Molecular signaling in feather morphogenesis (original) (raw)
. Author manuscript; available in PMC: 2015 Apr 22.
Published in final edited form as: Curr Opin Cell Biol. 2006 Oct 17;18(6):730–741. doi: 10.1016/j.ceb.2006.10.009
Abstract
The development and regeneration of feathers have gained much attention recently because of progress in the following areas. First, pattern formation. The exquisite spatial arrangement provides a simple model for decoding the rules of morphogenesis. Second, stem cell biology. In every molting, a few stem cells have to rebuild the entire epithelial organ, providing much to learn on how to regenerate an organ physiologically. Third, evolution and development (‘Evo-Devo’). The discovery of feathered dinosaur fossils in China prompted enthusiastic inquiries about the origin and evolution of feathers. Progress has been made in elucidating feather morphogenesis in five successive phases: macro-patterning, micro-patterning, intra-bud morphogenesis, follicle morphogenesis and regenerative cycling.
Introduction
Feathers are derived from a series of interactions between the epithelium and mesenchyme. Epithelium develops from the surface ectoderm, which initially consists of a multipotent single layer. These epithelial cells receive signals from the dermis and signal back to the dermis to form specialized appendages. The continuous reciprocal interactions between the epithelium and mesenchyme lead to one level of morphogenesis after another, reaching more and more complex morphologies [1–4,5••].
Here we review recent progress in the development and regeneration of feathers. Through the life cycle of a feather, we highlight the five phases of morphogenetic processes (Figure 1). In each phase, we emphasize major cellular events and try to link them with known molecular signaling.
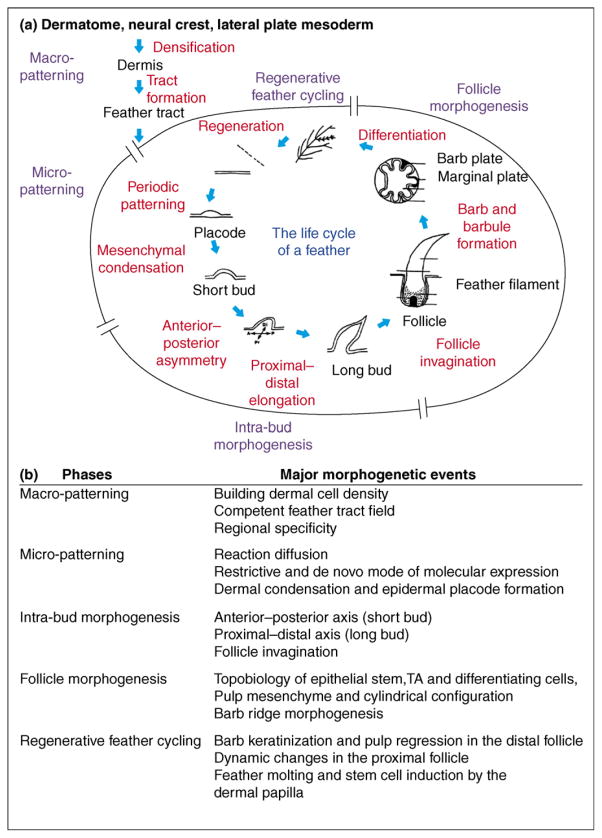
Figure 1.
Overview of feather morphogenesis. (a) The life cycle of a feather. The five phases of feather morphogenesis are shown in blue. Major morphological events are in red. (b) Five phases of feather morphogenesis and major events. Each event requires a network of molecular signaling pathways. Morphogenetic signaling pathways such as FGF, BMP, Shh and Wnt are used repetitively in different events. Therefore, perturbation experiments in feather morphogenesis can result in phenotypes which are time and context dependent [34•,49,78].
The classical period of feather research (the 1960s and 1970s) was highlighted by experimental embryology studies pioneered by Sengel [1] and Dhouailly (see below). Clear morphological changes were documented in Lucas and Stettenheim [6]. In the 1990s, the molecular basis of these classical phenomena began to be explored [6,7]. Dhouailly is the investigator whose research on feathers transcends both the classical and molecular periods. The special issue ‘Skin Development’ she edited contains excellent reviews from her and other groups [8]. Major reviews on evolution and development (‘Evo-Devo’) can be seen in the special issue on Development and Evolution of Amniote Integuments [9], and a detailed review by Prum and Brush [10]. Interested readers can consult these reviews for further information.
Macro-patterning
Skin regional specificities are quite obvious on the body surface of chickens and humans [11], although not so in the mouse. The process of forming these different skin regions (in chicken, different feather tracts and scale regions) is called macro-patterning [1,5••,12]. This process requires the formation of dermis and fate specification of that dermal region. In chicken embryos, the distinct skin regions are established by the dense dermis underlying the epidermis. Chicken dorsal dermis first forms at embryonic day (E)3–5 (stage 20–26) [5••]. Mesodermal segmentation forms the somites, which gives rise not only to vertebrae, but also to segmented dorsal dermis progenitors [13]. These dermal cells form a dense gradient at E6 and gradually expand from the midline of the dorsal trunk to the lateral regions in the chicken [5••]. With the propagation of the morphogenetic wave, the dense dermis forms, becoming a competent tract field to form skin appendages [14].
The mechanism of the macro-patterning is not well understood. It is generally agreed that the formation of dermis requires cell immigration. The origins of dermal cells in different regions have been traced. Quail–chicken chimera cell tracing and epithelial–mesenchymal recombination showed that different regions of dense dermis in the body come from different origins. For instance, the head and neck dermis are derived from neural crest cells [15], while the dorsal trunk dermis is generated by the dermomyotome of the somites [16] and the lateral and ventral body wall dermis comes from the lateral plate mesoderm [17–19]. What could initiate the process? Classical work showed that initiation of dorsal dermis formation requires the induction of the dorsal neural tube. Wnt1 was shown to be able to substitute for the neural tube in carrying out this role [18–20]. RCAS (replication-competent avian sarcoma virus) Wnt1 mis-expression in ovo induces Wnt11 expression, which decreases the expression of collagen type 2 and NCAM and induces cell migration.
It is generally agreed that densification is an important step in making the skin region competent to form skin appendages. To this end, cDermo-1 (also known as Twist 2) was found to be expressed in the subectodermal mesenchyme at a very early embryonic stage [21]. Over-expression of cDermo-1 induces dense dermis formation that consequently induces ectopic feathers and scales and makes existing feathers grow longer [22••]. This is in consistent with the in vitro data showing that when a fixed-size epithelium is faced with increasing numbers of dissociated mesenchymal cells, more feather buds form; when feathers reach a maximum density they develop faster [23]. What may induce c-Dermo-1? This group showed that BMP2 protein could induce ectopic feather tract formation at early stages (stage 17–21), probably via cDermo-1 [24]. It should be noted that overexpression of BMP2 in the next phase inhibits individual feather primordium formation [25,26]. This suggests that the signaling molecule can have different stage-dependent effects, as seen in other molecules. Another clue comes from the Ottawa naked chicken mutant, which fails to form dense dermis. Epithelial–mesenchymal recombination demonstrated that the defect is in the dermis [27]. With the advent of the avian genome [28], genetics may begin to provide a molecular answer.
On the epithelial side, expression of β-catenin is a reliable marker for the competence of epithelia to form feather primordia. Stabilization of excess β-catenin leads to ectopic bud formation [29] and even converts parts of scales to feather buds [30]. In a temporal sequence, BMP 7 appears nearly as early as β-catenin, and its presence is considered to be associated with the formation of ectodermal organs. Over-expressing BMP 7 can also lead to feathery scales [31]. Wnt 7a appears slightly later and also plays a critical role in feather formation [32,33].
Different feather tracts form with particular shapes, sizes and positioning on the chicken embryo surface, with apteric regions spaced between tracts. However, these patterns are not fixed and are actually plastic, since elimination of part of the spinal tract can lead to the ‘invasion’ of the adjacent femoral tract [12]. The sizes of feather tracts can be altered by experimental modulation of Wnt pathway members. Over-expression of Wnt 1 produced a truncated spinal tract and the neighboring femoral tracts expanded into the affected sacral–caudal region. Over-expressing Wnt3a expanded the size of the spinal tract [34•] and decreased Wnt11 expression. These data suggest Wnt members may be implicated in dermis formation and/or tract formation.
The specificity of skin regions is another mystery. In heterotopic transplantation experiments, the fate of the midventral apterium is not determined at E2 and different signals are required for the specification of dorsal and ventral feather dermal progenitors [18–20,27,35]. The different origins may lead them to express different morphogen profiles and to have different responses to morphogen gradient forming signals. Yet what sets up regional specificity remains unknown. In the limb bud, Lmx1 and engrailed-1 have been implicated in dorsal and ventral specification. Lmx1 can endow ventral foot dermis to form scutate scales, while En-1 blocks the competence of the dorsal dermis to form scutate scales [36]. In the plantar skin, engrailed-1 may inhibit Shh and Wnt 7a, and Shh and Wnt 7a can convert scutate scales to reticulate scales or to glabrous skin [37]. It has also been suggested that avian scales may be secondarily derived by inhibiting feather signaling networks with molecules such as En-1 [37].
We hypothesized that skin Hox codes may determine regional specificity of skin and skin appendages [2,38]. Results from studies on CHOX C-8 and D-13 are consistent with this thinking [39]. It was also found that one group of Hox proteins (Hox B4, A7 and C8) have a restricted expression on the chicken skin while expression of another group (Hox D4, D13, A11, C6) is unrestricted. The restricted group is expressed in temporal and spatial co-linearity with respect to their position in the Hox complex from E5–25 on the epidermis. Unrestricted group genes are expressed concomitantly [40]. More data on the whole picture and more functional experiments will be required to continue this line of research.
Another interesting thought comes from the fossil record showing that the feathered dinosaurs Sinosauropteryx exhibited similar skin appendages distributed all over the body. There is minimal or no regional specificity. It is not until later in Caudipteryx that multiple skin regions start to form. Eventually, the evolution of skin regional specificity allows different types of feathers (downy, contour, flight, etc), scales (reticulate, scutate, etc) to form in different body regions, making the integument much more diverse in its functions [41,42].
Micro-patterning
Following the formation of tract fields, a morphogenetic wave sweeps through each field, resulting in the previously homogenous field being converted into discrete buds separated by interbud spacing [43–45]. This is also called micro-patterning [1,5••,12]. During micro-patterning in the spinal tract, the primary bud (the first bud to form in a tract) forms at the lumbar region at the level of hind limbs and propagates bidirectionally along the midline body axis to form the primary row (the first row to form in a tract). Then the morphogenetic wave spreads symmetrically and bilaterally from the midline. In the femoral tract, the primary bud starts at the posterior limb bud/trunk junction, then spreads toward the anterior limb bud to form the primary row at the junction between the hind limb bud and trunk. This is followed by the asymmetric lateral spread of feather buds toward the trunk with only one row toward the limb [14].
The formation of the primary row is likely to be regulated differently from the secondary rows because scaleless chickens can still form the primary rows in spinal, femoral and scapular tracts [46]. Even the Ottawa naked mutant can form remnant feather buds in the primary row regions [27]. Propagation of the feather morphogenetic wave itself is not essential for periodic patterning because feather explants reconstituted in vitro from dissociated mesenchymal cells re-form periodically positioned buds simultaneously [23]. Thus the morphogenetic wave of feather primordium formation is a global process imposed on the local periodic patterning process. What underlies the morphogenetic wave in vivo is unknown. We think it represents the acquisition of competence in both the epithelia and the mesenchyme, but the cellular/molecular basis is still under investigation.
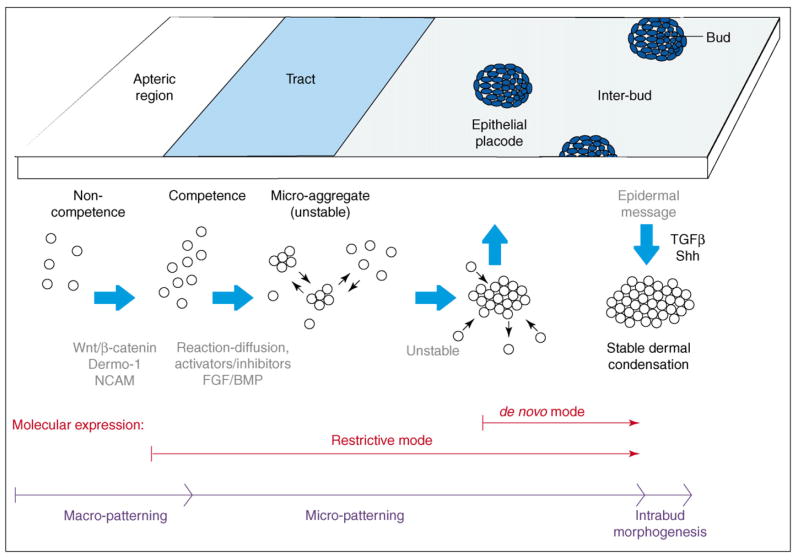
The local periodic patterning process is characterized by the re-distribution of the superficial dense dermis to periodic dermal condensations [26,47]. It has been implied that a molecular code may specify bud and interbud regions, particularly when some molecules are preferentially expressed in the bud, such as Shh [48,49], or in the interbud region, such as collagen I [50•]. We named this the de novo expression mode (Figure 2). However, to push the question to its origin, we must start at the point when the field is homogenous. Some molecules are expressed throughout the field and only later become restricted to the bud region, such as β-catenin [29,30] and Wnt 7a, [32], or to the interbud region, such as gremlin [51,52] and Wnt 11 [34•]. We named this the restrictive expression mode (Figure 2). It turns out that most molecules expressed in the restrictive mode are involved in the macro- or micro- patterning process, serving as competence factors or activators/inhibitors of bud induction. Molecules expressed in the de novo mode are involved in intra-bud morphogenesis (contributing to e.g. bud axis specification, growth or differentiation) or inter-bud morphogenesis (contributing to the formation of inter-follicular tendon, muscle and dermis). We propose to use a shorthand for molecular expression, designating the mode of expression followed by its location in the tract, placode and bud stage. Parentheses indicate whether the molecule is in the epithelium (E) or mesenchyme (M). For example, β-catenin can be recorded as: Restrictive: all tract (E) → placode (E) → posterior bud (M). Shh would be: De novo: none in tract → central placode (E) → posterior distal buds (E).
Figure 2.
Highlight of the periodic patterning process. Medium blue indicates the basal state; dark blue indicates bud domains; light blue is inter-bud state; white is apteric region (inter-tract regions). The timing for restrictive or de novo mode of molecular expression are also shown.
The feather reconstitution assay provides an experimental model in which the periodic patterning process starts from ground zero. Using a competent piece of β-catenin-positive epithelium of a fixed field size, we can vary the mesenchymal cell number (density). Results show that the cell density might be a key factor allowing the initiation of the patterning process [23]. Each cell has a certain intrinsic adhesive property based on its composition of adhesion molecules or growth factor receptors (which is genetically determined). The concentration of growth factors and matrix molecules also forms the micro-environment. On the basis of these basal conditions, epigenetic physical–chemical processes take place. The feather periodic patterning process may be composed of several mechanisms, and reaction-diffusion is involved [26,53]. The basal adhesive state allows the reaction and diffusion of activators and inhibitors, leading to the formation of unstable microaggregates. Through self-reinforcing epithelial–mesenchymal interactions, local activators are gradually enhanced to transform microaggregates into stable dermal condensations. This is a competitive equilibrium process and some initial aggregates are eliminated [23].
Many molecules are described as promoting or suppressing feather bud formation on the basis of RCAS-mediated molecular mis-expression studies. We can categorize these molecules into activator or inhibitor categories. The activators promoting bud formation are FGF2 and −4, noggin, follistatin [51,54–57], DN BMP receptors, BMP7 [31], TGFβ2 [58], β-catenin [29,30], Wnt 3a [34•] and Shh [48,49], among others. The inhibitors that suppress bud formation include BMP 2, 4 [23,25], Delta-1 [47,59], soluble FGFR [60•], Wnt11 [34•] and EGF [50•].
The story, of course, is not that simple. Promotion of the formation of bud domains can appear as an increase in the number of buds or as fused bud domains. This usually has to do with the timing of perturbation [49]. For example, when FGF2 or −4 was added earlier (i.e. in the periodic patterning phase), many small buds form. When FGF2 or −4 was added later (i.e. in the bud morphogenesis phase), the feather buds become fused. Different FGFs can have different effects. FGF8 is not present in feather buds (Chuong et al., unpublished). FGF10 has the unusual property of inhibiting feather bud formation but inducing epidermal thickening [61]. Unlike FGF, another major tyrosine receptor kinase member, EGF, appears to enhance interbud formation [50•]. Depending on the differences between existing intracellular molecular pathways in the downstream RTK pathway, activation may lead to different consequences — cell proliferation, migration or differentiation. An effort in this direction showed there may also be an EphA4–RhoB–actin-cytoskeleton relationship in bud formation [62•]. Since cell shape changes and arrangements are critical in placode morphogenesis, perturbing the cytoskeleton also disrupts feather patterns [63].
BMP activity appears to be adjusted by its antagonists follistatin and gremlin (drm). Follistatin is expressed in the restrictive mode: all tract (E) → circumference of placode (E) → posterior bud (M) [57], while gremlin is expressed in a restrictive mode: all tracts (M) → inter-placode (M) → posterior buds (M) [51,52]. Despite their different locations in the placode stage, they appear to work together to adjust BMP activity and modulate the response of cells to FGF 10 [60•].
There are other important pathways. Several Wnt and frizzled members are expressed in feather morphogenesis. Over-expressing Wnt 6 results in the formation of abnormally shaped buds with a ballooned outgrowth along the filament shaft. Over-expressing Wnt 3a produces big and irregularly shaped buds with a loss of tapering at the distal end. Over-expressing Wnt 11 makes buds with very thin diameters [34•,64]. Downstream to Wnt, the diverse effects observed may be due to the balance among canonical, JNK and planar cellular polarity pathways. Groucho-related genes may modulate Wnt signaling. cGrg2, −3 and −5 are first detected in the epidermal placode and then shift to the posterior and distal buds. Induction of Grg by β-catenin may play a negative feedback role [65].
Eda members are expressed following the restrictive mode in the bud region [66]. Eda is expressed in the inter-bud dermis but Edar and Edaradd are expressed in the feather bud epidermis. At later stages, Eda is expressed in the inter-bud dermis and at the apex of the dermis in feather buds, and Edar and Edaradd continue to be expressed in the epidermis of feather buds. Edar but not Eda can be induced by β-catenin. However, the BMP pathway suppresses Edar but not Eda [66]. In hairs, Eda pathway plays an induction role. In feathers, the function of Eda pathway remains to be investigated.
It is important to work out the regulatory relationship among molecular pathways. The Notch pathway plays a lateral inhibitory role in the periodic patterning of dermal cells [59], which requires the permissive action of the epidermis or of epidermally secreted FGF2 [47]. Dlx genes are considered to integrate information from FGF and BMP signaling [67]. Leucine zipper transcription factor TSC 22 is also considered to be downstream to FGF and BMP signaling [68]. Over-expressing Delta1 induces Notch 2 and suppresses Shh expression [59]. A homeobox gene Hex is considered to be upstream of Wnt 7a and Notch 1 [33,69,70].
The chicken genome project [28] and chicken microarrays from Affymetrix enable the use of chicken genetic mutants as another resource to identify unexpectedly related genes. For example, in scaleless mutant chickens, the dermal field develops normally [47,71] but is missing signals from the epidermis required to organize the dermis. The mutant can be rescued by FGF2 and −4 [47,54]. Without rescue, the periodic patterning process fails to proceed. β-catenin is present throughout the epidermis [30] and delta1 is continuously expressed throughout the entire dermis [47].
Intra-bud morphogenesis
Bud formation establishes the bud and inter-bud domains. Each will start a new level of morphogenesis. Intra-bud morphogenesis entails setting up the anterior–posterior (A–P) and proximal–distal (P–D) axes, as well as the transformation from a bud to a follicular structure. Inter-bud morphogenesis involves the mesenchymal patterning and differentiation of the dermal sheath, inter-follicular muscle and dermis [6]. We will not discuss inter-bud morphogenesis as very few molecular studies have been done on this.
In skin recombination experiments at stage 29, the locations and phenotypes of the new feather buds were determined according to the original dermal condensation [72,73], but the A–P axis was dictated by the epithelium [74,75]. Recombination at stage 33 led to two- or three-headed feather buds, suggesting that the A–P signals have been transferred to the mesenchyme and that the conflict leads to a split of the feather bud field [75]. Notch/Delta signaling is associated with the formation of the asymmetric A–P axis [75]. Notch1 is expressed in the center midline mesenchyme at the early radially symmetric stage, coincident with highly proliferating cells. Notch2 is expressed in the inter-bud at stage 30 and shifts to the feather bud epidermis at stage 31 [59,75]. This stripe of expression forms a gradient from the center to the posterior regions of the feather buds. As development progresses, the feather bud is transformed from having radial symmetry to having anterior–posterior asymmetry. The posterior feather bud contains Notch signaling activity at this stage because the Notch ligands, Delta1 and serrate1, are expressed in the posterior feather bud mesenchyme, adjacent to the Notch expression zone. Another Notch signaling modulator, l-fringe, is expressed in a dynamic pattern. Starting with a restrictive expression mode, it initially forms a ring surrounding each feather bud, then shifts to lateral regions of the feather bud and finally shifts to the posterior feather bud epithelium [76]. These patterns suggest the involvement of the Notch pathway in feather orientation.
Sonic Hedgehog (Shh) is a secreted protein expressed in the epidermis that has previously been implicated in mitogenic and morphogenetic processes throughout feather development [48,49,62•,77,78]. Shh is expressed following the restrictive mode in the initiation region of the dorsal tract and the de novo mode throughout the rest of the tract, appearing in the apex of early feather buds during feather morphogenesis [48]. Therefore, Shh is involved in morphogenetic processes including cell proliferation and mesenchymal condensation [48,49]. Forced Shh expression produces different effects, such as the disorganized epidermal growth at early stages and enhanced feather bud formation at later stages, which indicate that there are stage-specific effects of Shh signaling [49]. In chicken skin explant cultures, blocking Shh signaling with cyclopamine produced some interesting results. There are fused buds as well as new bud formation. The authors consider that Shh may maintain feather bud growth by positioning the source of Shh and localize dermal progenitors to that source. Suppression by cyclopamine results in dispersion of such activity, leading to fused flat buds and new buds (growth center) [62•]. In principle, this is consistent with the concept that a well positioned localized growth zone is critical for building organ architecture [7,64,79].
One Wnt member, Wnt7a, is expressed in the restrictive mode, where it becomes restricted to the posterior feather bud epithelium [32]. Feather reconstitution using RCAS Wnt 7a leads to plateau-shaped feather buds with a loss of A–P polarity, resulting in feathers that fail to elongate [32]. In addition, mis-expressed Wnt 7a also down-regulated tenascin expression and led to the diffuse expression of Notch and Delta within the feather bud, suggesting a posteriorization of feather buds. These data suggest that Wnt7a is involved in A–P axis formation.
Other processes important in the intra-bud morphogenesis phase include the formation of the follicle (Box 1) [41] and the achievement of the tubular organization [80]. The follicle forms through the invagination of epithelia surrounding the long bud. The tubular configuration is achieved through ‘loosening’ of the bud mesenchyme. The molecular mechanisms of theses processes are unknown.
Box 1. Developing the definition of a feather follicle.
- Has a follicle structure, with mesenchymal pulp wrapped inside to form a tubular organization during development.
- Has localized growth zone (LoGZ) of proliferating cells mainly positioned proximally, with a proximal–distal growth mode.
- Forms hierarchical branches of rachis, barbs and barbules. Barbs can be bilaterally or radially symmetric.
- At maturity, the two sides of the feather vane represent the previous basal and supra-basal layer respectively. The pulp is gone.
- Has stem cells and dermal papilla in the follicle, and is able to go through a molting cycle physiologically and to regenerate after plucking.
Adopted from [41].
Follicle morphogenesis
The uniqueness of the feather follicle structure is that it allows for sustained growth and can go through molting and regeneration throughout life [81]. To achieve this, it places its growth zone (transiently amplifying [TA] cells) [64] deep in the follicle and new cells are added from the proximal end [82••]. Feather filaments grow in length, and the distal filament is more differentiated. Then it goes through episodic molting and regeneration [6]. Birds and mammals evolved independently from reptiles and the mammalian hair follicle is the result of homologous evolution [42]. The hair follicle adopted a similar strategy of growth and regeneration. The follicles show many structural similarities but there are also significant differences. They are both ‘professional’ ectodermal organs with robust regenerative powers in adults, and much can be learned from how they ‘manage’ their stem cells under physiological conditions.
While hair stem cells are found in the bulge region [83••,84••], there are no such structures in feather follicles. Using long term label retention, DiI labeling and chicken/quail transplantation, it was found that feather stem cells are located within the follicle, in a collar bulge region, above the dermal papilla [82••] (Figure 3d). This stem cell region is surrounded by the collar region, which is full of TA cells. As cells move upward (in a distal direction), they start to differentiate to form barb ridges at the ramogenic zone. Barb ridge differentiation is shown schematically in Figure 3e.
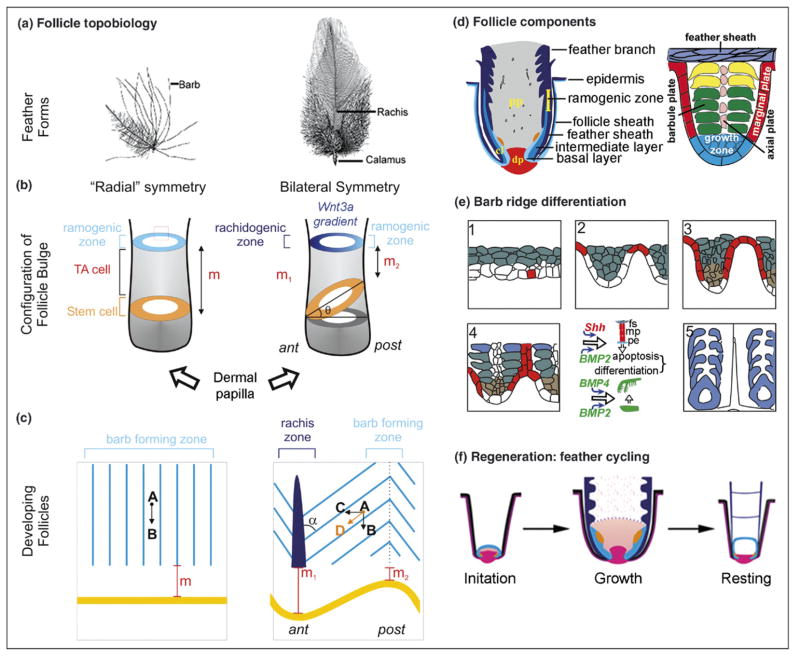
Figure 3.
Highlights of follicle morphogenesis. (a) Representative feather forms. (b) Idealized proximal follicle showing the topobiological relationship of stem cell, TA cell and differentiating ramogenic zone in radial and bilateral symmetric feathers. (c) Barb ridge orientation in an open follicle preparation. (d) Components of feather follicle (left) and a single barb ridge (right). (e) Barb ridge morphogenesis. A segment from (b) is enlarged. White, basal layer; red, marginal plate; green, suprabasal layer; blue, keratinized barbs. (f) Regenerative feather cycle. The initiation, growth and resting phases are depicted. Red, dermal papilla; blue, epithelia; orange, pulp.
The mesenchyme near the base of the long buds is packed tightly and becomes the dermal papilla. The rest of the mesenchyme is loosely packed and becomes the pulp, rich with blood vessels and extracellular matrix [6]. As a result, the feather filament is a cylindrical or tubular structure with a mesenchymal core. This tubular organization is key to the ability of the feather follicle to organize morphogenetic events in multiple independent axes [80]. In 3D, stem cells assume a ring configuration (Figure 3b). Interestingly, when we cut sections we found that the stem cell ring is horizontally placed in radially symmetric downy feathers, but tilted toward the anterior side (rachis side) in bilaterally symmetric feathers [82••]. This compelling difference prompted us to hypothesize a novel mechanism used by nature to convert organ radial symmetry to bilateral symmetry: simply tilting the stem cell ring. The tilting makes the distance to the ramogenic zone different between the anterior and posterior feather follicle and therefore may create an anterior–posterior molecular gradient. This can cause disparities in cell behavior, leading to the formation of a rachis on one side but not the other (Figure 3c).
Indeed, we found a Wnt 3a gradient across the anterior-posterior axis of a feather follicle. Further misexpression experiments with Wnt agonists and antagonists supported this model. In some specimens, feathers remain bilaterally symmetric but the position of the rachis is randomized. In most specimens, they become totally or partially radially symmetric [85••]. We think that a flat-tened gradient that can be experimentally induced by diffusely misexpressing exogenous genes dampens the slope of the molecular gradients, resulting in the conversion of bilaterally symmetric to radially symmetric feathers. How is the stem cell ring tilted? Transplanting the dermal papilla (DP) between breast and wing feathers showed that epithelial stem cells can be molded into different symmetries by the DP [85••].
In the formation of feather branches, RCAS BMP leads to the formation of a big rachis, as shown using feather plucking, regeneration and gene mis-expression. RCAS noggin leads to increased branching of barbs. RCAS anti-SHH or cyclopamine leads to webby branches [86]. Similar conclusions were obtained using late stage chicken and duck embryos [78]. For the periodic patterning of barb ridges, an elegant model based on reaction-diffusion was proposed [87•]. Barb ridge epithelia later segregate to become rami and barbules. Through these different levels of morphogenesis, hierarchical branching (rachis–barbs–barbules) is achieved. On the basis of the developmental sequence of events, we proposed that at an earlier evolutionary stage the early feathers were radially symmetric, lacking a rachis and composed of barb branches of equal length, similar to the downy feathers found today [4,86]. As a result of the limited size of the barb-forming field (the circumference of the calamus), the number of barbs is limited (usually <20). Later, through an evolutionary novelty, the stem cell ring was tilted, a rachis emerged and feathers became bilaterally symmetric. The new organization transformed bulky, fluffy feathers into a two dimensional flat vane, good for moving the air and providing flight.
The feather follicle morphogenesis offers a plethora of opportunities for evolutionary novelty, through the coopting of plesio-morphic molecular signaling modules or heterochrony of existing events [80]. During evolution, the complex forms of feathers today were not made in one day. They were made through numerous attempts via trial and error, probably over a span of ~50 million years [10,41,42,88]. Because of the intermediate stages, it has been debated which fossils of a skin appendage can be called a feather, a protofeather or a feather-like skin appendage. This Evo-Devo approach has enabled us to begin to define the characteristics of a feather (Box 1) [41].
By selecting six growth parameters, Prum and Williamson developed a theoretical model that can predict many feather shapes [89]. In the modern bird, individuals have plumulaceous radially symmetric downy feathers, pennaceous bilaterally symmetric and asymmetric flight and tail feathers, as well as plumulaceous and pennaceous bilaterally symmetric contour feathers. Detailed studies of these feather forms can inspire ideas on how feathers evolved [90]. In domestic birds, there are feather variants that could be a resource for identifying genes for morphogenesis [91]. In nature, there are also bizarrely shaped ornamental feathers (e.g. those on peacocks or birds of paradise). Studying the morphology of these feathers can bring in unexpected results. The bristle from a wild turkey beard does not form a follicle, but rather forms a tube-like filament with simple branching and expresses feather-type-β keratin [92]. In this case, it does not fulfill all five criteria in Box 1, but does share some characteristics of feathers. It also indicates that branching can form without follicular structure, but that a hollow or tubular structure is essential. Studies of different types of feathers from finches also provide interesting insights on feather morphogenesis [93,94]. In the future, it would be fruitful to identify the molecular basis of the growth parameters suggested by Prum and Williamson [89].
Regenerative feather cycling
Although both feathers and hairs go through a molting cycle, they do it in a somewhat different way. In pelage hairs, the lower follicle goes through apoptosis, so stem cells in the bulge have to be activated by the dermal papilla. The process is defined as anagen, catagen, telogen and exogen (phases of the hair cycle). In the feathers, the lower follicle is not destroyed. The feather cycle has been divided into growth, resting and initiation phases [6,81,82••], and feather follicles go through dynamic remodeling during these phases (Figure 3f). As feathers mature from the distal end, the basal epithelium, together with the mesenchymal pulp, retreats toward the proximal end and forms periodically positioned pulp caps [6]. This involves the apoptosis of pulp epithelium (derived from the basal layer) [95] and degeneration of pulp blood vessels, allowing the filament cylinder to open and the feather vane to form. Basal cell proliferation decreases, the intermediate layer epithelium undergoes terminal differentiation, and the upward cell flux in the follicle sheath epithelium slows down and eventually stops. Finally, TA cells and the collar mesenchyme are depleted. The mature feather (the part protruding out of the body surface composed of dead keratinized cells) is connected to the follicle through the proximal end of the calamus (feather shaft proximal to the vane, Figure 3a) where cells are still alive. Thus the growth phase transits to the resting phase.
Dislodging the mature feather is accomplished by the keratinization and the flaking off of keratinocytes at the base of the calamus. The collective adhesive force of cell junctions here is remarkable (as evidenced by the poultry industry’s search for strains with easily pluckable feathers). These processes allow stem cells to come into close contact with the DP and initiate the re-building of the follicle (Figure 3f) [82••]. For the next cycle, epithelial cells first form a thin covering over the DP, constituting the new feather blastema, and, upon signals from the DP, re-enter the growth phase. In the growth phase, feather epithelial cells proliferate actively in the collar region, and then enter branching morphogenesis in the ramogenic zone. The DP regains size and gives rise to the pulp mesenchyme. DiI labeling lineage tracing experiments show a vertical proximal-to-distal distribution [82••]. Feather length is determined by the duration of the growth phase. It can last weeks to months or even years. The resting phase can also range from several days to months. Unlike hair cycling, most molecular signals involved in the transition from resting, initiation and growth phases remain mostly unknown. Comparison of gene networks required for organ regeneration here can help us identify key molecular signals.
Feather molting is regulated by the season, and the prototype is twice a year, in spring and fall. This indicates that the regenerative cycle is under systematic control. Also, molting progresses in waves i_n vivo_, and flight feathers from left and right wings molt in coordination to keep a balance in flight. This indicates that there may be locally coordinated signals between adjacent feather follicles. Finally, while the morphology of feathers can remain constant, as dictated by the DP, DP specificity can change during the life of each bird. For example, the downy-type feathers in newborns are converted to contour feathers later in life. At puberty, tail feathers in roosters or peacocks are converted into feathers of spectacular size and color under the influence of sex hormones [96]. Thus feather morphogenesis is also regulated by systematic physiology, making this model even more informative.
Conclusions
The survey here shows how rich the feather model is. Recent cellular and molecular studies in feathers have been fruitful, providing many new insights linking molecular signaling and biological forms. The field is now going beyond basic molecular biology (e.g. molecule X is required for periodic patterning because X deletion shows a perturbed pattern), and pushing for a new level of understanding of morphogenesis at a systems biology level (e.g. how molecule X works with other molecular signaling pathways and physical-chemical principles to achieve periodic patterning) [53,97•,98]. Feathers, being simple and complex at the same time, may be one of the best available models for decoding epigenetic rules of morphogenesis.
Acknowledgments
We thank grant support from NIAMS AR2177, AR47364 (CMC) and NIAMS AR052397 (RW). We regret that the house style for Current Opinion in Cell Biology does not permit the bulleting and annotation of papers published earlier than the previous two years; otherwise, there were many excellent classic papers to which we would have liked to draw attention.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Sengel P. Morphogenesis of skin. In: Abercrombie M, Newth DR, Torrey JG, editors. Developmental and Cell Biology Series. Cambridge Univ. Press; Cambridge: 1976. [Google Scholar]
- 2.Chuong CM. The making of a feather: homeoproteins, retinoids and adhesion molecules. Bioessays. 1993;15:513–521. doi: 10.1002/bies.950150804. [DOI] [PubMed] [Google Scholar]
- 3.Prum RO. Development and evolutionary origin of feathers. J Exp Zool. 1999;285:291–306. [PubMed] [Google Scholar]
- 4.Chuong CM, Chodankar R, Widelitz RB, Jiang TX. Evo-devo of feathers and scales: building complex epithelial appendages. Curr Opin Genet Dev. 2000;10:449–456. doi: 10.1016/s0959-437x(00)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Dhouailly D, Olivera-Martinez I, Fliniaux I, Missier S, Viallet JP, Thelu J. Skin field formation: morphogenetic events. Int J Dev Biol. 2004;48:85–91. A rich reflection on experimental embryology studies of tract formation, and to bring them to modern biology. [PubMed] [Google Scholar]
- 6.Lucas AM, Stettenheim PR. Avian Anatomy: Integument. Washington, DC: United States Department of Agriculture; 1972. [Google Scholar]
- 7.Chuong CM, Wu P, Plikus M, Jiang TX, Widelitz R. Engineering stem cells into organs: topobiological transformations demonstrated by beak, feather, and other ectodermal organ morphogenesis. Curr Top Dev Biol. 2006;72:237–274. doi: 10.1016/S0070-2153(05)72005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhouailly D, editor. Skin development. Int J Dev Biol. 2004;48:75–270. special issue. [Google Scholar]
- 9.Chuong CM, Homberger DG, editors. Development and evolution of amniote integuments. J Exp Zool Part B: Mol Dev Evo. 2003;298B:1–180. doi: 10.1002/jez.b.23. special issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prum RO, Brush AH. The evolutionary origin and diversification of feathers. Q Rev Biol. 2002;77:261–295. doi: 10.1086/341993. [DOI] [PubMed] [Google Scholar]
- 11.Widelitz RB, Baker RE, Plikus M, Lin CM, Maini PK, Paus R, Chuong CM. Distinct mechanisms underlie pattern formation in the skin and skin appendages. In Embryo Today/Birth Defects Research. doi: 10.1002/bdrc.20075. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sengel P. Pattern formation in skin development. Int J Dev Biol. 1990;34:33–50. [PubMed] [Google Scholar]
- 13.Brand-Saberi B, Christ B. Evolution and development of distinct cell lineages derived from somites. Curr Top Dev Biol. 2000;48:1–42. doi: 10.1016/s0070-2153(08)60753-x. [DOI] [PubMed] [Google Scholar]
- 14.Mayerson PL, Fallon JF. The spatial pattern and temporal sequence in which feather germs arise in the white Leghorn chick embryo. Dev Biol. 1985;109:259–267. doi: 10.1016/0012-1606(85)90453-1. [DOI] [PubMed] [Google Scholar]
- 15.Couly G, LeDouarin NM. The fate map of the cephalic neural primordium at the presomitic to the 3-somite stage in the avian embryo. Development. 1988;103(Suppl):101–113. doi: 10.1242/dev.103.Supplement.101. [DOI] [PubMed] [Google Scholar]
- 16.Mauger A. The role of somitic mesoderm in the development of dorsal plumage in chick embryos. I. Origin, regulative capacity and determination of the plumage-forming mesoderm. J Embryol Exp Morphol. 1972;28:313–341. [PubMed] [Google Scholar]
- 17.Christ B, Jacob M, Jacob HJ. On the origin and development of the ventrolateral abdominal muscles in the avian embryo. An experimental and ultrastructural study. Anat Embryol (Berl) 1983;166:87–101. doi: 10.1007/BF00317946. [DOI] [PubMed] [Google Scholar]
- 18.Fliniaux I, Viallet JP, Dhouailly D. Signaling dynamics of feather tract formation from the chick somatopleure. Development. 2004;131:3955–3966. doi: 10.1242/dev.01263. [DOI] [PubMed] [Google Scholar]
- 19.Fliniaux I, Viallet JP, Dhouailly D. Ventral vs. dorsal chick dermal progenitor specification. Int J Dev Biol. 2004;48:103–106. [PubMed] [Google Scholar]
- 20.Olivera-Martinez I, Thelu J, Teillet MA, Dhouailly D. Dorsal dermis development depends on a signal from the dorsal neural tube, which can be substituted by Wnt-1. Mech Dev. 2001;100:233–244. doi: 10.1016/s0925-4773(00)00540-2. [DOI] [PubMed] [Google Scholar]
- 21.Scaal M, Fuchtbauer EM, Brand-Saberi B. cDermo-1 expression indicates a role in avian skin development. Anat Embryol (Berl) 2001;203:1–7. doi: 10.1007/pl00008244. [DOI] [PubMed] [Google Scholar]
- 22••.Hornik C, Krishan K, Yusuf F, Scaal M, Brand-Saberi B. cDermo-1 misexpression induces dense dermis, feathers, and scales. Dev Biol. 2005;277:42–50. doi: 10.1016/j.ydbio.2004.08.050. This study identifies roles of cDermo-1 in dermis formation and may give new understanding on the induction of tracts. [DOI] [PubMed] [Google Scholar]
- 23.Jiang TX, Jung HS, Widelitz RB, Chuong CM. Self organization is the initial event in periodic feather patterning: Roles of signaling molecules and adhesion molecules. Development. 1999;126:4997–5009. doi: 10.1242/dev.126.22.4997. [DOI] [PubMed] [Google Scholar]
- 24.Scaal M, Prols F, Fuchtbauer EM, Patel K, Hornik C, Kohler T, Christ B, Brand-Saberi B. BMPs induce dermal markers and ectopic feather tracts. Mech Dev. 2002;110:51–60. doi: 10.1016/s0925-4773(01)00552-4. [DOI] [PubMed] [Google Scholar]
- 25.Noramly S, Morgan BA. BMPs mediate lateral inhibition at successive stages in feather tract development. Development. 1998;125:3775–3787. doi: 10.1242/dev.125.19.3775. [DOI] [PubMed] [Google Scholar]
- 26.Jung HS, Francis-West PH, Widelitz RB, Jiang TX, Ting-Berreth S, Tickle C, Wolpert L, Chuong CM. Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Dev Biol. 1998;196:11–23. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- 27.Olivera-Martinez I, Viallet JP, Michon F, Pearton DJ, Dhouailly D. The different steps of skin formation in vertebrates. Int J Dev Biol. 2004;48:107–115. doi: 10.1387/ijdb.15272376. [DOI] [PubMed] [Google Scholar]
- 28.Burt DW. Chicken genome: current status and future opportunities. Genome Res. 2005;15:1692–1698. doi: 10.1101/gr.4141805. [DOI] [PubMed] [Google Scholar]
- 29.Noramly S, Freeman A, Morgan BA. β-catenin signaling can initiate feather bud development. Development. 1999;126:3509–3521. doi: 10.1242/dev.126.16.3509. [DOI] [PubMed] [Google Scholar]
- 30.Widelitz RB, Jiang T-X, Chuong J, LUCM β-catenin in epithelial morphogenesis: Conversion of part of avian foot scales into feather buds with a mutated β-catenin. Dev Biol. 2000;219:98–114. doi: 10.1006/dbio.1999.9580. [DOI] [PubMed] [Google Scholar]
- 31.Harris MP, Linkhart BL, Fallon JF. Bmp7 mediates early signaling events during induction of chick epidermal organs. Dev Dyn. 2004;231:22–32. doi: 10.1002/dvdy.20096. [DOI] [PubMed] [Google Scholar]
- 32.Widelitz RB, Jiang TX, Chen CW, Stott NS, Chuong CM. Wnt-7a in feather morphogenesis: involvement of anterior-posterior asymmetry and proximal-distal elongation demonstrated with an in vitro reconstitution model. Development. 1999;126:2577–2587. doi: 10.1242/dev.126.12.2577. [DOI] [PubMed] [Google Scholar]
- 33.Obinata A, Akimoto Y. Involvement of Hex in the initiation of feather morphogenesis. Int J Dev Biol. 2005;49:953–960. doi: 10.1387/ijdb.052079ao. [DOI] [PubMed] [Google Scholar]
- 34•.Chang CH, Jiang TX, Lin CM, Burrus LW, Chuong CM, Widelitz R. Distinct Wnt members regulate the hierarchical morphogenesis of skin regions (spinal tract) and individual feathers. Mech Dev. 2004;121:157–171. doi: 10.1016/j.mod.2003.12.004. A comprehensive study of the expression and roles of Wnt members in feather bud development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olivera-Martinez I, Missier S, Fraboulet S, Thelu J, Dhouailly D. Differential regulation of the chick dorsal thoracic dermal progenitors from the medial dermomyotome. Development. 2002;129:4763–4772. doi: 10.1242/dev.129.20.4763. [DOI] [PubMed] [Google Scholar]
- 36.Prin F, Logan C, D’Souza D, Ensini M, Dhouailly D. Dorsal versus ventral scales and the dorsoventral patterning of chick foot epidermis. Dev Dyn. 2004;229:564–578. doi: 10.1002/dvdy.20007. [DOI] [PubMed] [Google Scholar]
- 37.Prin F, Dhouailly D. How and when the regional competence of chick epidermis is established: feathers vs, scutate and reticulate scales, a problem en route to a solution. Int J Dev Biol. 2004;48:137–148. doi: 10.1387/ijdb.15272378. [DOI] [PubMed] [Google Scholar]
- 38.Chuong CM, Oliver G, Ting SA, Jegalian BG, Chen HM, De Robertis EM. Gradients of homeoproteins in developing feather buds. Development. 1990;110:1021–1030. doi: 10.1242/dev.110.4.1021. [DOI] [PubMed] [Google Scholar]
- 39.Kanzler B, Prin F, Thelu J, Dhouailly D. CHOXC-8 and CHOXD-13 expression in embryonic chick skin and cutaneous appendage specification. Dev Dyn. 1997;210:274–287. doi: 10.1002/(SICI)1097-0177(199711)210:3<274::AID-AJA8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 40.Reid AI, Gaunt SJ. Colinearity and non-colinearity in the expression of Hox genes in developing chick skin. Int J Dev Biol. 2002;46:209–215. doi: 10.1387/ijdb.011495. [DOI] [PubMed] [Google Scholar]
- 41.Chuong CM, Wu P, Zhang FC, Xu X, Yu M, Widelitz RB, Jiang TX, Hou L. Adaptation to the sky: Defining the feather with integument fossils from mesozoic China and experimental evidence from molecular laboratories. J Exp Zoolog B Mol Dev Evol. 2003;298:42–56. doi: 10.1002/jez.b.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu P, Hou L, Plikus M, Hughes M, Scehnet J, Suksaweang S, Widelitz R, Jiang TX, Chuong CM. Evo-Devo of amniote integuments and appendages. Int J Dev Biol. 2004;48:249–270. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linsenmayer TF. Control of integumentary patterns in the chick. Dev Biol. 1972;27:244–271. doi: 10.1016/0012-1606(72)90101-7. [DOI] [PubMed] [Google Scholar]
- 44.Davidson D. The mechanism of feather pattern development in the chick. 1. The time of determination of feather position. J Embryol Exp Morphol. 1983;74:245–259. [PubMed] [Google Scholar]
- 45.Davidson D. The mechanism of feather pattern development in the chick. II. Control of the sequence of pattern formation. J Embryol Exp Morphol. 1983;74:261–273. [PubMed] [Google Scholar]
- 46.Sawyer RH, Abbott UK. Defective histogenesis and morphogenesis in the anterior shank skin of the scaleless mutant. J Exp Zool. 1972;181:99–110. doi: 10.1002/jez.1401810111. [DOI] [PubMed] [Google Scholar]
- 47.Viallet JP, Prin F, Olivera-Martinez I, Hirsinger E, Pourquie O, Dhouailly D. Chick Delta-1 gene expression and the formation of the feather primordia. Mech Dev. 1998;72:159–168. doi: 10.1016/s0925-4773(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 48.Ting-Berreth SA, Chuong CM. Sonic Hedgehog in feather morphogenesis: induction of mesenchymal condensation and association with cell death. Dev Dyn. 1996;207:157–170. doi: 10.1002/(SICI)1097-0177(199610)207:2<157::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 49.Morgan BA, Orkin RW, Noramly S, Perez A. Stage-specific effects of sonic hedgehog expression in the epidermis. Dev Biol. 1998;201:1–12. doi: 10.1006/dbio.1998.8969. [DOI] [PubMed] [Google Scholar]
- 50•.Atit R, Conlon RA, Niswander L. EGF signaling patterns the feather array by promoting the interbud fate. Dev Cell. 2003;4:231–240. doi: 10.1016/s1534-5807(03)00021-2. This study shows receptor tyrosine kinase members can have distinct roles: FGF for the bud and EGF for the interbud. [DOI] [PubMed] [Google Scholar]
- 51.Ohyama A, Saito F, Ohuchi H, Noji S. Differential expression of two BMP antagonists, gremlin and Follistatin, during development of the chick feather bud. Mech Dev. 2001;100:331–333. doi: 10.1016/s0925-4773(00)00525-6. [DOI] [PubMed] [Google Scholar]
- 52.Bardot B, Lecoin L, Fliniaux I, Huillard E, Marx M, Viallet JP. Drm/Gremlin, a BMP antagonist, defines the interbud region during feather development. Int J Dev Biol. 2004;48:149–156. [PubMed] [Google Scholar]
- 53.Jiang TX, Widelitz RB, Shen WM, Will P, Wu DY, Lin CM, Jung HS, Chuong CM. Integument pattern formation involves genetic and epigenetic controls: feather arrays simulated by digital hormone models. Int J Dev Biol. 2004;48:117–135. doi: 10.1387/ijdb.041788tj. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song H, Wang Y, Goetinck PF. Fibroblast growth factor 2 can replace ectodermal signaling for feather development. Proc Natl Acad Sci USA. 1996;93:10246–10249. doi: 10.1073/pnas.93.19.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song HK, Lee SH, Goetinck PF. FGF-2 signaling is sufficient to induce dermal condensations during feather development. Dev Dyn. 2004;231:741–749. doi: 10.1002/dvdy.20243. [DOI] [PubMed] [Google Scholar]
- 56.Widelitz RB, Jiang T-X, Noveen A, Chen C-W, Chuong C-M. FGF induces new feather buds from developing avian skin. J Invest Dermatol. 1996;107:797–803. doi: 10.1111/1523-1747.ep12330553. [DOI] [PubMed] [Google Scholar]
- 57.Patel K, Makarenkova H, Jung HS. The role of long range, local and direct signalling molecules during chick feather bud development involving the BMP’s, follistatin and the Eph receptor tyrosine kinase Eph-A4. Mech Dev. 1999;86:51–62. doi: 10.1016/s0925-4773(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 58.Ting-Berreth SA, Chuong CM. Local delivery of TGF β2 can substitute for placode epithelium to induce mesenchymal condensation during skin appendage morphogenesis. Dev Biol. 1996;179:347–359. doi: 10.1006/dbio.1996.0266. [DOI] [PubMed] [Google Scholar]
- 59.Crowe R, Henrique D, Ish-Horowicz D, Niswander L. A new role for Notch and Delta in cell fate decisions: patterning the feather array. Development. 1998;125:767–775. doi: 10.1242/dev.125.4.767. [DOI] [PubMed] [Google Scholar]
- 60•.Mandler M, Neubuser A. FGF signaling is required for initiation of feather placode development. Development. 2004;131:3333–3343. doi: 10.1242/dev.01203. A nicely executed study that evaluates the roles of FGF members thoughtfully. [DOI] [PubMed] [Google Scholar]
- 61.Tao H, Yoshimoto Y, Yoshioka H, Nohno T, Noji S, Ohuchi H. FGF10 is a mesenchymally derived stimulator for epidermal development in the chick embryonic skin. Mech Dev. 2002;116:39–49. doi: 10.1016/s0925-4773(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 62•.McKinnell IW, Makarenkova H, de Curtis I, Turmaine M, Patel K. EphA4, RhoB and the molecular development of feather buds are maintained by the integrity of the actin cytoskeleton. Dev Biol. 2004;270:94–105. doi: 10.1016/j.ydbio.2004.02.007. This paper goes deeply into the Eph–Rho-cytoskeleton pathway. [DOI] [PubMed] [Google Scholar]
- 63.Kim JY, Cho SW, Song WC, Lee MJ, Cai J, Ohk SH, Song HK, Degan A, Jung HS. Formation of spacing pattern and morphogenesis of chick feather buds is regulated by cytoskeletal structures. Differentiation. 2005;73:240–248. doi: 10.1111/j.1432-0436.2005.00020.x. [DOI] [PubMed] [Google Scholar]
- 64.Chodankar R, Chang CH, Yue Z, Jiang TX, Suksaweang S, Burrus L, Chuong CM, Widelitz R. Shift of localized growth zones contributes to skin appendage morphogenesis: role of the Wnt/beta-catenin pathway. J Invest Dermatol. 2003;120:20–26. doi: 10.1046/j.1523-1747.2003.12008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Houghton L, Freeman A, Morgan BA. Expression and regulation of Groucho-related genes in the embryonic chicken feather bud. Dev Dyn. 2003;226:587–595. doi: 10.1002/dvdy.10268. [DOI] [PubMed] [Google Scholar]
- 66.Houghton L, Lindon C, Morgan BA. The ectodysplasin pathway in feather tract development. Development. 2005;132:863–872. doi: 10.1242/dev.01651. [DOI] [PubMed] [Google Scholar]
- 67.Rouzankina I, Abate-Shen C, Niswander L. Dlx genes integrate positive and negative signals during feather bud development. Dev Biol. 2004;265:219–233. doi: 10.1016/j.ydbio.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 68.Dohrmann CE, Noramly S, Raftery LA, Morgan BA. Opposing effects on TSC-22 expression by BMP and receptor tyrosine kinase signals in the developing feather tract. Dev Dyn. 2002;223:85–95. doi: 10.1002/dvdy.1236. [DOI] [PubMed] [Google Scholar]
- 69.Obinata A, Akimoto Y, Omoto Y, Hirano H. Expression of Hex homeobox gene during skin development: Increase in epidermal cell proliferation by transfecting the Hex to the dermis. Dev Growth Differ. 2002;44:281–292. doi: 10.1046/j.1440-169x.2002.00642.x. [DOI] [PubMed] [Google Scholar]
- 70.Obinata A, Akimoto Y. Expression of Hex during feather bud development. Int J Dev Biol. 2005;49:885–890. doi: 10.1387/ijdb.052037ao. [DOI] [PubMed] [Google Scholar]
- 71.Song HK, Sawyer RH. Dorsal dermis of the scaleless (sc/sc) embryo directs normal feather pattern formation until day 8 of development. Dev Dyn. 1996;205:82–91. doi: 10.1002/(SICI)1097-0177(199601)205:1<82::AID-AJA8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 72.Dhouailly D. Formation of cutaneous appendages in dermo-epidermal recombinations between reptiles, birds, and mammals. Wilhelm Roux Arch Dev Biol. 1975;177:323–340. doi: 10.1007/BF00848183. [DOI] [PubMed] [Google Scholar]
- 73.Chuong CM, Widelitz RB, Ting-Berreth S, Jiang TX. Early events during avian skin appendage regeneration: dependence on epithelial-mesenchymal interaction and order of molecular reappearance. J Invest Dermatol. 1996;107:639–646. doi: 10.1111/1523-1747.ep12584254. [DOI] [PubMed] [Google Scholar]
- 74.Novel G. Feather pattern stability and reorganization in cultured skin. J Embryol Exp Morphol. 1973;30:605–633. [PubMed] [Google Scholar]
- 75.Chen CW, Jung HS, Jiang TX, Chuong CM. Asymmetric expression of Notch/Delta/Serrate is associated with the anterior-posterior axis of feather buds. Dev Biol. 1997;188:181–187. doi: 10.1006/dbio.1997.8643. [DOI] [PubMed] [Google Scholar]
- 76.Chen CW, Chuong CM. Dynamic expression of lunatic fringe during feather morphogenesis: a switch from medial-lateral to anterior-posterior asymmetry. Mech Dev. 2000;91:351–354. doi: 10.1016/s0925-4773(99)00285-3. [DOI] [PubMed] [Google Scholar]
- 77.Nohno T, Kawakami Y, Ohuchi H, Fujiwara A, Yoshioka H, Noji S. Involvement of the Sonic hedgehog gene in chick feather formation. Biochem Biophys Res Commun. 1995;206:33–39. doi: 10.1006/bbrc.1995.1005. [DOI] [PubMed] [Google Scholar]
- 78.Harris MP, Fallon JF, Prum RO. Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J Exp Zool. 2002;294:160–176. doi: 10.1002/jez.10157. [DOI] [PubMed] [Google Scholar]
- 79.Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Science. 2004;305:1465–1466. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prum RO. Evolution of the morphological innovations of feathers. J Exp Zoolog B Mol Dev Evol. 2005;304:570–579. doi: 10.1002/jez.b.21073. [DOI] [PubMed] [Google Scholar]
- 81.Yu M, Yue Z, Wu P, Wu DY, Mayer JA, Medina M, Widelitz RB, Jiang TX, Chuong CM. The biology of feather follicles. Int J Dev Biol. 2004;48:181–191. doi: 10.1387/ijdb.031776my. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82••.Yue Z, Jiang TX, Widelitz RB, Chuong CM. Mapping stem cell activities in the feather follicle. Nature. 2005;438:1026–1029. doi: 10.1038/nature04222. This study identifies feather follicle stem cells. It also derives topobiological concept from stem cell configuration and feather symmetry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83••.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. A landmark paper characterizing hair bulge stem cells. [DOI] [PubMed] [Google Scholar]
- 84••.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. A landmark paper characterizing hair bulge stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85••.Yue Z, Jiang TX, Widelitz RB, Chuong CM. Wnt3a gradient converts radial to bilateral feather symmetry via topological arrangement of epithelia. Proc Natl Acad Sci USA. 2006;103:951–955. doi: 10.1073/pnas.0506894103. This study shows there is a Wnt 3a A–P gradient in the feather follicle. Flattening the gradient converts feathers from bilateral to radial symmetry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu M, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–312. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87•.Harris MP, Williamson S, Fallon JF, Meinhardt H, Prum RO. Molecular evidence for an activator-inhibitor mechanism in development of embryonic feather branching. Proc Natl Acad Sci USA. 2005;102:11734–11739. doi: 10.1073/pnas.0500781102. An elegant theoretical model for periodic patterning of barb ridges with the application of the reaction-diffusion mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sawyer RH, Knapp LW. Avian skin development and the evolutionary origin of feathers. J Exp Zoolog B Mol Dev Evol. 2003;298:57–72. doi: 10.1002/jez.b.26. [DOI] [PubMed] [Google Scholar]
- 89.Prum RO, Williamson S. Theory of the growth and evolution of feather shape. J Exp Zool. 2001;291:30–57. doi: 10.1002/jez.4. [DOI] [PubMed] [Google Scholar]
- 90.Alibardi L. Cell structure of barb ridges in down feathers and juvenile wing feathers of the developing chick embryo: barb ridge modification in relation to feather evolution. Ann Anat. 2006;188:303–318. doi: 10.1016/j.aanat.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 91.Bartels T. Variations in the morphology, distribution, and arrangement of feathers in domesticated birds. J Exp Zoolog B Mol Dev Evol. 2003;98:91–108. doi: 10.1002/jez.b.28. [DOI] [PubMed] [Google Scholar]
- 92.Sawyer RH, Washington LD, Salvatore BA, Glenn TC, Knapp LW. Origin of archosaurian integumentary appendages: the bristles of the wild turkey beard express feather-type β keratins. J Exp Zoolog B Mol Dev Evol. 2003;297:27–34. doi: 10.1002/jez.b.17. [DOI] [PubMed] [Google Scholar]
- 93.Alibardi L. Fine structure of juvenile feathers of the zebrafinch in relation to the evolution and diversification of pennaceous feathers. J Submicrosc Cytol Pathol. 2005;37:323–343. [PubMed] [Google Scholar]
- 94.Alibardi L, Sawyer RH. Cell structure of developing down feathers in the zebrafinch with emphasis on barb ridge morphogenesis. J Anat. 2006;208:621–642. doi: 10.1111/j.1469-7580.2006.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang CH, Yu M, Wu P, Jiang TX, Yu HS, Widelitz RB, Chuong CM. Sculpting skin appendages out of epidermal layers via temporally and spatially regulated apoptotic events. J Invest Dermatol. 2004;122:1348–1355. doi: 10.1111/j.0022-202X.2004.22611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mayer JA, Chuong CM, Widelitz R. Rooster feathering, androgenic alopecia, and hormone-dependent tumor growth: what is in common? Differentiation. 2004;72:474–488. doi: 10.1111/j.1432-0436.2004.07209003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97•.Forgacs G, Newman SA. Biological physics of the developing embryo. Cambridge: Cambridge University Press; 2005. A fresh perspective on how we look at embryo development. [Google Scholar]
- 98.Newman SA, Forgacs G, Muller GB. Before programs: the physical origination of multicellular forms. Int J Dev Biol. 2006;50:289–299. doi: 10.1387/ijdb.052049sn. [DOI] [PubMed] [Google Scholar]