Actin Dynamics in Growth Cone Motility and Navigation (original) (raw)
. Author manuscript; available in PMC: 2015 Apr 1.
Published in final edited form as: J Neurochem. 2013 Nov 17;129(2):221–234. doi: 10.1111/jnc.12506
Abstract
Motile growth cones lead growing axons through developing tissues to synaptic targets. These behaviors depend on the organization and dynamics of actin filaments that fill the growth cone leading margin (peripheral (P-) domain). Actin filament organization in growth cones is regulated by actin-binding proteins that control all aspects of filament assembly, turnover, interactions with other filaments and cytoplasmic components, and participation in producing mechanical forces. Actin filament polymerization drives protrusion of sensory filopodia and lamellipodia, and actin filament connections to the plasma membrane link the filament network to adhesive contacts of filopodia and lamellipodia with other surfaces. These contacts stabilize protrusions and transduce mechanical forces generated by actomyosin activity into traction that pulls an elongating axon along the path towards its target. Adhesive ligands and extrinsic guidance cues bind growth cone receptors and trigger signaling activities involving Rho GTPases, kinases, phosphatases, cyclic nucleotides and [Ca++] fluxes. These signals regulate actin binding proteins to locally modulate actin polymerization, interactions and force transduction to steer the growth cone leading margin towards the sources of attractive cues and away from repellent guidance cues.
Keywords: actin, growth cone, actin binding proteins, axon guidance
Introduction
At the tip of a growing axon is a motile growth cone, which was named in 1890 by the Nobel Laureate Santiago Ramon y Cajal. Cajal brilliantly recognized that growth cones navigate through developing tissues to their targets (Sotelo, 2002). This article discusses actin dynamics in growth cones, including actin filament (F-actin) functions in growth cone motility, actin binding proteins (ABPs) that regulate actin dynamics, and signaling mechanisms that mediate growth cone steering by extrinsic cues.
Growth Cone Behavior
The obvious activity of a growth cone is persistent protrusion and withdrawal of finger-like filopodia and broad lamellipodia from the actin-rich growth cone leading margin, called the peripheral or P-domain (Figure 1; Video 1). New protrusions bear membrane receptors at their tips that detect locally expressed adhesive ligands and extrinsic guidance cues. Although most filopodia range from 5-20 μm long, the small fraction that extend as far as 50 μm and longer suggests that individual growth cones can sample large area of their environment (Letourneau, 1979). These sensory functions of filopodia and lamellipodia are key to growth cone advance and navigation. Adhesive contacts stabilize the advancing P-domain, and coupled with the detection of guidance cues, provides the local signals that trigger growth cone turning for navigation. The constant advance and searching behavior of growth cones depends on the dynamic assembly, turnover, organization and protein associations of actin filaments in the P-domain (Dent et al., 2011; Lowery and van Vactor, 2009; Vitriol and Zheng, 2012; these reviews contain excellent schematic drawings).
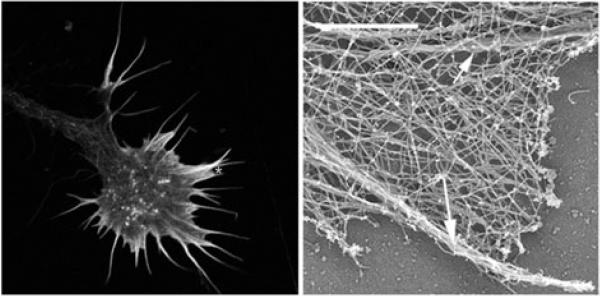
Figure 1.
Left panel is a confocal microscope image of a growth cone of a chick dorsal root ganglion neuron showing F-actin labeled with fluorescent phalloidin. Actin filaments fill the filopodia and small veil-like lamellipodia of the growth cone leading margin. The right panel is an electron micrograph of the branched network and bundles of actin filaments in the growth cone periphery in an area similar to that marked with an asterisk in the left panel. Right image is courtesy of Dr. Lorene Lanier, University of Minnesota.
Actin Dynamics and Actin Binding Proteins
Two aspects of actin filament dynamics are particularly significant to growth cone motility. 1) The polymerization and recycling of actin filaments provides the protrusive forces for the exploratory filopodia and lamellipodia. 2) Actin filaments in the P-domain interact with the motor protein myosin II to generate traction forces that pull the growth cone forward against adhesions and steer growth cone turning. The effects of inhibitors of actin polymerization and myosin II activity indicate that these actin functions are not essential for axonal elongation (Marsh and Letourneau 1984; Turney and Bridgman 2005), which still proceeds by microtubule advance and plasma membrane expansion. However, without actin dynamics, axonal elongation is slow and unresponsive to extrinsic cues. A growth cone without F-actin control is like a runaway vehicle without a driver to operate the brake, accelerator and steering wheel.
Growth cones are rich in actin, with a cytoplasmic concentration up to 100 μM, which is much higher than the 0.1 μM critical concentration at which actin filaments spontaneously polymerize (Pollard et al. 2003). Yet, about 50% of growth cone actin is unpolymerized monomer, because of the abundance of actin binding proteins (ABPs) that regulate every aspect of actin dynamics and organization (Korn, 1982; Pak et al. 2008; Revenu et al. 2004). ABP functions can be grouped into various roles. Actin polymerization and depolymerization are regulated by ABPs that nucleate actin polymerization, that bind to G-actin (globular) monomers, to barbed (+) F-actin ends, to pointed (−) F-actin ends, along F-actin, and that sever F-actin. F-actin organization is regulated by ABPs that crosslink F-actin into networks, create branched F-actin arrays, and bind F-actin into linear bundles. Actin interactions with other components are regulated by ABPs that bind F-actin to membrane proteins, microtubules, vesicles, and scaffolding proteins. F-actin-mediated mechanical force is generated by myosin motor proteins. The functions of ABPs in growth cones are discussed in the context of the motile activities of growth cone protrusion, regression and adhesion. This review does not include all ABPs identified in growth cones, but covers the roles that ABPs play in growth cone actin dynamics (See Table 1 in Dent and Gertler, 2003 and Table 1 in Dent et al., 2011 for extensive tables of ABPs in growth cones).
Roles of ABPs in Filopodial and Lamellipodial Protrusion
The force driving filopodial and lamellipodial protrusion is actin polymerization that pushes the plasma membrane forward at the growth cone leading margin, the P-domain, (Carlier and Pantaloni, 2007; Mogilner and Oster 2003; Pollard and Borisy, 2003; Yarmola and Bubb, 2009). This actin polymerization requires G-actin monomers and free F-actin barbed ends, where monomers are added (Figure 2). G-actin monomer availability at the leading margin involves two actin monomer binding proteins (Kiuchi et al. 2011; Lee et al. 2013). Profilin binds ADP-G-actin released from actin filament pointed ends, speeds nucleotide exchange to ATP-G-actin, and through protein-protein interactions, profilin-ATP-G-actin concentrates at the leading edge, making ATP-G-actin readily available for polymerization. ß-thymosin binds ATP-G-actin in a non-polymerizable form, but readily releases ATP-G-actin when the free concentration drops (Kiuchi et al. 2011). This sequestration may facilitate maintenance of a high ATP-G-actin concentration at the leading margin (Lee et al. 2013).
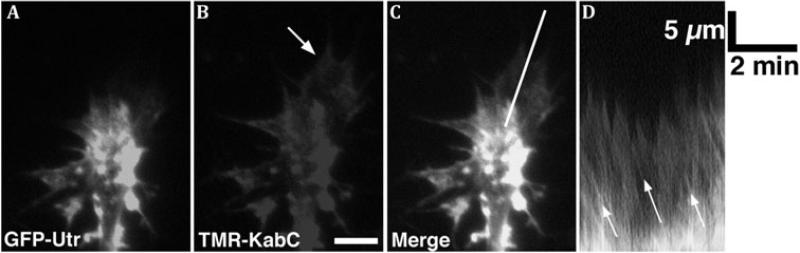
Figure 2.
A,B. GFP-Utrophin (GFP-Utr; F-actin probe) and TMR-Kabiramide C (TMR-KabC; F-actin barbed end binding) fluorescent images of a Xenopus growth cone on PDL-laminin collected with a TIRF microscope. Note bright TMR-KabC fluorescence at the periphery (arrow in B), indicating a high concentration of F-actin barbed ends. C. Merged image of the growth cone in (A,B) shows strong co-localization of GFP-Utr and TMR-KabC in central domain, but primarily TMR-KabC in the peripheral domain. Weak labeling of peripheral actin with GFP-Utr is consistent with the slow association rate of GFP-Utr onto recently polymerized F-actin. D. Single line kymograph constructed from region indicated by the white line in (C). The slope of the diagonal bands of GFP-Utr and TMR-KabC fluorescence (arrows) indicates a retrograde flow rate of ~ 4 μm/min. Scale bars, 5 μm or as indicated. With permission from Santiago-Medina et al. (2012) Dev. Neurobiol. 72, 585-599.
ABPs regulate the availability of free F-actin barbed ends at the leading margin. Capping proteins, like capZ, bind barbed ends and block monomer addition (Dent et al. 2011). When motility is low or when the P-domain retreats, barbed ends may be capped. Barbed end capping is inhibited by ABPs, such as ena, Vasp, and Evl (Bear et al., 2002; Breightsprecher et al., 2008; Dent et al, 2011). The anti-capping activity of these proteins is associated with continued polymerization of actin filaments, especially in filopodia. Two types of ABPs, Arp2/3 complex (Rotty et al. 2013) and formins (Kovar et al. 2006; Romero et al. 2004), nucleate G-actin to create new barbed ends for polymerization. The Arp2/3 complex binds at the side of an existing actin filament and nucleates a new filament that branches from the mother filament. Formins capture several actin monomers to nucleate a new filament and remain bound to the barbed end to facilitate polymerization of long filaments. Both Arp2/3 and formin individually bind profilin-ATP-G-actin, enhancing G-actin availability for nucleation. Besides the nucleation of new filaments, new barbed ends are created by the severing of existing actin filaments by the closely related ABPs, actin depolymerizing factor (ADF) and cofilin, which localizes to the growth cone leading edge (Dent et al., 2011; Marsick et al., 2012a; Sarmiere and Bamburg, 2004;). Excess ADF/cofilin activity can promote extensive filament breakdown, however, when G-actin concentrations are high, balanced ADF/cofilin activity can create limited F-actin severing, and the increase in barbed ends will trigger accelerated actin polymerization and protrusion.
Filopodial and lamellipodial shapes are determined by the relative activities of ABPs that regulate actin polymerization and filament interactions. High activities of the Arp2/3 complex and barbed end capping proteins lead to the highly branched arrays of short actin filaments that are assembled in lamellipodia (Pollard and Borisy, 2003). Filopodial protrusion is associated with nucleation by formins, which remain associated with barbed ends, together with anti-capping proteins Ena, Vasp and Evl, to allow polymerization of individual filaments to be maintained during filopodial protrusion (Dent et al. 2011; Mellor, 2010). However, protrusion shapes are influence by many factors, and Arp2/3-mediated actin nucleation may also contribute to filopodial protrusion (Mellor, 2010; Yang and Svitkina, 2011). ABPs that interconnect actin filaments also influence the shapes of protrusions. In the highly branched actin networks of lamellipodia the elongated actin crosslinking protein filamin can interconnect and stabilize Arp2/3-mediated dendritic arrays. In filopodia the bundling of long actin filaments by a short F-actin crosslinker, such as fascin, stabilizes the filament core of elongating filopodia.
The effective transformation of actin polymerization into protrusion involves F-actin connections to the plasma membrane. Several ABPs mediate F-actin links to membrane components. The ERM (ezrin-radixin-moesin) proteins bind F-actin to several plasmalemmal proteins. ERM proteins are concentrated in filopodia and lamellipodia, and L1, a major neuronal adhesion molecule, is a key partner in ERM-mediated linkage of F-actin to the plasmalemma (Figure 3; Marsick et al. 2012b, Mintz et al., 2003; Sakurai et al. 2008). When ERM proteins are knocked down or blocked, filopodial and lamellipodial protrusion is greatly reduced on L1 and other substrata (Marsick et al. 2012b). IRSp53 is another membrane-associated protein that localizes to the leading edge of filopodia and lamellipodia (Scita et al. 2008). The IRSp53 family includes multi-domain proteins that bind actin filaments, plasmalemmal phospholipids, and Rho GTPases, and their modulators, which regulate actin polymerization. IRSp53 and related proteins have important roles in mediating extrinsic regulation of growth cone motility.
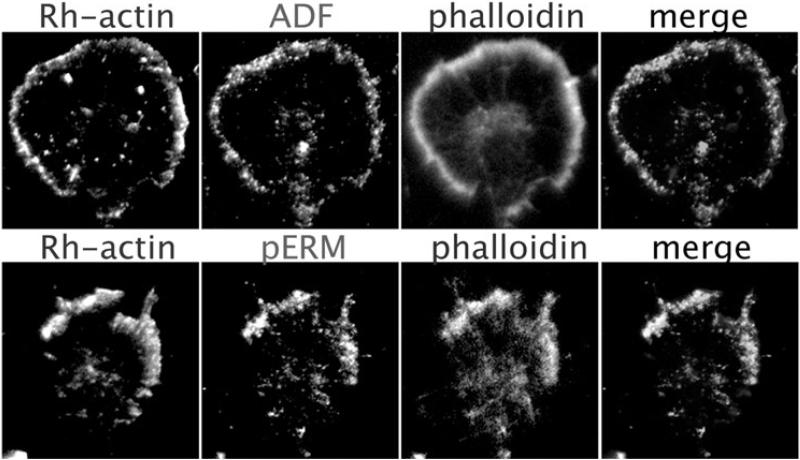
Figure 3.
Immunofluorescent images of two dorsal root ganglion growth cones multiple-labeled to show (upper panels) F-actin barbed ends (Rh-actin), ADF/cofilin, and F-actin (phalloidin) or (lower panels) barbed ends (Rh-actin, phosphorylated ERM proteins (pERM) and F-actin (phalloidin) in the growth cone leading margin after global exposure to an attractive guidance cue. With permission from Marsick et al., (2012b) J. Neurosci. 32, 282-196.
F-actin linkage to other growth cone receptors is mediated by distinct macromolecular complexes. For example, F-actin is coupled to the adhesion molecule N-cadherin by a complex of alpha- and ß-catenin (Bard et al. 2008; Giannone et al. 2009). Integrin receptors mediate growth cone adhesions to extracellular matrix molecules like laminin and fibronectin. Several ABPs are involved in linking F-actin to integrins. Talin plays a key role by directly connecting integrins to F-actin (Bard et al. 2008; Myers et al. 2011; Vicente-Manzanares et al. 2009). Other critical adaptors include vinculin, which binds F-actin and talin, while also binding PIP2 in the inner plasmalemmal layer, and alpha - actinin, which binds F-actin, vinculin, and also PIP2 in the plasmalemma. In addition, paxillin is a key adaptor protein in adhesions that is regulated by tyrosine phosphorylation (see below). Localized inactivation of talin and vinculin in filopodia interferes with filopodial extension (Sydor et al. 1996).
Roles of ABPs in F-actin Turnover and Force Generation
Turnover of actin filaments at the leading edge is equally important to growth cone motility. Most filopodia and lamellipodia are eventually withdrawn. At the side of a growth cone, protrusion ceases, as the P-domain is transformed to the central body of a growth cone (C-domain). To sustain protrusion and maintain growth cone shape, F-actin polymerized at the leading margin is recycled to release G-actin for re-polymerization at the leading margin. Actin polymerization is confined to the leading edge, but depolymerization occurs throughout the actin network, turning over the entire network within a few minutes (Van Goor et al. 2012). ABPs have roles in both promoting and inhibiting this F-actin turnover. ADF/cofilin proteins bind to the sides of actin filaments and induce filaments to break, as well as accelerating G-actin release from F-actin pointed ends. Gelsolin also severs F-actin, but its role in growth cone dynamics is minor compared to ADF/cofilin (Dent et al. 2011). As F-actin disassembles at filament pointed ends, the released monomer binds to ABPs profilin and thymosin, mentioned earlier, and returns to the leading edge by diffusion or other transport mechanisms. Tropomodulin and tropomyosin play important roles in regulating sarcomere structure and regulating actomyosin activity. Tropomodulin binds and stabilizes F-actin pointed ends, while tropomyosins are rod-shaped molecules that bind F-actin and block other ABPs, such as ADF/cofilin and myosin II, from binding F-actin. Neurons express multiple isoforms of tropomodulin and tropomyosin in growth cones (Schevzov et al. 2012). Although growth cone studies are limited, the effects of protein knockdown on neurite growth suggest that tropomodulin and tropomyosin have roles in regulating growth cone actin dynamics (Schevzov et al. 2012).
Filopodia and lamellipodia are protruded and withdrawn at rates of 1-4 μm/min. This rate reflects relative differences between protrusive activities, such as actin polymerization at the plasma membrane and actin crosslinking by ABPs, and anti-protrusive activities, such as actin depolymerization and forces that move the actin network back from the leading margin (Video 2). This retrograde flow has two components. As actin polymerization pushes against the plasma membrane, membrane tension resists and pushes the actin network back. In addition, the actin network of the P-domain is engaged by myosin-II motor molecules that pull the network backwards. Thus, during protrusion, actin polymerization exceeds retrograde flow and actin depolymerization, and when filopodial and lamellipodia are withdrawn, retrograde flow and/or F-actin breakdown exceed actin polymerization.
The dynamics of actin filament polymerization, depolymerization and linkage to other components of the P-domain are responsible for many aspects of growth cone motility. However, the production of mechanical forces within this actin system is another important component. Myosins are motor molecules that bind F-actin and move cargoes or exert tensions on the F-actin network. Several myosins participate in growth cone motility (Brown and Bridgman 2004). Myosin V moves vesicles towards F-actin barbed ends, and may move precursors to the plasmalemma for expansion and receptor insertion (Brown and Bridgman 2004). Myosin VI travels toward filament pointed ends and may move endocytic vesicles (Brown and Bridgman 2004; Hasson, 2003). Myosin X is a barbed end-directed motor that concentrates at filopodial tips. It may transport cargo during protrusion or otherwise promote F-actin polymerization (Kerber and Cheney 2011).
The most abundant myosin in the P-domain is the barbed end-directed motor myosin II (Figure 4). Myosin II has roles that both limit and promote growth cone migration and axon guidance. It powers retrograde flow in the P-domain by pulling the actin network back from the leading edge (Lin et al. 1996). This myosin II-driven movement concentrates and warps the actin network, promoting filament breakdown and recycling of G-actin to the leading edge (Medeiros et al. 2006). These retrograde forces bend and retract filopodial and lamellipodial protrusions that do not maintain adhesion to other cells or matrices. Finally, the myosin II-powered accumulation of F-actin in the proximal P-domain blocks advance of microtubules and other components of the growing axon (Medeiros et al., 2006; Zhou et al., 2002).
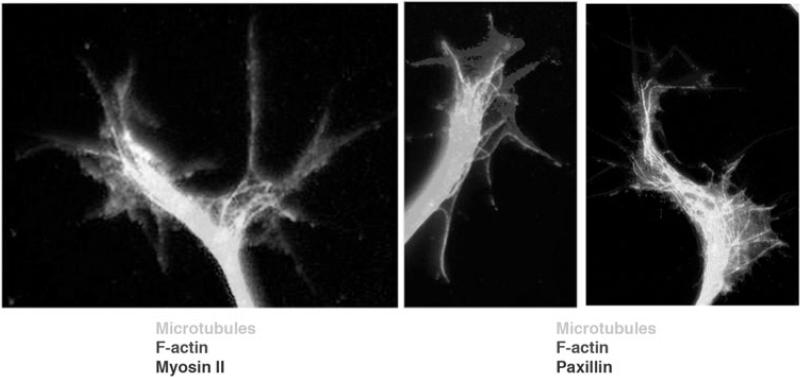
Figure 4.
Immunofluorescence images of chick dorsal root ganglion growth cones multiple-labeled to show (left panel) microtubules, F-actin and myosin II and (right panels) microtubules, F-actin and paxillin. Myosin II is the motor molecule that exerts tension on adhesive sites and growth cone structures, and paxillin marks adhesive contacts of filopodia and lamellipodia.
The Roles of ABPs in Growth Cone Adhesion and Migration
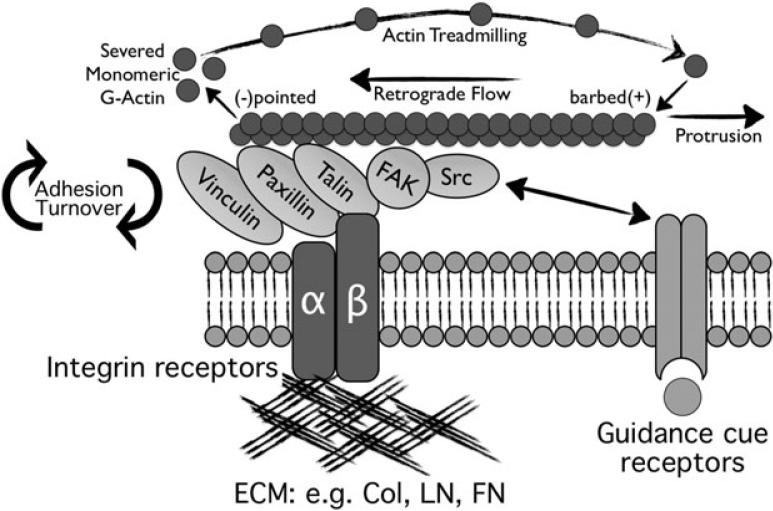
Filopodial and lamellipodial protrusion is converted to growth cone migration and axon elongation by adhesive contacts with other surfaces. The forces that drive retrograde flow of actin filaments are impeded by ABP-mediated connections between F-actin and the plasma membrane. Although these bonds may slip, they slow retrograde flow, promoting protrusion. However, more significant are F-actin connections to adhesion complexes at contact sites with other cells and extracellular matrices. These complexes involve the ABPs that mediate actin-adhesive molecule connections; ERM proteins and shootin1 (with L1), alpha- and ß- catenin (with N-cadherin) and talin, vinculin, alpha-actinin and paxillin (with integrins). These direct F-actin links to adhesive sites create a molecular “clutch” through which myosin II-derived force that pulls F-actin back from the leading edge is transduced into substratum-directed traction for growth cone advance (Figure 5; Jay 2000; Letourneau 1979, 1981, 1983; Suter and Forscher 2000).
Figure 5.
Key components of growth cone point contact adhesions. Integrin αβ heterodimeric receptors (dark blue lines) bind to extracellular matrix proteins, such as collagen, laminin and fibronectin. Integrin activation leads to the assembly of scaffolding proteins, such as talin, paxillin and vinculin with the cytoplasmic tail of integrins. In addition, kinases FAK and Src are activated, and they modulate the adhesions through phosphorylation of key residues that allow for assembly of additional proteins (not shown). Several proteins bind directly to actin filaments (red), which is believed to restrain retrograde flow and allow the force of actin polymerization to generate membrane protrusion. Guidance cue receptors (orange) can regulate adhesion-associated proteins through binding and activation of FAK and Src. Cross-talk through FAK/Src signaling modulates adhesion dynamics, as well as actin dynamics. With permission from Myers et al., (2011) Dev. Neurobiol. 71, 901-923.
Traction of individual filopodia and growth cones on substrata have been measured at >100 μdynes (Bridgman et al. 2001; Heidemann et al. 1990; Koch et al. 2012). The relationship of this “clutch” to retrograde actin flow is seen in studies showing that when growth cones exert greater traction on adhesive sites, the actin retrograde flow rate is reduced (Chan and Odde 2008; Koch et al. 2012). As previously stated, the rate of filopodial protrusion can be 1-4 μm/min. Maximal axonal elongation rates have been recorded at nearly the same velocity, up to 3 μm/min (Letourneau, observed). Presumably, in these cases adhesion and clutch activity is strong, while retrograde actin flow is negligible. The strength of the clutch (or inversely, its “slippage”) depends on how much F-actin is connected to adhesive sites, relative strengths of the links between F-actin, membrane proteins and extracellular ligands, and the force of retrograde flow. Thus, disruption of ABPs that contribute to this clutch interferes with growth cone traction and neurite elongation. When ERM or shootin proteins are knocked down, neurite elongation on an L1 substratum is greatly reduced, and the retrograde flow rate increases (Marsick et al. 2012b; Toriyama et al. 2013). Disruption of the N-cadherin- catenin link to F-actin reduces F-actin coupling to N-cadherin, weakening the clutch (Bard et al. 2008). Growth cone migration on a N-cadherin substratum is reduced, but there is no effect on axon growth on laminin. Depletion of talin does not interfere with cell spreading on an ECM substratum. However, talin depletion reduces substratum traction together with increased retrograde actin flow (Zhang et al. 2008).
This cycle of protrusion, adhesion and traction promotes axon elongation. Protrusion and adhesion of the P-domain expands cytoplasmic space for advancing the microtubule cytoskeleton. Myosin II-powered traction at adhesive sites counteracts intrinsic compressive forces and tensions that limit microtubule polymerization, microtubule advance and expansion of the plasmalemma. When F-actin barbed ends are well anchored at adhesive sites, like at a sarcomere Z-line, actin filaments and other structures associated with myosin II motors can be pulled toward the adhesive sites. Several ABPs might connect microtubules and the actin network in the P-domain. ACF7 (Drosophila Short Stop; Sanchez-Soriano et al. 2009), MAP1b (Noiges et al. 2002), CLASP1,2 (Marx et al. 2013; Svetkov et al. 2007), and drebrin (Geraldo et al. 2008; Worth et al. 2013) have all been shown to bind microtubules and actin filaments and have been localized in growth cones. These proteins have all been proposed to mediate microtubule-actin interactions that might direct the advance of microtubules in the growth cone P-domain (Figures 4, 6).
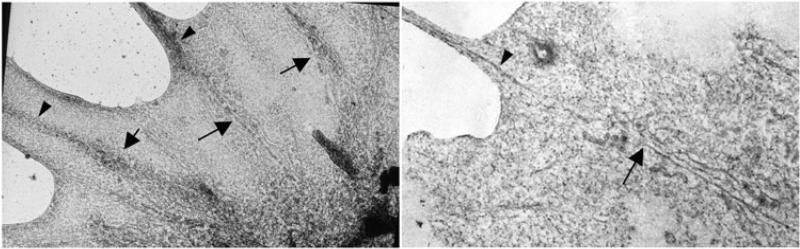
Figure 6.
Left panel is a whole-mount electron micrographs from the leading margin of a dorsal root ganglion growth cone. Right panel is a thin section from a similar region. These images show the relationship between microtubules and associated organelles that have advanced into the P-domain (arrows) and bundles of actin filaments (arrowheads), which might determine where the microtubules advance. Left panel with permission from Letourneau, P.C. (1979) Exp. Cell Res. 124, 127-138, right panel from Letourneau, P.C. (1983) J. Cell Biol. 97, 963-973.
Coordinating Actin Dynamics for Growth Cone Navigation
Growth cone navigation to synaptic targets occurs as protrusion, adhesion and traction are locally coordinated by interactions of growth cone receptors with adhesive ligands and guidance cues (Figure 7). In the following sections we discuss signaling mechanisms that act on ABPs to mediate growth cone navigation.
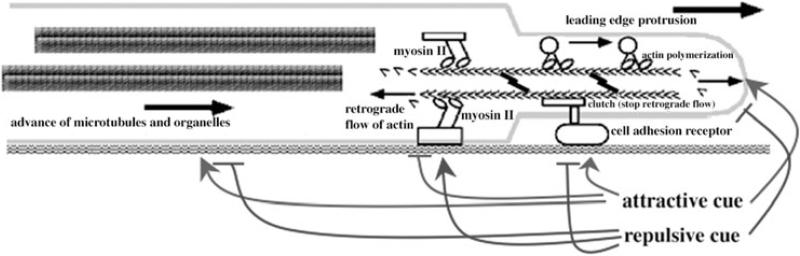
Figure 7.
A model of growth cone navigation. Actin polymerization pushes the leading margin of the growth cone forward. Forces generated by myosin II pull actin filaments backwards, where filaments are disassembled. When growth cone receptors make adhesive contacts, a ‘clutch’ links the adhesive contact to actin filaments, and the retrograde flow of actin filaments slows. This permits the advance of microtubules and organelles and promotes axonal elongation. Intracellular signals generated by attractive and repulsive axonal guidance cues interact with the mechanisms of actin polymerization, myosin II force generation, adhesive contacts, and microtubule advance to regulate the paths of growth cone migration.
While recent studies have begun to address the cellular and molecular details of growth cone navigation in vivo (Evans and Bashaw 2010, Quinn and Wadsworth 2008, Robles and Gomez 2006) many insights into mechanisms of growth cone navigation are known from extensive in vitro studies. When neurons are plated on a homogeneous substratum, growth cones exhibit stochastic brief turning, but generally migrate forward, pulling the trailing axon behind. However, growth cones can be directed by a chemical gradient of attractive or repulsive guidance cues released from a micropipette positioned ahead and at an angle to the orientation of axon outgrowth (Ming et al. 1999, Song et al. 1998, Zheng et al. 1994). In addition, neurons cultured on a substratum patterned with alternating stripes of adhesive and non-adhesive or repulsive molecules, growth cones migrate on the adhesive stripe, wandering from one edge of the stripe to the other, sampling, but not crossing, onto the non-adhesive or repulsive substratum (Knoll and Drescher 2004, Letourneau 1975, Snow et al. 1994).
Local stimulation with an attractive guidance cue promotes growth cone protrusion and adhesion in the P-domain region nearest to the guidance cue. Cytoplasmic signaling triggered by the cue regulates ABPs to locally increase actin polymerization and/or decrease retrograde actin flow (Figure 8; Vitriol and Zheng 2012). Increased growth cone point contacts by filopodia located toward the attractant stabilize filopodia and lamellipodia and support further protrusion (Myers and Gomez 2011). The turn is continued and completed by engagement of the molecular clutch at adhesion sites, which allows myosin II-powered force to be directed to advance microtubules and associated organelles, completing the turn (Figures 4, 6; Suter et al. 1998).
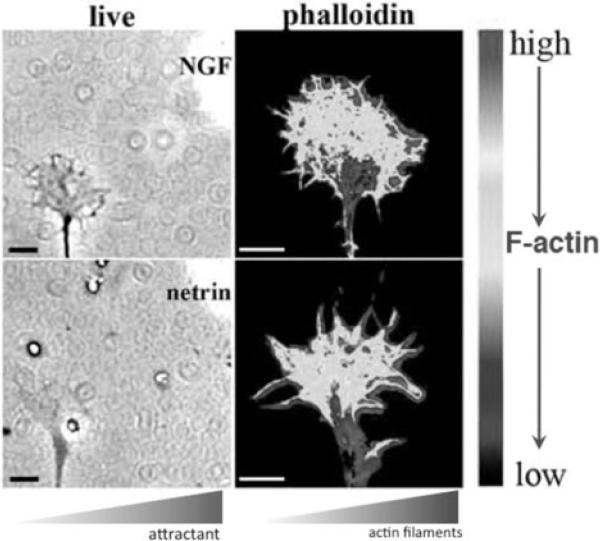
Figure 8.
Phase-contrast and psuedocolor images depicting the higher density of F-actin in the P-domain regions of growth cones that are closer to a micropipette releasing an attractive guidance cue. Upper panel shows a chick dorsal root ganglion growth cone exposed to nerve growth factor, and lower panel shows a chick retinal growth cone exposed to netrin. With permission from Marsick et al., (2010) Dev. Neurobiol. 70, 565-588.
Growth cones turn away from repulsive or repellent cues by stopping protrusion and/or losing adhesion on the side of the leading edge that is closer to the negative cue. As protrusion continues on the side away from the repellent, the growth cone turns away from the negative cue. Video records show that the negative cues slit3 and ephrin-A2 arrest leading edge protrusion, while retrograde flow continues within the collapsing P-domain (Video 3; Marsick et al. 2012a). This suggests actin polymerization is inhibited by signaling triggered by negative cues. Repulsive signaling may further stop protrusion by increasing retrograde flow or by inducing the removal or inactivation of plasmalemmal adhesion molecules (Woo and Gomez 2006).
Signal Transduction Pathways that Regulate Actin Filament Dynamics
Receptor activation on growth cones through binding of adhesive ligands and soluble axon guidance cues triggers local intracellular signals that modulate actin filament dynamics to control growth cone navigation (Quinn and Wadsworth 2008). In the following sections we discuss signaling mechanisms that act on ABPs to mediate growth cone navigation.
The Rho family GTPases, RhoA, Rac1 and Cdc42, are key signaling intermediates activated by growth factors, adhesive ligands and guidance cue receptors (Hall and Lalli 2010). Rho GTPases are activated when GDP is exchanged for GTP by guanine nucleotide exchange factors (GEFs) and are inactivated upon hydrolysis of GTP, which is facilitated by GTPase activating proteins (GAPs) (Etienne-Manneville and Hall 2002). Many adhesion and guidance cue receptors either contain intrinsic GTPase regulatory activity or regulate messengers that act on the neuronal GEFs and GAPs (Lowery and Van Vactor 2009). Both activation and inhibition of Rho GTPases by guidance cues has been detected in pull-down assays and by measurements in fixed or live growth cones, using antibodies and biosensors (Wong et al. 2001, Yuan et al. 2003). Live imaging is particular useful, as it allows local signaling to be correlated with real time motility during stimulation with guidance cues (Myers et al. 2012). Importantly, Rho GTPases signaling is necessary downstream of many adhesion molecules and axon guidance cues, because molecular and pharmacological inhibition of these actin regulators blocks growth cone turning in vitro and proper neuronal morphogenesis in vivo (Li et al. 2002, Yuan et al. 2003).
The activities of RhoA, Rac1 and Cdc42 regulate protrusion, retraction and adhesion through regulation of actin binding proteins that control actin filament polymerization, disassembly, and actomyosin contractility. In general, activities of Rac1 and Cdc42 are associated with attractive growth cone turning, and RhoA activity is associated with responses to repellent cues (Luo 2000). However, reality is more subtle, as it appears that tight spatial and temporal regulation of Rho GTPases contributes to both positive and negative turning responses to guidance cues.
One of the principal targets of RhoA activity is RhoA kinase (ROCK). ROCK activates contractility by phosphorylating the regulatory myosin light chain (MLC) and by inhibiting myosin light chain phosphatase (MLCP). This heightened myosin II activity may increase retrograde flow, thereby reducing leading edge protrusion. Several repulsive guidance cues strongly activate RhoA/ROCK signaling and actomyosin contraction (Shamah et al., 2001; Niederost et al., 2002; Swiercz et al., 2002), resulting in growth cone collapse and retraction (Jalink et al., 1994).
Another target of ROCK is LIM kinase, which phosphorylates ADF/cofilin at Serine3 and inhibits ADF/cofilin severing of F-actin (Sarmiere and Bamburg 2004). Such increased F-actin stability may promote protrusion, if actin polymerization is limited relative to F-actin turnover. In fact, during chemoattraction toward BDNF, Xenopus growth cones protruded and turned toward the region of the P-domain with reduced active cofilin (higher phospho- ADF/cofilin) in response to the attractive cue (Wen et al. 2007). On the other hand, in chick embryonic neurons, three repulsive cues, slit3, ephrin-A2, and semaphorin 3A, also inhibit cofilin (increase phospho-ADF/cofilin), consistent with RhoA activation by these cues (Marsick et al. 2012a). This decreased ADF/cofilin activity in slit3- or ephrin-A2-treated growth cones is associated with a reduction in F-actin barbed ends, which are seeds for actin polymerization. Further, the combination of reduced actin turnover and increased myosin II activity in repellent-treated growth cones stimulates growth cone retraction by increased actomyosin contractility. There are two possible explanations for these discrepant results. First, cofilin may function within an optimal set-point for promoting axon outgrowth, with the basal level in a particular situation determining the effect of inhibiting cofilin activity on the rate or direction of outgrowth. Alternatively, additional simultaneous signaling activated by positive and negative cues may modulate the effects of reduced cofilin activity. In support of the set-point hypothesis, partial inhibition of cofilin using a PAK inhibitory peptide was found to stimulate axon outgrowth, while full inhibition of cofilin caused axon retraction (Santiago-Medina et al. 2013).
Although growth cone collapse in response to negative cues involve RhoA, ROCK and myosin II contraction, RhoA, ROCK and myosin II also contribute to attractive growth cone turning. A modest level of ROCK activity and myosin-II-based contraction promotes integrin adhesion stabilization during protrusion (Arakawa et al. 2003, Woo and Gomez 2006) and ROCK directly phosphorylates ERM proteins, actin-membrane linkers, to promote cell-cell adhesion (Matsui et al. 1999). In addition, growth cone turning towards the attractive cue NGF is blocked by inhibiting ROCK, because of failure to suppress protrusive activity away from the NGF source (Loudon et al. 2006).
The GTPases Rac1 and Cdc42 are activated by attractive cues, such as Netrin and BDNF (Briancon-Marjollet et al. 2008, Myers et al. 2012, Shekarabi et al. 2005). Rac1 and Cdc42 signaling increases activities of several ABPs that promote actin filament polymerization to stimulate growth cone protrusion and turning toward attractive guidance cues. Principal targets of Rac1 and Cdc42 signaling are WAVE and N-WASP, respectively, which are activated by these GTPases resulting in Arp2/3-mediated polymerization of dendritic actin arrays (Hall and Lalli 2010). Another target of Rac1 signaling is the phosphatase Slingshot, which dephosphorylates and activates ADF/cofilin (Ng and Luo 2004). Netrin and NGF activate F-actin severing by ADF/cofilin to increase F-actin barbed ends and further stimulate actin polymerization, driving protrusion toward the source of positive cue (Marsick et al. 2010).
However, the actin polymerization activities of Rac1 may also contribute to turning away from repellent cues, such as semaphorin3A (Sema3A) and ephrin-A2 (Jurney et al. 2002; Marston et al. 2003, Vastrik et al. 1999). Actin polymerization is necessary at sites of membrane endocytosis, where filopodia and lamellipodia are retracted in response to repellents, and locally increased endocytosis is sufficient to promote repulsive turning (Hines et al. 2010). Interestingly, many axon guidance cues may regulate axon outgrowth through modulation of adhesion receptor function (e.g. trafficking, ligand affinity, clustering, cytoskeleton linkage). For example, MAG, Sema3A, Sema7A, Ephrin-A1, Slit and Netrin have all been shown to regulate integrin-dependent adhesion (Hines et al. 2010, Miao et al. 2000, Pasterkamp et al. 2003, Stevens and Jacobs 2002, Woo and Gomez 2006, Yebra et al. 2003).
The p21-activated kinase (PAK) is another effector downstream of Rac1 and Cdc42. At least three isoforms of PAK (PAK1-3) have been identified at distinct locations in growth cones (Santiago-Medina et al. 2013). These distinct localizations may target PAK to specific effectors. For example, in Xenopus neurons, PAK2 localizes to both paxillin-containing adhesions and to the tips of extending filopodia, suggesting a role in adhesion and actin polymerization at filopodial tips. Similar to ROCK, PAKs also activate myosin II and LIM kinase, but likely act differently in growth cone motility because of their specific localizations. For example, PAK2 and PAK3 bind the Rac1 GEF called PIX, which binds to paxillin at growth cone point contact adhesions to regulate adhesion formation. While PAK1 does not appear to localize specifically within Xenopus growth cones, PAK1 was recently found to phosphorylate Shootin1 in hippocampal neurons. Shootin1, like ERM proteins, mediates actin linkage to the adhesion molecule L1, reducing retrograde flow by increasing clutching forces on F-actin (Toriyama et al. 2013). Other targets of Rho GTPases that likely regulate the growth cone cytoskeleton include, actin nucleating formins, ena/Vasp anti-capping factors and F-bar containing membrane curving proteins (Hall and Lalli 2010).
Non-receptor tyrosine kinases, such as Focal Adhesion Kinase (FAK) and Src Family Kinases (SFKs), are another means by which guidance cues regulate actin dynamics, adhesion and motility (Figures 5, 9). FAK regulates Rho GTPases and acts downstream of several guidance cues. In non-neuronal cells, FAK activates the GTPase Regulator Associated with FAK (GRAF), which is a GAP for RhoA and Cdc42 (Hildebrand et al. 1996), and binds and activates both p190RhoGEF and p190RhoGAP, which have opposite effects on RhoA activity. FAK activates RhoA in some contexts via p190RhoGEF, as it does to control axon branching and synapse formation (Rico et al. 2004). In addition to Cdc42 inactivation via GRAF, FAK may also indirectly activate Cdc42 signaling. FAK is phosphorylated at Y861 by Src, and this creates a binding site for the scaffolding protein p130Cas (Cho and Klemke 2002). Subsequently, p130Cas associates with the Rac GEF DOCK180, which leads to Rac activation downstream of integrin engagement (Brugnera et al. 2002, Cote and Vuori 2002). Interestingly, p130Cas was found to activate both Rac1 and Cdc42 downstream of Netrin signaling, which is necessary for midline crossing by commissural interneurons (Liu et al. 2007). Therefore, it appears that FAK is capable of activating or inactivating both RhoA and Cdc42, depending on the particular GEF or GAP protein involved.
Figure 9.
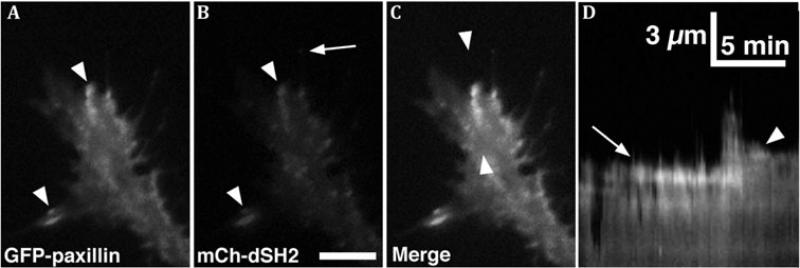
A, B. Total Internal Reflection Fluorescence (TIRF) microscopy images of paxillin-GFP and mCherry dual-Src homology 2 domain (mCh-dSH2) fluorescent images of a growth cone on PDL-laminin. Note that paxillin and phosphotyrosine (PY), as revealed with mChdSH2, colocalize at adhesion sites (arrowheads in A, B), whereas the tip of a growing filopodium has PY, without paxillin (arrow in B). C. Merged image of the growth cone in (A, B) shows co-localization at several peripheral adhesions. Note that mCh-dSH2 puncta within the central domain are mobile vesicles. D. Single line kymograph constructed from region between the arrowheads in C. Note a stable adhesion (arrow) that disassembles after a new protrusion extends forward, followed by the formation of a second adhesion (arrowhead), which stabilizes the receding protrusion. Scale bar, 10 μm in all images and as indicated in kymographs. With permission from Santiago-Medina et al. (2012) Dev. Neurobiol. 72, 585-599.
SFKs are complex signaling and scaffolding proteins that function in close association with FAK in the regulation of cell-matrix and cell-cell adhesion (Mitra et al. 2005). The complexity of SFK function in growth cones is exemplified by observations that SFKs are necessary downstream of both attractive and repellent guidance cues (Knoll and Drescher 2004, Li et al. 2004, Robles and Gomez 2006, Yam et al. 2009). Contributing to this complexity is the number of potential upstream activators and downstream targets for SFKs in growth cones. For example, SFKs function synergistically with Rho GTPases to modulate the activity of N-WASP and PAK (Renkema et al. 2002, Torres and Rosen 2003). Both N-WASP and PAK contain cryptic tyrosine residues for phosphorylation by SFKs, but these sites become accessible to phosphorylation only after binding active Cdc42. Phosphorylation of N-WASP and PAK by SFKs may alter the active state, localization or active lifetime of N-WASP and PAK. Another target of SFKs that regulates actin polymerization is cortactin, which promotes actin polymerization through linking Arp2/3 to F-actin in lamellipodia and invadopodia (Kirkbride et al. 2011). However, little is known about cortactin function in growth cones (Decourt et al. 2009, Kurklinsky et al. 2011). Lastly, cytosolic [Ca2+] fluctuations in growth cones regulate the actin cytoskeleton in several ways that affect growth cone guidance (Gomez and Zheng 2006, Henley and Poo 2004). Many proteins that regulate actin, either directly as ABPs or indirectly, as kinases, phosphatases, proteases, etc. are regulated allosterically by [Ca2+]. In addition, the particular source of Ca2+ signals can have varying and even opposite effects on growth cone motility. For example, blocking certain Ca2+ channels with specific antagonists stimulates neurite outgrowth, while blocking other Ca2+ channels has no effect or slows neurite outgrowth (Jacques-Fricke et al. 2006). Channel-specific effects of Ca2+ influx are possible since [Ca2+] functions in distinct microdomains (Augustine et al. 2003), highly localized sites of Ca2+ influx or release that are linked to specific [Ca2+]-sensitive effector functions. Consistent with this notion, both positive and negative guidance cues have been shown to control growth cone motility by stimulating Ca2+ influx and release from intracellular stores (Henley et al. 2004, Jin et al. 2005, Shim et al. 2005, Jacques-Fricke et al. 2006, Wen et al. 2007, Kaczmarek et al. 2012). Some [Ca2+]-activated effectors are ABPs, including lpha-actinin, gelsolin and troponin. Other [Ca2+]-activated effectors control Rho GTPase signals. For example, BDNF and Netrin activate [Ca2+]-dependent calmodulin kinase II (CaMKII), which increases Rac1/Cdc42 and decreases RhoA activity to promote axon outgrowth (Jin et al. 2005). However, [Ca2+] signaling through calmodulin also activates myosin II contractility. There is extensive crosstalk between [Ca2+] and cAMP signaling in regulating growth cone navigation (Nicol et al. 2011, Forbes et al. 2012). cAMP regulates Ca2+ channel activity and IP3-dependent Ca2+ release, while [Ca2+] regulates adenyl cyclase activity. In addition, cAMP signaling modulates downstream effectors of Ca2+ signaling. The switching of growth cone turning responses from attractive to repulsive and the reverse are the result of [Ca2+]/cAMP crosstalk and downstream regulation of ABPs (Song and Poo 1999).
One class of Ca2+ channel that may be a common target of both positive and negative guidance cues are members of the Trp family of heterotetrameric cation channels. Channels that contain TrpC subunits are necessary for attractive and repulsive turning toward different guidance cues and can also be activated by mechanical forces (Kerstein et al. 2013, Li et al. 2009, Li et al. 2005, Shim et al. 2005, Wang and Poo 2005, Wen et al. 2007). In kidney podocytes, TrpC5 subunit-containing channels associate with and activate Rac1 to promote cell motility, while TrpC6 subunit-containing channels associate with and activate RhoA to induce stress fibers and cell contractility (Tian et al. 2010). Although not tested in growth cones, similar TrpC subunit-specific effects likely regulate axon guidance. Another direct Ca2+ effector in growth cones is the protease calpain, which can inhibit axon extension by cleaving target proteins, including proteins that regulate cell adhesion and motility (Kaczmarek et al. 2012, Kerstein et al. 2013, Robles et al. 2003). For example, one principal target of calpain is the ABP talin, which both activates integrin receptors and links integrins to the actin cytoskeleton. In non-neuronal cells, talin plays key roles in the dynamics of adhesion turnover and cell motility (Franco et al. 2004). Talin targets to the tips of growth cone filopodia, where it likely activates integrin receptors (Calderwood et al. 1999) and initiates point contact assembly to stabilize protrusions (Kerstein et al. 2013). Co-localization of talin with pY118-paxillin in some filopodia suggests these are nascent adhesions and are regulated by Ca2+ influx through mechanosensitive channels. As rapid point contact turnover is associated with efficient growth cone migration (Myers and Gomez 2011), Ca2+/calpain activity may disrupt adhesion assembly or maturation through cleavage of talin (Bate et al. 2012; Kerstein et al. 2013).
Future Directions
Much has been learned about growth cone actin dynamics, since Ramon y Cajal deduced growth cone behaviors from fixed sections of chick embryos. The key roles of ABPs in regulating growth cone behaviors are recognized, and the complex events that link guidance cues to actin dynamics are beginning to be revealed. However, much remains to be learned. Not all ABPs and signaling components that regulate actin dynamics in growth cones are identified. It is still unclear how the diverse and sometimes redundant activities of ABPs are coordinated and how growth cones integrate simultaneous signaling from multiple guidance cues, which is the in vivo state. The interactions of F-actin, the driver of growth cone motility, and microtubules, the driver of axon elongation, remain unclear. Clinical applications may arise from the better understanding of actin dynamics in growth cones. Mental retardation, autism, and other developmental brain disorders may arise from defective growth cone actin dynamics and navigation to and branching in target areas (Bernstein et al. 2012; Engle, 2010; Izzi and Charron 2011; Ramakers, 2002). Recovery from nervous system injuries and diseases may require axonal growth to regenerate or replace lost neuronal connections (Saijilafu et al., 2013). Better understanding of actin dynamics in growth cones can promote therapies that return or replace lost protein functions or regulatory signaling in growth cones, so neural circuits develop more normally or be more fully repaired after disease or injury.
How can new technologies and approaches be used to further understand growth cone actin dynamics? Advances in genetics, fluorescence chemistry, illumination systems, and microscopes are sharpening the dissection of the functions of individual ABPs and elements of the signaling pathways that mediate growth cone guidance. Expression of fluorescent protein analogs and biosensors allows the location of proteins and signaling activities in live growth cones to be detected at high resolution. Light activated fluorescence or the use of structured illumination improves resolution further. Targeted mutations of individual ABPs or signaling components can be combined with expression of fluorescent analogues with engineered light activated properties. Light activation provides tight temporal and spatial control of protein function, either positive or negative, which is limited by the more common approach of overexpression of dominant-negative or constitutively active proteins, or with knock-downs or knock-outs, which allow for adaptation. Super-resolution microscopes offer resolution to 100 nm or less for proteins and biosensors in fixed and live growth cones. Nanofabrication and microfluidics offers precise control of the presentation of adhesive ligands and guidance cues to couple with the above approaches to analyzing growth cone function. These new technologies can also be used for improved modeling of in vivo environments, while still allowing high spatial and temporal resolution of actin dynamics. Most growth cone studies are done on inflexible, two-dimensional substrata coated on glass for maximal microscopic resolution. However, growth cones in vivo travel in complex three-dimensional spaces. These new technologies can be used to probe growth cone mechanisms in matrices that better approximate in vivo environments.
Supplementary Material
Supp Video S1
Supp Video S2
Supp Video S3
Acknowledgements
The authors would like to thank the members of their laboratories whom have conducted the research that is described from the Gomez and Letourneau laboratories. Miguel Santiago-Medina prepared the drawing in Figure 5. The ARRIVE guidelines for animal research were followed in the laboratories of the authors. The authors have no conflicts of interest to declare. Research in the Gomez laboratory is supported by NIH grant NS41564, and research in the Letourneau laboratory is supported by NIH grant HD19950.
References
- Arakawa Y, Bito H, Furuyashiki T, Tsuji T, Takemoto-Kimura S, Kimura K, Nozaki K, Hashimoto N, Narumiya S. Control of axon elongation via an SDF-1alpha/Rho/mDia pathway in cultured cerebellar granule neurons. J. Cell Biol. 2003;161:381–391. doi: 10.1083/jcb.200210149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- Bard L, Boscher C, Lambert M, Mège RM, Choquet D, Thoumine O. A molecular clutch between the actin flow and N-cadherin adhesions drives growth cone migration. J. Neurosci. 2008;28:5879–5890. doi: 10.1523/JNEUROSCI.5331-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate N, Gingras AR, Bachir A, Horwitz R, Ye F, Patel B, Goult BT, Critchley DR. Talin contains a C-terminal calpain2 cleavage site important in focal adhesion dynamics. PLoS One. 2012;7:e34461. doi: 10.1371/journal.pone.0034461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, Gertler FB. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–521. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Shaw AE, Minamide LS, Pak CW, Bamburg JR. Incorporation of cofilin into rods depends on disulfide intermolecular bonds: implications for actin regulation and neurodegenerative disease. J. Neurosci. 2012;32:6670–6681. doi: 10.1523/JNEUROSCI.6020-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briancon-Marjollet A, Ghogha A, Nawabi H, Triki I, Auziol C, Fromont S, Piche C, Enslen H, Chebli K, Cloutier JF, Castellani V, Debant A, Lamarche-Vane N. Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol. Cell Biol. 2008;28:2314–2323. doi: 10.1128/MCB.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D, Kiesewetter AK, Linkner J, Urbanke C, Resch GP, Small JV, Faix J. Clustering of VASP actively drives processive, WH2 domain-mediated actin filament elongation. EMBO J. 2008;27:2943–2954. doi: 10.1038/emboj.2008.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnera E, Haney L, Grimsley C, Lu M, Walk SF, Tosello-Trampont AC, Macara IG, Madhani H, Fink GR, Ravichandran KS. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat. Cell Biol. 2002;4:574–582. doi: 10.1038/ncb824. [DOI] [PubMed] [Google Scholar]
- Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO, Ginsberg MH. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J. Biol Chem. 1999;274:28071–28074. doi: 10.1074/jbc.274.40.28071. [DOI] [PubMed] [Google Scholar]
- Carlier MF, Pantaloni D. Control of actin assembly dynamics in cell motility. J Biol. Chem. 2007;282:23005–23009. doi: 10.1074/jbc.R700020200. [DOI] [PubMed] [Google Scholar]
- Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Sci. 2008;322:1687–1691. doi: 10.1126/science.1163595. [DOI] [PubMed] [Google Scholar]
- Cho SY, Klemke RL. Purification of pseudopodia from polarized cells reveals redistribution and activation of Rac through assembly of a CAS/Crk scaffold. J. Cell Biol. 2002;156:725–736. doi: 10.1083/jcb.200111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote JF, Vuori K. Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J. Cell Sci. 2002;115:4901–4913. doi: 10.1242/jcs.00219. [DOI] [PubMed] [Google Scholar]
- Decourt B, Munnamalai V, Lee AC, Sanchez L, Suter DM. Cortactin colocalizes with filopodial actin and accumulates at IgCAM adhesion sites in Aplysia growth cones. J. Neurosci. Res. 2009;87:1057–1068. doi: 10.1002/jnr.21937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–227. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a001800. 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle EC. Human Genetic Disorders of Axon Guidance. Cold Spr. Harb. Perspect. Biol. 2010;2(3):a001784. doi: 10.1101/cshperspect.a001784. doi: 10.1101/cshperspect.a001784 PMCID: PMC2829956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Evans TA, Bashaw GJ. Axon guidance at the midline: of mice and flies. Curr. Opin. Neurobiol. 2010;20:79–85. doi: 10.1016/j.conb.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EM, Thompson AW, Yuan J, Goodhill GJ. Calcium and cAMP levels interact to determine attraction versus repulsion in axon guidance. Neuron. 2012;74:490–503. doi: 10.1016/j.neuron.2012.02.035. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Rodgers MA, Perrin BJ, Han JW, Bennin DA, Critchley DR, Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- Geraldo S, Khanzada UK, Parsons M, Chilton JK, Gordon-Weeks PR. Targeting of the F-actin-binding protein drebrin by the microtubule plus-tip protein EB3 is required for neuritogenesis. Nat. Cell Biol. 2008;10:1181–1189. doi: 10.1038/ncb1778. [DOI] [PubMed] [Google Scholar]
- Giannone G, Mège RM, Thoumine O. Multi-level molecular clutches in motile cell processes. Trends Cell Biol. 2009;19:475–486. doi: 10.1016/j.tcb.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat. Rev. Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb. Perspect. Biol. 2010;2:a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarback JA, Letourneau PC. Neurite extension across regions of low cell-substratum adhesivity: Implications for the guidepost hypothesis of axonal pathfinding. Dev. Biol. 1986;117:655–662. doi: 10.1016/0012-1606(86)90334-9. [DOI] [PubMed] [Google Scholar]
- Hasson T. Myosin Vi: Two distinct roles in endocytosis. J. Cell Sci. 2003;116:3453–3461. doi: 10.1242/jcs.00669. [DOI] [PubMed] [Google Scholar]
- Henley J, Poo MM. Guiding neuronal growth cones using Ca2+ signals. Trends in Cell Biol. 2004;14:320–330. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JR, Huang KH, Wang D, Poo MM. Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron. 2004;44:909–916. doi: 10.1016/j.neuron.2004.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JD, Taylor JM, Parsons JT. An SH3 domain-containing GTPase-activating protein for Rho and Cdc42 associates with focal adhesion kinase. Mol. Cell Biol. 1996;16:3169–3178. doi: 10.1128/mcb.16.6.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines JH, Abu-Rub M, Henley JR. Asymmetric endocytosis and remodeling of beta1-integrin adhesions during growth cone chemorepulsion by MAG. Nat. Neurosci. 2010;13:829–837. doi: 10.1038/nn.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzi L, Charron F. Midline axon guidance and human genetic disorders. Clin. Genet. 2011;80:226–234. doi: 10.1111/j.1399-0004.2011.01735.x. [DOI] [PubMed] [Google Scholar]
- Jacques-Fricke BT, Seow Y, Gottlieb PA, Sachs F, Gomez TM. Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth in opposition to other influx pathways and release from intracellular stores. J. Neurosci. 2006;26:5656–5664. doi: 10.1523/JNEUROSCI.0675-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J. Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay DG. The clutch hypothesis revisited: ascribing the roles of actin-associated proteins in filopodial protrusion in the nerve growth cone. J. Neurobiol. 2000;44:114–125. doi: 10.1002/1097-4695(200008)44:2<114::aid-neu3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Jin M, Guan CB, Jiang YA, Chen G, Zhao CT, Cui K, Song YQ, Wu CP, Poo MM, Yuan XB. Ca2+-dependent regulation of Rho GTPases triggers turning of nerve growth cones. J. Neurosci. 2005;25:2338–2347. doi: 10.1523/JNEUROSCI.4889-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurney WM, Gallo G, Letourneau PC, McLoon SC. Rac1 mediated endocytosis during ephrin-A2 and semaphorin 3A induced growth cone collapse. J. Neurosci. 2002;22:6019–6028. doi: 10.1523/JNEUROSCI.22-14-06019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek JS, Riccio A, Clapham DE. Calpain cleaves and activates the TRPC5 channel to participate in semaphorin 3A-induced neuronal growth cone collapse. Proc. Natl. Acad. Sci. USA. 2012;109:7888–7892. doi: 10.1073/pnas.1205869109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerber ML, Cheney RE. Myosin-X: a MyTH-FERM myosin at the tips of filopodia. J. Cell Sci. 2011;124:3733–3741. doi: 10.1242/jcs.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstein PC, Jacques-Fricke BT, Rengifo J, Mogen BJ, Williams JC, Gottlieb PA, Sachs F, Gomez TM. Mechanosensitive TRPC1 channels promote calpain proteolysis of talin to regulate spinal axon outgrowth. J. Neurosci. 2013;33:273–285. doi: 10.1523/JNEUROSCI.2142-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi T, Nagai T, Ohashi K, Mizuno K. Measurements of spatiotemporal changes in G-actin concentration reveal its effect on stimulus-induced actin assembly and lamellipodium extension. J. Cell Biol. 2011;193:365–380. doi: 10.1083/jcb.201101035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkbride KC, Sung BH, Sinha S, Weaver AM. Cortactin: a multifunctional regulator of cellular invasiveness. Cell Adhesion & Migration. 2011;5:187–198. doi: 10.4161/cam.5.2.14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll B, Drescher U. Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J. Neurosci. 2004;24:6248–6257. doi: 10.1523/JNEUROSCI.0985-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch D, Rosoff WJ, Jiang J, Geller HM, Urbach JS. Strength in the periphery: growth cone biomechanics and substrate rigidity response in peripheral and central nervous system neurons. Biophys. J. 2012;102:452–460. doi: 10.1016/j.bpj.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn ED. Actin polymerization and its regulation by proteins from non-muscle cells. Physiol. Rev. 1982;62:672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Kovar DR, Harris ES, Mahaffy R, Higgs HN, Pollard TD. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell. 2006;124:423–435. doi: 10.1016/j.cell.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Kurklinsky S, Chen J, McNiven MA. Growth cone morphology and spreading are regulated by a dynamin-cortactin complex at point contacts in hippocampal neurons. J. Neurochem. 2011;117:48–60. doi: 10.1111/j.1471-4159.2011.07169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Vitriol EA, Shim S, Wise AL, Velayutham RP, Zheng JQ. Dynamic localization of G-actin during membrane protrusion in neuronal motility. Curr Biol. 2013;23:1046–1056. doi: 10.1016/j.cub.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau PC. Cell-to-substratum adhesion and guidance of axonal elongation. Dev. Biol. 1975;44:92–101. doi: 10.1016/0012-1606(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Letourneau PC. Cell-substratum adhesion of neurite growth cones and its role in neurite elongation. Exp. Cell Res. 1979;124:127–138. doi: 10.1016/0014-4827(79)90263-5. [DOI] [PubMed] [Google Scholar]
- Letourneau PC. Immunocytochemical evidence for colocalization in neurite growth cones of actin and myosin and their relationship to cell-substratum adhesions. Dev. Biol. 1981;85:113–122. doi: 10.1016/0012-1606(81)90240-2. [DOI] [PubMed] [Google Scholar]
- Letourneau PC. Differences in the distribution of actin filaments between the growth cones and the neurites of cultured chick sensory neurons. J. Cell Biol. 1983;97:963–973. doi: 10.1083/jcb.97.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hutchins BI, Kalil K. Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J. Neurosci. 2009;29:5873–5883. doi: 10.1523/JNEUROSCI.0183-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Lee J, Vikis HG, Lee SH, Liu G, Aurandt J, Shen TL, Fearon ER, Guan JL, Han M, Rao Y, Hong K, Guan KL. Activation of FAK and Src are receptor-proximal events required for netrin signaling. Nat. Neurosci. 2004;7:1213–1221. doi: 10.1038/nn1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ, Yuan XB. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- Li Z, Aizenman CD, Cline HT. Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron. 2002;33:741–750. doi: 10.1016/s0896-6273(02)00621-9. [DOI] [PubMed] [Google Scholar]
- Lin CH, Espreafico EM, Mooseker MS, Forscher P. Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron. 1996;16:769–782. doi: 10.1016/s0896-6273(00)80097-5. [DOI] [PubMed] [Google Scholar]
- Liu G, Li W, Gao X, Li X, Jurgensen C, Park HT, Shin NY, Yu J, He ML, Hanks SK, Wu JY, Guan KL, Rao Y. p130CAS is required for netrin signaling and commissural axon guidance. J. Neurosci. 2007;27:957–968. doi: 10.1523/JNEUROSCI.4616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon RP, Silver LD, Yee HF, Gallo G. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J. Neurobiol. 2006;66:847–867. doi: 10.1002/neu.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat. Rev. Mol. Cell Biol. 2009;10:332–343. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L. Rho GTPases in neuronal morphogenesis. Nature Rev. NeuroscI. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- Marsick BM, Flynn KC, Santiago-Medina M, Bamburg JR, Letourneau PC. Activation of ADF/cofilin mediates attractive growth cone turning toward nerve growth factor and netrin-1. Dev. Neurobiol. 2010;70:565–588. doi: 10.1002/dneu.20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsick BM, Roche FK, Letourneau PC. Repulsive axon guidance cues ephrin-A2 and slit3 stop protrusion of the growth cone leading margin concurrently with inhibition of ADF/cofilin and ERM proteins. Cytoskeleton. 2012a;69:496–505. doi: 10.1002/cm.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsick BM, San Miguel-Ruiz J, Letourneau PC. Activation of Ezrin/radixin/moesin mediates attractive growth cone guidance through regulation of growth cone actin and adhesion receptors. J. Neurosci. 2012b;32:282–296. doi: 10.1523/JNEUROSCI.4794-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat. Cell Biol. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- Marx A, Godinez WJ, Tsimashchuk V, Bankhead P, Rohr K, Engel U. Xenopus cytoplasmic linker-associated protein 1 (XCLASP1) promotes axon elongation and advance of pioneer microtubules. Mol. Biol Cell. 2013;24:1544–1558. doi: 10.1091/mbc.E12-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Yonemura S, Tsukita S, Tsukita S. Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr. Biol. 1999;9:1259–1262. doi: 10.1016/s0960-9822(99)80508-9. [DOI] [PubMed] [Google Scholar]
- Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat. Cell Biol. 2006;8:215–26. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- Mellor H. The role of formins in filopodia formation. Bioc. Biophys. Acta. 2010;1803:191–200. doi: 10.1016/j.bbamcr.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat. Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- Ming G, Song H, Berninger B, Inagaki N, Tessier-Lavigne M, Poo MM. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron. 1999;23:139–148. doi: 10.1016/s0896-6273(00)80760-6. [DOI] [PubMed] [Google Scholar]
- Mintz CD, Dickson TC, Gripp ML, Salton SR, Benson DL. ERMs colocalize transiently with L1 during neocortical axon outgrowth. J. Comp. Neurol. 2003;464:438–448. doi: 10.1002/cne.10809. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Mogilner A, Oster G. Polymer motors: pushing out the front and pulling up the back. Curr Biol. 2003;13:R721–33. doi: 10.1016/j.cub.2003.08.050. [DOI] [PubMed] [Google Scholar]
- Myers JP, Gomez TM. Focal adhesion kinase promotes integrin adhesion dynamics necessary for chemotropic turning of nerve growth cones. J. Neurosci. 2011;31:13585–13595. doi: 10.1523/JNEUROSCI.2381-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JP, Robles E, Ducharme-Smith A, Gomez TM. Focal adhesion kinase modulates Cdc42 activity downstream of positive and negative axon guidance cues. J. Cell Sci. 2012 doi: 10.1242/jcs.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JP, Santiago-Medina M, Gomez TM. Regulation of axonal outgrowth and pathfinding by integrin-ECM interactions. Dev Neurobiol. 2011;71:901–923. doi: 10.1002/dneu.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng J, Luo L. Rho GTPases regulate axon growth through convergent and divergent signaling pathways. Neuron. 2004;44:779–793. doi: 10.1016/j.neuron.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Nicol X, Hong KP, Spitzer NC. Spatial and temporal second messenger codes for growth cone turning. Proc. Natl. Acad. Sci. USA. 2011;108:13776–13781. doi: 10.1073/pnas.1100247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiges R, Eichinger R, Kutschera W, Fischer I, Nemeth Z, Wiche G, Propst F. Microtubule-associated protein 1A (MAP1A) and MAP1B: light chains determine distinct functional properties. J. Neurosci. 2002;22:2106–2114. doi: 10.1523/JNEUROSCI.22-06-02106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak CW, Flynn KC, Bamburg JR. Actin-binding proteins take the reins in growth cones. Nat. Rev. Neurosci. 2008;9:136–47. doi: 10.1038/nrn2236. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 2000;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- Quinn CC, Wadsworth WG. Axon guidance: asymmetric signaling orients polarized outgrowth. Trends Cell Biol. 2008;18:597–603. doi: 10.1016/j.tcb.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers GJA. Rho proteins, mental retardation and the cellular basis of cognition. Trends in Neurosci. 2002;25:191–199. doi: 10.1016/s0166-2236(00)02118-4. [DOI] [PubMed] [Google Scholar]
- Renkema GH, Pulkkinen K, Saksela K. Cdc42/Rac1-mediated activation primes PAK2 for superactivation by tyrosine phosphorylation. Mol. Cell Biol. 2002;22:6719–6725. doi: 10.1128/MCB.22.19.6719-6725.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenu C, Athman R, Robine S, Louvard D. The co-workers of actin filaments: from cell structures to signals. Nat. Rev. Mol. Cell Biol. 2004;5:635–646. doi: 10.1038/nrm1437. [DOI] [PubMed] [Google Scholar]
- Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nat. Neurosci. 2004;7:1059–1069. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat. Neurosci. 2006;9:1274–1283. doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- Robles E, Huttenlocher A, Gomez TM. Filopodial calcium transients regulate growth cone motility and guidance through local activation of calpain. Neuron. 2003;38:597–609. doi: 10.1016/s0896-6273(03)00260-5. [DOI] [PubMed] [Google Scholar]
- Romero S, Le Clainche C, Didry D, Egile C, Pantaloni D, Carlier MF. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell. 2004;119:419–429. doi: 10.1016/j.cell.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Rotty JD, Wu C, Bear JE. New insights into the regulation and cellular functions of the Arp2/3 complex. Nat. Rev. Mol. Cell. Biol. 2013;14:7–12. doi: 10.1038/nrm3492. [DOI] [PubMed] [Google Scholar]
- Saijilafu, Zhang BY, Zhou FQ. Signaling pathways that regulate axon regeneration. Neurosci. Bull. 2013;29:411–420. doi: 10.1007/s12264-013-1357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Soriano N, Travis M, Dajas-Bailador F, Goncalves-Pimentel C, Whitmarsh AJ, Prokop A. Mouse ACF7 and drosophila short stop modulate filopodia formation and microtubule organisation during neuronal growth. J. Cell Sci. 2009;122:2534–2542. doi: 10.1242/jcs.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Medina M, Gregus KA, Gomez TM. PAK-PIX interactions regulate adhesion dynamics and membrane protrusion to control neurite outgrowth. J. Cell Sci. 2013;126:1122–1133. doi: 10.1242/jcs.112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmiere PD, Bamburg JR. Regulation of the neuronal actin cytoskeleton by ADF/cofilin. J. Neurobiol. 2004;58:103–117. doi: 10.1002/neu.10267. [DOI] [PubMed] [Google Scholar]
- Schevzov G, Curthoys NM, Gunning PW, Fath T. Functional diversity of actin cytoskeleton in neurons and its regulation by tropomyosin. Int. Rev. Cell Mol. Biol. 2012;298:33–94. doi: 10.1016/B978-0-12-394309-5.00002-X. [DOI] [PubMed] [Google Scholar]
- Scita G, Confalonieri S, Lappalainen P, Suetsugu S. IRSp53: crossing the road of membrane and actin dynamics in the formation of membrane protrusions. Trends Cell Biol. 2008;18:52–60. doi: 10.1016/j.tcb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, Greenberg ME. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- Shekarabi M, Moore SW, Tritsch NX, Morris SJ, Bouchard JF, Kennedy TE. Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J. Neurosci. 2005;25:3132–3141. doi: 10.1523/JNEUROSCI.1920-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim S, Goh EL, Ge SY, Sailor K, Yuan JP, Roderick HL, Bootman MD, Worley PF, Song H, Ming GL. XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat. Neurosci. 2005;8:730–735. doi: 10.1038/nn1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow DM, Atkinson P, Hassinger T, Kater SB, Letourneau PC. Growth cone intracellular calcium levels are elevated upon contact with sulfated proteoglycans. Dev. Biol. 1994;166:87–100. doi: 10.1006/dbio.1994.1298. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo MM. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr. Opin. Neurobiol. 1999;9:355–363. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- Sotelo C. The chemotactic hypothesis of Cajal: A Century Behind. Progr. Brain Res. 2002;136:11–20. doi: 10.1016/s0079-6123(02)36004-7. [DOI] [PubMed] [Google Scholar]
- Stevens A, Jacobs JR. Integrins regulate responsiveness to slit repellent signals. J. Neurosci. 2002;22:4448–4455. doi: 10.1523/JNEUROSCI.22-11-04448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter DM, Errante LD, Belotserkovsky V, Forscher P. The Ig superfamily cell adhesion molecule, apCAM, mediates growth cone steering by substrate-cytoskeletal coupling. J. Cell Biol. 1998;141:227–240. doi: 10.1083/jcb.141.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter DM, Forscher P. Substrate-cytoskeletal coupling as a mechanism for the regulation of growth cone motility and guidance. J. Neurobiol. 2000;44:97–113. [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Behrens J, Offermanns S. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron. 2002;35:51–63. doi: 10.1016/s0896-6273(02)00750-x. [DOI] [PubMed] [Google Scholar]
- Sydor AM, Su AL, Wang FS, Xu A, Jay DG. Talin and Vinculin Play Distinct Roles in Filopodial Motility in the Neuronal Growth Cone. J. Cell Biol. 1996;134:1197–1207. doi: 10.1083/jcb.134.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T, Pavenstadt H, Hsu HH, Schlondorff J, Ramos A, Greka A. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Science Signaling. 2010;3:ra77. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriyama M, Kozawa S, Sakumura Y, Inagaki N. Conversion of a signal into forces for axon outgrowth through Pak1-mediated shootin1 phosphorylation. Curr. Biol. 2013;23:529–534. doi: 10.1016/j.cub.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Torres E, Rosen MK. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Molecular Cell. 2003;11:1215–1227. doi: 10.1016/s1097-2765(03)00139-4. [DOI] [PubMed] [Google Scholar]
- Tsvetkov AS, Samsonov A, Akhmanova A, Galjart N, Popov SV. Microtubule-binding proteins CLASP1 and CLASP2 interact with actin filaments. Cell Motil. Cytoskel. 2007;64:519–30. doi: 10.1002/cm.20201. [DOI] [PubMed] [Google Scholar]
- Turney SG, Bridgman PC. Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nat. Neurosci. 2005;8:717–719. doi: 10.1038/nn1466. [DOI] [PubMed] [Google Scholar]
- Worth DC, Daly CN, Geraldo S, Oozeer F, Gordon-Weeks PR. Drebrin contains a cryptic F-actin-bundling activity regulated by Cdk5 phosphorylation. J. Cell Biol. 2013 Aug 26; doi: 10.1083/jcb.201303005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goor D, Hyland C, Schaefer AW, Forscher P. The role of actin turnover in retrograde actin network flow in neuronal growth cones. PLoS One. 2012;7(2):e30959. doi: 10.1371/journal.pone.0030959. doi: 10.1371/journal.pone.0030959. Epub 2012 Feb 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastrik I, Eickholt BJ, Walsh FS, Ridley A, Doherty P. Sema3A-induced growth-cone collapse is mediated by Rac1 amino acids 17-32. Curr. Biol. 1999;9:991–998. doi: 10.1016/s0960-9822(99)80447-3. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration – the actin connection. J. Cell Sci. 122:199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitriol EA, Zheng JQ. Growth Cone Travel in Space and Time: the Cellular Ensemble of Cytoskeleton, Adhesion, and Membrane. Neuron. 2012;73:1068–1081. doi: 10.1016/j.neuron.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GX, Poo MM. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature. 2005;434:898–904. doi: 10.1038/nature03478. [DOI] [PubMed] [Google Scholar]
- Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, Tang H, Wen L, Brady-Kalnay SM, Mei L, Wu JY, Xiong WC, Rao Y. Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell. 2001;107:209–221. doi: 10.1016/s0092-8674(01)00530-x. [DOI] [PubMed] [Google Scholar]
- Woo S, Gomez TM. Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J. Neurosci. 2006;26:1418–1428. doi: 10.1523/JNEUROSCI.4209-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- Yang C, Svitkina T. Filopodia initiation: focus on the Arp2/3 complex and formins. Cell Adh. Migr. 2011;5:402–408. doi: 10.4161/cam.5.5.16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmola EG, Bubb MR. How depolymerization can promote polymerization: the case of actin and profilin. Bioessays. 2009;31:1150–1160. doi: 10.1002/bies.200900049. [DOI] [PubMed] [Google Scholar]
- Yebra M, Montgomery AM, Diaferia GR, Kaido T, Silletti S, Perez B, Just ML, Hildbrand S, Huford R, Florkiewicz E, Tessier-Lavigne M, Cirulli V. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev. Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- Yuan XB, Jin M, Xu X, Song YQ, Wu CP, Poo MM, Duan S. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nat. Cell Biol. 2003;5:38–45. doi: 10.1038/ncb895. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat. Cell Biol. 2008;10:1062–1068. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JQ, Felder M, Conner JA, Poo M. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- Zhou FQ, Waterman-Storer CM, Cohan CS. Focal loss of actin bundles causes microtubule redistribution and growth cone turning. J. Cell Biol. 2002;157:839–849. doi: 10.1083/jcb.200112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Video S1
Supp Video S2
Supp Video S3