Tumor Microenvironment Complexity: Emerging Roles in Cancer Therapy (original) (raw)
. Author manuscript; available in PMC: 2013 May 14.
Abstract
The tumor microenvironment (TME) consists of cells, soluble factors, signaling molecules, extracellular matrix, and mechanical cues that can promote neoplastic transformation, support tumor growth and invasion, protect the tumor from host immunity, foster therapeutic resistance, and provide niches for dormant metastases to thrive. An American Association for Cancer Research (AACR) special conference held on November 3–6, 2011, addressed five emerging concepts in our understanding of the TME: its dynamic evolution, how it is educated by tumor cells, pathways of communication between stromal and tumor cells, immunomodulatory roles of the lymphatic system, and contribution of the intestinal microbiota. These discussions raised critical questions on how to include the analysis of the TME in personalized cancer diagnosis and treatment.
Introduction
The tumor microenvironment (TME) has received growing attention from an increasing number of investigators in the United States and abroad (1) and also by research organizations including the National Cancer Institute (NCI) and the American Association for Cancer Research (AACR) over the last decade. As our understanding of the role of the TME in cancer continues to evolve, the complexity of the interactions between neoplastic tumor cells and their microenvironment has become increasingly apparent, and at the same time, the number of agents entering clinical trials that specifically target interactive pathways between neoplastic and stromal cells has increased. On November 3–6, 2011, the AACR, in conjunction with its Tumor Microenvironment Working Group, organized a special conference entitled “Tumor Microenvironment Complexity: Emerging Roles in Cancer Therapy” that brought together in Orlando, FL, more than 280 participants including 42 speakers to discuss recent progress made in the field and to identify future directions of the highest priority. A workshop chaired by Suresh Mohla (NCI, Bethesda, MD) also presented the new TME network (TMEN) program at the NCI funded by U54 and U01 grants that brings together 11 centers and whose mission is to promote and facilitate interdisciplinary collaborations in understanding the host stroma in tumorigenesis (2).
Many of the presentations focused on the interactions between tumor cells and their surrounding environment. How such interactions are critical for tumor progression was well illustrated by the work of Luis Parada (University of Texas Southwest Medical Center, Dallas, TX), who gave the opening keynote lecture. Using mouse models of neurofibromatosis type 1 (Nf1), he showed that loss of Nf1 heterozygosity in Schwann cells that give rise to plexiform neurofibroma is insufficient for neurofibroma formation but rather that Nf1 haploinsufficiency in mast cells is also required for tumor formation. Furthermore, cKIT in mast cells was critical for their recruitment and protumor effects. These elegant studies have led to clinical studies evaluating imatinib (Gleevec) in patients with Nf1 that have thus far indicated a favorable response rate and have bolstered enthusiasm for targeting stromal cells in a diversity of solid tumors.
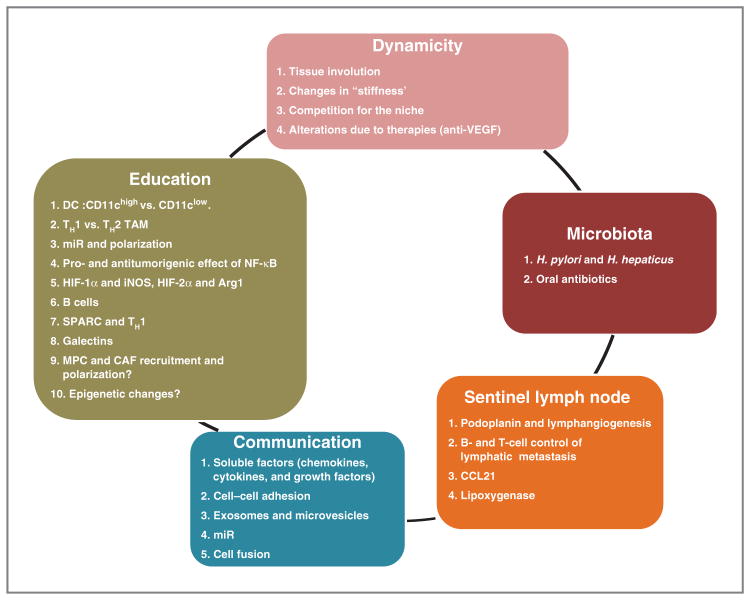
The present article summarizes the meeting in 5 new emerging concepts (Fig. 1) and 2 critical questions that were the subject of discussion during the conference.
Figure 1.
The diagram summarizes the 5 emerging themes that were the subject of presentations and discussions at the special AACR conference entitled “Tumor Microenvironment Complexity: Emerging Roles in Cancer Therapy” that took place in Orlando, FL, on November 3–6, 2011.
Emerging concept 1: The TME is a dynamic milieu
The TME is in constant evolution as a result of tissue remodeling, metabolic alterations in the tumor, and changes in the recruitment of stromal cells including a diversity of immune cells. Tissue remodeling that occurs in the post-partum breast during mammary gland involution, for example, perpetuates an increased risk of breast cancer (3). Pepper Schedin (University of Colorado, Aurora, CO) described how such remodeling creates a tumor-promoting inflammatory environment similar to the environment of a wound, characterized by an influx of T-helper (TH)2-type macrophages, abundant fibrillar collagen, and increased COX-2 activity. Human mammary tumor cells implanted into mouse mammary fat pads formed tumors more readily, with increased metastatic potential, when implanted in involuting (postlactation) mammary glands, rather than during pregnancy, whereas COX-2 inhibition during weaning slowed tumor growth and limited metastasis. These results raise the intriguing possibility that short-term anti-inflammatory treatment during the post-partum period may decrease breast cancer risk, similar to results in colon cancer (4).
Another important feature of the TME is the content and organization of the extracellular matrix (ECM), whose mechanical properties affect neoplastic cell differentiation and invasiveness. Increased stromal stiffness in breast tissue is a known risk factor of breast cancer in humans, and Valerie Weaver (University of California San Francisco, San Francisco, CA) described how inhibition of collagen cross-linking by lysyl oxidase (LOX) in murine models delayed and decreased tumor invasion (5). ECM stiffening promoted activation of ROCK, a Rho kinase effector, which increased collagen deposition by mechanisms associated with increased Wnt signaling, activation of STAT3, and expression of inflammatory cytokines, including CCL2 and granulocyte macrophage colony-stimulating factor (GM-CSF), that recruited bone marrow (BM)-derived cells into the TME. These compelling data indicate that changes in the biomechanical function of the TME impact neoplastic cell proliferation and migration, as well as secretion of immunomodulatory factors. Using second harmonic generation microscopy, Ryan Burke (University of Rochester, New York, NY) from the laboratory of Edward Brown showed that altering the alignment of collagen fibers in solid tumors impacted malignant cell invasion and their metastatic properties, in part, via regulation of TNF-α and macrophages. Thus, as similarly illustrated in P. Shedin’s presentation in the involuting mammary gland not only the amount of stromal collagen but also its organization are drivers of the malignant process.
Dynamic interactions between tumor cells and cells of the osteoblastic niche affect malignant evolution. It has been appreciated for some time that in patients with prostate cancer, increased presence of circulating hematopoietic stem cells (HSC) is an indicator of bone metastasis. Russell Taichman (University of Michigan, Ann Arbor, MI) showed that prostate carcinomas seeded into BM remained dormant and insensitive to drug treatment (6). During bone metastasis, these cells competed with HSCs for occupation of the osteoblastic niche. Conversely, altering the osteoblastic niche in mice by pretreatment with parathormone or by clearing HSCs with a CXCR4 inhibitor promoted prostatic bone metastasis.
Anticancer therapies alter the TME in ways that either promote or inhibit tumorigenicity, depending on a diverse array of heterotypic mechanisms. This concept was elegantly illustrated for the case of anti-VEGF therapy by Gabriele Bergers (University of California San Francisco) who showed that glioblastoma-bearing mice treated with Avastin exhibited a transient beneficial therapeutic response that was followed by tumor revascularization and enhanced invasiveness (7) associated with increased c-Met expression and epithelial-to-mesenchymal transformation (EMT). This is explained by the observation that VEGF receptor (VEGFR)2 and c-Met were antagonistically associated, where c-Met signaling became dominant in the presence of VEGFR2 signaling blockade and vice versa. Thus, combined therapy targeting both signaling pathways may be required for efficient, durable antitumor responses.
Emerging concept 2: Significance of immune and stromal cell education in the TME
Emerging studies indicate that reciprocal interactions between the diverse assemblages of stromal cells and evolving neoplastic cells fundamentally regulate tumor progression. Adaptive and innate immune cells represent a significant component of the TME. Largely dependent on soluble cytokines and chemokines, immune cells become variably polarized toward TH1- (generally antitumor) or TH2-type (generally protumor) phenotypes. While initially described for CD4+T cells, it is now clear that TH1- and TH2-type factors regulate the phenotype and bioactivity of essentially all immune cells subtypes. Michael Shurin (University of Pittsburgh, Pittsburgh, PA) showed that conventional dendritic cells (DC) exposed to tumor-derived factors polarize into regulatory DCs (regDC) that suppressed the proliferation of preactivated T cells and were phenotypically and functionally different from their precursors and from immature conventional DCs (8). In particular, CD11clow CD11bhigh DCs exhibited immunosuppressive activity toward implanted 3LL tumors, whereas CD11chigh DCs instead promoted their metastasis dependent on RhoGTPase. In the presence of Clostridium difficile toxin, DCs failed to polarize and exhibited altered activity and effector functions. Related to DCs, the significance of macrophage TH2 polarization was reported by Lisa Coussens (Oregon Health and Science University, Portland, OR). TH2-type tumor-associated macrophages (TAM) are common constituents of many solid tumor types and not only provide proangiogenic and proinvasive factors to growing tumors but also suppress CD8+T-cell–mediated antitumor immunity. Accordingly, blocking recruitment of macrophages into mammary tumors by treating mice with agents that blocked CSF1R signaling not only diminished tumor vascularity and slowed primary tumor development but also reduced formation of pulmonary metastases and improved survival, when given in combination with chemotherapy, by CD8+T-cell–dependent mechanisms (9). These preclinical data highlight the multifunctional role of macrophages in solid tumors and importantly reveal that TAMs blunt cell killing by CD8+T cells as well as by various forms of chemotherapy suggesting a novel combinatorial anticancer approach. A therapeutic CSF1R kinase inhibitor, PLX3397, is currently being tested with eribulin in a phase Ib/II clinical trial in patients with metastatic triple-negative breast cancer.
Macrophage polarization is also modulated by endogenous microRNAs (miR). Michele de Palma (San Raffaele Scientific Institute, Milan, Italy) presented data identifying miR-511-3p, an miRNA encoded by the mannose receptor Mrc1 gene that was specifically upregulated in F4/80+MRC1+CD11c+TAMs and functioned as a negative regulator of TAM protumoral polarization. Also, critical for macrophage phenotype and bioactivity is the expression of the NF-κB. Using mice in which expression of a constitutive activator of NF-κB was induced, Fiona Yull (Vanderbilt University, Nashville, TN) reported that NF-κB activation in macrophages variably affected carcinoma cell metastasis dependent on spatial/temporal features (10). When activated in the presence of circulating tumor cells, NF-κB exerted antitumorigenic activities whereas when activated later in tumor progression, for example, in secondary sites of metastasis, protumorigenic activities on macrophages predominated.
Hypoxia can also affect immune cell education in the TME depending on the type of hypoxia-inducible factor (HIF) involved in myeloid cells (11). Jessica Shay (University of Pennsylvania, Philadelphia, PA) of Celeste Simon’s laboratory reported that whereas HIF-1α fostered TH1 polarization, HIF-2α instead favored TH2 polarization of immune cells. Experimentally, when HIF-2α was either inhibited via acriflavine or genetically deleted, CD68+ macrophage infiltration into colons of mice challenged with dextran sodium sulphate (DSS) was decreased, and carcinogenesis was reduced. Randall Johnson (University of California San Diego, La Jolla, CA) presented complementary data showing that HIF regulated inducible nitric oxide synthase (iNOS) and arginase 1 (Arg1) expression. In the presence of low IFN-γ, HIF-2α induced the expression of Arg1, reducing the production of NO and fostering a TH2 phenotype. Under conditions of high IFN-γ, HIF-1α dominated and iNOS was induced converting arginine into NO and promoting a TH1 phenotype.
Tumor recruitment of myelocytic cells is also regulated by B cells. Using a mouse model of squamous cell carcinoma induced by K-Ras expression in basal keratinocytes, Andrew Gunderson (Pennsylvania State University, University Park, PA) of Adam Glick’s laboratory reported that K-Ras activation led to cutaneous inflammation, including expansion of immunosuppressive myeloid cells. However, when B cells were deleted, myeloid suppressor cells were ablated, indicating the requirement of B cells to stimulate the recruitment and suppressive activity of these myeloid cells. Along these same lines, Tiziana Schioppa (Barts Cancer Institute, London, United Kingdom) from Frances Balkwill’s laboratory presented data showing that the clinical efficacy of anti–TNF-α therapy may, in part, be due to its effect on protumorigenic B cells (12). Development of 7,12-dimethylbenz(a)anthracene (DMBA)/12-_O_-tetradecanoylphorbol-l3-acetate (TPA)-induced papillomas was significantly reduced in either TNF-α– deficient or B-cell–deficient mice; transfer of B cells from DMBA-treated mice into TNF-α −/− recipient mice reinstated papilloma development. Notably, B regulatory cell expression of interleukin (IL)-10 was critically dependent on TNF-α expression, suggesting that anti–TNF-α therapy promotes antitumor immunity by suppressing B regulatory expression of IL-10.
ECM proteins also contribute to local immunoregulation. Sabina Sangaletti (Fondazione IRCCS Instituto Nazionale Tumori, Milan, Italy) from the laboratory of Mario Colombo showed that the matricellular secreted protein acidic rich in cysteine (SPARC) with profibrotic actions was expressed in remodeling tissues and in tumors and promoted TH1-type polarization by regulating expression and activation of TGF-β1 and in turn modulating macrophage production of TNF-α (13). In the absence of SPARC, macrophages did not down-modulate TNF-α in response to TGF-β1, and thus fostered fibrosis. SPARC could thus be a potential therapeutic target to render the TME unsuitable for cancer cell proliferation.
Galectins, a family of evolutionarily conserved glycan-binding proteins, play an important function in educating immune cells and controlling angiogenesis. Gabriel Rabinovich (Instituto de Biologica y Medicina Experimental, Buenos Aires, Argentina) discussed how galectin-1 associated with VEGFR2 in tumor-associated endothelial cell stimulated VEGFR2-mediated signaling and angiogenesis in the absence of VEGF (14). Accordingly, a monoclonal antibody (mAb) against galectin-1 inhibited growth of Kaposi sarcoma and B16 melanomas in mice with increased recruitment of TH17-type lymphocytes and decreased tumor vascularization.
In addition to polarization, programmed death-1 (PD-1), an inhibitory coreceptor expressed on T and B cells, plays an important role in providing immune-inhibitory signals in the TME. Drew Pardoll (Johns Hopkins University, Baltimore, MD) showed that PD-1 was expressed by activated T and B cells and monocytes and interacted with the ligand B7-H1 expressed by DCs and many tumor cells, providing them with adaptive resistance and an immune escape mechanism. Accordingly, a therapeutic mAb against B7H1 is currently being tested in a phase I clinical trial in patients with advanced solid tumors and preliminary data suggest clinical activity against melanoma and non–small cell lung cancer (15).
Nonimmune mesenchymal cells, such as fibroblasts, myofibroblasts, or adipocytes, play an important role in TMEs where they are “educated” by neoplastic cells. Frank Marini (Wake Forest Comprehensive Cancer Center, Winston-Salem, NC) used mice transplanted with EGFP-labeled BM cells to show that BM-derived mesenchymal progenitor cells (MPC) were recruited into primary tumors where they differentiated into cancer-associated fibroblasts (CAF), expressing fibroblast activation protein (FAP) and fibroblast-specific protein (FSP). The presence of these cells in the tumor affected growth and promoted immune escape. Deletion of CD44 led to a loss of FAP/FSP-producing cells in the tumor, suggesting that CD44 was critical for their recruitment. When in the TME, MPCs and CAFs interact with tumor cells by a variety of mechanisms. One mechanism is activation of the hedgehog (HH) pathway. Yunjung Choi (University of Michigan, Ann Arbor, MI) of Ronald Buckanovich’s laboratory showed that HH activation in tumoral stem cells led to induction of bone morphogenic protein (BMP)-2 and -4 (among other factors) that stimulate proliferation of ALDH+ovarian cancer stem cells. MPCs and CAFs are a source of multiple growth factors and chemokines/cytokines including IL-6 and FAP. Yves DeClerck (University of Southern California, Los Angeles, CA) reported that IL-6 expression in MPCs was increased in the presence of tumor (neuroblastoma) cells and that it activated STAT3, which by upregulating expression of survivin, Mcl-1, and BclxL in neoplastic cells, increased their resistance to cytotoxic drugs. Interestingly, TAMs collaborated with MPCs by being a source of the agonistic soluble IL-6 receptor enhancing STAT3 activation. Targeting CAFs may be an attractive therapeutic target; however, it may have a toxic effect as these cells are present in normal tissue. Ed Roberts (University of Cambridge, Cambridge, United Kingdom) from Douglas Fearon’s group provided data showing that FAP+cells were present in normal tissues and that their depletion in nontumor-bearing mice induced a state of cachexia and metabolic waste with a loss in skeletal muscle of follistatin, an inhibitor of procatabolic mediators. Using intravital microscopy and 3-dimensional cell–based models, Eric Sahai (London Research Institute, London, United Kingdom) showed that CAFs contributed to tumor cell migration by locally remodeling the ECM to generate routes used by “following” carcinoma cells (16). ECM remodeling by CAFs depended on Rho-Rock signaling that occurred under the influence of neoplastic cells via IL-6 that induced actomyosin polymerization in CAFs by STAT3 activation (17).
Genetic factors can also contribute to education of the stroma in cancer. Germ line mutations that affect formation of carcinomas such as in the case of familial adenomatous polyposis coli (FAPC) affect mesenchymal cells in the TME. For example, Monica Bertagnolli (Harvard Medical College, Boston, MA) presented data showing that in ApcMin/+ mice, Wnt signaling was also deregulated in mesenchymal cells and desmoid tumors formed as the result of a COX-2–dependent activation of the mesenchyme associated with an increased production of collagens (18). Furthermore, epigenetic factors may contribute. Benjamin Tycko (Columbia University, New York, NY) showed that in pancreatic intraepithelial neoplasia, there was a decrease in global methylation not only in malignant epithelial cells but also in CAFs. DNA methylation further decreased as lesions progressed from in situ to invasive carcinoma. Interestingly, in transgenic mice prone to develop pancreatic cancers, treatment with the hypomethylating agent 5-azacytidine led to a hypomethylation crisis associated with reduction in tumor growth and upregulation of a subset of IFN target genes affecting cell proliferation.
Emerging concept 3: The mechanisms of communication between tumor cells and the microenvironment are diverse: emerging role of exosomes and cell fusion
Two novel mechanisms potentially supporting the communication between tumor cells and stromal cells were the subject of presentations at the meeting. A first mechanism consisted of exosomes (19). Initially considered to be primarily responsible for release of unwanted material by cells, exosomes are now recognized as active entities involved in regulating a variety of extracellular signals. Exosomes have been isolated from the plasma of patients with cancer, and their concentration and protein content correlated with tumor stage and clinical outcome. David Lyden (Cornell University Weill Medical College, New York, NY) presented data suggesting that tumor exosomes, which package not only tumor antigens and immunosuppressive molecules but also miRs, were involved in mobilizing BM-derived cells to premetastatic niches (20). Preconditioning of BM cells with exosomes purified from metastatic melanoma cells, but not from non-malignant cells, transplanted into lethally irradiated recipient mice significant-ly increased metastasis. Tumor cells within the TME are not the sole source of exosomes, and Ngai-Na Chloe Co (University of Texas MD Anderson Cancer Center, Houston, TX) from Samuel Mok’s laboratory presented work showing that CAFs and cancer-associated adipocytes from ovarian tumors released miR21 containing exosomes that in co-culture, transferred miR21 to tumor cells promoting migration and invasion. Exosomes thus appear to be involved in a 2-way communication between tumor cells and stromal cells. A second newly reported mechanism of communication between tumor and stromal cells is cell fusion. Melissa Wong (Oregon Health and Science University) presented in vitro and in vivo data showing that functional fusion between TAMs and neoplastic cells occurred and altered the transcriptome by introducing the expression of macrophage-specific genes (21). These macrophage–carcinoma cell fusion hybrid cells may be more prone to migrate and metastasize due to their ability to mimic migratory behaviors of macrophages.
Emerging concept 4: The sentinel lymph node is an active part of the TME
Many tumors metastasize first to the sentinel lymph node after entering lymphatic vessels around the tumor. Although tumor-associated lymphatic vessels were previously considered passive transporters of fluid, molecules, and cells, the last decade has seen numerous reports correlating lymphatic growth factors in the TME with metastatic potential (22). Furthermore, observations that sentinel lymph nodes undergo lymphangiogenesis before metastasis led to the notion that lymph node lymphangiogenesis may be involved in the premetastatic niche. Five presentations illustrated how tumor-associated lymphatic vessels, lymph flow, and the sentinel lymph nodes promote immune tolerance and distant metastasis.
Michael Detmar (ETC Zurich Institute of Pharmaceutical Sciences, Zurich, Switzerland) discussed how podoplanin, expressed by lymph node stromal cells in the T-cell zone, can be present in the tumor stroma and how its presence correlated with tumor lymphangiogenesis (23). One mechanism by which podoplanin-induced lymphangiogenesis occurred is via endothelin-1 upregulation. In vitro, podoplanin increased tumor cell motility as well as lymphatic endothelial cell (LEC) migration and tubulogenesis. Podoplanin upregulation may be induced by increased lymph flow to the draining lymph nodes, which occurs in lymphangiogenic tumors unless metastasis is extensive enough to be obstructive (24). Interestingly, both increased lymph flow from the tumor as well as lymph node metastasis appear to depend on tumor-draining lymph node lymphangiogenesis, according to new evidence presented by Alanna Ruddell (Fred Hutchison Cancer Research Center, Seattle, WA). She showed that lymphangiogenesis in the tumor-draining lymph nodes was dependent on B cells and that normally metastatic tumors grown in B-cell–deficient mice failed to provoke lymph node lymphangiogenesis, to increase flow or metastasis. Furthermore, in Eμ-c-Myc mice with B-cell expansion in the lymph node, melanoma and lymphoma metastasis to the sentinel lymph node was increased and more rapid, and hematogeneous metastasis also increased (25). However, metastatic colonization of the same tumors after intravenous injection was unchanged in these mice, supporting the hypothesis that B-cell–driven lymph node lymphangiogenesis affects lymphatic spread of lymphoma and melanoma and that hematogeneous spread occurs after lymphatic spread.
Why does sentinel lymph node lymphangiogenesis promote metastasis? Melody Swartz (Swiss Federal Institute of Technology, Lausanne, Switzerland) presented data suggesting that lymphatic involvement by tumors and lymph node lymphangiogenesis promoted tolerance from host immunity (26). B16 melanoma expression of VEGF-C protected tumors against preexisting, vaccine-induced immunity. VEGF-C upregulated CCL21 in the tumor stroma, which attracted naive T cells and promoted their education in the regulatory chemokine environment (27). In addition, LEC in the sentinel lymph node could cross-present tumor antigen MHC class I molecules leading to CD8+T-cell deletion, supporting a new role for LECs in tolerance. On the other hand, CCL21 in tumors and lymphoid stroma drove antitumor as well as protumor effects by attracting naive and regulatory T cells along with antigen-presenting cells. David Peske (University of Virginia, Charlot-tesville, VA) from the laboratory of Victor Engelhart showed that CCL21 expression in the stroma of ovalbumin-expressing melanomas could attract adoptively transferred naive ovalbumin-specific CD8+T cells after adoptive transfer from T-cell receptor transgenic mice and activated them in the TMEs. In contrast, naturally arising host CD8+T cells proliferated from a rare population of naive CD8+T cells and existed in balance with regulatory T cells. These studies highlight the importance of context and timing for both antitumor immune responses and tumor tolerance to develop.
The session closed with Dontscho Kerjaschki (University of Vienna, Wien, Austria) describing new work on mammary carcinomas, whose lymph node metastasis correlated with lymphangiogenesis in the sentinel lymph nodes and with metastatic tumors but not with the primary tumor. Histopathology of invasive mammary tumors revealed large gaps in lymphatic vessels where tumor cells entered, and this was consistent with in vitro data showing tumor spheroids forming gaps in LEC monolayers. Invasive but not benign tumors induced this gap formation, and only in lymphatic but not blood endothelial cells, in a lipoxygenase-dependent manner. In mice with lipoxygenase knockdown, metastasis was prevented, even in VEGF-C–overexpressing tumors. These exciting new data identify lipoxygenase as a potential new drug target to prevent lymphatic spread of mammary carcinomas (28).
Emerging concept 5: The microbial flora can be friend or foe of cancer
The recent possibility of examining the entire microbiome in the gut is shedding new light on the role of commensal/pathogenic bacteria in cancer initiation and progression. One of the burning questions is how the microbiota regulates the inflammatory components of the TME and affects inflammation-associated carcinogenesis (29). The protumorigenic role of gut Helicobacter hepaticus in extra-intestinal carcinogenesis was discussed by Susan Erdman (Massachusetts Institute of Technology, Cambridge, MA) who reported that introduction of H. hepaticus to ApcMin/+Rag2−/− mice led to development of colitis and intestinal tumors but also to mammary gland tumors heavily infiltrated with macrophages (30). Systemic anti–TNF-α treatment or adoptive transfer of IL-10 producing CD4+T regulatory cells abolished both intestinal and mammary tumorigenesis. Because Rag2−/− mice lack both T and B cells, inflammation-associated carcinogenesis found in ApcMin/+ Rag2−/− mice was mediated by cells of the innate immune system. The presence of mammary tumors in these mice suggest that H. hepaticus inhabiting the gut elicits a systemic inflammatory reaction driven by innate immune cells that is also carcinogenic in tissues not directly in contact with the pathogens. Noriho Iida (NCI, Frederick, MD) from Giorgio Trinchieri’s group explored the potential ability of intestinal commensal bacteria to augment antitumor immune responses in treatment-induced acute inflammation. They used a model in which mice subcutaneously implanted with melanoma cells and treated with an anti–IL-10R antibody and intratumoral injection of CpG oligonucleotides developed an intratumoral hemorrhagic necrosis due to production of TNF-α by macrophages and exhibited prolonged survival. He showed that depletion of gut commensal bacteria by administration of antibiotics impaired production of TNF-α in the tumor and decreased survival in treated colon carcinoma and melanoma-bearing mice. Thus, proper activation of innate myeloid cells by CpG nucleotides requires an intact intestinal microbiota. These two presentations illustrate the opposite roles that the microbial flora may have on cancer initiation and progression as a function of the type of inflammation present.
Critical question 1: How should we include the TME in the initial evaluation of a tumor and in preclinical cancer models?
Typically, genomic and transcriptomic analyses of tumors are conducted on DNA and RNA extracted from entire tumors and it is generally assumed that genetic and epigenetic alterations and changes in gene expression observed reflect modifications in the tumor cells. This may not necessarily be the case. Morag Park (McGill University, Montreal, Quebec, Canada) reported results from an extensive transcriptomic analysis of breast cancer specimens in which samples were microdissected to separate stroma from malignant epithelium that were individually analyzed (31). She described stromal-specific signatures that allowed classification of breast cancers into 5 TME-based subgroups. Her laboratory also identified 26 stromal-derived genes that predicted outcomes with a power better than the 70 genes used in the mammaprint test (32). Furthermore, data generated from transgenic mice in which oncogenes were targeted in the mammary epithelium suggested that oncogenes produce distinct patterning changes in the mouse mammary stroma similar to those observed in human breast tumors.
Another important question is the development of preclinical cancer mouse models that take the TME into consideration. The important role played by immune cells in the TME indicates that xenotransplanted tumor models in immunodeficient mice are of limited value in the study of TME. Genetically engineered mice have the advantage to recapitulate an oncogenic event within the context of a competent immune system but they represent murine and not human cancer models. Alana Welm (University of Utah, Salt Lake City, UT) discussed a mouse model where fresh human breast cancer samples were xenografted into immunodeficient mice along with a component of the human stroma to achieve a high rate of engraftment and spontaneous metastasis (33). The pathology of these tumors faithfully reflected the organization of human tumors. Engraftment was also a prognosticator of outcome as women whose tumors engrafted had a poorer survival than women whose tumors did not engraft. Interestingly, although cotransplantation of a stromal component or of human mesenchymal stem cells increased the efficiency of the xenograft, the mouse stroma was quickly recruited to reshape the TME.
Critical question 2: What should be the strategies to target the microenvironment in cancer therapy?
A fundamental question raised by many of the presentations at the meeting is related to the application of our knowledge of the TME to the design of therapeutic strategies. Therapies solely targeted at the TME are unlikely to eradicate cancer, although they could have the potential to convert cancer into a chronic disease. The major focus of ongoing efforts have thus been on strategies that combine targeting tumor cells and the microenvironment and several presentations showed that targeting the microenvironment in combination with therapies aimed at tumor cells is a valuable approach. A first strategy is the use of agents blocking pathways responsible for the recruitment and activation of stromal cells in the TME as first shown for Avastin, that is now part of the armamentarium to combat colon cancer and glioblastoma (34). William Dougall (Amgen, Seattle, WA) presented data illustrating the efficacy of targeting receptor activator of NF-κB ligand (RANKL) in bone metastasis with denosumab, a fully human mAb against RANKL (35). Three phase III clinical studies with denosumab recently completed in patients with bone metastasis from breast cancer, castration-resistant prostate cancer (CRPC), and other advanced malignancies showed effective inhibition of bone remodeling and the superiority of denosumab over Zometa (zoledronic acid) in decreasing the number of skeletal-related events in these patients. Denosumab was also effective in delaying the development of bone metastasis in men with CRPC, showing that targeting the TME can also have a preventive effect on metastasis. RANKL is also a mediator of the mitogenic activity of progesterone in mouse mammary epithelium and pharmacologic inhibition of RANKL in progesterone-dependent mouse mammary tumors attenuated tumorigenesis. A second strategy is based on the concept that the TME modulates tumor susceptibility to therapy. Combination therapies that include agents targeting pathways affecting tumor cell resistance to drugs with standard chemotherapy or targeted therapy have garnered renewed excitement. William Dalton (H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL) discussed environment-mediated drug resistance (EMDR) as a mechanism where the interaction between tumor cells and the BM environment allows for discrete tumor populations to survive as minimal residual disease and emerge as drug-resistant clones (36). Interfering with these mechanisms may increase cancer response to therapy and prevent resistance. In myeloma, in which such resistance has been extensively studied, the mechanisms involve activation of specific pathways such as IL-6/STAT3, SDF1/CXCR4, Notch, or TRAIL and miR that provide tumor cells with a survival advantage. Several inhibitors of such pathways are currently in clinical trials. The ECM can also be a factor of therapeutic resistance and thus a target for therapeutic intervention. Sunil Hingorani (Fred Hutchinson Cancer Research Center) showed that the formation of a hyaluronic acid–rich stroma in pancreatic tumors resulted in unper-fused blood vessels that provided a drug-free sanctuary for tumor cells. Treatment of the stroma with hyaluronidase in transgenic mice prone to develop pancreatic cancer was followed by a rapid reperfusion of the blood vessels that led to decreased proliferation, increased apoptosis, increased response to therapy, and improved survival.
A third strategy consists of targeting protumorigenic inflammatory pathways, an approach taken by Frances Balkwill’s (Barts Cancer Institute) laboratory. She identified TNF, IL-6, and CXCL12 as a TNF network of 3 key cytokine mediators of cancer-related inflammation, having a paracrine action on angiogenesis, infiltration with myeloid cells, and Notch signaling. She reported that siltuximab, an anti-human IL-6 antibody inhibited IL-6 signaling (STAT3 activation) in cancer cells and had therapeutic effects in xenograft models (37). A phase II clinical trial of siltuximab as single agent in platinum-resistant ovarian cancer indicated that it was well-tolerated and had some therapeutic effects.
Conclusion
This conference illustrated well the high level of complexity of the TME and the challenges ahead in our attempt to ultimately identify therapeutic agents that target the TME. This aspect was illustrated by Michael Karin (University of California San Diego), who gave the closing keynote lecture. His work shows how in certain cases, inflammation can suppress antitumor immunity but can also be used to enhance the efficacy of cancer immunotherapy. Central to inflammation is NF-κB that can have pro- and anti-inflammatory functions (38). Evidence showing its activation in many inflammatory conditions associated with cancer such as inflammatory bowel disease, rheumatoid arthritis, or psoriasis raised enthusiasm about NF-κB and IKKβ as therapeutic targets in chronic inflammation, autoimmune diseases, and cancer. However, it was a disappointment to later realize that inhibition of NF-κB increased or even caused inflammation under certain circumstances. In cancer, NF-κB can have a pro- or antitumorigenic activity depending on the cancer type and also the mechanism of carcinogenesis involved. To understand this complexity will be critical to avoid premature testing in clinical trials of agents whose activity is not entirely understood, which could result in a premature abandonment of these agents as effective anti-cancer agents. We must remember the lessons learned from the clinical testing of inhibitors of matrix metalloproteinases in the late 1990s when the complexity of their role in cancer was not entirely appreciated at the time they were clinically tested (39). This is therefore a challenging but also a very exciting time for investigators studying the TME.
Acknowledgments
The authors thank Amy Baran, Amy Flynn, and Kacie Sheppeck for their assistance in the planning and conduct of the meeting. They also thank J. Rosenberg for her assistance in the preparation of the manuscript.
Grant Support
The meeting was supported by Genentech, Teva Pharmaceuticals, Amgen, Bristol Myers Squibb, and Hypoxygen, and a conference grant, R13 CA165812, from the NIH to Y.A. DeClerck and the AACR.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential confiicts of interests were disclosed.
Authors’ Contributions
Conception and design: M.A. Swartz, M.H. Wong, Y.A. DeClerck
Writing, review, and/or revision of the manuscript: M.A. Swartz, N. Iida, E. W. Roberts, S. Sangaletti, M.H. Wong, F.E. Yull, L.M. Coussens, Y.A. DeClerck
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): Y.A. DeClerck
Y.A. DeClerck chaired the editorial committee that was responsible for summarizing the presentations at the meeting. M.A. Swartz, N. Iida, E.W. Roberts, S. Sangaletti, M.H. Wong, F.E. Yull, and Y.A. DeClerck equally contributed to the summary of the various sessions of the meeting.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Tumor Microenvironment Network [homepage on the Internet] Bethesda, MD: NCI; Available from: http://tmen.nci.nih.gov/ [Google Scholar]
- 3.Borges VF, Schedin PJ. Pregnancy-associated breast cancer: an entity needing refinement of the definition. Cancer. 2011 Nov 15; doi: 10.1002/cncr.26643. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koehne CH, DuBois RN. COX-2 inhibition and colorectal cancer. Semin Oncol. 2004;31:12–21. doi: 10.1053/j.seminoncol.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 5.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol. 2011;12:308–19. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel LR, Camacho DF, Shiozawa Y, Pienta KJ, Taichman RS. Mechanisms of cancer cell metastasis to the bone: a multistep process. Future Oncol. 2011;7:1285–97. doi: 10.2217/fon.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dvorak HF, Weaver VM, Tlsty TD, Bergers G. Tumor microenvironment and progression. J Surg Oncol. 2011;103:468–74. doi: 10.1002/jso.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shurin GV, Ouellette CE, Shurin MR. Regulatory dendritic cells in the tumor immunoenvironment. Cancer Immunol Immunother. 2011;61:223–30. doi: 10.1007/s00262-011-1138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connelly L, Barham W, Onishko HM, Chen L, Sherrill TP, Zabuawala T, et al. NF-kappaB activation within macrophages leads to an anti-tumor phenotype in a mammary tumor lung metastasis model. Breast Cancer Res. 2011;13:R83. doi: 10.1186/bcr2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, et al. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662–7. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sangaletti S, Tripodo C, Cappetti B, Casalini P, Chiodoni C, Piconese S, et al. SPARC oppositely regulates inflammation and fibrosis in bleomycin-induced lung damage. Am J Pathol. 2011;179:3000–10. doi: 10.1016/j.ajpath.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soldati R, Berger E, Zenclussen AC, Jorch G, Lode HN, Salatino M, et al. Neuroblastoma triggers an immunoevasive program involving galectin-1-dependent modulation of T cell and dendritic cell compartments. Int J Cancer. 2011 Oct 23; doi: 10.1002/ijc.26498. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29:4828–36. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calvo F, Sahai E. Cell communication networks in cancer invasion. Curr Opin Cell Biol. 2011;23:621–9. doi: 10.1016/j.ceb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Sanz-Moreno V, Gaggioli C, Yeo M, Albrengues J, Wallberg F, Viros A, et al. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell. 2011;20:229–45. doi: 10.1016/j.ccr.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Carothers AM, Rizvi H, Hasson RM, Heit YI, Davids JS, Bertagnolli MM, et al. Mesenchymal stromal cell mutations and wound healing contribute to the etiology of desmoid tumors. Cancer Res. 2012;72:346–55. doi: 10.1158/0008-5472.CAN-11-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 20.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for premetastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21:139–46. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Powell AE, Anderson EC, Davies PS, Silk AD, Pelz C, Impey S, et al. Fusion between intestinal epithelial cells and macrophages in a cancer context results in nuclear reprogramming. Cancer Res. 2011;71:1497–505. doi: 10.1158/0008-5472.CAN-10-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2011 Dec 19; doi: 10.1038/onc.2011.602. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Kitano H, Kageyama S, Hewitt SM, Hayashi R, Doki Y, Ozaki Y, et al. Podoplanin expression in cancerous stroma induces lymphangiogenesis and predicts lymphatic spread and patient survival. Arch Pathol Lab Med. 2010;134:1520–7. doi: 10.1043/2009-0114-OA.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proulx ST, Detmar M. Watching lymphatic vessels grow by making them glow. Cell Res. 2011;22:12–3. doi: 10.1038/cr.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruddell A, Harrell MI, Furuya M, Kirschbaum SB, Iritani BM. B lymphocytes promote lymphogenous metastasis of lymphoma and melanoma. Neoplasia. 2011;13:748–57. doi: 10.1593/neo.11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund AW, Swartz MA. Role of lymphatic vessels in tumor immunity: passive conduits or active participants? J Mammary Gland Biol Neoplasia. 2010;15:341–52. doi: 10.1007/s10911-010-9193-x. [DOI] [PubMed] [Google Scholar]
- 27.Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010;328:749–52. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- 28.Kerjaschki D, Bago-Horvath Z, Rudas M, Sexl V, Schneckenleithner C, Wolbank S, et al. Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J Clin Invest. 2011;121:2000–12. doi: 10.1172/JCI44751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–31. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 30.Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4:22–30. doi: 10.1038/mi.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertos NR, Park M. Breast cancer - one term, many entities? J Clin Invest. 2011;121:3789–96. doi: 10.1172/JCI57100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glas AM, Floore A, Delahaye LJ, Witteveen AT, Pover RC, Bakx N, et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics. 2006;7:278. doi: 10.1186/1471-2164-7-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17:1514–20. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrara N. From the discovery of vascular endothelial growth factor to the introduction of avastin in clinical trials - an interview with Napoleone Ferrara by Domenico Ribatti. Int J Dev Biol. 2011;55:383–8. doi: 10.1387/ijdb.103216dr. [DOI] [PubMed] [Google Scholar]
- 35.Dougall WC. Osteoclast-dependent and-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res. 2012;18:326–35. doi: 10.1158/1078-0432.CCR-10-2507. [DOI] [PubMed] [Google Scholar]
- 36.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–74. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 37.Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA, et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17:6083–96. doi: 10.1158/1078-0432.CCR-11-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–23. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 39.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–92. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]