SLEEP DURATION IN MIDLIFE AND LATER LIFE IN RELATION TO COGNITION (original) (raw)
. Author manuscript; available in PMC: 2015 Jun 1.
Published in final edited form as: J Am Geriatr Soc. 2014 May 1;62(6):1073–1081. doi: 10.1111/jgs.12790
Abstract
Background/Objectives
Sleep might influence brain health in older adults; however, epidemiologic research in this area is limited. We evaluated associations of sleep duration at midlife and later life, and change in sleep duration over time, with cognitive function in older women.
Design
Participants reported sleep duration in 1986 and 2000, and a subgroup of older participants began cognitive testing in 1995–2001; follow-up testing was conducted three times, at two-year intervals.
Setting
Prospective Nurses’ Health Study cohort.
Participants
15,385 female nurses, aged ≥70 years, free of stroke and depression at the initial cognitive assessment.
Measurements
Validated, telephone-based cognitive battery to measure cognitive function; we averaged the four repeated assessments over a six-year period to estimate overall cognition at older ages, and also evaluated trajectories of cognitive change over follow up.
Results
Extreme sleep durations in later life were associated with worse average cognition (p-value for the quadratic term<0.001 for a global score averaging all six cognitive tests). For example, women sleeping ≤5 hours/day had worse global cognition than those sleeping 7 hours/day, as did women sleeping ≥9 hours/day; differences were equivalent to nearly two additional years of age. Associations were similar, though slightly attenuated, for sleep duration in midlife. Also, women whose sleep duration changed by ≥2 hours/day over time had worse cognition than women with no change in sleep duration (e.g., for the global score, p-value for the quadratic term<0.001). Sleep duration was not associated with women’s trajectories of cognitive function over six years (i.e., cognitive decline), which might be attributable to relatively short follow up for detecting cognitive decline.
Conclusions
Extreme sleep durations at both midlife and later life, and extreme changes in sleep duration over time, appear to be associated with worse cognitive status in older women.
Keywords: sleep, cognition, epidemiology, cohort study
INTRODUCTION
Approximately 30% of U.S. adults have usual sleep durations outside of 7–8 hours/day—a percentage that increases with advancing age1. Yet, growing epidemiologic evidence suggests that shorter or longer sleep durations are associated with higher risks of cardiovascular disease2 and type 2 diabetes3, both of which have been strongly linked to cognitive impairment and Alzheimer’s disease4; thus, there is increasing interest in examining the relation of sleep duration and cognitive function in older adults. In addition to indirect effects of sleep duration on cognitive health via vascular mechanisms, there might be a direct effect on the brain: experimental studies in mice have demonstrated that shorter sleep can cause accumulation of beta-amyloid in the brain5—a hallmark Alzheimer’s disease pathology. Several epidemiologic studies have evaluated sleep duration and cognitive function in later life, with varying results6–19. However, most previous studies have been cross sectional, and no studies have investigated sleep duration in both midlife and later life. Thus, in the Nurses’ Health Study, we evaluated sleep duration at both of these time points—and change in sleep duration between these two points—in relation to cognitive performance in later life.
METHODS
The Nurses’ Health Study (NHS) began in 1976, when 121,701 female nurses, aged 30–55 years, returned a mailed questionnaire on health and lifestyle. Biennial questionnaires are used to update this information, and follow up is 90%. Women reported their sleeping habits in 1986 (i.e., midlife) and 2000 (i.e., later life); in 1995–2001, a telephone-based cognitive study was initiated for participants, aged ≥70 years old, with no stroke history. Among eligible women, 19,415 (92%) completed an initial cognitive assessment, which was repeated three times at two-year intervals (median time between the first and fourth interview=6.4 years); >90% of these women completed at least one follow-up assessment. The institutional review board of Brigham and Women’s Hospital (Boston, Massachusetts) approved this study. Participants provided implied consent by returning the questionnaires, and oral consent for the cognitive study.
Population for analysis
Of 19,415 women who completed the initial cognitive assessment, 1,825 were excluded because they had depression (potentially a strong confounding factor) prior to the initial cognitive assessment; another 2,103 nursing home residents were excluded because sleep duration might be influenced by an institutional environment. Of 15,487 remaining participants, 2,435 women did not report sleep duration in midlife, leaving 13,052 women for analyses of sleep duration in midlife and cognition; 224 women did not report sleep duration in later life, which left 15,263 women for analyses of sleep duration in later life and cognition. Key characteristics were similar among participants who were included in the analytic sample vs. excluded because they lacked sleep data (e.g., mean age=74.2 vs. 74.4 years, and mean body-mass index=25.6 vs. 26.1 kg/m2, respectively).
Ascertainment of sleep duration
Women reported their usual hours of sleep in a 24-hour period on the 1986 and 2000 questionnaires; response categories were ≤5, 6, 7, 8, 9, 10, and ≥11 hours. In a validation study, 260 NHS participants completed a sleep diary over six days; a single questionnaire-based report of sleep duration correlated highly with sleep duration recorded in these diaries (ρ=0.79)20.
Ascertainment of cognitive function
We initially administered the Telephone Interview of Cognitive Status (TICS), a telephone version of the Mini-Mental State Examination (MMSE); these tests are highly correlated (ρ=0.97)21. After establishing high participation rates, we added five additional tests: East Boston Memory Test—immediate and delayed recalls, category fluency, delayed recall of the TICS 10-word list, and digit span backward22–24. In a validation study, our telephone-based cognitive battery performed well compared to detailed, one-hour, in-person interviews conducted by a neuropsychologist among 61 women aged ≥70 years (ρ=0.81 comparing the two assessment modes)25. Inter-interviewer reliability was high across ten interviewers each scoring the same cognitive interview (ρ>0.95 for each cognitive test).
Three primary outcomes were selected a priori: two measures of general cognition (a global score averaging all six cognitive tests, and the TICS score) and a verbal memory score, averaging the immediate and delayed recalls of the East Boston Memory Test and TICS 10-word list. Verbal memory is the strongest predictor of Alzheimer’s disease26. Because our cognitive tests are scaled differently, we used z-scores to create composite measures by calculating the difference between each participant’s score and the mean score for our study population, divided by the population standard deviation; we then averaged relevant z-scores to calculate the global and verbal scores. Individual cognitive test scores were considered as secondary outcomes.
Statistical analysis
We employed two approaches to modeling our repeated measures of cognitive function. First, we evaluated sleep duration in midlife (measured in 1986, when women were 56–66 years old), sleep duration in later life (measured in 2000, when women were 70–80 years old), and change in sleep duration (between midlife and later life) in relation to “average” cognitive performance, which was calculated by averaging all four repeated cognitive assessments. Because these assessments were ascertained across a relatively short period (i.e., six years), this outcome provides the most statistically stable estimate of the women’s overall cognitive status in later life. For these analyses, we used multivariable-adjusted linear regression to estimate mean differences in average cognition across categories of sleep duration (≤5, 6, 7, 8, and ≥9 hours/day); the reference category was 7 hours of sleep/day. Second, we examined the associations of sleep duration and change in sleep duration with cognitive decline over six years of follow up. We found nonlinearity in cognitive trajectories due to “learning effects,” primarily between the first and second interviews (i.e., there was an average increase in cognitive scores between the first and second assessments due to participants’ familiarity with the tests, followed by an average decline in scores at the third and fourth interviews). Thus, we averaged test scores at the first and second cognitive assessments to obtain a “robust baseline” score (as described previously27); this method yielded linear cognitive trajectories over time. We then utilized multivariable-adjusted mixed linear regression to estimate rates of cognitive decline across the same exposure categories described above. Specifically, we used repeated-measures modeling with random intercepts and slopes, which permits description of individual cognitive trajectories over time, and provides explicit tests of association between sleep duration and rates of cognitive decline. We calculated 95% confidence intervals (CI) for all effect estimates, and a quadratic term was used to test for inverted, U-shaped associations. The partial F test was used to evaluate the relative contribution of sleep duration in midlife vs. later life in predicting cognitive performance28.
We considered many possible confounding factors, including: age (in years), education (registered nursing degree, bachelor’s degree, graduate degree), shift work history (never, 1–9, 10–19, ≥20 years), smoking status (never, past, current), alcohol intake (none, 1–14 grams/day, ≥15 grams/day), physical activity (in quintiles of metabolic-equivalent-hours/week), body-mass index (BMI) (<22, 22–24, 25–29, ≥30 kg/m2), history of high blood pressure (yes, no), SF-36 mental health score (dichotomized as: ≤52 points indicating poor mental health, >52 points indicating normal mental health), living alone (yes, no), and tranquilizer use (yes, no).
We conducted several secondary analyses. First, we stratified our analyses of change in sleep duration and cognitive function by midlife sleep duration to examine whether associations were strongest among women who initially reported “normal” sleep duration (i.e., 7 hours/day). Second, we excluded women who reported a history of shift work because shift work might have strongly influenced sleep duration, and perhaps cognitive function; therefore, shift work history might be a substantial confounding factor. Third, we conducted secondary analyses of sleep duration in later life and cognition excluding women in the bottom 10% of the cognitive score distribution. These analyses were intended to address concern about the cross-sectional nature of our analyses focused on sleep in later life; specifically, because individuals with greater cognitive impairments are more likely to have sleep disturbances, it is possible that any observed associations might reflect the influence of pathologic processes in the brain on sleep duration in these individuals (i.e., reverse causation). Therefore, we excluded women with the worst cognitive scores (i.e., bottom 10% of the score distribution) to evaluate whether associations remained after removing these women from the analyses.
Analyses were performed using SAS, version 6.0 (SAS Institute, Inc., Cary, NC).
RESULTS
Table 1 shows participant characteristics in midlife across categories of sleep duration in midlife. We observed few meaningful differences in these characteristics; however, women with shorter and longer sleep durations had somewhat lower physical activity levels compared to women with 7 hours of sleep/day. Table 1 also shows participant characteristics in later life across categories of sleep duration in later life. We found few substantial differences, although women with shorter and longer sleep durations had higher body-mass index and lower physical activity levels compared to women who reported 7 hours of sleep/day. Women with the shortest sleep durations were also more likely to live alone compared to other women in this study.
Table 1.
Key characteristics of study participants across categories of sleep duration in both midlife and later life a
| Characteristics | Sleep duration and characteristics in midlife (reported in 1986) | ||||
|---|---|---|---|---|---|
| ≤5 hours/day (n=577) | 6 hours/day (n=3,302) | 7 hours/day (n=5,432) | 8 hours/day (n=3,356) | ≥9 hours/day (n=626) | |
| Mean age, in years | 60.9 | 61.0 | 61.0 | 61.2 | 61.5 |
| Highest education, % master’s or doctoral degree | 7 | 7 | 7 | 6 | 5 |
| Smoking, % never | 48 | 47 | 48 | 49 | 45 |
| Alcohol intake, % none | 39 | 37 | 34 | 37 | 35 |
| Mean body-mass index, in kg/m2 | 25.4 | 25.3 | 25.0 | 25.4 | 25.6 |
| Median physical activity level, in | 6.6 (2.3–18.3) | 7.9 (2.9–20.0) | 8.8 (3.2–20.2) | 7.9 (2.9–20.2) | 7.7 (2.7–17.2) |
| MET-hours/weekb | |||||
| History of high blood pressure, % | 34 | 34 | 32 | 34 | 36 |
| Poor mental health score on the SF-36, % | 7 | 4 | 4 | 4 | 5 |
| Living alone, % | 20 | 20 | 17 | 15 | 16 |
| Use of tranquilizers, % | 4 | 2 | 2 | 2 | 4 |
| Characteristics | Sleep duration and characteristics in later life (reported in 2000) | ||||
|---|---|---|---|---|---|
| ≤5 hours/day (n=920) | 6 hours/day (n=3,484) | 7 hours/day (n=5,379) | 8 hours/day (n=4,257) | ≥9 hours/day (n=1,223) | |
| Mean age, in years | 74.3 | 74.3 | 74.1 | 74.2 | 74.5 |
| Highest education, % master’s or doctoral degree | 4 | 5 | 6 | 6 | 8 |
| Smoking, % never | 47 | 46 | 48 | 47 | 45 |
| Alcohol intake, % none | 55 | 49 | 47 | 49 | 52 |
| Mean body-mass index, in kg/m2 | 26.0 | 25.5 | 25.3 | 25.8 | 26.3 |
| Median physical activity level, in | 7.9 (2.3–20.2) | 9.9 (3.4–22.5) | 10.9 (3.7–22.9) | 10.2 (3.2–22.2) | 7.6 (2.1–18.5) |
| MET-hours/weekb | |||||
| History of high blood pressure, % | 60 | 55 | 52 | 55 | 60 |
| Poor mental health score on the SF-36, % | 8 | 4 | 2 | 2 | 4 |
| Living alone, % | 37 | 36 | 32 | 29 | 28 |
| Use of tranquilizers, % | 8 | 5 | 4 | 4 | 6 |
In general, models adjusted for age and education yielded similar results to models adjusted for multiple potential confounding factors. Here, we present results from fully adjusted models only.
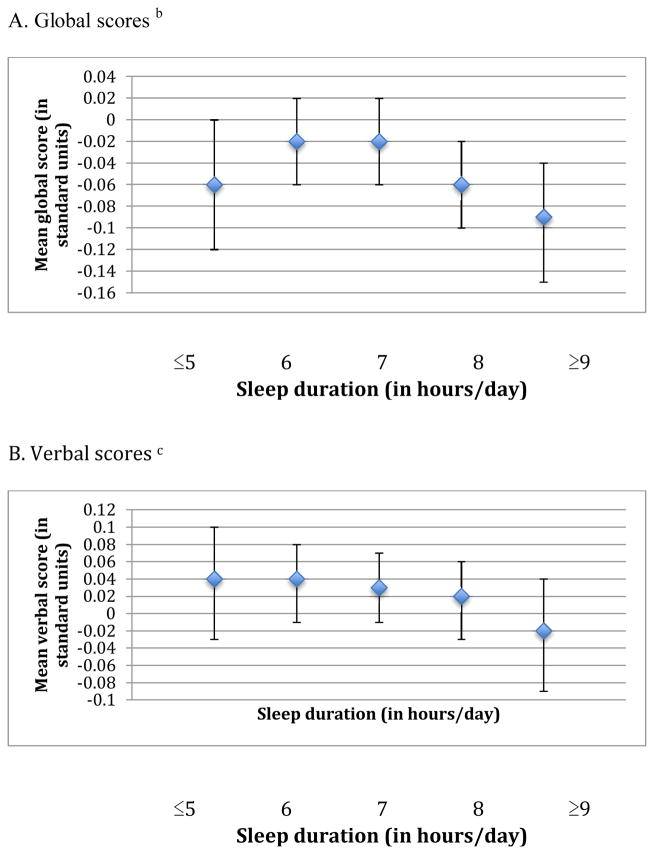
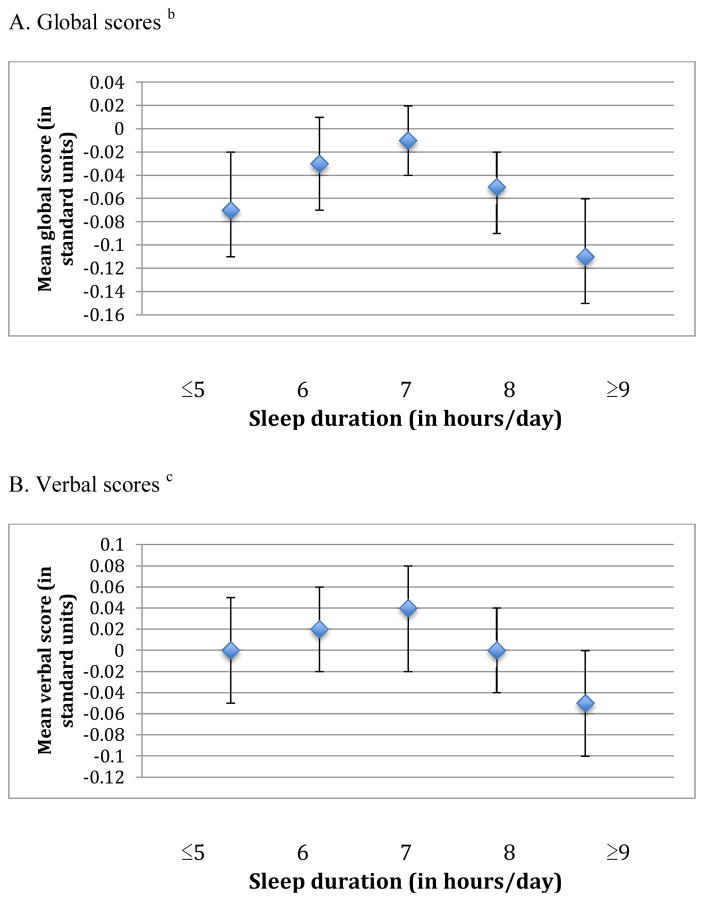
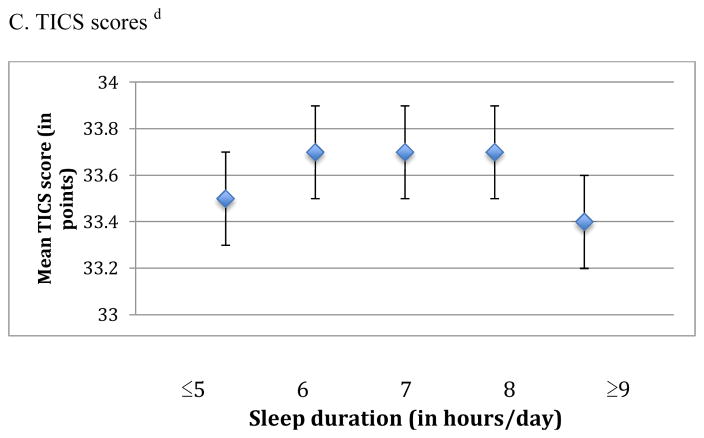
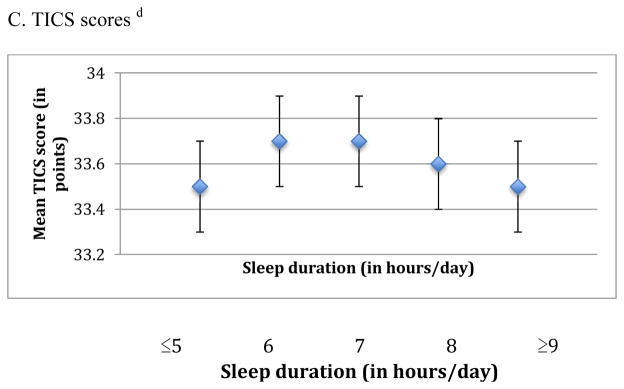
When we examined the association of sleep duration in midlife with cognition, we observed an inverted U-shaped association for the global score and the TICS score (p-values for the quadratic terms were 0.002 and <0.001, respectively) (Figure 1). For example, women reporting ≤5 hours of sleep/day had average global scores that were lower by 0.04 standard units compared to those with 7 hours of sleep/day (mean scores= −0.06 standard units [95% CI= −0.12, 0.00] vs. −0.02 standard units [95% CI= −0.06, 0.02]), whereas women with ≥9 hours of sleep had average scores that were 0.07 standard units lower than those sleeping 7 hours per day (mean scores= −0.09 standard units [95% CI= −0.15, −0.04] vs. −0.02 standard units [95% CI= −0.06, 0.02]). Associations were observed across all individual cognitive tests, except for those contributing to the verbal memory score. Similar associations, but slightly stronger, were found for sleep duration in older age and average cognition across all three primary outcomes (p-values for the quadratic term were <0.001 for the global, verbal, and TICS scores) (Figure 2). For example, women who slept ≤5 hours/day had average global scores that were 0.06 standard units lower than women sleeping 7 hours/day (mean scores= −0.07 standard units [95% CI= −0.11, −0.02] vs. −0.01 standard units [95% CI= −0.04, 0.02]); in addition, women sleeping ≥9 hours/day had worse global scores by 0.10 standard units compared to women with 7 hours of sleep/day (mean scores= −0.11 standard units [95% CI= −0.15, −0.06] vs. −0.01 standard units [95% CI= −0.04, 0.02]). These associations were evident across all six of our cognitive tests. When sleep duration in midlife and later life were included in the same model, sleep duration in later life contributed significant information to the model (F4,∞=6.09 > 2.37, p<0.001) but sleep duration in midlife did not (F4,∞=1.33 < 2.37, p=0.3); thus, sleep duration in later life was more strongly associated with cognitive performance in later life than sleep duration in midlife. To help interpret differences between sleep duration groups, we find that each one year of age in our cohort is associated with a decrease of 0.05 standard units on the global score; thus, the observed mean score differences of 0.04 to 0.10 standard units comparing extreme categories of sleep duration to 7 hours of sleep/day were equivalent to 1 to 2 years of cognitive aging.
Figure 1.
Means scores representing average cognition in later life across categories of sleep duration in midlife (reported in 1986) a
a Estimates are adjusted for age (continuous), education (registered nurse, bachelor’s degree, graduate degree), shift work history (never, 1–9, 10–19, ≥20 years), smoking status (never, past, current), alcohol intake (none 1–14 grams/day, ≥15 grams/day, missing), physical activity (in quintiles of MET-hours/week, missing), body-mass index (<22, 22–24, 25–29, ≥30 kg/m2), history of high blood pressure (yes, no), mental health score on the SF-36 (poor, normal, missing), living alone (yes, no), and tranquilizer use (yes, no).
b For each participant, the global score at each cognitive interview is calculated by averaging z-scores for each of the six tests in our cognitive battery. The mean global score representing average cognition in later life is further calculated by averaging global scores across repeated cognitive interviews for each participant.
c For each participant, the verbal score at each cognitive interview is calculated by averaging z-scores for each of the four tests of verbal memory in our cognitive battery. The mean verbal score representing average cognition in later life is further calculated by averaging verbal scores across repeated cognitive interviews for each participant.
d The mean TICS score representing average cognition in later life is calculated by averaging TICS scores across repeated cognitive interviews for each participant.
Figure 2.
Mean scores representing average cognition in later life across categories of sleep duration in later life (reported in 2000) a
a Estimates are adjusted for age (continuous), education (registered nurse, bachelor’s degree, graduate degree), shift work history (never, 1–9, 10–19, ≥20 years), smoking status (never, past, current), alcohol intake (none 1–14 grams/day, ≥15 grams/day, missing), physical activity (in quintiles of MET-hours/week, missing), body-mass index (<22, 22–24, 25–29, ≥30 kg/m2), history of high blood pressure (yes, no), mental health score on the SF-36 (poor, normal, missing), living alone (yes, no), and tranquilizer use (yes, no).
b For each participant, the global score at each cognitive interview is calculated by averaging z-scores for each of the six tests in our cognitive battery. The mean global score representing average cognition in later life is further calculated by averaging global scores across repeated cognitive interviews for each participant.
c For each participant, the verbal score at each cognitive interview is calculated by averaging z-scores for each of the four tests of verbal memory in our cognitive battery. The mean verbal score representing average cognition in later life is further calculated by averaging verbal scores across repeated cognitive interviews for each participant.
d The mean TICS score representing average cognition in later life is calculated by averaging TICS scores across repeated cognitive interviews for each participant.
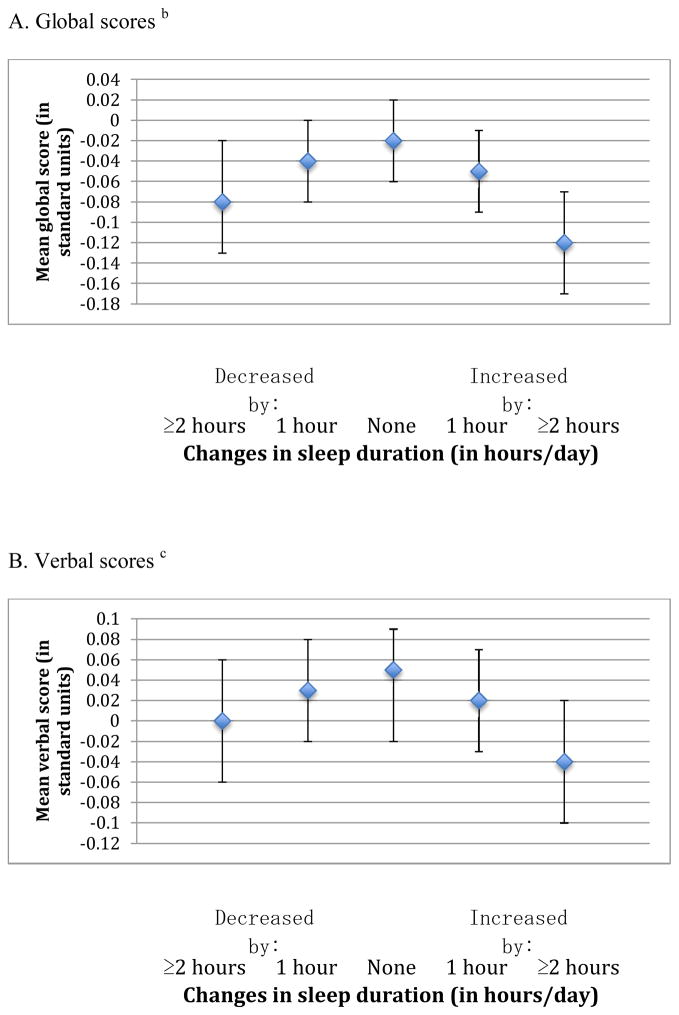
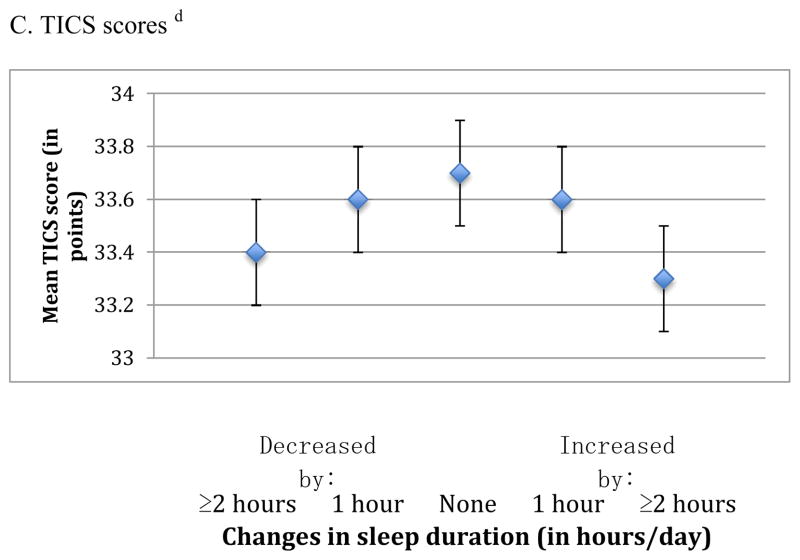
In the analysis of change in sleep duration (from middle to older age) and average cognition, we also observed an inverted U-shaped association for all three cognitive outcomes (p-values for the quadratic terms were <0.001 for the global, verbal, and TICS scores) (Figure 3). For example, the mean global score was lower by 0.06 standard units comparing women who reported changes in sleep duration that increased or decreased by ≥2 hours over time (i.e., 13% of the cohort) vs. women reporting no changes in sleep duration (i.e., 42% of the cohort) (e.g., mean scores= −0.08 standard units [95% CI= −0.13, −0.02] and −0.12 standard units [95% CI= −0.17, −0.07] vs. −0.02 standard units [95% CI= −0.06, 0.02]). Associations were consistent across all individual cognitive tests, and the magnitude of these effects was approximately equivalent to 1–2 years of cognitive aging.
Figure 3.
Mean scores representing average cognition in later life across categories of change in sleep duration from 1986 to 2000 a
a Estimates are adjusted for age (continuous), education (registered nurse, bachelor’s degree, graduate degree), sleep duration in middle age (obtained in 1986) (≤5, 6, 7, 8, ≥9 hours/day), shift work history (never, 1–9, 10–19, ≥20 years), smoking status (never, past, current), alcohol intake (none 1–14 grams/day, ≥15 grams/day, missing), physical activity (in quintiles of MET-hours/week, missing), body-mass index (<22, 22–24, 25–29, ≥30 kg/m2), history of high blood pressure (yes, no), mental health score on the SF-36 (poor, normal, missing), living alone (yes, no), and tranquilizer use (yes, no).
b For each participant, the global score at each cognitive interview is calculated by averaging z-scores for each of the six tests in our cognitive battery. The mean global score representing average cognition in later life is further calculated by averaging global scores across repeated cognitive interviews for each participant.
c For each participant, the verbal score at each cognitive interview is calculated by averaging z-scores for each of the four tests of verbal memory in our cognitive battery. The mean verbal score representing average cognition in later life is further calculated by averaging verbal scores across repeated cognitive interviews for each participant.
d The mean TICS score representing average cognition in later life is calculated by averaging TICS scores across repeated cognitive interviews for each participant.
When we analyzed cognitive trajectories in later life (i.e., change in cognitive performance over the four assessments), there were no associations of sleep duration in midlife, sleep duration in later life, or change in sleep duration from midlife to later life and cognitive decline.
In secondary analyses, associations between extreme sleep durations and worse cognitive function were strongest among women who reported normal sleep durations in midlife (i.e., 7 hours/day) (e.g., mean global scores= −0.14 standard units [95% CI= −0.24, −0.03] vs. −0.01 standard units [95% CI= −0.07, 0.05]) for women with ≥2 hour decreases in sleep/day vs. no change in sleep duration, and mean global scores= −0.14 standard units [95% CI= −0.22, −0.05] vs. −0.01 standard units [95% CI= −0.07, 0.05]) for women with ≥2 hour increases in sleep/day, compared to those with no changes in sleep duration). Analyses excluding women with a history of shift work did not change our results materially, nor did analyses that excluded women in the bottom 10% of the score distribution in analyses of sleep duration in older age.
DISCUSSION
We found that, in women, shorter or longer sleep durations compared to normal sleep duration (i.e., 7 hours/day) in midlife and in later life were associated with worse average cognition in later life. However, sleep duration in later life was more strongly associated with cognition than sleep duration in midlife. Changes in sleep duration of ≥2 hours/day, between middle and older age, were also related to lower cognitive performance in later life. In general, we observed that having shorter or longer sleep durations was cognitively equivalent to aging by 1 to 2 years. No associations were observed for sleep duration or change in sleep duration in relation to rates of cognitive decline over time, which might be due to relatively short follow up; therefore, average cognitive function may be a better measure of cognitive status in this cohort.
To our knowledge, this is the first study to examine the association of sleep duration in both midlife and later life with cognitive performance in older adults. Our findings are consistent with some, but not all, epidemiologic studies where sleep duration and cognition have been measured in later life. Most studies have been modest in size and cross-sectional, and findings have been mixed but tend to suggest that shorter7, 8, 19 or longer10–12 sleep durations, or both14–16, are associated with worse cognitive function. To date, the largest of these studies is a cross-sectional investigation of 28,670 older Chinese persons that found shorter and longer sleep durations were associated with worse scores on a delayed word recall test (multivariable-adjusted p-trend<0.0001 across categories of sleep duration from 3–4 to 7 hours/day, and p-trend<0.0001 across categories of sleep duration from 7 to ≥10 hours/day)16. In contrast, one prospective study (n=1,664) was less clear, finding that short sleep durations were associated with a greater risk of cognitive impairment in men (OR=2.91, 95% CI=1.24, 6.82 comparing ≤5 vs. >5 to <9 hours of sleep/night), but long sleep durations were related to a higher risk of cognitive impairment in women (OR=2.10, 95% CI=1.10, 4.00 comparing ≥9 vs. >5 to <9 hours of sleep/night)18.
In addition, two previous studies have reported on change in sleep duration over time and cognition in older adults13, 15. In the German HeiDE study (n=409), participants whose sleep duration increased from 7–8 to >8 hours of sleep/night, over ten years, had higher odds of cognitive impairment at the end of follow up compared to those with no change in sleep duration13. The Whitehall study (n=1,457) found a similar association with cognitive function for individuals with increased sleep duration vs. no change in sleep duration over five years, although individuals with decreased sleep duration appeared to have worse cognitive function as well15. Both studies are consistent with our findings, in that, greater changes in sleep duration over time were related to worse cognition; thus, existing data on this association generally support our results, although evidence remains somewhat limited.
Several lines of evidence suggest biologic links between sleep duration and cognition. First, experimental studies involving mice have demonstrated that sleep deprivation can lead to increased accumulation of amyloid beta in the brain – a hallmark pathology of Alzheimer’s disease5. These findings indicate that sleep duration may have direct effects on the brain, although human studies have shown that sleep deprivation also has adverse effects on cardio-metabolic factors (e.g., increasing blood pressure and insulin resistance)29, 30—and both cardiovascular and metabolic health have been associated with cognitive health in older adults4. Thus, there is the potential for direct and indirect effects of sleep duration to influence cognitive status in later life. Another possibility is that self-reported sleep duration might be a marker of sleep quality, as suggested by a study that found participants self reporting extreme sleep durations also had the most objectively-measured sleep fragmentation. This study reported that, although extreme sleep durations and greater sleep fragmentation were both associated with poor cognitive function in separate models, only sleep fragmentation remained significantly associated with cognition in models simultaneously adjusted for both variables. Thus, in the only large-scale, epidemiologic study that has evaluated these measures simultaneously, poor sleep quality appears to explain much of the association between subjective sleep duration and cognitive function14. Moreover, a recent clinical study found that poor sleep quality is likely a contributing factor to cognitive impairment in later life31. Alternatively, extreme sleep durations and extreme changes in sleep duration over time could be markers of underlying circadian disruption, which have increasingly been related to cognitive decrements. For example, several studies have found that job-related circadian disruptions are associated with poorer short-term cognitive function32–34, and possibly structural brain atrophy33, and emerging investigations of objectively measured circadian rhythms have observed relations between disturbed rhythms and cognitive impairment in older adults35–37. These studies support the biologic plausibility of our findings, although we acknowledge the relationship between sleep duration and cognition is likely complex and may be bidirectional.
Several limitations of this study should be considered. First, this is an observational study, and therefore we cannot rule out the possibility that residual confounding explains the observed associations. We controlled for a wide variety of possible confounding factors, which did not change our effect estimates substantially, suggesting that residual confounding by measured factors is less likely to explain our findings; however, unmeasured factors related to health status could still explain our results. For example, we did not measure sleep-disordered breathing in this cohort, and this condition might influence both sleep duration and risk of cognitive impairment38. The prevalence of sleep-disordered breathing is relatively low among women, and even lower among younger women39, suggesting that lack of adjustment for this variable probably had a limited effect. This might have caused us to slightly underestimate associations of sleep duration and cognition, particularly those involving sleep duration in later life because sleep-disordered breathing is somewhat more prevalent in later life. Second, sleep duration was self reported by our participants, which probably led to some random misclassification. However, a previous validation study found that our participants self reported usual sleep duration well compared to sleep durations derived from sleep diaries20. In addition, several other studies in NHS have reported that sleep duration is associated with mortality20, coronary heart disease40, and type 2 diabetes41—providing reassurance that our measurement of sleep duration contains important information for predicting health outcomes in this cohort. Third, although our analyses of sleep duration in midlife and cognition were prospective (i.e., the exposure was measured prior to outcome assessment), the evaluation of sleep duration in later life and cognition could be subject to reverse causation bias, if underlying cognitive health influenced sleep duration in these older women. However, our results did not change when we excluded women with the worst cognitive scores in later life (i.e., women most likely to have underlying brain pathology that could affect sleep), indicating that reverse causation is not likely to be completely responsible for observed associations. Finally, the period over which we measured cognitive decline was relatively short (i.e., six years), and we observed no associations between sleep duration and cognitive decline over six years, which stands in contrast to the associations that we observed between sleep duration and average cognitive status in later life; thus, follow up may have been too short to capture the amount of decline necessary to detect significant associations. As noted above, average cognitive status may provide a better reflection of women’s later-life cognition in this cohort.
In conclusion, we found that extreme sleep durations were associated with worse cognition in later life, including sleeping habits in midlife. More extreme changes in sleep duration from middle age to older age were related to worse cognitive function as well. If future research supports our findings, then clinical interventions based on sleep therapy should be examined for the prevention of cognitive impairment. Future research is necessary to better understand the mechanisms by which sleep duration might be associated with cognitive function, including measurement of objective sleep assessments among middle- and older- aged individuals.
Acknowledgments
Funding source: The Nurses’ Health Study is funded by a grant from the National Institutes of Health (P01 CA87969).
Sponsor’s role: The sponsor played no role in the design, methods, subject recruitment, data collection, analysis, and preparation of this article.
Footnotes
Previous presentation: A preliminary version of these data were presented, in part, during an oral presentation at the Alzheimer’s Association International Conference in July 14–19, 2012 (Vancouver, Canada).
Conflict of interest: (To be determined by the editorial office based on COI information).
Author contributions: E.E.D. was responsible for conception and design of the study, analysis and interpretation of data, and drafting the article and revising it for important intellectual content. F.G. was responsible for conception and design of the study, acquisition and interpretation of data, and revising the article for important intellectual content. J.F.D. was responsible for interpretation of data and revising the article for important intellectual content. M.J.S. was responsible for conception and design of the study, acquisition, analysis, and interpretation of data, and revising the article for important intellectual content. C.A.C. was responsible for conception and design of the study, analysis and interpretation of data, and revising the article for important intellectual content. E.S.S. was responsible for conception and design of the study, analysis and interpretation of data, and revising the article for important intellectual content. All authors have given final approval of the version of this article to be published.
References
- 1.Schoenborn CA, Adams PE. Health behaviors of adults: United States, 2005–2007. Vital Health Stat. 2010;10:1–132. [PubMed] [Google Scholar]
- 2.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 3.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley DJ, Masaki K, White L, Larkin EK, Monjan A, Redline S. Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep. 2003;26:596–599. doi: 10.1093/sleep/26.5.596. [DOI] [PubMed] [Google Scholar]
- 7.Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28:981–989. [PubMed] [Google Scholar]
- 8.Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20:41–48. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 9.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 10.Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx aging study. Behav Sleep Med. 2007;5:39–56. doi: 10.1207/s15402010bsm0501_3. [DOI] [PubMed] [Google Scholar]
- 11.Faubel R, Lopez-Garcia E, Guallar-Castillon P, Graciani A, Banegas JR, Rodriguez-Artalejo F. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res. 2009;18:427–435. doi: 10.1111/j.1365-2869.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 12.Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18:436–446. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 13.Loerbroks A, Debling D, Amelang M, Sturmer T. Nocturnal sleep duration and cognitive impairment in a population-based study of older adults. Int J Geriatr Psychiatry. 2010;25:100–109. doi: 10.1002/gps.2305. [DOI] [PubMed] [Google Scholar]
- 14.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34:1347–1356. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34:565–573. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L, Jiang CQ, Lam TH, et al. Short or long sleep duration is associated with memory impairment in older Chinese: the Guangzhou Biobank Cohort Study. Sleep. 2011;34:575–580. doi: 10.1093/sleep/34.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auyeung TW, Lee JS, Leung J, et al. Cognitive deficit is associated with phase advance of sleep-wake rhythm, daily napping, and prolonged sleep duration-a cross-sectional study in 2,947 community-dwelling older adults. Age (Dordr) 2012 doi: 10.1007/s11357-011-9366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potvin O, Lorrain D, Forget H, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35:491–499. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keage HA, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13:886–892. doi: 10.1016/j.sleep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 21.Brandt J, Spencer M, Folstein M. The telephone interview of cognitive status. Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 22.Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer’s disease. A longitudinal study. Brain. 1991;114 (Pt 6):2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- 23.Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 24.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 25.Evans DA, Grodstein F, Loewenstein D, Kaye J, Weintraub S. Reducing case ascertainment costs in U.S. population studies of Alzheimer’s disease, dementia, and cognitive impairment-Part 2. Alzheimers Dement. 2011;7:110–123. doi: 10.1016/j.jalz.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locascio JJ, Growdon JH, Corkin S. Cognitive test performance in detecting, staging, and tracking Alzheimer’s disease. Arch Neurol. 1995;52:1087–1099. doi: 10.1001/archneur.1995.00540350081020. [DOI] [PubMed] [Google Scholar]
- 27.Devore EE, Kang JH, Stampfer MJ, Grodstein F. The Association of Antioxidants and Cognition in the Nurses’ Health Study. American journal of epidemiology. 2012 doi: 10.1093/aje/kws202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosner B. Fundamentals of Biostatistics. 5. Pacific Grove, CA: Duxbury; 2000. [Google Scholar]
- 29.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best practice & research Clinical endocrinology & metabolism. 2010;24:731–743. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buxton OM, Cain SW, O’Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra143. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mander BA, Rao V, Lu B, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013 doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho K, Ennaceur A, Cole JC, Suh CK. Chronic jet lag produces cognitive deficits. J Neurosci. 2000;20:RC66. doi: 10.1523/JNEUROSCI.20-06-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- 34.Machi MS, Staum M, Callaway CW, et al. The relationship between shift work, sleep, and cognition in career emergency physicians. Acad Emerg Med. 2012;19:85–91. doi: 10.1111/j.1553-2712.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- 35.Cochrane A, Robertson IH, Coogan AN. Association between circadian rhythms, sleep and cognitive impairment in healthy older adults: an actigraphic study. J Neural Transm. 2012 doi: 10.1007/s00702-012-0802-2. [DOI] [PubMed] [Google Scholar]
- 36.Oosterman JM, van Someren EJ, Vogels RL, Van Harten B, Scherder EJ. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res. 2009;18:129–135. doi: 10.1111/j.1365-2869.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 37.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Annals of neurology. 2011;70:722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. Jama. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased Prevalence of Sleep-Disordered Breathing in Adults. American journal of epidemiology. 2013 doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–209. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 41.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]