Innate Immune Activation Enhances HIV Acquisition in Women, Diminishing the Effectiveness of Tenofovir Microbicide Gel (original) (raw)
Abstract
The antiretroviral agent, tenofovir, formulated as a vaginal microbicide gel, reduces human immunodeficiency virus (HIV) acquisition by 39% in women. This study assessed the role of preexisting immune activation in HIV acquisition in women from the CAPRISA 004 trial, to identify potential strategies to increase the effectiveness of tenofovir gel. Systemic cytokine and cellular immune mediators (platelets and natural killer [NK] cells) were assessed in women at high risk for HIV assigned to either tenofovir or placebo gel in the CAPRISA 004 trial. Notwithstanding tenofovir gel use, women who acquired HIV had significantly higher systemic innate immune activation prior to infection than women who remained uninfected. Activation of both soluble (cytokine) and cellular (NK cells) immune mediators were associated with HIV acquisition, individually or in combination. Hence, an innate immune activation suppressant could be added to tenofovir gel as a potential combination gel strategy in developing the next generation of higher efficacy antiretroviral microbicides.
Women comprise just over half of the 33.3 million people living with human immunodeficiency virus (HIV) globally [1]. Each year there are 2.6 million new infections [1]. The highest prevalence is in sub-Saharan Africa, where young women (15–24 years) bear the brunt of this epidemic [2]. Reducing HIV infection rates in young women is key to altering current epidemic trajectories and is the impetus for developing prevention modalities for women [3]. Results from preexposure prophylaxis (PrEP) studies of both oral and topical formulations of antiretroviral drugs signal new hope for preventing sexual transmission in young women [4]. Notwithstanding the 2 oral PrEP trials [5, 6] that have been stopped for futility, the 3 positive trials involving women [7, 8] demonstrate partial protection that ranges from 39% to 73%. New technologies that further enhance the efficacy of antiretroviral agents are therefore needed.
The CAPRISA 004 study was a phase IIb, randomized, placebo-controlled clinical trial to assess the safety and effectiveness of 1% tenofovir gel in preventing HIV infection in women [9, 10]. The trial showed a 39% reduction in HIV infection. Even in the most adherent of women, protection was no higher than 54%. Thus, the occurrence of infections even in women using tenofovir gel highlights the need to identify strategies to enhance the effectiveness of tenofovir gel.
Some recent studies have suggested that higher levels of systemic immune activation are associated with the risk of HIV acquisition and concluded that a “quiescent immune” phenotype is protective [11–14], but others conclude that activation is protective [15–19]. To resolve this question we investigated the role of immune activation in HIV acquisition in the CAPRISA 004 trial.
METHODS
Subjects
This case–control study was nested in the CAPRISA004 Tenofovir gel trial [9, 10] (see supplementary methods). Samples from 44 HIV acquirers (cases) were studied at the last preinfection visit for which cryopreserved plasma and peripheral blood mononuclear cells (PBMCs) were available (mean, 12.1 months post–trial enrollment). Samples from 37 women were selected as controls if they reported the highest sexual exposure but remained uninfected during the trial (HIV nonacquirers), at the visit at which they reported the greatest frequency of sex in the preceding month (mean, 7.8 months post–trial enrollment). This study was approved by the University of KwaZulu-Natal Biomedical Research Ethics Committee (BE073/010). Participants gave informed consent for their samples to be used for these studies.
Study Procedure
HIV serostatus, safety, sexual behavior, and gel and condom use were assessed at monthly follow-up visits for up to 30 months postenrollment In addition, plasma and PBMCs were obtained and cryopreserved at 3, 12, and 24 months postenrollment.
Plasma Cytokine Quantitation
Plasma samples from cases and controls were assessed using a high-sensitivity human cytokine premixed 13-plex kit (Millipore, Billerica, Massachusetts) for 13 cytokines: GMCSF, interferon gamma (IFN-γ), interleukin (IL)-1β, IL-2 ,-4, -5, -6, -7, -8, -10, -12(p70), -13, and tumor necrosis factor α (TNF-α) per the manufacturer's protocol. Plasma was assayed in duplicate or triplicate after a single thaw, without dilution. Cytokine measures beneath the detection limit of the assay were given a value of the midpoint between zero and the lower detection limit of the assay and were included in the analysis.
Phenotypic Analysis
Natural killer (NK) cell and T-cell activation was measured in cryopreserved PBMCs in batch analyses using optimized flow cytometry methods [20]. Cryopreserved PBMCs were rapidly thawed in warm media, washed, and rested for 2 hours. Sample viability and cell counts were determined by staining with Viacount (Millipore) and run on a Guava PCA (Millipore). PBMCs were stained with yellow viability dye (Invitrogen), anti-CD14 and -CD20 antibodies (to exclude nonviable cells, monocytes, and B cells, respectively). In addition, the cells were stained with anti-CD3, -CD16, -CD38, -CD56, -CD69, -CD158a, -CD158b (BD Biosciences) and-CD158e1/e2 (Beckman Coulter) antibodies. Stained cells were run on a BD LSRII, and the data were analyzed using Flowjo (Treestar). NK cells were defined as viable, CD14neg, CD20neg, CD3neg, and CD16/56pos lymphocytes. The frequency of NK cells that were CD69pos, HLA-DRpos, or CD38pos was determined using fluorescent minus one (FMO) gates. The frequency of T cells, defined as viable, CD14neg, CD20neg, CD3pos cells, expressing CD69 or both CD38 and HLA-DR was similarly determined.
NK-cell and T-cell effector functions were measured using cryopreserved PBMCs in batched analyses. Each of the following 4 NK-cell effector functions were quantified in the absence of exogenous stimulation, excepting IL-2 in the culture media: degranulation (using CD107a expression as a surrogate [21]), cytokine secretion (IFN-γ), activation (CD69), and recent cell division (using Ki-67 as a surrogate [22]). Cryopreserved PBMC were rapidly thawed in warm media, washed, and rested for 12 hours in media containing 50 U/mL IL-2. For experiments in which cells were stimulated with K562 cells (Supplementary Figure 1), NK cells from HIV-uninfected healthy donors were cultured with equal numbers of K562 cells. Cells were washed, resuspended in medium containing an antibody directed against CD107a, and cultured for a further 6 hours. Samples were processed for flow cytometry as detailed above with the following modification. Cells were surface stained with anti-CD3, -CD8, -CD56, -CD69 (BD Biosciences), and -CD7 (Beckman Coulter) antibodies, then washed, fixed, and permeabilized before staining with anti-Ki-67 and -IFN- γ (BD Biosciences) antibodies. To account for NK cells that have lost CD16 expression due to activation [23], CD7 expression was used to classify viable, CD14neg, CD20neg, and CD3neg cells as NK cells [24]. FMO gates were used to determine the proportion of cells responding with ≥1 of the 4 effector functions measured (CD107a, CD69, IFN-γ, or Ki-67). Boolean gates were used to enumerate the proportions of NK cells or CD8 T cells expressing any particular combination of the 4 effector functions. Spice (v5.2) and Pestle (v1.6.2) software (kindly provided by M. Roederer) were used to analyze these data.
Statistical Methods
Comparisons Between Cases and Controls
Assays were conducted blinded. All except the cytokine assays were conducted prior to the unblinding of the CAPRISA004 trial arm allocation. Comparisons were made using a nonparametric Mann–Whitney U test in GraphPad Prism v5 (GraphPad). The cytokine analysis was corrected for multiple comparisons using a false-discovery step-down procedure. The heatmap presented in Figure 1 was assembled in GeneCluster and visualized in TreeStar (Michael Eisen, Stanford University). All other statistical analyses were conducted in SAS v9.2 (SAS Institute, Cary, North Carolina). Logistic regression analyses were implemented in SAS v9.2 with the “Proc logistic” procedure.
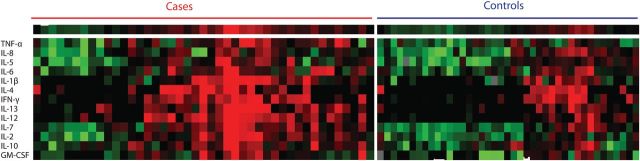
Figure 1.
Heat map representation of 13 plasma cytokines measured in cases and controls. Color changes in the spectrum green to red denote increasing levels of cytokines relative to the median for all participants.
Principal Component Analysis
Principal component analysis (PCA) was run on all variables that differed between cases and controls. Orthogonally rotated (varimax) components that had an eigenvalue of >1 (see Supplementary Figure 2 for the scree plot) and had at least 3 factors loading on it each with a loading score of >0.4, were included. The principal components (PCs), their eigenvalues, variance, and the important variables that composed a PC are shown in Supplementary Table 1.
RESULTS
Behavioral and Biological Risk Factors for HIV Acquisition in the CAPRISA 004 Trial
In the CAPRISA 004 trial population of 889 high-risk women (445 randomized to 1% tenofovir gel, 444 randomized to placebo gel), neither coital frequency (protected or unprotected by condoms), nor the number of sexual partners, nor symptomatic sexually transmitted infections (STIs) were strong predictors of HIV acquisition (data not shown). In contrast, younger age and prevalent herpes simplex virus type 2 (HSV-2) infection were predictive of HIV acquisition (data not shown). But taken together, these risk factors did not entirely explain HIV acquisition risk in this high-risk cohort of young women.
Nested Case-Control Analysis of Immunological Risk Factors for HIV Acquisition
To identify immunological risk factors for HIV acquisition in high-risk women from the CAPRISA 004 trial, we selected 44 cases who acquired HIV (cases), and 37 controls who, despite being at the highest sexual risk in the trial, did not acquire HIV. Baseline demographic characteristics, sexual behaviors during the trial and HSV-2 serostatus by group are detailed in Table 1. Controls had a higher average number of self-reported sex acts per month and a congruently higher average number of used vaginal gel applicators returned per month than cases. Cases were, on average, younger than controls. In total, 33 of 44 cases and 22 of 37 controls were in the Tenofovir treatment arm (p-0.41).
Table 1.
Baseline Demographic Characteristics, Sexual Behavior, and Herpes Simplex Type 2 (HSV-2) Serostatus
| Baseline | Cases (n = 44 women) | Controls (n = 37 women) | P |
|---|---|---|---|
| Demographic characteristics | |||
| Mean age, years | 23.3 | 27.6 | <.001 |
| Mean days preinfection at sample collection (range) | 127 (15–404) | ||
| Sexual behavior | |||
| Mean no. of sex acts/month | 5.7 | 11 | <.001 |
| Mean no. of returned used applicators/month | 6 | 10.7 | <.001 |
| Mean no. of partners | 0.9 | 1.2 | .059 |
| Overall mean self-reported condom use, % | 87.1 | 84.6 | .650 |
| HSV-2 serostatus, no. (%) | |||
| HSV-2 positive at baseline | 28 (63.6) | 21 (56.8) | .788 |
| HSV-2 positive at exit | 33 (75.0) | 25 (67.6) | .693 |
| Gel arm comparison group, no. (%) | |||
| Tenofovir arm | 33 (75) | 22 (60) | .417 |
Cases Had a Proinflammatory Signature of Plasma Cytokine Expression
Cases had a distinctive pattern of plasma inflammatory cytokines prior to infection compared with controls (Figure 1). The cytokine profile (regardless of treatment arm assignment in the trial) of cases was marked mainly by higher concentrations of the proinflammatory and T-cell homeostatic cytokines TNF-α, IL-2, IL-7, and IL-12p70 (Table 2). In an unsupervised hierarchical cluster analysis, cases were clustered separately from controls (data not shown). To probe for the source or functional consequences of these cytokine differences, the cellular profiles of peripheral blood collected at the same timepoints were studied.
Table 2.
Plasma Cytokine Levels, NK-Cell and T-Cell Activation, and Peripheral White Blood Cell Counts
| Variable | Cases (n = 44 women) | Controls (n = 37 women) | P |
|---|---|---|---|
| Median plasma cytokine, pmol/L (IQR) | |||
| IL-10 | 7.82 (4.50–18.93) | 5.00 (2.46–9.18) | .101a |
| IFN-γ | 0.36 (0.15–2.84) | 0.15 (0.15–0.95) | .101a |
| TNF-α | 2.16 (1.26–3.17) | 1.21 (1.01–1.98) | .017a |
| IL-2 | 0.30 (0.15–1.07) | 0.10 (0.06–0.28) | .003a |
| IL-4 | 0.07 (0.07–2.02) | 0.07 (0.07–1.36) | .213a |
| IL-6 | 0.67 (0.28–1.51) | 0.48 (0.24–1.12) | .338a |
| IL-7 | 0.55 (0.21–1.09) | 0.12 (0.06–0.34) | .003a |
| IL-1β | 0.03 (0.03–0.11) | 0.03 (0.03–0.05) | .084a |
| IL-5 | 0.08 (0.03–0.19) | 0.08 (0.08–0.14) | .445a |
| IL-12p70 | 0.68 (0.06–3.19) | 0.06 (0.06–0.11) | .007a |
| IL-13 | 0.53 (0.24–1.52) | 0.24 (0.24–0.24) | .053a |
| IL-8 | 0.60 (0.26–0.99) | 0.59 (0.21–1.15) | .873a |
| GMCSF | 0.23 (0.15–0.49) | 0.23 (0.23–0.25) | .062a |
| Median peripheral blood cell counts (IQR) | |||
| Platelets (×109/L) | 345 (277–383) | 289 (257–355) | .038 |
| WBC | 6.76 (4.79–8.01) | 5.89 (4.99–7.41) | .763 |
| Neutrophil | 3.50 (2.39–4.57) | 3.08 (2.31–4.36) | .647 |
| Lymphocyte | 2.11 (1.91–2.65) | 2.32 (1.6–2.78) | .289 |
| Median NK-cell activation (IQR) | |||
| %HLA-DRpos | 7.43 (4.81–11.10) | 4.08 (2.37–5.61) | .0001 |
| %CD38pos | 46.40 (35.00–55.90) | 67.0 (49.8–76–95) | <.0001 |
| %CD69pos | 0.07 (0.4–0.15) | 0.04 (0.01–0.09) | .049 |
| Median (total) T-cell activation (IQR) | |||
| %CD38pos and HLA-DRpos | 0.93 (0.66–1.14) | 1.08 (0.77–1.56) | .370 |
| %CD69pos | 0.17 (0.10–0.31) | 0.15 (0.7–0.30) | .350 |
Cases Had Higher Numbers of Platelets Just Prior to HIV Acquisition
Consistent with a proinflammatory cytokine profile in plasma, cases had significantly higher platelet counts than did controls (P = .04, Table 2). Cases and controls had similar neutrophil and lymphocyte counts (Table 2). In contrast, there was no difference in the platelet count between cases and controls at study enrollment (data not shown). Next, we explored whether the differences in platelet counts extended to other components of the innate immune system.
Cases Had Higher Levels of Systemic Innate Immune Activation
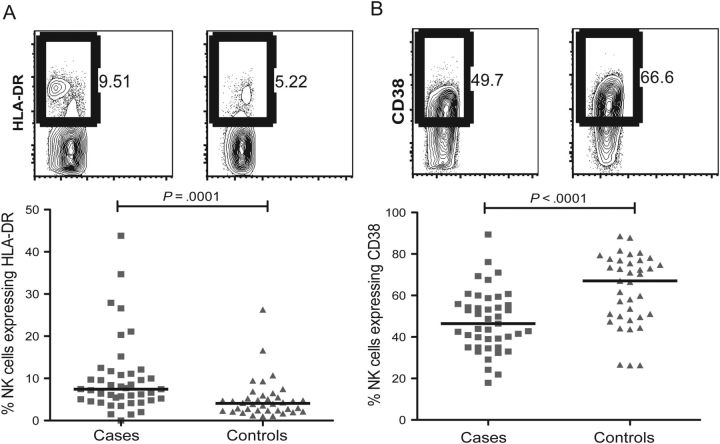
Cases had significantly higher proportions of activated NK cells than did controls. The proportions of NK cells that expressed HLA-DR, a marker of chronic activation and CD69, a marker of recent activation were raised in cases relative to controls (P = .0001 and P = .05, respectively, Figure 2A and Table 2). In addition, cases had markedly lower proportions of CD38 expressing NK cells than controls (P < .0001, Figure 2B and Table 2). In an independent analysis, we observed CD38 down-regulation following in vitro stimulation with K562 cells (Supplementary Figure 1). Thus, lower CD38 expression on NK cells is consistent with higher activation, unlike T cells.
Figure 2.
Cases had higher levels of natural killer (NK) cell activation in blood. A_–_B, Proportion of NK cells expressing (A) HLA-DR or (B) CD38 shown as representative flow cytometry plots from a case (left) in comparison to a control (right) and summary graphs of the same (below). Squares, cases; triangles, controls.
To validate these findings as of functional relevance, the effector function of NK cells in cases and controls was studied. In the absence of in vitro stimulation excepting for IL-2 in the culture medium, cases had a greater proportion of degranulating NK cells than controls (median % CD107apos NK cells in cases: 8.5%, interquartile range [IQR], 6.08%–11.58% vs controls: 4.8%, IQR 2.52%–7.48%: P = .0004). Cases also had fewer recently divided NK cells than controls (median, %Ki-67pos NK cells in cases: 8.94%, IQR 3.99%–18.85% vs controls: 17.1%, IQR 10.0%–24.03%: P = .003), but the proportion of unstimulated NK cells secreting IFN-γ was similar (median, %IFN- γpos NK cells in cases: 2.43%; IQR, 1.65%–4.95% vs controls: 3.32%; IQR, 0.83%–8.04%, P = .93).
To account for the observed differences in NK-cell activation between cases and controls, NK-cell maturation status and killer immunoglobulin–like receptor (KIR) genotypes and phenotypes were examined. There was no difference in the proportion of NK cells expressing CD57, a marker of lymphocyte differentiation (mean %CD57+ NK cells, 56.7% vs 50.7% for cases and controls, respectively; P = .16) Furthermore, there were no differences in KIR genotypes between cases and controls in this study. In this study only 1 donor possessed the KIR3DS1 gene, which has been previously implicated in HIV disease pathogenesis [25, 26]. Finally, there was no difference between cases and controls in the proportion of NK cells expressing KIRs for which commercial antibodies for flow cytometric staining were available (data not shown). Although others have shown that HSV-2 infection is associated with elevated white blood cell counts and increased NK cell degranulation in HIV-coinfected individuals [27], there was no difference in NK cell activation between HSV-2 seropositive and seronegative individuals in this study (data not shown).
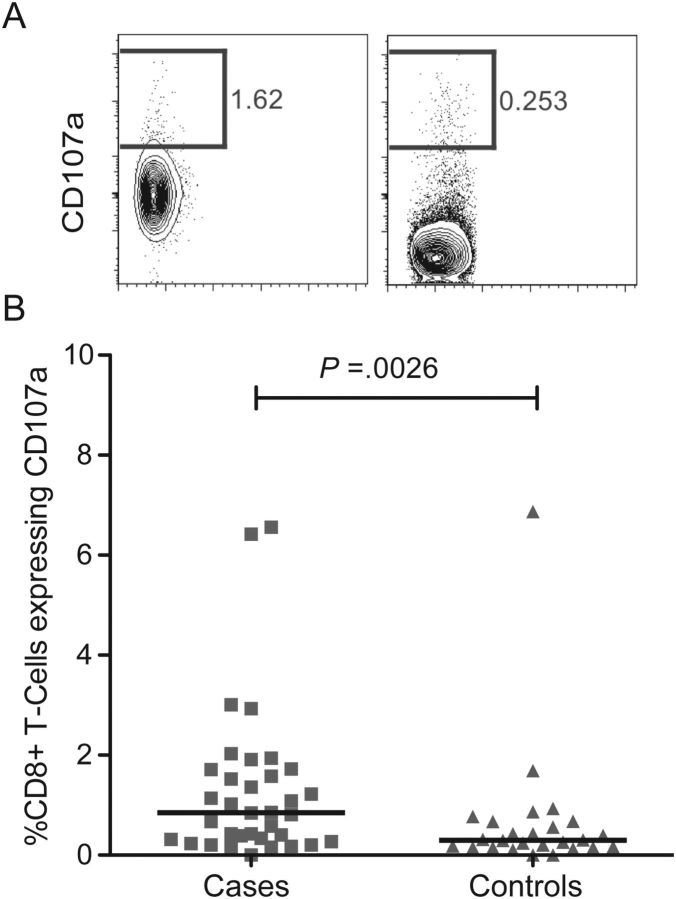
Cases Had Higher Levels of Spontaneous Cytotoxic T-Cell Degranulation
The degree of bulk T-cell activation using conventional markers of activation (coexpression of CD38 and HLA-DR) was similar between cases and controls (P = .37, Table 2). We did not measure activation of T-cell subsets. In contrast, the proportion of degranulating cytotoxic (CD8) T cells in the absence of in vitro stimulation (excepting for IL-2 in the culture medium) was significantly higher among cases compared to controls (P = .0026, Figure 3).
Figure 3.
Cases had higher levels of spontaneous CD8 T-cell degranulation. A_–_B, Proportion of CD8pos T cells expressing CD107a shown as (A) representative flow cytometry plots from a case (left) in comparison to a control (right), and (B) a summary graph of the same. Squares, cases; triangles, controls.
In a Principal Component Analysis of Immunological Drivers of HIV Acquisition, Innate Immune Activation Was Predictive of Acquisition
To conduct a more comprehensive analysis, the putative factors related to acquisition were subjected to a principal components analysis. Four PCs with eigenvalues >1 were extracted (Supplementary Figure 2) and collectively explained almost 70% of the variability in activation measures. Each PC measured a qualitatively different immune response, which were labeled accordingly (Supplementary Table 1): PC1-T-cell homeostatic cytokines, PC2-innate immune activation, PC3-innate immune quiescence, and PC4-innate immune activation (independent of CD8pos T-cell degranulation). The PCs were included in an adjusted logistic regression model of HIV acquisition.
In this logistic regression analysis, adjusted for treatment arm, age and HSV-2 serostatus, PC2-innate immune activation was a significant risk factor for HIV acquisition (OR, 11.27; 95% CI: 1.84–69.09, P = .009, Table 3). Conversely and in congruence, PC3-innate immune quiescence was a significant independent protective factor (OR, 0.06; 95% CI: .013–.33, P = .001, Table 3).
Table 3.
Logistic Regression Model for HIV Acquisition in Case–Control Analysis Including Principal Components
| Component | Adjusted Odds Ratio (95% CI) for HIV Acquisition | P |
|---|---|---|
| Placebo vs tenofovir | 7.15 (1.02–50.28) | .048 |
| HSV-2pos vs HSV-2neg serostatus | 21.85 (1.31–364.45) | .031 |
| Age (per year increase) | 0.72 (.58–.88) | .001 |
| PC 1: “T-cell homeostatic cytokines” | 9.36 (.35–247.90) | .181 |
| PC 2: “Innate immune activation” | 11.27 (1.84–69.09) | .009 |
| PC 3: “Innate immune quiescence” | 0.06 (.013–.33) | .001 |
| PC 4: “NK-cell activation independent of CD8+ T-cell degranulation” | 1.65 (.69–3.95) | .260 |
Finally, to exclude tenofovir treatment as a confounding effect, and to determine whether immune activation enhances HIV acquisition over and above the protective effect of tenofovir gel, the logistic regression was repeated focusing on the 2 measures of activation most divergent between cases and controls in the stratum of women who were using tenofovir gel. In this analysis, which was also adjusted for age, HSV-2 infection, and HLA-DR expressing NK cells, a reduced proportion of CD38-expressing NK cells was independently associated with HIV acquisition among women who were using tenofovir gel (OR, 0.90; 95% CI: .82–.97, P = .005, Table 4). In summary, after adjusting for multiple potential confounders, systemic innate immune activation measured collectively in a principal component or by a reduced proportion of CD38 expressing NK cells in blood was associated with HIV acquisition.
Table 4.
Logistic Regression Model for HIV Acquisition: Tenofovir Gel Arm Only Including Individual Measures of NK-Cell Activation
| Component | Adjusted Odds Ratio (95% CI) for HIV Acquisition | P |
|---|---|---|
| HSV-2pos vs HSV-2neg | 77.33 (.90–999) | .056 |
| Age (per year increase) | 0.67 (.48–.91) | .011 |
| %CD38pos NK cells (per 1% decrease) | 1.11 (1.03–1.22) | .005 |
| %HLA-DRpos NK cells (per 1% increase) | 1.38 (.99–1.92) | .058 |
DISCUSSION
In this study systemic innate immune activation was associated with HIV acquisition, even among women using tenofovir gel. Relative to women at high HIV risk who remained uninfected, women who acquired HIV had higher levels of proinflammatory cytokines, platelets, activated NK cells, and spontaneous cytotoxic T-cell degranulation in blood. Many of these various measures of immune activation were predictors of HIV acquisition independent of other risk factors but when these factors were assembled into principal components, innate immune activation was a strong predictor of HIV acquisition. We infer from these collective data that systemic innate immune activation enhances HIV acquisition, and thus modulating activation may provide a new strategy for improving the efficacy of antiretroviral agents in preventing HIV acquisition.
The search for risk factors for HIV has yielded an array of genetic, innate, humoral, and cellular immune response factors that differentiate highly exposed seronegative (HESN) individuals from healthy controls [28]. However, many promising correlates have not been reproducible: some studies conclude that a quiescent immune phenotype is protective [11–14], whereas others conclude that activation is protective [15–19]. These divergent findings may be because of differences in comparison groups (HESN to healthy controls—women with low/no sexual risk for HIV—or HIV-infected patients) and the definition of exposure used. We propose that subsequent HIV acquisition is a more accurate measure of HIV exposure. In the CAPRISA 004 trial of tenofovir gel, conventional risk factors for HIV acquisition [29] did not fully account for overall HIV risk. This confirmed the relative homogeneity in the risk profiles of the trial participants and the underlying population [2]. Further, it validated the search for additional immunological risk factors by comparing preinfection samples from women who acquired HIV (cases) to samples from those who did not (controls).
Women who acquired HIV had higher levels of systemic innate immune activation than those who remained HIV negative despite high risk exposure. Platelets, which are closely intertwined with the innate immune system [30], were also more abundant in the blood of cases than controls, consistent with our data from a similar population in a previous study [31]. NK cells are the first line of defense against a variety of intracellular viral pathogens and are potently equipped to kill HIV-infected cells or to recruit other cells [32]. Thus it was reasoned that their basal state of activation broadly portrays the extent of innate immune activation and thereby may correlate with HIV endpoints. Using several different measures of activation, NK cells from cases were more activated and more avidly degranulated than those from controls. An activated NK-cell phenotype associated with higher levels of degranulation and lower proliferation is reminiscent of a more differentiated or exhausted NK cell. But the finding that a similar proportion of NK cells in cases and controls expressed CD57, a marker of differentiation [33], suggests that this does not account for differences in NK-cell activation and function in this study.
Our data suggest reduced CD38 expression on NK cells is an activation phenotype associated with HIV. Ligation of CD38 on NK cells results in proliferation, cytokine production, and HLA-DR expression [34–36] but because CD38 colocalizes with CD16 [37, 38] and CD16 is rapidly down-regulated on NK-cell activation [23], we conclude that in this context, lower frequencies of CD38 expressing NK cells are consistent with prior NK-cell activation. But the role of CD38 in HIV-infection is complex [39].
Although this study had a similar sample size to other studies that have found specific KIR or KIR-HLA compound genotypes associated with protection from HIV acquisition [40–42], we did not find evidence to support this. High genetic diversity of KIR genes has been described among sub-Saharan Africans [43]. Thus, power to detect genetic associations in this study was limited.
Two limitations of this study warrant attention: its small size and the paucity of longitudinal measures of activation. Despite the increased Type II error because of a small sample size, we found marked differences that remain statistically significant after correcting for multiple comparisons. These differences withstood rigorous testing in adjusted regression models. Second, because of the rarity of stored samples (particularly PBMCs) in the CAPRISA 004 trial, the availability of and access to longitudinal samples was limited. Nevertheless, for women who did not acquire HIV, NK-cell and T-cell activation was similar at the highest sex visit (reported here) compared to the last visit in the trial for controls (data not shown). Hence, it could be inferred that the single timepoint measurement accurately portrayed underlying activation.
A particular strength of this analysis is that it compares women who acquire HIV to women who do not acquire HIV followed prospectively. The participants were drawn from a clinical trial population conducted in a community with high background HIV incidence rates. Jennes and colleagues have reported an immune quiescence phenomenon in highly exposed seronegative African women in comparison to healthy women [14]. Our data are consistent with these observations and suggest that prior to infection, women have a potentially modifiable set of immunological parameters that are strongly associated with their risk of HIV acquisition. The temporal relationship between the presence of inflammation prior to infection and HIV acquisition and the high degree of consistency across multiple measures of systemic immune activation increase the likelihood that systemic immune activation is causally related to HIV acquisition. But what biological plausibility is there for immune activation as a driver of acquisition?
There are at least 2 potential mechanisms by which innate immune activation may drive acquisition. First, activated T cells are more susceptible to HIV infection and support higher rates of viral replication [44]. We did not observe evidence of bulk T-cell activation, but importantly we did not measure activation on T-cell subsets (CD4pos or CD8pos T cells) in peripheral blood, nor at the site of HIV exposure (genital tract). But an increased frequency of innate lymphocytes that activate these cells at the genital tract mucosa or regional lymph node level may (1) recruit potential target cells to the site of infection [45] or (2) increase the likelihood that the initial viral reproductive rate _R_0 will exceed 1 (_R_0 > 1), thereby establishing productive infection [46]. In additional concordant studies done in this study population, reported higher cervicovaginal cytokine concentrations in women who acquired HIV [47]. Further work to measure T-cell subset activation and the crosstalk between genital and systemic activation is warranted. Second, in the presence of generalized systemic immune activation, the ability to generate a protective immune response may be compromised. Immune activation is associated with greater rates of immunosenescence, disruption of anatomic niches, and excessive bystander apoptosis [48]. Consequently, these effects may impair defense against HIV, aside from their effects on defense against other pathogens.
Perhaps the most promising finding from this study is the identification of a potentially modifiable risk factor for HIV. If methods to dampen immune activation were available, the efficacy of tenofovir gel may be improved. One approach is to identify and target the upstream drivers of immune activation. The finding of systemic activation suggests that a multipotent factor may be driving activation, perhaps a common pathogen. In chronic HIV infection, there is evidence that herpes viruses, such as human cytomegalovirus (CMV), may drive immune activation, and reducing CMV replication with valganciclovir reduces activation [49]. In our study population, almost all adults are infected with CMV (A Kharsany, personal communication) so is unlikely to be distributed unequally in cases and controls so as to account for differences in activation. But a search for other pathogens is nonetheless warranted.
An alternative approach to targeted treatment of specific pathogens is to develop and test methods that directly dampen inflammation. There are recent data to suggest that reducing inflammation may prevent HIV. In a monkey model of HIV acquisition, the modulation of Toll-like receptor (TLR) signaling events at the genital tract mucosa protects from infection [50].
Future studies will seek to identify pathogens that drive activation prior to HIV and to determine whether innate immune activation in women who acquire HIV has an underlying host genetic component. Incorporating an intervention that dampens innate immune activation among the existing HIV prevention methods may meanwhile provide additional protection for young African women, even in the tenofovir gel era. More research is needed before the results can be confidently translated into clinical applications.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Data
Notes
Acknowledgments. We thank the participants of the CAPRISA 004 study; women whose dedication and commitment to improving their and their peers' health and donating samples during the conduct of the trial made this research possible. We gratefully acknowledge the technical assistance of Dr Rachel Simmons for advice on cytokine assays, Drs Clive Gray and Debra de Assis Rosa for KIR and HLA typing, and Lise Werner and Anneke Grobler for assisting with statistical analysis. We also thank Dr Thumbi Ndung'u for providing access and support for using the BD LSRII flow cytometer located at the Doris Duke Medical Research Institute.
Financial support. This work was supported by the South African HIV/AIDS Research Platform (SHARP), and US National Institutes for Health FIC K01-TW007793. The parent trial (CAPRISA 004) was supported by the US Agency for International Development (USAID), Family Health International (FHI) cooperative agreement GPO-A-00-05-00022-00, contract 132119, and LIFELab, a biotechnology centre of the South African Department of Science and Technology. These studies were also supported by the TRAPS (Tenofovir gel Research for AIDS Prevention Science) Program, which is funded by CONRAD cooperative grant GP00-08-00005-00, subproject agreement PPA-09-046. We thank the US National Institutes for Health's Comprehensive International Program of Research on AIDS (CIPRA grant AI51794) for the research infrastructure. V. N. was supported by LIFELab and the Columbia University-South Africa Fogarty AIDS International Training and Research Program (AITRP D43 TW000231). M. A. is a Distinguished Clinical Scientist of the Doris Duke Charitable Foundation. W. H. C. was supported by a Massachusetts General Hospital Physician Scientist Development Award.
Potential conflicts of interest. Salim Abdool Karim and Quarraisha Abdool Karim were the co-Principal Investigators of the CAPRISA 004 trial of tenofovir gel. Q. A. K. is co-Principal Investigator of the HIV Prevention Trials Network, which is undertaking HPTN 052 trial of treatment for prevention. S. S. A. K. is an executive committee member of the Microbicide Trials Network, which is undertaking the VOICE trial of oral and topical PrEP. S. S. A. K. and Q. A. K. are also coinventors of 2 pending patents (61/354.050 and 61/357.892) of tenofovir gel against HSV-1 and HSV-2 with scientists from Gilead Sciences.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. 2010 [Google Scholar]
- 2.Abdool Karim Q, Kharsany AB, Frohlich JA, et al. Stabilizing HIV prevalence masks high HIV incidence rates amongst rural and urban women in KwaZulu-Natal, South Africa. Int J Epidemiol. 2011;40(4):922–30. doi: 10.1093/ije/dyq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdool Karim Q, Sibeko S, Baxter C. Preventing HIV infection in women: a global health imperative. Clin Infect Dis. 2010;50(Suppl 3):S122–9. doi: 10.1086/651483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdool Karim SS, Abdool Karim Q. Antiretroviral prophylaxis: a defining moment in HIV control. Lancet. 2011;378(9809):e23–5. doi: 10.1016/S0140-6736(11)61136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FHI. Press Release: FHI to Initiate Orderly Closure of FEM-PrEP. 2011 [Google Scholar]
- 6.MTN. Press Release: MTN statement on decision to discontinue use of oral tenofovir tablets in VOICE, a Major HIV Prevention Study in Women. 2011 [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Press Release: CDC trial and another major study find PrEP can reduce risk of HIV infection among heterosexuals. 2011 [Google Scholar]
- 8.University of Washington International Clinical Research Center. Press Release: Partners PrEP Study. Pivotal study finds that HIV medications are highly effective as prophylaxis against HIV infection in men and women in Africa. 2011 [Google Scholar]
- 9.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdool Karim Q, Kharsany AB, Frohlich JA, et al. Recruitment of high risk women for HIV prevention trials: baseline HIV prevalence and sexual behavior in the CAPRISA 004 tenofovir gel trial. Trials. 2011;12:67. doi: 10.1186/1745-6215-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Begaud E, Chartier L, Marechal V, et al. Reduced CD4 T cell activation and in vitro susceptibility to HIV-1 infection in exposed uninfected Central Africans. Retrovirology. 2006;3:35. doi: 10.1186/1742-4690-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLaren PJ, Ball TB, Wachihi C, et al. HIV-exposed seronegative commercial sex workers show a quiescent phenotype in the CD4+ T cell compartment and reduced expression of HIV-dependent host factors. J Infect Dis. 2010;202(Suppl 3):S339–44. doi: 10.1086/655968. [DOI] [PubMed] [Google Scholar]
- 13.Pancino G, Saez-Cirion A, Scott-Algara D, Paul P. Natural resistance to HIV infection: lessons learned from HIV-exposed uninfected individuals. J Infect Dis. 2010;202(Suppl 3):S345–50. doi: 10.1086/655973. [DOI] [PubMed] [Google Scholar]
- 14.Jennes W, Evertse D, Borget MY, et al. Suppressed cellular alloimmune responses in HIV-exposed seronegative female sex workers. Clin Exp Immunol. 2006;143:435–44. doi: 10.1111/j.1365-2249.2006.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biasin M, Caputo SL, Speciale L, et al. Mucosal and systemic immune activation is present in human immunodeficiency virus-exposed seronegative women. J Infect Dis. 2000;182:1365–74. doi: 10.1086/315873. [DOI] [PubMed] [Google Scholar]
- 16.Jennes W, Sawadogo S, Koblavi-Deme S, et al. Cellular human immunodeficiency virus (HIV)-protective factors: a comparison of HIV-exposed seronegative female sex workers and female blood donors in Abidjan, Cote d'Ivoire. J Infect Dis. 2003;187:206–14. doi: 10.1086/346049. [DOI] [PubMed] [Google Scholar]
- 17.Suy A, Castro P, Nomdedeu M, et al. Immunological profile of heterosexual highly HIV-exposed uninfected individuals: predominant role of CD4 and CD8 T-cell activation. J Infect Dis. 2007;196:1191–201. doi: 10.1086/521193. [DOI] [PubMed] [Google Scholar]
- 18.Tomescu C, Duh FM, Lanier MA, et al. Increased plasmacytoid dendritic cell maturation and natural killer cell activation in HIV-1 exposed, uninfected intravenous drug users. AIDS. 2011;24:2151–60. doi: 10.1097/QAD.0b013e32833dfc20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran HK, Chartier L, Troung LX, et al. Systemic immune activation in HIV-1-exposed uninfected Vietnamese intravascular drug users. AIDS Res Hum Retroviruses. 2006;22:255–61. doi: 10.1089/aid.2006.22.255. [DOI] [PubMed] [Google Scholar]
- 20.Naranbhai V, Bartman P, Ndlovu D, et al. Impact of blood processing variations on natural killer cell frequency, activation, chemokine receptor expression and function. J Immunol Methods. 2011;366:28–35. doi: 10.1016/j.jim.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Soares A, Govender L, Hughes J, et al. Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation. J Immunol Methods. 2010;362:43–50. doi: 10.1016/j.jim.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison D, Phillips JH, Lanier LL. Involvement of a metalloprotease in spontaneous and phorbol ester-induced release of natural killer cell-associated Fc gamma RIII (CD16-II) J Immunol. 1991;147:3459–65. [PubMed] [Google Scholar]
- 24.Milush JM, Long BR, Snyder-Cappione JE, et al. Functionally distinct subsets of human NK cells and monocyte/DC-like cells identified by coexpression of CD56, CD7, and CD4. Blood. 2009;114:4823–31. doi: 10.1182/blood-2009-04-216374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alter G, Martin MP, Teigen N, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204:3027–36. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–34. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 27.Long BR, Erickson AE, Chapman JM, et al. Increased number and function of natural killer cells in human immunodeficiency virus 1-positive subjects co-infected with herpes simplex virus 2. Immunology. 2010;129:186–96. doi: 10.1111/j.1365-2567.2009.03170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazawa M, Lopalco L, Mazzotta F, Lo Caputo S, Veas F, Clerici M. The ‘immunologic advantage’ of HIV-exposed seronegative individuals. AIDS. 2009;23:161–75. doi: 10.1097/QAD.0b013e3283196a80. [DOI] [PubMed] [Google Scholar]
- 29.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–29. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delvaeye M, Conway EM. Coagulation and innate immune responses: can we view them separately? Blood. 2009;114:2367–74. doi: 10.1182/blood-2009-05-199208. [DOI] [PubMed] [Google Scholar]
- 31.Ramsuran V, Kulkarni H, He W, et al. Duffy-Null-associated low neutrophil counts influence HIV-1 susceptibility in high-risk South African black women. Clin Infect Dis. 2011;52:1248–56. doi: 10.1093/cid/cir119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol. 2006;118:1–10. doi: 10.1016/j.clim.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Verges S, Milush JM, Pandey S, et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–74. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malavasi F, Deaglio S, Funaro A, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev. 2008;88:841–86. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- 35.Mallone R, Funaro A, Zubiaur M, et al. Signaling through CD38 induces NK cell activation. Int Immunol. 2001;13:397–409. doi: 10.1093/intimm/13.4.397. [DOI] [PubMed] [Google Scholar]
- 36.Sconocchia G, Titus JA, Mazzoni A, et al. CD38 triggers cytotoxic responses in activated human natural killer cells. Blood. 1999;94:3864–71. [PubMed] [Google Scholar]
- 37.Deaglio S, Zubiaur M, Gregorini A, et al. Human CD38 and CD16 are functionally dependent and physically associated in natural killer cells. Blood. 2002;99:2490–8. doi: 10.1182/blood.v99.7.2490. [DOI] [PubMed] [Google Scholar]
- 38.Funaro A, Reinis M, Trubiani O, Santi S, Di Primio R, Malavasi F. CD38 functions are regulated through an internalization step. J Immunol. 1998;160:2238–47. [PubMed] [Google Scholar]
- 39.Savarino A, Bottarel F, Malavasi F, Dianzani U. Role of CD38 in HIV-1 infection: an epiphenomenon of T-cell activation or an active player in virus/host interactions? AIDS. 2000;14:1079–89. doi: 10.1097/00002030-200006160-00004. [DOI] [PubMed] [Google Scholar]
- 40.Boulet S, Sharafi S, Simic N, et al. Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. AIDS. 2008;22:595–9. doi: 10.1097/QAD.0b013e3282f56b23. [DOI] [PubMed] [Google Scholar]
- 41.Jennes W, Verheyden S, Demanet C, et al. Cutting edge: resistance to HIV-1 infection among African female sex workers is associated with inhibitory KIR in the absence of their HLA ligands. J Immunol. 2006;177:6588–92. doi: 10.4049/jimmunol.177.10.6588. [DOI] [PubMed] [Google Scholar]
- 42.Ravet S, Scott-Algara D, Bonnet E, et al. Distinctive NK-cell receptor repertoires sustain high-level constitutive NK-cell activation in HIV-exposed uninfected individuals. Blood. 2007;109:4296–305. doi: 10.1182/blood-2006-08-040238. [DOI] [PubMed] [Google Scholar]
- 43.Norman PJ, Abi-Rached L, Gendzekhadze K, et al. Unusual selection on the KIR3DL1/S1 natural killer cell receptor in Africans. Nat Genet. 2007;39:1092–9. doi: 10.1038/ng2111. [DOI] [PubMed] [Google Scholar]
- 44.Shapira-Nahor O, Kalinkovich A, Weisman Z, et al. Increased susceptibility to HIV-1 infection of peripheral blood mononuclear cells from chronically immune-activated individuals. AIDS. 1998;12:1731–3. [PubMed] [Google Scholar]
- 45.Jaspan HB, Liebenberg L, Hanekom W, et al. Immune activation in the female genital tract during HIV Infection predicts mucosal CD4 depletion and HIV shedding. J Infect Dis. 2011;204:1550–6. doi: 10.1093/infdis/jir591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–23. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 47.Roberts L, Passmore J, Williamson C, et al. Genital tract inflammation in women participating in the CAPRISA TFV Microbicide Trial who became infected with HIV: A mechanism for breakthrough infection? 18th Conference on Retroviruses and Opportunistic Infections. (Boston, MA, USA) [Google Scholar]
- 48.d'Ettorre G, Paiardini M, Ceccarelli G, Silvestri G, Vullo V. HIV-associated immune activation: from bench to bedside. AIDS Res Hum Retroviruses. 2011;27:355–64. doi: 10.1089/aid.2010.0342. [DOI] [PubMed] [Google Scholar]
- 49.Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T-cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–83. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data