colistin (9) | 9/0 | |||||
| Tigecycline-aminoglycoside (4) | 4/0 | |||||
| Carbapenem-tigecycline (1) | 1/0 | |||||
| Carbapenem-tigecycline-colistin (2) | 2/0 | |||||
| Tigecycline-colistin-aminoglycoside (1) | 1/0 | |||||
| No active drug (18) | 7/11 |
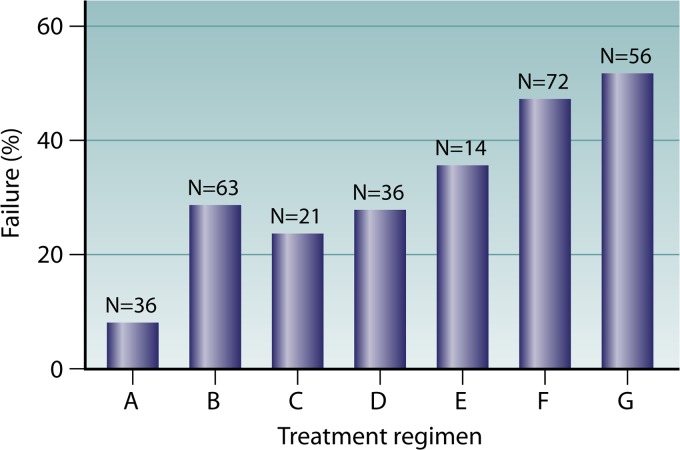
Fig 2.
Outcomes of infections caused by carbapenemase-producing Klebsiella pneumoniae, according to treatment regimen. Regimen A, combination therapy with ≥2 active drugs, one of which was a carbapenem; regimen B, combination therapy with ≥2 active drugs, not including a carbapenem; regimen C, monotherapy with an aminoglycoside; regimen D, monotherapy with a carbapenem; regimen E, monotherapy with tigecycline; regimen F, monotherapy with colistin; regimen G, inappropriate therapy. Regimen A was superior to regimens B, E, F, and G (for A versus B, E, F, and G, the P value was 0.02, 0.03, <0.0001, and <0.0001, respectively). Regimens B, C, and D were superior to regimen G (for B versus G, P = 0.014; for C versus G, P = 0.04; and for D versus G, P = 0.03).
The lowest failure rate (8.3%) was observed for patients who received combination therapies including a carbapenem (regimen A). In addition, the therapeutic efficacy of this regimen was superior to those of regimens B, E, F, and G (for A versus B, the P value is 0.02, the odds ratio [OR] is 4.4, and the 95% confidence interval [95% CI] is 1.19 to 16.19; for A versus E, the P value is 0.03, the OR is 6.11, and the 95% CI is 1.22 to 30.58; for A versus F, the P value is <0.0001, the OR is 9.84, and the 95% CI is 2.76 to 35.03; and for A versus G, the P value is <0.0001, the OR is 11.81, and the 95% CI is 3.24 to 43.06). Combination therapy not including a carbapenem (regimen B), as well as monotherapy with either an aminoglycoside (regimen C) or a carbapenem (regimen D), was nevertheless effective compared to inappropriate therapy (for B versus G, the P value is 0.014, the OR is 2.68, and the 95% CI is 1.26 to 5.73; for C versus G, the P value is 0.04, the OR is 3.44, and the 95% CI is 1.11 to 10.67; and for D versus G, the P value is 0.03, the OR is 2.79, and the 95% CI is 1.14 to 6.86). On the other hand, treatment with tigecycline and colistin as single active agents resulted in failure rates comparable to that observed for patients who received inappropriate therapy (Fig. 2). These observations raise concerns about the use of tigecycline or colistin as a single agent in the treatment of serious carbapenemase-producing K. pneumoniae infections and support the notion of administering drug combinations preferentially including a carbapenem when susceptibility data allow.
The limited efficacy of tigecycline revealed by the present analysis is in line with the recent warning issued by the U.S. Food and Drug Administration (FDA) against the use of this agent for serious infections (91a). The FDA, in a pooled analysis of 13 clinical trials, found an increased mortality risk associated with the use of tigecycline compared to other drugs to treat a variety of serious infections. A higher mortality rate was seen most clearly for patients treated for ventilator-associated pneumonia and bacteremia (9/18 [50.0%] tigecycline-treated patients versus 1/13 [7.7%] comparator drug-treated patients). The cause of excess death in these trials most likely was related to progression of the infection. Similarly, in a recent meta-analysis including 15 randomized clinical trials, the overall mortality was higher for patients treated with tigecycline than for those treated with other antibacterial agents, including levofloxacin, carbapenems, ceftriaxone, and ampicillin-sulbactam (267).
The decreased clinical effectiveness of tigecycline in severe infections could be attributed partly to the pharmacokinetic/pharmacodynamic (PK/PD) profile of the drug. Tigecycline demonstrates mainly bacteriostatic activity against Gram-negative organisms, and the attainable drug concentrations at several anatomic sites are suboptimal. The peak serum concentrations achieved with the standard dosing regimen of the drug (50 mg twice daily) range from 0.6 to 0.9 μg/ml, while those attained in the urine and in the epithelial lining fluid are severalfold lower (2, 36, 88, 210). The drug concentrations attainable by this standard dosing regimen, combined with this drug's MIC profile for current CPE isolates, render it unlikely for tigecycline to cure CPE infections at anatomic sites where drug concentrations are suboptimal. Therefore, this drug should be used with caution against CPE, preferentially in combination with another active agent and after due consideration of the attainable drug concentration at the anatomic site of infection and of the MIC for the infecting organism.
Rather disappointing results were also observed with colistin monotherapy, since 34 of 72 (47.2%) colistin-treated patients had adverse outcomes. The poor performance of colistin monotherapy against CPE infections has also been noticed previously (112). Nevertheless, when colistin was combined with tigecycline or an aminoglycoside, the failure rate decreased to 32% (17 of 53 patients failed treatment). More impressively, however, when it was combined with a carbapenem, the failure rate decreased dramatically, to 5% (1 of 17 patients failed treatment). The inferior clinical efficacy of colistin monotherapy may be associated, among other factors, with a suboptimal dosing regimen of the drug. In a retrospective study that evaluated patients with multidrug-resistant Gram-negative infections who received several daily dosages of colistin, multivariate analysis of survival data showed that a lower total daily dosage of intravenous colistin was associated with increased mortality (90). It is therefore critical to administer an adequate total daily dosage of colistin to critically ill patients, particularly to those who are on renal replacement therapy, in order to accomplish efficacious levels according to current recommendations (97). An additional factor that could be detrimental to a patient's outcome is the delay in attaining an efficacious drug concentration with the standard treatment regimen of colistin. This could be overcome by administering a loading dose of the drug (211).
Although colistin has been used extensively in critically ill patients infected with multidrug-resistant Gram-negative organisms, its optimum dosing regimen remains to be defined. Animal infection models have shown that the ratio of the area under the concentration-time curve for the free, unbound fraction of the drug (_f_AUC) to the MIC is the PK/PD index that is linked most strongly to an antibacterial effect, indicating the importance of achieving adequate time exposure to colistin across the day by administering the drug twice or three times a day (77, 78). In contrast, however, several features of this drug, such as its prolonged half-life, its concentration-dependent killing, and a phenomenon known as “adaptive resistance” that has not been appreciated adequately (50, 68, 92, 236), favor a once-daily dosing regimen, provided that such a scheme is not proven to be more nephrotoxic. Thus, a better understanding of the complex PK/PD features of colistin will be essential in devising dosing regimens with improved efficacy against CPE infections.
Among the publications available in MEDLINE, we were able to identify 15 studies reporting on 50 patients infected with carbapenemase-positive K. pneumoniae, all of whom had received carbapenem monotherapy (meropenem or imipenem). Twenty-nine of the respective isolates exhibited carbapenem MICs of ≤2 μg/ml. In seven and six isolates, the MICs were equal to 4 and 8 μg/ml, respectively. The remaining eight isolates were inhibited in vitro by carbapenem concentrations of >8 μg/ml. Note that, as indicated by the reported outcomes of these patients, the therapeutic efficacy of carbapenems increased from 25% for a MIC of >8 μg/ml to 66.7% for a MIC of 8 μg/ml, 71.4% for a MIC of 4 μg/ml, and 72.4% for a MIC of 2 μg/ml or less (Table 5). Clinical experience with carbapenem monotherapy is indeed limited. Yet we may consider the above data to indicate that carbapenems could provide some therapeutic benefit in infections caused by carbapenemase-producing K. pneumoniae, even for strains with intermediate susceptibility to carbapenems. It should be pointed out here that these observations do not contradict the findings of the experimental infection models discussed herein or those of human PK/PD studies (34, 118, 131, 145). Carbapenems display time-dependent bactericidal killing when free drug concentrations remain above the MIC for 40 to 50% of the time between dosing intervals. The probability of attaining a 50% _T_MIC target for an isolate with a MIC of 4 μg/ml is 69% for the traditional dosing regimen (e.g., 30-min infusion of 1 g every 8 h for meropenem) and increases to 100% for the high-dose/prolonged-infusion regimen (e.g., 3-h infusion of 2 g every 8 h for meropenem). For a MIC of 8 μg/ml, only the high-dose/prolonged-infusion regimen displays a relatively high probability (85%) of bactericidal target attainment (131).
Table 5.
Results of carbapenem monotherapy in 50 CPE-infected patients from 15 studiesa
| MIC of carbapenem (μg/ml) | No. of patients | No. of successes | No. of failures | % Failure |
|---|---|---|---|---|
| ≤1 | 17 | 12 | 5 | 29.4 |
| 2 | 12 | 9 | 3 | 25.0 |
| 4 | 7 | 5 | 2 | 28.6 |
| 8 | 6 | 4 | 2 | 33.3 |
| Subtotal | 42 | 30 | 12 | 28.6b |
| >8 | 8 | 2 | 6 | 75.0b |
| Total | 50 | 32 | 18 | 36 |
Whether we can use carbapenems in the presence of a carbapenemase is an issue that remains to be answered (65, 245). However, faced with the daily challenge of managing critically ill patients and the dearth of alternative therapeutic options, some of which have not been investigated satisfactorily and/or whose efficacy in certain situations remains questionable, use of a carbapenem against an organism with a MIC of ≤4 or even ≤8 μg/ml, using a high-dose/prolonged-infusion regimen and in combination with another active agent, preferentially gentamicin or colistin, seems reasonable.
The number of CPE isolates exhibiting resistance to almost all available agents is worryingly high in various settings (85). Given that fosfomycin displays good in vitro activity against most CPE, this agent could be selected as salvage therapy in situations where therapeutic options are very limited (89). Although the main indication of fosfomycin remains the treatment of lower urinary tract infection, some investigators have included this drug in various combination schemes to treat systemic infections caused by CPE (87, 164). Available data, however, are too limited to allow a sound hypothesis as to its efficacy. Also, the potential of fosfomycin to rapidly select resistant mutants during therapy is a matter of consideration (188).
The clinical data reviewed here allow for some reasonable notions but not for solid conclusions, since it was not possible to measure and adjust for certain important variables (e.g., host-related factors, severity of infections, and dosing and timing of initiation of treatment). Thus, we cannot exclude the possibility that our analysis, in some cases, might have resulted in biased associations between antimicrobial treatment and outcome. Nevertheless, given that the majority of patients infected with CPE are debilitated, with various underlying diseases, and that more than 90% of them have severe infections (BSIs or pneumonias), it is unlikely that residual confounding could account to an appreciable extent for the significantly different failure rates between treatment groups.
CPE IN HEALTH CARE SETTINGS
Epidemiology
The prevalence of CPE, primarily K. pneumoniae, in several institutions in areas of endemicity may vary between 20 and 40% (28, 99, 133, 233). Initially, CPE appeared to cause hospital-acquired infections, mainly in ICU patients (28, 29, 67, 233, 265). More recently, however, they have spread in different health care settings, including long-term care facilities (LTCF) (16, 53, 83, 161, 249).
Several investigators have evaluated the factors associated with increased risk for acquisition of CPE in the hospital setting (69, 91, 98, 106, 117, 132, 205, 233, 261). Investigators from Israel have shown that poor functional status of the host, prior antibiotic therapy, and stay in the ICU are independent risk factors for colonization or infection with carbapenem-resistant K. pneumoniae (233). Other factors that have been associated with CPE include solid organ or stem cell transplantation (122, 204), presence of a biliary catheter (120), multiple invasive devices (157), prior surgery, and the presence of wounds (106). Antibiotic selection pressure may be an additional factor that influences colonization with these organisms. Case-control studies have shown that almost every class of antibiotics can select for CPE (69, 91, 98, 117, 120, 132, 203, 261). What appears to be more important, however, is the cumulative number of prior antibiotic exposures rather than the use of a specific class of antibiotics (69, 205).
Since members of the Enterobacteriaceae constitute part of the human enteric flora, once CPE colonize the intestinal tract, carriage may persist for a long time (232). Based on limited experience (90, 116, 207), it appears that colonization with CPE is prolonged and lasts at least several months. A prolonged duration of colonization means a larger reservoir of colonized patients, exerting more colonization pressure, which in turn will result in higher rates of patient-to-patient transmission. CPE cross-transmission occurs more efficiently in health care settings where infection control practices are poor. Indeed, in a surgical unit where hand hygiene compliance was 21%, the probability of a patient becoming colonized with CPE was 7.1% per week of hospitalization, and the incidence of new acquisitions was 9.1/1,000 patient-days (39). It can be supported that once the first case of CPE infection is recognized in a health care facility, these organisms may have already spread widely and colonized a substantial number of patients. Colonization may be extensive and pass largely unnoticed in institutions located in regions of endemicity, as evident in several studies. Calfee et al. (41) reported that 37% of patients with carbapenem-resistant K. pneumoniae colonization were first identified by surveillance cultures. During an outbreak of carbapenemase-producing K. pneumoniae in Israel, a point prevalence survey demonstrated that 16 (5.4%) of 298 patients screened were colonized with carbapenemase producers, and notably, 11 (69%) of these carriers would have remained undetected without the performance of active surveillance cultures (261).
More importantly, CPE colonization may evolve to infection, with detrimental effects for the host (23, 41, 261). Although data regarding the infection/colonization ratio are very limited, it is estimated that a proportion of colonized patients (10 to 30%) will develop CPE infection. This proportion is probably related to the severity of the underlying disease of the host and appears to be higher for severely immunocompromised patients (e.g., patients in induction chemotherapy for acute myelogenous leukemia or post-allogeneic stem cell transplantation).
Infection Control Strategies
The rapid and worldwide dissemination of a variety of CPE reflects, to various extents, increased antibiotic selection pressure, carriage of the acquired carbapenemase genes by mobile genetic units, and probably the enhanced spreading potential of specific clones, such as K. pneumoniae ST258. Notwithstanding these factors, the fact that CPE outbreaks occur principally in settings where infection control practices are inadequate shows that there remains plenty of room for curbing CPE spread.
CPE seem to have a high potential for spread not only from patient to patient within a health care facility but also through “cycling” of patients between institutions in the same region (263) and/or across borders from high- to low-prevalence countries, which, for instance, has happened repeatedly in Europe (107), threatening every health care system. To address this public health threat, it is imperative to formulate a preparedness plan before CPE have the opportunity to become endemic. In areas where this has already happened, on the other hand, control measures must include a multifaceted approach coordinated by the national health authorities, as indicated in a recent study from Israel (234).
In response to this need, both the Centers for Disease Control and Prevention (CDC) and a group of experts from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) have published guidelines for interventions to control CPE transmission in acute health care facilities (44, 48). These recommendations are based primarily on experience with other MDR organisms (www.cdc.gov/hicpac/mdro/mdro_0.html) and typically include detection, isolation or cohorting, and other enhanced infection control measures. It is expected that the increasing number of studies dealing with CPE epidemiology will soon lead to guidelines specifically targeting CPE.
Tracing of Carriers
Critical to the success of interrupting cross-transmission of CPE in a health care facility is the timely identification of colonized and/or infected patients. Thus, every clinical microbiology laboratory should establish a reliable detection methodology. Additionally, resources and trained personnel should be readily available to carry out point prevalence surveys as well as active surveillance cultures, a demanding yet highly effective approach for detecting carriers (15, 53, 177). The most suitable anatomic sites for surveillance cultures appear to be the perianal area and the rectum (31, 261). In patients with surgical wounds, decubitus ulcers, a urinary catheter, or bronchial secretions, the respective sites could also be screened. Several versions of culture-based techniques have been described. In some of them, differences are limited to the concentration of carbapenem used for the initial screening, with the lowest being that proposed by EUCAST (0.25 μg/ml of meropenem), based on an epidemiologic cutoff. Additionally, several PCR-based techniques for active surveillance have been described (111, 135, 231). PCR assays are rapid and usually more sensitive than culture-based methods. However, their main disadvantages are that they do not provide information on the carbapenemase-producing species and can assess the presence of only already known resistance genes.
In settings with low CPE prevalence, laboratories should monitor clinical culture results to determine whether CPE have been isolated in the facility (48). If a CPE is identified by clinical culture, a point prevalence survey should be performed in selected wards (e.g., ICUs and units where CPE have been identified). Detection of additional CPE carriers should then be followed by active surveillance covering a wider range of patients with potential epidemiological links to persons from whom CPE have been isolated [e.g., patients from the same unit and patients cared for by the same health care worker(s)]. Active surveillance should be continued until no new CPE cases are identified.
In areas where CPE are endemic, an increased likelihood exists for importation of CPE into a previously CPE-free health care facility. Upon admission of patients at increased risk of CPE carriage (such as residency in an LTCF, previous stay in an ICU, prolonged hospitalization in the previous 6 months, or presence of indwelling devices), preemptive isolation while awaiting surveillance culture results can prevent early transmission events (19, 43, 53, 122). Performing a surveillance culture before the discharge of a patient into the community or an LTCF may also be useful to avoid transfer of CPE to additional niches (83, 122). It is also important to communicate the results of screening and to provide alerts for previously identified CPE carriers for every readmitted patient. The surveillance strategy should be defined clearly for each setting and evaluated periodically according to the current situation and available resources (19, 48).
Intervention
When a CPE carrier is identified, the infection control personnel should be notified immediately. Isolation or cohorting of CPE carriers seems to be the main prevention measure (43, 83, 106, 110, 122, 127, 176, 177, 234). Assignment of dedicated health care workers and use of separate equipment for carriers are additional interventions that have been employed successfully in several outbreaks (19, 43, 122, 176, 177, 234). In the Israeli experience, physical separation of carriers from noncarriers and assignment of dedicated nursing staff to care for carriers on all shifts were the most important components of the intervention measures in halting transmission (234).
In addition to isolation, personnel in acute health care facilities should use contact precautions (wearing a gown and gloves) when caring for patients colonized or infected with CPE in order to minimize indirect transmission of the organism (44, 48; www.cdc.gov/hicpac/mdro/mdro_0.html). In LTCFs, it is only practical to apply contact precautions for those patients who are severely ill and have conditions that may facilitate transmission (e.g., diarrhea or decubitus ulcers) (83). The success of the intervention should be monitored constantly, and when failure is observed, a root cause analysis should be performed (19).
Environmental cleaning and decolonization of patients.
Cleaning of the inanimate environment and equipment in proximity to a CPE carrier, along with daily antiseptic (chlorhexidine) baths to cleanse patients' skin, were included in the bundles of intervention measures that successfully controlled two recent outbreaks in the United States (176, 177). Available data do not allow for an assessment of the usefulness of the systematic application of these practices. Indeed, persisting environmental contamination with Enterobacteriaceae is limited compared to that of other organisms (144). In addition, culturing of surfaces and equipment to investigate their role in the transmission chain is not usually required, unless the inanimate environment or shared equipment is potentially linked to an outbreak (43). Such an event, however, does not seem to occur frequently with CPE. On the other hand, daily antiseptic baths have been proven efficacious in preventing central vascular catheter-associated bloodstream infections (178). This practice therefore appears to warrant further evaluation with respect to CPE-positive settings.
Selective decolonization of the gut by use of oral gentamicin in patients undergoing chemotherapy and allogeneic stem cell transplantation has achieved a 66% eradication rate of the CPE carrier state (278). This approach should be adopted cautiously, however, as gentamicin is commonly used for the treatment of CPE infections, and its excessive use may select CPE that are resistant to this drug as well.
Judicious antimicrobial use.
The role of restriction of antibiotics in controlling CPE needs further evaluation. As mentioned previously, almost every antimicrobial class can select for CPE. In this regard, cumulative exposure to antibiotics is likely to be more important than selection exerted by a specific agent. Therefore, formulary interventions should focus on reduction of overall antibiotic usage (19).
Success Stories
There have been various successful attempts to control CPE outbreaks in both endemic and nonendemic settings. The relevant studies (summarized in Table 6) concern single-center outbreaks, except for one describing a countrywide epidemic in Israel (234). Although some differences in approach did exist, the interventions implemented were largely based on the rationale of infection control strategies mentioned previously, with their main components being surveillance cultures, isolation and cohorting, contact precautions, and assignment of dedicated staff.
Table 6.
Synopsis of 11 successful infection control studies for CPE infections in nonendemic and endemic settings
| Reference | Study design | Health care setting and geographic region | Infection control measures | Outcome | |
|---|---|---|---|---|---|
| Baseline | Additional | ||||
| Studies in regions where CPE are not endemic | |||||
| 110 | Retrospective | 36-bed ICU in a tertiary care hospital, Melbourne, Australia | 1. Surveillance of culture results | 1. Universal contact precautions in ICU | Decrease of CPE cases from 3 to 1 per month |
| 2. Standard precautions | 2. Single-room isolation of CPE patients (all wards) | ||||
| 3. Environmental cleaning | 3. Restriction of carbapenem use | ||||
| 122 | Retrospective | Abdominal surgery center, Paris, France | 1. Active surveillance for ESBLs | 1. Preemptive isolation of contact patients and newly admitted patients | Rapid control of the outbreak |
| 2. Isolation of CPE patients | 2. Dedicated nursing staff | ||||
| 3. Contact precautions | 3. Limited transfer of CPE patients | ||||
| 4. Environmental disinfection | 4. Antibiotic restriction policy (imipenem) | ||||
| 5. Active surveillance | 5. Screening campaign targeting contact patients discharged from the hospital | ||||
| 43 | Retrospective | 7 hospitals, Paris, France | 1. National early warning system for multiresistant isolates | 1. Cohorting of CPE cases and contacts | Rapid control of the outbreak |
| 2. Active surveillance for ESBLs | 2. Flagging of CPE cases | ||||
| 3. Active screening of contact patients | 3. Dedicated health care workers | ||||
| 4. Evaluation of duodenoscope disinfection practices | 4. Reinforcing hand hygiene and contact precautions | ||||
| 5. Limited transfer of CPE cases and contacts | |||||
| 6. Revision of duodenoscope disinfection procedure | |||||
| Studies in regions of endemicity | |||||
| 127 | Retrospective | 10-bed ICU in a tertiary care hospital, New York, NY | 1. Contact isolation of CPE patients | 1. Active surveillance for CPE on admission to ICU and weekly thereafter | Incidence decreased from 9.7 ± 2.2 to 3.7 ± 1.6 CPE cases per 1,000 patient-days |
| 2. Environmental cleaning | 2. ICU closure and disinfection | ||||
| 3. Infection control supervising | 3. Cohorting of CPE patients | ||||
| 4. Active surveillance for vancomycin-resistant enterococci and carbapenem-resistant Acinetobacter | 4. Dedicated nursing staff5. Promotion of hand hygiene | ||||
| 176 | Retrospective | 20-bed surgical ICU in a tertiary care hospital, Miami, FL | No data | 1. Point prevalence surveillance | Control of CPE spread |
| 2. Isolation and contact precautions for CPE patients | |||||
| 3. Dedicated nursing staff | |||||
| 4. Daily chlorhexidine baths on all patients | |||||
| 5. Environmental cleaning after every shift and evaluation with environmental cultures | |||||
| 6. Educational campaigns | |||||
| 177 | Retrospective | 70-bed long-term acute care hospital, Chicago, IL | 1. Active surveillance on admission | 1. Active surveillance cultures for CPE and point prevalence surveys during the intervention | Colonization prevalence of CPE decreased progressively, from 21% to 12, 6, 3, and 0% |
| 2. Baseline point prevalence surveillance | 2. Isolation and contact precautions for CPE patients | ||||
| 3. Preemptive isolation of high-risk patients | |||||
| 4. Environmental cultures and enhanced environmental cleaning | |||||
| 5. Daily chlorhexidine baths for all patients | |||||
| 6. Educational campaign | |||||
| 83 | Retrospective | Long-term acute care hospital, South Florida | No data | 1. Active surveillance culture | Termination of the outbreak |
| 2. Point prevalence survey | |||||
| 3. Isolation and contact precautions for CPE patients | |||||
| 4. Dedicated nursing staff and equipment | |||||
| 106 | Retrospective | Tertiary care hospital, Puerto Rico | No data | 1. Contact precautions for CPE patients | Control of the outbreak |
| 2. Cohorting of CPE patients | |||||
| 3. Dedicated nursing staff | |||||
| 4. Hand hygiene audits | |||||
| 5. ICU closure | |||||
| 6. Restriction of broad-spectrum antibiotics | |||||
| 7. Active surveillance on admission to high-risk units (ICU, diabetes ward) and weekly thereafter | |||||
| 15 | Retrospective | Tertiary care hospital, Tel Hashomer, Israel | 1. Contact precautions for CPE cases | 1. Active surveillance on admission to ICU and in step-down units and weekly thereafter | Incidence decreased from 6.93 to 1.8 CPE cases per 10,000 patient-days |
| 2. In other departments, active surveillance of patients with epidemiologic links to CPE carriers | |||||
| 3. Daily reporting of CPE cases to hospital manager and the national coordinator | |||||
| 53 | Prospective intervention study | Tertiary care hospital, Rehovot, Israel | No data | 1. Active surveillance on admission to ICU, in roommates of new CPE cases or carriers, and in patients at high risk for carriage | Incidence decreased from 8.2 to 0.5 CPE case per 10,000 patient-days |
| 2. Isolation-cohorting and contact precautions for CPE cases and carriers | |||||
| 3. Dedicated nursing staff | |||||
| 4. Environmental cleaning and disinfecting during hospital stay and after discharge | |||||
| 5. Education and training to all medical staff members, patients, and caregivers | |||||
| 6. Automatic warning system | |||||
| 234 | Prospective intervention study | 27 acute care hospitals, Israel | No data | 1. Isolation-cohorting and contact precautions for CPE patients and carriers | Monthly incidence decreased from 55.5 to 11.7 CPE cases per 100,000 patient-days |
| 2. Dedicated nursing staff and equipment | |||||
| 3. Mandatory reporting to public health authorities of every CPE case | |||||
| 4. Establishment of Task Force on Antimicrobial Resistance and Infection Control |
The design of these studies does not allow us to accurately classify measures according to their effectiveness. Critical interpretation of the published data, however, suggests that application of a bundle of infection control measures may be required for maximum containment of CPE. Controlled studies and mathematical modeling of CPE transmission and prevention are needed to specify the most appropriate procedures for containment or even eradication of CPE.
NOVEL AGENTS AGAINST CPE
Antibiotics
Not unexpectedly, many new antibiotics under development target multidrug-resistant bacteria. The relatively small number of new antibacterials active against MDRs does not necessarily mean a lack of industry interest but rather reflects the countless difficulties posed from the early stages of designing to the introduction into clinical practice. Although not specifically focused on CPE, some of these experimental antibacterials, belonging to diverse antimicrobial classes, exhibit high activity against these microorganisms. We present below a few indicative compounds, preferentially those that are under advanced clinical testing (Table 7).
Table 7.
Experimental antimicrobial agents active against carbapenemase-producing Enterobacteriaceae
| Drug | Compound type | Relevant target | Reference |
|---|---|---|---|
| BAL30072 | Siderophore-containing sulfactam | Enterobacteriaceae, including MβL producers | 194 |
| Plazomicin (ACHN-490) | Sisomicin derivative | Gram-negative organisms, including carbapenemase producers | 149 |
| GSK2251052 | Leucyl-tRNA synthetase inhibitor | Gram-negative organisms, including carbapenemase producers | 5 |
Sulfactams.
The sulfactams comprise a distinct series of monocyclic β-lactams (with tigemonam being the first member) exhibiting potent activity against the Enterobacteriaceae. Additionally, other structurally related monocyclic compounds, such as aztreonam, are virtually unaffected by the hydrolytic activity of MβLs. Of special interest is a novel group of siderophore-coupled sulfactams, represented by BAL30072 (194); the coupled siderophores enhance activity against MβL producers by facilitating the entrance rate through the outer membrane, even in strains with defective permeability (195). Animal experiments have further supported the therapeutic potential of BAL30072 against MβL-positive enterobacteria (168).
Plazomicin.
Formerly known as ACHN-490, plazomicin is a sisomicin derivative with substitutions at positions 1 and 6 (a hydroxyaminobutyric and a hydroxyethyl group, respectively). This antibiotic resists most aminoglycoside-modifying enzymes (but not 16S rRNA methylases) and exhibits potent bactericidal activity against many MDRs, including CPE (149). It is currently undergoing a phase 2 study for use against complicated urinary tract infections (276).
Aminoacyl-tRNA synthetase inhibitors.
Until recently, the aminoacyl-tRNA synthetase inhibitors included only mupirocin, an isoleucyl-tRNA synthetase inhibitor of limited clinical use. Development of novel boron-containing aminoacyl-tRNA synthetase inhibitors added compounds with kinetics suitable for systematic use that are also active against Gram-negative organisms. A promising compound, GSK2251052, which inhibits the leucyl-tRNA synthetase and is effective against CPE and other MDRs, is currently under clinical evaluation (5).
Carbapenemase Inhibitors
Despite almost 3 decades of efforts to develop β-lactamase inhibitors, only three, clavulanic acid, tazobactam, and sulbactam, have been made available as therapeutics. Fortunately, a number of potential β-lactamase inhibitors are under evaluation (for a comprehensive review, see reference 76). The majority of currently tested compounds are β-lactam derivatives, though a number of diverse non-β-lactam substances also exhibit significant inhibitory activity. Only compounds that also happen to inhibit acquired carbapenemases (mainly KPCs and MβLs) at clinically relevant concentrations are mentioned briefly here (Table 8).
Table 8.
Experimental β-lactamase inhibitors active against carbapenemases from Enterobacteriaceae
| Inhibitor | Compound type | Inhibition spectrum (β-lactamase classes) | Susceptible carbapenemasesa | Reference(s) |
|---|---|---|---|---|
| BLI-489 | Penem | A, C, D | KPC type | 209 |
| J-110,411 and J-111,225 | 1-β-Methyl carbapenem | A, C, B | IMP type | 183, 184 |
| Mercaptomethyl sulfones | C-6-substituted penicillin sulfone | B | VIM and IMP types | 38 |
| 2,3-(S,S)-Disubstituted succinic acids | Succinic acid | B | IMP type | 170 |
| Thiomandelic acids | Thiol | B | VIM and IMP types | 147, 268 |
| Avibactam (NLX104) | Diazabicyclo-octanone | A, C, D | KPC type | 22 |
Penem derivatives.
The penem derivatives include the prototype, BRL 42715, and a series of heterocyclic methylidene penems with significant inhibitory activity against serine β-lactamases (molecular classes A, C, and D). BLI-489, a bicyclic derivative, has been shown to be capable of reducing the MICs of piperacillin against enterobacteria producing KPC enzymes (209).
1-β-Methylcarbapenems.
1-β-Methylcarbapenems have been synthesized by various substitutions in the carbapenem nucleus, which contains a methyl group at position C-1 (the same nucleus as that in doripenem). Interesting members of the group are compounds J-110,411 and J-111,225, which inhibit class A and C β-lactamases, as well as IMP-type MβLs, at low concentrations (183, 184).
Sulfones.
C-6-substituted penicillin sulfones are primarily inhibitors of class A β-lactamases. However, compounds with a mercaptomethyl substituent at C-6 exhibit strong inhibitory activity against MβLs (38).
Succinic acids (non-β-lactams).
Various succinic acid derivatives [2,3-(S,S)-disubstituted succinic acids] exhibit potent inhibitory activity against the IMP-type MβLs, restoring carbapenem susceptibility in members of the Enterobacteriaceae producing these enzymes (170).
Thiols (non-β-lactams).
Thiol compounds, such as thiomandelic acids, are considered effective inhibitors of MβLs, especially those of the VIM and IMP types. They act by bridging the zinc ions of the active site, thus displacing the catalytic water molecule (147, 268).
Avibactam.
Formerly known as NXL104, avibactam, a non-β-lactam compound, is likely to be the most promising experimental inhibitor and is expected to be introduced soon into antimicrobial chemotherapy. It is a bridged diazabicyclo (EC 3.2.1) octanone with excellent activity against virtually all serine β-lactamases but no activity against molecular class B enzymes (22). In the current era of carbapenemases, the ability of avibactam to inhibit KPC β-lactamases at very low concentrations (50% inhibitory concentration [IC50] of 38 nM) is important (22, 242). MICs of various newer β-lactams tested against KPC producers in the presence of avibactam have clearly shown the in vitro efficacy of these combinations (82). Most importantly, the therapeutic efficacy of the ceftazidime-avibactam combination has been documented for murine infection models (259).
CONCLUDING REMARKS AND PERSPECTIVES
The current public health crisis due to the international spread of carbapenemase-producing multidrug-resistant enterobacteria has caught us unprepared, despite clear signs of this problem arising years ago. Most of the recent papers describing yet another emergence of a CPE or a CPE outbreak conclude, almost invariably, with the urgent need for measures to contain these microorganisms. This, however, begs the question: precisely what measures are to be taken? First of all, a clearer and more accurate picture of the situation at the global level, based on data that are valid for comparisons, is necessary. For instance, in developed countries, it appears that many studies have been conducted in single institutions or a small number of tertiary care hospitals and cover limited times. Therefore, biased sampling is probable, at least in some cases. Moreover, systematic reports from many African countries, the Balkans, the Middle East, and vast areas in Asia are scarce, if they exist at all. The collection of epidemiological data that are as complete as possible is imperative for the implementation of effective yet affordable and sustainable measures against CPE, especially in the most affected countries. In this respect, if the expressed international concern is indeed genuine, resources from international public health organizations should be mobilized and allocated appropriately.
Some new drugs active against CPE are indeed in an advanced stage of development, but very few of them are expected to be clinically available soon. Thus, in the foreseeable future, we shall continue to rely on the available antibiotics. Nevertheless, the studies summarized above show that there is yet room to improve our therapeutic approaches. Colistin and tigecycline are among the most frequently used agents in the treatment of CPE infections. However, as discussed here, laboratory and clinical data supporting this practice are insufficient. Although it has to be admitted that alternative options have not yet been documented solidly, well-designed clinical trials aiming to (i) determine the optimum dosing regimen of colistin, (ii) define CPE infections that could be controlled effectively by tigecycline, (iii) exploit the PK/PD features of carbapenems, and (iv) unravel the most effective drug combinations may prove valuable.
Biographies

Leonidas S. Tzouvelekis, Pharm.D., Ph.D., is Associate Professor of Microbiology at the School of Medicine, National and Kapodistrian University of Athens, Athens, Greece, and a research collaborator at the Laboratory of Bacteriology, Hellenic Pasteur Institute, Athens, Greece. His main research interests include various aspects of beta-lactamase-mediated resistance in enterobacterial species. He is also involved in projects regarding the epidemiology of hospital- and community-acquired antibiotic-resistant pathogens.

Antonios Markogiannakis, Pharm.D., Ph.D., obtained his bachelor's degree in pharmacy at the University of Messina, Italy, before obtaining a master's degree in public health at the National School of Public Health, Athens, Greece, and subsequently a Ph.D. in the Department of Microbiology, Medical School, National and Kapodistrian University of Athens, Athens, Greece. He works as a clinical pharmacist at Laiko General Hospital of Athens. His main research interests focused on the mechanisms of bacterial resistance to antibiotics. He also participates in antibiotic audit projects.

Mina Psichogiou, M.D., Ph.D., is a Lecturer of Internal Medicine and Infectious Diseases at the National and Kapodistrian University of Athens, Athens, Greece. After her qualification in medicine, she worked in the Department of Hygiene and Epidemiology, where she obtained her Ph.D. Dr. Psichogiou had her clinical training in internal medicine and infectious diseases at the First Department of Propaedeutic Medicine, Laiko General Hospital, Athens, Greece, and then worked at the Hellenic Center for Disease Control and Prevention. During the past 6 years, she has gained broad experience in the clinical aspects, epidemiology, and treatment of multidrug-resistant infections. Her research interests include health care-associated infections and the epidemiology of infectious diseases.

Panayiotis T. Tassios, B.Sc., Ph.D., is Assistant Professor in Molecular Microbiology at the Department of Microbiology, Medical School, National and Kapodistrian University of Athens, Athens, Greece. Since 1994, he has been working on the epidemiology and molecular typing of bacteria causing both health care-associated and community-acquired infections. He has been both Secretary (2003 to 2005) and Chairperson (2005 to 2007) of the ESCMID Study Group on Epidemiological Markers. Since 2003, he has collaborated as an expert with the European Center for Disease Prevention and Control (ECDC), the French National Health Institute (Institut de Veille Sanitaire), the French Ministry of Research and New Technologies, and the Italian Ministry of Education, Universities and Research.

George L. Daikos, M.D., Ph.D., is Associate Professor of Medicine and Infectious Diseases at the School of Medicine, National and Kapodistrian University of Athens, Athens, Greece. He is Director of the Antimicrobial Chemotherapy Research Laboratory at the First Department of Propaedeutic Medicine at this institution. Dr. Daikos performed his residency in internal medicine at Wayne State University, Detroit, MI, and a fellowship in infectious diseases at University of Illinois, Chicago, IL. He is board certified in both internal medicine and infectious diseases. His research focuses on health care-associated infections, antimicrobial resistance, and the molecular epidemiology of infectious diseases.
REFERENCES
- 1.Adams-Haduch JM, Potoski BA, Sidjabat HE, Paterson DL, Doi Y. 2009. Activity of temocillin against KPC-producing Klebsiella pneumoniae and Escherichia coli. Antimicrob. Agents Chemother. 53:2700–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agwuh KN, MacGowan A. 2006. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J. Antimicrob. Chemother. 58:256–265 [DOI] [PubMed] [Google Scholar]
- 3.Aktas Z, Bal C, Midilli K, Poirel L, Nordmann P. 2006. First IMP-1-producing Klebsiella pneumoniae isolate in Turkey. Clin. Microbiol. Infect. 12:695–696 [DOI] [PubMed] [Google Scholar]
- 4.Alba J, Ishii Y, Thomson K, Moland ES, Yamaguchi K. 2005. Kinetics study of KPC-3, a plasmid-encoded class A carbapenem-hydrolyzing β-lactamase. Antimicrob. Agents Chemother. 49:4760–4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alley D. 2011. Novel boron-containing aminoacyl-tRNA synthetase inhibitors with Gram-negative bacterial activity, abstr 1071. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL [Google Scholar]
- 6.Anthony KB, et al. 2008. Clinical and microbiological outcomes of serious infections with multidrug-resistant Gram-negative organisms treated with tigecycline. Clin. Infect. Dis. 46:567–570 [DOI] [PubMed] [Google Scholar]
- 7.Antoniadou A, et al. 2007. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: first report of a multiclonal cluster. J. Antimicrob. Chemother. 59:786–790 [DOI] [PubMed] [Google Scholar]
- 8.Aubert D, Naas T, Héritier C, Poirel L, Nordmann P. 2006. Functional characterization of IS_1999_, an IS_4_ family element involved in mobilization and expression of β-lactam resistance genes. J. Bacteriol. 188:6506–6514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avlami A, et al. 2010. Detection of metallo-β-lactamase genes in clinical specimens by a commercial multiplex PCR system. J. Microbiol. Methods 83:185–187 [DOI] [PubMed] [Google Scholar]
- 10.Babouee B, et al. 2011. Emergence of four cases of KPC-2 and KPC-3-carrying Klebsiella pneumoniae introduced to Switzerland, 2009–10. Euro Surveill. 16:19817http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19817 [DOI] [PubMed] [Google Scholar]
- 11.Bagley ST. 1985. Habitat association of Klebsiella species. Infect. Control 6:52–58 [DOI] [PubMed] [Google Scholar]
- 12.Baraniak A, et al. 2009. The emergence of Klebsiella pneumoniae ST258 with KPC-2 in Poland. Antimicrob. Agents Chemother. 53:4565–4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bebrone C. 2007. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem. Pharmacol. 74:1686–1701 [DOI] [PubMed] [Google Scholar]
- 14.Ben-David D, et al. 2012. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin. Microbiol. Infect. 18:54–60 [DOI] [PubMed] [Google Scholar]
- 15.Ben-David D, et al. 2010. Potential role of active surveillance in the control of a hospital-wide outbreak of carbapenem-resistant Klebsiella pneumoniae infection. Infect. Control Hosp. Epidemiol. 31:620–626 [DOI] [PubMed] [Google Scholar]
- 16.Ben-David D, et al. 2011. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect. Control Hosp. Epidemiol. 32:845–853 [DOI] [PubMed] [Google Scholar]
- 17.Benenson S, et al. 2009. Carbapenem-resistant Klebsiella pneumoniae endocarditis in a young adult. Successful treatment with gentamicin and colistin. Int. J. Infect. Dis. 13:e295–e298 [DOI] [PubMed] [Google Scholar]
- 18.Berçot B, Poirel L, Nordmann P. 2011. Updated multiplex polymerase chain reaction for detection of 16S rRNA methylases: high prevalence among NDM-1 producers. Diagn. Microbiol. Infect. Dis. 71:442–445 [DOI] [PubMed] [Google Scholar]
- 19.Bilavsky E, Schwaber MJ, Carmeli Y. 2010. How to stem the tide of carbapenemase-producing Enterobacteriaceae?: proactive versus reactive strategies. Curr. Opin. Infect. Dis. 23:327–331 [DOI] [PubMed] [Google Scholar]
- 20.Bisiklis A, Papageorgiou F, Frantzidou F, Alexiou-Daniel S. 2007. Specific detection of _bla_VIM and _bla_IMP metallo-β-lactamase genes in a single real-time PCR. Clin. Microbiol. Infect. 13:1201–1203 [DOI] [PubMed] [Google Scholar]
- 21.Bogdanovich T, et al. 2011. Colistin-resistant, Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae belonging to the international epidemic clone ST258. Clin. Infect. Dis. 53:373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnefoy A, et al. 2004. In vitro activity of AVE1330A, an innovative broad-spectrum non-β-lactam β-lactamase inhibitor. J. Antimicrob. Chemother. 54:410–417 [DOI] [PubMed] [Google Scholar]
- 23.Borer A, et al. 8 September 2011. Risk factors for developing clinical infection with carbapenem-resistant Klebsiella pneumoniae in hospital patients initially only colonized with carbapenem-resistant K. pneumoniae. Am. J. Infect. Control [Epub ahead of print.] doi:10.1016/j.ajic.2011.05.022 [DOI] [PubMed] [Google Scholar]
- 24.Borer A, et al. 2009. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect. Control Hosp. Epidemiol. 30:972–976 [DOI] [PubMed] [Google Scholar]
- 25.Bradford PA, et al. 2004. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 β-lactamases in New York City. Clin. Infect. Dis. 39:55–60 [DOI] [PubMed] [Google Scholar]
- 26.Bratu S, et al. 2007. Detection and spread of Escherichia coli possessing the plasmid-borne carbapenemase KPC-2 in Brooklyn, New York. Clin. Infect. Dis. 44:972–975 [DOI] [PubMed] [Google Scholar]
- 27.Bratu S, Landman D, Alam M, Tolentino E, Quale J. 2005. Detection of KPC carbapenem-hydrolyzing enzymes in Enterobacter spp. from Brooklyn, New York. Antimicrob. Agents Chemother. 49:776–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bratu S, et al. 2005. Rapid spread of carbapenem-resistant Klebsiella pneumoniae in New York City: a new threat to our antibiotic armamentarium. Arch. Intern. Med. 165:1430–1435 [DOI] [PubMed] [Google Scholar]
- 29.Bratu S, et al. 2005. Emergence of KPC-possessing Klebsiella pneumoniae in Brooklyn, New York: epidemiology and recommendations for detection. Antimicrob. Agents Chemother. 49:3018–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bratu S, et al. 2005. Carbapenemase-producing Klebsiella pneumoniae in Brooklyn, NY: molecular epidemiology and in vitro activity of polymyxin B and other agents. J. Antimicrob. Chemother. 56:128–132 [DOI] [PubMed] [Google Scholar]
- 31.Buehlmann M, Fankhauser H, Laffer R, Bregenzer T, Widmer AF. 2010. The inguinal skin: an important site of colonization with extended-spectrum β-lactamase-producing Enterobacteriaceae. Infect. Control Hosp. Epidemiol. 31:427–428 [DOI] [PubMed] [Google Scholar]
- 32.Bulik CC, et al. 2010. Comparison of the activity of a human simulated, high-dose, prolonged infusion of meropenem against Klebsiella pneumoniae producing the KPC carbapenemase versus that against Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 54:804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bulik CC, et al. 2010. Comparison of meropenem MICs and susceptibilities for carbapenemase-producing Klebsiella pneumoniae isolates by various testing methods. J. Clin. Microbiol. 48:2402–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulik CC, Nicolau DP. 2010. In vivo efficacy of simulated human dosing regimens of prolonged-infusion doripenem against carbapenemase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 54:4112–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burckhardt I, Zimmermann S. 2011. Using matrix-assisted laser desorption ionization–time of flight mass spectrometry to detect carbapenem resistance within 1 to 2.5 hours. J. Clin. Microbiol. 49:3321–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkhardt O, et al. 2009. Tigecycline possibly underdosed for the treatment of pneumonia: a pharmacokinetic viewpoint. Int. J. Antimicrob. Agents 34:101–102 [DOI] [PubMed] [Google Scholar]
- 37.Bush K, Jacoby GA. 2010. Updated functional classification of β-lactamases. Antimicrob. Agents Chemother. 54:969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buynak JD, et al. 2004. Penicillin-derived inhibitors that simultaneously target both metallo- and serine-β-lactamases. Bioorg. Med. Chem. Lett. 14:1299–1304. [DOI] [PubMed] [Google Scholar]
- 39.Buzala G, et al. 2011. Risk factors for carbapenem resistant Enterobacteriaceae carriage on admission to a surgical unit and acquisition rate during hospitalization, abstr P687. Abstr. 21st Eur. Congr. Clin. Microbiol. Infect. Dis., Milan, Italy [Google Scholar]
- 40.Cagnacci S, et al. 2008. Bloodstream infections caused by multidrug-resistant Klebsiella pneumoniae producing the carbapenem-hydrolysing VIM-1 metallo-β-lactamase: first Italian outbreak. J. Antimicrob. Chemother. 61:296–300 [DOI] [PubMed] [Google Scholar]
- 41.Calfee D, Jenkins SG. 2008. Use of active surveillance cultures to detect asymptomatic colonization with carbapenem-resistant Klebsiella pneumoniae in intensive care unit patients. Infect. Control Hosp. Epidemiol. 29:966–968 [DOI] [PubMed] [Google Scholar]
- 42.Carattoli A, et al. 2010. Complete nucleotide sequence of the IncN plasmid pKOX105 encoding VIM-1, QnrS1 and SHV-12 proteins in Enterobacteriaceae from Bolzano, Italy compared with IncN plasmids encoding KPC enzymes in the U. S. A. J. Antimicrob. Chemother. 65:2070–2075 [DOI] [PubMed] [Google Scholar]
- 43.Carbonne A, et al. 2010. Control of a multi-hospital outbreak of KPC-producing Klebsiella pneumoniae type 2 in France, September to October 2009. Euro Surveill. 15:19734http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19734 [DOI] [PubMed] [Google Scholar]
- 44.Carmeli Y, et al. 2010. Controlling the spread of carbapenemase-producing gram-negatives: therapeutic approach and infection control. Clin. Microbiol. Infect. 16:102–111 [DOI] [PubMed] [Google Scholar]
- 45.Carrër A, et al. 2008. Spread of OXA-48-positive-carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob. Agents Chemother. 52:2950–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carrër A, et al. 2010. Spread of OXA-48-encoding plasmid in Turkey and beyond. Antimicrob. Agents Chemother. 54:1369–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castanheira M, et al. 2011. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY Antimicrobial Surveillance Program, 2006–2007. Antimicrob. Agents Chemother. 55:1274–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention (CDC) 2009. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb. Mortal. Wkly. Rep. 58:256–260 [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention (CDC) 2010. Detection of Enterobacteriaceae isolates carrying metallo-β-lactamase—United States, 2010. MMWR Morb. Mortal. Wkly. Rep. 59:750. [PubMed] [Google Scholar]
- 50.Cheng HY, Chen YF, Peng HL. 2010. Molecular characterization of the PhoPQ-PmrD-PmrAB mediated pathway regulating polymyxin B resistance in Klebsiella pneumoniae CG43. J. Biomed. Sci. 17:60–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chu YW, et al. 2001. IMP-4, a novel metallo-β-lactamase from nosocomial Acinetobacter spp. collected in Hong Kong between 1994 and 1998. Antimicrob. Agents Chemother. 45:710–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung KP, et al. 2011. Arrival of Klebsiella pneumoniae carbapenemase (KPC)-2 in Taiwan. J. Antimicrob. Chemother. 66:1182–1184 [DOI] [PubMed] [Google Scholar]
- 53.Ciobotaro P, Oved M, Nadir E, Bardenstein R, Zimhony O. 2011. An effective intervention to limit the spread of an epidemic carbapenem-resistant Klebsiella pneumoniae strain in an acute care setting: from theory to practice. Am. J. Infect. Control 39:671–677 [DOI] [PubMed] [Google Scholar]
- 54.Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing: 19th informational supplement. CLSI document M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 55.Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement (June 2010 update). CLSI document M100-S20-U. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 56.Cobo J, et al. 2008. Use of tigecycline for the treatment of prolonged bacteremia due to a multiresistant VIM-1 and SHV-12 β-lactamase-producing Klebsiella pneumoniae epidemic clone. Diagn. Microbiol. Infect. Dis. 60:319–322 [DOI] [PubMed] [Google Scholar]
- 57.Cohen Stuart J, et al. 2010. Guideline for phenotypic screening and confirmation of carbapenemases in Enterobacteriaceae. Int. J. Antimicrob. Agents 36:205–210 [DOI] [PubMed] [Google Scholar]
- 58.Cohen Stuart J, Voets G, Scharringa J, Fluit AC, Leverstein-Van Hall MA. 1 March 2012. Detection of carbapenemase producing Enterobacteriaceae with a commercial DNA Microarray. J. Med. Microbiol. [Epub ahead of print.] doi:10.1099/jmm.0.041673-0 [DOI] [PubMed] [Google Scholar]
- 59.Colinon C, Miriagou V, Carattoli A, Luzzaro F, Rossolini GM. 2007. Characterization of the IncA/C plasmid pCC416 encoding VIM-4 and CMY-4 β-lactamases. J. Antimicrob. Chemother. 60:258–262 [DOI] [PubMed] [Google Scholar]
- 60.Cornaglia G, et al. 2007. Metallo-β-lactamases as emerging resistance determinants in Gram-negative pathogens: open issues. Int. J. Antimicrob. Agents 29:380–388 [DOI] [PubMed] [Google Scholar]
- 61.Cornaglia G, Giamarellou H, Rossolini GM. 2011. Metallo-β-lactamases: a last frontier for β-lactams? Lancet Infect. Dis. 11:381–393 [DOI] [PubMed] [Google Scholar]
- 62.Cuzon G, Naas T, Lesenne A, Benhamou M, Nordmann P. 2010. Plasmid-mediated carbapenem-hydrolysing OXA-48 β-lactamase in Klebsiella pneumoniae from Tunisia. Int. J. Antimicrob. Agents 36:91–93 [DOI] [PubMed] [Google Scholar]
- 63.Cuzon G, et al. 2010. Worldwide diversity of Klebsiella pneumoniae that produce β-lactamase _bla_KPC-2 gene. Emerg. Infect. Dis. 16:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daikos GL, et al. 2007. VIM-1-producing Klebsiella pneumoniae bloodstream infections: analysis of 28 cases. Int. J. Antimicrob. Agents 29:471–473 [DOI] [PubMed] [Google Scholar]
- 65.Daikos GL, Markogiannakis A. 2011. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin. Microbiol. Infect. 17:1135–1141 [DOI] [PubMed] [Google Scholar]
- 66.Daikos GL, et al. 2007. Activity of imipenem against VIM-1 metallo-β-lactamase-producing Klebsiella pneumoniae in the murine thigh infection model. Clin. Microbiol. Infect. 13:202–205 [DOI] [PubMed] [Google Scholar]
- 67.Daikos GL, et al. 2009. Prospective observational study of the impact of VIM-1 metallo-β-lactamase on the outcome of patients with Klebsiella pneumoniae bloodstream infections. Antimicrob. Agents Chemother. 53:1868–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daikos GL, et al. 2010. Serum bactericidal activity of three different dosing regimens of colistin with implications for optimum clinical use. J. Chemother. 22:175–178 [DOI] [PubMed] [Google Scholar]
- 69.Daikos GL, et al. 2010. Risk factors for bloodstream infection with Klebsiella pneumoniae producing VIM-1 metallo-β-lactamase. J. Antimicrob. Chemother. 65:784–788 [DOI] [PubMed] [Google Scholar]
- 70.Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 65:490–495 [DOI] [PubMed] [Google Scholar]
- 71.Daly MW, Riddle DJ, Ledeboer NA, Dunne WM, Ritchie DJ. 2007. Tigecycline for treatment of pneumonia and empyema caused by carbapenemase-producing Klebsiella pneumoniae. Pharmacotherapy 27:1052–1057 [DOI] [PubMed] [Google Scholar]
- 72.Daoud Z, Hobeika E, Choucair A, Rohban R. 2008. Isolation of the first metallo-β-lactamase producing Klebsiella pneumoniae in Lebanon. Rev. Esp. Quimioter. 21:123–126 [PubMed] [Google Scholar]
- 73.Docquier JD, et al. 2009. Crystal structure of the OXA-48 β-lactamase reveals mechanistic diversity among class D carbapenemases. Chem. Biol. 16:540–547 [DOI] [PubMed] [Google Scholar]
- 74.Docquier JD, et al. 2003. On functional and structural heterogeneity of VIM-type metallo-β-lactamases. J. Antimicrob. Chemother. 51:257–266 [DOI] [PubMed] [Google Scholar]
- 75.Doi Y, et al. 2008. Simple disk-based method for detection of Klebsiella pneumoniae carbapenemase-type β-lactamase by use of a boronic acid compound. J. Clin. Microbiol. 46:4083–4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drawz SM, Bonomo RA. 2010. Three decades of beta-lactamase inhibitors. Clin. Microbiol. Rev. 23:160–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dudhani RV, et al. 2010. Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob. Agents Chemother. 54:1117–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dudhani RV, Turnidge JD, Nation RL, Li JJ. 2010. fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J. Antimicrob. Chemother. 65:1984–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elemam A, Rahimian J, Doymaz M. 2010. In vitro evaluation of antibiotic synergy for polymyxin B-resistant carbapenemase-producing Klebsiella pneumoniae. J. Clin. Microbiol. 48:3558–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elemam A, Rahimian J, Mandell W. 2009. Infection with panresistant Klebsiella pneumoniae: a report of 2 cases and a brief review of the literature. Clin. Infect. Dis. 49:271–274 [DOI] [PubMed] [Google Scholar]
- 81.Endimiani A, et al. 2008. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing _bla_KPC in the United States. Antimicrob. Agents Chemother. 52:2680–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Endimiani A, Choudhary Y, Bonomo RA. 2009. In vitro activity of NXL104 in combination with β-lactams against Klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob. Agents Chemother. 53:3599–3601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Endimiani A, et al. 2009. Emergence of _bla_KPC-containing Klebsiella pneumoniae in a long-term acute care hospital: a new challenge to our healthcare system. J. Antimicrob. Chemother. 64:1102–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Endimiani A, et al. 2009. Characterization of _bla_KPC-containing Klebsiella pneumoniae isolates detected in different institutions in the eastern U. S. A. J. Antimicrob. Chemother. 63:427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Endimiani A, et al. 2010. In vitro activity of fosfomycin against _bla_KPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob. Agents Chemother. 54:526–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Evagelopoulou P, Myrianthefs P, Markogiannakis A, Baltopoulos G, Tsakris A. 2008. Multidrug-resistant Klebsiella pneumoniae mediastinitis safely and effectively treated with prolonged administration of tigecycline. Clin. Infect. Dis. 46:1932–1933 [DOI] [PubMed] [Google Scholar]
- 87.Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. 2008. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin. Infect. Dis. 46:1069–1077 [DOI] [PubMed] [Google Scholar]
- 88.Falagas ME, Karageorgopoulos DE, Nordmann P. 2011. Therapeutic options for infections with Enterobacteriaceae producing carbapenem-hydrolyzing enzymes. Future Microbiol. 6:653–666 [DOI] [PubMed] [Google Scholar]
- 89.Falagas ME, et al. 2010. Antimicrobial susceptibility of multidrug-resistant (MDR) and extensively drug-resistant (XDR) Enterobacteriaceae isolates to fosfomycin. Int. J. Antimicrob. Agents 35:240–243 [DOI] [PubMed] [Google Scholar]
- 90.Falagas ME, et al. 2010. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int. J. Antimicrob. Agents 35:194–199 [DOI] [PubMed] [Google Scholar]
- 91.Falagas ME, et al. 2007. Risk factors of carbapenem-resistant Klebsiella pneumoniae infections: a matched case control study. J. Antimicrob. Chemother. 60:1124–1130. [DOI] [PubMed] [Google Scholar]
- 91a.FDA 1 September 2010, posting date FDA drug safety communication: increased risk of death with Tygacil (tigecycline) compared to other antibiotics used to treat similar infections. http://www.fda.gov/Drugs/DrugSafety/ucm224370.htm
- 92.Fernández L, Breidenstein EB, Hancock RE. 2011. Creeping baselines and adaptive resistance to antibiotics. Drug Resist. Updat. 14:1–21 [DOI] [PubMed] [Google Scholar]
- 93.Franceschini N, et al. 2000. Purification and biochemical characterization of the VIM-1 metallo-β-lactamase. Antimicrob. Agents Chemother. 44:3003–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fukigai S, et al. 2007. Nosocomial outbreak of genetically related IMP-1 β-lactamase-producing Klebsiella pneumoniae in a general hospital in Japan. Int. J. Antimicrob. Agents 29:306–310 [DOI] [PubMed] [Google Scholar]
- 95.Gacar GG, et al. 2005. Genetic and enzymatic properties of metallo-β-lactamase VIM-5 from a clinical isolate of Enterobacter cloacae. Antimicrob. Agents Chemother. 49:4400–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gales AC, Jones RN, Sader HS. 2011. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program (2006–09). J. Antimicrob. Chemother. 66:2070–2074 [DOI] [PubMed] [Google Scholar]
- 97.Garonzik SM, et al. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 55:3284–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gasink LB, Edelstein PH, Lautenbach E, Synnestvedt M, Fishman NO. 2009. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect. Control Hosp. Epidemiol. 30:1180–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Giakkoupi P, et al. 2011. An update of the evolving epidemic of _bla_KPC-2-carrying Klebsiella pneumoniae in Greece (2009–10). J. Antimicrob. Chemother. 66:1510–1513 [DOI] [PubMed] [Google Scholar]
- 100.Giakkoupi P, et al. 2009. Emerging Klebsiella pneumoniae isolates coproducing KPC-2 and VIM-1 carbapenemases. Antimicrob. Agents Chemother. 53:4048–4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Giakkoupi P, et al. 2005. Discrepancies and interpretation problems in susceptibility testing of VIM-1-producing Klebsiella pneumoniae isolates. J. Clin. Microbiol. 43:494–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Giakoupi P, et al. 2009. Greek system for the surveillance of antimicrobial resistance. KPC-2-producing Klebsiella pneumoniae infections in Greek hospitals are mainly due to a hyperepidemic clone. Euro Surveill. 14:19218http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19218 [DOI] [PubMed] [Google Scholar]
- 103.Giani T, et al. 2009. Emergence in Italy of Klebsiella pneumoniae sequence type 258 producing KPC-3 carbapenemase. J. Clin. Microbiol. 47:3793–3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giske CG, et al. 2011. A sensitive and specific phenotypic assay for detection of metallo-β-lactamases and KPC in Klebsiella pneumoniae with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin. Clin. Microbiol. Infect. 17:552–556 [DOI] [PubMed] [Google Scholar]
- 105.Goren MG, Navon-Venezia S, Chmelnitsky I, Carmeli Y. 2010. Carbapenem-resistant KPC-2-producing Escherichia coli in a Tel Aviv medical center, 2005 to 2008. Antimicrob. Agents Chemother. 54:2687–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gregory CJ, et al. 2010. Outbreak of carbapenem-resistant Klebsiella pneumoniae in Puerto Rico associated with a novel carbapenemase variant. Infect. Control Hosp. Epidemiol. 31:476–484 [DOI] [PubMed] [Google Scholar]
- 107.Grundmann H, et al. 2010. Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Euro Surveill. 15:19711http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19711 [DOI] [PubMed] [Google Scholar]
- 108.Halaby T. 13 February 2012. A case of New Delhi metallo-β-lactamase 1 (NDM-1)-producing Klebsiella pneumoniae from the Balkan region in the Netherlands with putative secondary transmission. Antimicrob. Agents Chemother. [Epub ahead of print.] doi:10.1128/AAC.00111-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hall BG, Barlow M. 2005. Revised Ambler classification of β-lactamases. J. Antimicrob. Chemother. 55:1050–1051 [DOI] [PubMed] [Google Scholar]
- 110.Herbert S, et al. 2007. Large outbreak of infetion and colonization with gram-negative pathogens carrying the metallo-β-lactamase gene _bla_IMP-4 at a 320-bed tertiary hospital in Australia. Infect. Control Hosp. Epidemiol. 28:98–101 [DOI] [PubMed] [Google Scholar]
- 111.Hindiyeh M, et al. 2008. Rapid detection of _bla_KPC carbapenemase genes by real-time PCR. J. Clin. Microbiol. 46:2879–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hirsch EB, Tam VH. 2010. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J. Antimicrob. Chemother. 65:1119–1125 [DOI] [PubMed] [Google Scholar]
- 113.Ho VP, et al. 2011. Use of meropenem by continuous infusion to treat a patient with a Bla(kpc-2)-positive Klebsiella pneumoniae blood stream infection. Surg. Infect. (Larchmt.) 12:325–327 [DOI] [PubMed] [Google Scholar]
- 114.Ho PL, et al. 2011. Complete sequencing of pNDM-HK encoding NDM-1 carbapenemase from a multidrug-resistant Escherichia coli strain isolated in Hong Kong. PLoS One 21:e17989 doi:10.1371/journal.pone.0017989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hrabák J, Walková R, Studentová V, Chudácková E, Bergerová T. 2011. Carbapenemase activity detection by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J. Clin. Microbiol. 49:3222–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Humphries RM, et al. 2010. Successful treatment of pan-resistant Klebsiella pneumoniae pneumonia and bacteraemia with a combination of high-dose tigecycline and colistin. J. Med. Microbiol. 59:1383–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hussein K, et al. 2009. Carbapenem resistance among Klebsiella pneumoniae isolates: risk factors, molecular characteristics, and susceptibility patterns. Infect. Control Hosp. Epidemiol. 30:666–671 [DOI] [PubMed] [Google Scholar]
- 118.Jaruratanasirikul S, Sriwiriyajan S, Punyo J. 2005. Comparison of the pharmacodynamics of meropenem in patients with ventilator-associated pneumonia following administration by 3-hour infusion or bolus injection. Antimicrob. Agents Chemother. 49:1337–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jarvis WR, Munn VP, Highsmith AK, Culver DH, Hughes JM. 1985. The epidemiology of nosocomial infections caused by Klebsiella pneumoniae. Infect. Control 6:68–74 [DOI] [PubMed] [Google Scholar]
- 120.Jeon MH, et al. 2008. Risk factors for the acquisition of carbapenem-resistant Escherichia coli among hospitalized patients. Diagn. Microbiol. Infect. Dis. 62:402–406 [DOI] [PubMed] [Google Scholar]
- 121.Karabinis A, et al. 2004. Colistin for Klebsiella pneumoniae-associated sepsis. Clin. Infect. Dis. 38:e7–e9 [DOI] [PubMed] [Google Scholar]
- 122.Kassis-Chikhani N, et al. 2010. Extended measures for controlling an outbreak of VIM-1 producing imipenem-resistant Klebsiella pneumoniae in a liver transplant centre in France, 2003–2004. Euro Surveill. 15:19713http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19713 [DOI] [PubMed] [Google Scholar]
- 123.Ke W, Bethel CR, Thomson JM, Bonomo RA, van den Akker F. 2007. Crystal structure of KPC-2: insights into carbapenemase activity in class A β-lactamases. Biochemistry 46:5732–5740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim S-Y, Hong SG, Moland ES, Thomson KS. 2007. Convenient test using a combination of chelating agents for detection of metallo-β-lactamases in the clinical laboratory. J. Clin. Microbiol. 45:2798–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim MN, et al. 2012. Nosocomial clustering of NDM-1-producing Klebsiella pneumoniae sequence type 340 strains in four patients at a South Korean tertiary care hospital. J. Clin. Microbiol. 50:1433–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kitchel B, et al. 2009. Molecular epidemiology of KPC-producing Klebsiella pneumoniae in the United States: clonal expansion of MLST sequence type 258. Antimicrob. Agents Chemother. 53:3365–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kochar S, et al. 2009. Success of an infection control program to reduce the spread of carbapenem-resistant Klebsiella penumoniae. Infect. Control Hosp. Epidemiol. 30:447–452 [DOI] [PubMed] [Google Scholar]
- 128.Kontopoulou K, et al. 2010. Hospital outbreak caused by Klebsiella pneumoniae producing KPC-2 β-lactamase resistant to colistin. J. Hosp. Infect. 76:70–73 [DOI] [PubMed] [Google Scholar]
- 129.Ktari S, et al. 2006. Emergence of multidrug-resistant Klebsiella pneumoniae isolates producing VIM-4 metallo-β-lactamase, CTX-M-15 extended-spectrum β-lactamase, and CMY-4 AmpC β-lactamase in a Tunisian university hospital. Antimicrob. Agents Chemother. 50:4198–4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kumarasamy KK, et al. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kuti JL, Dandekar PK, Nightingale CH, Nicolau DP. 2003. Use of Monte Carlo simulation to design an optimized pharmacodynamic dosing strategy for meropenem. J. Clin. Pharmacol. 43:1116–1123 [DOI] [PubMed] [Google Scholar]
- 132.Kwak YG, et al. 2005. Risk factors for the acquisition of carbapenem-resistant Klebsiella pneumoniae among hospitalized patients. Microb. Drug Resist. 11:165–169 [DOI] [PubMed] [Google Scholar]
- 133.Landman D, et al. 2007. Evolution of antimicrobial resistance among Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae in Brooklyn, NY. J. Antimicrob. Chemother. 60:78–82 [DOI] [PubMed] [Google Scholar]
- 134.Landman D, Bratu S, Quale J. 2009. Contribution of OmpK36 to carbapenem susceptibility in KPC-producing Klebsiella pneumoniae. J. Med. Microbiol. 58:1303–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Landman D, Salvani JK, Bratu S, Quale J. 2005. Evaluation of techniques for detection of carbapenem-resistant Klebsiella pneumoniae in stool surveillance cultures. J. Clin. Microbiol. 43:5639–5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Laraki N, et al. 1999. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 43:902–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lassaux P, et al. 2011. Biochemical and structural characterization of the subclass B1 metallo-β-lactamase VIM-4. Antimicrob. Agents Chemother. 55:1248–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leão RS, et al. 2011. KPC-2 carbapenemase-producing Klebsiella pneumoniae isolates from patients with cystic fibrosis. J. Cyst. Fibros. 10:140–142 [DOI] [PubMed] [Google Scholar]
- 139.Leavitt A, Navon-Venezia S, Chmelnitsky I, Schwaber MJ, Carmeli Y. 2007. Emergence of KPC-2 and KPC-3 in carbapenem-resistant Klebsiella pneumoniae strains in an Israeli hospital. Antimicrob. Agents Chemother. 51:3026–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lederman ER, Crum NF. 2005. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am. J. Gastroenterol. 100:322–331 [DOI] [PubMed] [Google Scholar]
- 141.Lee K, et al. 2010. Improved performance of the modified Hodge test with MacConkey agar for screening carbapenemase-producing Gram-negative bacilli. J. Microbiol. Methods 83:149–152 [DOI] [PubMed] [Google Scholar]
- 142.Lee J, Patel G, Huprikar S, Calfee DP, Jenkins SG. 2009. Decreased susceptibility to polymyxin B during treatment for carbapenem-resistant Klebsiella pneumoniae infection. J. Clin. Microbiol. 47:1611–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee NY, et al. 2004. Clinical experience of bacteremia caused by metallo-β-lactamase-producing gram-negative organisms. J. Microbiol. Immunol. Infect. 37:343–349 [PubMed] [Google Scholar]
- 144.Lemmen SW, Häfner H, Zolldann D, Stanzel S, Lütticken R. 2004. Distribution of multi-resistant Gram-negative versus Gram-positive bacteria in the hospital inanimate environment. J. Hosp. Infect. 56:191–197 [DOI] [PubMed] [Google Scholar]
- 145.Li C, Kuti JL, Nightingale CH, Nicolau DP. 2006. Population pharmacokinetic analysis and dosing regimen optimization of meropenem in adult patients. J. Clin. Pharmacol. 46:1171–1178 [DOI] [PubMed] [Google Scholar]
- 146.Li B, et al. 2011. First report of Klebsiella oxytoca strain coproducing KPC-2 and IMP-8 carbapenemases. Antimicrob. Agents Chemother. 55:2937–2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lienard BM, et al. 2008. Structural basis for the broad-spectrum inhibition of metallo-β-lactamases by thiols. Org. Biomol. Chem. 6:2282–2294 [DOI] [PubMed] [Google Scholar]
- 148.Lincopan N, et al. 2005. First isolation of metallo-β-lactamase-producing multiresistant Klebsiella pneumoniae from a patient in Brazil. J. Clin. Microbiol. 43:516–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Livermore DM, et al. 2011. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. J. Antimicrob. Chemother. 66:48–53 [DOI] [PubMed] [Google Scholar]
- 150.Livermore DM, Walsh TR, Toleman M, Woodford N. 2011. Balkan NDM-1: escape or transplant? Lancet Infect. Dis. 11:164. [DOI] [PubMed] [Google Scholar]
- 151.Livermore DM, et al. 2011. What remains against carbapenem-resistant Enterobacteriaceae? Evaluation of chloramphenicol, ciprofloxacin, colistin, fosfomycin, minocycline, nitrofurantoin, temocillin and tigecycline. Int. J. Antimicrob. Agents 37:415–419 [DOI] [PubMed] [Google Scholar]
- 152.Loli A, et al. 2006. Sources of diversity of carbapenem resistance levels in Klebsiella pneunoniae carrying _bla_VIM-1. J. Antimicrob. Chemother. 58:669–672 [DOI] [PubMed] [Google Scholar]
- 153.Lomaestro BM, Tobin EH, Shang W, Gootz T. 2006. The spread of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae to upstate New York. Clin. Infect. Dis. 43:e26–e28 [DOI] [PubMed] [Google Scholar]
- 154.Maltezou HC, et al. 2009. Outbreak of infections due to KPC-2-producing Klebsiella pneumoniae in a hospital in Crete (Greece). J. Infect. 58:213–219 [DOI] [PubMed] [Google Scholar]
- 155.Marcano D, et al. 2008. First isolation of a VIM-producing Klebsiella pneumoniae from a seven-year-old child in Venezuela. J. Infect. Dev. Ctries. 2:241–244 [DOI] [PubMed] [Google Scholar]
- 156.Marchaim D, et al. 2011. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob. Agents Chemother. 55:593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Marchaim D, Navon-Venezia S, Schwaber MJ, Carmeli Y. 2008. Isolation of imipenem-resistant Enterobacter species: emergence of KPC-2 carbapenemase, molecular characterization, epidemiology, and outcomes. Antimicrob. Agents Chemother. 52:1413–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Marschall J, et al. 2009. Presence of the KPC carbapenemase gene in Enterobacteriaceae causing bacteremia and its correlation with in vitro carbapenem susceptibility. J. Clin. Microbiol. 47:239–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mathers AJ, et al. 2009. Fatal cross infection by carbapenem-resistant Klebsiella in two liver transplant recipients. Transpl. Infect. Dis. 11:257–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mavroidi A, et al. 2012. Emergence of Escherichia coli sequence type 410 (ST410) with KPC-2 β-lactamase. Int. J. Antimicrob. Agents 39:247–250 [DOI] [PubMed] [Google Scholar]
- 161.McGuinn M, Hershow RC, Janda WM. 2009. Escherichia coli and Klebsiella pneumoniae carbapenemase in long-term care facility, Illinois, USA. Emerg. Infect. Dis. 15:988–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mendes RE, et al. 2008. Carbapenem-resistant isolates of Klebsiella pneumoniae in China and detection of a conjugative plasmid (_bla_KPC-2 plus qnrB4) and a _bla_IMP-4 gene. Antimicrob. Agents Chemother. 52:798–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mendes RE, et al. 2007. Rapid detection and identification of metallo-β-lactamase-encoding genes by multiplex real-time PCR assay and melt curve analysis. J. Clin. Microbiol. 45:544–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Michalopoulos A, et al. 2010. Intravenous fosfomycin for the treatment of nosocomial infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: a prospective evaluation. Clin. Microbiol. Infect. 16:184–186 [DOI] [PubMed] [Google Scholar]
- 165.Miriagou V, Carattoli A, Tzelepi E, Villa L, Tzouvelekis LS. 2005. IS_26_-associated In4-type integrons forming multiresistance loci in enterobacterial plasmids. Antimicrob. Agents Chemother. 49:3541–3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Miriagou V, et al. 2010. Acquired carbapenemases in Gram-negative bacterial pathogens: detection and surveillance issues. Clin. Microbiol. Infect. 16:112–122 [DOI] [PubMed] [Google Scholar]
- 167.Miriagou V, et al. 2010. Sequence of pNL194, a 79.3-kilobase IncN plasmid carrying the _bla_VIM-1 metallo-β-lactamase gene in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 54:4497–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Miriagou V, et al. 2010. Efficacy of BAL30072, alone and combined with meropenem, against VIM-producing enterobacteria in a murine thigh infection model, abstr P1240. Abstr. 20th Eur. Congr. Clin. Microbiol. Infect. Dis., Vienna, Austria [Google Scholar]
- 169.Mochon AB, et al. 2011. New Delhi metallo-β-lactamase (NDM-1)-producing Klebsiella pneumoniae: case report and laboratory detection strategies. J. Clin. Microbiol. 49:1667–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Moloughney JG, Thomas JD, Toney JH. 2005. Novel IMP-1 metallo-β-lactamase inhibitors can reverse meropenem resistance in Escherichia coli expressing IMP-1. FEMS Microbiol. Lett. 243:65–71 [DOI] [PubMed] [Google Scholar]
- 171.Monteiro J, Fernandes Santos A, Asensi MD, Peirano G, Gales AC. 2009. First report of KPC-2-producing Klebsiella pneumoniae strains in Brazil. Antimicrob. Agents Chemother. 53:333–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Monteiro J, Widen RH, Pignatari AC, Kubasek C, Silbert S. 2012. Rapid detection of carbapenemase genes by multiplex real-time PCR. J. Antimicrob. Chemother. 67:906–909 [DOI] [PubMed] [Google Scholar]
- 173.Moquet O, et al. 2011. Class D OXA-48 carbapenemase in multidrug-resistant enterobacteria, Senegal. Emerg. Infect. Dis. 17:143–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moulds NM, Thomson KS, Hanson ND. 2011. IMP-27, a novel metallo-β-lactamase (MBL) associated with a class II integron identified in an isolate of Proteus mirabilis, abstr C1-1212. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL [Google Scholar]
- 175.Mouloudi E, et al. 2010. Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect. Control Hosp. Epidemiol. 31:1250–1256 [DOI] [PubMed] [Google Scholar]
- 176.Munoz-Price LS, et al. 2010. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect. Control Hosp. Epidemiol. 31:1074–1077 [DOI] [PubMed] [Google Scholar]
- 177.Munoz-Price LS, et al. 2010. Successful control of an outbreak of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae at a long-term acute care hospital. Infect. Control Hosp. Epidemiol. 31:341–347 [DOI] [PubMed] [Google Scholar]
- 178.Munoz-Price LS, Hota B, Stemer A, Weinstein RA. 2009. Prevention of bloodstream infections by use of daily chlorhexidine baths for patients at a long-term acute care hospital. Infect. Control Hosp. Epidemiol. 30:1031–1035 [DOI] [PubMed] [Google Scholar]
- 179.Naas T, Cuzon G, Bogaerts P, Glupczynski Y, Nordmann P. 2011. Evaluation of a DNA microarray (Check-MDR CT102) for rapid detection of TEM, SHV, and CTX-M extended-spectrum β-lactamases and of KPC, OXA-48, VIM, IMP, and NDM-1 carbapenemases. J. Clin. Microbiol. 49:1608–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Naas T, et al. 2008. Genetic structures at the origin of acquisition of the β-lactamase _bla_KPC gene. Antimicrob. Agents Chemother. 52:1257–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Naas T, Nordmann P, Vedel G, Poyart C. 2005. Plasmid-mediated carbapenem-hydrolyzing β-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob. Agents Chemother. 49:4423–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nadkarni AS, Schliep T, Khan L, Zeana CB. 2009. Cluster of bloodstream infections caused by KPC-2 carbapenemase producing Klebsiella pneumoniae in Manhattan. Am. J. Infect. Control 37:121–126 [DOI] [PubMed] [Google Scholar]
- 183.Nagano R, Adachi Y, Hashizume T, Morishima H. 2000. In vitro antibacterial activity and mechanism of action of J-111,225, a novel 1β-methylcarbapenem, against transferable IMP-1 metallo-β-lactamase producers. J. Antimicrob. Chemother. 45:271–276 [DOI] [PubMed] [Google Scholar]
- 184.Nagano R, et al. 1999. Carbapenem derivatives as potential inhibitors of various β-lactamases, including class B metallo-β-lactamases. Antimicrob. Agents Chemother. 43:2497–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Neonakis IK, et al. 2010. Resistance status and evolution trends of Klebsiella pneumoniae isolates in a university hospital in Greece: ineffectiveness of carbapenems and increasing resistance to colistin. Chemotherapy 56:448–452 [DOI] [PubMed] [Google Scholar]
- 186.Neonakis IK, Stylianou K, Daphnis E, Maraki S. 2011. First case of resistance to tigecycline by Klebsiella pneumoniae in a European university hospital. Indian J. Med. Microbiol. 29:78–79 [DOI] [PubMed] [Google Scholar]
- 187.Neuner EA, et al. 2011. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn. Microbiol. Infect. Dis. 69:357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nilsson A, Berg OG, Aspevall O, Kahlmeter G, Andersson DI. 2003. Biological costs and mechanisms of fosfomycin resistance in Escherichia coli. Antimicrob. Agents Chemother. 47:2850–2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nordmann P, Boulanger AE, Poirel L. 2012. NDM-4 metallo-β-lactamase with increased carbapenemase activity from Escherichia coli. Antimicrob. Agents Chemother. 56:2184–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nordmann P, Couard JP, Sansot D, Poirel L. 2012. Emergence of an autochthonous and community-acquired NDM-1-producing Klebsiella pneumoniae in Europe. Clin. Infect. Dis. 54:150–151 [DOI] [PubMed] [Google Scholar]
- 191.Nordmann P, Cuzon G, Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228–236 [DOI] [PubMed] [Google Scholar]
- 192.Nordmann P, Poirel L, Toleman MA, Walsh TR. 2011. Does broad-spectrum β-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by Gram-negative bacteria? J. Antimicrob. Chemother. 66:689–692 [DOI] [PubMed] [Google Scholar]
- 193.Osano E, et al. 1994. Molecular characterization of an enterobacterial metallo-β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob. Agents Chemother. 38:71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Page MG, Dantier C, Desarbre E. 2010. In vitro properties of BAL30072, a novel siderophore sulfactam with activity against multiresistant gram-negative bacilli. Antimicrob. Agents Chemother. 54:2291–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Page MG, Heim J. 2009. New molecules from old classes: revisiting the development of beta-lactams. IDrugs 12:561–565 [PubMed] [Google Scholar]
- 196.Panagea T, et al. 2011. Evaluation of CHROMagar KPC for the detection of carbapenemase-producing Enterobacteriaceae in rectal surveillance cultures. Int. J. Antimicrob. Agents 37:124–128 [DOI] [PubMed] [Google Scholar]
- 197.Panagiotakopoulou A, et al. 2007. Comparative in vitro killing of carbapenems and aztreonam against Klebsiella pneumoniae producing VIM-1 metallo-β-lactamase. Int. J. Antimicrob. Agents 29:360–362 [DOI] [PubMed] [Google Scholar]
- 198.Papagiannitsis CC, et al. 2011. Characterization of metallo-β-lactamase VIM-27, an A57S mutant of VIM-1 associated with Klebsiella pneumoniae ST147. Antimicrob. Agents Chemother. 55:3570–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pasteran F, Lucero C, Soloaga R, Rapoport M, Corso A. 2011. Can we use imipenem and meropenem Vitek 2 MICs for detection of suspected KPC and other carbapenemase producers among species of Enterobacteriaceae? J. Clin. Microbiol. 49:697–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pasteran F, Mendez T, Guerriero L, Rapoport M, Corso A. 2009. Sensitive screening tests for suspected class A carbapenemase production in species of Enterobacteriaceae. J. Clin. Microbiol. 47:1631–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pasteran FG, et al. 2008. Klebsiella pneumoniae carbapenemase-2, Buenos Aires, Argentina. Emerg. Infect. Dis. 14:1178–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Patel G, Bonomo RA. 2011. Status report on carbapenemases: challenges and prospects. Expert Rev. Anti Infect. Ther. 9:555–570 [DOI] [PubMed] [Google Scholar]
- 203.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. 2008. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect. Control Hosp. Epidemiol. 29:1099–1106 [DOI] [PubMed] [Google Scholar]
- 204.Patel G, Perez F, Bonomo RA. 7 October 2010. Carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii: assessing their impact on organ transplantation. Curr. Opin. Organ Transplant. [Epub ahead of print.] doi:10.1097/MOT.0b013e3283404373 [DOI] [PubMed] [Google Scholar]
- 205.Patel N, et al. 2011. Clinical epidemiology of carbapenem-intermediate or -resistant Enterobacteriaceae. J. Antimicrob. Chemother. 66:1600–1608 [DOI] [PubMed] [Google Scholar]
- 206.Peleg AY, Franklin C, Bell JM, Spelman DW. 2005. Dissemination of the metallo-β-lactamase gene _bla_IMP-4 among gram-negative pathogens in a clinical setting in Australia. Clin. Infect. Dis. 41:1549–1556 [DOI] [PubMed] [Google Scholar]
- 207.Perez F, et al. 2010. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J. Antimicrob. Chemother. 65:1807–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Perry JD, et al. 2011. Prevalence of faecal carriage of Enterobacteriaceae with NDM-1 carbapenemase at military hospitals in Pakistan, and evaluation of two chromogenic media. J. Antimicrob. Chemother. 66:2288–2294 [DOI] [PubMed] [Google Scholar]
- 209.Petersen PJ, et al. 2009. Establishment of in vitro susceptibility testing methodologies and comparative activities of piperacillin in combination with the β-lactamase inhibitor BLI-489. Antimicrob. Agents Chemother. 53:370–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Peterson LR. 2008. A review of tigecycline—the first glycylcycline. Int. J. Antimicrob. Agents 32(Suppl 4): S215–S222 [DOI] [PubMed] [Google Scholar]
- 211.Plachouras D, et al. 2009. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob. Agents Chemother. 53:3430–3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11:589–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Poeylaut-Palena AA, et al. 2007. A minimalistic approach to identify substrate binding features in B1 metallo-β-lactamases. Bioorg. Med. Chem. Lett. 17:5171–5174 [DOI] [PubMed] [Google Scholar]
- 214.Poirel L, Benouda A, Hays C, Nordmann P. 2011. Emergence of NDM-1-producing Klebsiella pneumoniae in Morocco. J. Antimicrob. Chemother. 66:2781–2783 [DOI] [PubMed] [Google Scholar]
- 215.Poirel L, Bonnin RA, Nordmann P. 2011. Analysis of the resistome of a multidrug-resistant NDM-1-producing Escherichia coli strain by high-throughput genome sequencing. Antimicrob. Agents Chemother. 55:4224–4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Poirel L, et al. 2011. OXA-163, an OXA-48-related class D β-lactamase with extended activity toward expanded-spectrum cephalosporins. Antimicrob. Agents Chemother. 55:2546–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Poirel L, Héritier C, Tolün V, Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Poirel L, Lagrutta E, Taylor P, Pham J, Nordmann P. 2010. Emergence of metallo-β-lactamase NDM-1-producing multidrug-resistant Escherichia coli in Australia. Antimicrob. Agents Chemother. 54:4914–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Poirel L, Walsh TR, Cuvillier V, Nordmann P. 2011. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 70:119–123 [DOI] [PubMed] [Google Scholar]
- 220.Potron A, Kalpoe J, Poirel L, Nordmann P. 2011. European dissemination of a single OXA-48-producing Klebsiella pneumoniae clone. Clin. Microbiol. Infect. 17:E24–E26 [DOI] [PubMed] [Google Scholar]
- 221.Potron A, et al. 2011. Characterization of OXA-181, a carbapenem-hydrolyzing class D β-lactamase from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 55:4896–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pournaras S, et al. 2011. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae strains by time-kill assay. Int. J. Antimicrob. Agents 37:244–247 [DOI] [PubMed] [Google Scholar]
- 223.Prior AR, et al. 2010. First identified case of VIM-producing carbapenem-resistant Klebsiella pneumoniae in the Republic of Ireland associated with fatal outcome. Euro Surveill. 15:19752http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19752 [PubMed] [Google Scholar]
- 224.Psichogiou M, et al. 2008. Ongoing epidemic of _bla_VIM-1-positive Klebsiella pneumoniae in Athens, Greece: a prospective survey. J. Antimicrob. Chemother. 61:59–63 [DOI] [PubMed] [Google Scholar]
- 225.Queenan AM, Bush K. 2007. Carbapenemases: the versatile β-lactamases. Clin. Microbiol. Rev. 20:440–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rodríguez-Avial C, Rodríguez-Avial I, Merino P, Picazo JJ. 2012. Klebsiella pneumoniae: development of a mixed population of carbapenem and tigecycline resistance during antimicrobial therapy in a kidney transplant patient. Clin. Microbiol. Infect. 18:61–66 [DOI] [PubMed] [Google Scholar]
- 227.Rodriguez-Martinez JM, Nordmann P, Fortineau N, Poirel L. 2010. VIM-19, a metallo-β-lactamase with increased carbapenemase activity from Escherichia coli and Klebsiella pneumoniae. Antimicrob. Agents Chemother. 54:471–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Samonis G, Maraki S, Karageorgopoulos DE, Vouloumanou EK, Falagas ME. 31 July 2011. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolates. Eur. J. Clin. Microbiol. Infect. Dis. [Epub ahead of print.] doi:10.1007/s10096-011-1360-5 [DOI] [PubMed] [Google Scholar]
- 229.Samra Z, et al. 2008. Evaluation of CHROMagar KPC for rapid detection of carbapenem-resistant Enterobacteriaceae. J. Clin. Microbiol. 46:3110–3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Samuelsen O, et al. 2009. Emergence of clonally related Klebsiella pneumoniae isolates of sequence type 258 producing plasmid-mediated KPC carbapenemase in Norway and Sweden. J. Antimicrob. Chemother. 63:654–658 [DOI] [PubMed] [Google Scholar]
- 231.Schechner V, et al. 2009. Evaluation of PCR-based testing for surveillance of KPC-producing carbapenem-resistant members of the Enterobacteriaceae family. J. Clin. Microbiol. 47:3261–3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Schwaber MJ, Carmeli Y. 2008. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA 300:2911–2913 [DOI] [PubMed] [Google Scholar]
- 233.Schwaber MJ, et al. 2008. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob. Agents Chemother. 52:1028–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Schwaber MJ, et al. 2011. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin. Infect. Dis. 52:848–855 [DOI] [PubMed] [Google Scholar]
- 235.Senda K, et al. 1996. PCR detection of metallo-β-lactamase gene (_bla_IMP) in gram-negative rods resistant to broad-spectrum β-lactams. J. Clin. Microbiol. 34:2909–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Skiada A, Markogiannakis A, Plachouras D, Daikos GL. 2011. Adaptive resistance to cationic compounds in Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 37:187–193 [DOI] [PubMed] [Google Scholar]
- 237.Souli M, et al. 2010. An outbreak of infection due to β-lactamase Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae in a Greek university hospital: molecular characterization, epidemiology and outcomes. Clin. Infect. Dis. 50:364–373 [DOI] [PubMed] [Google Scholar]
- 238.Souli M, et al. 2011. In vitro interactions of antimicrobial combinations with fosfomycin against KPC-2-producing Klebsiella pneumoniae and protection of resistance development. Antimicrob. Agents Chemother. 55:2395–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Souli M, et al. 2011. Efficacy of carbapenems against a metallo-β-lactamase-producing Escherichia coli clinical isolate in a rabbit intra-abdominal abscess model. J. Antimicrob. Chemother. 66:611–617 [DOI] [PubMed] [Google Scholar]
- 240.Souli M, et al. 2008. Clinical experience of serious infections caused by Enterobacteriaceae producing VIM-1 metallo-β-lactamase in a Greek university hospital. Clin. Infect. Dis. 46:847–854 [DOI] [PubMed] [Google Scholar]
- 241.Souli M, et al. 2009. Does the activity of the combination of imipenem and colistin in vitro exceed the problem of resistance in metallo-β-lactamase-producing Klebsiella pneumoniae isolates? Antimicrob. Agents Chemother. 53:2133–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Stachyra T, et al. 2009. In vitro activity of the β-lactamase inhibitor NXL104 against KPC-2 carbapenemase and Enterobacteriaceae expressing KPC carbapenemases. J. Antimicrob. Chemother. 64:326–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Suh JY, et al. 2010. Nonclonal emergence of colistin-resistant Klebsiella pneumoniae isolates from blood samples in South Korea. Antimicrob. Agents Chemother. 54:560–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tenover FC, et al. 2006. Carbapenem resistance in Klebsiella pneumoniae not detected by automated susceptibility testing. Emerg. Infect. Dis. 12:1209–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Thomson KS. 2010. Extended-spectrum-β-lactamase, AmpC, and carbapenemase issues. J. Clin. Microbiol. 48:1019–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Thomson KS, Robledo IE, Vázquez GJ, Moland ES. 2011. KPC screening by updated BD Phoenix and Vitek 2 automated systems. J. Clin. Microbiol. 49:3386–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Tóth A, et al. 2010. Emergence of a colistin-resistant KPC-2-producing Klebsiella pneumoniae ST258 clone in Hungary. Eur. J. Clin. Microbiol. Infect. Dis. 29:765–769 [DOI] [PubMed] [Google Scholar]
- 248.Tsakris A, et al. 2009. Use of boronic acid disk tests to detect extended-spectrum β-lactamases in clinical isolates of KPC carbapenemase-possessing Enterobacteriaceae. J. Clin. Microbiol. 47:3420–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Urban C, et al. 2008. Carbapenem-resistant Escherichia coli harboring Klebsiella pneumoniae carbapenemase β-lactamases associated with long-term care facilities. Clin. Infect. Dis. 46:e127–e130 [DOI] [PubMed] [Google Scholar]
- 250.Urban C, Mariano N, Rahal JJ. 2010. In vitro double and triple bactericidal activities of doripenem, polymyxin B, and rifampin against multidrug-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli. Antimicrob. Agents Chemother. 54:2732–2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Vatopoulos A. 2008. High rates of metallo-β-lactamase-producing Klebsiella pneumoniae in Greece—a review of the current evidence. Euro Surveill. 13:8023http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=8023 [PubMed] [Google Scholar]
- 252.Villegas MV, et al. 2006. First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob. Agents Chemother. 50:2880–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Voets GM, et al. 2011. A set of multiplex PCRs for genotypic detection of extended-spectrum β-lactamases, carbapenemases, plasmid-mediated AmpC β-lactamases and OXA β-lactamases. Int. J. Antimicrob. Agents 37:356–359 [DOI] [PubMed] [Google Scholar]
- 254.Walsh TR, Toleman MA, Poirel L, Nordmann P. 2005. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Walsh TR, Weeks J, Livermore DM, Toleman MA. 2011. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect. Dis. 11:355–362 [DOI] [PubMed] [Google Scholar]
- 256.Walther-Rasmussen J, Høiby N. 2006. OXA-type carbapenemases. J. Antimicrob. Chemother. 57:373–383 [DOI] [PubMed] [Google Scholar]
- 257.Wei ZQ, et al. 2007. Plasmid-mediated KPC-2 in a Klebsiella pneumoniae isolate from China. Antimicrob. Agents Chemother. 51:763–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Weisenberg SA, Morgan DJ, Espinal-Witter R, Larone HD. 2009. Clinical outcomes of patients with KPC-producing Klebsiella pneumoniae following treatment with imipenem or meropenem. Diagn. Microbiol. Infect. Dis. 64:233–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Weiss WJ, et al. 2009. Efficacy of NXL104 in combination with ceftazidime in murine infection models, abstr B-1339. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA [Google Scholar]
- 260.Wernli D, et al. 2011. A call for action: the application of The International Health Regulations to the global threat of antimicrobial resistance. PLoS Med. 8:e1001022 doi:10.1371/journal.pmed.1001022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wiener-Well Y, et al. 2010. Carriage rate of carbapenem-resistant Klebsiella pneumoniae in hospitalised patients during a national outbreak. J. Hosp. Infect. 74:344–349 [DOI] [PubMed] [Google Scholar]
- 262.Wiskirchen DE, Koomanachai P, Nicasio AM, Nicolau DP, Kuti JL. 2011. In vitro pharmacodynamics of simulated pulmonary exposures of tigecycline alone and in combination against Klebsiella pneumoniae isolates producing a KPC carbapenemase. Antimicrob. Agents Chemother. 55:1420–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Won SY, et al. 2011. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin. Infect. Dis. 53:532–540 [DOI] [PubMed] [Google Scholar]
- 264.Woodford N, et al. 2007. Ertapenem resistance among Klebsiella and Enterobacter submitted in the UK to a reference laboratory. Int. J. Antimicrob. Agents 29:456–459 [DOI] [PubMed] [Google Scholar]
- 265.Woodford N, et al. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A β-lactamase, KPC-3, in a New York medical center. Antimicrob. Agents Chemother. 48:4793–4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Woodford N, et al. 2008. Arrival of Klebsiella pneumoniae producing KPC carbapenemase in the United Kingdom. J. Antimicrob. Chemother. 62:1261–1264 [DOI] [PubMed] [Google Scholar]
- 267.Yahav D, Lador A, Paul M, Leibovici L. 2011. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J. Antimicrob. Chemother. 66:1963–1971 [DOI] [PubMed] [Google Scholar]
- 268.Yamaguchi Y, et al. 2007. Crystallographic investigation of the inhibition mode of a VIM-2 metallo-β-lactamase from Pseudomonas aeruginosa by a mercaptocarboxylate inhibitor. J. Med. Chem. 50:6647–6653 [DOI] [PubMed] [Google Scholar]
- 269.Yan JJ, Ko WC, Tsai SH, Wu HM, Wu JJ. 2001. Outbreak of infection with multidrug-resistant Klebsiella pneumoniae carrying _bla_IMP-8 in a university medical center in Taiwan. J. Clin. Microbiol. 39:4433–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yigit H, et al. 2001. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45:1151–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Yigit H, et al. 2003. Carbapenem-resistant strain of Klebsiella oxytoca harboring carbapenem-hydrolyzing β-lactamase KPC-2. Antimicrob. Agents Chemother. 47:3881–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yong D, et al. 2006. Increasing prevalence and diversity of metallo-β-lactamases in Pseudomonas spp., Acinetobacter spp., and Enterobacteriaceae from Korea. Antimicrob. Agents Chemother. 50:1884–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yong D, et al. 2009. Characterization of a new metallo-β-lactamase gene, _bla_NDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 53:5046–5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zarkotou O, et al. 2011. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin. Microbiol. Infect. 17:1798–1803 [DOI] [PubMed] [Google Scholar]
- 275.Zarkotou O, et al. 2010. Risk factors and outcomes associated with acquisition of colistin-resistant KPC-producing Klebsiella pneumoniae: a matched case-control study. J. Clin. Microbiol. 48:2271–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Zhanel GG. 2011. New aminoglycosides for Gram-negative infections, abstr 1070. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL [Google Scholar]
- 277.Zhang R, Cai JC, Zhou HW, Nasu M, Chen GX. 2011. Genotypic characterization and in vitro activities of tigecycline and polymyxin B for Enterobacteriaceae with decreased susceptibility to carbapenems. J. Med. Microbiol. 60:1813–1819 [DOI] [PubMed] [Google Scholar]
- 278.Zuckerman T, et al. 2011. SCT in patients with carbapenem resistant Klebsiella pneumoniae: a single center experience with oral gentamicin for the eradication of carrier state. Bone Marrow Transplant. 46:1226–1230 [DOI] [PubMed] [Google Scholar]