Antibody against CD44s Inhibits Pancreatic Tumor Initiation and Post-Radiation Recurrence in Mice (original) (raw)
. Author manuscript; available in PMC: 2015 Apr 1.
Published in final edited form as: Gastroenterology. 2014 Jan 5;146(4):1108–1118.e12. doi: 10.1053/j.gastro.2013.12.035
Abstract
Background & Aims
CD44s is a surface marker of tumor-initiating cells (TICs); high tumor levels correlate with metastasis and recurrence, as well as poor outcomes of patients. Monoclonal antibodies against CD44s might eliminate TICs with minimal toxicity. This strategy is unclear for treatment of pancreatic cancer, and little is known about how anti-CD44s affect pancreatic cancer initiation or recurrence after radiotherapy.
Methods
192 pairs of human pancreatic adenocarcinoma and adjacent non-tumor pancreatic tissues were collected from patients undergoing surgery. We measured CD44s levels in tissue samples and pancreatic cancer cell lines by immunohistochemistry, real-time PCR and immunoblot; levels were correlated with patient survival times. We studied the effects of anti-CD44s in mice with human pancreatic tumor xenografts, and used flow cytometry to determine effects on TICs. Changes in CD44s signaling were examined by real-time PCR, immunoblot, reporter assay, and in vitro tumorsphere formation assays.
Results
Levels of CD44s were significantly higher in pancreatic cancer than adjacent non-tumor tissues. Patients whose tumors expressed high levels of CD44s had a median survival of 10 months, compared to 43 months for those with low levels. Anti-CD44s reduced growth, metastasis, and post-radiation recurrence of pancreatic xenograft tumors in mice. The antibody reduced the number of TICs in cultured pancreatic cancer cells and in xenograft tumors, as well as their tumorigenicity. In cultured pancreatic cancer cell lines, anti-CD44s downregulated the stem cell self-renewal genes Nanog, Sox-2, and Rex-1 and inhibited STAT3-mediated cell proliferation and survival signaling.
Conclusions
The TIC marker CD44s is upregulated in human pancreatic tumors and associated with patient survival time. CD44s is required for initiation, growth, metastasis, and post-radiation recurrence of xenograft tumors in mice. Anti-CD44s eliminated bulk tumor cells as well as TICs from the tumors. Strategies to target CD44s might be developed to block pancreatic tumor formation and post-radiotherapy recurrence in patients.
Keywords: cancer stem cell, H4C4, tumorigenesis, progression
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related deaths with an overall 5-year survival less than 5% 1. At the time of diagnosis, over 80% of the patients are not eligible for curative surgery resection and 70–80% of patients die with widespread metastatic disease 2. Patients with locally advanced (unresectable) disease treated with chemoradiation have a median survival time of only 10–12 months due to both local disease progression and metastasis 3, 4. Therfore, it is critical to develop new therapeutic strategies based on a new understanding of the biological mechanisms of resistance and recurrence.
Emerging evidence suggest that cancer originates from tumor initiating cells (TICs) or cancer stem cells (CSCs), which are a small subpopulation of cells capable of self-renewal and differentiation into multiple cell types 5. TICs lie dormant during the process of conventional radiotherapy and chemotherapy that mainly eliminate the more rapidly proliferating cells, and regrow after conventional therapy has discontinued 6. The persistence of TICs may be the cause of the high frequency of relapse and failure after current therapies. Therefore, optimal therapeutic strategies should not only focus on eliminating proliferating cancer cells that form the bulk tumor mass but also on eradicating TICs 7.
CD44 is highly expressed in both bulk tumor cells and TICs 5, representing a promising candidate as a therapeutic target. CD44, as a major receptor for extracellular components, such as hyaluronic acid (HA), is involved in many cellular mechanisms, including cell adhesion, migration, proliferation, survival and chemo-resistance 5. Upregulation of CD44 is correlated with tumor progression and metastatic phenotype in a broad range of cancers, including pancreatic cancer 5, 8. CD44 has also been linked to radiotherapy resistance in larynx and prostate cancer 9, 10. Additionally, CD44 has extensively been studied as a marker for TICs in breast, gastric, head and neck, ovarian, colon, and pancreatic cancer 5, 6. We recently identified CD44+ subpopulation such as CD44+CD24+ESA+ and CD133+CD44+ as the putative pancreatic TICs 8, 11. CD44+ cells are more resistant to chemotherapy in pancreatic and gastric cancer 12, 13. However, the role of CD44 in the resistance of radiotherapy in pancreatic cancer remains undefined.
There are two types of CD44 proteins: the standard isoform (CD44s) is expressed predominantly in hematopoietic cells, normal epithelial cells and TICs, and the variant isoforms (CD44v) are expressed in some epithelial cells during embryonic development, activated lymphocytes and on several types of cancer cells 5, 14. Although the importance of CD44v, especially CD44v6, in tumor progression has been demonstrated in many tumors, the identification of CD44 as a TIC marker is based on CD44s 5, 15, 16. CD44s is essential for cells undergoing epithelial-mesenchymal transition (EMT) and is required for the formation of EMT-associated recurrent breast tumors 17. CD44s has been shown to regulate the mesenchymal phenotype in hepatocellular carcinoma cells 14. As EMT is considered a characteristic of stem cell-like cells 18 and is associated with tumor recurrence and metastasis 17, CD44s may play an important role in TICs and TICs-related tumor recurrence and metastasis. Highly expressed CD44s has been reported in pancreatic cancer, however the sample size of these studies is relatively small (fewer than 52 cases) 19, 20. Furthermore, neither the effect of CD44s expression level nor the role of CD44s-associated TICs, has been investigated in the context of pancreatic tumor recurrence (especially post-radiation recurrence) 19, 20.
During epithelial tumor cell activation, Nanog and signal transducer and activator of transcription 3 (STAT3) are both structurally linked and functionally coupled in HA/CD44 signalling, and they mediate the chemo-resistance effect of CD44 in stem cell-like cells 21, 22. HA/CD44 signalling increases Nanog phosphorylation and translocation to the nucleus, thus initiating the upregulation of the inhibitor of apoptosis (IAP) proteins and multidrug-resistant protein 1 (MDR1). This could be one of the mechanisms through which CD44 contributes to TICs resistance to chemotherapy 5, 21. CD44 has also been reported to activate STAT3 signalling by interacting with Nanog 21, 22. STAT3 plays an important role in regulating cancer cell growth and survival, and has key functions in the maintenance of self-renewal of embryonic stem cells which are required for growth of CD44+CD24− stem cell-like breast cancer cells 23.
We have previously shown that CD44+ TICs are capable of initiating pancreatic tumorigenesis 8, 11. In this study, we show that CD44s, highly expressed in both bulk tumor cells and TICs, acts as an independent prognostic factor and therapeutic target for pancreatic cancer. The anti-CD44s monoclonal antibody, H4C4 24, inhibits post-radiation recurrence in mice by reducing pancreatic TICs through modulating the expression and activity of the stem cell gene, Nanog. H4C4 also promotes cell death in pancreatic cancer cells and inhibits pancreatic tumor growth and metastasis, at least in part, by regulating STAT3 signaling. These results suggest that targeting CD44s by a specific antibody may become a promising therapeutic strategy to block pancreatic tumor initiation and post-radiotherapy recurrence.
Materials and Methods
Antibodies and Reagents
Reagents details are provided in the Supplementary Table S1. The detailed methods are described in online supplemental data.
Patient Samples and TMA
Thirty-six pairs of fresh human pancreatic adenocarcinoma specimens and adjacent non-tumor pancreatic tissues were collected from patients who underwent surgery at the University of Michigan Comprehensive Cancer Center (UMCCC) (Ann Arbor, MI, USA) and the National Engineering Center for Biochip (NECB) (Shanghai, China). Tissue microarrays (TMA) comprised of 156 paired human pancreatic adenocarcinoma specimens and adjacent non-tumor tissues (including normal pancreas and chronic pancreatitis) within the edge of 5 cm were obtained from NECB. The clinical, pathological, and treatment information, together with follow-ups and the consent forms were also obtained for these 156 patients. This study was reviewed and approved by the Institutional Review Board of the Fourth Military Medical University and University of Michigan Cancer Center.
For TMA, four micrometer sections of tissue were transferred to an adhesive-coated slide; immunohistochemical staining was performed 25. The number of positively stained cells and the intensity of positive staining was scored by two pathologists independently, and averaged to obtain a final score for the tissue. Scoring was based on the percentage of positively stained cells: score 0 had no positive cells; scores 1, 2, and 3 had 1–25%, 26–75% and > 75% positive cells, respectively. The intensity of positively stained cells was assessed as: score 0 displayed no visible difference as compared to the negative control sample; the positively stained cells of scores 1, 2 and 3 were light brown (positive staining can be observed clearly under 400X magnification), mid-brown (positive staining can be observed clearly under 200X) and dark brown (positive staining can be observed clearly under 100X), respectively, with the same intensity covering more than 75% of the staining area. The immunostaining of each tissue was assessed in five areas of the acquired images of each tissue section and the average of these five scores was calculated.
Cell lines, cell culture and tretament
Human pancreatic cancer cell lines (PANC-1, MIA PaCa-2, BxPC3, L3.6pL, Hs-766T, AsPC-1, Panc03.27 and COLO357) were purchased from American Type Culture Collection and cultured in DMEM or RPMI 1640 (HyClone, Logan, UT), supplemented with 10% fetal bovine serum (HyClone). Cells were incubated with 0–20 μg/ml H4C4 or normal mouse IgG (nIgG) in DMEM medium containing 10% fetal bovine serum at 37°C for 1 hour. The antibody doses used were based on the dose giving the maximal inhibition of cell growth and are also achievable in vivo. Treated cells were used for the following experiments: quantitative Real-Time RT-PCR (qRT-PCR), immunoblot assay, in vitro assays of cell growth, cell invasion, sphere/colony formation, apoptosis, and Stem Cell PCR Array. The detailed methods are described in online supplemental data (Supplementary Table S2).
Statistical Analysis
The Kaplan-Meier method and the log-rank test were used to compare overall survival, defined as the time from surgery until death (living patients were censored at the time of their last follow-up). A χ2 test and multivariable Cox proportional hazards model were used to study associations of all variables with patient survival as well as independent associations between CD44s positivity, tumor size greater than 3 cm, tumor stage or lymph node metastasis. One-way ANOVA and two-tailed _t_-tests were employed to analyze in vitro and in vivo data using GraphPad Prism 5.0 (GraphPad Software, http://www.graphpad.com). All data shown are the mean ± SD of triplicate values from three independent experiments. * P < 0.05, ** P < 0.01, and *** P < 0.001 are considered to be statistically significant as indicated.
Results
CD44s is Upregulated in Human Pancreatic Adenocarcinoma and Associated with Patients’ Overall Survival
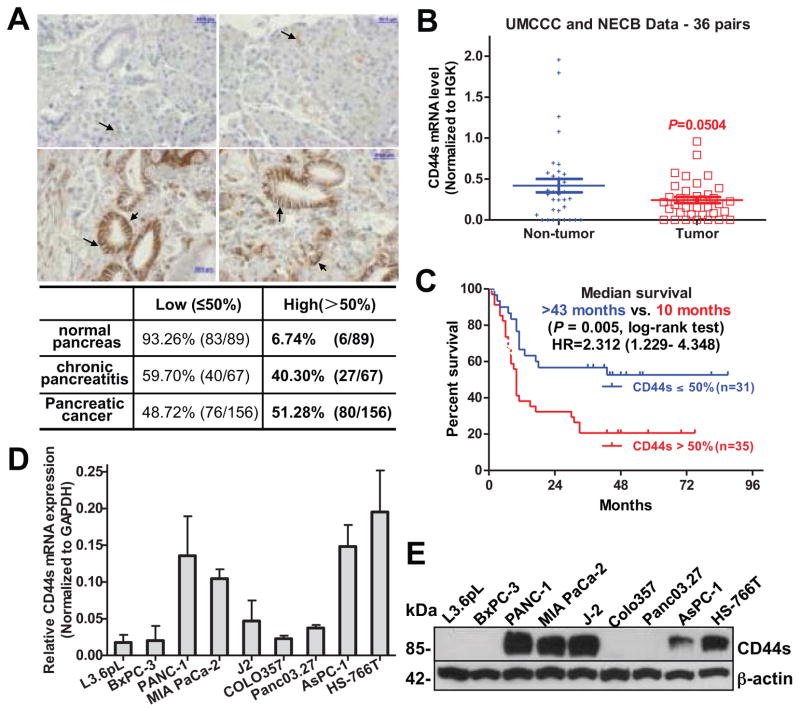
We first investigated the CD44s expression by immunohistochemistry in resected primary tumors from 156 paired pancreatic cancer and adjacent non-tumor tissues. We found that CD44s has negative to weak expression in normal pancreas (87.6%); while moderately to strongly expressed in chronic pancreatitis (59.7%) and pancreatic cancer (72.4%, Supplementary Table S3). The ratio of strong CD44s positivity was significantly higher in cancer as compared to normal pancreas (33.98% vs. 3.37%, P < 0.001) and chronic pancreatitis (33.98% vs. 19.40%, P = 0.029). To be more objective, we divided CD44s expression into high and low groups according to a positive ratio of 50%. CD44s expression in cancer tissues was significantly higher than in adjacent non-tumor pancreatic tissues (51.28% vs. 6.74%, P < 0.001, Figure 1A). Similar results were also observed in CD44 splicing variant, CD44v6. Compared with CD44s, the expression of CD44v6 was significantly higher both in cancer tissues and in paired adjacent non-tumor pancreas tissues (Supplementary Tables S6 & S7). Unexpectedly, CD44s mRNA levels tended to be lower in pancreatic cancer tissues as compared to the paired adjacent non-tumor pancreas tissues, but this difference did not reach statistical significance (0.2426 ± 0.0378 vs. 0.4196 ± 0.0822, P = 0.0504, n = 36) (Figure 1B).
Figure 1. CD44s is upregulated in human pancreatic cancer and is correlated with patients’ overall survival.
(A) Representative CD44s immunohistochemistry staining in pancreatic cancer (lower panel) and adjacent non-tumor tissues (top panel). Bar, 50 μm. Magnification: 400X. Estimated by χ2 test, P < 0.001, compared to normal pancreas. (B) Relative CD44s mRNA levels in 36 pairs of pancreatic cancer and adjacent non-tumor tissues. Data was normalized to 18sRNA/GAPDH. (C) Kaplan-Meier survival analysis of overall survival in 66 patients comparing high and low CD44s expression groups. (D) CD44s mRNA expression in human pancreatic cancer cell lines, tested using qRT-PCR. (E) Western blot of CD44s protein in human pancreatic cancer cell lines. β-actin: loading control.
Next, we examined the correlation of CD44s expression with patients’ survival of 66 pancreatic cancers that had survival data available, by Kaplan-Meier survival analysis. Patients with high expression of CD44s had a median survival of 10 months as compared with more than 43 months for the patients with low expression of CD44s [hazard ratio (HR) =2.31, 95% confidence interval (CI) = 1.23 to 4.35, P = 0.005] (Figure 1C). However, unlike CD44s protein, CD44v6 protein and CD44s mRNA levels were not significantly associated with the patients’ survival (Figures S5–S6). In an effort to explore the correlation between CD44 expression and clinicopathologic characteristics, we observed that CD44s expression was significantly associated with tumor grade (P < 0.001) and tumor location (P = 0.014) (Supplementary Table S4). In a multivariable Cox proportional hazards model, patients with high CD44s expression had worse overall survival as compared to patients with low CD44s expression (HR = 2.79, 95% CI = 1.07 to 7.27, P = 0.036) (Table 1), after adjusting for tumor type, size, grade, location and lymph node metastasis. This data suggest that the CD44s protein level in pancreatic cancer acts as an independent prognostic factor for patient survival.
Table 1.
Multivariable Cox proportional hazards analysis of overall survival
| Characteristic | HR (95% CI) | P value |
|---|---|---|
| No. of involved lymph nodes | ||
| 0 | 1.00 (referent) | |
| ≥1 | 5.281 (0.613 to 45.476) | 0.130 |
| Tumor grade | ||
| I+II | 1.00 (referent) | |
| III | 3.053 (1.306 to 7.138) | 0.010 |
| Tumor CD44s expression | ||
| ≤50% | 1.00 (referent) | |
| >50% | 2.792 (1.072 to 7.272) | 0.036 |
H4C4 Inhibits Pancreatic Tumor Initiation, Metastasis and Post-radiation Recurrence
To select optimal in vitro cell models for exploring the CD44-targeted cancer therapy, we examined CD44s expression in a series of human pancreatic cancer cell lines. We observed that CD44s was highly expressed in PANC-1, MIA PaCa-2, AsPC-1 and HS-766T cell lines, as well as in human pancreatic cancer primary tumor early passage xenograft, J-2 (Figure 1D–E). Based on this result and our previous observation 11, 26 that PANC-1 and MIA PaCa-2 are highly tumorigenic in vivo and reproducibly form tumorspheres in vitro, these two cell lines as well as early passage xenograft, J-2, were selected for the current study.
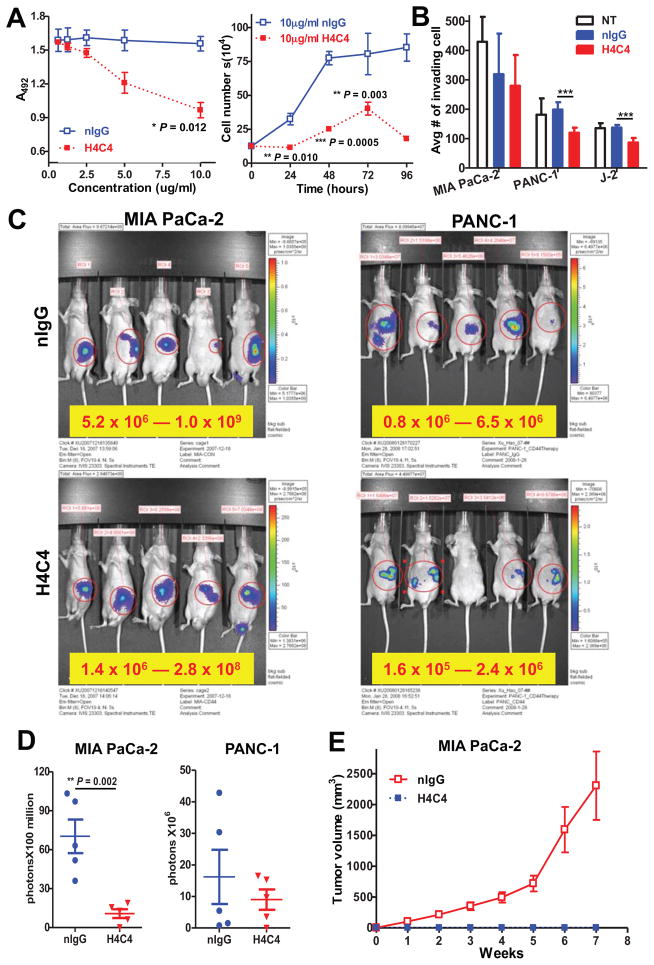
We tested the therapeutic potential of H4C4 on pancreatic cancer cell growth and invasion in vitro. We observed that H4C4 decreased cell growth and cell invasion in a dose- and time-dependent manner as compared to the nIgG control, with a maximal decrease at the concentration of 10 μg/mL when treated for 96 hours (Figures 2A–B, S1C).
Figure 2. H4C4 inhibits pancreatic tumor growth in vitro and in vivo.
(A) In vitro cell growth assay. Cells were treated with H4C4 and nIgG at different doses (0–10μg/mL) and time points (24–96 hours). Cell viability was determined by WST-8 assay (left) and cell counting (right). (B) In vitro cell invasion assay. Cells were treated by 10 μg/mL H4C4 or nIgG. NT, no treatment. (C–D) In vivo tumor growth assay. MIA PaCa-2-luc (left) and PANC-1-luc (right) cells were injected into the tail of pancreas of nude mice (2×106 cells/site, n = 5), 4 mg/kg H4C4 or nIgG were injected i.v. the following day, three times per week for 8 weeks. (C) Bioluminescence imaging analysis of the xenograft mice. Quantifiable photon emission from in vivo luciferase signaling was recorded using IVIS system. Note the color scale bars are different in scale, as marked in yellow box. (D) Change of tumor growth as monitored by BLI luciferase activity at 4 weeks. (E) In vivo tumor formation and tumor growth in mice injected with Mia PaCa-2 cells pre-treated with 8 μg/mL of H4C4 or nIgG (1×106 cells/site, n=10).
Using in vivo luciferase bioluminescence imaging of MIA PaCa-2-Luc and PANC-1-Luc cells, we observed that H4C4 significantly decreased tumor formation and tumor growth as compared to the nIgG control (Figures 2C–D, S2A–B). Furthermore, no tumor metastases were observed in H4C4-treated mice, while 3 of 5 mice developed spleen metastatic lesions in nIgG-treated mice (data not shown). When transplanting H4C4 pre-treated MIA PaCa-2 cells into nude mice, no tumor was formed in H4C4-treated mice, while tumor formation was observed in all nIgG-treated mice (Figure 2E). Histologically, tumor cell necrosis and reduced CD44s immunostaining was observed in H4C4-treated mice, but not in the nIgG control mice (Figure S2D).
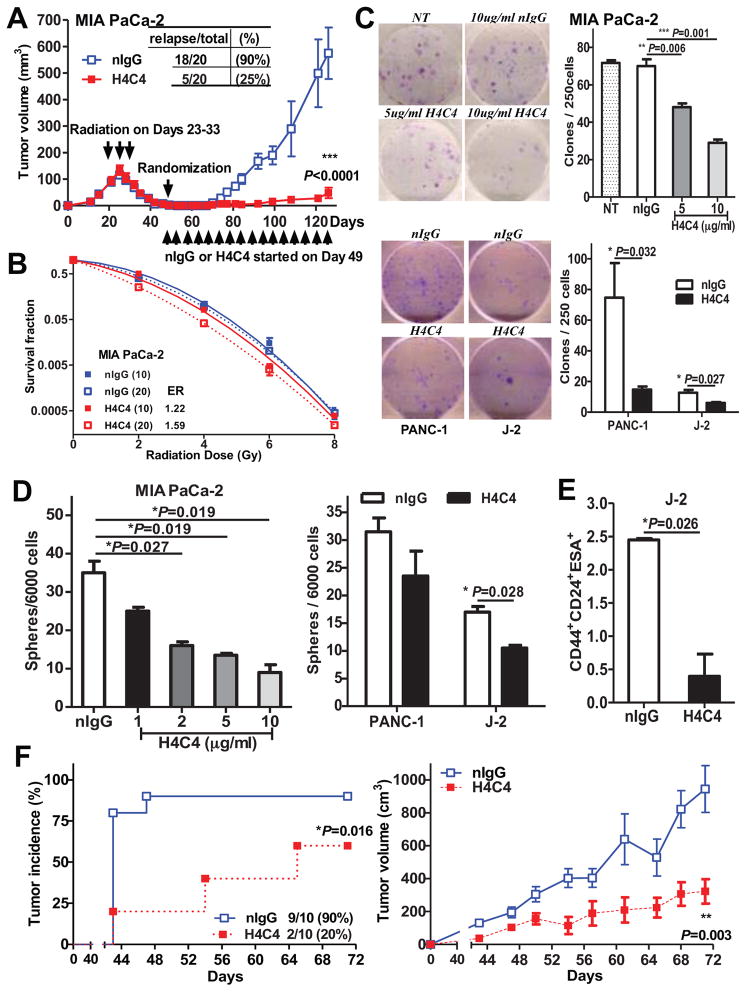
To examine the effect of H4C4 on TICs, we used a post-radiation tumor recurrence model, where the bulk tumor cells were eliminated by radiation and only the CD44+ TICs were left when H4C4 therapy started. H4C4 treatment greatly reduced the post-radiation tumor recurrence as compared to nIgG-treated group [H4C4: 5/20 (25%) tumor relapse vs. nIgG: 18/20 (90%)], and significantly decreased tumor growth (P < 0.001, Figure 3A). We also observed that CD44s expression was increased in tumors that recurred post-radiation (Figure S3A). When MIA PaCa-2 cells were irradiated 5 Gy in vitro, the CD44+/CD133+ TICs subpopulation better survived radiation and increased from 0.69 ± 0.06% to 4.09 ± 0.45% after radiation (P = 0.002, Figure S3B).
Figure 3. H4C4 inhibits post-radiation tumor recurrence by inhibiting pancreatic TICs.
(A) H4C4-mediated inhibition of post-radiation tumor recurrence. MIA PaCa-2 s.q. xenografts at 150 mm3 received daily 2 Gy X-ray radiation (while shielding the rest of the body), total 20 Gy. On day 49, the mice with no palpable tumors were randomized into two groups and started treatment with either H4C4 or nIgG, 4 mg/kg i.v., twice a week. (B) Clonogenic survival assay of MIA PaCa-2 cells treated by 10 and 20 μg/ml H4C4 or nIgG. Survival fractions were plotted and the enhancement ratios (ER) by H4C4 were calculated vs. nIgG. (C) Colony formation assay of MIA PaCa-2 cells treated 5 and 10 μg/ml H4C4 or 10 μg/ml nIgG. Representative pictures of clones were shown. (D) Tumorphere formation assay of 1, 2, 5, 10 μg/ml H4C4 or 10 μg/ml nIgG treated cells. (E) Flow cytometry analysis of CD44+CD24+ESA+ subpopulation in human primary pancreatic cancer early passage J2 xenografts treated with H4C4 or nIgG. (F) Tumor formation and tumor growth evaluation in NOD-SCID mice injected with the sorted CD44+CD24+ESA+ subpopulation of J-2 xenografts treated with 4 mg/kg of H4C4 or nIgG.
We investigated the histological or gross toxicity of H4C4 by conducting histology staining of organs from H4C4 and nIgG treated mice. H4C4 treatment caused no significant impairment of major organs including the heart, lung, liver, spleen and kidney (Figure S2C) and no body weight loss (Figure S3C), indicating a safety profile as a therapeutic agent. Since H4C4 does not bind to mouse CD44, we employed an anti-mouse CD44 antibody for i.v. injection into the SCID mice with same dose/schedule and did not observe any toxicity or body weight loss. Thus, systemic administration of anti-CD44 antibody is safe for syngeneic host.
H4C4 Eliminates TICs
H4C4 sensitized MIA PaCa-2 cells to radiation and inhibited clonogenic growth in a dose-dependent manner as compared to nIgG (Figure 3B–C). In PANC-1 and J-2 cells, H4C4 significantly decreased the number of colonies by 80% and 50%, respectively. H4C4 also significantly inhibited the anchorage-independent growth of pancreatic cancer cells in a dose-dependent manner in MIA PaCa-2 cells (Figure 3D). Radiation resistance, colony formation capabilities and anchorage-independent growth are all characteristics of TICs, thus the data supports our hypothesis that H4C4 negatively affects TICs function.
We also examined the number of TICs and their tumorigenicity by isolating pancreatic TICs using putative markers and flow cytometery analysis 8. H4C4 treatment significantly reduced the CD44+CD24+ESA+ subpopulation in J-2 xenograft tumors in vivo (P = 0.026) and its tumor-initiation capability (P = 0.016, n=10) (Figure 3E–F). Furthermore, the tumors formed in the H4C4 group were much smaller and had a longer latent period than nIgG control. In summary, H4C4 reduced the number of TICs in vitro and in vivo as well as their tumorigenicity. Thus our data provide a strong proof-of-principle that anti-CD44s antibody may be developed as a potential therapeutic agent for eliminating TICs in pancreatic cancer.
H4C4 Eliminates TICs via inhibiting Nanog Signaling
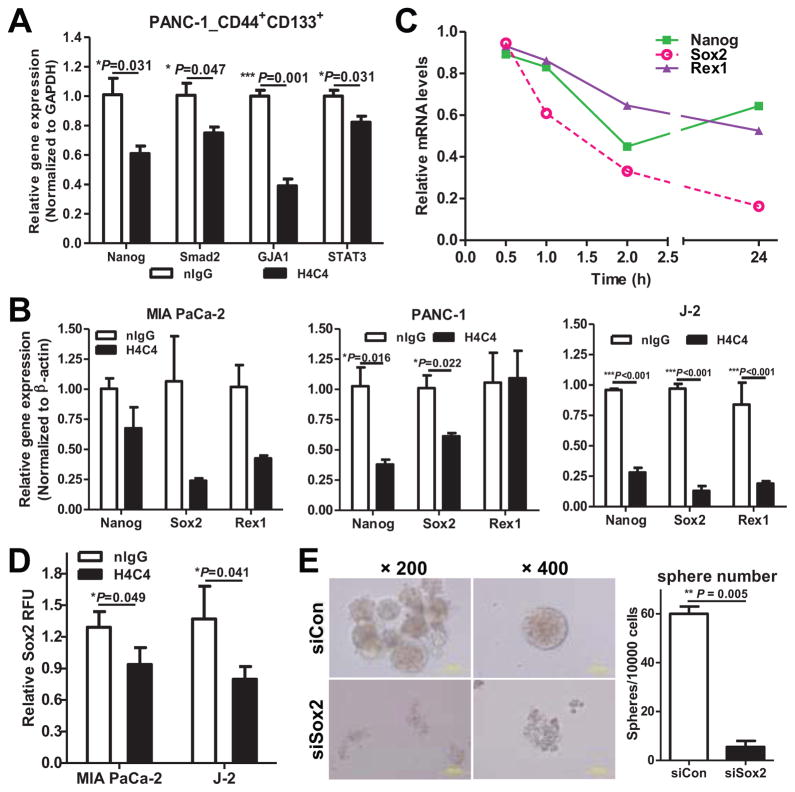
To explore whether H4C4 eliminates TICs through inhibiting stem cell genes or other targets, we carried out qRT-PCR analysis. We observed that H4C4 significantly decreased the expression of Nanog, S_mad2_, GJA1 and STAT3 in H4C4-treated, CD44+CD133+ sorted cells (Figure 4A). H4C4 also reduced the mRNA levels of Nanog as well as its downstream targets Sox2 and Rex1 in pancreatic cancer cells, in a time-dependent manner (Figure 4B–C).
Figure 4. H4C4 inhibits TICs by regulating Nanog signaling.
(A) Nanog, Smad2, GJA1 and STAT3 mRNA expression in the CD44+CD133+ PANC-1 cells after H4C4 or nIgG treatment. Data were normalized to GAPDH. (B) Nanog, Sox2 and Rex1 mRNA expression in MIA PaCa-2, PANC-1 and J-2 cells after H4C4 or nIgG treatment. Data was normalized to β-actin. (C) Time-course of Nanog, Sox2, and Rex1 mRNA expression in J-2 cells after H4C4 treatment. (D) Sox2 transcriptional activity in MIA PaCa-2 and J-2 cells. Cells were treated by 10 μg/mL H4C4 or nIgG. Dual Luciferase assay was performed, a Renilla reporter was used for internal normalization. (E) Tumorphere formation assay in Sox2 knock-down cells.
Functional Sox2 reporter assay showed that H4C4 treatment significantly decreased Sox2 transcriptional activity, as compared to the nIgG control (Figure 4D). Furthermore, Sox2 knock-down phenocopied the inhibitive effect of H4C4 on tumorsphere formation, with a significant decrease of the tumorsphere number (Figure 4E) and size (573.5 ± 95.5 vs. 220.5 ± 26.5 cells/sphere, P = 0.07). In conclusion, this data indicates that H4C4 eliminates pancreatic TICs via inhibiting Nanog signalling and Sox2 transcriptional activity.
We further screened the Human Stem Cell Primer Library to explore other potential stem cell genes affected by H4C4 (Figure S4A, Table S5). We found that 20 out of 88 total stem cell genes (23%) had altered expression (>2.5-fold) upon H4C4 treatment, including: 10 embryonic stem cell markers (COMMD3, GYLTL1B, IFITM1, IFITM2, LEFTY1, LEFTY2, LIN28, NR5A2, NR6A1, NTS), 1 ectoderm marker (VIM), 2 endoderm markers (GATA6, GCG), 3 mesoderm markers (COL1A1, COL2A1, HAND1), 1 trophoblast marker (EOMES), and GAL, PTEN, REXO1. Besides LIN28, REXO1 and GATA6, most of these genes had decreased expression upon H4C4 treatment. Thus, our data suggest that H4C4 treatment eliminates pancreatic TICs by decreasing the expression of a wide spectrum of stem cell genes besides Nanog, Sox2 and Rex1.
H4C4 Inhibits STAT3 Mediated Cell Survival and Anchorage-Independent Growth
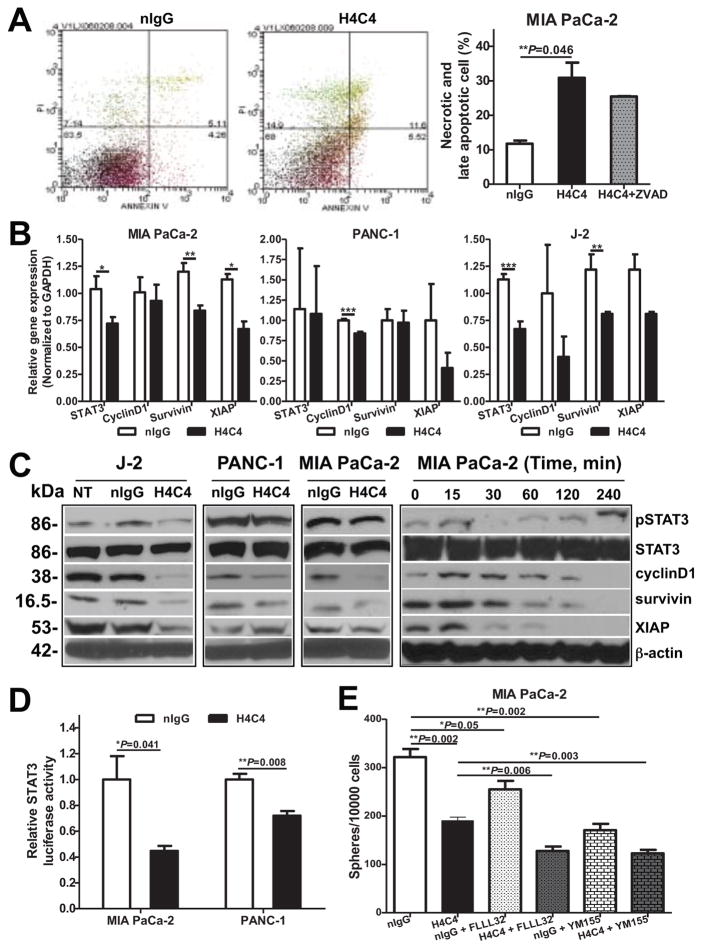
To determine whether the effects of H4C4 on cancer cells’ response to radiation and clonogenic survival were due to apoptosis, we carried out Annexin V/PI staining 27. As shown in Figures 5A & S4B, 5 μg/mL H4C4 treatment for 24 hours significantly increased the percentage of necrotic and late stage apoptotic cells, and this effect was partly attenuated when cells were treated with the apoptosis inhibitor, Z-VAD. This data supports that H4C4 treatment increases both apoptotic and necrotic cell death in pancreatic cancer cells.
Figure 5. H4C4 inhibits bulk tumor cells by regulating STAT3 signaling.
(A) Apoptosis analysis in MIA PaCa-2 cells treated with H4C4 or nIgG using Annexin V-FITC/PI staining. (B) STAT3, cyclin D1, survivin and XIAP mRNA expression in MIA PaCa-2, PANC-1 and J-2 cells after H4C4 or nIgG treatment. Data were normalized to GAPDH. (C) Protein levels of pSTAT3, total STAT3, cyclin D1, survivin and XIAP in MIA PaCa-2, PANC-1 and J-2 cells after H4C4 or nIgG treatment. Cells were collected at 0, 15, 30, 60, 120, and 240 minutes post-treatment. (D) STAT3 transcriptional activity in MIA PaCa-2 and PANC-1 cells after H4C4 or nIgG treatment. The luciferase activity was measured using the Bright-Glo™ Luciferase Assay System, with β-galactosidase as internal control. (E): Tumorsphere formation assay in MIA PaCa-2 cells after the treatment with STAT3 inhibitor FLLL32 (5 μM) or survivin inhibitor YM155 (0.1 μM) alone or in combination with H4C4 (10 μg/ml).
To explore whether H4C4 induces apoptosis through inhibiting STAT3 signaling, we carried out qRT-PCR and immunobloting analysis. As shown in Figure 5B, H4C4 treatment reduced the mRNA and protein levels of STAT3, cyclin D1, survivin and XIAP. At the protein level, a time-dependent inhibition of pSTAT3 and its downstream targets were observed after H4C4 treatment (Figure 5C). The time point of 50% reduction in pSTAT3 and XIAP (30 mins) occurred earlier than that in pSTAT3 specific target genes, cyclin D1 and survivin (120 mins). Functional reporter assay showed that H4C4 significantly inhibited the STAT3-specific transcriptional activity in PANC-1 and MIA PaCa-2 cells (Figure 5D).
We further examined whether inhibiting STAT3-survivin signalling would enhance the effect of H4C4 on the anchorage-independent growth of MIA PaCa-2 cells. As shown in Figure 5E, FLLL32, a specific STAT3 inhibitor 26, 28 that moderately inhibits tumorsphere formation alone, significantly enhanced the H4C4-induced tumorsphere inhibition, from 40.8 ± 0.009% inhibition by H4C4 alone to 60.3 ± 0.021% inhibition when combined with H4C4 (P < 0.001). Similarly, the specific survivin inhibitor YM155 also enhanced H4C4-mediated tumorsphere inhibition (P < 0.001).
Discussion
In this study, we show that the upregulated CD44s protein expression in human pancreatic cancer acts as an independent prognostic factor for patient survival. H4C4, a CD44s-specific antibody, decreases pancreatic tumor growth and metastasis and sensitizes pancreatic cancer cells to radiotherapy. Our data suggest a critical role for CD44s in promoting tumor-initiation and post-radiation recurrence through directly affecting TICs. CD44s specific antibody may provide a promising therapeutic strategy to overcome radiation resistance and might be beneficial for pancreatic cancer patients.
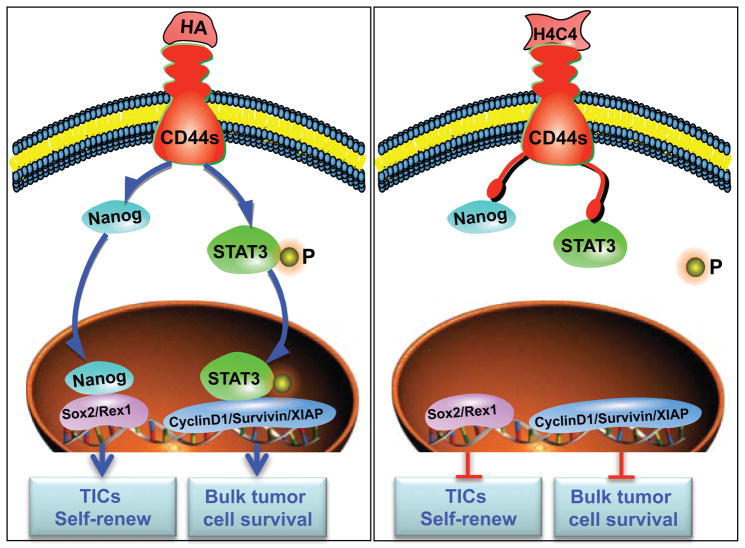
Conventional cancer therapies mainly focus on eradicating the rapidly proliferating bulk tumor cells, however, most therapies are ineffective against the relatively quiescent/dormant TICs 6, 8. In this study, we show that CD44s, being expressed both on bulk tumor cells and TICs 5, could be a promising therapeutic target for pancreatic cancer. Furthermore, we show that H4C4 decreased pancreatic tumor growth and metastasis by deactivating anti-apoptosis and pro-survival mechanisms partly by inhibiting STAT3 signalling. Treating cells with H4C4 reduced post-radiation tumor recurrence through dramatically decreasing the TICs number and their capacity for tumor initiation by downregulating the stem cell self-renewal gene Nanog (Figure 6). Thus, H4C4 may be used as a potential therapeutic agent that targets both bulk tumor cells and TICs. The anti-cancer effects of H4C4 may be improved further when combined with chemotherapy and radiation therapy, which needs to be further studied for clinical application.
Figure 6. A proposed working model for H4C4.
H4C4 deactivates bulk tumor cell survival partly by inhibiting STAT3 signalling pathway, and reduces TICs and its capacity for tumor initiation by downregulating stem cell self-renewal genes Nanog/Sox2/Rex1.
Current cancer therapeutics and target selection are mainly concentrated on one frequently mutated oncogene or on a single signaling pathway that act as key drivers of oncogenesis. However, these approaches are easily evaded by cancer cells due to their enhanced ability to sustain new genetic and epigenetic alterations 29. Therefore, an optimal strategy is needed to identify broad-spectrum regulators like CD44s, which is upregulated during tumor progression and exerts multiple functions, such as activating pro-survival mechanisms, promoting self-renewal and tumor initiation. It is possible that cancer cells will be sensitized to conventional therapy and thus are not able to fully recover from such an impact by inhibiting a multi-functional protein 5.
CD44 correlates with radiation resistance 9, 10, 30 and chemo-resistance 21, 22. However, due to the usage of antibodies against total CD44 (including CD44s or CD44v), the molecular pattern of CD44, i.e., whether the standard form or variants play a role in radiation sensitivity, has not been well defined. In this study, we provide evidence that CD44s promotes radiation resistance of pancreatic TICs. Furthermore, anti-CD44s antibody has the potential to sensitize cancer cells to radiotherapy by inhibiting pancreatic TICs.
It has been reported that CD44s plays an important role in regulating EMT and EMT-associated tumor recurrence 14, 23. A shift in CD44 expression from CD44v to CD44s was essential for cells to undergo EMT 17, indicating a critical role of CD44s in the modulation of TICs properties. In this study, we showed that CD44s plays a role in regulating TICs and its anchorage-independent growth capability. CD44s contributes to the activation of stem cell genes (e.g., Nanog/Sox2/Rex1) and participates in the maintenance of TICs features (e.g., metastasis and radiation resistance). However, Su et al. 31 reported that nuclear translocation of CD44s play an active role in transforming cancer cells to a TICs-like phenotype, indicating that the role of CD44 in modulation of TICs properties may not be due to full length CD44s but due to a nuclear CD44-Intracytoplasmic Domain (CD44-ICD). Therefore, the molecular form of CD44s that contributes to TICs needs to be further studied.
Inhibition of CD44 could be through ligand mimetics, competitive peptides, short interfering RNA or microRNA mediated approach, and by specific antibodies 5, 6, 13, 32. The antibody therapeutic approach has experienced a clinical and commercial success over the past 30 years 33, while peptides and short interfering RNA or microRNA approaches have limited success due to delivery problems 34. TICs are natural targets of antibody therapeutics since they have an ability to initiate tumor formation and tumor recurrence. Actually, several monoclonal antibodies are currently in clinical trials for targeting TICs, such as CD44 monoclonal antibody H90. H90 markedly reduced the ratio of leukaemia stem cells in vivo as the result of inhibition of proper homing of stem cells to their microenvironment niche 5, 35. As the most established and common TICs marker, CD44s isoform is a target of high interest for tumor therapy. Here, we show that CD44s antibody, H4C4, significantly decreased the TICs population in solid tumors, which is a novel mechanism of action as compared to previous report in haematological cancer 35.
One potential pitfall of the CD44-targeted therapeutic strategies is that CD44s is a marker shared by TICs and adult stem cells (ASCs). Certainly, further investigations need to explore the influence of CD44 antibodies on normal stem cells populations. However, there may exist a therapeutic window that allows for an attack of TICs without affecting ASCs under the condition of CD44s overexpression in TICs as a result of p53 mutation 5. Thus, being cell context dependent, the distinct expression profile of tumour suppressor genes, oncogenes, CD44 partner molecules and/or its downstream signaling in the TICs versus ASCs in a particular patient may dictate this therapeutic window, which needs to be studied further before clinical applications can be achieved.
In summary, we show that CD44s is highly expressed in pancreatic cancer and acts as a poor prognostic factor on patient survival. Targeting CD44s with H4C4 reduced pancreatic tumor growth, metastasis and post-radiation tumor recurrence by dramatically influencing both bulk tumor cells and TICs, especially decreasing TICs capacity of self-renewal and tumor initiation. Our data suggest that targeting CD44s by specific antibodies may provide a promising therapeutic strategy to reduce pancreatic TICs and sensitize cells to radiotherapy for pancreatic cancer patients.
Materials and Methods
Antibodies and Reagents
Details are provided in the Supplementary Table S1. Anti-human CD44s monoclonal antibody was prepared by our lab from hybridoma H4C4 (University of Iowa Developmental Studies Hybridoma Bank) [1]. FLLL32 was a kind gift from Dr. Jiayuh Lin as previously described [2, 3].
CD44s antibody H4C4 preparation
Briefly, H4C4 hybridoma cells were cultured in RPMI medium containing 10% fetal bovine serum (FBS) and then injected into Balb/C mice for antibody production for additional 14 days. The ascites fluid was retrieved, and the anti-CD44s monoclonal antibody H4C4 was purified on a Protein A column according to the manufacturer’s guide (GE Healthcare Biosciences) (Supplementary Figure S1A). The binding activity of H4C4 to CD44s expressed in MIA PaCa-2 cells was measured using cellular ELISA [4] (Supplementary Figure S1B). Control normal mouse nIgG was prepared similarly from Balb/C mouse serum.
Cellular ELISA
Cellular ELISA was used to measure the binding activity of H4C4 to CD44s expressed on MIA PaCa-2 cells. Briefly, cells cultivated in 96 well plate were fixed with 10% buffered formalin for 15 minutes, blocked with 2% bovine serum albumin (BSA) at room temperature for 2 h, incubated with 1% BSA diluted ascites fluid or controls at 37°C for 2 h, and followed by HRP-conjugated goat anti-mouse IgG at 37°C for 1 h. Finally, plates were detected with 3,3′5,5′-tetramethyl benzidine (Sigma, St. Louis, MO) for 20 minutes at room temperature. A490 was measured using a POLARstar OPTIMA Microplate Reader (BMG LABTECH, Cary, NC)
Quantitative Real-Time RT-PCR (qRT-PCR)
Total RNA extraction and cDNA synthesis were carried out as previously described [5]. qRT-PCR was carried out using an ABI 7700® real-time PCR system (Applied Biosystems) with gene specific primers for CD44, STAT3, CyclindD1, survivin, XIAP, Nanog, Sox2, Rex1, β-actin, GAPDH or 18sRNA (Supplementary Table 2). Individual genes of interest (GOI) were normalized to housekeeping genes (HKG): β-actin, 18s RNA or GAPDH. Relative mRNA levels are presented as unit values of 2−ΔCt = 2−(Ct (HKG) −Ct (GOI)).
Immunoblot assay
Immunoblot assays were performed using standard methods [5]. Cells were treated with H4C4 or normal mouse nIgG as indicated in each assay. Membranes were probed with total and phosphorylated antibodies as detailed in Supplementary Table S1.
Cell growth assay
Cells were plated at a density of 8 × 104/ml with 1 ml per well in 24-well plates in complete media, collected and counted every 24 hours for 4 days using a haemocytometer. A cell growth curve was drawn according to the cell numbers at the specified incubation time.
In vitro matrigel invasion assay
Cells were seeded into upper chambers of 8 μm pore transwells coated with matrigel and then allowed to invade the matrigel for 36 hours. Invaded cells were stained with Diff-Quik™ stain (Allegiance), and the invasive potential of the cells was determined by counting the number of cells that had invaded to the lower surface of the filter in 10 different areas under an inverted light microscope (Olympus BX41).
Flow cytometry CD44+CD133+ or CD44+CD24+ESA+ analysis and sorting
Cells were incubated on ice with anti-CD133/1-PE, anti-CD24-PE, anti-CD44-APC and anti-ESA-FITC (Miltenyi Biotec, Aubum, CA, USA) in 2% FBS/HBSS while protected from light for 20 min. Isotype-matched mouse immunoglobulin served as control. Antibody labelled cells were then analysed using a FACS Calibur flow cytometer and CellQuest software (BD Biosciences, San Jose, CA, USA). For cell sorting, cells were stained with the indicated antibodies, washed and resuspended in 2% FBS/HBSS with 1 μg/ml DAPI (Sigma-Aldrich) for gating viable cells. Positive and negative cells were sorted on a BD FACS Vantage SE (BD Biosciences). To isolate human pancreatic cancer CD44+CD133+ cells from the irradiated xenografts, tumors were excised and single-cell suspensions were prepared [6, 7]. Mouse-derived cells were gated using anti-mouse CD31, mouse lineage cocktail, and mouse H-2Kd biotin-conjugated antibodies. The purity of sorted cells was determined as >90% positive. For the positive and negative population, only the top 25% most brightly stained cells or the bottom 20% most dimly stained cells were collected for analysis.
Tumorsphere culture
H4C4 or nIgG treated cells were suspended in DMEM/F12 serum-free medium containing 1% N2, 2% B27, 1% antibiotic-antimycotic, 20 ng/ml human FGF-2, 100 ng/ml EGF, and were seeded in 6-well ultra-low attachment plates (Corning) in triplicates, 10,000 cells per well. 7–10 days later, plates were quantified for sphere numbers under an inverted microscope (Olympus). For subsequent quantification of sphere size, spheres were collected with a 40 μm sieve (BD Biosciences) and disassociated with TrypLE™ to make single cell suspension. The viable cells were then counted using trypan blue exclusion [6].
Colony formation and clonogenic assays
For colony formation, H4C4 or nIgG treated cells were seeded with 250 cells per well into a 6-well plate. For clonogenic assays, H4C4 or nIgG treated cells were seeded with different cell numbers for each radiation dose group (200~10,000 cells/well), and then subjected to X-ray radiation (0, 2, 4, 6, or 8 Gy). Cells were incubated for 2–3 weeks in complete medium and were subsequently stained with 0.1% crystal violet. The colonies with >50 cells were counted manually with the aid of an Olympus INT-2 inverted microscope. Data from radiation treated cells were normalized against the untreated cells. Plating efficiencies and survival fractions were calculated to obtain survival parameters and plot cell survival curves as described [8].
Sox2 Reporter Assays
Transcriptional activity of the Sox2 transcription factor was analyzed according to Cignal Sox2 Reporter (luc) Kit (QIAGEN) according to the manufacturer’s protocol. Briefly, cells were transfected with 0.1 μg Sox2-luc reporter constructs, 0.1 μg negative or positive control constructs using Lipofectamine 2000. Cells were cultivated for 24 hours and then treated with 10 μg/ml H4C4 or nIgG for 1 hour. The luciferase activity was measured in cell lysates by a POLARstar OPTIMA Microplate Reader using the Dual-Luciferase Reporter System (Promega). For comparison, the constitutively expressing Renilla luciferase reporter was used as an internal control for normalizing transfection efficiencies and monitoring cell viability.
STAT3 Reporter Assays
STAT3 transcriptional activity was detected by STAT3-luc reporter plasmid using the Bright-Glo™ Luciferase Assay System (Promega) according to the manufacturer’s protocol [3]. Cells were seeded in 48-well plates and were then transfected with 0.5μg STAT3-luc reporter constructs or 0.5 μg of a GL3 vector control using Lipofectamine 2000. Sixteen hours after transfection, cells were treated 10 μg/mL H4C4 or nIgG for 1 hour. Luciferase activity was then measured in cell lysates by a POLARstar OPTIMA Microplate Reader using the Bright-Glo™ Luciferase Assay System. For comparison, luciferase activity was normalized to β-galactosidase expression using the Beta-Glo® Assay System (Promega).
Apoptosis Assay
Apoptosis was detected using Annexin V-FITC and propidium iodide (PI) double staining kit (Trevigen, Gaithersburg, MD) as described [8]. Cells were analyzed with a flow cytometer (FACSCalibur; BD Biosciences) in the Flow Cytometry Core at the University of Michigan Cancer Center.
Stem Cell PCR Array
Total RNAs isolated from MIA PaCa-2 cells treated 10μg/mL H4C4 or nIgG were reverse transcribed into cDNA, and amplified by Human Stem Cell Primer Library (HSCL-I) from RealTime Primers LLC (Elkins Park, PA, USA), which contains 88 primer sets directed against stem cell-related genes and 8 housekeeping gene primer sets (ACTB, B2M, GAPD, GUSB, HPRT1, PGK1, PPIA, RPL13A) (Supplementary Table 5). The detailed PCR conditions were as described previously [2]. Individual genes of interest (GOI) were normalized to housekeeping genes (HKG). The PCR array data were validated by qRT-PCR using TaqMan Universal PCR Master Mix in an ABI 7700® real-time PCR system (Applied Biosystems).
Animal studies
Animal experiments were done according to the protocol approved by University of Michigan Guidelines for Use and Care of Animals. Five- to six-week old female athymic NCr-nu/nu nude mice or NOD-SCID mice were transplanted with human pancreatic cancer cells as previously described [5, 8, 9]. Briefly, 1–2×106 MIA PaCa-2 or PANC-1 cells or 2×104 sorted J-2 cells in 0.2 ml cell suspension were collected and inoculated either subcutaneously or into pancreas tail of mice (5–20 mice/group). H4C4 (4 mg/kg) was intravenous injected (i.v.) the following day, and maintained 2–3 times per week for 2–10 weeks. nIgG was included as a control. In the post-radiation tumor recurrence experiment, MIA PaCa-2 cells were injected subcutaneously into nude mice (1×106 cells/site, n = 20). Xenograft tumors were daily X-ray irradiated at 2 Gy on days 23–33 (total 20 Gy), while shielding the rest of the body [8]. Then on day 49, when all tumors were completely regressed as indicated by no palpable tumors, mice were randomized into two groups and treated with H4C4 or nIgG, 4 mg/kg i.v., twice a week. Tumor size and body weight were measured twice weekly and plotted as previously described [5]. Tumor volume was calculated using the formula: (length × width2)/2. At the end of experiments, tumors were dissected to measure tumor weight, and heart, lung, liver, spleen, kidney were removed from the mice for histopathological examination. The tissues were fixed in 10% neutral formalin, and paraffin-embedded blocks were sectioned at a thickness of 6 μm. The sections were dewaxed and stained with hematoxylin and eosin. For bioluminescence imaging (BLI) analysis of the orthotopic pancreatic cancer models, MIA PaCa-2-luc/PANC-1-luc cells were used, and quantifiable photon emission from in vivo luciferase bioluminescent signals were recorded using IVIS system as we described previously [8, 9].
Supplementary Material
Acknowledgments
We wish to thank Medjaden Bioscience Limited for editing and proofreading of this manuscript, the University of Michigan Comprehensive Cancer Center (UMCCC) Flow Cytometry Core for flow cytometry analysis and cell sorting, and the University of Michigan Unit of Laboratory Animal Medicine for help with animal experiments.
Abbreviations
TICs
tumor initiating cells
CSCs
cancer stem cells
HA
Hyaluronic Acid
EMT
epithelial-mesenchymal transition
MDR1
multidrug-resistant protein 1
STAT3
signal transducer and activator of transcription 3
TMA
tissue microarrays
ASCs
adult stem cells
Footnotes
†
Grant Support: This study was supported in part by National Institutes of Health grants (R01 CA121830 S1 and CA134655 to L. X.); NIH University of Michigan Cancer Center Support Grant (5 P30 CA46592); K-INBRE (P20 GM103418) Bridging Grant and Kansas Bioscience Authority Rising Star Award (to L. X.), National Science Foundation of China grant 81160260 (to X. H.), and China National Science and Technology Major Project 2013ZX09301301 (to Z.N. C.). The sponsors had no role in the study design in the collection, analysis, and interpretation of data.
‡
Author disclosures: The authors declare no conflicts of interest.
Author Contributions:
Study concept and design: LL, LX, TSL, ZNC.
Acquisition of data: LL, XH, JQ, WT, FH, MZ, AS.
Analysis and interpretation of data: LL, JQ, XTQ, WT.
Contributed reagents/materials/analysis tools: XH, JQ, FH, DMS.
Drafting and editing of the manuscript: LL, LX, AS.
Critical revision of the manuscript: LX, TSL, DMS, ZNC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet. 2011;378:607–20. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–13. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutt R, Liauw SL, Weichselbaum RR. The role of radiotherapy in locally advanced pancreatic carcinoma. Nat Rev Gastroenterol Hepatol. 2010;7:437–47. doi: 10.1038/nrgastro.2010.98. [DOI] [PubMed] [Google Scholar]
- 4.Trakul N, Koong AC, Maxim PG, et al. Modern radiation therapy techniques for pancreatic cancer. Gastroenterol Clin North Am. 2012;41:223–35. doi: 10.1016/j.gtc.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Zller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nature Reviews Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Fu L. Targeting cancer stem cells: a new therapy to cure cancer patients. Am J Cancer Res. 2012;2:340–56. [PMC free article] [PubMed] [Google Scholar]
- 7.McCubrey JA, Steelman LS, Abrams SL, et al. Targeting the cancer initiating cell: the ultimate target for cancer therapy. Current pharmaceutical design. 2012;18:1784–95. doi: 10.2174/138161212799859701. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 9.de Jong MC, Pramana J, van der Wal JE, et al. CD44 expression predicts local recurrence after radiotherapy in larynx cancer. Clin Cancer Res. 2010;16:5329–38. doi: 10.1158/1078-0432.CCR-10-0799. [DOI] [PubMed] [Google Scholar]
- 10.Xiao W, Graham PH, Power CA, et al. CD44 is a biomarker associated with human prostate cancer radiation sensitivity. Clin Exp Metastasis. 2012;29:1–9. doi: 10.1007/s10585-011-9423-7. [DOI] [PubMed] [Google Scholar]
- 11.Ji Q, Hao X, Zhang M, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takaishi S, Okumura T, Tu S, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–20. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong SP, Wen J, Bang S, et al. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125:2323–31. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 14.Mima K, Okabe H, Ishimoto T, et al. CD44s Regulates the TGF-beta-Mediated Mesenchymal Phenotype and Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma. Cancer Res. 2012;72:3414–23. doi: 10.1158/0008-5472.CAN-12-0299. [DOI] [PubMed] [Google Scholar]
- 15.Hao JL, Cozzi PJ, Khatri A, et al. CD147/EMMPRIN and CD44 are potential therapeutic targets for metastatic prostate cancer. Curr Cancer Drug Targets. 2010;10:287–306. doi: 10.2174/156800910791190193. [DOI] [PubMed] [Google Scholar]
- 16.Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer. 2010;46:1271–7. doi: 10.1016/j.ejca.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Brown RL, Reinke LM, Damerow MS, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121:1064–74. doi: 10.1172/JCI44540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Immervoll H, Hoem D, Steffensen OJ, et al. Visualization of CD44 and CD133 in normal pancreas and pancreatic ductal adenocarcinomas: non-overlapping membrane expression in cell populations positive for both markers. J Histochem Cytochem. 2011;59:441–55. doi: 10.1369/0022155411398275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomaszewska R, Nowak K, Stachura J. CD44 isoforms expression in intraductal and invasive pancreatic cancer and its correlation to p53 gene mutations. Pol J Pathol. 1999;50:145–53. [PubMed] [Google Scholar]
- 21.Bourguignon LY, Earle C, Wong G, et al. Stem cell marker (Nanog) and Stat-3 signaling promote MicroRNA-21 expression and chemoresistance in hyaluronan/CD44-activated head and neck squamous cell carcinoma cells. Oncogene. 2012;31:149–60. doi: 10.1038/onc.2011.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourguignon LY, Peyrollier K, Xia W, et al. Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem. 2008;283:17635–51. doi: 10.1074/jbc.M800109200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marotta LL, Almendro V, Marusyk A, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell-like breast cancer cells in human tumors. J Clin Invest. 2011;121:2723–35. doi: 10.1172/JCI44745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penno MB, August JT, Baylin SB, et al. Expression of CD44 in human lung tumors. Cancer Res. 1994;54:1381–7. [PubMed] [Google Scholar]
- 25.Dai Y, DeSano JT, Meng Y, et al. Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:1217–25. doi: 10.1016/j.ijrobp.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Tang W, Wu X, Karnak D, et al. HAb18G/CD147 Promotes pSTAT3-Mediated Pancreatic Cancer Development via CD44s. Clin Cancer Res. 2013;19:6703–15. doi: 10.1158/1078-0432.CCR-13-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai Y, Liu M, Tang W, et al. Molecularly targeted radiosensitization of human prostate cancer by modulating inhibitor of apoptosis. Clin Cancer Res. 2008;14:7701–10. doi: 10.1158/1078-0432.CCR-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin L, Hutzen B, Zuo M, et al. Novel STAT3 phosphorylation inhibitors exhibit potent growth-suppressive activity in pancreatic and breast cancer cells. Cancer Res. 70:2445–54. doi: 10.1158/0008-5472.CAN-09-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, Stamenkovic I, Yu Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 2010;70:2455–64. doi: 10.1158/0008-5472.CAN-09-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin JT, Chang TH, Chang CS, et al. Prognostic value of pretreatment CD44 mRNA in peripheral blood of patients with locally advanced head and neck cancer. Oral Oncol. 2010;46:e29–33. doi: 10.1016/j.oraloncology.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Su YJ, Lai HM, Chang YW, et al. Direct reprogramming of stem cell properties in colon cancer cells by CD44. EMBO J. 2011;30:3186–99. doi: 10.1038/emboj.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deonarain MP, Kousparou CA, Epenetos AA. Antibodies targeting cancer stem cells: a new paradigm in immunotherapy? MAbs. 2009;1:12–25. doi: 10.4161/mabs.1.1.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 34.Pan X, Thompson R, Meng X, et al. Tumor-targeted RNA-interference: functional non-viral nanovectors. Am J Cancer Res. 2010;1:32–49. [PMC free article] [PubMed] [Google Scholar]
- 35.Jin L, Hope KJ, Zhai Q, et al. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–74. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
Supplementary References
- 1.Dai Y, Liu M, Tang W, et al. Molecularly targeted radiosensitization of human prostate cancer by modulating inhibitor of apoptosis. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:7701–10. doi: 10.1158/1078-0432.CCR-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin L, Hutzen B, Zuo M, et al. Novel STAT3 phosphorylation inhibitors exhibit potent growth-suppressive activity in pancreatic and breast cancer cells. Cancer Res. 70:2445–54. doi: 10.1158/0008-5472.CAN-09-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Tang W, Wu X, Karnak D, et al. HAb18G/CD147 Promotes pSTAT3-Mediated Pancreatic Cancer Development via CD44s. Clin Cancer Res. 2013;19:6703–15. doi: 10.1158/1078-0432.CCR-13-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang C, Takayanagi A, Yoshida T, et al. Screening of scFv-displaying phages recognizing distinct extracellular domains of EGF receptor by target-guided proximity labeling method. J Immunol Methods. 2011;372:127–36. doi: 10.1016/j.jim.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Lian J, Wu X, He F, et al. A natural BH3 mimetic induces autophagy in apoptosis-resistant prostate cancer via modulating Bcl-2-Beclin1 interaction at endoplasmic reticulum. Cell death and differentiation. 2011;18:60–71. doi: 10.1038/cdd.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji Q, Hao X, Zhang M, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 8.Dai Y, DeSano JT, Meng Y, et al. Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:1217–25. doi: 10.1016/j.ijrobp.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia L, Yang J, Hao X, et al. Validation of SAG/RBX2/ROC2 E3 Ubiquitin Ligase as an Anticancer and Radiosensitizing Target. Clinical Cancer Research. 2010;16:814–24. doi: 10.1158/1078-0432.CCR-09-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.