New approaches in angiogenic targeting for colorectal cancer (original) (raw)
Abstract
Colorectal carcinoma (CRC) is one of the leading causes of cancer death worldwide. In the last decade, the addition of irinotecan and oxaliplatin to standard fluorouracil-based chemotherapy regimens have set the new benchmark of survival for patients with metastatic CRC at approximately 20 mo. Despite these advances in the management of CRC, there is a strong medical need for more effective and well-tolerated therapies. The dependence of tumor growth and metastasis on blood vessels makes angiogenesis a rational target for therapy. One of the major pathways involved in this process is the vascular endothelial growth factor (VEGF) and its receptors (VEGFR). In 2004, the first agent targeting angiogenesis, bevacizumab (BV), was approved as an adjunct to first-line cytotoxic treatment of metastatic CRC. The role of BV as part of adjuvant treatment and in combination with other targeted therapies is the subject of ongoing trials. However, BV is associated with an increase in the risk of arterial thromboembolic events, hypertension and gastrointestinal perforations and its use must be cautious. Novel VEGFR TK inhibitors with different ranges of nanomolar potencies, selectivities, and pharmacokinetic properties are entering phase III trials for the treatment of cancer. Conversely, one of these novel agents, vatalanib, has been shown not to confer survival benefit in first and second-line treatment of advanced CRC. The basis of these findings is being extensively evaluated. Ongoing and new well-designed trials will define the optimal clinical application of the actual antiangiogenic agents, and, on the other hand, intensive efforts in basic research will identify new agents with different antiangiogenic approaches for the treatment of CRC. In this review we discuss and highlight current and future approaches in angiogenic targeting for CRC.
Keywords: Angiogenesis inhibitors, Vascular endothelial growth factor, VEGF receptors, Bevacizumab, Vatalanib, Colorectal carcinoma
INTRODUCTION
Colorectal carcinoma (CRC) is one of the leading causes of cancer death worldwide despite progressive improvements in preventive, diagnostic, and therapeutic approaches[1]. Approximately 50 percent of patients who undergo potentially curative surgery alone ultimately relapse and die of metastatic disease[2]. From the late 50 s, 5-fluorouracil (5-FU) was the only drug approved for the treatment of advanced CRC with an overall response rate (RR) and median survival of 10% and 10 mo, respectively[3,4]. This RR was improved to nearly 25% when leucovorin (LV) was used to modulate 5-FU[5]. Recently, irinotecan and oxaliplatin have been added to the armamentarium of agents with activity in CRC. The addition of these two cytotoxic agents to the standard 5-FU/LV-based regimens improves not only RR, but also overall survival (OS) over 5-FU/LV alone, setting the new benchmark of survival for patients with unresectable advanced CRC at around 20 mo[6-10]. Despite these advances in the management of CRC, there is a strong medical need for more effective and well-tolerated therapies and further improvements in survival are anticipated with the introduction of novel targeted therapies both as single agents and in combination. Among them, anti-angiogenesis agents have become a new therapeutic approach in the metastatic setting. In this review we will discuss and highlight current and future approaches in angiogenic targeting for CRC.
ANGIOGENIC TARGETING
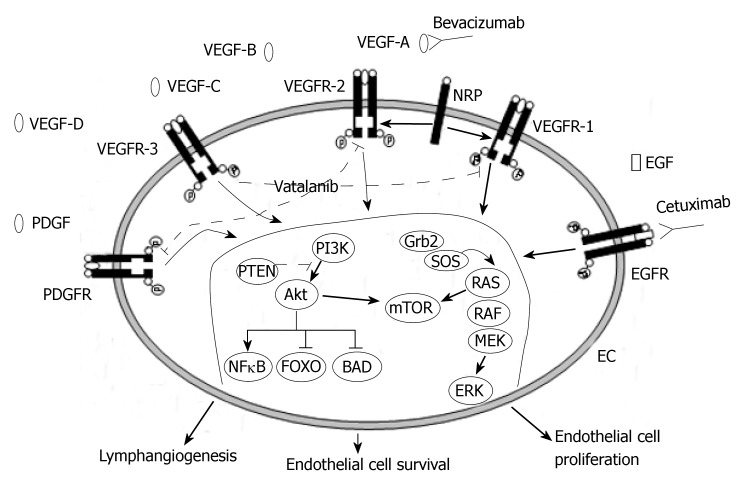
The dependence of tumor growth and metastasis on blood vessels makes angiogenesis one of the fundamental hallmarks of cancer[11] and a rational target for[12]. Several growth factor receptor pathways have been implicated in the promotion of tumor angiogenesis. One of the major pathways involved in this process is the vascular endothelial growth factor (VEGF) family of proteins, also known as vascular permeability factors, and its receptors (Figure 1). The VEGF pathway plays a crucial role in normal and pathologic angiogenesis, triggering multiple signaling networks that result in endothelial cell survival, migration, mitogenesis, differentiation, and vascular permeability[13]. The VEGF-related gene family of angiogenic and lymphangiogenic growth factors comprises six secreted glycoproteins referred to as VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and placenta growth factor (PlGF) 1 and 2. The primary effects of VEGF ligands are mediated through binding to the VEGF tyrosine kinase receptors (VEGFR): VEGFR-1, which binds VEGF-A, VEGF-B, and PlGF-1; VEGFR-2, which binds VEGF-A, VEGF-C, VEGF-D, and VEGF-E; and VEGFR-3, which binds VEGF-C and VEGF-D, and its expression is limited to the lymphatic endothelial cells. In addition to these receptors, VEGF interacts with neuropilins, a family of activating coreceptors without an intracellular signaling domain[14,15]. VEGFR-1 and VEGFR-2 have seven extracellular immunoglobulin-like domains, a single transmembrane region and a consensus kinase sequence that is interrupted by a kinase-insert domain[16]. Once bound by VEGF, two receptors dimerize, and the tyrosine kinase domain of each receptor “autophosphorylates” the other, leading to an active receptor that initiates a signaling cascade. The VEGF pathway is upregulated by hypoxia[17] and by several growth factors, such as epidermal growth factor (EGF)[18], platelet-derived growth factors (PDGFs)[19,20], hepatocyte growth factor[21] and other cytokines.
Figure 1.
Vascular endothelial growth factor (VEGF) signaling network and novel targeted therapies. VEGFR: Vascular endothelial growth factor receptor; PDGF: Plateled-derived growth factor; PDGFR: Plateled-derived growth factor receptor; EGF: Epidermal growth factor; EGFR: Epidermal growth factor receptor; NRP: Neuropilin; EC: Endothelial cell.
Overexpression of VEGF has been associated with tumor progression and poor prognosis in several tumor systems, including CRC[22,23]. Preoperative serum VEGF have also been shown to correlate with advanced tumor stage or nodal status at the time of surgery[24]. Furthermore, intense expression of VEGF mRNA is detected in human liver metastases from primary colon or rectal carcinomas[25]. In 1993, Kim et al[26] reported that antibodies to VEGF exert a potent inhibitory effect on the growth of several tumor cell lines in nude mice. In addition, the combination of anti-VEGF antibody and chemotherapy in nude mice injected with human cancer xenografts has an increased antitumor effect compared with antibody or chemotherapy treatment alone[27]. It is, therefore, not surprising that most of the antiangiogenesis treatment strategies focus on inhibition of the VEGF pathway and its regulators. However, the mechanisms of action of anti-VEGF therapy in cancer patients are still far from being fully understood.
In December 2004 the first agent targeting angiogenesis, bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA), was approved to be given intravenously as a combination treatment along with standard chemotherapy drugs for metastatic CRC, increasing RR, progression-free survival (PFS) and overall survival (OS) with limited toxicity[28]. Gradually, many other antiangiogenic agents that target the VEGF pathway are entering the clinic. These novel targeted agents inhibit the VEGF pathway by targeting the VEGF ligand, its receptors or by blocking downstream signaling pathway components. Antiangiogenic agents include antibodies, low-molecular-weight tyrosine kinase (TK) inhibitors, antisense oligonucleotides and aptamers (Table 1).
Table 1.
Anti-VEGF agents currently in clinical development
| Agent | Targets | Phase of development |
|---|---|---|
| Specific anti-VEGF antibodies | ||
| Bevacizumab (Avastin) | VEGF-A | Phase III |
| IMC-C1121b | VEGFR-2 | PhaseI-II |
| VEGF Trap | VEGF, PlGF, VEGF-B | Phase I |
| Agents that target VEGF receptors tyrosine kinase | ||
| Vatalanib (PTK787/ZK 222584) | VEGFR1, VEGFR2, VEGFR3, PDGFR-β, c-Kit | Phase III |
| Sorafenib (BAY 43-9006) | VEGFR-2, PDGFR-β, FLT3, c-Kit, Raf | Phase III |
| Sunitinib (SU11248) | VEGFR2, PDGFR-β, FLT3, c-Kit | Phase III |
| Semaxanib (SU5416) | VEGFR1, VEGFR2 | Stopped |
| AZD2171 | VEGFR1, VEGFR2, VEGFR3, PDGFR-β, c-Kit | PhaseI-II |
| CEP-7055 | VEGFR1, VEGFR2, VEGFR3 | PhaseI-II |
| CHIR258 | VEGFR1, VEGFR2, FGFR1, FGFR3 | |
| CP-547632 | VEGFR2 | PhaseI-II |
| GW786034 | VEGFR2 | PhaseI-II |
| OSI-930 | VEGFR, c-Kit | PhaseI-II |
| ZK-CDK | VEGFRs, PDGFR, CDKs | PhaseI-II |
| AG013736 | VEGFR, PDGFR-β, c-Kit | PhaseI-II |
| AMG706 | VEGFR1, VEGFR2, PDGFR-β, c-Kit | PhaseI-II |
| KRN-951 | VEGFR1, VEGFR2, VEGFR3, PDGFR-β, c-Kit | PhaseI-II |
| BMS-582664 | VEGFR2, FGFR | PhaseI-II |
| XL999 | FGFR, VEGFRs, PDGFR, FLT3 | PhaseI-II |
| Zactima (ZD6474) | VEGFR2, EGFR, RET | PhaseI-II |
| AEE788 | VEGFR1, VEGFR2, EGFR | PhaseI-II |
| Antisense oligonucleotides | ||
| Veglin (VEGF-AS) | VEGF, VEGF-C, VEGF-D | PhaseI |
| Aptamer | ||
| Aplidin (Dehydrodidemnin B) | VEGF | PhaseI |
BEVACIZUMAB IN CRC
Bevacizumab (BV) is a recombinant humanized monoclonal antibody that binds to all isoforms of VEGF-A with a reported half-life of 17-21 d[29]. In phase Itrials, BV was generally well tolerated and did not demonstrate dose-limiting toxicity or interactions with commonly used chemotherapy regimens[30,31]. Based on the data obtained in these phaseItrials, Kabbinavar et al[32] conducted a randomized, phase II trial comparing the safety and efficacy of BV (at two dose levels, 5 and 10 mg/kg every 2 wk) plus 5-FU (500 mg/m2)/LV (500 mg/m2) versus 5-FU/LV alone as first-line therapy for metastatic CRC(Table 2). One hundred and two patients were included. Administration of BV at low-dose and high-dose every 2 wk resulted in a significant increase of 3.8 mo and 2.0 mo, respectively, in the estimated progression-free survival (PFS) compared with 5-FU/LV alone. Treatment with 5-FU/LV/BV at both dose levels compared with 5-FU/LV resulted in higher RR [control arm, 17%, (95% CI, 7% to 34%); low-dose arm, 40%, (95% CI, 24% to 58%); high-dose arm, 24%, (95% CI, 12% to 43%)]. Although median survival was 7.7 and 2.3 mo higher in the 5-mg/kg arm and 10-mg/kg arm, respectively, it was not statistically significant. These findings contrast with the effective higher dose administered in other tumors like non-small cell lung cancer (15 mg/kg every three weeks)[33], breast cancer (10 mg/kg every two weeks)[34] and renal cancer (10 mg/kg every two weeks)[35]. Nevertheless, the majority of subsequent CRC studies administered a BV dose of 5 mg/kg. Potential safety concerns observed in this phase II study were thrombosis, hypertension, proteinuria, and epistaxis.
Table 2.
Completed trials for Bevacizumab with chemotherapy in metastatic CRC
| REF | Regimen | Pts | RR (%) | P | PFS or TTP (mo) | P | OS (mo) | P |
|---|---|---|---|---|---|---|---|---|
| 28 | IFL | 411 | 35 | 0.004 | 6.2 | < 0.001 | 15.6 | < 0.001 |
| IFL + BV | 402 | 45 | 10.6 | 20.3 | ||||
| 32 | 5-FU/LV | 35 | 17 | - | 5.2 | - | 13.6 | - |
| 5-FU/LV + BV-low | 35 | 40 | 0.029 | 9 | 0.005 | 21.5 | 0.137 | |
| 5-FU/LV + BV-high | 32 | 24 | 0.434 | 7.2 | 0.217 | 16.1 | 0.582 | |
| 41 | 5-FU/LV | 105 | 15 | 0.055 | 5.5 | 0.0002 | 12.9 | 0.16 |
| 5-FU/LV + BV | 104 | 26 | 9.2 | 16.6 | ||||
| 43 | FOLFOX | 289 | 9 | < 0.001 | 4.8 | < 0.001 | 10.7 | 0.0018 |
| FOLFOX + BV | 290 | 22 | 7.2 | 12.5 | ||||
| 44 | FOLFOX/bFOL/XELOX | 147 | 22-43 | NR | 6.1-8.7 | NR | 18.2 | NR |
| FOLFOX/bFOL/XELOX + BV | 213 | 41-53 | 8.3-10.3 | 24.4 | ||||
| 45 | FOLFOX/XELOX | 701 | 49 | 0.99 | 8.5 | < 0.001 | - | - |
| FOLFOX/XELOX + BV | 699 | 47 | 11 | - |
In 2004, a large (813 patients) phase III, double-blind, randomized trial in patients with untreated metastatic CRC demonstrated that the addition of BV to IFL (irinotecan/5-FU/LV) chemotherapy prolonged OS by 4.7 mo compared with IFL alone (20.3 vs 15.6 mo; HR = 0.66, P < 0.001)[28]. The one-year survival rate was 74.3% in the group given IFL plus BV and 63.4% in the group given IFL plus placebo (P < 0.001). All secondary efficacy end points were also improved with the addition of BV to the chemotherapeutic regimen: PFS increased from 6.2 to 10.6 mo (hazard ratio HR = 0.54; P < 0.001), RR increased from 34.8% to 44.8% (P = 0.004), and median duration of the response increased from 7.1 to 10.4 mo (HR = 0.62; P = 0.001). Grade 3 hypertension was more common during treatment with IFL plus BV than with IFL plus placebo (11.0 percent vs 2.3 percent, P < 0.01) but it was easily managed with medical treatment. Although the overall incidence of grade 3 or 4 adverse events was higher among patients receiving the combined treatment, the study did not identify hemorrhage, thromboembolism, and proteinuria as possible BV-associated adverse events. Uncommon but serious side-effects of BV included the appearance of gastrointestinal perforations (1.5%), in some instances with fatal outcome[28].
Toxicity derived from antiangiogenic therapy is a main concern in the management of CRC. BV is associated with a two-fold increase in the risk of arterial thromboembolic events, from 2.5% to 5% (P < 0.01)[36]. These events consist primarily of acute coronary syndrome, transient ischemic attack and stroke. Patients at risk for these events are those with a prior history of arterial thromboembolism and age older than 65 years. Moreover, BV administration can result in the development of wound dehiscence. However, the risk of wound healing is not increased if the administration of BV with or without chemotherapy is delayed until 28-60 d after primary care surgery[37].
Although the addition of BV to 5-FU-based combination chemotherapy resulted in statistically significant and clinically meaningful improvement in RR, PFS and OS among patients with metastatic CRC, previous studies have suggested that the benefit observed with irinotecan-based schedules might be limited to patients with a performance status (PS) of 0[38]; and certain subgroups, including those with advanced age, impaired PS, low serum albumin, and prior pelvic radiotherapy, may experience significant toxicities when adding irinotecan to 5-5-FU/LV regimens[39]. In this particular population, the combination of BV and 5-FU/LV would remain a potentially useful therapeutic alternative. Two studies led by Kabbinavar et al[40,41] addressed this question enrolling patients who were not candidates for irinotecan because of advanced age or poor PS. The results suggested that 5-FU/LV (Roswell Park Schedule[42]) plus BV seems as effective as IFL and might have a better safety profile. Based on all of the previous data, BV became the first anti-VEGF agent to be approved by the FDA for cancer patients.
On June 2006, the FDA granted approval for a labelling extension for BV in combination with intravenous 5-FU-based chemotherapy for the second-line treatment of metastatic CRC. This decision was based on the preliminary results of the E3200 phase III trial of the Eastern Cooperative Oncology Group (ECOG). The aim of this randomized, three-arm, multicenter study was to determine the efficacy of infusional 5-FU/LV/oxaliplatin (FOLFOX) with or without BV (10 mg/kg every two weeks) in 829 patients with irinotecan-refractory advanced CRC not previously treated with BV[43]. The median age was 61 years, 49% had an ECOG performance status of 0, and 80% received prior adjuvant chemotherapy. The combination therapy showed an improvement in the OS by 2.1 mo (12.5 vs 10.7 mo; P = 0.0024) without a significant difference in the toxicity profile. The BV-alone arm was closed at the interim analysis due to a low RR and an apparent lack of activity in this setting. Final analyses of this trial are forthcoming.
Whether the combination of BV with oxaliplatin/5-FU/LV-based chemotherapy regimens will be the best option for first-line therapy for CRC is under investigation in the TREE study[44] and NO16966[45]. The TREE study was previously designed to assess the safety, tolerability and efficacy of each of three oxaliplatin plus fluoropyrimidine regimens without (TREE1 cohort) or with (TREE2 cohort) BV. In the TREE-2 cohort, BV was added to each regimen. With a follow-up of 27 mo, median OS with infusional 5-FU/LV and oxaliplatin (mFOLFOX-6) plus BV was 26.0 mo, 20.7 mo with bolus 5-FU/LV and oxaliplatin (bFOL) plus BV, and 27.0 mo with capecitabine and oxaliplatin (CapeOX) plus BV. Median OS with oxaliplatin-containing regimens without BV in sequential historical cohorts (TREE-1 study), reached 18.2 mo[44]. However, the first large, randomized, multicenter phase III trial to evaluate the efficacy of BV in combination with the standard chemotherapy regimen FOLFOX and the XELOX regimen in the first-line treatment of metastatic CRC is the NO16966[45]. Interestingly, in the general treated population, the addition of BV to FOLFOX did not significantly improve PFS (HR = 0.89, P = 0.1871). However, 50% of patients discontinued treatment for reasons unrelated to progression of disease. Further analyses focusing on the on-treatment subgroup population revealed that median PFS for XELOX-BV and FOLFOX-BV was 10.4 mo compared to 8.1 mo for XELOX-Placebo and FOLFOX-Placebo (HR = 0.63, P < 0.0001). These results demonstrated that the addition of BV to oxaliplatin-based chemotherapy regimens significantly improves PFS. In addition, continuation of BV until disease progression could be necessary to optimize the contribution of BV to PFS[45].
The activity shown by BV in the metastatic setting justified the evaluation of this antibody in the adjuvant scenario. In the first trial, the National Surgical Adjuvant Breast and Bowel Project C-08 phase III trial[46], 2632 patients with stage II or III colorectal cancer have been randomized to receive mFOLFOX-6 for 12 cycles with or without BV. Patients assigned to BV plus chemotherapy also received an additional 6 mo of BV alone. This trial has already completed accrual. In a second trial recently finished, the AVANT phase III study[47], patients with stage II or III colorectal cancer were randomized to three combination chemotherapy regimens (FOLFOX-4 vs FOLFOX-4 plus BV vs capecitabine/oxaliplatin plus BV). In addition, a phase II clinical trial, the Eastern Cooperative Oncology Group (ECOG) E5202[48], is evaluating the addition of BV in combination with FOLFOX on patients with stage II colon cancer at high-risk for recurrence. In conclusion, at this point in time, no evidence supports the actual use of BV in the adjuvant setting in order to prolong survival. The results of these important clinical trials are eagerly awaited.
VATALINIB IN CRC
A second antiangiogenic approach is to target both cancer cells and endothelial cells with small molecules. Similar to BV, VEGFR multitargeted TK inhibitors have been evaluated in combination with chemotherapy in phase III trials. The first agent, semaxinib (SU5416, Pharmacia, San Francisco, California) which targets VEGFR-1, VEGFR-2 VEGFR-3, and PDGFR-β did not show any survival benefit when added intravenously to standard chemotherapy in metastatic CRC. In addition worse toxicity in the semaxinib arm was observed[49]. Finally, in a phaseItrial that evaluated the combination of semaxinib with cisplatin/gemcitabine in solid tumors, an unexpected high incidence of thromboembolic events was observed which discouraged overall further investigation of this agent[50].
Another novel synthetic agent, with orally bioavai-lability, vatalanib (PTK787/ZK222584, Novartis, Basel, Switzerland) belongs to the chemical class of amino-phthalazines[51]. It is a potent inhibitor of all known VEGFR tyrosine kinases (TK) with greater potency against VEGFR-1 and VEGFR-2[52,53] (Figure 1). It also inhibits other kinases, such as platelet-derived growth factor receptor beta (PDGFR-β) and c-Kit tyrosine kinase. In preclinical studies, vatalanib has shown antitumor activity in subcutaneously implanted human tumor xenografts in nude mice[53]. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and pharmacokinetic (PK) data indicated that vatalanib ≥ 1000 mg total daily dose is the biologically active dose[54] with a terminal half-life of about 6 h. In view of the short half-life of the drug, a phaseIstudy with vatalanib given twice daily was conducted to exploit the theoretical advantage of maintaining constant drug levels[55]. PK data from this study showed that at equivalent daily doses, drug exposure is comparable with the previous once-daily-dosing schedule[54]; however, the trough levels are significantly higher with the bid dosing. Whether this will translate into improved efficacy is unknown at this time.
Vatalanib has been evaluated in two phaseI/II studies as a single daily-dose in combination with FOLFOX or FOLFIRI, as first-line treatment for patients with metastatic CRC[56,57]. In both studies, vatalanib was safe and well tolerated at doses of 1250 mg/d. Ataxia, expressive dysphasia and dizziness were seen at higher doses when administered in combination with FOLFOX and these were considered dose-limiting toxicities. The combination of vatalanib with chemotherapy significantly affected the PK parameters of SN38, the active metabolite of irinotecan. Indeed, the concentration-time curve (AUC) of SN38 was decreased when vatalanib was added to the FOLFIRI regimen. The relevance of this finding needs further investigation.
Two phase III studies have evaluated the administration of vatalanib (single daily-dose of 1250 mg/d) in combination with chemotherapy in CRC (Table 3). A first randomized phase III trial (CONFIRM-1) compared the efficacy of vatalanib in combination with FOLFOX versus FOLFOX alone in 1168 patients for first-line treatment of metastatic CRC[58]. The results of the primary endpoint of this trial, PFS, showed a modest benefit of adding vatalanib to FOLFOX without achieving statistical significance (HR = 0.88; P = 0.118). OS has not been reported. The adverse events attributable to vatalanib (hypertension, deep-vein thrombosis, diarrhea and dizziness) were generally reversible and similar to other VEGF pathway inhibitors. No increase in bleeding or bowel perforation compared to placebo was observed. The second phase III trial (CONFIRM-2) evaluated the efficacy of vatalanib in combination with FOLFOX versus FOLFOX alone in 855 patients with irinotecan-refractory advanced CRC[59,60]. PFS was 1.4 mo significantly longer in the vatalanib arm (5.5 mo vs 4.1 mo, HR = 0.83; P = 0.026). No improvement in OS was demonstrated. In the CONFIRM-2 trial, the most frequent grade 3/4 events associated with vatalanib were again hypertension (21% vs 5%), diarrhea (16% vs 8%), fatigue (14.5% vs 6.9%), nausea (11% vs 5%), vomiting (9% vs 5%) and dizziness (9% vs 1%). Two hypotheses have been tried to explain why survival was not affected when adding vatalanib in first and second-line therapy. The first one deals with the short half-life of vatalanib. The once-daily administration of the drug might not be the optimal schedule to maintain constant blood levels of vatalanib, although another study refutes this hypothesis[54]. A second one would be the “off-target” effects, such as targeting PDGFR-β. The inhibition of PDGFR-β could interfere with vascular normalization by blocking perivascular cell recruitment and thus impeding the delivery of chemotherapeutics to chemoresponsive tumors[61].
Table 3.
Trials for Vatalanib with chemotherapy in metastatic CRC
| REF | Regimen | Pts | RR (%) | P | PFS or TTP (mo) | P | OS (mo) | P |
|---|---|---|---|---|---|---|---|---|
| 58 | FOLFOX-4 | 583 | 46 | NS | 7.6 | 0.118 | NR | - |
| FOLFOX-4 + Vatalanib | 585 | 42 | 7.7 | |||||
| 59-60 | FOLFOX-4 | 429 | 18 | NR | 4.1 | 0.026 | 11.8 | 0.511 |
| FOLFOX-4 + Vatalanib | 426 | 19 | 5.5 | 12.1 |
Major et al[62] reported a metanalysis by pooling preplanned strata in CONFIRM-1 (C1) and CONFIRM-2 (C2) trials and showed that patients with high LDH (> 1.5 X ULN) experienced the greatest improvement in PFS for C1 (HR = 0.67; P = 0.01) and for C2 (HR = 0.63; P < 0.001). This finding brings forward an eventual role of LDH in angiogenesis-dependent tumor growth and progression in CRC. Previously, the expression of LDH-5, a LDH isoform, has been linked with distant metastases in CRC and with the expression of hypoxia inducible factor (HIF)[63]. Furthermore, evidence of a biologic link between tumor LDH, hypoxia and activated VEGF pathway has been described in CRC[64]. LDH, being regulated by the same pathway as VEGF, is expected to reflect a subset of tumors with a high likelihood to bear an activated VEGF signalling pathway. Nevertheless, whether LDH can be used as a surrogate marker for screening patients for TK inhibitor therapy remains an open question. Thus, validation of biomarkers of efficacy of anti-VEGF therapy with the aim of identifying responsive patients and predict the optimal biological dose are imperative.
TARGETED THERAPY COMBINATIONS
Growth factors and their receptors play a pivotal role in the regulation of cancer progression and neovascularization[65], stimulating downstream signaling cascades involved in cell proliferation, survival and antiapoptosis. The expression or activation of epidermal growth factor receptor (EGFR) and ErbB2 are altered in many epithelial tumors, and clinical studies indicate that they have an important role in tumor progression[66]. Inhibiting signaling pathways through EGFR and ErbB2 has become a cornerstone in the treatment of a subgroup of patients with non-small cell lung cancer and breast cancer, respectively. In CRC, cetuximab (IMC C225, Erbitux, ImClone, New York, NY), a monoclonal antibody targeting EGFR[67], has been shown to induce apoptosis of CRC cells[68], and cetuximab in combination with irinotecan (in irinotecan-refractory and EGFR expressing metastatic CRC) was found to reverse resistance to irinotecan, producing a 22.9% RR (BOND-1 Trial)[69,70]. These findings have led to the approval of cetuximab for irinotecan-refractory advanced CRC in the Unites States and, more recently, in Europe.
As it is known, the expression of proangiogenic molecules by tumor cells can be stimulated by EGFR receptor signaling[71]. Furthermore, several studies have shown that EGFR inhibitors reduce VEGF and microvessel density in tumors that regress upon EGFR blockade[72,73]. These results provide a strong rationale for combinations of anti-EGFR agents with angiogenesis inhibitors in CRC.
The safety and efficacy of concurrent administration of BV and cetuximab has been evaluated in a randomized phase II trial in patients with irinotecan-refractory metastatic CRC (BOND-2 trial)[74]. Seventy-five patients were assigned to receive either irinotecan/cetuximab/BV (5 mg/kg every other week) or cetuximab/BV. This study presents a similar design to BOND-1 trial with BV included in both arms. The combination of cetuximab/BV, alone or with irinotecan, is tolerable, and RR and median TTP seen with the addition of BV to either arm appear favorable compared to historical controls of the BOND-1 trial. The results of the BOND-2 trial validate the design of the planned intergroup trial CALGB/SWOG 80405[75], which plans to randomize 2289 patients to receive standard chemotherapy with the addition of cetuximab, BV, or both monoclonal antibodies in first-line metastatic CRC. The primary end-point of this trial will be to detect differences in overall median survival.
SMALL-MOLECULE TK INHIBITORS IN CRC
Finally, novel VEGFR and/or PDGFR TK inhibitors with different ranges of nanomolar potencies, selectivities, and pharmacokinetic properties are entering phaseI/II trials for the treatment of cancer[76-78]. In addition, there are now available a series of TK inhibitors that block both the EGFR and the downstream signalling molecules on the one hand and the VEGF receptor TK on the other (Table 1). Zactima (ZD6474, AstraZeneca Pharmaceuticals, Cheshire, UK) is an orally bioavailable, anilinoquinazoline derivative, multitargeted tyrosine kinase inhibitor that targets VEGFR-2, EGFR, and RET tyrosine kinases, and is currently in phaseI/II evaluation for the treatment of cancer[79,80]. Another broad spectrum multitargeted agent, AEE788 (Novartis, Basel, Switzerland), is an oral small-molecule inhibitor of both EGFR and VEGFR tyrosine kinases[81,82]. In preclinical studies, this agent has shown growth and metastases inhibition of human colon carcinoma in an orthotopic nude mouse model[83]. Sorafenib (BAY 43-9006; Nexavar®, Bayer Aktiengesellschaft, Leverkusen-Bayerwerk, Germany, and Onyx Pharmaceuticals Inc., Emeryville, CA) targets VEGFR2 and VEGFR3, PDGFR-β, c-Kit and FLT3 (fms-related tyrosine kinase 3) and the downstream signalling molecule of EGFR known as Raf[84]. This agent efficiently inhibits both tumor-cell proliferation and angiogenesis in preclinical models, and monotherapy treatment has shown efficacy in a phase III trial in patients with cytokine-refractory advanced renal carcinoma, which led in 2005 to the approval by the FDA for this indication[85]. In contrast with BV, the monotherapy efficacy demonstrated by Sorafenib could mimic the synergistic effect of the combination of an anti-VEGF antibody and chemotherapy[86]. The activity of Sorafenib and similar agents in the treatment of CRC needs further development. In addition, whether it will be better to target the EGFR and VEGF receptor with two compounds, each targeting one system, or to use these new class of oral duals or broad-spectrum inhibitors, is not known at this time[87].
SUMMARY AND CONCLUDING REMARKS
The increased knowledge of the VEGF signaling network and its implication in the development and progression of CRC, together with the initial positive clinical results observed with anti-VEGF therapies, makes angiogenic targeting an appropriate cancer treatment strategy. Based on the results of the completed phase III trials, BV can increase survival when combined with standard chemotherapy in first and second-line therapy of advanced CRC. These findings have led to the approval of BV for the treatment of metastatic CRC. Simultaneously, the activity of BV in combination with 5-FU/LV-based chemotherapy regimens is being evaluated in early disease, a period when angiogenesis might be particularly critical. Results of these trials are eagerly awaited. The initial positive results of anti-VEGF therapy are not accomplished without added toxicity. Side effects of anti-VEGF agents are usually moderate compared with other therapies, but the etiology is poorly understood. Major safety concerns have been raised by increased morbidity, and a number of treatment-related deaths from bowel perforations and cardiovascular events. Modest elevations in blood pressure occur occasionally and are easily managed with standard antihypertensive medications.
Since multiple growth-controlling pathways may be altered in cancer cells, combination antibody strategies are being explored in advanced CRC. BV is being assessed in combination with cetuximab in irinotecan-refractory metastatic CRC, based on the positive results of anti-EGFR therapies in this context. Preliminary data for this combination shows remarkable results without substantial differences about toxicity. New clinical trials with both targeted strategies in first-line metastatic CRC are recruiting patients. Combination of BV with novel VEGFR and broad-spectrum TK inhibitors also needs to be assessed in the treatment of CRC. One of these VEGFR TK inhibitors, vatalanib, combined with standard chemotherapy has been shown not to improve survival in first and second-line treatment of advanced CRC in both phase III trials. New broad-spectrum TK inhibitors, such as Sorafenib, oppositely to the VEGF antibody, have shown promising monotherapy activity in other tumors. The basis of these findings is being extensively evaluated, and the identification of biomarkers to predict therapeutic response and optimal doses of anti-VEGF therapy is urgently needed in order to identify patients who will benefit from antiangiogenic therapy.
Angiogenesis research moves in two directions. In one hand, ongoing and new, well-designed trials will define the optimal clinical application of the actual antiangiogenic agents, and, on the other, intensive efforts in basic research will identify new agents with different antiangiogenic approaches for the treatment of CRC.
Footnotes
S- Editor Liu Y L- Editor Alpini GD E- Editor Wang HF
References
- 1.Cancer facts and figures 2006. Atlanta: American Cancer Society, 2006. Available from: http: //www.cancer.org/downloads/STT/CAFF2006PWSecured.pdf.
- 2.Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum. 1997;40:15–24. doi: 10.1007/BF02055676. [DOI] [PubMed] [Google Scholar]
- 3.Petrelli N, Douglass HO, Herrera L, Russell D, Stablein DM, Bruckner HW, Mayer RJ, Schinella R, Green MD, Muggia FM. The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. Gastrointestinal Tumor Study Group. J Clin Oncol. 1989;7:1419–1426. doi: 10.1200/JCO.1989.7.10.1419. [DOI] [PubMed] [Google Scholar]
- 4.Poon MA, O'Connell MJ, Moertel CG, Wieand HS, Cullinan SA, Everson LK, Krook JE, Mailliard JA, Laurie JA, Tschetter LK. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989;7:1407–1418. doi: 10.1200/JCO.1989.7.10.1407. [DOI] [PubMed] [Google Scholar]
- 5.Piedbois P, Buyse M, Rustum Y, Machover D, Erlichman C, Carlson R, Valone F, Labianca R, Doroshow J, Petrelli N. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. Advanced Colorectal Cancer Meta-Analysis Project. J Clin Oncol. 1992;10:896–903. doi: 10.1200/JCO.1992.10.6.896. [DOI] [PubMed] [Google Scholar]
- 6.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 7.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 9.Grothey A, Sargent D. Overall survival of patients with advanced colorectal cancer correlates with availability of fluorouracil, irinotecan, and oxaliplatin regardless of whether doublet or single-agent therapy is used first line. J Clin Oncol. 2005;23:9441–9442. doi: 10.1200/JCO.2005.04.4792. [DOI] [PubMed] [Google Scholar]
- 10.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 14.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 15.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 16.Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Böhlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 18.Bruns CJ, Harbison MT, Davis DW, Portera CA, Tsan R, McConkey DJ, Evans DB, Abbruzzese JL, Hicklin DJ, Radinsky R. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res. 2000;6:1936–1948. [PubMed] [Google Scholar]
- 19.Cao R, Bråkenhielm E, Li X, Pietras K, Widenfalk J, Ostman A, Eriksson U, Cao Y. Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-alphaalpha and -alphabeta receptors. FASEB J. 2002;16:1575–1583. doi: 10.1096/fj.02-0319com. [DOI] [PubMed] [Google Scholar]
- 20.Reinmuth N, Liu W, Jung YD, Ahmad SA, Shaheen RM, Fan F, Bucana CD, McMahon G, Gallick GE, Ellis LM. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB J. 2001;15:1239–1241. doi: 10.1096/fj.00-0693fje. [DOI] [PubMed] [Google Scholar]
- 21.Van Belle E, Witzenbichler B, Chen D, Silver M, Chang L, Schwall R, Isner JM. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation. 1998;97:381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- 22.Lee JC, Chow NH, Wang ST, Huang SM. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer. 2000;36:748–753. doi: 10.1016/s0959-8049(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995;55:3964–3968. [PubMed] [Google Scholar]
- 24.Kumar H, Heer K, Lee PW, Duthie GS, MacDonald AW, Greenman J, Kerin MJ, Monson JR. Preoperative serum vascular endothelial growth factor can predict stage in colorectal cancer. Clin Cancer Res. 1998;4:1279–1285. [PubMed] [Google Scholar]
- 25.Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest. 1995;95:1789–1797. doi: 10.1172/JCI117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 27.Borgström P, Gold DP, Hillan KJ, Ferrara N. Importance of VEGF for breast cancer angiogenesis in vivo: implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer Res. 1999;19:4203–4214. [PubMed] [Google Scholar]
- 28.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 29.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 30.Gordon MS, Margolin K, Talpaz M, Sledge GW, Holmgren E, Benjamin R, Stalter S, Shak S, Adelman D. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 31.Margolin K, Gordon MS, Holmgren E, Gaudreault J, Novotny W, Fyfe G, Adelman D, Stalter S, Breed J. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J Clin Oncol. 2001;19:851–856. doi: 10.1200/JCO.2001.19.3.851. [DOI] [PubMed] [Google Scholar]
- 32.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 33.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF, Gaudreault J, Damico LA, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 34.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 35.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratner M. Genentech discloses safety concerns over Avastin. Nat Biotechnol. 2004;22:1198. doi: 10.1038/nbt1004-1198. [DOI] [PubMed] [Google Scholar]
- 37.Scappaticci FA, Fehrenbacher L, Cartwright T, Hainsworth JD, Heim W, Berlin J, Kabbinavar F, Novotny W, Sarkar S, Hurwitz H. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173–180. doi: 10.1002/jso.20301. [DOI] [PubMed] [Google Scholar]
- 38.Knight R, Miller L, Pirotta N. First-line irinotecan (C), fluorouracil (F), leucovorin (L) especially improves survival (OS) in metastatic colorectal cancer (MCRC) patients (PT) with favorable prognostic indicators. Proc Am Soc Clin Oncol. 2000:255a. [Google Scholar]
- 39.Bleiberg H, Cvitkovic E. Characterisation and clinical management of CPT-11 (irinotecan)-induced adverse events: the European perspective. Eur J Cancer. 1996;32A Suppl 3:S18–S23. doi: 10.1016/0959-8049(96)00293-6. [DOI] [PubMed] [Google Scholar]
- 40.Kabbinavar F, Schulz J, McCleod M, Patel T, Hamm J, Hecht J, Perrou B, Griffing S, Nelson B, Novotny W. Bevacizumab (a monoclonal antibody to vascular endothelial growth factor) to prolong progression-free survival in first-line colorectal cancer (CRC) in subjects who are not suitable candidates for first-line CPT-11. Proc Am Soc Clin Oncol. 2004:249a. [Google Scholar]
- 41.Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B, Novotny WF. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 42.Wolmark N, Rockette H, Fisher B, Wickerham DL, Redmond C, Fisher ER, Jones J, Mamounas EP, Ore L, Petrelli NJ. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11:1879–1887. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 43.Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 44.Hochster HS, Hart LL, Ramanathan RK, Hainsworth JD, Hedrick EE, Childs BH. Safety and efficacy of oxaliplatin/fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer (mCRC): Final analysis of the TREE-Study. Proc Am Soc Clin Oncol. 2006:3510a. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 45.Saltz LB, Clarke S, Diaz-Rubio W, Scheithauer A, Figer A, Wong R, Koski S, Lichinitser M, Yang T, Cassidy J. Bevacizumab in combination with XELOX or FOLFOX4: Efficacy results from XELOX-1/NO16966, a randomized phase III trial in the first-line treatment of metastatic colorectal cancer (MCRC) Gastrointestinal Cancers Symposium. 2007:238a. [Google Scholar]
- 46.Fluorouracil, leucovorin, and oxaliplatin with or without bevacizumab in treating Patients who have undergone surgery for stage II or III colon cancer. Available from: http: //www.clinicaltrials.gov/show/NCT00096278.
- 47.Combination chemotherapy with or without bevacizumab in treating patients who have undergone surgery for stage II or III colon cancer. Available from: http: //www.clinicaltrials.gov/show/NCT00112918.
- 48.Oxaliplatin, leucovorin, and fluorouracil with or without Bevacizumab in Treating Patients who have undergone surgery for stage II colon cancer. Available from: http: //www.clinicaltrials.gov/show/NCT00217737.
- 49.Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist. 2006;11:753–764. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- 50.Kuenen BC, Rosen L, Smit EF, Parson MR, Levi M, Ruijter R, Huisman H, Kedde MA, Noordhuis P, van der Vijgh WJ, et al. Dose-finding and pharmacokinetic study of cisplatin, gemcitabine, and SU5416 in patients with solid tumors. J Clin Oncol. 2002;20:1657–1667. doi: 10.1200/JCO.2002.20.6.1657. [DOI] [PubMed] [Google Scholar]
- 51.Jost LM, Gschwind HP, Jalava T, Wang Y, Guenther C, Souppart C, Rottmann A, Denner K, Waldmeier F, Gross G, et al. Metabolism and disposition of vatalanib (PTK787/ZK-222584) in cancer patients. Drug Metab Dispos. 2006;34:1817–1828. doi: 10.1124/dmd.106.009944. [DOI] [PubMed] [Google Scholar]
- 52.Drevs J, Müller-Driver R, Wittig C, Fuxius S, Esser N, Hugenschmidt H, Konerding MA, Allegrini PR, Wood J, Hennig J, et al. PTK787/ZK 222584, a specific vascular endothelial growth factor-receptor tyrosine kinase inhibitor, affects the anatomy of the tumor vascular bed and the functional vascular properties as detected by dynamic enhanced magnetic resonance imaging. Cancer Res. 2002;62:4015–4022. [PubMed] [Google Scholar]
- 53.Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O'Reilly T, et al. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178–2189. [PubMed] [Google Scholar]
- 54.Morgan B, Thomas AL, Drevs J, Hennig J, Buchert M, Jivan A, Horsfield MA, Mross K, Ball HA, Lee L, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol. 2003;21:3955–3964. doi: 10.1200/JCO.2003.08.092. [DOI] [PubMed] [Google Scholar]
- 55.Thomas AL, Morgan B, Horsfield MA, Higginson A, Kay A, Lee L, Masson E, Puccio-Pick M, Laurent D, Steward WP. Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J Clin Oncol. 2005;23:4162–4171. doi: 10.1200/JCO.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 56.Steward WP, Thomas A, Morgan B, Wiedenmann B, Bartel C, Vanhoefer U, Trarbach T, Junker U, Laurent D, Lebwohl D. Expanded phase I/II study of PTK787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in combination with FOLFOX-4 as first-line treatment for patients with metastatic colorectal cancer. Proc Am Soc Clin Oncol. 2004:3556a. [Google Scholar]
- 57.Trarbach T, Schleucher N, Tewes M, Seeber S, Junker U, Laurent D, Vanhoefer U, Masson E, Lebwohl D. Phase I/II study of PTK787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor in combination with FOLFIRI as first-line treatment for patients with metastatic colorectal cancer (CRC) Proc Am Soc Clin Oncol. 2005:3605a. [Google Scholar]
- 58.Hecht JR, Trarbach T, Jaeger E, Hainsworth J, Wolff R, Lloyd K, Bodoky G, Borner M, Laurent D, Jacques C. A randomized, double-blind, placebo-controlled, phase III study in patients (Pts) with metastatic adenocarcinoma of the colon or rectum receiving first-line chemotherapy with oxaliplatin/5-fluorouracil/leucovorin and PTK787/ZK 222584 or placebo (CONFIRM-1) Proc Am Soc Clin Oncol. 2005:3a. [Google Scholar]
- 59.Koehne C, Bajetta E, Lin E, Valle J, Van Cutsem E, Hecht J, Moore M, Germond C, Meinhardt G, Jacques C. Final results of CONFIRM 2: A multinational, randomized, double-blind, phase III study in 2nd line patients (pts) with metastatic colorectal cancer (mCRC) receiving FOLFOX4 and PTK787/ZK 222584 (PTK/ZK) or placebo. Proc Am Soc Clin Oncol. 2007:4033a. [Google Scholar]
- 60.Koehne C, Bajetta E, Lin E, Van Cutsem E, Hecht J, Douillard J, Moore M, Germond C, Laurent D, Jacques C. Results of an interim analysis of a multinational randomized, double-blind, phase III study in patients (pts) with previously treated metastatic colorectal cancer (mCRC) receiving FOLFOX4 and PTK787/ZK 222584 (PTK/ZK) or placebo (CONFIRM 2) Proc Am Soc Clin Oncol. 2006:3508a. [Google Scholar]
- 61.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 62.Major P, Trarbach T, Lenz H, Kerr D, Pendergrass K, Douillard J, Chen B, Laurent D, Jacques C, Van Cutsem E. A meta-analysis of two randomized, double-blind, placebo-controlled, phase III studies in patients (pts) with metastatic colorectal cancer (mCRC) receiving FOLFOX4 and PTK/ZK to determine clinical benefit on progression-free survival (PFS) in high LDH pts. Proc Am Soc Clin Oncol. 2006:3529a. [Google Scholar]
- 63.Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis. 2005;22:25–30. doi: 10.1007/s10585-005-2343-7. [DOI] [PubMed] [Google Scholar]
- 64.Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL. Lactate dehydrogenase 5 expression in operable colorectal cancer: strong association with survival and activated vascular endothelial growth factor pathway--a report of the Tumour Angiogenesis Research Group. J Clin Oncol. 2006;24:4301–4308. doi: 10.1200/JCO.2006.05.9501. [DOI] [PubMed] [Google Scholar]
- 65.Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- 66.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 67.Ciardiello F, Bianco R, Damiano V, De Lorenzo S, Pepe S, De Placido S, Fan Z, Mendelsohn J, Bianco AR, Tortora G. Antitumor activity of sequential treatment with topotecan and anti-epidermal growth factor receptor monoclonal antibody C225. Clin Cancer Res. 1999;5:909–916. [PubMed] [Google Scholar]
- 68.Liu B, Fang M, Schmidt M, Lu Y, Mendelsohn J, Fan Z. Induction of apoptosis and activation of the caspase cascade by anti-EGF receptor monoclonal antibodies in DiFi human colon cancer cells do not involve the c-jun N-terminal kinase activity. Br J Cancer. 2000;82:1991–1999. doi: 10.1054/bjoc.2000.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 70.Prewett MC, Hooper AT, Bassi R, Ellis LM, Waksal HW, Hicklin DJ. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002;8:994–1003. [PubMed] [Google Scholar]
- 71.Wang D, Huang HJ, Kazlauskas A, Cavenee WK. Induction of vascular endothelial growth factor expression in endothelial cells by platelet-derived growth factor through the activation of phosphatidylinositol 3-kinase. Cancer Res. 1999;59:1464–1472. [PubMed] [Google Scholar]
- 72.Ciardiello F, Caputo R, Bianco R, Damiano V, Fontanini G, Cuccato S, De Placido S, Bianco AR, Tortora G. Inhibition of growth factor production and angiogenesis in human cancer cells by ZD1839 (Iressa), a selective epidermal growth factor receptor tyrosine kinase inhibitor. Clin Cancer Res. 2001;7:1459–1465. [PubMed] [Google Scholar]
- 73.Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 74.Saltz LB, Lenz HJ, Hochster H, Wadler S, Hoff P, Kemeny N, Hollywood E, Gonen M, Wetherbee S, Chen H. Randomized phase II trial of cetuximab/bevacizumab/irinotecan (CBI) versus cetuximab/bevacizumab (CB) in irinotecan-refractory colorectal cancer. Proc Am Soc Clin Oncol. 2005:3508a. doi: 10.1200/JCO.2007.12.0949. [DOI] [PubMed] [Google Scholar]
- 75.Cetuximab and/or bevacizumab combined with combination chemotherapy in treating patients with metastatic colorectal cancer. Available from: http: //clinicaltrials.gov/ct/show/NCT00265850.
- 76.Beebe JS, Jani JP, Knauth E, Goodwin P, Higdon C, Rossi AM, Emerson E, Finkelstein M, Floyd E, Harriman S, et al. Pharmacological characterization of CP-547,632, a novel vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for cancer therapy. Cancer Res. 2003;63:7301–7309. [PubMed] [Google Scholar]
- 77.Ruggeri B, Singh J, Gingrich D, Angeles T, Albom M, Yang S, Chang H, Robinson C, Hunter K, Dobrzanski P, et al. CEP-7055: a novel, orally active pan inhibitor of vascular endothelial growth factor receptor tyrosine kinases with potent antiangiogenic activity and antitumor efficacy in preclinical models. Cancer Res. 2003;63:5978–5991. [PubMed] [Google Scholar]
- 78.Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–4400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- 79.Ciardiello F, Bianco R, Caputo R, Caputo R, Damiano V, Troiani T, Melisi D, De Vita F, De Placido S, Bianco AR, et al. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin Cancer Res. 2004;10:784–793. doi: 10.1158/1078-0432.ccr-1100-03. [DOI] [PubMed] [Google Scholar]
- 80.Wedge SR, Ogilvie DJ, Dukes M, Kendrew J, Chester R, Jackson JA, Boffey SJ, Valentine PJ, Curwen JO, Musgrove HL, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62:4645–4655. [PubMed] [Google Scholar]
- 81.Baselga J, Rojo F, Dumez H, Mita A, Takimoto CH, Tabernero J, Dilea C, Parker K, Dugan M, van Oosterom AT, et al. Phase I study of AEE788, a novel multitargeted inhibitor of ErbB and VEGF receptor family tyrosine kinases: A pharmacokinetic (PK)-pharmacodynamic (PD) study to identify the optimal therapeutic dose regimen. Proc Am Soc Clin Oncol. 2005;3028a [Google Scholar]
- 82.Traxler P, Allegrini PR, Brandt R, Brueggen J, Cozens R, Fabbro D, Grosios K, Lane HA, McSheehy P, Mestan J, et al. AEE788: a dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2004;64:4931–4941. doi: 10.1158/0008-5472.CAN-03-3681. [DOI] [PubMed] [Google Scholar]
- 83.Yokoi K, Thaker PH, Yazici S, Rebhun RR, Nam DH, He J, Kim SJ, Abbruzzese JL, Hamilton SR, Fidler IJ. Dual inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation by AEE788 reduces growth and metastasis of human colon carcinoma in an orthotopic nude mouse model. Cancer Res. 2005;65:3716–3725. doi: 10.1158/0008-5472.CAN-04-3700. [DOI] [PubMed] [Google Scholar]
- 84.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 85.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 86.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 87.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–2459. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]