Long-Lived Poxvirus Immunity, Robust CD4 Help, and Better Persistence of CD4 than CD8 T Cells (original) (raw)
Abstract
The currently used smallpox vaccine is associated with a high incidence of adverse events, and there is a serious need for a safe and effective alternative vaccine. Here, we carried out a longitudinal evaluation of vaccinia virus-specific CD4 and CD8 T cells in smallpox-vaccinated individuals by using a highly sensitive intracellular cytokine staining assay. Our results demonstrate that, in addition to the CD8 response, the smallpox vaccinations raised a robust CD4 response with a Th1-dominant cytokine profile. These CD4 T cells were stable and exhibited only a twofold contraction between peak effector and memory phases compared with an approximate sevenfold contraction for CD8 cells. A significant proportion of vaccinated individuals lost detectable CD8 memory while maintaining CD4 memory. After a booster immunization, these individuals generated a robust CD8 response, which some of them rapidly lost. Thus, the current smallpox vaccine provides long-lasting CD4 help that may be critical for long-lived B-cell memory. We suggest that the provision of adequate CD4 help for CD8 and humoral effector functions will be critical to the success of the next generation of smallpox vaccines.
In May of 1980, the World Health Organization certified that smallpox had been eradicated from the world. This triumph is now threatened by bioterrorists reintroducing smallpox, against which vaccination is no longer routine (9, 11). The reintroduction of vaccination for smallpox has been countermanded by the relatively high incidence of adverse events associated with the currently used smallpox vaccine (7). Thus, for vaccination to become routine in an unexposed population, smallpox vaccines with fewer side effects need to be developed (23). Human smallpox is caused by the variola virus, which belongs to the family Poxviridae and the genus Orthopoxvirus. The natural smallpox infections were eliminated successfully worldwide by vaccination with the Orthopoxvirus relative, vaccinia virus. Impressively, eradication of smallpox was achieved with very little knowledge about the protective immunity raised after vaccination with vaccinia virus. A thorough analysis of protective immunity that is generated by the current smallpox vaccine and understanding the correlates for protection are critical for the development of safer vaccines against smallpox.
The correlates for protection against smallpox have not clearly been defined, but they likely involve both humoral and cellular immunity. Exposed individuals with high titers of vaccinia virus-specific neutralizing antibodies exhibit resistance to smallpox, demonstrating a critical role for humoral immunity in protection (14, 18). A recent study in mice evaluating the mechanisms of protection demonstrated that vaccinia virus-specific antibody responses are essential for protection (2). Evidence for a critical role of cellular immunity comes from studies in individuals with T-cell immunodeficiency. Such individuals are at high risk for developing disseminated vaccinia virus infection following smallpox vaccinations (16, 17).
The cellular immunity generated by smallpox vaccinations is long lasting and has been shown to persist for more than 35 years after primary vaccination (5, 13). However, little is known about the temporal dynamics and the differentiation into memory of CD4 and CD8 T cells following either primary or booster vaccination. Studies evaluating cellular immunity after primary vaccination have focused on vaccinia virus-specific CD8 T cells with certain epitope specificity (22) or used techniques that do not distinguish CD4 response from a CD8 response (6, 8, 23). These studies have suggested that the majority of primary response could be CD8 specific. In this report, we longitudinally evaluate the magnitude, kinetics, and effector function of vaccinia virus-raised CD4 and CD8 T cells by using the intracellular cytokine staining (ICS) assay. Our results demonstrate that a significant proportion of the cellular immunity generated by smallpox vaccinations is a CD4 response with a Th1-dominant profile. We also show that CD4 T cells show better persistence than CD8 T cells and can be detected for more than 55 years after vaccination.
MATERIALS AND METHODS
Study population.
A cohort of 20 vaccinees that were vaccinated against smallpox were studied. Sixteen of the volunteers were Native Americans and four were Southeast Asians. Sixteen of the volunteers had been vaccinated only once, in childhood, 30 years before this study. Thirteen of these were boosted with the current smallpox vaccine, Dryvax. Four volunteers received Dryvax vaccination for the first time. The Dryvax was given by scarification, as recommended by the Centers for Disease Control and Prevention. Ten unvaccinated controls were included in the study. All study volunteers were employees of Emory University. The study was approved by the Emory University Institutional Review Board, and signed informed consents were obtained from all individuals before enrollment in the study.
ICS assay.
ICS assays were performed as described previously with a few modifications (1, 20). Approximately 106 peripheral blood mononuclear cells (PBMCs) were stimulated in 5-ml polypropylene tubes in RPMI medium containing 10% fetal bovine serum (FBS), and anti-human CD28, anti-human CD49d (1 μg per ml each; Pharmingen, Inc., San Diego, Calif.) in a volume of 100 μl. Approximately 107 PFU of vaccinia virus strain WR (multiplicity of infection [MOI], 10) was added in a volume of 100 μl. This MOI was used based on a previous titration done for a volunteer who had been recently vaccinated. Initial experiments comparing both live and killed vaccinia virus as the stimulating agent revealed that live antigens are superior to killed preparations in terms of stimulating both CD4 and CD8 T-cell responses. After 12 h of incubation at 37°C, 900 μl of RPMI medium containing 10% FBS and monensin (10 μg/ml) was added to enrich the intracellular cytokine levels by blocking the secretion of cytokine, and cells were cultured for an additional 3 h at 37°C at an angle of 5 degrees. Cells were surface stained with fluorochrome-conjugated antibodies to CD8 (clone SK1; Becton Dickinson) at 8 to 10°C for 30 min, washed once with cold phosphate-buffered saline (PBS) containing 2% FBS, and fixed and permeabilized with Cytofix/Cytoperm solution (Pharmingen, Inc.). Cells were then incubated with fluorochrome-conjugated antibodies to human CD3 (clone UCHT1; Beckman Coulter), gamma interferon (IFN-γ) (clone B27; Pharmingen), and interleukin 2 (IL-2) (clone MQ1-17H12; Pharmingen) in Perm wash solution (Pharmingen) for 30 min at 4°C. To detect tumor necrosis factor alpha (TNF-α)- or IL-13-positive cells, antibody to TNF-α (clone Mab11; Pharmingen) or IL-13 (clone JES10-5A2; Pharmingen) was used, respectively, in place of IL-2. Cells were washed twice with Perm wash, washed once with plain PBS, and resuspended in 1% formalin in PBS. Approximately 200,000 lymphocytes were acquired on the FACScalibur and analyzed using FloJo software (Treestar, Inc., San Carlos, Calif.). Lymphocytes were identified based on their scatter patterns; CD3+, CD8− cells were considered CD4-positive T cells, and CD3+, CD8+ cells were considered CD8-positive T cells. Using this assay, we could detect vaccinia virus-specific CD4 and CD8 T cells at levels as low as 0.01% of the respective total cells. In unvaccinated controls, the frequencies of vaccinia virus-specific CD4 and CD8 T cells were below 0.01%.
ELISPOT analysis.
MULTISCREEN 96-well filtration plates (Millipore, Inc., Bedford, Mass.) were coated overnight with the respective anticytokine capture antibody at a concentration of 2 μg/ml in PBS at 8 to 10°C overnight. Plates were washed two times with RPMI medium and then blocked for 1 h with complete medium (RPMI containing 10% FBS) at 37°C. Plates were washed five more times with plain RPMI medium, and cells were seeded in duplicate in 100 μl of complete medium at concentrations ranging from 2 × 104 to 5 × 105 cells per well. Approximately 2 × 106 PFU of vaccinia virus strain WR (MOI, 2) was added in a volume of 100 μl in complete medium. This MOI was used based on a previous titration done for a volunteer who had been recently vaccinated. Cells were cultured at 37°C for about 36 h under a 5% CO2 atmosphere. Plates were washed six times with wash buffer (PBS with 0.05% Tween 20) and then incubated with 1 μg of respective biotinylated anticytokine antibody diluted in wash buffer containing 2% FBS. Plates were incubated for 2 h at 37°C and washed six times with wash buffer. Avidin-horseradish peroxidase (Vector Laboratories, Inc., Burlingame, Calif.) was added to each well and incubated for 60 min at 37°C. Plates were washed six times with wash buffer, and spots were developed with stable 1,4-diamino-2-butanone (DAB) used as substrate (Research Genetics, Inc., Huntsville, Ala.). Spots were counted by using an automated enzyme-linked immunospot (ELISPOT) reader (CTL). An ovalbumin peptide (SIINFEKL) was included as a control in each analysis. Background spots for the ovalbumin peptide were generally less than 5 for 5 × 105 PBMCs. This background, when normalized for 106 PBMCs, was less than 10. Only ELISPOT counts of twice the background level (≥20) were considered significant. The following capture and detection antibody pairs were used: anti-human IFN-γ capture antibody (Clone B27; Pharmingen) and anti-human IFN-γ detection antibody (clone 7-86-1; Diapharma Group, Inc., West Chester, Ohio); anti-human IL-4 capture antibody (clone MP4-25D2; Pharmingen) and anti-human IL-4 detection antibody (clone 860F10H12; Biosource International, Camarillo, Calif.); and anti-human IL-13 capture antibody (AF 213; R&D Systems, Minneapolis, Minn.) and anti-human IL-13 detection antibody (BAF 213; R&D Systems).
Statistical analysis.
A paired t test on log-transformed values was used to compare the magnitude of decreases in the frequencies of CD4 and CD8 T cells over time. The decreases were expressed as ratios of the week 2 to week 12 levels.
RESULTS
Robust CD4 and CD8 T-cell responses after primary vaccination.
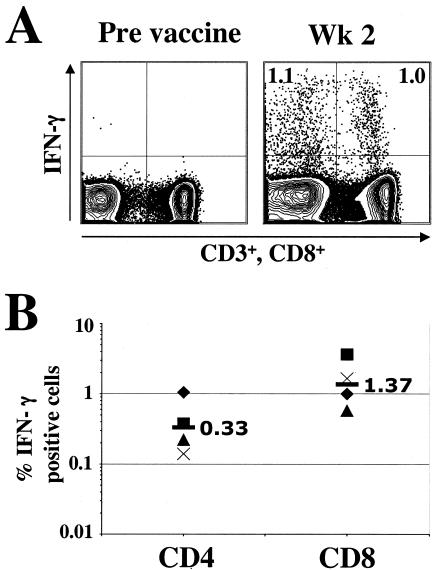
To evaluate the magnitude of vaccinia virus-specific cellular immunity following a primary vaccination, we used an ICS assay that allowed us to simultaneously evaluate CD4 and CD8 T-cell responses. Vaccinia virus-specific T cells were measured at 2 weeks postvaccination in four volunteers that were vaccinated with Dryvax (Fig. 1). The frequencies of vaccinia virus-specific CD4 and CD8 cells prior to vaccination in these individuals were below our detection limit (data not shown). At 2 weeks after vaccination, a robust expansion of vaccinia virus-specific CD4 and CD8 T cells was observed in all four volunteers (Fig. 1). At this time, the magnitude of vaccinia virus-specific CD4 cells ranged from 0.14 to 1.1% of total CD4 cells and had a geometric mean frequency of 0.33%; in addition, the magnitude of vaccinia virus-specific CD8 cells ranged from 0.4 to 3.7% of total CD8 cells and had a geometric mean frequency of 1.37%. In general, at the peak vaccine response, the magnitude of CD8 response was two- to fourfold higher than the magnitude of CD4 response.
FIG. 1.
Cellular immune responses after primary vaccination. (A) ICS assay to measure vaccinia virus-specific CD4 and CD8 T cells. PBMCs were stimulated with vaccinia virus as described in Materials and Methods and stained for CD3, CD8, IFN-γ, and IL-2. Cells were gated on lymphocytes based on the scatter pattern, analyzed for CD3 expression, and analyzed for the expression of CD8 and IFN-γ. Cells in the right quadrants represent CD8 cells, and those in the left quadrants represent CD4 cells (CD3 positive, CD8 negative). The frequencies in the upper quadrants are data for IFN-γ-producing cells expressed as the percentage of total CD4 cells (left quadrants) or total CD8 cells (right quadrants). (B) Frequency of vaccinia virus-specific CD4 and CD8 T cells at 2 weeks postvaccination. The numbers inside the graph represent the respective geometric mean values.
Vaccinia virus-raised CD4 help is Th1 dominant.
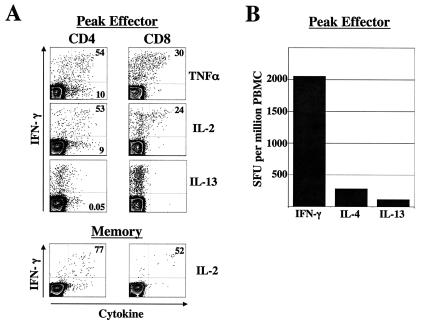
The quality of cellular immunity, in addition to the magnitude thereof, is critical for the control of many viral infections. We next characterized the peak vaccinia virus-specific CD4 and CD8 cells with respect to their ability to coproduce other Th1 and Th2 cytokines such as TNF-α, IL-2, and IL-13 during the peak effector (week 2) and memory (week 12) phases. At the peak, about 50% of the IFN-γ-positive CD4 cells (Fig. 2A, left panel) also produced TNF-α and IL-2, and only about 20 to 30% of IFN-γ-positive CD8 cells produced these cytokines (Fig. 2A, right panel). A small proportion of CD4 cells produced only TNF-α or IL-2, and this population was absent among CD8 cells. In addition, a small proportion of CD4 cells produced exclusively the Th2 cytokine IL-13, whereas none of the CD8 cells produced this cytokine. We also measured IFN-γ-, IL-4-, and IL-13-secreting cells by using an ELISPOT assay and obtained similar results (Fig. 2B). In this assay, the geometric mean frequency of IL-4- and IL-13-positive cells was 10 to 15 times lower than the geometric mean frequency of IFN-γ-positive cells. In the memory phase, the proportion of IFN-γ-positive cells that also produced IL-2 was higher than the peak effector cells, reaching frequencies greater than 70% (Fig. 2A). These results demonstrate that the primary response was biased toward type I cytokines and that the CD4 and CD8 cells had overlapping, yet distinctive, patterns of cytokine production.
FIG. 2.
Cytokine expression profile of vaccinia virus-specific T cells. (A) ICS analysis at 2 weeks (peak effector) and 12 weeks (memory) postvaccination. The numbers in the upper quadrants represent the frequencies of the respective cytokine-producing cells as the percentage of IFN-γ-producing cells, and the cells numbers in the lower quadrants represent the frequencies of the respective cytokine-producing cells expressed as the percentage of total CD4 cells. (B) ELISPOT analysis at 2 weeks postvaccination. Data represent the geometric mean frequency of three vaccinated individuals. SFU, spot-forming units.
Long-lived memory and preferential loss of CD8 cells.
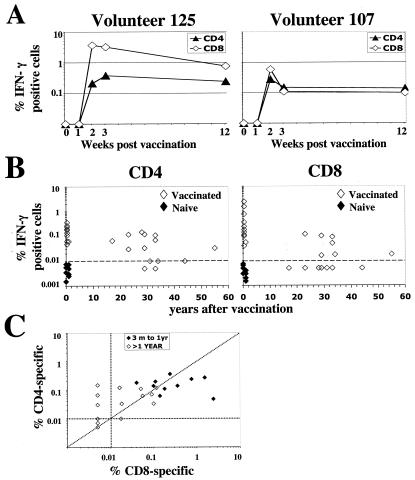
To better understand the temporal dynamics of vaccinia virus-specific CD4 and CD8 T cells, vaccinia virus-specific T cells were measured at various times early after vaccination in two of the four volunteers that received primary vaccination (Fig. 3A). Both CD4 and CD8 responses were not readily detectable at 1 week after vaccination. However, they were detectable by 2 weeks and peaked by 2 to 3 weeks after vaccination. By 12 weeks after vaccination, as the effector cells differentiated into memory cells, the CD8 response contracted about sevenfold, whereas the response of CD4 cells contracted less than twofold, suggesting that the dynamics of vaccinia virus-specific CD4 and CD8 T cell contraction are different.
FIG. 3.
Longevity of vaccinia virus-specific T-cell memory. (A) Longitudinal analysis of CD4 and CD8 T cells after primary vaccination in two individuals. (B) Cross-sectional analysis of vaccinia virus-specific CD4 and CD8 T cells over time. The solid symbols represent data for the responses in naïve individuals. (C) Comparison of vaccinia virus-specific CD4 and CD8 responses within each vaccinated individual.
To evaluate the longevity of memory CD4 and CD8 T cells, we measured vaccinia virus-specific T cells in 16 individuals that were vaccinated against smallpox 15 to 60 years before. Impressively, vaccinia virus-specific T cells were readily detectable in 14 out of the 16 individuals and remained detectable in an individual who had been vaccinated 55 years before the time of assay (Fig. 3B). The magnitude of vaccinia virus-specific CD4 cells ranged from 0.01 to 0.15% of total CD4 cells and had a geometric mean frequency of 0.03%; in addition, the magnitude of vaccinia virus-specific CD8 cells ranged from 0.01 to 0.09% of total CD8 cells and had a geometric mean frequency of 0.02%. Comparison of CD4 and CD8 responses within each individual revealed that while all positive individuals maintained detectable levels of CD4 response, a significant proportion of them (7 out of 14) selectively lost their CD8 response (Fig. 3B). The magnitude of CD4 and CD8 T-cell response in individuals that were vaccinated more than once were not significantly different when compared to one-time-vaccinated individuals (data not shown). In addition, between 3 months and 1 year after vaccination, the magnitude of CD8 response was generally higher than that of the CD4 response, whereas by 20 years after vaccination, the CD4 response showed better persistence than that of the CD8 response (Fig. 3C). These results indicate that vaccinia virus-specific immunity is long lived and suggest that a significant proportion of vaccinated individuals preferentially lose their CD8 memory.
Similar expansion of CD4 and CD8 cells but better persistence of CD4 following booster immunization.
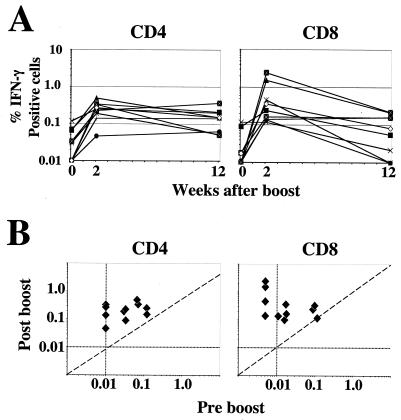
To learn more about the temporal dynamics of vaccinia virus-specific CD4 and CD8 T cells after booster vaccinations, we analyzed the magnitude of vaccinia virus-specific CD4 and CD8 T cells in 10 individuals that had received a booster vaccination (Fig. 4). Prior to the boost, all individuals had detectable levels of CD4 cells, whereas only six had detectable levels of CD8 cells. By 2 weeks after the boost, both CD4 and CD8 T cells underwent a robust expansion in all vaccinated individuals (Fig. 4A). At this time, the magnitude of CD4 cells ranged from 0.05 to 0.5% of the total CD4 cells and had a geometric mean frequency of 0.22%; in addition, the magnitude of vaccinia virus-specific CD8 cells ranged from 0.12 to 2.4% of the total CD8 cells and had a geometric mean frequency of 0.34%. No significant correlation was observed between the magnitude of the memory response prior to the boost and the magnitude of the peak effector response after the boost for CD4 or CD8 cells (Fig. 4B). In general, at 2 weeks after the boost, the magnitude of the CD8 response was, overall, similar to the magnitude of CD4 response. However, by 12 weeks after the boost, the CD8 responses had undergone a 5.5-fold contraction, whereas the CD4 cells had contracted less than twofold (P = 0.01). Interestingly, CD8 responses in some individuals had fallen below our detection limit by this time, despite being present at high frequencies at the peak response.
FIG. 4.
Cellular immunity after booster immunization. (A) Longitudinal analysis of vaccinia virus-specific CD4 and CD8 T cells after booster vaccination. Each symbol represents data for a boosted individual. (B) Comparison of the frequencies of vaccinia virus-specific T cells before and after the boost within each individual. The vertical and the horizontal dotted lines represent the sensitivity of the assay.
DISCUSSION
Our results clearly demonstrate that primary vaccinations against smallpox generate a robust and long-lived vaccinia virus-specific CD4 response. This response was strongly biased toward type 1 cytokines. Impressively, our studies also revealed the presence of long-lived vaccinia virus-specific CD4 T cells that persisted even 55 years after a primary vaccination. The ex vivo ICS assay used in our study revealed frequencies of vaccinia virus-specific CD4 cells of ∼0.03% of total CD4 cells or 20 specific cells per 65,000 CD4 cells. This estimate is 20 times higher than the calculated frequencies estimated in in vitro restimulation assays used in prior studies (5) but is similar to the results in a recent report that also described the detection of long-lived vaccinia virus-specific CD4 and CD8 T cells using an ICS assay (10).
Comparison of the magnitude of memory CD4 and CD8 T cells within individual vaccinees revealed that CD8 T cells were being selectively lost in a significant proportion of vaccinees by 20 years after vaccination. When boosted, these individuals could generate a robust CD8 response, indicating that they were not compromised in the ability to raise a CD8 response following smallpox vaccination. The kinetics of CD8 T-cell expansion after the boost suggested that this CD8 response might represent a primary response (detectable only by 2 weeks postvaccination) raised by the booster immunization rather than the expansion of memory CD8 cells (data not shown). Interestingly, some of these boosted individuals rapidly lost their CD8 response while maintaining the CD4 response.
Our longitudinal studies are consistent with a recently published cross-sectional study (10), strongly suggesting that vaccinia virus-specific CD4 cells show preferential persistence over vaccinia virus-specific CD8 T cells and that this phenomenon is more pronounced in some individuals than in others. In addition, our study is unique compared to the recently published and other previous studies for the following important reasons. (i) To our knowledge, our study represents the first study that evaluated the magnitude and cytokine expression profile of both CD4 and CD8 T cells at the peak response after vaccination. This analysis was critical (a) to demonstrate that the primary vaccinations raise robust Th1-dominated CD4 T cells in addition to the CD8 T cells and (b) to strongly suggest that the preferential loss of vaccinia virus-specific CD8 T cells may occur very early after vaccination and that this preferential loss may not be due to a difference in the half-life of memory CD4 and CD8 T cells. (ii) In our study, we measured the vaccinia virus-specific CD4 and CD8 T cells before and after a booster immunization from the same individuals and clearly demonstrated that those individuals that selectively lost CD8 T cells after the primary immunization could generate a robust CD8 T-cell response following booster immunization, a finding signifying that these individuals were not compromised in their ability to raise a CD8 response following smallpox vaccination.
What is the significance of this robust and persistent CD4 T-cell response primed by the smallpox vaccine? CD4 T cells play a crucial role in generating functional memory CD8 T cells (3). Recent experiments in mice demonstrate that CD8 T-cell priming in the absence of CD4 help results in generation of impaired CD8 memory (12, 19, 21). CD4 T cells also play a critical role in generation of B-cell memory (4, 15) and the affinity maturation of antibody. It is reasonable to speculate that the robust CD4 help that is primed during smallpox vaccinations is critical for the generation of long-lived CD8 and B-cell memory and contributes significantly to the protection against smallpox. In addition, our results strongly suggest that the approaches that are currently under development for an alternative vaccine for smallpox should carefully evaluate the magnitude and the quality of the CD4 as well as the CD8 and humoral responses.
Acknowledgments
We thank all volunteers that participated in the study for their generous enthusiasm and valuable time. We are indebted to Harriet L. Robinson for valuable suggestions throughout the course of the study and for critical reading of the manuscript. We thank James Herndon and Lakashmi Chennareddi for help with statistical analysis and Janet McNicoll and Inger Damon for helpful discussions. We are grateful to Maureen Thompson and Maureen Mittler for help with coordination of the study, and we thank Helen Drake-Perrow for outstanding administrative support. We are thankful to the Yerkes Division of Research Resources for the consistent excellence of veterinary care and pathology support.
This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases grant number R21 AI53488 and the Yerkes National Primate Research Center base grant P51 RR00165 to R.R.A.
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292**:**69-74. [DOI] [PubMed] [Google Scholar]
- 2.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 100**:**9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourgeois, C., H. Veiga-Fernandes, A. M. Joret, B. Rocha, and C. Tanchot. 2002. CD8 lethargy in the absence of CD4 help. Eur. J. Immunol. 32**:**2199-2207. [DOI] [PubMed] [Google Scholar]
- 4.Crotty, S., E. N. Kersh, J. Cannons, P. L. Schwartzberg, and R. Ahmed. 2003. SAP is required for generating long-term humoral immunity. Nature 421**:**282-287. [DOI] [PubMed] [Google Scholar]
- 5.Demkowicz, W. E., Jr., R. A. Littaua, J. Wang, and F. A. Ennis. 1996. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J. Virol. 70**:**2627-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ennis, F. A., J. Cruz, W. E. Demkowicz, Jr., A. L. Rothman, and D. J. McClain. 2002. Primary induction of human CD8+ cytotoxic T lymphocytes and interferon gamma-producing T cells after smallpox vaccination. J. Infect. Dis. 185**:**1657-1659. [DOI] [PubMed] [Google Scholar]
- 7.Enserink, M. 2002. Bioterrorism. In search of a kinder, gentler vaccine. Science 296**:**1594.. [DOI] [PubMed] [Google Scholar]
- 8.Frey, S. E., F. K. Newman, J. Cruz, W. B. Shelton, J. M. Tennant, T. Polach, A. L. Rothman, J. S. Kennedy, M. Wolff, R. B. Belshe, and F. A. Ennis. 2002. Dose-related effects of smallpox vaccine. N. Engl. J. Med. 346**:**1275-1280. [DOI] [PubMed] [Google Scholar]
- 9.Gani, R., and S. Leach. 2001. Transmission potential of smallpox in contemporary populations. Nature 414**:**748-751. [DOI] [PubMed] [Google Scholar]
- 10.Hammarlund, E., M. W. Lewis, S. G. Hansen, L. I. Strelow, J. A. Nelson, G. J. Sexton, J. M. Hanifin, and M. K. Slifka. 2003. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 9**:**1131-1137. (First published 17 August 2003; 10.1038/nm917.) [DOI] [PubMed] [Google Scholar]
- 11.Henderson, D. A. 1999. The looming threat of bioterrorism. Science 283**:**1279-1282. [DOI] [PubMed] [Google Scholar]
- 12.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421**:**852-856. [DOI] [PubMed] [Google Scholar]
- 13.Littaua, R. A., A. Takeda, J. Cruz, and F. A. Ennis. 1992. Vaccinia virus-specific human CD4+ cytotoxic T-lymphocyte clones. J. Virol. 66**:**2274-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mack, T. M., J. Noble, Jr., and D. B. Thomas. 1972. A prospective study of serum antibody and protection against smallpox. Am. J. Trop. Med. Hyg. 21**:**214-218. [DOI] [PubMed] [Google Scholar]
- 15.McHeyzer-Williams, M. G., and R. Ahmed. 1999. B cell memory and the long-lived plasma cell. Curr. Opin. Immunol. 11**:**172-179. [DOI] [PubMed] [Google Scholar]
- 16.O'Connell, C. J., D. T. Karzon, A. L. Barron, M. E. Plaut, and V. M. Ali. 1964. Progressive vaccinia with normal antibodies: a case possibly due to deficient cellular immunity. Ann. Intern. Med. 60**:**282-289. [DOI] [PubMed] [Google Scholar]
- 17.Redfield, R. R., D. C. Wright, W. D. James, T. S. Jones, C. Brown, and D. S. Burke. 1987. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N. Engl. J. Med. 316**:**673-676. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar, J. K., A. C. Mitra, and M. K. Mukherjee. 1975. The minimum protective level of antibodies in smallpox. Bull. W. H. O. 52**:**307-311. [PMC free article] [PubMed] [Google Scholar]
- 19.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300**:**337-339. [DOI] [PubMed] [Google Scholar]
- 20.Speller, S. A., and A. P. Warren. 2002. Ex vivo detection and enumeration of human antigen-specific CD8+ T lymphocytes using antigen delivery by a recombinant vaccinia expression vector and intracellular cytokine staining. J. Immunol. Methods 262**:**167-180. [DOI] [PubMed] [Google Scholar]
- 21.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300**:**339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terajima, M., J. Cruz, G. Raines, E. D. Kilpatrick, J. S. Kennedy, A. L. Rothman, and F. A. Ennis. 2003. Quantitation of CD8+ T cell responses to newly identified HLA-A*0201-restricted T cell epitopes conserved among vaccinia and variola (smallpox) viruses. J. Exp. Med. 197**:**927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weltzin, R., J. Liu, K. V. Pugachev, G. A. Myers, B. Coughlin, P. S. Blum, R. Nichols, C. Johnson, J. Cruz, J. S. Kennedy, F. A. Ennis, and T. P. Monath. 2003. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat. Med. 9**:**1125-1130. [DOI] [PubMed] [Google Scholar]