Dietary intake of minerals and risk of esophageal squamous cell carcinoma: results from the Golestan Cohort Study (original) (raw)
Abstract
Background: Dietary factors have been hypothesized to affect the risk of esophageal cancer via different mechanisms, but the intake of minerals is understudied and the evidence is conflicting.
Objective: The objective was to evaluate the associations of dietary intake of minerals with risk of esophageal squamous cell carcinoma (ESCC).
Design: We used data from the Golestan Cohort Study, which was launched in a high-risk region for esophageal cancer in Iran. Participants were enrolled in 2004–2008 and were followed to 2014. Intakes of minerals were assessed with a validated food-frequency questionnaire. A Cox proportional hazards model was used to estimate HRs and 95% CIs of ESCC for dietary intakes of selected minerals.
Results: We identified 201 ESCC cases among 47,405 subjects. Calcium intake was significantly inversely associated with the risk of ESCC (HR per 100-mg/d increase: 0.88; 95% CI: 0.81, 0.96; P = 0.005; quartile 4 vs. quartile 1 HR: 0.49; 95% CI: 0.29, 0.82; _P_-trend = 0.013). Zinc intake was also inversely associated with ESCC, but the quartile association did not reach significance (HR per 1-mg/d increase: 0.87; 95% CI: 0.77, 0.98; P = 0.027; quartile 4 vs. quartile 1 HR: 0.56; 95% CI: 0.28, 1.12; _P_-trend = 0.097). The relations between dietary intakes of selenium, magnesium, and copper and risk of ESCC were nonlinear (_P_-nonlinear trend = 0.001, 0.016, and 0.029, respectively). There was no relation between dietary intake of manganese and the risk of ESCC.
Conclusion: The results suggest that higher intakes of calcium and zinc are associated with a lower risk of ESCC in a high-risk region of Iran.
Keywords: esophageal cancer, minerals, calcium, zinc, selenium, magnesium, copper, manganese
INTRODUCTION
Esophageal cancer (EC)12 is the eighth most common cause of cancer and the sixth most common cause of cancer death in the world (1). EC still has a 5-y survival of <20% in the United States (1, 2) and 3.3% in Iran (3). Northeastern Iran is one of the high-incidence areas for EC in the world, and the rate of EC has been reported to be >100 per 100,000 population/y, whereas in the low-incidence areas of the world, the incidence rate decreased to <10 per 100,000/y (4). More than 90% of EC cases diagnosed in northeastern Iran represent esophageal squamous cell carcinoma (ESCC) (4), which is also the most prevalent type of EC in the world (5).
Opium use, hot drinks, tobacco smoking, low socioeconomic status, polycyclic aromatic hydrocarbons, and nutritional deficiency are possible etiologic factors. Among nutritional factors, low intakes of fruit and vegetables have been associated with higher risk of this cancer (6, 7). When specific minerals were investigated, the protective effects of zinc and selenium against EC were in animal studies (8, 9). However, there are few studies on the relation of mineral intakes and ESCC in humans. A case-control study in Iran identified that low intakes of calcium, zinc, and selenium are associated with increased risk of EC (10). On the other hand, a null effect of zinc and calcium was reported in a case-control study in the United States (11). Calcium has been associated with a decreased risk of ESCC in men in a cohort study in the United States (12). Despite poor survival rates and a relatively high incidence in certain regions of the world, the impacts of mineral intake on the etiology of ESCC are unknown.
The primary aim of this study was therefore to evaluate the association between calcium, zinc, and selenium and the risk of ESCC in a cohort study in northeastern Iran. We also investigated the intakes of other minerals (magnesium, copper, and manganese), because they were suspected ESCC risk factors in previous studies (13–15).
METHODS
Study population
The Golestan Cohort Study was performed in the eastern portion of the Caspian Sea littoral in northeastern Iran to investigate the causes of ESCC. Details of the study methods were published previously (16). In brief, ∼50,000 healthy persons aged 40–75 y were recruited in 2004–2008. For this analysis, participants who had extreme daily energy intake (n = 796), defined as >99th percentile or less than the first percentile, or had ≥30 missing responses on the food-frequency questionnaire (FFQ; n = 933) and participants with other cancer (except for nonmelanoma skin cancer) diagnosed at baseline or during follow-up (n = 966) were excluded (17).
An ESCC risk factor questionnaire, an FFQ, and written informed consent were completed at baseline. Physical activity at work was used to calculate a physical activity score, because this included most of the physical activities that individuals performed (18). Multiple correspondence analysis was used to calculate a wealth score on the basis of household appliances, vehicles, and other variables associated with wealth (19). Marital status was categorized as single or married. Divorced or widowed individuals were uncommon, so they were considered in a single category. Height and weight were measured before FFQ completion. The study protocol was approved by the ethical review committee of the Digestive Diseases Research Institute.
Dietary intake
To assess dietary intake, we used a 116-item semiquantitative FFQ at baseline. The FFQ consisted of a list of foods with standard serving sizes, and participants were asked to report their frequency of consumption of each food item during the previous year on a daily, weekly, or monthly basis. For standardization purposes, portion sizes were converted from household measurements to grams. Nutrient intakes were calculated by multiplying the frequency of each food item by the nutrient content of each food (20). The FFQ was previously found to be valid and reliable in this population (21). Mineral intakes were adjusted for energy intake by using 2 methods: the residual approach and the multivariate nutrient density model (22). Because supplement use is not common in this region, only minerals from food sources were reported.
Ascertainment of endpoints
ESCC was the endpoint in our analyses. All participants were contacted by telephone once every year. Case review questionnaires were completed during each phone call, and any occurrence of disease or hospital admissions that occurred since the previous follow-up contact were recorded. After a report of cancer or upper gastrointestinal endoscopy, a team visited the subject’s home and the medical centers in which any major diagnostic was performed. The team collected all clinical reports, pathology reports, and hospital records and any tumor samples that were available. Reports of upper gastrointestinal cancer were reviewed and verified by an Endpoint Review Committee composed of experts from the Digestive Diseases Research Institute of Tehran University. Any reported death was followed by a physician visit, and death certificates were evaluated and recorded. During the period of analysis, only 369 participants were lost to follow-up. For this study, person-years were calculated as the time from the completion of the FFQ to one of the following events: 1) ESCC diagnosis, 2) emigration from Golestan, 3) death, or 4) the end of follow-up for this analysis (1 January 2014).
Statistical analysis
HRs and 95% CIs were estimated by using Cox proportional hazards regression models. The proportional hazards assumption was verified by using Aalen plots and the Schoenfeld residuals test. Age was used as the underlying time metric. All suspected confounders were investigated, including age, sex, place of residence (urban or rural), smoking, wealth score, ethnicity (non-Turkmen or Turkmen), opiate use (never or ever), BMI, education (illiterate or formal education), marital status, physical activity score, and daily intake of fruit, vegetables, and calories. Because only a few individuals reported drinking alcohol, the model was not adjusted for alcohol drinking. Primary studies have shown that alcohol is not a risk factor in this region (23).
Multivariate HRs were reported within quartiles, with the lowest quartile being used as the reference category. For linear trend tests, the median value of each quartile was used. Linear continuous change in intake of minerals was also evaluated. HRs for the continuous scale were reported for each 100-mg/d increase in calcium and magnesium intake, each 1-mg/d increase in zinc and manganese intake, each 0.1-mg/d increase in copper intake, and each 100-μg/d increase in selenium intake (according to the range of intakes of each mineral in the population). In addition, we used restricted cubic spline (RCS) functions to plot and test the association of each mineral and ESCC risk. In the RCS functions, we used 5 knots and set the median of the first quartile of intake as the reference point for each mineral. Overall and nonlinear associations were tested by using 4 and 3 df tests, respectively (24).
We conducted a lag analysis by excluding the first 2 y of follow-up. We also conducted stratified analyses by age, sex, BMI, ethnicity, and residence area. We conducted further analyses to adjust for dietary factors that may affect mineral bioavailability: intakes of dietary fiber, calcium, selenium, zinc, iron, copper, histidine, and saturated fat. Statistical analyses were carried out by using STATA software (version 12; StataCorp). Reported P values are 2-sided.
RESULTS
In total, 47,405 subjects were analyzed (19,969 men and 27,436 women). The mean ± SD age of the participants at enrollment was 51.9 ± 8.8 y. During a median follow-up time of 7.2 y (IQR: 6.4–8 y), 201 ESCC cases were diagnosed.
Baseline characteristics are shown according to quartile of calcium intake (Table 1). Compared with individuals in the lowest quartile, participants in the highest quartile of calcium intake were more likely to consume more total calories but were otherwise similar in baseline characteristics (Table 1). Calcium was the only mineral with a median intake below the US Recommended Dietary Allowance in all quartiles (<1000–1200 mg/d).
TABLE 1.
Baseline characteristics of subjects in the Golestan Cohort Study, by quartile of dietary calcium intake1
| Quartile | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | P | |
| Calcium, mg/d | 409 ± 842 | 594 ± 42 | 748 ± 51 | 1048 ± 200 | |
| Male sex, n (%) | 5043 (42) | 4941 (41) | 4988 (42) | 4993 (42) | 0.69 |
| Age, y | 52 ± 8.8 | 51.8 ± 8.8 | 51.8 ± 8.7 | 52 ± 8.9 | 0.33 |
| Energy, kcal | 1632 ± 376 | 2040 ± 359 | 2283 ± 402 | 2649 ± 505 | <0.001 |
| Vegetables, g/d | 113 (77–159)3 | 112 (76–158) | 113 (76–158) | 113 (77–159) | 0.78 |
| Fruit, g/d | 110 (64–181) | 112 (65–183) | 111 (65–182) | 110 (64–181) | 0.42 |
| BMI, kg/m2 | 26.7 ± 5.4 | 26.7 ± 5.4 | 26.6 ± 5.4 | 26.6 ± 5.4 | 0.97 |
| Smoker, n (%) | 2023 (17.0) | 2000 (16.8) | 2030 (17.1) | 2031 (17.1) | 0.94 |
| Wealth score, n (%) | 0.78 | ||||
| Low | 4438 (37) | 4462 (38) | 4483 (38) | 4471 (38) | |
| Medium | 3418 (29) | 3314 (28) | 3319 (28) | 3379 (28) | |
| High | 4013 (34) | 4068 (34) | 4040 (34) | 3994 (34) | |
| Physical activity score | 1.4 ± 0.6 | 1.4 ± 0.6 | 1.5 ± 0.7 | 1.4 ± 0.6 | 0.12 |
| Rural place of residence, n (%) | 9487 (79.9) | 9488 (80) | 9489 (80) | 9450 (79.8) | 0.92 |
| Turkmen ethnicity, n (%) | 8693 (73) | 8818 (74) | 8783 (74) | 8807 (74) | 0.12 |
| Opium user, n (%) | 1988 (16.7) | 1957 (16.5) | 1999 (16.8) | 1990 (16.8) | 0.89 |
| Marital status, single, n (%) | 1424 (11) | 1393 (12) | 1450 (11) | 1420 (12) | 0.72 |
| No formal education, n (%) | 8253 (69) | 8267 (69) | 8324 (70) | 8316 (70) | 0.54 |
Dietary calcium intake was inversely associated with ESCC risk in the continuous and quartile models (Tables 2 and 3). Further adjustment for intakes of dietary fiber, selenium, zinc, and saturated fat did not significantly change the HRs (data not shown).
TABLE 2.
HRs (95% CIs) of esophageal squamous cell carcinoma, by quartile of mineral intake1
| Quartile | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | _P_-trend | |
| Calcium | |||||
| Intake, mg/d | 428 (100–520)2 | 594 (521–666) | 745 (667–845) | 991 (846–2700) | |
| Person-years | 84,269 | 84,827 | 84,315 | 84,551 | |
| Cases, n | 70 | 45 | 43 | 43 | |
| HR (95% CI)3 | 1 | 0.64 (0.44, 0.93) | 0.62 (0.42, 0.91) | 0.60 (0.41, 0.89) | 0.01 |
| HR (95% CI)4 | 1 | 0.58 (0.38, 0.86) | 0.55 (0.35, 0.86) | 0.49 (0.29, 0.82) | 0.01 |
| Zinc | |||||
| Intake, mg/d | 6.8 (2.46–8) | 8.9 (8.1–9.6) | 10.5 (9.7–11.4) | 12.9 (11.5–33.2) | |
| Person-years | 84,250 | 84,447 | 84,175 | 85,067 | |
| Cases, n | 67 | 42 | 44 | 48 | |
| HR (95% CI)3 | 1 | 0.64 (0.44, 0.95) | 0.66 (0.45, 0.96) | 0.71 (0.49, 1.03) | 0.07 |
| HR (95% CI)4 | 1 | 0.60 (0.39, 0.93) | 0.54 (0.32, 0.91) | 0.56 (0.28, 1.12) | 0.10 |
| Selenium | |||||
| Intake, μg/d | 93 (20–116) | 133 (117–148) | 162 (149–175) | 197 (176–389) | |
| Person-years | 84,450 | 84,189 | 84,236 | 85,064 | |
| Cases, n | 64 | 42 | 36 | 59 | |
| HR (95% CI)3 | 1 | 0.66 (0.47, 0.98) | 0.56 (0.35, 0.85) | 0.92 (0.64, 1.31) | 0.45 |
| HR (95% CI)4 | 1 | 0.73 (0.47, 1.11) | 0.61 (0.37, 0.99) | 1.12 (0.64, 1.94) | 0.97 |
| Magnesium | |||||
| Intake, mg/d | 295 (101–359) | 406 (360–450) | 486 (451–525) | 583 (526–1084) | |
| Person-years | 84,317 | 84,587 | 84,236 | 84,799 | |
| Cases, n | 60 | 49 | 31 | 61 | |
| HR (95% CI)3 | 1 | 0.81 (0.55, 1.18) | 0.51 (0.33, 0.79) | 1.00 (0.70, 2.43) | 0.59 |
| HR (95% CI)4 | 1 | 0.90 (0.59, 1.38) | 0.58 (0.34, 0.98) | 1.38 (0.75, 2.54) | 0.70 |
| Copper | |||||
| Intake, mg/d | 1.1 (0.4–1.2) | 1.4 (1.3–1.5) | 1.7 (1.6–1.8) | 2.1 (1.9–6.5) | |
| Person-years | 83,353 | 82,784 | 85,468 | 86,221 | |
| Cases, n | 60 | 51 | 41 | 49 | |
| HR (95% CI)3 | 1 | 0.86 (0.52, 1.25) | 0.66 (0.36, 0.99) | 0.78 (0.34, 1.13) | 0.13 |
| HR (95% CI)4 | 1 | 0.79 (0.59, 1.21) | 0.60 (0.44, 1.01) | 0.67 (0.53, 1.30) | 0.20 |
| Manganese | |||||
| Intake, mg/d | 6 (1.4–7) | 8 (7.1–9) | 10 (9.1–10.8) | 12 (10.9–32.6) | |
| Person-years | 83,983 | 84,555 | 84,530 | 84,870 | |
| Cases, n | 53 | 43 | 45 | 60 | |
| HR (95% CI)3 | 1 | 0.81 (0.54, 1.21) | 0.83 (0.56, 1.24) | 1.11 (0.77, 2.62) | 0.52 |
| HR (95% CI)4 | 1 | 0.88 (0.57, 1.35) | 1.00 (0.63, 1.57) | 1.49 (0.91, 2.45) | 0.09 |
TABLE 3.
HRs (95% CIs) of esophageal squamous cell carcinoma for continuous mineral intake1
| HR (95% CI) | P | |
|---|---|---|
| Calcium, per 100-mg/d increase | 0.88 (0.81, 0.96) | 0.005 |
| Zinc, per 1-mg/d increase | 0.87 (0.77, 0.98) | 0.027 |
| Selenium, per 10-μg/d increase | 0.98 (0.93, 1.00) | 0.499 |
| Magnesium, per 100-mg/d increase | 0.98 (0.79, 1.21) | 0.901 |
| Copper, per 0.1-mg/d increase | 0.96 (0.90, 1.01) | 0.189 |
| Manganese, per 1-mg/d increase | 1.05 (0.98, 1.11) | 0.150 |
An inverse association was also observed for zinc intake and ESCC risk. On the linear continuous scale, the association was significantly inverse (Table 3), but in the quartile model the HR for quartile 4 vs. quartile 1 intake and the P value for linear trend were not significant (Table 2). However, the magnitude of the HR was similar in quartile 3 vs. quartile 1, which shows the protective effect of zinc. The inclusion of factors in the model that may affect zinc bioavailability, such as fiber, iron, copper, and histidine, did not affect HRs (data not shown).
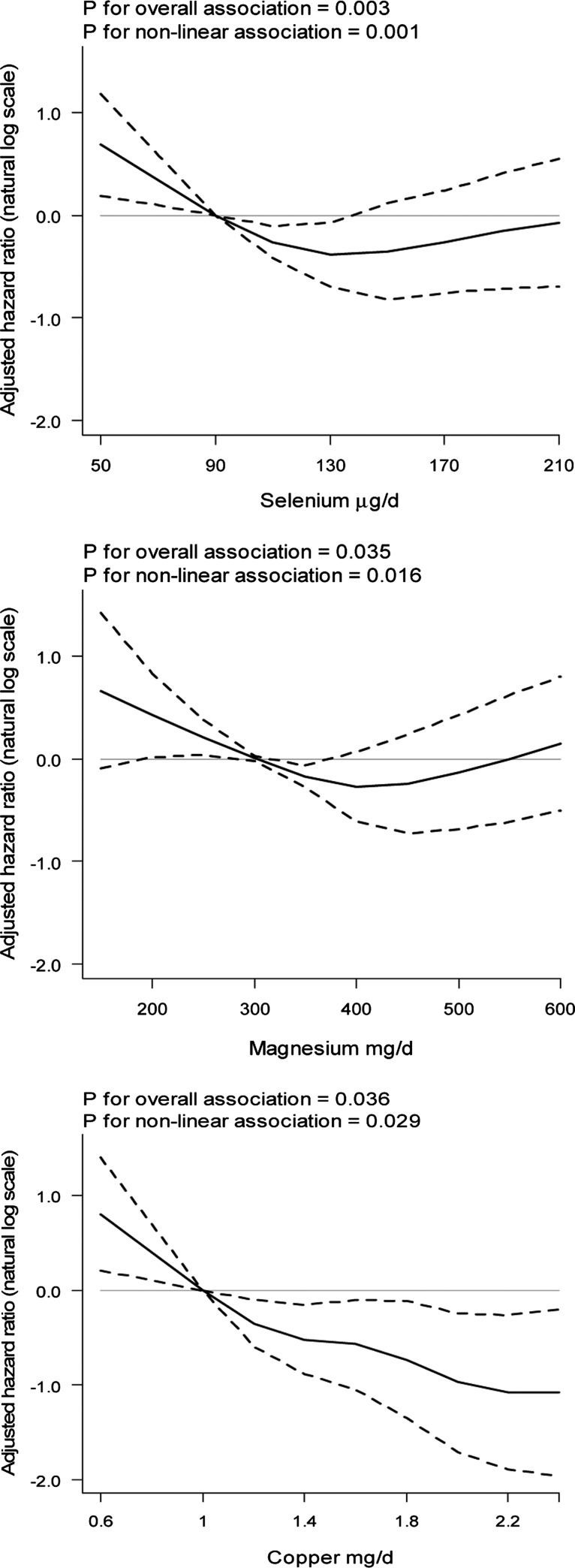
No associations were found in the continuous and quartile models between intakes of other minerals and risk of ESCC (Tables 2 and 3). However, the RCS models showed that there were significant overall and nonlinear associations between intakes of selenium, magnesium, and copper and ESCC risk. The RCS plots show that the associations between selenium and magnesium were U-shaped (Figure 1).
FIGURE 1.
Associations between intakes of selenium, magnesium, and copper and risk of esophageal squamous cell carcinoma. Curved solid lines represent adjusted HRs and dashed lines indicate their 95% CIs based on restricted cubic splines for amounts of intake for each mineral (n = 47,405).
The exclusion of cancers diagnosed in the first 2 y of follow-up (n = 55) did not affect our results (Supplemental Table 1). On stratification by age, sex, BMI, ethnicity, and residence area, we did not find any consistent modifications of our findings. The use of the residual method for adjusting intakes of minerals for energy did not change the results obtained by using the multivariate nutrient density model.
DISCUSSION
In this study, we found a significant inverse association between calcium and zinc intake and risk of ESCC, and U-shaped associations were also suggested for selenium and magnesium intakes and the risk of developing ESCC. No previous studies have suggested these U-shaped associations between mineral intake and risk of ESCC.
Calcium
We found a significant linear inverse association between calcium intake and risk of ESCC, which is consistent with 2 case-control studies in Iran and Germany (10, 13). However, some studies reported no association (11, 25, 26).Our finding is in agreement with 2 other prospective studies, which suggested a protective effect of calcium against ESCC (RR: 0.67; 95% CI: 0.38, 1.17) (27) and esophageal adenocarcinomas (EACs) in men (quintile 5 vs. quintile 1 HR: 0.66; 95% CI: 0.49, 0.90) (12).
A second analysis from the second study showed that the use of calcium supplements was associated with a higher risk of EAC (HR: 1.27; 95% CI: 1.06, 1.52), but not of ESCC (HR: 0.92; 95% CI: 0.67, 1.25) (28). This association may have been confounded by higher calcium intakes in the form of antacids to treat gastroesophageal reflux symptoms.
Dairy foods, as a main source of calcium in our population, are not fortified with vitamin D in Iran, so these calcium intakes are not correlated with vitamin D status, which is an important concern in other studies examining the effect of calcium intake sources (12). Our findings are also consistent with previous animal and in vitro studies. In 1 study in mice, a calcium-enriched diet inhibited tumor formation, and the authors also showed that calcium inhibited cell growth, invasion, and migration in a human colon cancer cell culture system (29). Calcium may suppress the cell cycle, promote apoptosis, and reduce the formation of tumors (30, 31).
Zinc
We also found an inverse association between zinc intake and risk of ESCC, which is in agreement with previous case-control studies in Iran and China (10, 25), although Mayne et al. (11) reported no association in the United States. The other 2 prospective studies reported that zinc intake (17) and zinc concentrations in esophageal tissue biopsies were inversely associated with risk of EC (quintile 5 vs. quintile 1 HR: 0.21; 95% CI: 0.065, 0.68) (32), which is in agreement with the findings of our study. A recent systematic review found that the evidence is not still strong enough to conclude a protective role of zinc in EC (33). However, there is biological plausibility for this inverse association. Zinc is necessary for proper immune function and for transcription factors that control cell proliferation (34), apoptosis, and signaling pathways (35).
Selenium
We found a suggestion of a U-shaped association between intake of selenium and risk of ESCC. Two case-control studies in Iran and China reported that a low intake of selenium is related to increased risk of EC (10, 25); however, the results from prospective studies in Finland and China, which investigated plasma selenium, were inconsistent (36, 37). In a trial in the same cohort in China, ESCC deaths were not significantly different between those who did and did not receive a combination of selenium, vitamin E, and β-carotene (38).
It could be concluded that different intake profiles may contribute to the discrepancies in the results of different studies. For example, a linear protective association was reported between selenium intake and ESCC risk in a study in a population with selenium deficiency (25), which is consistent with our findings in the descending part of the U-shaped curve. Furthermore, in Ireland, which has sufficient selenium intake, this mineral was not related to EAC, which is in agreement with our results (39). An ecologic case-control study showed that selenium deficiency is not common and is thus not a major risk factor for ESCC in northeastern Iran (40), which was confirmed by our results. We hypothesize that the risk of cancer may increase in a population with excessive intakes of selenium (the ascending part of the curve). And a recent ecologic study in Golestan Province showed that the selenium concentration of drinking water increased in villages with a gradient from low to high risk of EC (41). This U-shaped dose-response relation was also suggested in animal studies. In dogs, selenium reduced DNA damage in prostate tissue, but excessive intake of selenium may cause oxidative damage and increase DNA damage (42–44).
Magnesium, copper, and manganese
We found a suggestion of U-shaped associations between the intake of magnesium and risk of ESCC. There are conflicting data on the role of magnesium in the development of EC in case-control studies (13, 45). We could not find any previous prospective study that evaluated the relation between the intake of magnesium and risk of ESCC.
For copper, although there was a nonlinear association with ESCC risk, no clear trend of intake in relation to ESCC was found. Case-control studies showed inconsistent results (14, 46). In a cohort study in China, copper in tissue sections of the esophagus was inversely but not significantly associated with risk of ESCC (32). We hypothesize that the association between copper intake and ESCC risk depends on the overall copper intake in that population.
This study is unique in its evaluation of the possible association between manganese intake and risk of ESCC. We found no association between manganese intake and ESCC; however, the importance of this mineral in the risk of ESCC will require further study.
In this study, calcium was the only mineral with a median intake less than the US Recommended Dietary Allowance in all quartiles. The stronger inverse association between calcium and ESCC than between the other minerals and ESCC might be related to the overall low calcium intake in our population.
General poor nutrition, such as low intakes of some food items, may contribute to the susceptibility to ESCC risk, rather than intakes of a specific nutrient. The earliest studies also concluded that the increased susceptibility in this region could be due to poor nutritional status, with low intakes of fruit and vegetables (47).
A major advantage of the current study is its prospective design. In addition, maximum insight into the diet and disease associations can be achieved by examining the data with several methods of energy adjustment. We used 2 methods for energy adjustment in this study, because the residual approach generally has more power to detect associations when the exposure variable is categorized (22). This study also has some limitations. The moderate sample size or short follow-up period may have precluded the detection of a main effect with other minerals. Because there were correlations between intakes of minerals, the associations we found may be due to residual confounding by other nutritional and nonnutritional factors. The use of an FFQ is a suboptimal method of assessing nutrient intakes (48). In addition, we used a single baseline FFQ for assessing dietary intake and repeated measures may have more accurately estimated intake.
In conclusion, we found that increased calcium and zinc intake was associated with a lower risk of ESCC. The relation between the dietary intake of selenium and magnesium and risk of ESCC was nonlinear, and probably U-shaped.
Supplementary Material
Supplemental data
Acknowledgments
The authors’ responsibilities were as follows—CCA, PB, SMD, PJB, PP, FK, and RM: designed the research; HP, AE, and RM: conducted the research; MH and MS: analyzed data; MH and AH: wrote the manuscript; CCA, PB, SMD, PJB, PP, AE, and FK: critically revised the manuscript for important intellectual content; and AH and RM: had primary responsibility for final content. All authors read and approved the final manuscript. No conflicts of interest were declared.
Footnotes
12
Abbreviations used: EAC, esophageal adenocarcinoma; EC, esophageal cancer; ESCC, esophageal squamous cell carcinoma; FFQ, food-frequency questionnaire; RCS, restricted cubic spline.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 3.Aghcheli K, Marjani HA, Nasrollahzadeh D, Islami F, Shakeri R, Sotoudeh M, Abedi-Ardekani B, Ghavamnasiri MR, Razaei E, Khalilipour E, et al. Prognostic factors for esophageal squamous cell carcinoma—a population-based study in Golestan Province, Iran, a high incidence area. PLoS ONE 2011;6:e22152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Islami F, Kamangar F, Nasrollahzadeh D, Moller H, Boffetta P, Malekzadeh R. Oesophageal cancer in Golestan Province, a high-incidence area in northern Iran—a review. Eur J Cancer 2009;45(18):3156–65. [DOI] [PubMed] [Google Scholar]
- 5.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut . 2015;64(3):381–7. [DOI] [PubMed]
- 6.Kamangar F, Chow W-H. Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am 2009;38:27–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Cancer Research Fund/American Institute for Cancer Research.Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington (DC): AICR, 2007:11–2.
- 8.Fong LY, Zhang L, Jiang Y, Farber JL. Dietary zinc modulation of COX-2 expression and lingual and esophageal carcinogenesis in rats. J Natl Cancer Inst 2005;97:40–50. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Fang J, Jia X, Han C, Chen X, Yang CS, Li N. Chemopreventive effects of early-stage and late-stage supplementation of vitamin E and selenium on esophageal carcinogenesis in rats maintained on a low vitamin E/selenium diet. Carcinogenesis 2011;32(3):381–8. [DOI] [PMC free article] [PubMed]
- 10.Jessri M, Rashidkhani B, Hajizadeh B, Gotay C. Macronutrients, vitamins and minerals intake and risk of esophageal squamous cell carcinoma: a case-control study in Iran. Nutr J 2011;10:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TL, Farrow DC, Schoenberg JB, Stanford JL, Ahsan H, et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev 2001;10:1055–62. [PubMed] [Google Scholar]
- 12.Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med 2009;169(4):391–401. [DOI] [PMC free article] [PubMed]
- 13.Wolfgarten E, Rosendahl U, Nowroth T, Leers J, Metzger R, Holscher AH, Bollschweiler E. Coincidence of nutritional habits and esophageal cancer in Germany. Onkologie 2001;24:546–51. [DOI] [PubMed] [Google Scholar]
- 14.Dar NA, Mir MM, Salam I, Malik MA, Gulzar GM, Yatoo GN, Ahmad A, Shah A. Association between copper excess, zinc deficiency, and TP53 mutations in esophageal squamous cell carcinoma from Kashmir Valley, India—a high risk area. Nutr Cancer 2008;60:585–91. [DOI] [PubMed] [Google Scholar]
- 15.Hajizadeh B, Jessri M, Akhoondan M, Moasheri SM, Rashidkhani B. Nutrient patterns and risk of esophageal squamous cell carcinoma: a case-control study. Dis Esophagus 2012;25:442–8. [DOI] [PubMed] [Google Scholar]
- 16.Pourshams A, Khademi H, Malekshah AF, Islami F, Nouraei M, Sadjadi AR, Jafari E, Rakhshani N, Salahi R, Semnani S, et al. Cohort profile: the Golestan Cohort Study—a prospective study of oesophageal cancer in northern Iran. Int J Epidemiol 2010;39(1):52–9. [DOI] [PMC free article] [PubMed]
- 17.Lee DH, Anderson KE, Folsom AR, Jacobs DR Jr. Heme iron, zinc and upper digestive tract cancer: the Iowa Women's Health Study. Int J Cancer 2005;117:643–7. [DOI] [PubMed] [Google Scholar]
- 18.Golozar A, Khademi H, Kamangar F, Poutschi H, Islami F, Abnet CC, Freedman ND, Taylor PR, Pharoah P, Boffetta P, et al. Diabetes mellitus and its correlates in an Iranian adult population. PLoS One 2011;6(10):e26725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islami F, Kamangar F, Nasrollahzadeh D, Aghcheli K, Sotoudeh M, Abedi-Ardekani B, Merat S, Nasseri-Moghaddam S, Semnani S, Sepehr A, et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol 2009;38(4):978–88. [DOI] [PMC free article] [PubMed]
- 20.U.S. Department of Agriculture, Agricultural Research Service. 2010 USDA National Nutrient Database for Standard Reference. Release 23. Nutrient Data Laboratory Home Page. [cited 2015 Apr 18]. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl.
- 21.Malekshah AF, Kimiagar M, Saadatian-Elahi M, Pourshams A, Nouraie M, Goglani G, Hoshiarrad A, Sadatsafavi M, Golestan B, Yoonesi A, et al. Validity and reliability of a new food frequency questionnaire compared to 24 h recalls and biochemical measurements: pilot phase of Golestan cohort study of esophageal cancer. Eur J Clin Nutr 2006;60:971–7. [DOI] [PubMed] [Google Scholar]
- 22.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(4 Suppl):1220S–8S; discussion 9S–31S. [DOI] [PubMed]
- 23.Hormozdiari H, Day NE, Aramesh B, Mahboubi E. Dietary factors and esophageal cancer in the Caspian littoral of Iran. Cancer Res 1975;35:3493–8. [PubMed] [Google Scholar]
- 24.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037–57. [DOI] [PubMed] [Google Scholar]
- 25.Lu H, Cai L, Mu LN, Lu QY, Zhao J, Cui Y, Sul JH, Zhou XF, Ding BG, Elashoff RM, et al. Dietary mineral and trace element intake and squamous cell carcinoma of the esophagus in a Chinese population. Nutr Cancer 2006;55:63–70. [DOI] [PubMed] [Google Scholar]
- 26.Brown LM, Swanson CA, Gridley G, Swanson GM, Silverman DT, Greenberg RS, Hayes RB, Schoenberg JB, Pottern LM, Schwartz AG, et al. Dietary factors and the risk of squamous cell esophageal cancer among black and white men in the United States. Cancer Causes Control 1998;9:467–74. [DOI] [PubMed] [Google Scholar]
- 27.Chyou PH, Nomura AM, Stemmermann GN. Diet, alcohol, smoking and cancer of the upper aerodigestive tract: a prospective study among Hawaii Japanese men. Int J Cancer 1995;60:616–21. [DOI] [PubMed] [Google Scholar]
- 28.Dawsey SP, Hollenbeck A, Schatzkin A, Abnet CC. A prospective study of vitamin and mineral supplement use and the risk of upper gastrointestinal cancers. PLoS One 2014;9(2):e88774. [DOI] [PMC free article] [PubMed]
- 29.Ju J, Kwak Y, Hao X, Yang CS. Inhibitory effects of calcium against intestinal cancer in human colon cancer cells and Apc(Min/+) mice. Nutr Res Pract 2012;6:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipkin M. Application of intermediate biomarkers to studies of cancer prevention in the gastrointestinal tract: introduction and perspective. Am J Clin Nutr 1991;54(1 Suppl):188S–92S. [DOI] [PubMed] [Google Scholar]
- 31.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer 2003;3(8):601–14. [DOI] [PubMed]
- 32.Abnet CC, Lai B, Qiao YL, Vogt S, Luo XM, Taylor PR, Dong ZW, Mark SD, Dawsey SM. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst 2005;97(4):301–6. [DOI] [PubMed]
- 33.Hashemian M, Hekmatdoost A, Poustchi H, Mohammadi Nasrabadi F, Abnet CC, Malekzadeh R. Systematic review of zinc biomarkers and esophageal cancer risk. Middle East J Dig Dis 2014;6:177–85. [PMC free article] [PubMed] [Google Scholar]
- 34.Fong LY, Li JX, Farber JL, Magee PN. Cell proliferation and esophageal carcinogenesis in the zinc-deficient rat. Carcinogenesis 1996;17:1841–8. [DOI] [PubMed] [Google Scholar]
- 35.Liu CG, Zhang L, Jiang Y, Chatterjee D, Croce CM, Huebner K, Fong LY. Modulation of gene expression in precancerous rat esophagus by dietary zinc deficit and replenishment. Cancer Res 2005;65:7790–9. [DOI] [PubMed] [Google Scholar]
- 36.Knekt P, Aromaa A, Maatela J, Alfthan G, Aaran RK, Nikkari T, Hakama M, Hakulinen T, Teppo L. Serum micronutrients and risk of cancers of low incidence in Finland. Am J Epidemiol 1991;134:356–61. [DOI] [PubMed] [Google Scholar]
- 37.Mark SD, Qiao YL, Dawsey SM, Wu YP, Katki H, Gunter EW, Fraumeni JF Jr, Blot WJ, Dong ZW, Taylor PR. Prospective study of serum selenium levels and incident esophageal and gastric cancers. J Natl Cancer Inst 2000;92:1753–63. [DOI] [PubMed] [Google Scholar]
- 38.Qiao YL, Dawsey SM, Kamangar F, Fan JH, Abnet CC, Sun XD, Johnson LL, Gail MH, Dong ZW, Yu B, et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst 2009;101:507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy SJ, Anderson LA, Ferguson HR, Johnston BT, Watson PR, McGuigan J, Comber H, Reynolds JV, Murray LJ, Cantwell MM. Dietary antioxidant and mineral intake in humans is associated with reduced risk of esophageal adenocarcinoma but not reflux esophagitis or Barrett's esophagus. J Nutr 2010;140(10):1757–63. [DOI] [PubMed]
- 40.Nouarie M, Pourshams A, Kamangar F, Sotoudeh M, Derakhshan MH, Akbari MR, Fakheri H, Zahedi MJ, Caldwell K, Abnet CC, et al. Ecologic study of serum selenium and upper gastrointestinal cancers in Iran. World J Gastroenterol 2004;10:2544–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keshavarzi B, Moore F, Najmeddin A, Rahmani F, Malekzadeh A. Quality of drinking water and high incidence rate of esophageal cancer in Golestan province of Iran: a probable link. Environ Geochem Health 2012;34:15–26. [DOI] [PubMed] [Google Scholar]
- 42.Waters DJ, Shen S, Cooley DM, Bostwick DG, Qian J, Combs GF Jr, Glickman LT, Oteham C, Schlittler D, Morris JS. Effects of dietary selenium supplementation on DNA damage and apoptosis in canine prostate. J Natl Cancer Inst 2003;95:237–41. [DOI] [PubMed] [Google Scholar]
- 43.Waters DJ, Shen S, Glickman LT, Cooley DM, Bostwick DG, Qian J, Combs GF Jr, Morris JS. Prostate cancer risk and DNA damage: translational significance of selenium supplementation in a canine model. Carcinogenesis 2005;26(7):1256–62. [DOI] [PubMed]
- 44.Schiar VP, Dos Santos DB, Paixao MW, Nogueira CW, Rocha JB, Zeni G. Human erythrocyte hemolysis induced by selenium and tellurium compounds increased by GSH or glucose: a possible involvement of reactive oxygen species. Chem Biol Interact 2009;177(1):28–33. [DOI] [PubMed]
- 45.Yang CY, Chiu HF, Tsai SS, Wu TN, Chang CC. Magnesium and calcium in drinking water and the risk of death from esophageal cancer. Magnes Res 2002;15:215–22. [PubMed] [Google Scholar]
- 46.Witkowski K, Kozlowski A, Pardela M, Piecuch J, Walichiewicz P. [Level of copper in plasma and tissue of patients with esophageal and large bowel cancer.] Wiad Lek 1993;46:586–8 (in Polish). [PubMed] [Google Scholar]
- 47.Kamangar F, Malekzadeh R, Dawsey SM, Saidi F. Esophageal cancer in northeastern Iran: a review. Arch Iran Med 2007;10(1):70–82. [PubMed]
- 48.Schatzkin A, Kipnis V, Carroll RJ, Midthune D, Subar AF, Bingham S, Schoeller DA, Troiano RP, Freedman LS. A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int J Epidemiol 2003;32:1054–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data