Trans-epithelial antigen delivery in the small intestine: different paths, different outcomes (original) (raw)
. Author manuscript; available in PMC: 2014 Mar 1.
Published in final edited form as: Curr Opin Gastroenterol. 2013 Mar;29(2):112–118. doi: 10.1097/MOG.0b013e32835cf1cd
Abstract
Purpose of the review
The intestinal epithelium is a dynamic barrier protecting the body from the multitudes of luminal micro-organisms present in the gut. However, this barrier is not impermeable and mechanisms exist that allow small amounts of antigen to traverse the epithelium in controlled manner to maintain tolerance and to mount immune responses. This review will summarize our current understanding of how luminal antigens traverse the small intestine epithelium without disrupting the epithelial barrier and how these antigen delivery pathways might influence the resulting immune responses.
Recent findings
Recent findings have revealed four pathways for trans-epithelial antigen delivery in the absence of barrier disruption. We propose that during homeostasis, antigen introduced through microfold (M) cells induces IgA responses, antigen delivered by goblet cell associated antigen passages (GAPs) contributes to peripheral tolerance, and antigen delivered by paracellular leak initiates immune responses in the MLN. In contrast dendritic cell transepithelial dendrites (TEDs) may play an important role in host protection during pathogen infection, but do not appear to play a role in antigen capture by lamina propria dendritic cells in the steady-state.
Summary
These observations indicate that the route by which antigen crosses the epithelium directs the outcome of the subsequent immune response.
Keywords: Epithelium, antigen, dendritic cells
Two roads diverged in a wood, and I
I took the one less traveled by,
And that has made all the difference
…Robert Frost
Introduction
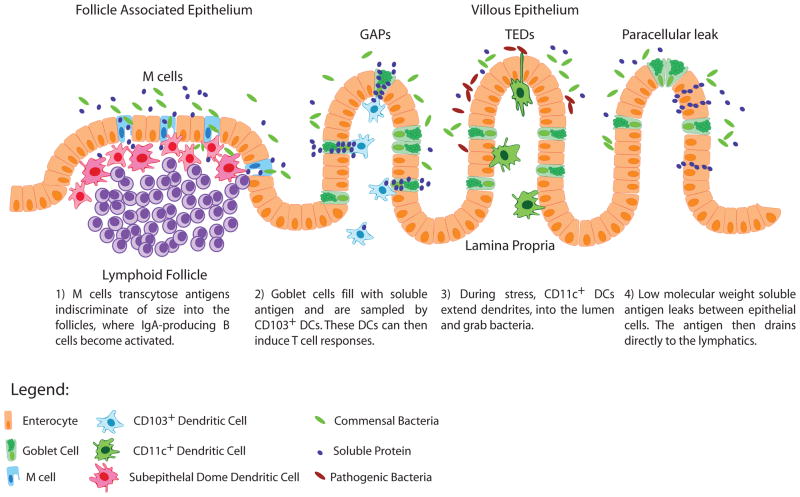
The principal function of the small intestine is the absorption of dietary nutrients. This role necessitates that the small intestine epithelium not be an absolute barrier isolating the nutrients and microbiota in the lumen from the body, and it dictates that the underlying mucosal immune system must continually monitor this environment to appropriately guide immune responses and maintain homeostasis. For antigens to be recognized by the mucosal immune system, they must be delivered to antigen presenting cells (APCs) with the capacity to deal with the different types of luminal antigens, as unchecked bacteria traversing the epithelium could quickly result in an infection, and inappropriate responses to food antigens could result in pathogenic inflammation. Furthermore these APCs must physically interact with lymphocytes to initiate and guide host protective immune responses. Studies have identified multiple pathways in which luminal antigen traverses the small intestine epithelium and can be captured by APCs. Moreover, APC subtypes have different capacities for microbial phagocytosis and killing, inflammatory cytokine secretion and tolerance induction. Incorporating recent findings with prior studies, we propose that each of these trans-epithelial antigen delivery pathways has a specific role in shaping the phenotype of intestinal immune responses (Figure 1).
Figure 1.
Models for trans-epithelial antigen delivery. The four described pathways of trans-epithelial antigen delivery that do not result in barrier disruption are shown incorporating their different locations, sizes of delivered antigen, cells involved, and proposed physiological outcomes. Not shown is antigen delivery via villous M cells, which is presumed to have similar characteristics to antigen delivery via M cells in the follicle associated epithelium.
M Cells: a pathway promoting IgA Responses
Microfold (M) cell-mediated transcytosis is the most studied pathway delivering antigen across the small intestine epithelium. M cells are differentiated epithelial cells commonly found in the follicle associated epithelium (FAE) that overlies Peyer’s patches (PPs) and isolated lymphoid follicles (ILFs) and less commonly on the villous epithelium [1,2]. M cells use pinocytosis, macropinocytosis, and receptor-mediated endocytosis [3,4] to continuously transcytose luminal antigens ranging from small soluble particles to whole bacteria. M cells deliver these antigens to dendritic cells (DCs) residing directly underneath the epithelium in subepithelial dome of the lymphoid tissue [5] or in the villous lamina propria (LP) [2]. Because the majority of M cells are found in the FAE overlying the PPs and ILFs, most of the antigen transcytosed by M cells is delivered to lymphoid tissues, and accordingly the role of M cells in intestinal immunity is intrinsically linked to the function of the mucosal lymphoid tissues. Multiple observations indicate that mucosal lymphoid tissues, and by extension M cells, support the production of IgA [6]. IgA is the most abundantly produced antibody isotype, and a large portion of IgA is secreted into the intestinal lumen. IgA is the prevailing immunoglobulin produced in response to a large variety of antigens: proteins, carbohydrates, and lipids, large or small, which can be introduced through M cells. PPs and ILFs house follicles containing cellular components of the immune system specialized for promoting IgA responses [7]. CD103+ DCs within PPs are better at inducing B-cells to become IgA producing plasma cells when compared to their systemic counterparts [8]. ILFs can support T cell independent IgA production via cytokines produced by lymphoid tissue inducer (LTi) cells and ILF DCs [9]. Moreover mice lacking PPs and ILFs have diminished luminal IgA and IgA producing plasma cells in the LP [10,11]. Together these observations indicate that antigen traversing the FAE via M cells in the homeostatic condition will be the target for IgA responses (Figure 1). Interestingly some enteric pathogens have usurped this pathway and use it as a portal of entry into the host [12]. Immune responses to these pathogens within PPs will obviously deviate from the homeostatic preference for IgA production [13,14].
The differentiation and function of M cells has been covered in great detail in other reviews [15,16], but several key discoveries have been made recently regarding the developmental requirements of M cells. First, Spi-B has been identified as the transcription factor controlling the development of M cells including the expression of M cell specific receptors [17,18]. Secondly, glycoprotein 2 (GP-2) has been shown to be a receptor for the FimH protein found in the outer membrane of enterobacteria like E. coli and Salmonella typhimurium [4]. In the intestine, GP2 is only found on the apical side of M cells, and is important for receptor-mediated endocytosis of bacteria [4]. Finally, it’s been shown the development of M cells is regulated by the TNF family cytokine member RANKL, Receptor Activator of NFκB Ligand, [19], which is found on the stromal cells directly below the epithelium in PP and ILF [20]. Epithelial cells express the receptor for RANKL, RANK, and M cells require the continued interaction between RANK and RANKL to develop on the follicle-associated epithelium, FAE [19]. Within the intestine, RANKL is found in the FAE of PP and ILF promoting M cells development over lymphoid structures providing a pathway for luminal antigen to be delivered for initiation of IgA responses.
The Spi-B−/− and RANKL−/− mice provide models to study the outcome of immune responses when M cells are absent, but PPs and ILFs are present, while the GP2−/− mice provide a model to study the outcome of immune responses when a specific receptor necessary for receptor mediated endocytosis by M cells is missing. RANKL−/− mice have decreased fecal IgA levels [19], and GP2−/− mice fail to mount specific IgA or CD4 T cells in response to salmonella typhimurium [4]. In Spi-B−/− mice, transferred CD4 T cells specific for Salmonella fail to proliferate in response to intralumenal infections with Salmonella typhimurium [4]. As these are all global knockouts, further studies need to be performed demonstrate that these outcomes are M cell intrinsic, but these experiments as well as the studies of PP and ILF functions indicate that antigen transported through M cells initiate IgA responses, including the generation of antigen specific CD4 T cells supporting IgA production.
Because of their antigen delivery function, M cells have been identified as an ideal target for developing oral-based vaccines to promote strong IgA responses [21] [22,23]. Indeed, targeting antigen to M cells by coating the antigen in carbohydrates that bind M cells, like NKM 16-2-4 [24] or UEA-I [25] results in antigen-specific IgA responses. Naturally, M cells may be able to reinforce IgA responses by binding and transcytosing IgA-antigen complexes, including antigen bound commensal bacteria, and transferring them to DCs [26,27], though it has not been shown if this results in higher affinity IgA responses.
Though IgA responses are initiated in the PPs, antigen delivered through M cells can also be found in the MLN. Transport of commensal Enterobacter cloacae across the epithelium occurs solely by PP M cell mediated transport [28]. However, after traversing the epithelium, E. cloacae can be found within PP and MLN DCs [29], but not in LP DCs, suggesting the bacteria delivered by M cells can be transported by PP DCs to the MLN. Moreover, the DCs that contained E. cloacae were able to initiate IgA production by B cells [29], consistent with M cell-mediated antigen delivery playing a central role in promoting IgA responses.
While the vast majority of M cells are found as part of the FAE, rare M cells can be found as part of the villous epithelium in a “diffuse” pattern as discrete individual cells or in some cases in a “dense” pattern covering the majority of the villus [2]. The diffuse villous M cells develop on less than 10% of the villi [19], and the dense villous M cells develop even more rarely on approximately 40–50 villi [2]. Since RANKL is found below the epithelium of ILFs [20] and is sufficient to induce M cell development [19], dense villous M cells could potentially overlay ILF, however this has yet to be determined. Like their PP counterparts, villous M cells are able to transcytose particles as large as whole bacteria [2,19], and pathogenic bacteria adhere preferentially to M cells, as opposed to enterocytes [2]. Due to the small number of naturally occurring villous M cells, their role in antigen acquisition in the LP for initiating immune responses to luminal antigen is largely unknown.
Goblet-cell-Associated Antigen Passages (GAPs): a pathway delivering antigen to lamina propria DCs to promote intestinal T cell responses at homeostasis
While M cells are the best studied transepithelial antigen delivery pathway, the FAE overlying PP and ILFs represents only a small proportion of the surface area of the intestine. The LP underlies the vast majority of the intestinal epithelium and contains a substantial population of DCs and T cells. Accordingly how antigen is introduced to the cellular immune system in the diffuse LP is an area significant interest.
Recently, a novel mechanism of antigen delivery in the small intestine was described [30], which starred an unexpected epithelial cell, the goblet cell (GC), suggesting there’s more to GCs than mucus secretion. In vivo two-photon imaging of mice showed that luminal fluorescent dextrans and small proteins such as bovine serum albumin and ovalbumin entered GCs and were passed to DCs residing in the LP beneath the epithelium, a phenomenon termed goblet cell associated antigen passages (GAPs). GAPs were common in a wide range of inbred mouse strains and were also present in healthy human small intestine. Intriguingly, not every GC appeared to function as GAPs in these experiments. This heterogenetity among GCs for forming GAPs could be explained by the dynamics of GC secretion, since GAP numbers increased dramatically when GC secretion was induced by cholinergic agonists. Although GAPs readily delivered a variety of low weight soluble antigens across the epithelium, transport of molecules larger than 70kD or inert beads as small as 0.02μm was inefficient, indicating that GAPs are best suited to deliver small soluble antigens, such as those derived from the diet. Since IgA responses against dietary antigens are not normally mounted [31], antigens delivered via GAPs may preferentially induce homeostatic responses such as the generation of T regulatory responses. Consistent with this idea, GAPs were found to preferentially deliver antigen to CD103+ LP DCs, which have unique ‘intestinal’ properties. In mice, CD103+ LP DCs, but not CD103− LP DCs can migrate to the MLN [32] to initiate immune responses. In addition, CD103+ LP DCs generate retinoic acid [33] [34], which is essential to imprint the expression of the gut homing receptors CCR9 and α4β7 on lymphocytes [35], to promote the generation of Foxp3+ T regulatory cells, and to promote IgA production [32–34,36]. Similar functions have been attributed to human CD103+ DCs [37], implicating a role for CD103+ DCs, and by extension GAPs, in the maintenance of intestinal immune homeostasis in humans. In addition, CD103+ LP DCs contained the goblet cell protein cytokeratin-18 despite the fact that the DCs did not express cytokeratin-18 mRNA, indicating that when the CD103+ DCs sample from GAPs they acquire both luminal antigens and goblet cell proteins [30]. Exactly how antigen and GC proteins are transferred to the CD103+ LP DCs is unclear, but observations suggest it is linked to the process of GC secretion [30] and can appear similar to the “nibbling” function observed in some DCs [38]. The significance of epithelial protein transfer to LP DCs is not known, but could be a mechanism to endow DCs with unique properties specific to the intestine. CD103+ LP DCs were able to induce antigen specific T cell responses following the acquisition of luminal antigen, indicating that antigens acquired by this pathway are functional to induce immune responses [30,32,37]. Importantly, when GCs were deleted, GAPs were absent and luminal antigen delivery to LP DCs was undetectable, indicating that GAPs are a major luminal antigen delivery pathway to LP DCs in the basal state.
Transepithelial Dendrites (TEDs): a pathway to sample potential pathogens
Several studies have reported that DCs can penetrate the epithelium with their dendrites and sample the lumen of the intestine, a phenomenon referred to as trans-epithelial dendrites (TEDs). When cultured on a model monolayer of intestinal epithelial cells, bone-marrow derived DCs expressed tight junction proteins and extended their dendrites through the the epithelium to sample bacteria [39]. Moreover, two-photon imaging of exteriorized small intestine demonstrated that CD11c-YFP expressing LP DCs formed TEDs in response to TLR signaling [40]. Whether TED formation is restricted to one LP DC population or is a property that all LP DCs is unclear. In a study examining DC sampling behavior in explanted intestines, the property of TED formation was found to be restricted to the CX3CR1+ monocytic DCs and driven by CX3CL1/Fractalkine expressed on epithelial cells [41]. However, other studies suggest a broader population of LP DCs can form TEDs and that this might be driven by other chemokines, such as CCL20 [40]. The phenomenon of TED formation may not be restricted to LP DCs. PP DCs, similar to CX3CR1+ LP DCs, can extend dendrites around M cells to capture bacteria or particles [42]. The size and chemical characteristics of material that are sampled via TEDs has not been systematically explored, since most studies have focused on investigating to the role of TEDs in sampling the luminal microbiota.
It was initially hypothesized that DCs used TEDs to monitor the the luminal contents and acquire antigen for the maintenance tolerance. However, this idea has subsequently been challenged by a number of studies. TEDs have been reported to increase after infection and with TLR-stimulation [43]. Moreover, a recent in vivo imaging study revealed that LP DC TED formation was absent in the healthy intestine and only rarely occurred following infection with Salmonella [30]. Additionally, as noted above, when GCs and GAPs were deleted, no antigen was delivered to LP DCs, indicating that GAPs, not TEDs are a major pathway delivering antigen to the LP DCs at homeostasis.
Though they may not contribute to the acquisition of luminal antigen in the homeostatic state, TEDs may be a mechanism for DCs to acquire potential pathogens and avoid impending infections. CX3CR1+ LP DCs, which can form TEDs, are an inflammatory population of intestinal APCs capable of initiating Th17 responses [44,45]. In contrast to the CD103+ LP DCs, CX3CR1+ LP DCs have poor migratory capacity, even when stimulated [32], suggesting that a function of these DCs could be to contain pathogens within the LP. In support of this, CX3CR1 deficient mice quickly succumb to Salmonella infection after oral challenge and show increased dissemination of bacteria to the liver [41,46]. During pathogenic infections, CX3CR1+ DCs could form TEDs, grab pathogens, and quickly initiate inflammatory responses before the barrier is breached (Figure 1).
Paracellular Leak: a direct pathway to the lymphatics
The intestinal epithelium has been described as a selective permeable barrier, allowing water, solutes, small molecules and ions through, yet preventing larger proteins from entering the body [47]. The epithelial monolayer maintains this protective characteristic through the formation of tight junctions between cells [48]. Tight junctions are comprised of multiple proteins binding the cells together, similar to plastic rings around a six-pack of soda cans [49]. Most of the tight junctions are sealed shut and only allow small molecules less than 4 angstroms to pass through pores in the claudin proteins [48]. However, paracellular leak can occur when larger pores form in the tight junctions due to the expression of different claudin proteins, allowing macromolecules like proteins and carbohydrates to traverse the epithelium [50]. Claudin-2 is one such tight-junction protein that is associated with increased epithelial permeability and is increased in patients with Crohn’s Disease and Ulcerative Colitis [50]. Most reports have linked states of inflammation to increased tight-junction permeability, which could augment the incidence of diarrhea [51]. In the IL-10−/− mouse model of spontaneous colitis, increased intestinal permeability measured as an increase in dietary sugars found in the urine, occurs prior to the onset of overt inflammation, suggesting that increased epithelial permeability may predispose mice to colitis[52].
Paracellular leak of low molecular weight dextran has been visualized in healthy intestine using real-time in vivo two-photon imaging [53]. Paracellular leak was seen to occur independently in some villi, passing between epithelial cells and collecting just beneath the epithelium. Interestingly, surrounding villi did not show evidence of paracellular leak, suggesting this phenomenon was localized and regulated, and not due to global tissue damage or inflammation associated with the imaging procedure. Dextran was flushed from the base of the epithelium over several minutes during tissue contraction and dextran appeared to flow through prelymphactic channels and lacteals in the villus [53], which drain to the MLN (Figure 1). In contrast to M cells and GAPs (above), paracellular leak preferentially delivered much smaller (< 10kD) soluble antigens and these substances were not captured efficiently by LP DCs as detected by two-photon imaging [30]. Once in the MLN, antigen delivered via paracellular leak could be picked up by resident DCs to initiate T cells responses. However, additional studies are needed to understand how paracellular leak is regulated locally and how antigens delivered via paracellular leak might be presented by DCs to influence immunity and tolerance.
Conclusions
The small intestine has multiple pathways that deliver a wide range of luminal antigens across the epithelium. Distinct transport pathways may provide and mechanism to deliver luminal antigens to DCs in a context specific manner and allow the immune system to respond appropriately to harmless antigens by inducing tolerance and potential pathogens by initiating protective host immune responses. Much remains to be known about how these pathways are regulated and altered during homeostasis and during stress and the contributions of each pathway to transepithelial antigen delivery and immune responses.
Bullet points.
- Four pathways deliver antigen across the small intestine epithelium without disrupting the barrier; M cells, goblet cell associated antigen passages (GAPs), trans-epithelial dendrites (TEDs), and paracellular leak.
- Each pathway preferentially delivers antigens with specific characteristics; M cells deliver particulates, GAPs deliver soluble antigens < 70 kD, TEDs deliver micro-organisms, and paracellular leak delivers low molecular weight solutes <10kD.
- Each pathway may be associated with specific immune outcomes; M cells with IgA production, GAPs with delivering antigen to CD103+ LP-DCs, TEDs with capturing potential pathogens, and paracellular leak with delivering antigen to the lymphatics.
Acknowledgments
This work was supported in part by NIH grants DK064798 (R.D.N.), AI077600 (M.J.M.), AI095550 (R.D.N. and M.J.M.), and DK097317 (R.D.N and M.J.M).
Footnotes
Conflicts of interest:
The authors declare no other conflicts of interest.
References
- 1.Bockman DE, Cooper MD. Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer’s patches. An electron microscopic study. Am J Anat. 1973;136:455–477. doi: 10.1002/aja.1001360406. [DOI] [PubMed] [Google Scholar]
- 2.Jang MH, Kweon MN, Iwatani K, Yamamoto M, Terahara K, Sasakawa C, Suzuki T, Nochi T, Yokota Y, Rennert PD, et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc Natl Acad Sci U S A. 2004;101:6110–6115. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyrer PC, Ruth Foxwell A, Kyd JM, Otczyk DC, Cripps AW. Receptor mediated targeting of M-cells. Vaccine. 2007;25:3204–3209. doi: 10.1016/j.vaccine.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 4**.Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, Kadokura K, Tobe T, Fujimura Y, Kawano S, et al. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. Through the demonstartion that an M cell specific receptor is required for endocytosis and sampling of bacteria, this work clearly describes the importance of M cells in mounting specific responses against enteric bacteria. [DOI] [PubMed] [Google Scholar]
- 5.Neutra MR, Frey A, Kraehenbuhl JP. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 6.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 7.Lorenz RG, Newberry RD. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann N Y Acad Sci. 2004;1029:44–57. doi: 10.1196/annals.1309.006. [DOI] [PubMed] [Google Scholar]
- 8**.Mora JR, Iwata M, Eksteen B, Song S-Y, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of Gut-Homing IgA-Secreting B Cells by Intestinal Dendritic Cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. This report was the first to show retinoic acid provided by intestinal Dendritic Cells is required to induce IgA producing B cells, and that RA promotes a gut homing phenotype within the B cells. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 11.Newberry RD, McDonough JS, McDonald KG, Lorenz RG. Postgestational lymphotoxin/lymphotoxin beta receptor interactions are essential for the presence of intestinal B lymphocytes. J Immunol. 2002;168:4988–4997. doi: 10.4049/jimmunol.168.10.4988. [DOI] [PubMed] [Google Scholar]
- 12.Sansonetti PJ, Phalipon A. M cells as ports of entry for enteroinvasive pathogens: Mechanisms of interaction, consequences for the disease process. Seminars in Immunology. 1999;11:193–203. doi: 10.1006/smim.1999.0175. [DOI] [PubMed] [Google Scholar]
- 13.Hutchings AB, Helander A, Silvey KJ, Chandran K, Lucas WT, Nibert ML, Neutra MR. Secretory Immunoglobulin A Antibodies against the σ1 Outer Capsid Protein of Reovirus Type 1 Lang Prevent Infection of Mouse Peyer’s Patches. Journal of Virology. 2004;78:947–957. doi: 10.1128/JVI.78.2.947-957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinoli C, Chiavelli A, Rescigno M. Entry Route of Salmonella typhimurium Directs the Type of Induced Immune Response. Immunity. 2007;27:975–984. doi: 10.1016/j.immuni.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000;16:301–332. doi: 10.1146/annurev.cellbio.16.1.301. [DOI] [PubMed] [Google Scholar]
- 16.Mach J, Hshieh T, Hsieh D, Grubbs N, Chervonsky A. Development of intestinal M cells. Immunol Rev. 2005;206:177–189. doi: 10.1111/j.0105-2896.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 17*.Kanaya T, Hase K, Takahashi D, Fukuda S, Hoshino K, Sasaki I, Hemmi H, Knoop KA, Kumar N, Sato M, et al. The Ets transcription factor Spi-B is essential for the differentiation of intestinal microfold cells. Nat Immunol. 2012 doi: 10.1038/ni.2352. This report, along with the next citation, identify the key transcription factor required for the differentiation of M cells, SpiB. A better genetic understading of M cells could lead to more robust vaccination strategies by targeting M cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.de Lau W, Kujala P, Schneeberger K, Middendorp S, Li VSW, Barker N, Martens A, Hofhuis F, DeKoter RP, Peters PJ, et al. Peyer’s Patch M cells derive from Lgr5+ stem cells require SpiB and are induced by RankL in cultured ‘organoids’. Molecular and Cellular Biology. 2012 doi: 10.1128/MCB.00434-12. This report, along with the previous citation, identify the key transcription factor required for the differentiation of M cells, SpiB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, Akiba H, Yagita H, Kiyono H, Williams IR. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol. 2009;183:5738–5747. doi: 10.4049/jimmunol.0901563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor RT, Patel SR, Lin E, Butler BR, Lake JG, Newberry RD, Williams IR. Lymphotoxin-independent expression of TNF-related activation-induced cytokine by stromal cells in cryptopatches, isolated lymphoid follicles, and Peyer’s patches. J Immunol. 2007;178:5659–5667. doi: 10.4049/jimmunol.178.9.5659. [DOI] [PubMed] [Google Scholar]
- 21.Jepson MA, Clark MA, Foster N, Mason CM, Bennett MK, Simmons NL, Hirst BH. Targeting to intestinal M cells. J Anat. 1996;189 (Pt 3):507–516. [PMC free article] [PubMed] [Google Scholar]
- 22.Brayden DJ, Baird AW. Apical membrane receptors on intestinal M cells: potential targets for vaccine delivery. Advanced Drug Delivery Reviews. 2004;56:721–726. doi: 10.1016/j.addr.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 23.Azizi A, Kumar A, Diaz-Mitoma F, Mestecky J. Enhancing Oral Vaccine Potency by Targeting Intestinal M Cells. PLoS Pathog. 2010;6:e1001147. doi: 10.1371/journal.ppat.1001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nochi T, Yuki Y, Matsumura A, Mejima M, Terahara K, Kim DY, Fukuyama S, Iwatsuki-Horimoto K, Kawaoka Y, Kohda T, et al. A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J Exp Med. 2007;204:2789–2796. doi: 10.1084/jem.20070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chionh Y-T, Wee JLK, Every AL, Ng GZ, Sutton P. M-cell targeting of whole killed bacteria induces protective immunity against gastrointestinal pathogens. Infect Immun. 2009 doi: 10.1128/IAI.01522-08. IAI.01522-01508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rey J, Garin N, Spertini F, Corthésy B. Targeting of Secretory IgA to Peyer’s Patch Dendritic and T Cells after Transport by Intestinal M Cells. The Journal of Immunology. 2004;172:3026–3033. doi: 10.4049/jimmunol.172.5.3026. [DOI] [PubMed] [Google Scholar]
- 27.Rol N, Favre L, Benyacoub J, Corthésy B. The role of secretory immunoglobulin A in the natural sensing of commensal bacteria by mouse Peyer’s patch dendritic cells. Journal of Biological Chemistry. 2012 doi: 10.1074/jbc.M112.405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Man AL, Prieto-Garcia ME, Nicoletti C. Improving M cell mediated transport across mucosal barriers: do certain bacteria hold the keys? Immunology. 2004;113:15–22. doi: 10.1111/j.1365-2567.2004.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 30*.McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA, Newberry RD, Miller MJ. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. This work describes a novel antigen acquistion pathway specific to the lamina properia of the intestine through goblet cells, and has major implications into how homeostatic responses to luminal antigen are initiated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strobel S, Mowat AM. Immune responses to dietary antigens: oral tolerance. Immunology Today. 1998;19:173–181. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 32.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. This report, along with the next citation, identified the intestinal CD103+ Dendritic cells as the population of DCs which stimulate regulatory T cell development necessary for intestinal homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Sun C-M, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. The Journal of Experimental Medicine. 2007;204:1775–1785. doi: 10.1084/jem.20070602. This report, along with the previous citation, identified the intestinal CD103+ Dendritic cells as the population of DCs which stimulate regulatory T cell development necessary for intestinal homeostasis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song S-Y. Retinoic Acid Imprints Gut-Homing Specificity on T Cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. This report was the first to identify the key molecule on intestinal Dendritic Cells, retinoic acid, that is required for imprint lymphocytes with a gut tropism phenotype. [DOI] [PubMed] [Google Scholar]
- 36.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell–mediated regulation of experimental colitis. The Journal of Experimental Medicine. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaensson E, Uronen-Hansson H, Pabst O, Eksteen B, Tian J, Coombes JL, Berg P-L, Davidsson T, Powrie F, Johansson-Lindbom B, et al. Small intestinal CD103+ dendritic cells display unique functional properties that are conserved between mice and humans. The Journal of Experimental Medicine. 2008;205:2139–2149. doi: 10.1084/jem.20080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic Cells Acquire Antigens from Live Cells for Cross-Presentation to CTL. The Journal of Immunology. 2001;166:3717–3723. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 39.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 40*.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. This study used two-photon imaging to show dendritic cells extending dendrites past the epithelium to acquire bacteria in the lumen. This study concludes TLR signaling through Myd88 is required for TED formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA, Vyas JM, Boes M, Ploegh HL, Fox JG, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. This study used two-photon imaging to show dendritic cells extending dendrites past the epithelium to acquire bacteria in the lumen. This study concludes TED formation is a function of the CX3CR1+ DCs. [DOI] [PubMed] [Google Scholar]
- 42.Lelouard H, Fallet M, de Bovis B, Méresse S, Gorvel JP. Peyer’s Patch Dendritic Cells Sample Antigens by Extending Dendrites Through M Cell-Specific Transcellular Pores. Gastroenterology. 2012;142:592–601.e593. doi: 10.1053/j.gastro.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 43.Vallon-Eberhard A, Landsman L, Yogev N, Verrier B, Jung S. Transepithelial Pathogen Uptake into the Small Intestinal Lamina Propria. The Journal of Immunology. 2006;176:2465–2469. doi: 10.4049/jimmunol.176.4.2465. [DOI] [PubMed] [Google Scholar]
- 44.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 45.Niess JH, Adler G. Enteric Flora Expands Gut Lamina Propria CX3CR1+ Dendritic Cells Supporting Inflammatory Immune Responses under Normal and Inflammatory Conditions. The Journal of Immunology. 2010;184:2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- 46.Nicoletti C, Arques JL, Bertelli E. CX3CR1 is critical for Salmonella-induced migration of dendritic cells into the intestinal lumen. Gut Microbes. 2010;1:131–134. doi: 10.4161/gmic.1.3.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Machen TE, Erlij D, Wooding FBP. PERMEABLE JUNCTIONAL COMPLEXES. The Journal of Cell Biology. 1972;54:302–312. doi: 10.1083/jcb.54.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight Junction Pore and Leak Pathways: A Dynamic Duo. Annual Review of Physiology. 2011;73:283–309. doi: 10.1146/annurev-physiol-012110-142150. This review clearly examines the field of paracellular leak and how different states of inflammation affects the dynamics of the tight junctions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.JMD The epithelial junction: bridge, gate, and fence. Physiologist. 1977;20:10–18. [PubMed] [Google Scholar]
- 50.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 51.Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. Targeted Epithelial Tight Junction Dysfunction Causes Immune Activation and Contributes to Development of Experimental Colitis. Gastroenterology. 2009;136:551–563. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut. 2009;58:41–48. doi: 10.1136/gut.2008.150888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller MJ, McDole JR, Newberry RD. Microanatomy of the intestinal lymphatic system. Annals of the New York Academy of Sciences. 2010;1207:E21–E28. doi: 10.1111/j.1749-6632.2010.05708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]