Adapting to new threats: the generation of memory by CRISPR-Cas immune systems (original) (raw)
. Author manuscript; available in PMC: 2015 Jul 1.
Published in final edited form as: Mol Microbiol. 2014 Jun 4;93(1):1–9. doi: 10.1111/mmi.12640
Summary
Clustered, regularly interspaced, short palindromic repeats (CRISPR) loci and their associated genes (cas) confer bacteria and archaea with adaptive immunity against phages and other invading genetic elements. A fundamental requirement of any immune system is the ability to build a memory of past infections in order to deal more efficiently with recurrent infections. The adaptive feature of CRISPR-Cas immune systems relies on their ability to memorize DNA sequences of invading molecules and integrate them in between the repetitive sequences of the CRISPR array in the form of “spacers”. The transcription of a spacer generates a small antisense RNA that is used by RNA-guided Cas nucleases to cleave the invading nucleic acid in order to protect the cell from infection. The acquisition of new spacers allows the CRISPR-Cas immune system to rapidly adapt against new threats and is therefore termed “adaptation”. Recent studies have begun to elucidate the genetic requirements for adaptation and have demonstrated that rather than being a stochastic process, the selection of new spacers is influenced by several factors. We review here our current knowledge of the CRISPR adaptation mechanism.
Keywords: CRISPR/Cas systems, spacer acquisition, adaptation, bacteriophage, adaptive immunity, horizontal gene transfer
Introduction
Bacteria and archaea have evolved to thrive in hostile environments under the constant threat of viral (phage) attack. As a result, these organisms have devised numerous strategies to prevent phage infection, including abortive infection, surface exclusion and restriction modification systems (Thomas & Nielsen, 2005, Bikard & Marraffini, 2012). While highly effective, these innate defense strategies provide non-specific immunity. In contrast, the CRISPR-Cas immune system provides an adaptive defense mechanism against phages and other mobile genetic elements (Mojica et al., 2005, Makarova et al., 2006, Barrangou et al., 2007).
Since their discovery in Escherichia coli in 1987 (Ishino et al., 1987), CRISPR systems have proven to be widespread among bacteria and archaea (Marraffini, 2013, Jansen et al., 2002, Sorek et al., 2008). Generally, a CRISPR locus contains the CRISPR-associated (cas) genes and the CRISPR array. The cas genes encode a diverse family of Cas proteins carrying predicted functional domains of proteins that participate in nucleic acids transactions, such as DNA-binding proteins, nucleases, polymerases and helicases (Haft et al., 2005, Makarova et al., 2006). The CRISPR array consists of identical nonadjacent sequences (repeats) interspaced by similarly sized variable sequences (spacers). An AT-rich leader sequence located upstream of the first repeat promotes the transcription of the CRISPR array (Agari et al., 2010, Pougach et al., 2010) and is essential for spacer acquisition (Yosef et al., 2012). Repeats are usually conserved within the same locus, and in most cases contain partially palindromic sequences (Kunin et al., 2007). Spacers are highly diverse even among closely related strains and were therefore initially exploited for strain typing purposes (Kamerbeek et al., 1997). In 2005, three independent bioinformatics studies revealed homology between spacer sequences and mobile genetic elements (Pourcel et al., 2005, Mojica et al., 2005, Bolotin et al., 2005, Shah et al., 2009). This observation led to the hypothesis that CRISPR may provide protection against invading phages and plasmids (Makarova et al., 2006). Soon, spacers were confirmed to provide sequence-specific interference against all prokaryotic routes of horizontal gene transfer, including bacteriophage infection (Barrangou et al., 2007, Horvath et al., 2008, Deveau et al., 2008), plasmid conjugation (Marraffini & Sontheimer, 2008) and transformation (Bikard et al., 2012, Zhang et al., 2013).
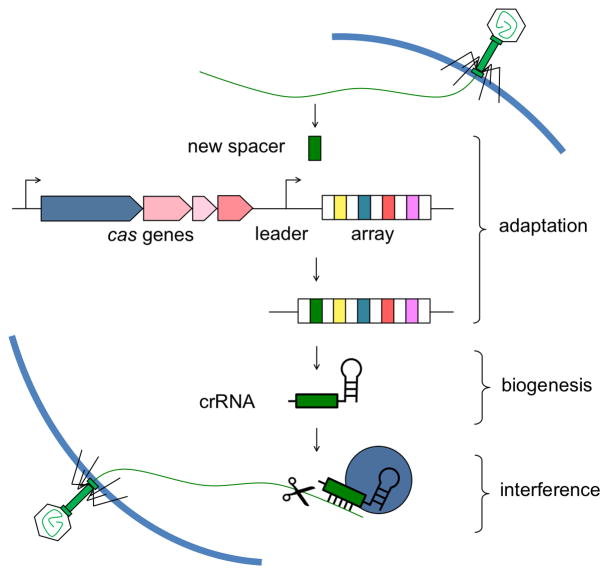
CRISPR-Cas systems provide immunity against phages through a three-step defense pathway (Figure 1). First, a fragment of the invading nucleic acid (protospacer) is incorporated into the CRISPR array along with a synthesis of an additional repeat unit. This process is known as adaptation and is responsible for the unique adaptive features of CRISPR (Barrangou et al., 2007). Second, during the crRNA biogenesis phase, the CRISPR locus is transcribed and then processed into mature guide RNAs (crRNAs) (Haurwitz et al., 2010). Third, crRNAs recruit effector complexes and guide them to their target by base pairing with the invading nucleic acids (Brouns et al., 2008, Westra et al., 2012). This last step of CRISPR immunity is known as interference and ends with the cleavage of the exogenous genetic element (Garneau et al., 2010). Despite this general mode of action, CRISPR systems have been classified into three types (I–III), each of them with several subtypes, depending on the gene composition and architecture of the respective cas operons (Makarova et al., 2011). While studies of the mechanisms of crRNA biogenesis and interference are well advanced, CRISPR adaptation, also known as immunization or spacer acquisition, perhaps the most puzzling and fascinating aspect of these systems, remains poorly understood (Fineran & Charpentier, 2012, Kiro et al., 2013).
Figure 1.
The three stages of CRISPR immunity. CRISPR loci consist of clusters of repeats (white rectangles) and spacers (colored rectangles) that are in proximity of the upstream leader sequence and CRISPR-associated (cas) genes. During adaptation, new spacers derived from the genome of the invading virus are incorporated into the CRISPR array along with a new repeat unit. During crRNA biogenesis, the array is transcribed and the precursor transcript is processed by Cas endoribonucleases in order to generate small crRNAs. During interference, the crRNA guides a complex of Cas proteins to the matching target to initiate nucleolytic cleavage (scissors) of the invading nucleic acid.
Spacer acquisition was first demonstrated under laboratory conditions in 2007 for the type II-A system of Streptococcus thermophilus (Barrangou et al., 2007). In these studies, investigators examined the CRISPR array of phage-immunized bacteria and found the addition of new repeat-spacer units, with all new spacers perfectly matching regions of the genome of the challenging phage. Mutants that acquired spacers targeting sequences shared between two phages were resistant to both viruses. These results established CRISPR-Cas systems as an adaptive, sequence-specific immune system against phages and were corroborated in other bacteria and archaea containing different CRISPR-Cas Types: Escherichia coli type I-E (Datsenko et al., 2012, Swarts et al., 2012, Yosef et al., 2012), Pseudomonas aeruginosa type I-F (Cady et al., 2012), Streptococcus agalactiae type II-A (Lopez-Sanchez et al., 2012), Haloarcula hispanica type I-B (Li et al., 2013), and Sulfolobus solfataricus type I-A and III-B (Erdmann & Garrett, 2012) (Table 1).
Table 1.
Summary of CRISPR systems in which spacer acquisition has been experimentally observed.
| Organism | Type | PAM | Notable Observations | Reference |
|---|---|---|---|---|
| S. thermophilus | II-A | NGGNG + NNAGAAW | Phage challenge results in acquisition of new spacers matching the viral genome. | Barrangou et al. (2007) |
| High throughput spacer analysis reveals a strong bias in the phage genome locations from which spacers derive. | Paez-Espino et al. (2013) | |||
| E. coli | I-E | AAG | Cas1 and Cas2 are sufficient for spacer acquisition. | Yosef et al. (2012) |
| Priming is first observed against the M13 lytic phage. | Datsenko et al. (2013) | |||
| Priming is confirmed against plasmids. | Savitskaya et al. (2013) | |||
| DNA motifs affect spacer incorporation. | Yosef et al. (2013) | |||
| Integration of spacers in the wrong orientation gives insight into the aquisition mechanism | Shmakov et al. (2014) | |||
| Characterization of priming requirements | Fineran et al. (2014) | |||
| AWG / AAG | Interaction between the leader sequence and Cas proteins determines spacer orientation | Diez-Villasenor et al. (2013) | ||
| S. solfataricus | III-B | No PAM | Spacer acquisition occurs in the middle of the array. | Garrett et al. (2012) |
| I-A | CCN | Spacer acquisition occurs at the leader-end of the array. | ||
| P. aeruginosa | I-F | CC | Spacers are acquired in response to phage challenge. | Cady et al. (2012) |
| S. agalactiae | II-A | NGG | Newly acquired spacers are highly diverse. Rare acquisition events in the wrong orientation lead to non-functional spacers. | Lopez-Sanchez et al. (2012) |
| H. hispanica | I-B | TTC | Priming is required for adaptation in a native system. | Li et al. (2013) |
Target requirements: can any sequence of the invader’s genome become a CRISPR spacer?
When investigators aligned newly acquired spacers from the S. thermophilus CRISPR-Cas system in search for common motifs, they found something unexpected. Instead of a common sequence within the spacers they found a conserved sequence outside the target (also known as protospacer), which was termed Protospacer Adjacent Motif (PAM) (Deveau et al., 2008). This suggested that the adaptation machinery only acquires spacers that have adjacent PAMs. It therefore became clear early on that not all phage sequences are equal for the CRISPR-Cas system.. The PAM is not only important for the acquisition of spacer sequences, it is also required for the interference phase of CRISPR immunity since PAM mutations in types I and II prevent Cas nuclease cleavage (Deveau et al., 2008, Jiang et al., 2013, Jinek et al., 2012, Semenova et al., 2011, Westra et al., 2013, Fineran et al., 2014). This interference requirement is readily exploited by phages, which can avoid CRISPR immunity by mutating the PAM sequence (Deveau et al., 2008). The PAM is fundamental to avoid auto-immunity. If CRISPR immunity relied only on base-pair interactions between the crRNA and the target DNA, then the spacer sequence on the CRISPR array would be a target for the crRNA as well. Since the flanking sequences of a spacer in the CRISPR array are the CRISPR repeat, which lack a proper PAM, auto-immunity is prevented and only protospacers that are flanked with the correct PAM can be cleaved. Type III CRISPR-Cas systems, however, seem to be an exception, as no PAM is evident from the alignment of protospacer sequences acquired by these systems, nor it is required for target cleavage (Hale et al., 2009, Marraffini & Sontheimer, 2010). As a consequence, Type III systems developed a different mechanism to prevent auto-immunity (Marraffini & Sontheimer, 2010).
While the recognition of a PAM by the acquisition machinery is essential for a protospacer to be selected, it appears that other mechanisms further influence the protospacer choice. Studies of spacer acquisition by the E. coli type I-E (Savitskaya et al., 2013, Yosef et al., 2013) and the S. thermophilus type II-A (Paez-Espino et al., 2013) CRISPR-Cas systems reported unequal distributions of protospacers across various targets. The expansion of the CRISPR arrays from S. thermophilus was monitored using DNA deep-sequencing upon infection with a lytic phage (Paez-Espino et al., 2013) and roughly half a million phage-derived spacer sequences were analyzed. Surprisingly, the top 10% most overrepresented spacers accounted for 99% of the identified sequences. In contrast, some candidate protospacers that could have been theoretically acquired from the target based on PAM compatibility were never sampled. Due to partial sequence similarities between some endogenous spacers and the target, Paez-Espino et al. (Paez-Espino et al., 2013) propose priming (see below) as a possible explanation for the strong overrepresentation of certain protospacers. In a similar study in type I-E, all potential protospacer sequences adjacent to a PAM were used as spacer donors, but the frequencies were indeed highly unequal (Savitskaya et al., 2013). While no correlation was observed between the frequency of protospacer incorporation and its nucleotide sequence, melting temperature, GC content, ssDNA secondary structure, or transcription pattern, other investigators detected additional sequence motifs besides the PAM that influence the acquisition efficiency of a protospacer (Yosef et al., 2013). By exchanging nucleotide blocks of various lengths upstream or downstream of one high-acquisition and one low-acquisition protospacer, investigators were able to reverse their rates of acquisition, suggesting that DNA motifs located at both ends of the highly acquired protospacer were responsible for its frequent incorporation. More specifically, in addition to the PAM, a dinucleotide AA motif termed Acquisition Affecting Motif (AAM) located at the 3′ end of the protospacer can boost the rate of incorporation of a given protospacer. Sequences of more than two million spacers confirmed the overrepresentation of the AA motif in highly sampled protospacers. Nonetheless, the AAM was not present in all highly sampled protospacers, indicating that other unidentified DNA motifs might influence the sampling frequencies of protospacers. In a different study, the AAM was not confirmed among plasmid-derived spacers (Shmakov et al., 2014).
Other observations suggest that yet unknown mechanisms influence the spacer choice. In an experiment where Cas1 and Cas2 were overexpressed in the absence of the interference machinery, Yosef et al. (Yosef et al., 2012) observed a 200-fold preferential acquisition of spacers matching the Cas1+2 overexpression plasmid rather than the chromosome. This suggests that a mechanisms might exist to limit the acquisition of self-targeting spacers. This hypothesis however is not supported by a more recent study where preferential acquisition of plasmid-derived protospacers was also observed, but only from a plasmid carrying the cas genes and not from another reported plasmid present in the cells (Diez-Villasenor et al., 2013). These observations rather suggest that cas1 and cas2 preferentially capture protospacers in close proximity to their coding region.
The machinery responsible for spacer acquisition: naïve versus primed adaptation
Cas1 and Cas2 are the only Cas proteins universally conserved across all types and subtypes of CRISPR-Cas systems (Haft et al., 2005, Makarova et al., 2011). Initial studies on the role of Cas proteins revealed that mutations or deletions of Cas1 and Cas2 did not impact interference and crRNA maturation in type I (Brouns et al., 2008), type II (Sapranauskas et al., 2011, Deltcheva et al., 2011), and type III (Hatoum-Aslan et al., 2011, Hatoum-Aslan et al., 2014). These observations led to the hypothesis that the two universal Cas proteins might be involved in adaptation. Indeed, in E. coli, overexpression of both cas1 and cas2 alone in the absence of the other cas genes was sufficient to acquire new plasmid-derived or host-derived spacers (Yosef et al., 2012, Datsenko et al., 2012). Yosef et al. (2012) also found that Cas1/2 mediate the preferential acquisition of spacers with a correct PAM, demonstrating that there is a mechanism to select spacer sequences flanking this motif, as opposed to the random acquisition of DNA sequences followed by the selection of those with correct PAMs. The biochemical properties of Cas1 support its role in spacer acquisition. Pseudomonas aeruginosa Cas1 has been shown to bind dsDNA in a sequence-independent manner with high affinity, and to work as a metal-dependent endonuclease which cleaves dsDNA into short fragments, that might serve as precursors for new spacers (Han et al., 2009, Wiedenheft et al., 2009). Nonetheless, it is still unclear whether new spacers are cut or copied from the invading molecule. Because Cas1 also has the ability to resolve Holliday junctions and thus promote DNA integration and recombination events, it could promote the integration of spacer sequences into the repeat-spacer array. While many Cas2 crystal structures have been solved and studied biochemically (Beloglazova et al., 2008, Samai et al., 2010, Nam et al., 2012), a general consensus regarding its activity has not been reached.
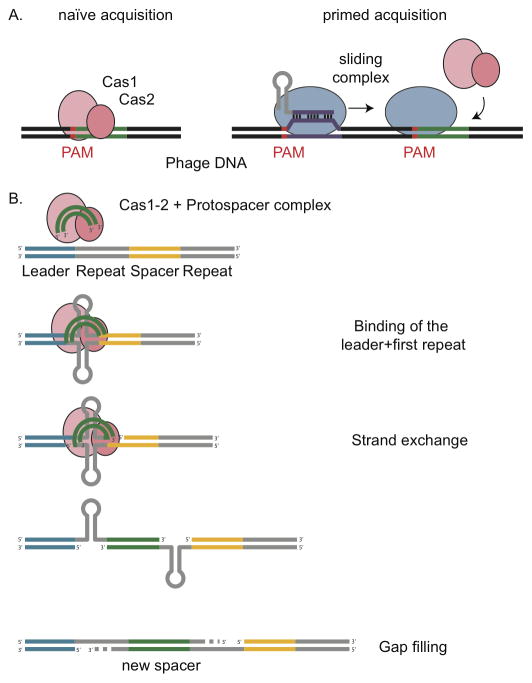
The Cas1/2-mediated acquisition can add repeat-spacer units to a minimal CRISPR locus consisting of only one repeat sequence (Yosef et al., 2012). This indicates that this mechanism of acquisition does not require the presence of any other spacers, i.e. a previous exposure to the same or related phages and therefore is referred as “naïve” acquisition. This is in contrast to “primed” acquisition, where the presence of spacers with a full or partial match to the target DNA increases the frequency of acquiring another spacer (Fig. 2A).
Figure 2.
A model for the acquisition of new spacers. (A) Naïve vs. primed spacer acquisition. Upon lytic infection with a phage previously not encountered, incorporation of a new spacer into the CRISPR array ensures cell survival. Only protospacers adjacent to PAMs are sampled. Naïve adaptation (left) requires the concerted action of Cas1 and Cas2 alone. Primed acquisition (right) presupposes the existence of a non-targeting crRNA with partial homology to a region of the infecting phage (purple). Following the low-affinity target recognition by the interference machinery, the complex slides along the target DNA and, aided by Cas1 and Cas2, recruits spacers from the same strand at a high rate. (B) A model for spacer integration onto the CRISPR array. The protospacer (green) is acquired from the viral genome and inserted into the CRISPR array at the leader-proximal end. Upon integration, the first pre-existent repeat serves as a template for the new repeat. In this model we speculate that the palindromic sequence of the repeat allows it to fold into DNA hairpins (grey).
Primed acquisition has been studied in E. coli, which CRISPR-Cas system harbors the genes encoding the Cascade (CRISPR associated complex for antiviral defense) complex that contains the crRNA guide and is responsible for target recognition (Brouns et al., 2008) and the Cas3 nuclease responsible for target cleavage (Westra et al., 2012), in addition to Cas1 and Cas2. One study showed that this CRISPR system can acquire spacers from a plasmid present in the cell, resulting in plasmid curing (Swarts et al., 2012). While the acquisition of a single spacer is enough to cure the plasmid, multiple spacers were frequently incorporated into the CRISPR array. Interestingly these were always acquired from the same strand of DNA. This led to the hypothesis that the acquisition of one spacer can trigger the acquisition of additional spacers from the same strand of the target DNA (Swarts et al., 2012). These authors hypothesize that the Cascade complex is directed to bind the foreign nucleic acid by a spacer already present in the array. If the match is good enough to trigger interference, Cas3 will degrade the dsDNA and these cleavage products can be used by Cas1 and Cas2 as precursors for new spacers recruited from the same strand.
Another study reported primed adaptation during infection of E. coli with the M13 phage (Datsenko et al., 2012). The acquisition of new spacers was much more frequent when a spacer already targeting the M13 phage was present in the array. The authors showed how the orientation of the priming target determines the orientation of new protospacers. Interestingly, priming events occurred when mismatches that abolish interference were present between the crRNA and its target. This suggests that degradation of the target DNA by CRISPR interference is not necessary to prime adaptation. It is hypothesized that the Cascade complex can bind an imperfect target and trigger the acquisition of new spacers from the same molecule. Mutagenesis of the cas genes showed that in addition to Cas1 and Cas2, primed adaptation requires the Cascade complex and Cas3. Subsequent high-throughput analysis of spacer acquisition in E. coli confirmed that spacers are preferentially acquired from the primed strand with a 10-fold bias (Savitskaya et al., 2013). Assuming that spacers acquired from the non-primed strand are due to naïve adaptation, the authors conclude that primed adaptation occurs at much higher rates than naïve adaptation. Recently, a comprehensive study performed in type I-E showed that priming is nucleotide-dependent, as well as sensitive to the number of mutations and their locations with the target (Fineran et al., 2014). Accordingly, high-throughput plasmid-loss assays revealed that priming tolerates up to 13 mutations within the PAM and protospacer. While the nucleotide-dependence of priming appears to be a more complex mechanism that needs to be further characterized, it appears that G-rich spacers are more likely to prime a better adaptation response.
These observations led to the “sliding” hypothesis for primed acquisition: after a low-affinity target recognition by the Cascade-crRNA complex, the complex slides along the target DNA randomly stopping at PAM sequences to recruit more spacers from the same strand (Fig. 2A). This hypothesis was tested by several studies with results that both corroborated or challenged it. In a recent study of the E. coli type I-E system, Savitskaya et al. (Savitskaya et al., 2013) argue that sliding from the priming position should lead to a preferential acquisition of nearby spacers, producing a gradient in spacer acquisition frequency relative to the priming location, which they did not observe. Moreover, insertion of poly-PAM blocks on the target molecule next to the priming site failed to halt the putative sliding acquisition machinery. In contrast, a recent study of Haloarcula hispanica type I-B system showed that protospacers in close-proximity of the priming protospacers were sampled more often than protospacers located farther away (Li et al., 2013), thus supporting the sliding hypothesis. Regions located both upstream and downstream of the priming protospacer were highly sampled, but from opposite strands. Therefore the authors proposed that sliding also involves stochastic Cas3 flipping from one strand of the DNA to the other. However, these results are not necessarily in contradiction. It is possible for instance that the observation of a gradient in the Savitskaya experiment was hindered by the presence of multiple priming sites or the small size (~ 3kb) of the plasmids used. Indeed, a Cascade that slides long distances relative to the plasmid size would prevent the generation of a positional gradient of acquired spacers.
The priming mechanism has likely evolved as a way to counteract phage mutants that escape CRISPR immunity by single point mutations in the target sequence. The spacers matching mutated targets cannot direct cleavage but can still be used to trigger the acquisition of new spacers and adapt against an evolving threat. Furthermore, priming favors the acquisition of multiple spacers targeting the same DNA molecule, which reduces the probability of escape and strengthens resistance. Notwithstanding the benefits of primed spacer acquisition, naïve adaptation remains crucial to detect unknown foreign molecules and is probably a universal feature of CRISPR systems.
Insertion of new spacers into the CRISPR array
Once a target has been selected on the invading genome, it has to be incorporated into the CRISPR array. During this process, not only the spacer is incorporated, but also a new repeat is added to the array. Spacer insertion is polar, since the vast majority of new spacers are incorporated at the 5′ end of the array, upstream of the first repeat (Barrangou et al., 2007, Andersson & Banfield, 2008, Horvath et al., 2008, Tyson & Banfield, 2008, Horvath et al., 2009, Pride et al., 2011, Pride et al., 2012, Yosef et al., 2012). Little is known about this process; however the first repeat and the region immediately upstream of it, known as the leader sequence, seem to play a role.
The 200–500 bp region located upstream of the first repeat contains an A/T rich leader sequence that usually harbors the promoter of the CRISPR array but it is also involved in adaptation. Deleting or scrambling the 60 nucleotides immediately adjacent to the array of the type I-E CRISPR-Cas system of E. coli prevents spacer acquisition (Yosef et al., 2012). This indicates that the leader contains specific sequence motifs essential for adaptation. Interestingly, deleting the −10 promoter element required for the transcription of the array does not prevent spacer acquisition (Yosef et al., 2012), suggesting that transcription is not essential for the adaptation process. It is believed that the leader sequence is recognized by the acquisition machinery. Evidence supporting this hypothesis comes from a study showing that Cas1 and Cas2 from the E. coli K12 strain can direct spacer acquisition in the CRISPR array of the O157:H7 strain, which carries a different leader sequence (Diez-Villasenor et al., 2013). However, this artificial leader-Cas combination led to frequent abnormal acquisition events where the spacers were integrated in the wrong orientation. This suggests that the interaction between Cas proteins and the leader sequence determines the orientation of newly acquired spacers. Furthermore, in some instances, the insertion site was shifted by 2 bases, suggesting that the acquisition complex is anchored at the leader-repeat boundary where a first cut is made, and uses a ruler mechanism to cut the other strand on the other side of the repeat. The nucleotide content of the spacer is also thought to impact the orientation of newly acquired spacers. In a recent high-throughput study of spacers acquired by the type I-E system, Shmakov et al. observed that spacers are frequently inserted in the wrong orientation in the CRISPR array, resulting in pairs of complementary spacers in the dataset (Shmakov et al., 2014). Interestingly, integration happened in the proper orientation ~99.5% of the time when a proper PAM was present, but that preference for a given orientation was much lower for sequences lacking a PAM. This suggests that eventhough the PAM itself is not integrated in the CRISPR array, it is able to influence the orientation of the integrated spacer in a way that other sequences cannot do.
The presence of a single repeat has been shown to be necessary and sufficient for both naïve and primed adaptation in the type I-E CRISPR system (Yosef et al., 2012, Datsenko et al., 2012), and the presence of additional repeats does not increase the rate of acquisition of new spacers (Yosef et al., 2013). Interestingly, spacers incorporated into a minimal CRISPR array (one repeat, no preexisting spacers) have the correct length (Yosef et al., 2012), suggesting that the protein machinery, rather than preexistent repeat-spacer units, dictates the size of additional spacers. In type I-E systems, the new repeat (29 nt long) is copied from the first repeat in the array since point mutations introduced in the first repeat are replicated in newly incorporated spacer-repeats units (Datsenko et al., 2012, Yosef et al., 2012). Interestingly, mutations of the last nucleotide of the repeat were not passed on to new repeats, indicating that only bases 1 through 28 of the repeat serve as a template for new repeats. In contrast, the 29th base originates from the protospacer and represents the last nucleotide of the PAM (Goren et al., 2012, Datsenko et al., 2012). While the last nucleotide of the 5′-AWG-3′ PAM is highly conserved in E. coli, this is not the case in many other systems where this mode of repeat duplication remains to be determined. Based on these results and on the known mechanisms of insertion of transposable elements (Goryshin & Reznikoff, 1998) and retroviruses (Merkel et al., 2009) a model for spacer acquisition has emerged (Fig. 2B). The first repeat sequence of the CRISPR locus is subjected to ssDNA nicking at the 3′ end of each repeat strand. This cleavage could be facilitated by the stem-loop structure that can form on most repeats due to their partially palindromic sequences. Proximity to the leader would provide recognition of the first repeat and/or help recruit the spacer acquisition machinery. The free 3′-ends of repeats are ligated to the 5′ end of viral fragments, leading to the insertion of a new spacer and the generation of a staggered intermediate. The gaps are filled by DNA polymerase I, thus adding a new repeat to the array. Current research across different laboratories is testing this model.
Concluding remarks
Although recent studies have established molecular requirements as well as a general mechanism for the acquisition phase, many details of CRISPR adaptation are still poorly understood. An extra layer of complexity is added by the many different types of CRISPR-Cas systems, some of which could have different mechanisms of spacer acquisition. Indeed, variations in the way spacers are acquired from the target likely exist between CRISPR types and subtypes. In the type I-A of the crenarchaeon Thermoproteus tenax, Cas1 and Cas2 are fused as a single protein that forms the CRISPR-associated complex for the integration of spacers (Cascis) together with Csa1 and Cas4 (Plagens et al., 2012). In type II, Csn2 was reported to be required for adaptation (Barrangou et al., 2007). Structural studies have revealed that this protein forms a ring-like structure around DNA, suggesting that it might recruit other proteins to the protospacer and could form a sliding clamp that facilitates primed acquisition (Arslan et al., 2013). In type III-B, the effector Cmr complex cleaves RNA rather than DNA (Hale et al., 2009), yet spacers are incorporated as DNA fragments.
We believe that future research will focus on understanding how the leader sequence is recognized, how the first repeat is cleaved and the new spacer ligated in a way that allows the generation of an additional repeat, and whether the length of the array is regulated. In addition, it is still unknown why spacers are acquired preferentially from certain molecules or certain positions on a given molecule, and whether any mechanisms truly exists to prevent, or at least limit, the acquisition of self-targeting spacers. An interesting question also arises from the observation that the PAM motif is recognized both during acquisition and interference, and that the motif might be recognized by different protein complexes in each of these stages of the CRISPR immunity pathway. As the exact sequence requirements might be different for these two functions, it has been proposed to use the term Spacer Acquisition Motif (SAM) when referring to the sequence recognized by the acquisition machinery and the term Target Interference Motif (TIM) when referring to the sequence recognized by the interference machinery (Almendros et al., 2012). A mutation that affects SAM recognition might lead to the acquisition of spacers that will not be effective during the interference stage. Conversely, a mutation that affects the TIM recognition might render preexisting as well as newly acquired spacers useless. In the presence of this apparent evolutionary bottleneck, PAM sequences might be expected to be highly conserved, yet an extensive diversity has been described. How these two facts can be reconciled remains to be investigated.
Acknowledgments
D.B. is supported by the Bettencourt Schuller Foundation. L.A.M is supported by the Searle Scholars Program, the Rita Allen Scholars Program, an Irma T. Hirschl Award, a Sinsheimer Foundation Award and a NIH Director’s New Innovator Award (1DP2AI104556-01).
References
- Agari Y, Sakamoto K, Tamakoshi M, Oshima T, Kuramitsu S, Shinkai A. Transcription Profile of Thermus thermophilus CRISPR Systems after Phage Infection. J Mol Biol. 2010;395:270–281. doi: 10.1016/j.jmb.2009.10.057. [DOI] [PubMed] [Google Scholar]
- Almendros C, Guzman NM, Diez-Villasenor C, Garcia-Martinez J, Mojica FJ. Target motifs affecting natural immunity by a constitutive CRISPR-Cas system in Escherichia coli. PLoS One. 2012;7:e50797. doi: 10.1371/journal.pone.0050797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson AF, Banfield JF. Virus population dynamics and acquired virus resistance in natural microbial communities. Science. 2008;320:1047–1050. doi: 10.1126/science.1157358. [DOI] [PubMed] [Google Scholar]
- Arslan Z, Wurm R, Brener O, Ellinger P, Nagel-Steger L, Oesterhelt F, Schmitt L, Willbold D, Wagner R, Gohlke H, Smits SH, Pul U. Double-strand DNA end-binding and sliding of the toroidal CRISPR-associated protein Csn2. Nucleic Acids Res. 2013;41:6347–6359. doi: 10.1093/nar/gkt315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. doi: 10.1126/science.1138140. [DOI] [PubMed] [Google Scholar]
- Beloglazova N, Brown G, Zimmerman MD, Proudfoot M, Makarova KS, Kudritska M, Kochinyan S, Wang S, Chruszcz M, Minor W, Koonin EV, Edwards AM, Savchenko A, Yakunin AF. A novel family of sequence-specific endoribonucleases associated with the clustered regularly interspaced short palindromic repeats. J Biol Chem. 2008;283:20361–20371. doi: 10.1074/jbc.M803225200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikard D, Hatoum-Aslan A, Mucida D, Marraffini LA. CRISPR interference can prevent natural transformation and virulence acquisition during in vivo bacterial infection. Cell Host Microbe. 2012;12:177–186. doi: 10.1016/j.chom.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Bikard D, Marraffini LA. Innate and adaptive immunity in bacteria: mechanisms of programmed genetic variation to fight bacteriophages. Curr Opin Immunol. 2012;24:15–20. doi: 10.1016/j.coi.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology. 2005;151:2551–2561. doi: 10.1099/mic.0.28048-0. [DOI] [PubMed] [Google Scholar]
- Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, Dickman MJ, Makarova KS, Koonin EV, van der Oost J. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science. 2008;321:960–964. doi: 10.1126/science.1159689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady KC, Bondy-Denomy J, Heussler GE, Davidson AR, O’Toole GA. The CRISPR/Cas Adaptive Immune System of Pseudomonas aeruginosa Mediates Resistance to Naturally Occurring and Engineered Phages. J Bacteriol. 2012;194:5728–5738. doi: 10.1128/JB.01184-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Pougach K, Tikhonov A, Wanner BL, Severinov K, Semenova E. Molecular memory of prior infections activates the CRISPR/Cas adaptive bacterial immunity system. Nat Commun. 2012;3:945. doi: 10.1038/ncomms1937. [DOI] [PubMed] [Google Scholar]
- Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao Y, Pirzada ZA, Eckert MR, Vogel J, Charpentier E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature. 2011;471:602–607. doi: 10.1038/nature09886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveau H, Barrangou R, Garneau JE, Labonte J, Fremaux C, Boyaval P, Romero DA, Horvath P, Moineau S. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J Bacteriol. 2008;190:1390–1400. doi: 10.1128/JB.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Villasenor C, Guzman NM, Almendros C, Garcia-Martinez J, Mojica FJ. CRISPR-spacer integration reporter plasmids reveal distinct genuine acquisition specificities among CRISPR-Cas I-E variants of Escherichia coli. RNA Biol. 2013;10:792–802. doi: 10.4161/rna.24023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann S, Garrett RA. Selective and hyperactive uptake of foreign DNA by adaptive immune systems of an archaeon via two distinct mechanisms. Mol Microbiol. 2012;85:1044–1056. doi: 10.1111/j.1365-2958.2012.08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fineran PC, Charpentier E. Memory of viral infections by CRISPR-Cas adaptive immune systems: acquisition of new information. Virology. 2012;434:202–209. doi: 10.1016/j.virol.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Fineran PC, Gerritzen MJ, Suarez-Diez M, Kunne T, Boekhorst J, van Hijum SA, Staals RH, Brouns SJ. Degenerate target sites mediate rapid primed CRISPR adaptation. Proc Natl Acad Sci USA. 2014;111:E1629–1638. doi: 10.1073/pnas.1400071111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau JE, Dupuis ME, Villion M, Romero DA, Barrangou R, Boyaval P, Fremaux C, Horvath P, Magadan AH, Moineau S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 2010;468:67–71. doi: 10.1038/nature09523. [DOI] [PubMed] [Google Scholar]
- Goren M, Yosef I, Edgar R, Qimron U. The bacterial CRISPR/Cas system as analog of the mammalian adaptive immune system. RNA Biol. 2012;9:549–554. doi: 10.4161/rna.20177. [DOI] [PubMed] [Google Scholar]
- Goryshin IY, Reznikoff WS. Tn5 in vitro transposition. J Biol Chem. 1998;273:7367–7374. doi: 10.1074/jbc.273.13.7367. [DOI] [PubMed] [Google Scholar]
- Haft DH, Selengut J, Mongodin EF, Nelson KE. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput Biol. 2005;1:e60. doi: 10.1371/journal.pcbi.0010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale CR, Zhao P, Olson S, Duff MO, Graveley BR, Wells L, Terns RM, Terns MP. RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell. 2009;139:945–956. doi: 10.1016/j.cell.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Lehmann K, Krauss G. SSO1450 - a CAS1 protein from Sulfolobus solfataricus P2 with high affinity for RNA and DNA. FEBS Lett. 2009;583:1928–1932. doi: 10.1016/j.febslet.2009.04.047. [DOI] [PubMed] [Google Scholar]
- Hatoum-Aslan A, Maniv I, Marraffini LA. Mature clustered, regularly interspaced, short palindromic repeats RNA (crRNA) length is measured by a ruler mechanism anchored at the precursor processing site. Proc Natl Acad Sci USA. 2011;108:21218–21222. doi: 10.1073/pnas.1112832108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatoum-Aslan A, Maniv I, Samai P, Marraffini LA. Genetic Characterization of Antiplasmid Immunity through a Type III-A CRISPR-Cas System. J Bacteriol. 2014;196:310–317. doi: 10.1128/JB.01130-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haurwitz RE, Jinek M, Wiedenheft B, Zhou K, Doudna JA. Sequence- and structure-specific RNA processing by a CRISPR endonuclease. Science. 2010;329:1355–1358. doi: 10.1126/science.1192272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath P, Coute-Monvoisin AC, Romero DA, Boyaval P, Fremaux C, Barrangou R. Comparative analysis of CRISPR loci in lactic acid bacteria genomes. Int J Food Microbiol. 2009;131:62–70. doi: 10.1016/j.ijfoodmicro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- Horvath P, Romero DA, Coute-Monvoisin AC, Richards M, Deveau H, Moineau S, Boyaval P, Fremaux C, Barrangou R. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. J Bacteriol. 2008;190:1401–1412. doi: 10.1128/JB.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 1987;169:5429–5433. doi: 10.1128/jb.169.12.5429-5433.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R, Embden JD, Gaastra W, Schouls LM. Identification of genes that are associated with DNA repeats in prokaryotes. Mol Microbiol. 2002;43:1565–1575. doi: 10.1046/j.1365-2958.2002.02839.x. [DOI] [PubMed] [Google Scholar]
- Jiang W, Bikard D, Cox D, Zhang F, Marraffini LA. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat Biotechnol. 2013;31:233–239. doi: 10.1038/nbt.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiro R, Goren MG, Yosef I, Qimron U. CRISPR adaptation in Escherichia coli subtypeI-E system. Biochem Soc Trans. 2013;41:1412–1415. doi: 10.1042/BST20130109. [DOI] [PubMed] [Google Scholar]
- Kunin V, Sorek R, Hugenholtz P. Evolutionary conservation of sequence and secondary structures in CRISPR repeats. Genome Biol. 2007;8:R61. doi: 10.1186/gb-2007-8-4-r61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wang R, Zhao D, Xiang H. Adaptation of the Haloarcula hispanica CRISPR-Cas system to a purified virus strictly requires a priming process. Nucleic Acids Res. 2013 doi: 10.1093/nar/gkt1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sanchez MJ, Sauvage E, Da Cunha V, Clermont D, Ratsima Hariniaina E, Gonzalez-Zorn B, Poyart C, Rosinski-Chupin I, Glaser P. The highly dynamic CRISPR1 system of Streptococcus agalactiae controls the diversity of its mobilome. Mol Microbiol. 2012;85:1057–1071. doi: 10.1111/j.1365-2958.2012.08172.x. [DOI] [PubMed] [Google Scholar]
- Makarova KS, Grishin NV, Shabalina SA, Wolf YI, Koonin EV. A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol Direct. 2006;1:7. doi: 10.1186/1745-6150-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova KS, Haft DH, Barrangou R, Brouns SJ, Charpentier E, Horvath P, Moineau S, Mojica FJ, Wolf YI, Yakunin AF, van der Oost J, Koonin EV. Evolution and classification of the CRISPR-Cas systems. Nat Rev Microbiol. 2011;9:467–477. doi: 10.1038/nrmicro2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffini LA. CRISPR-Cas Immunity against Phages: Its Effects on the Evolution and Survival of Bacterial Pathogens. PLoS Pathog. 2013;9:e1003765. doi: 10.1371/journal.ppat.1003765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffini LA, Sontheimer EJ. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science. 2008;322:1843–1845. doi: 10.1126/science.1165771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffini LA, Sontheimer EJ. Self versus non-self discrimination during CRISPR RNA-directed immunity. Nature. 2010;463:568–571. doi: 10.1038/nature08703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel G, Andrake MD, Ramcharan J, Skalka AM. Oligonucleotide-based assays for integrase activity. Methods. 2009;47:243–248. doi: 10.1016/j.ymeth.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60:174–182. doi: 10.1007/s00239-004-0046-3. [DOI] [PubMed] [Google Scholar]
- Nam KH, Ding F, Haitjema C, Huang Q, DeLisa MP, Ke A. Double-stranded endonuclease activity in Bacillus halodurans clustered regularly interspaced short palindromic repeats (CRISPR)-associated Cas2 protein. J Biol Chem. 2012;287:35943–35952. doi: 10.1074/jbc.M112.382598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Espino D, Morovic W, Sun CL, Thomas BC, Ueda K, Stahl B, Barrangou R, Banfield JF. Strong bias in the bacterial CRISPR elements that confer immunity to phage. Nat Commun. 2013;4:1430. doi: 10.1038/ncomms2440. [DOI] [PubMed] [Google Scholar]
- Plagens A, Tjaden B, Hagemann A, Randau L, Hensel R. Characterization of the CRISPR/Cas subtype I-A system of the hyperthermophilic crenarchaeon Thermoproteus tenax. J Bacteriol. 2012;194:2491–2500. doi: 10.1128/JB.00206-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pougach K, Semenova E, Bogdanova E, Datsenko KA, Djordjevic M, Wanner BL, Severinov K. Transcription, processing and function of CRISPR cassettes in Escherichia coli. Mol Microbiol. 2010;77:1367–1379. doi: 10.1111/j.1365-2958.2010.07265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcel C, Salvignol G, Vergnaud G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology. 2005;151:653–663. doi: 10.1099/mic.0.27437-0. [DOI] [PubMed] [Google Scholar]
- Pride DT, Salzman J, Relman DA. Comparisons of clustered regularly interspaced short palindromic repeats and viromes in human saliva reveal bacterial adaptations to salivary viruses. Environ Microbiol. 2012;14:2564–2576. doi: 10.1111/j.1462-2920.2012.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pride DT, Sun CL, Salzman J, Rao N, Loomer P, Armitage GC, Banfield JF, Relman DA. Analysis of streptococcal CRISPRs from human saliva reveals substantial sequence diversity within and between subjects over time. Genome Res. 2011;21:126–136. doi: 10.1101/gr.111732.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samai P, Smith P, Shuman S. Structure of a CRISPR-associated protein Cas2 from Desulfovibrio vulgaris. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66:1552–1556. doi: 10.1107/S1744309110039801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapranauskas R, Gasiunas G, Fremaux C, Barrangou R, Horvath P, Siksnys V. The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Res. 2011 doi: 10.1093/nar/gkr606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitskaya E, Semenova E, Dedkov V, Metlitskaya A, Severinov K. High-throughput analysis of type I-E CRISPR/Cas spacer acquisition in E. coli. RNA Biol. 2013;10:716–725. doi: 10.4161/rna.24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova E, Jore MM, Datsenko KA, Semenova A, Westra ER, Wanner B, van der Oost J, Brouns SJ, Severinov K. Interference by clustered regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence. Proc Natl Acad Sci USA. 2011;108:10098–10103. doi: 10.1073/pnas.1104144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SA, Hansen NR, Garrett RA. Distribution of CRISPR spacer matches in viruses and plasmids of crenarchaeal acidothermophiles and implications for their inhibitory mechanism. Biochem Soc Trans. 2009;37:23–28. doi: 10.1042/BST0370023. [DOI] [PubMed] [Google Scholar]
- Shmakov S, Savitskaya E, Semenova E, Logacheva MD, Datsenko KA, Severinov K. Pervasive generation of oppositely oriented spacers during CRISPR adaptation. Nucleic Acids Res. 2014 doi: 10.1093/nar/gku226. In print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek R, Kunin V, Hugenholtz P. CRISPR--a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat Rev Microbiol. 2008;6:181–186. doi: 10.1038/nrmicro1793. [DOI] [PubMed] [Google Scholar]
- Swarts DC, Mosterd C, van Passel MW, Brouns SJ. CRISPR interference directs strand specific spacer acquisition. PLoS One. 2012;7:e35888. doi: 10.1371/journal.pone.0035888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CM, Nielsen KM. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Microbiol. 2005;3:711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- Tyson GW, Banfield JF. Rapidly evolving CRISPRs implicated in acquired resistance of microorganisms to viruses. Environ Microbiol. 2008;10:200–2007. doi: 10.1111/j.1462-2920.2007.01444.x. [DOI] [PubMed] [Google Scholar]
- Westra ER, Semenova E, Datsenko KA, Jackson RN, Wiedenheft B, Severinov K, Brouns SJ. Type I-E CRISPR-cas systems discriminate target from non-target DNA through base pairing-independent PAM recognition. PLoS Genetics. 2013;9:e1003742. doi: 10.1371/journal.pgen.1003742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westra ER, van Erp PB, Kunne T, Wong SP, Staals RH, Seegers CL, Bollen S, Jore MM, Semenova E, Severinov K, de Vos WM, Dame RT, de Vries R, Brouns SJ, van der Oost J. CRISPR immunity relies on the consecutive binding and degradation of negatively supercoiled invader DNA by Cascade and Cas3. Mol Cell. 2012;46:595–605. doi: 10.1016/j.molcel.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenheft B, Zhou K, Jinek M, Coyle SM, Ma W, Doudna JA. Structural basis for DNase activity of a conserved protein implicated in CRISPR-mediated genome defense. Structure. 2009;17:904–912. doi: 10.1016/j.str.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Yosef I, Goren MG, Qimron U. Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli. Nucleic Acids Res. 2012;40:5569–5576. doi: 10.1093/nar/gks216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosef I, Shitrit D, Goren MG, Burstein D, Pupko T, Qimron U. DNA motifs determining the efficiency of adaptation into the Escherichia coli CRISPR array. Proc Natl Acad Sci USA. 2013;110:14396–14401. doi: 10.1073/pnas.1300108110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Heidrich N, Ampattu BJ, Gunderson CW, Seifert HS, Schoen C, Vogel J, Sontheimer EJ. Processing-Independent CRISPR RNAs Limit Natural Transformation in Neisseria meningitidis. Mol Cell. 2013;50:488–503. doi: 10.1016/j.molcel.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]