DNA double-strand breaks promote methylation of histone H3 on lysine 9 and transient formation of repressive chromatin (original) (raw)
Significance
Double-strand break (DSB) repair initiates dynamic changes in histone modifications that are required to maintain genome stability. Methylation of histone H3 lysine-9 (H3K9me3) is critical for activating ataxia telangiectasia-mutated (ATM) kinase, but how H3K9 methylation is regulated at DSBs is unknown. We show that a complex containing the suv39h1 methyltransferase is rapidly recruited to DSBs, where it directs H3K9 methylation on large chromatin domains adjacent to the DSB. This process results in transient formation of repressive chromatin and serves to both stabilize the chromatin structure and promote activation of DSB-signaling proteins, including ATM kinase. Dynamic changes in H3K9 modification in euchromatin by suv39h1 are therefore one of the earliest signaling events required for processing and remodeling of the damaged chromatin template.
Keywords: histone methylation, homologous recombination
Abstract
Dynamic changes in histone modification are critical for regulating DNA double-strand break (DSB) repair. Activation of the Tip60 acetyltransferase by DSBs requires interaction of Tip60 with histone H3 methylated on lysine 9 (H3K9me3). However, how H3K9 methylation is regulated during DSB repair is not known. Here, we demonstrate that a complex containing kap-1, HP1, and the H3K9 methyltransferase suv39h1 is rapidly loaded onto the chromatin at DSBs. Suv39h1 methylates H3K9, facilitating loading of additional kap-1/HP1/suv39h1 through binding of HP1’s chromodomain to the nascent H3K9me3. This process initiates cycles of kap-1/HP1/suv39h1 loading and H3K9 methylation that facilitate spreading of H3K9me3 and kap-1/HP1/suv39h1 complexes for tens of kilobases away from the DSB. These domains of H3K9me3 function to activate the Tip60 acetyltransferase, allowing Tip60 to acetylate both ataxia telangiectasia-mutated (ATM) kinase and histone H4. Consequently, cells lacking suv39h1 display defective activation of Tip60 and ATM, decreased DSB repair, and increased radiosensitivity. Importantly, activated ATM rapidly phosphorylates kap-1, leading to release of the repressive kap-1/HP1/suv39h1 complex from the chromatin. ATM activation therefore functions as a negative feedback loop to remove repressive suv39h1 complexes at DSBs, which may limit DSB repair. Recruitment of kap-1/HP1/suv39h1 to DSBs therefore provides a mechanism for transiently increasing the levels of H3K9me3 in open chromatin domains that lack H3K9me3 and thereby promoting efficient activation of Tip60 and ATM in these regions. Further, transient formation of repressive chromatin may be critical for stabilizing the damaged chromatin and for remodeling the chromatin to create an efficient template for the DNA repair machinery.
DNA double-strand breaks (DSBs) are toxic and must be repaired to maintain genomic stability. Detection of DSBs requires recruitment of the mre11–rad50–nbs1 (MRN) complex to the DNA ends (1). MRN then recruits and activates the ataxia telangiectasia-mutated (ATM) kinase (2, 3) through a mechanism that also requires the Tip60 acetyltransferase (3). Tip60 directly acetylates and activates ATM’s kinase activity (4–6) and functions, in combination with MRN, to promote ATM-dependent phosphorylation of DSB repair proteins (3), including histone H2AX. This process creates domains of phosphorylated H2AX (γH2AX) extending for hundreds of kilobases along the chromatin (7, 8). Mdc1 then binds to γH2AX, providing a landing pad for other DSB repair proteins, including the RNF8/RNF168 ubiquitin ligases (1, 3, 9, 10). Tip60 also plays a critical role in regulating chromatin structure at DSBs as part of the NuA4–Tip60 complex (11). NuA4-Tip60 catalyzes histone exchange (via the p400 ATPase subunit) and acetylation of histone H4 (by Tip60) at DSBs (12–15), leading to the formation of open, flexible chromatin domains adjacent to the break (12, 13). These open chromatin structures then facilitate histone ubiquitination, the loading of brca1 and 53BP1, and repair of the DSB (13, 16). The ordered acetylation and ubiquitination of the chromatin and loading of DNA repair proteins is therefore critical for DSB repair.
Activation of Tip60’s acetyltransferase activity requires interaction between Tip60’s chromodomain and histone H3 methylated on lysine 9 (H3K9me3) on nucleosomes at the break (4, 6). This interaction, in combination with tyrosine phosphorylation of Tip60 (17), increases Tip60’s acetyltransferase activity and promotes acetylation of both the ATM kinase and histone H4 (4–6, 17). Consequently, loss of H3K9me2/3 leads to failure to activate the ATM signaling pathway, loss of H4 acetylation during DSB repair, disruption of heterochromatin, genomic instability, and defective DSB repair (4, 17–19). H3K9me3s therefore play a critical role in linking chromatin structure at DSBs to the activation of DSB signaling proteins such as Tip60 and ATM.
How Tip60 gains access to H3K9me3 and how H3K9me3 levels at DSBs are regulated is not known. H3K9me3 is concentrated in heterochromatin domains, where it recruits HP1, kap-1, and H3K9 methyltransferases (20, 21) to maintain the silent, compact conformation of heterochromatin (20). This implies that Tip60’s acetyltransferase activity can only be activated at DSBs in regions of high H3K9me3 density, such as heterochromatin. Alternatively, H3K9 methylation may be actively increased at DSBs in regions of low H3K9me3 density to allow for Tip60 activation and efficient DSB repair in euchromatin. Understanding the dynamics of H3K9 methylation at DSBs is therefore critical to understanding how Tip60 activity is regulated by the local chromatin architecture. Here, we show that the suv39h1 methyltransferase is recruited to DSBs in euchromatin as part of a larger kap-1/HP1/suv39h1 complex. Suv39h1 increases H3K9me3 at DSBs, activating Tip60’s acetyltransferase activity and promoting the subsequent acetylation and activation of ATM. Further, loss of inducible H3K9me3 at DSBs leads to defective repair and increased radiosensitivity. Finally, loading of the kap-1/HP1/suv39h1 complex is transient, and the complex is rapidly released from the chromatin through a negative feedback loop driven by ATM-dependent phosphorylation of the kap-1 protein.
Results
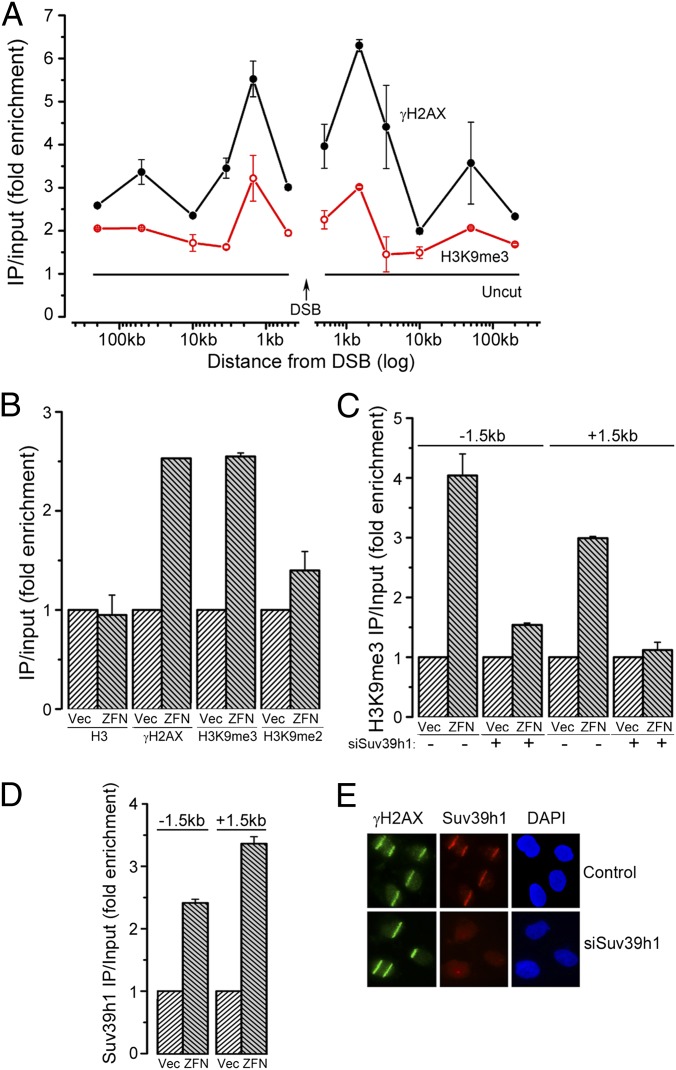
Initially, we determined whether H3K9me3 participates in DSB repair in chromatin domains that lack endogenous H3K9me2/3. The p84–zinc finger nuclease (p84-ZFN) creates a DSB in intron 1 of the PPP1R12C gene (12, 13). PPP1R12C lacks significant H3K9me2/3 but is rich in marks associated with transcription (ENCODE database, http://encodeproject.org/ENCODE/). Chromatin IP (ChIP) demonstrated increased phosphorylation of H2AX (γH2AX) at the p84-ZFN DSB (Fig. 1_A_). ChIP with H3K9me3 antibody (Fig. S1_A_) demonstrated increased H3K9me3 on either side of the DSB (±1.5 kb), with lower levels of H3K9me3 extending >200 kb away from the DSB (Fig. 1_A_). A small increase in H3K9me2 was also detected (Fig. 1_B_). Histone H3 levels were unchanged (Fig. 1_B_), indicating increased methylation rather than changes in H3 content. Further, no change in either H3K36me3 or H4K20me2 was seen at the DSB (Fig. S1_B_). Finally, H3K9me3 was not increased at a distal chromosome site (Fig. S1_C_), indicating that the increased H3K9me3 is restricted to the chromatin domain adjacent to the DSB.
Fig. 1.
Suv39h1 promotes H3K9 methylation at DSBs. (A) 293T cells transfected with p84-ZFN were processed for ChIP by using γH2AX or H3K9me3 antibodies, followed by quantitative RT-PCR (RT-qPCR) with primer pairs located at the indicated positions. Results are fold enrichment relative to uncut DNA, which is assigned a value of 1 (solid black line; Uncut). Results are ±SD (n = 3). (B) 293T cells transfected with p84-ZFN (ZFN) or vector (Vec) were processed for ChIP by using antibodies to H3, γH2AX, H3K9me3, or H3K9me2 and primer pairs located 1.5 kb to the right of the DSB. Results are expressed as fold enrichment relative to uncut DNA. Results are ±SD (n = 3). (C) 293T cells were transfected with nonspecific (−) or siRNA targeting suv39h1 (+). Forty-eight hours later, cells were transfected with vector (Vec) or p84-ZFN (ZFN) and processed for ChIP by using antibody against H3K9me3 and primers located 1.5 kb to either side of the DSB. Results are ±SD (n = 3). (D) 293T cells were transfected with vector (Vec) or p84-ZFN (ZFN) and processed for ChIP with suv39h1 antibody and primers located 1.5 kb to either side of the DSB. Results are ±SD (n = 3). (E) U2OS cells were transfected with nonspecific (control) or suv39h1-specific (siSuv39h1) siRNA. Forty-eight hours later, focused regions of DNA damage were produced by using a scanning laser system. Cells were fixed for immunofluorescent staining by using antibodies to γH2AX (green) or suv39h1 (red). Nuclei were stained with DAPI (blue).
The H3K9 methyltransferase suv39h1 has been implicated in DSB repair (4, 18, 19). Depletion of suv39h1 with siRNA (Fig. S1_D_) significantly reduced H3K9me3 at the p84-ZFN DSB (Fig. 1_C_). Furthermore, ChIP demonstrated that suv39h1 was loaded onto the chromatin at the DSB (Fig. 1_D_ and Fig. S1_E_). Finally, suv39h1 was rapidly (within 5 min) recruited to regions of DNA damage created with laser microirradiation (Fig. 1_E_). The suv39h1 methyltransferase is therefore recruited to DSBs and increases local H3K9me3 in response to DSBs.
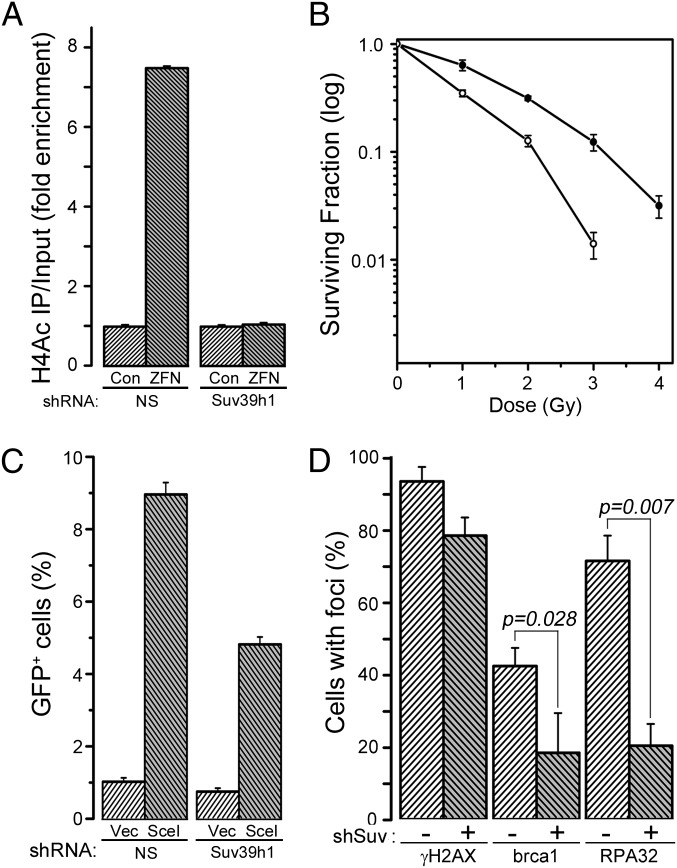
Interaction between Tip60 and H3K9me3 (4) promotes acetylation of ATM (5, 6, 17) and histone H4 (12–14) by Tip60. Depletion of suv39h1 reduced inducible H3K9me3 (Fig. 1_C_) and inhibited acetylation of histone H4 by Tip60 (Fig. 2_A_). Furthermore, depletion of suv39h1 attenuated ATM activation (Fig. S2_A_) and reduced phosphorylation of kap-1 (Fig. S2_B_). This finding is consistent with methylation of H3K9 by suv39h1 playing an essential role in activation of Tip60’s acetyltransferase activity and the subsequent acetylation and activation of the ATM kinase. Furthermore, cells lacking suv39h1 have increased radiation sensitivity (Fig. 2_B_) and reduced homologous recombination (HR)-mediated repair (Fig. 2_C_). Finally, recruitment of brca1 and RPA32 to DSBs (Fig. 2_D_) were reduced following loss of suv39h1, consistent with the decrease in HR-mediated repair in suv39h1-deficient cells (Fig. 2_C_). However, nonhomologous end-joining (NHEJ) activity was not significantly altered by loss of suv39h1 (Fig. S2_C_), implying that regulation of the Ku70/80 complex and DNA–PKcs activity does not require H3K9 methylation. These results are consistent with previous studies demonstrating increased genomic instability in mice and other experimental systems (4, 18, 19) in which suv39h1 was inactivated. Methylation of H3K9 by suv39h1 at DSBs therefore plays a critical role in activating Tip60, controlling ATM signaling, and in directing DSB repair and maintaining genomic stability.
Fig. 2.
Suv39h1 regulates Tip60 activity, genomic stability, and homologous recombination (HR)-mediated repair. (A) 293T cells expressing nonspecific shRNA (control) or shRNA targeting suv39h1 were transfected with vector (Vec) or p84-ZFN (ZFN), followed by ChIP with antibody to H4Ac. RT-qPCR was carried out using primer pairs located to 1.5 kb to the right of the DSB. Results are ±SD (n = 3). (B) 293T cells expressing nonspecific shRNA (●) or shRNA targeting suv39h1 (○) were irradiated, and clonogenic cell survival assays were carried out. Results are ±SD (n = 3). (C) U2OS cells containing a GFP–HR reporter were stably transfected with nonspecific (NS) or shRNA targeting suv39h1, followed by transient transfection with vector (Vec) or the I-Sce1 endonuclease. GFP-positive cells were counted by FACS. Results are ±SD (n = 4 biological replicates). (D) U2OS cells expressing nonspecific (−) or shRNA targeting suv39h1 (+) were irradiated (2 Gy), allowed to recover for 30 min, and stained with antibodies to γH2AX, brca1, or RPA32. Cells with more than five foci were scored as positive. Results are ±SD (n = 3). P value was determined by using a t test.
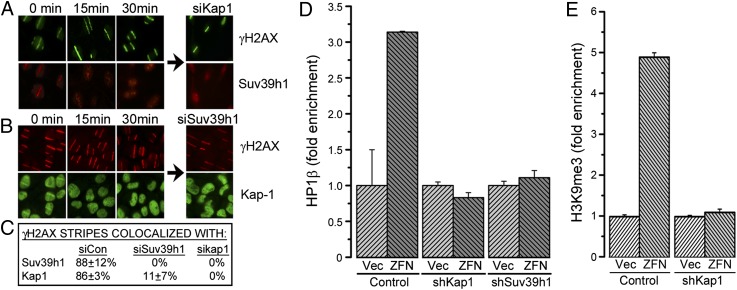
Two heterochromatin-binding proteins, kap-1 and HP1, are corecruited to sites of DNA damage (22, 23), although how they impact DSB repair is not clear. However, suv39h1 can interact with kap-1/HP1 repressor complexes (20, 21, 24), suggesting that kap-1 and HP1 may recruit suv39h1 to DSBs. Initially, we confirmed that kap-1 interacts with the HP1α, HP1β, and suv39h1 and that this interaction was not altered by DNA damage (Fig. S3 B and C). When laser “striping” was used to create focused regions of DNA damage, suv39h1 (Fig. 3_A_) and kap-1 (Fig. 3_B_) were recruited to sites of DNA damage with similar kinetics, such that ∼90% of γH2AX stripes were colocalized with suv39h1 and kap-1 (Fig. 3_C_). Furthermore, ChIP demonstrated that HP1β was also recruited to sites of DNA damage (Fig. 3_D_). Importantly, suv39h1, kap-1, and HP1 were only transiently retained at damage sites (Fig. 3 A and B), indicating that they function during the initial few minutes following DSB production. Furthermore, depletion of kap-1 (Fig. S3_A_) blocked recruitment of both suv39h1 (Fig. 3 A and C) and HP1β (Fig. 3_D_) to DSBs. Similarly, depletion of suv39h1 blocked recruitment of kap-1 (Fig. 3 B and C and Fig. S3_D_) and HP1β (Fig. 3_D_) to DSBs. Loss of any one of suv39h1, kap-1, or HP1 therefore prevents loading of all three proteins at the DSB. Finally, depletion of kap-1, which blocks recruitment of both suv39h1 and HP1 to DSBs, abolished the increase in H3K9me3 at DSBs (Fig. 3_E_). Combining these data with previous work demonstrating direct interaction between kap-1, HP1 family members, and suv39h1 (24–28) implies that kap-1, HP1, and suv39h1 are recruited to DSBs as a single kap-1/HP1/suv39h1 complex and that it is this complex that directs H3K9 methylation at DSBs.
Fig. 3.
Recruitment of suv39h1 to DSBs requires kap-1 and HP1. (A) U2OS cells were transfected with control siRNA or siRNA to kap-1. DNA damage was created by using a laser, and cells were allowed to recover for 0, 15, or 30 min. Cells were costained with antibody to suv39h1 (red) or γH2AX (green). (B) U2OS cells were transfected with control siRNA or siRNA to suv39h1. DNA damage was created by using a laser, and cells were allowed to recover for 0, 15, or 30 min. Cells were costained with antibody to kap1 (green) or γH2AX (red). (C) Quantitation of results in A and B. The percentage of γH2AX laser stripes that colocalized with either suv39h1 or kap-1 stripes were noted. Results are ±SD (n = 25–60 cells). (D) 293T cells expressing nonspecific shRNA (control) or shRNA to kap-1 or suv39h1 were transfected with vector (Vec) or p84-ZFN (ZFN), followed by ChIP using HP1β antibody and primers 1.5 kb to the right of the DSB. Results are ±SD (n = 3). (E) 293T cells expressing nonspecific shRNA (control) or shRNA against kap-1 (shKap1) were transfected with p84-ZFN (ZFN) or vector (Vec) followed by ChIP using H3K9me3 antibody and primers located 1.5 kb to the right of the DSB. Results are ±SD (n = 3).
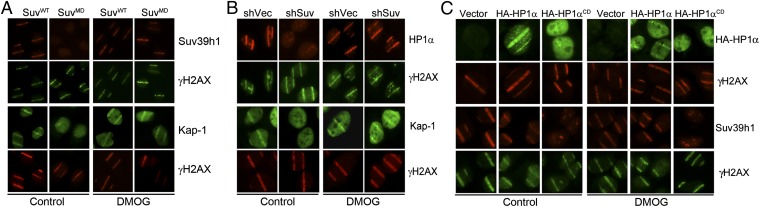
Next, we examined suv39h1’s methyltransferase activity. Suv39h1MD, containing a point mutation in the catalytic domain, was efficiently incorporated into the kap-1/HP1/suv39h1 complex (Fig. S4_A_). However, suv39h1MD was not recruited to regions of laser damage, and, furthermore, it blocked recruitment of kap-1 (Fig. 4_A_), such that <5% of γH2AX stripes in suv39h1MD cells contained either kap-1 or suv39h1 (see Fig. S4_E_ for quantitation). Dimethyloxalylglycine (DMOG), a pan-specific inhibitor of H3K9 demethylases (29), was then used to increase global H3K9me3 levels (Fig. S4 B and D). DMOG rescued recruitment of both suv39h1MD and kap-1 to regions of laser damage (Fig. 4_A_), such that >80% of γH2AX stripes in suv39h1MD cells then colocalized with suv39h1 and kap-1 (Fig. S4_E_). Further, DMOG rescued recruitment of kap-1 and HP1α, even in cells lacking expression of suv39h1 (Fig. 4_B_). This finding demonstrates that H3K9me3, rather than suv39h1, mediates retention of kap-1, HP1, and suv39h1 on the chromatin. Because the chromodomain of HP1α binds to H3K9me3, we deleted HP1α’s chromodomain (Fig. S4_D_). Loss of HP1α’s chromodomain did not alter interaction of HP1α with either suv39h1 or kap-1 (Fig. S4 F and G). Importantly, HP1αCD was not recruited to sites of laser microirradiation and inhibited corecruitment of suv39h1 (Fig. 4_C_). Furthermore, increasing H3K9me3 with DMOG did not restore loading of HP1 or suv39h1 in HP1αCD cells (Fig. 4_C_), demonstrating that both HP1α's chromodomain and H3K9me3 are required to retain kap-1/HP1/suv39h1 at DSBs. This finding suggests a model in which the initial positioning of kap-1/HP1/suv39h1 at DSBs promotes H3K9 methylation by suv39h1. This “priming” event then recruits additional kap-1/HP1/suv39h1 complexes, which are retained through interaction between HP1’s chromodomain and newly synthesized H3K9me3. This kap-1/HP1/suv39h1 then bridges to more distal nucleosomes, promoting additional cycles of H3K9 methylation and kap-1/HP1/suv39h1 binding, which spreads H3K9me3 along the chromatin away from the DSB. Spreading therefore requires both suv39h1 (to create H3K9me3) and HP1 (which binds to H3K9me3). Consequently, loss of HP1, suv39h1, or kap-1 prevents spreading of H3K9me3 and therefore loading of the complex onto the chromatin.
Fig. 4.
HP1’s chromodomain is required to recruit kap-1, HP1, and suv39h1 to DSBs. (A) U2OS cells expressing myc-suv39h1 (SuvWT) or catalytically inactive myc-suv39h1 (SuvMD) were exposed to laser damage and stained with antibody to myc and γH2AX or kap-1 and γH2AX. Some cells were preincubated in dimethyloxalylglycine (DMOG) (1 mM/1 h). (B) U2OS cells expressing nonspecific shRNA (shVec) or shRNA to suv39h1 (shSuv) were exposed to laser damage and costained with antibodies to HP1α and γH2AX or kap-1 and γH2AX. Some cells were preincubated in DMOG (1 mM/1 h). (C) U2OS cells expressing vector, HP1α (HA-HP1α), or HP1α with the chromodomain deleted (HA-HP1αCD) were exposed to laser damage and costained with antibodies for HA and γH2AX or suv39h1 and γH2AX. Some cells were preincubated in DMOG (1 mM/1 h).
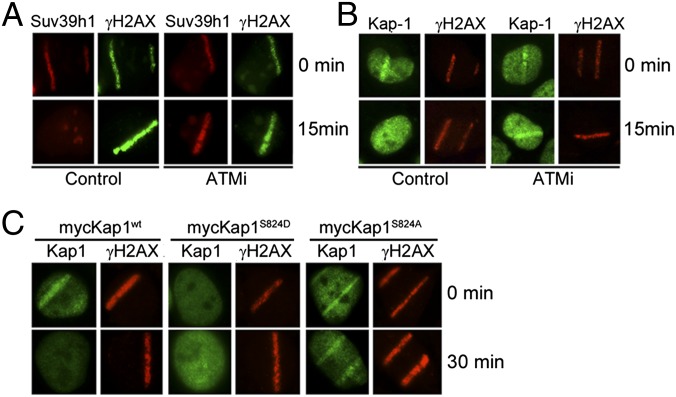
Kap-1, HP1, and suv39h1 are only retained at the DSB during the first few minutes after damage (Figs. 3 and 4). Because the ATM-dependent phosphorylation of kap-1 weakens kap-1’s interaction with chromatin (30, 31), we examined whether ATM was required to release kap-1/HP1/suv39h1 from DSBs. The ATM inhibitor Ku-55933 (ATMi) did not alter interaction between kap-1 and suv39h1 (Fig. S5_A_). Suv39h1 (Fig. 5_A_) and kap-1 (Fig. 5_B_) were recruited to DSBs in the presence of ATMi. However, ATMi blocked release of suv39h1 and kap-1 (Fig. 5 A and B) and increased retention of kap-1 at p84-ZFN–generated DSBs (Fig. S5_B_). Similarly, inactivation of the MRN complex, which is required for ATM activation (2), blocked phosphorylation of kap-1 by ATM (Fig. S5_D_) and prevented release of suv39h1 from sites of DNA damage (Fig. S5_C_). Phosphorylation of serine 824 of kap-1 by ATM weakens kap-1 interaction with chromatin (30–32). Kap-1wt, kap-1S824A, and kap-1S824D (containing a phospho-mimic) were expressed in U2OS cells (Fig. S5_E_). Kap-1wt was transiently recruited to and released from regions of DNA damage, whereas the nonphosphorylatable kap-1S824A was recruited to DSBs but retained at DSBs for an extended time (Fig. 5C and Fig. S5_F_). This result is consistent with kap-1 phosphorylation promoting release of kap-1/HP1/suv39h1 from DSBs. Intriguingly, the phospho-mimic kap-1S824D was poorly retained at both sites of laser damage (Fig. 5_C_) and at DSBs created by the p84-ZFN (Fig. S5_F_). We conclude that the rapid release of kap-1/HP1/suv39h1 from DSBs requires the ATM-dependent phosphorylation of serine 824 of kap-1.
Fig. 5.
Phosphorylation of kap-1 by ATM releases suv39h, kap-1, and HP1 from DSBs. (A and B) U2OS cells were preincubated with ATMi (100 μM) or solvent for 60 min. After laser microirradiation, cells were immediately fixed (0 min) or allowed to recover for 15 min and then costained with antibodies to suv39h1 and γH2AX (A) or kap-1 and γH2AX (B). (C) U2OS cells expressing wild-type kap-1 (mycKap1wt), kap-1 with an alanine mutation in the ATM phosphorylation site (mycKap1S824D), or kap-1 with a phospho-mimic in the same site (mycKap1S824D) were exposed to laser microirradiation and mycKap1 and γH2AX detected with myc or γH2AX antibody.
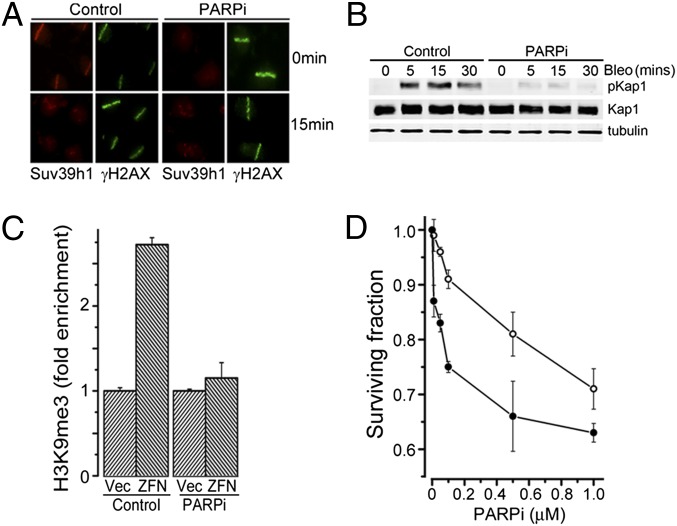
Because many early responses to DNA damage require poly(ADP-ribose) polymerase (PARP) family members, which catalyze synthesis of PAR chains on the chromatin at DSBs (33), we examined whether the rapid recruitment of kap-1/HP1/suv39h1 to DSBs required PARP activity. The parp inhibitor olaparib (PARPi) did not alter interaction between kap-1, HP1α, and suv39h1 (Fig. S5_A_). However, inhibition of PARP blocked the rapid recruitment of suv39h1 (Fig. 6_A_) and kap-1 (Fig. S6_B_) to sites of DNA damage. Chromatin PARylation after DNA damage was not altered in cells lacking suv39h1 (Fig. S6_A_), demonstrating that PARylation is upstream of the kap-1/HP1α/suv39h1 complex. Furthermore, siRNA to parp1 also blocked recruitment of suv39h1 (Fig. S6_C_), implying that parp1, rather than other parp family members, is required for recruitment of kap-1/HP1/suv39h1 to damaged chromatin. PARPi led to a significant reduction in H3K9me3 at DSBs (Fig. 6_C_), consistent with the failure to recruit suv39h1 to DSBs in the presence of PARPi (Fig. 6_A_) or parp1 siRNA (Fig. S6_C_). In addition, because H3K9me3 is important for full activation of ATM (4), PARPi also inhibited phosphorylation of kap-1 by ATM after DNA damage (Fig. 6_B_). Finally, depletion of suv39h1 increased cell sensitivity to PARPi (Fig. 6D), consistent with previous reports that cells lacking ATM are sensitive to PARP inhibition (34, 35).
Fig. 6.
Chromatin PARylation recruits kap-1, HP1, and suv39h1 to DSBs. (A) U2OS cells were preincubated with PARPi (olaparib; 1 h/20 μM), followed by laser microirradiation. Cells were either fixed (0 min) or allowed to recover for 15 min, and then costained with antibody to suv39h1 and γH2AX. (B) U2OS cells were preincubated in PARPi (olaparib; 20 μM) for 1 h, and then exposed to bleomycin (5 μM) for the indicated times. Kap1, pkap1, and tubulin were monitored by Western blot analysis. (C) 293T cells were transfected with vector (Vec) or p84-ZFN (ZFN), followed by solvent (control) or PARPi (olaparib; 20μM). ChIP was carried out by using H3K9me3 antibody and primers located 1.5 kb to the right of the DSB. Results are ±SD (n = 3). (D) 293T cells expressing nonspecific shRNA (○) or shRNA targeting suv39h1 (●) were incubated with PARPi for 24 h, and clonogenic cell survival assays were carried out. Results are ±SE (n = 3).
Discussion
We have shown that the suv39h1 methyltransferase is rapidly recruited to DSBs, where it functions to create domains of H3K9me3 adjacent to DSBs. Previous work demonstrated that the repressive HP1 and kap-1 proteins were also recruited to DSBs (4, 22, 23, 32, 36), although how these proteins contributed to DSB repair was not clear. Kap-1, HP1 family members, and suv39h1 can form large repressive complexes (24–28). Here, we show that loading of suv39h1, kap-1, and HP1 at DSBs was interdependent, with loss of any one protein inhibiting recruitment of the other two. This finding is consistent with the idea that kap-1, HP1, and suv39h1 are recruited to DSBs as a single kap-1/HP1/suv39h1 complex. We propose a model in which loading of the kap-1/HP1/suv39h1 complex at DSBs increases H3K9me3 methylation on nucleosomes on either side of the DSB. This promotes recruitment of additional kap-1/HP1/suv39h1 (through interaction of HP1’s chromodomain with H3K9me3), which then methylates H3K9 on nucleosomes further from the DSB. This process leads to cycles of kap-1/HP1/suv39h1 loading and H3K9 methylation, which catalyzes the spreading of H3K9me3 (and kap-1/HP1/suv39h1) along the chromatin. This model is similar to the spreading of heterochromatin, in which an initial nucleation event positions HP1 complexes containing H3K9 methyltransferases on the chromatin (20, 37). Subsequent cycles of H3K9 methylation and loading of HP1 complexes result in the spreading of heterochromatin along the chromatin (26, 38, 39). In this way, an initial nucleation event at DSBs can spread H3K9me3 and kap-1/HP1/suv39h1 along the chromatin domains flanking the DSB, leading to the rapid formation of repressive chromatin at the DSB.
The initial nucleation event that recruits kap-1/HP1/suv39h1 to DSBs required parp1. Several PARP family members are recruited to DSBs where they rapidly create PAR chains. PAR provides docking sites for several proteins implicated in DSB repair, including macroH2A and the ALC1 and NuRD remodeling complexes (40–44). However, neither kap-1 nor HP1 nor suv39h1 contain conserved PAR binding motifs or undergo changes in PARylation after DNA damage. Thus, whether the kap-1/HP1α/suv39h1 complex binds directly to PAR chains on the chromatin, or whether the complex contains additional PAR-binding subunits, remains to be determined. Alternatively, PARylation may alter nucleosome structure at DSBs, thereby facilitating H3K9 methylation by the kap-1/HP1/suv39h1 complex. In either case, both parp1 activity and H3K9me3 spreading are required to stably, but transiently, load kap-1/HP1α/suv39h1 onto the chromatin.
H3K9me3 is required for activation of the Tip60 acetyltransferase (4, 17). However, because H3K9me3 is primarily located within silent, heterochromatic regions (20, 37, 38), this requirement suggests that Tip60 activity during DNA repair may be restricted to chromatin domains with a high density of H3K9me2/3. Here, we demonstrate that transient loading of kap-1/HP1/suv39h1 at DSBs provides a mechanism for rapidly increasing H3K9me3 in open (euchromatin) domains that lack preexisting H3K9me3. Furthermore, reducing H3K9me3 by targeting either suv39h1 or PARP activity inhibited Tip60’s acetyltransferase activity and attenuated activation of ATM by DSBs. This finding is consistent with published studies in which loss of MRN (2), Tip60 (4, 6), or acute PARP inhibition (35) led to defective activation of ATM’s kinase activity. Increased H3K9 methylation by the kap-1/HP1/suv39h1 complex is therefore critical for full activation of Tip60 and ATM and for the repair of DSBs within open, euchromatic regions of the genome.
The recruitment of kap-1/HP1/suv39h1 and the increase in H3K9me3 may reflect a need to temporarily stabilize and “heterochromatinize” DSBs in open regions. Other repressive complexes, such as NuRD and histone deacetylases (HDACs) (43–46), also transiently accumulate at DSBs, supporting this idea. The formation of repressive structures at DSBs in open chromatin has parallels with DSB repair in heterochromatin. DSB repair in heterochromatin requires the ATM-dependent phosphorylation of kap-1 (30, 31), which releases the repressive CHD3 chromatin remodeler (47) and promotes chromatin relaxation and facilitates DSB repair (31, 32, 47). Thus, immediately after DNA damage, both euchromatin and heterochromatin domains have similar structural organization, including high density of H3K9me3 and the presence of repressive complexes, such as kap-1, HP1α, methyltransferases, HDACs, and CHD3/CHD4 remodeling ATPases (31, 32, 43–47). This rapid, but temporary, formation of repressive chromatin may inhibit local transcription, compact the local chromatin structure, and rewrite the local epigenetic landscape, stabilizing open chromatin structures and limiting DSB mobility during the initial moments following DSB production. However, because repressive chromatin inhibits DSB repair (30, 31, 47), it is important that these repressive structures are rapidly dismantled. As the damage response unfolds, H3K9 methylation increases, leading to Tip60 activation and increased ATM kinase activity. ATM then phosphorylates kap-1, releasing the repressive kap-1/HP1/suv39h1 from the chromatin and thereby providing a negative feedback loop that regulates both H3K9 methylation and loading of the kap-1/HP1/suv39h1 complex at DSBs. In heterochromatin, kap-1 phosphorylation releases the CHD3 complex (47), leading to relaxation of the chromatin structure (31), although kap-1 remains associated with the heterochromatin. The retention of phosphorylated kap-1 in heterochromatin may result from the presence of Kruppel-associated box zinc finger proteins (21), which anchor kap-1 to heterochromatin, but which are absent from open, euchromatin regions. In this way, the compact structure of heterochromatin and the transient establishment of repressive chromatin at DSBs in open regions can be reversed through a common mechanism dependent on the ATM kinase. This structure then allows further chromatin processing to create open, flexible chromatin structures, which are essential for DSB repair (11).
Dynamic methylation of histones and phosphorylation of kap-1 during DSB repair therefore provide a regulated mechanism for increasing compaction of open chromatin and decreasing the compaction of heterochromatin, so that DSBs in both regions begin to have similar epigenetic and structural organization. This process is critical for establishing H3K9me3 for activation of Tip60 and the ATM kinase, as well as for contributing to the early processing of the chromatin at DSBs. These dynamic changes in chromatin organization are therefore critical for creating a common chromatin template that is an efficient substrate for the HR or NHEJ DSB repair pathways.
Materials and Methods
Details on cell growth, HR assays, transfection, antibodies, plasmid construction, Western blot, mRNA analysis, and shRNA/siRNA are in SI Materials and Methods.
ChIP.
For ChIP assays (12, 13), cross-linked chromatin was sonicated, and equivalent amounts were incubated with primary antibody and protein G agarose beads precoated with sperm DNA. After washing, immunopurified chromatin was eluted and digested with proteinase K, and purified DNA was quantified by quantitative RT-PCR (RT-qPCR) using the Step One Plus real-time PCR system (Applied Biosystems). Results are expressed as fold increase in signal relative to uncut sample. Detailed protocols, primer pairs, and ChIP grade antibodies are described in SI Materials and Methods.
Laser Microirradiation and Immunofluorescence.
Laser damage was produced by using a 30-mW, 405-nm diode laser focused through the 40×-Plan Apochromat/1.25-N.A. oil objective (Leica TCS SP5; Leica Microsystems) in combination with Hoechst 33258 (12). The time between the initial laser exposure and termination by fixation was 5 min, which is referred to as time 0. At least 50 nuclei were microirradiated per slide. For recruitment of brca1 and RPA32 to ionizing radiation induced foci, cells were cultured on coverslips and irradiated in a Cs137 irradiator. Cells were fixed and incubated with primary and secondary antibodies as described in SI Materials and Methods and imaged by using a Zeiss AxioImager Z1 microscope.
Supplementary Material
Supporting Information
Acknowledgments
We thank Sangamo Biosciences for p84-ZFN and Dipanjan Chowdhury for the kap1 constructs. This work was supported by National Institutes of Health Grants CA64585, CA93602, and CA177884 (to B.D.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Ciccia A, Elledge SJ. The DNA damage response: Making it safe to play with knives. Mol Cell. 2010;40(2):179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiloh Y, Ziv Y. The ATM protein kinase: Regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol. 2013;14(4):197–210. [PubMed] [Google Scholar]
- 3.Sun Y, Jiang X, Price BD. Tip60: Connecting chromatin to DNA damage signaling. Cell Cycle. 2010;9(5):930–936. doi: 10.4161/cc.9.5.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Y, et al. Histone H3 methylation links DNA damage detection to activation of the tumour suppressor Tip60. Nat Cell Biol. 2009;11(11):1376–1382. doi: 10.1038/ncb1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y, Xu Y, Roy K, Price BD. DNA damage-induced acetylation of lysine 3016 of ATM activates ATM kinase activity. Mol Cell Biol. 2007;27(24):8502–8509. doi: 10.1128/MCB.01382-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci USA. 2005;102(37):13182–13187. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146(5):905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iacovoni JS, et al. High-resolution profiling of gammaH2AX around DNA double strand breaks in the mammalian genome. EMBO J. 2010;29(8):1446–1457. doi: 10.1038/emboj.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson SP, Durocher D. Regulation of DNA damage responses by ubiquitin and SUMO. Mol Cell. 2013;49(5):795–807. doi: 10.1016/j.molcel.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Fradet-Turcotte A, et al. 53BP1 is a reader of the DNA-damage-induced H2A Lys 15 ubiquitin mark. Nature. 2013;499(7456):50–54. doi: 10.1038/nature12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price BD, D’Andrea AD. Chromatin remodeling at DNA double-strand breaks. Cell. 2013;152(6):1344–1354. doi: 10.1016/j.cell.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, et al. Histone H2A.Z controls a critical chromatin remodeling step required for DNA double-strand break repair. Mol Cell. 2012;48(5):723–733. doi: 10.1016/j.molcel.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, et al. The p400 ATPase regulates nucleosome stability and chromatin ubiquitination during DNA repair. J Cell Biol. 2010;191(1):31–43. doi: 10.1083/jcb.201001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murr R, et al. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nat Cell Biol. 2006;8(1):91–99. doi: 10.1038/ncb1343. [DOI] [PubMed] [Google Scholar]
- 15.Soria G, Polo SE, Almouzni G. Prime, repair, restore: The active role of chromatin in the DNA damage response. Mol Cell. 2012;46(6):722–734. doi: 10.1016/j.molcel.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Tang J, et al. Acetylation limits 53BP1 association with damaged chromatin to promote homologous recombination. Nat Struct Mol Biol. 2013;20(3):317–325. doi: 10.1038/nsmb.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaidi A, Jackson SP. KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling. Nature. 2013;498(7452):70–74. doi: 10.1038/nature12201. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Peters AH, et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107(3):323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 19.Peng JC, Karpen GH. Heterochromatic genome stability requires regulators of histone H3 K9 methylation. PLoS Genet. 2009;5(3):e1000435. doi: 10.1371/journal.pgen.1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grewal SI, Jia S. Heterochromatin revisited. Nat Rev Genet. 2007;8(1):35–46. doi: 10.1038/nrg2008. [DOI] [PubMed] [Google Scholar]
- 21.Iyengar S, Farnham PJ. KAP1 protein: An enigmatic master regulator of the genome. J Biol Chem. 2011;286(30):26267–26276. doi: 10.1074/jbc.R111.252569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldeyron C, Soria G, Roche D, Cook AJ, Almouzni G. HP1alpha recruitment to DNA damage by p150CAF-1 promotes homologous recombination repair. J Cell Biol. 2011;193(1):81–95. doi: 10.1083/jcb.201101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luijsterburg MS, et al. Heterochromatin protein 1 is recruited to various types of DNA damage. J Cell Biol. 2009;185(4):577–586. doi: 10.1083/jcb.200810035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fritsch L, et al. A subset of the histone H3 lysine 9 methyltransferases Suv39h1, G9a, GLP, and SETDB1 participate in a multimeric complex. Mol Cell. 2010;37(1):46–56. doi: 10.1016/j.molcel.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Lechner MS, Begg GE, Speicher DW, Rauscher FJ., 3rd Molecular determinants for targeting heterochromatin protein 1-mediated gene silencing: Direct chromoshadow domain-KAP-1 corepressor interaction is essential. Mol Cell Biol. 2000;20(17):6449–6465. doi: 10.1128/mcb.20.17.6449-6465.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen AL, et al. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 1999;18(22):6385–6395. doi: 10.1093/emboj/18.22.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, et al. SUMOylation of the transcriptional co-repressor KAP1 is regulated by the serine and threonine phosphatase PP1. Sci Signal. 2010;3(119):ra32. doi: 10.1126/scisignal.2000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanov AV, et al. PHD domain-mediated E3 ligase activity directs intramolecular sumoylation of an adjacent bromodomain required for gene silencing. Mol Cell. 2007;28(5):823–837. doi: 10.1016/j.molcel.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayrapetov MK, et al. Activation of Hif1α by the prolylhydroxylase inhibitor dimethyoxalyglycine decreases radiosensitivity. PLoS ONE. 2011;6(10):e26064. doi: 10.1371/journal.pone.0026064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noon AT, et al. 53BP1-dependent robust localized KAP-1 phosphorylation is essential for heterochromatic DNA double-strand break repair. Nat Cell Biol. 2010;12(2):177–184. doi: 10.1038/ncb2017. [DOI] [PubMed] [Google Scholar]
- 31.Goodarzi AA, et al. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell. 2008;31(2):167–177. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 32.Ziv Y, et al. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat Cell Biol. 2006;8(8):870–876. doi: 10.1038/ncb1446. [DOI] [PubMed] [Google Scholar]
- 33.Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nat Rev Mol Cell Biol. 2012;13(7):411–424. doi: 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- 34.Williamson CT, et al. ATM deficiency sensitizes mantle cell lymphoma cells to poly(ADP-ribose) polymerase-1 inhibitors. Mol Cancer Ther. 2010;9(2):347–357. doi: 10.1158/1535-7163.MCT-09-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haince JF, et al. Ataxia telangiectasia mutated (ATM) signaling network is modulated by a novel poly(ADP-ribose)-dependent pathway in the early response to DNA-damaging agents. J Biol Chem. 2007;282(22):16441–16453. doi: 10.1074/jbc.M608406200. [DOI] [PubMed] [Google Scholar]
- 36.Ayoub N, Jeyasekharan AD, Bernal JA, Venkitaraman AR. HP1-beta mobilization promotes chromatin changes that initiate the DNA damage response. Nature. 2008;453(7195):682–686. doi: 10.1038/nature06875. [DOI] [PubMed] [Google Scholar]
- 37.Tamaru H. Confining euchromatin/heterochromatin territory: Jumonji crosses the line. Genes Dev. 2010;24(14):1465–1478. doi: 10.1101/gad.1941010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hathaway NA, et al. Dynamics and memory of heterochromatin in living cells. Cell. 2012;149(7):1447–1460. doi: 10.1016/j.cell.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheutin T, et al. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science. 2003;299(5607):721–725. doi: 10.1126/science.1078572. [DOI] [PubMed] [Google Scholar]
- 40.Ahel D, et al. Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science. 2009;325(5945):1240–1243. doi: 10.1126/science.1177321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu C, Xu Y, Gursoy-Yuzugullu O, Price BD. The histone variant macroH2A1.1 is recruited to DSBs through a mechanism involving PARP1. FEBS Lett. 2012;586(21):3920–3925. doi: 10.1016/j.febslet.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timinszky G, et al. A macrodomain-containing histone rearranges chromatin upon sensing PARP1 activation. Nat Struct Mol Biol. 2009;16(9):923–929. doi: 10.1038/nsmb.1664. [DOI] [PubMed] [Google Scholar]
- 43.Polo SE, Kaidi A, Baskcomb L, Galanty Y, Jackson SP. Regulation of DNA-damage responses and cell-cycle progression by the chromatin remodelling factor CHD4. EMBO J. 2010;29(18):3130–3139. doi: 10.1038/emboj.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chou DM, et al. A chromatin localization screen reveals poly (ADP ribose)-regulated recruitment of the repressive polycomb and NuRD complexes to sites of DNA damage. Proc Natl Acad Sci USA. 2010;107(43):18475–18480. doi: 10.1073/pnas.1012946107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smeenk G, et al. The NuRD chromatin-remodeling complex regulates signaling and repair of DNA damage. J Cell Biol. 2010;190(5):741–749. doi: 10.1083/jcb.201001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller KM, et al. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol. 2010;17(9):1144–1151. doi: 10.1038/nsmb.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodarzi AA, Kurka T, Jeggo PA. KAP-1 phosphorylation regulates CHD3 nucleosome remodeling during the DNA double-strand break response. Nat Struct Mol Biol. 2011;18(7):831–839. doi: 10.1038/nsmb.2077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information