Medulloblastoma Stem Cells (original) (raw)
. Author manuscript; available in PMC: 2015 Jul 21.
Published in final edited form as: J Clin Oncol. 2008 Jun 10;26(17):2821–2827. doi: 10.1200/JCO.2007.15.2264
Abstract
Medulloblastoma and other embronal brain tumors are similar in appearance and differentiation potential to neural stem and progenitor cells. Expression studies performed using human tumor samples, as well as the analysis of murine transgenic models, suggest that both multipotent cerebellar stem cells and lineage-restricted progenitors of the external germinal layer can be transformed into medulloblastoma by genetic alterations. These molecular changes frequently involve constitutive activation of signaling pathways such as Wnt, Hedgehog, and Notch, which play a key role in non-neoplastic neural stem cells. Pharmacologic blockade of the Hedgehog and Notch pathways suppresses the growth of medulloblastoma in culture and in vivo and may prove effective in targeting the small cancer stem-cell subpopulation required for tumor initiation and long-term propagation.
INTRODUCTION
Medulloblastoma are tumors in which the majority of cells have an undifferentiated stem- or progenitor-like appearance. Signs of differentiation into neurons, glia, and other cell types can be detected, however, providing support for the concept that medulloblastoma have properties of multipotent stem cells. Indeed, their primitive appearance led long ago to the suggestion that medulloblastoma arise from CNS stem cells. Recent studies have sought to more precisely define stem-cell subtypes in the cerebellum and their relationship to medulloblastoma. Other types of embryonal brain tumors, most of which fall into the general category of CNS primitive neuroectodermal tumors (PNET) in the current WHO classification scheme,1 also likely derive from stem-cell populations in the brain. In this review, we discuss our growing understanding of the stem-like phenotype of embryonal brain tumors such as medulloblastoma.
We focus on three issues: first, the potential origin of medulloblastoma from cerebellar stem and progenitor cells; second, the signaling pathways required in both neural stem cells and medulloblastoma; and third, the role of so-called cancer stem cells in long-term propagation of medulloblastoma and the susceptibility of these rare cells to therapies targeting developmentally critical signaling pathways, such as Notch. Novel therapies are clearly needed, for although the 5-year survival rate for average-risk children with these aggressive neoplasms now approaches 90%, the outcome in patients with high-risk disease or in those too young for radiation therapy is much worse.2 In addition, current therapies often result in serious long-term neurocognitive difficulties and other adverse effects.2
CEREBELLAR STEM CELLS AND MEDULLOBLASTOMA HISTOGENESIS
Brain tumor classification has historically been based on the morphologic or functional similarity of tumors to non-neoplastic cells types in the brain. Interest in the cellular origin of medulloblastoma therefore extends back to their initial description by the great neurosurgeon Harvey Cushing and his associate Percival Bailey. They named the tumor after the putative medulloblast, an undifferentiated cell on the cerebellar surface that was thought at that time to mature into both neurons and glia.3 Our understanding of how various stem and progenitor populations give rise to the cerebellum has evolved greatly since then, but it remains unclear what percentage of medulloblastoma and other embryonal brain tumors derive from true stem cells, lineage committed progenitors, or perhaps even fully differentiated cells (Fig. 1). By achieving a better understanding of the cellular origin of these tumors, we hope to also gain insight into what signals they require to survive and grow, permitting more effective targeted therapies to be developed.
Fig. 1.
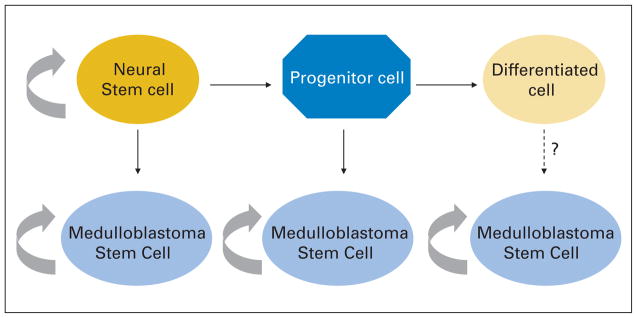
Only true stem cells can self-renew indefinitely, and they give rise to a transiently proliferating group of progenitors that eventually differentiate. Evidence exists for transformation of both neural stem and progenitor cells into medulloblastoma, and it is possible that genetic changes could generate these tumors from a fully differentiated cell as well. It is not clear whether cancer stem cells in medulloblastoma derived from these various origins would have unique properties.
The two primary germinal epithelia of the cerebellum are the deep-seated ventricular zone (VZ) of the posterior medullary vellum and the superficial external germinal layer (EGL), which covers the cerebellar surface (reviewed in Sotelo,4 Goldowitz and Hamre,5 and Chizhikov and Millen6). The midline VZ germinal matrix gives rise to many neuronal and glial cell types in the cerebellum. The EGL, in contrast, is thought to only produce cerebellar granule cells, the most numerous class of neurons in the entire brain. Peak cerebellar growth occurs relatively late compared to the rest of the brain, driven primarily by proliferation of EGL cells. In the mouse, the period of greatest growth occurs during the 2 weeks after birth, while in humans, the analogous proliferative peak occurs in utero during the third trimester, although EGL remnants can persist for up to a year after birth.7
In addition to these two relatively well characterized populations, other cerebellar stem cells have been described that could be a source of medulloblastoma. Lee et al8 recently isolated CD133-positive stem cells from the postnatal cerebellum, concentrated in the white matter, which have the ability to self-renew and display multilineage differentiation potential both in vitro and in vivo. Two other groups have identified novel stem/progenitor-cell populations in the rhombic lip that give rise to nuclei of the deep cerebellar white matter.9,10 Finally, Bergmann glia, like other radial glia, have some properties of stem cells.11
What evidence directly connects these stem- and progenitor-cell populations to medulloblastoma? In humans, the data are merely correlative. Expression of Calbindin-D, a ventricular zone marker not found in the EGL, is present in less than half of medulloblastomas, mainly those of the classic (non-nodular) subtype.12 In contrast, nodular/desmoplastic medulloblastoma were found to commonly express the EGL marker p75, whereas classic lesions did not, giving rise to a dual origin hypothesis, which proposes that tumors arise from either the VZ or EGL.13 Global gene expression profiling also suggests a kinship between medulloblastoma and cerebellar stem/precursor cells. Two groups have shown that at least a subset of human tumors display expression profiles similar to murine cerebellar EGL.14,15 In addition, as discussed below, the evidence for origin of medulloblastoma from the EGL is strong in murine models, with aberrant activation of Hedgehog (Hh) and other pathways.
One might ask how committed neuronal progenitors such as granule cell precursors of the EGL can give rise to multipotent medulloblastoma that also express glial markers. Interestingly, when unipotent EGL precursors are immortalized by SV40 Large T antigen, or exposed to sonic hedgehog and bone morphogenetic proteins in culture, they gain the ability to differentiate into multiple cells types, including glia, perhaps explaining how multipotent tumors can derive from EGL.16,17 As perhaps the most extreme example of multipotency in an EGL-derived cell, it has been reported that medulloblastoma cells from Patched (PTCH) heterozygous mice can direct development of mouse embryos after nuclear transfer.18 Such studies, however, call into question the strength of conclusions one can draw from expression of molecular markers when inferring histogenesis, as oncogenic transformation can clearly alter the molecular signature and cellular phenotype of restricted progenitors, causing them to take on a multipotent stem-cell phenotype.
A more radical concept is that fully differentiated cells give rise to medulloblastoma, acquiring a primitive, multipotent phenotype in the process. No data supporting this hypothesis has been shown for embryonal brain tumors. A recent report, however, suggests that embryonal tumors in the eye can form in just this fashion in a mouse model. Ajioka et al19 have shown that transgenic Rb(−/−);p107(±); p130(−/−) mice develop retinoblastoma, with the earliest lesions arising from differentiated horizontal interneurons that re-enter the cell cycle and clonally expand. It therefore seems premature to rule out the possibility that some medulloblastoma might be formed by fully differentiated cells.
SIGNALING PATHWAYS IMPORTANT IN BOTH STEM CELLS AND MEDULLOBLASTOMA
It is clear that many of the pathways required in neural stem cells are also aberrantly activated in medulloblastoma. Indeed, this tumor has more mutations and other genetic changes in developmental genes than in canonical regulators of the cell cycle such as p53.1 Examination of the signaling pathways dysregulated in various medulloblastoma subtypes has also allowed further speculation about the relationship between tumor-initiating stem- or progenitor-cell populations and early molecular events in oncogenesis (Fig. 2). It is hoped that a better understanding of the molecular link between cerebellar development and tumor formation will lead to new therapies specifically targeting medulloblastoma cells. Listed on the next page are some of the better-understood signaling pathways active in both neural stem cells and in medulloblastoma.
Fig. 2.
Mutations activating Wnt have been identified primarily in the classic medulloblastoma subtype, which is thought to derive primarily from stem and/or progenitor cells of the ventricular zone. The external germinal layer (EGL), in contrast, is thought to give rise to nodular/desmoplastic medulloblastoma commonly associated with Hedgehog (Hh) pathway activation. Other cerebellar stem and progenitor cells have been described and may also give rise to medulloblastoma (modified from Louis et al1). MB, medulloblastoma; PNET, primitive neuroectodermal tumor.
Hedgehog
The Hh pathway has been shown to regulate neural stem-cell development in many species and plays a particularly important role in the cerebellar EGL. After their migration from the rhombic lip to the EGL, granule cell precursors are stimulated by Purkinje cell-secreted sonic hedgehog ligand to undergo a rapid burst of proliferation.20–22 Hh signaling also seems to be required for maintaining stem cells in the subventricular zone (SVZ) and hippocampus in the postnatal brain.23–25 Mutations in the PTCH receptor, which result in activation of the Hh pathway, have been identified in both sporadic medulloblastoma and in tumors arising in patients with Gorlin’s syndrome, an autosomal dominant disorder in which patients present with various neoplasms including basal cell carcinoma and medulloblastoma.26,27 Approximately 15% of sporadic human medulloblastomas, particularly those of the nodular/desmoplastic subtype, harbor PTCH mutations.28,29 Global gene expression analyses have also associated Hh pathway activation with medulloblastoma-containing nodules.30,31
Murine medulloblastoma arising as a result of constitutive activation of Hh signaling represent the most common transgenic models of this tumor. Approximately 14% of mice heterozygous for PTCH develop medulloblastomas.32,33 Detailed flow cytometric and molecular analyses have confirmed that granule cell precursors from the EGL are transformed into medulloblastoma-like tumors in these animals by loss of PTCH function, which causes constitutive activation of the Hh pathway.34 The Hh pathway genes SMO and SUFU are also occasionally mutated in human medulloblastoma, and have been used to develop murine tumor models.35,36 Preliminary reports from the groups of Wechsler-Reya and Rowitch, published as meeting abstracts, indicate that activation of Hh signaling in multipotent murine stem cells in vivo can also result in medulloblastoma formation. This suggests that not only granule cell precursors are susceptible to transformation by this signaling pathway.37,38
Interestingly, it has been shown that medulloblastoma models in mice initiated by genetic changes to genes outside the Hh pathway also result in upregulation of Hh signaling.15,39,40 Indeed, in one such model based on Cxcr6 inactivation, the tumors that resulted were susceptible to Hh pathway inhibitors in vivo.40 Such results buttress the argument that by understanding the cellular origin and signaling pathways associated with medulloblastoma formation, new therapies may be discovered.
Wnt
A second canonical signaling pathway involved in both sporadic and syndromic medulloblastoma is Wingless/Wnt. The Wnt signaling pathway regulates proliferation of stem and progenitor cells in the fetal VZ, as well as the postnatal SVZ and hippocampus.41–44 Loss of Wnt1 or the key pathway effector beta-catenin causes severe abnormalities in the development of the midbrain and cerebellum, but despite this important role in early cerebellar growth, Wnt does not seem to regulate EGL precursors.45–47 It is therefore interesting the Wnt has been found to be activated predominantly in medulloblastoma of the classic subtype, supporting the concept that these tumors derive from stem/progenitor-cell populations outside the EGL, such as those in the VZ (Fig. 2). Consistent with this concept, constitutive activation of Wnt in VZ cells results in cortical overgrowth and some hyperproliferative masses histologically reminiscent of CNS PNET.41
The first Wnt pathway mutations to be identified in medulloblastoma were in patients with Turcot’s syndrome, an autosomal recessive disease caused by loss of adenomatous polyposis coli function.48 Mutations activating the Wnt pathway member beta-catenin are more common than adenomatous polyposis coli loss in sporadic medulloblastoma and are found in 5% to 10% of cases.49–51 Mutation or reduced expression of Axin2, a negative regulator of Wnt signaling, has also been detected in a small fraction of medulloblastoma.52 Nuclear translocation of beta-catenin, a sign of Wnt pathway activation, is found in up to 25% of medulloblastoma,51,53 and is associated with better clinical outcomes.53 Activation of Wnt is seen predominantly in classic tumors not showing signs of Hh signaling or chromosome 17 aberrations, suggesting they represent a unique subset of medulloblastoma (Fig. 2).31,54 It is not clear, however, if Wnt activity is particularly important in cancer stem cells within medulloblastoma, and therapeutic applications of Wnt blockade in neuro-oncology await the development of drugs that can effectively target this pathway in patients.
Notch
Like the Hh pathway, Notch signaling is active in neural stem and progenitor cells in both the embryonic VZ and rhombic lip,55,56 as well as the postnatal SVZ57 and EGL.58 During CNS development, activation of Notch signaling generally promotes proliferation and/or survival of stem cells while inhibiting their differentiation. The presence in mammals of four Notch receptors, as well as multiple ligands and effectors, adds to the complexity of this pathway, which is notoriously cell and context dependent in its effects. For example, Notch2 seems to promote proliferation of cerebellar granule neuron progenitors, whereas Notch1 expression is associated with cell cycle exit and differentiation in these cells.58 A similar paradigm may hold in medulloblastoma, as we found that Notch2 was overexpressed in human primary tumors, and promotes tumor proliferation, whereas Notch1 is largely undetectable and suppresses the growth of tumor cells in culture.59 Some human medulloblastoma and CNS PNET showed chromosomal gains including the Notch2 locus, and expression of the pathway target Hes1 was correlated with poor clinical outcome.59 Others have also found evidence of Notch pathway dysregulation in human and murine medulloblastoma, along with evidence that Notch activity can be driven by Hh signaling in these tumors.36,60 Finally, hypoxia has been shown to promote neural stem-cell proliferation via Notch, and a similar regulatory loop could be operational in cancer stem cells in medulloblastoma.61,62
Other Pathways
A number of other genes important in cerebellar stem and progenitor cells are dysregulated in medulloblastoma. Many of these have been linked to the three canonical developmental signaling pathways discussed above. REN promotes differentiation of granule cell precursors in the cerebellum at least in part by suppressing Hh signaling, and is located in a region of chromosome 17 frequently deleted in medulloblastoma.63,64 The oncogene N-myc plays an important role in the growth of the cerebellum, and is a key target of Hh signaling in cerebellar granule cells and in medulloblastoma.65–68 Amplification or overexpression of myc oncogenes is associated with the large-cell/anaplastic medulloblastoma subtype and worse clinical outcomes69,70 and sometime occurs as tumors progress from less aggressive subtypes (Fig. 2). The development of drugs such as the quassinoid NBT-272 that target Myc proteins in medulloblastoma cells is therefore promising and could potentially affect stem-like cells in the tumors.71 The transcription factor RE-1 silencing transcription factor, a repressor of neuronal differentiation, is also highly expressed in both neural stem cells and in medulloblastoma and is able to transform c-myc immortalized granule cell precursors into medulloblastoma.72,73 OTX2 and BMI1 represent additional transcription factors that seem to regulate both neural stem/progenitor cells and medulloblastoma growth.74–77
DO MEDULLOBLASTOMA CONTAIN CANCER STEM CELLS?
It seems that developmental stem-cell hierarchies similar to those in fetal brain are maintained in some brain tumors, including medulloblastoma. The cancer stem-cell hypothesis holds that not all cells within a neoplasm are equal in terms of their potential for self-renewal and long-term growth. Rather, as is the case in development of the brain, only a small fraction of stem-like cancer cells are capable of unlimited self-renewal, whereas the rest of the neoplasm is composed of progenitors of limited (albeit rapid) proliferative potential or fully differentiated cells (reviewed in Wang and Dick,78 Tan et al,79 and Reya et al80). Although it seems likely that these cancer stem cells are the progeny of non-neoplastic stem or progenitor cells that are transformed by genetic and/or epigenetic alterations, it is also possible that better-differentiated cells give rise to tumors and acquire stem-like properties after transformation. The issues of histogenesis and hierarchy are therefore potentially, but not necessarily, linked.
Several pieces of evidence support the concept that medulloblastoma cells are functionally heterogeneous and that only a subset have a stem-like phenotype that drives tumor growth. First, nodular/desmoplastic medulloblastoma contain two karyotypically similar compartments,81 one comprised of rapidly proliferating, undifferentiated cells, and a second (the nodules) composed of largely nonproliferative cells that have differentiated into neurons. Extensively nodular tumors that lack the stem or progenitor-like component are known to be less aggressive clinically.1 This suggests that by modulating the number of primitive or stem-like cells in a medulloblastoma, clinical outcome can be affected. Second, it has been shown that medulloblastoma contain a subset of stem-like multipotent cells that can be maintained as neurospheres under the same conditions used to grow non-neoplastic neural stem cells.82,83 Third, the stem-cell marker CD133 is expressed in only a fraction of medulloblastoma cells, and some have reported that these cells alone are able to form multipotent neurospheres in vitro or engraft in vivo.84,85 We have also found that CD133-positive cells from some established medulloblastoma cell lines have a greatly increased ability to form tumor xenografts.86 Other groups, however, have recently reported that CD133 negative cells can propagate some glioblastoma lines,87 suggesting that the CD133 marker may not identify all cancer stem cells in brain tumors. Indeed, in a recently published meeting abstract, Wechsler-Reya et al88 reported that CD133 negative, CD15 positive cells are cancer-propagating cells in medulloblastoma derived from PTCH heterozygous mice.
TARGETING CANCER STEM CELLS IN MEDULLOBLASTOMA
If the cancer stem-cell hypothesis is true, stem-like cells within medulloblastoma must be removed if a patient is to be cured. However, it is thought that most cancer stem cells are resistant to standard therapies.89 The mechanisms for such resistance are beginning to be elucidated in some CNS tumors, although relatively little is known in medulloblastoma. It has been suggested that cancer stem cells in malignant gliomas are radiation resistant because of an intrinsic ability to induce the DNA damage response.90 At least one group also documented increased radiation resistance in CD133-positive stem-like cells in medulloblastoma.91 Regarding chemotherapy, it is thought that cancer stem cells have an increased ability to efflux harmful chemicals because of their expression of the adenosine triphosphate–binding cassette–type transporters.92 Although no direct evidence supporting this has been generated in medulloblastoma, it has been reported that stem-like side population cells are resistant to mitoxantrone in a similar tumor, the neuroblastoma.93
Hh inhibitors represent one class of therapies that may deplete cancer stem cells. Hh signaling seems to be elevated in the more primitive-appearing, progenitor-like regions of nodular medulloblastoma.94 Cyclopamine, an inhibitor of Hh signaling, has been shown to reduce medulloblastoma growth in vitro and prevent tumor propagation in vivo as a result of reduced proliferation and induced apoptosis.95,96 Other Hh inhibitors also showed encouraging results in vivo in transgenic models.97 In neither case, however, was it clear whether the stem-like tumor fraction was effectively targeted, as was recently shown using cyclopamine to treat malignant glioma.25,98 It is also not clear whether Hh inhibitors will only prove effective in tumors with extremely high levels of pathway activity, or if even medulloblastoma with lower levels of signaling will be susceptible. In one experiment, freshly resected cells from all seven medulloblastoma tested proved sensitive to Hh blockade, suggesting that even tumors without high-level Hh activity might need this pathway.96
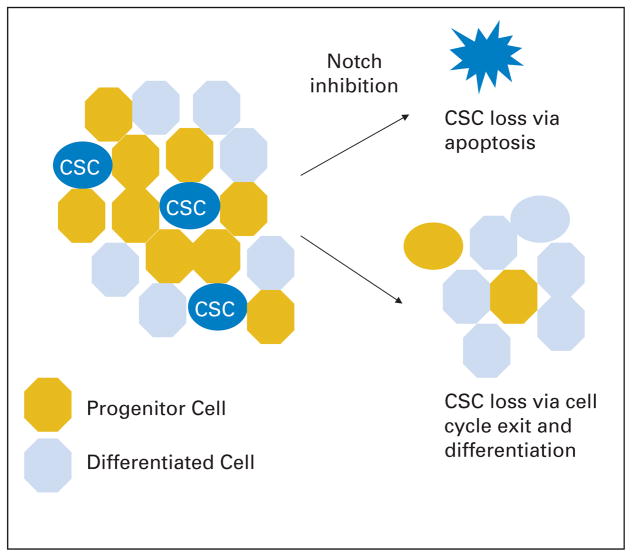
The Notch pathway represents a second signaling cascade for which cancer stem cells might maintain a requirement present in their non-neoplastic cognate cells. When we separated cells from established medulloblastoma lines into CD133 positive and negative fractions, the level of Notch signaling was several-fold greater in the former than in the latter.99 After Notch signaling blockade in medulloblastoma lines using pharmacologic inhibitors of gamma-secretase, the cancer stem-cell population marked by CD133, side population, and Nestin was markedly reduced or abolished. Moreover, the viable cells remaining after Notch blockade were unable to efficiently form colonies in soft agar or xenografts in nude mice.99 Although the mechanism by which these stem-like cancer cells were depleted was not entirely clear, cell cycle exit and neuronal differentiation were observed in the cultures treated with gamma-secretase inhibitors. In addition, double immunolabeling for Nestin and apoptotic markers revealed that the primitive cells were more than 10 times more sensitive to apoptotic induction after Notch blockade than better-differentiated ones (Fig. 3). Hallahan et al36 have also shown that gamma-secretase inhibition caused reduced proliferation and increased apoptosis in medulloblastoma cells. Consistent with the idea that Notch is required in tumor-propagating cancer stem cells, we found that after gamma-secretase treatment, the remaining viable cells could proliferate for a time in culture but were no longer able to form xenografts in immunocompromised mice.99 This experimental evidence supports the hypothesis that reagents that target cancer stem cells, most likely in combination with standard therapies, may eventually help to completely treat medulloblastoma.
Fig. 3.
Notch pathway blockade seems to preferentially deplete stem-like medulloblastoma cells, leaving behind better-differentiated cells no longer able to form tumor masses. Both differentiation and cell death are involved in loss of the putative cancer stem cell (CSC) subpopulation.
Another avenue by which cancer stem cells in medulloblastoma and other tumors might be depleted is through ablation of the so-called niche required for their survival. The stem-cell niche is a micro-environment composed of supportive cells, extracellular matrix, and other factors required for stem-cell self-renewal.100 The nature of the niche likely varies during various phases of neural development and in different regions of the brain. In the SVZ, for example, astrocytes, neuroblasts, and endothelial cells all help form the niche by providing factors required for neural stem-cell self-renewal.101,102 In the developing cerebellum, the niche required for the various stem- and progenitor-cell fractions is largely undefined. It is not yet clear whether cancer stem cells require a niche similar to that present in their non-neoplastic counterparts, but a recent report by Calabrese et al103 supports this notion. They showed that CD133-positive putative cancer stem cells in medulloblastoma are more commonly found near endothelial cells and small vessels, suggesting the latter may function as a niche and provide critical growth factors and stromal support required for self-renewal. This raises the possibility that antiangiogenic therapy could have the added benefit of depriving stem-like cells of the niche signals they require for survival.
In summary, much remains to be defined regarding the relationship between medulloblastoma and the stem cells that form the cerebellum. However, it seems clear that at least some medulloblastoma derive from stem or progenitor populations, and that the same signaling pathways required in these non-neoplastic cells are frequently activated in embryonal brain tumors. These molecular pathways may represent an Achilles heel for cancer stem cells in medulloblastoma, and by targeting these cells, we may be able to circumvent their presumed resistance to standard therapies. Inhibition of Hh, Notch, and other developmental pathways therefore represents an exciting new tool in the therapeutic armamentarium, although detrimental effects on non-neoplastic stem cells will have to be closely monitored as new agents are moved into the clinic.
Footnotes
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Xing Fan, Charles G. Eberhart
Collection and assembly of data: Xing Fan, Charles G. Eberhart
Data analysis and interpretation: Xing Fan, Charles G. Eberhart
Manuscript writing: Xing Fan, Charles G. Eberhart
Final approval of manuscript: Xing Fan, Charles G. Eberhart
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. WHO Classification of Tumours of the Central Nervous System. Lyon, France: IARC Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crawford JR, Macdonald TJ, Packer RJ. Medulloblastoma in childhood: New biological advances. Lancet Neurol. 2007;6:1073–1085. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- 3.Kershman J. The medulloblast and medulloblastoma: A study of human embryos. Arch Neurol Psychiatry. 1938;40:937–967. [Google Scholar]
- 4.Sotelo C. Cellular and genetic regulation of the development of the cerebellar system. Prog Neurobiol. 2004;72:295–339. doi: 10.1016/j.pneurobio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Goldowitz D, Hamre K. The cells and molecules that make a cerebellum. Trends Neurosci. 1998;21:375–382. doi: 10.1016/s0166-2236(98)01313-7. [DOI] [PubMed] [Google Scholar]
- 6.Chizhikov V, Millen KJ. Development and malformations of the cerebellum in mice. Mol Genet Metab. 2003;80:54–65. doi: 10.1016/j.ymgme.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Abrahám H, Tornoczky T, Kosztolanyi G, et al. Cell formation in the cortical layers of the developing human cerebellum. Int J Dev Neurosci. 2001;19:53–62. doi: 10.1016/s0736-5748(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 8.Lee A, Kessler JD, Read TA, et al. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fink AJ, Englund C, Daza RA, et al. Development of the deep cerebellar nuclei: Transcription factors and cell migration from the rhombic lip. J Neurosci. 2006;26:3066–3076. doi: 10.1523/JNEUROSCI.5203-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43. doi: 10.1016/j.neuron.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Alcock J, Scotting P, Sottile V. Bergmann glia as putative stem cells of the mature cerebellum. Med Hypotheses. 2007;69:341–345. doi: 10.1016/j.mehy.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Katsetos CD, Herman MM, Krishna L, et al. Calbindin-D28k in subsets of medulloblastomas and in the human medulloblastoma cell line D283 Med. Arch Pathol Lab Med. 1995;119:734–743. [PubMed] [Google Scholar]
- 13.Bühren J, Christoph AH, Buslei R, et al. Expression of the neurotrophin receptor p75NTR in medulloblastomas is correlated with distinct histological and clinical features: Evidence for a medulloblastoma subtype derived from the external granule cell layer. J Neuropathol Exp Neurol. 2000;59:229–240. doi: 10.1093/jnen/59.3.229. [DOI] [PubMed] [Google Scholar]
- 14.Kho AT, Zhao Q, Cai Z, et al. Conserved mechanisms across development and tumorigenesis revealed by a mouse development perspective of human cancers. Genes Dev. 2004;18:629–640. doi: 10.1101/gad.1182504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee Y, Miller HL, Jensen P, et al. A molecular fingerprint for medulloblastoma. Cancer Res. 2003;63:5428–5437. [PubMed] [Google Scholar]
- 16.Gao WQ, Hatten ME. Immortalizing oncogenes subvert the establishment of granule cell identity in developing cerebellum. Development. 1994;120:1059–1070. doi: 10.1242/dev.120.5.1059. [DOI] [PubMed] [Google Scholar]
- 17.Okano-Uchida T, Himi T, Komiya Y, et al. Cerebellar granule cell precursors can differentiate into astroglial cells. Proc Natl Acad Sci U S A. 2004;101:1211–1216. doi: 10.1073/pnas.0307972100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Connelly MC, Wetmore C, et al. Mouse embryos cloned from brain tumors. Cancer Res. 2003;63:2733–2736. [PubMed] [Google Scholar]
- 19.Ajioka I, Martins RA, Bayazitov IT, et al. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell. 2007;131:378–390. doi: 10.1016/j.cell.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahmane N, Ruiz i Altaba A. Sonic hedgehog regulates the growth and patterning of the cerebellum. Development. 1999;126:3089–3100. doi: 10.1242/dev.126.14.3089. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 22.Wallace VA. Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr Biol. 1999;9:445–448. doi: 10.1016/s0960-9822(99)80195-x. [DOI] [PubMed] [Google Scholar]
- 23.Machold R, Hayashi S, Rutlin M, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 24.Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007;27:5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahmane N, Sanchez P, Gitton Y, et al. The Sonic Hedgehog-Gli pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128:5201–5212. doi: 10.1242/dev.128.24.5201. [DOI] [PubMed] [Google Scholar]
- 26.Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 27.Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 28.Raffel C, Jenkins RB, Frederick L, et al. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57:842–845. [PubMed] [Google Scholar]
- 29.Pietsch T, Waha A, Koch A, et al. Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res. 1997;57:2085–2088. [PubMed] [Google Scholar]
- 30.Pomeroy SL, Tamayo P, Gaasenbeek M, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415:436–442. doi: 10.1038/415436a. [DOI] [PubMed] [Google Scholar]
- 31.Thompson MC, Fuller C, Hogg TL, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–1931. doi: 10.1200/JCO.2005.04.4974. [DOI] [PubMed] [Google Scholar]
- 32.Goodrich LV, Milenkovic L, Higgins KM, et al. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 33.Wetmore C, Eberhart DE, Curran T. Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res. 2001;61:513–516. [PubMed] [Google Scholar]
- 34.Oliver TG, Read TA, Kessler JD, et al. Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development. 2005;132:2425–2439. doi: 10.1242/dev.01793. [DOI] [PubMed] [Google Scholar]
- 35.Lee Y, Kawagoe R, Sasai K, et al. Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene. 2007;26:6442–6447. doi: 10.1038/sj.onc.1210467. [DOI] [PubMed] [Google Scholar]
- 36.Hallahan AR, Pritchard JI, Hansen S, et al. The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004;64:7794–7800. doi: 10.1158/0008-5472.CAN-04-1813. [DOI] [PubMed] [Google Scholar]
- 37.Yang Z, Ellis T, Markant S, et al. The cellular origin of Patched associated medulloblastoma. Neuro-Oncology. 2007;9:559. [Google Scholar]
- 38.Rowitch DH. Developmental origins of medulloblastoma from regionally restricted multipotent progenitors in the mammalian central nervous system. Neuro-oncology. 2007;9:562. [Google Scholar]
- 39.Tong WM, Ohgaki H, Huang H, et al. Null mutation of DNA strand break-binding molecule poly(ADP-ribose) polymerase causes medulloblastomas in p53(−/−) mice. Am J Pathol. 2003;162:343–352. doi: 10.1016/S0002-9440(10)63825-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasai K, Romer JT, Kimura H, et al. Medulloblastomas derived from Cxcr6 mutant mice respond to treatment with a smoothened inhibitor. Cancer Res. 2007;67:3871–3877. doi: 10.1158/0008-5472.CAN-07-0493. [DOI] [PubMed] [Google Scholar]
- 41.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 42.Adachi K, Mirzadeh Z, Sakaguchi M, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 43.Zhou CJ, Borello U, Rubenstein JL, et al. Neuronal production and precursor proliferation defects in the neocortex of mice with loss of function in the canonical Wnt signaling pathway. Neuroscience. 2006;142:1119–1131. doi: 10.1016/j.neuroscience.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Lie DC, Colamarino SA, Song HJ, et al. Wnt signaling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 45.Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990;346:847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- 46.McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- 47.Schüller U, Rowitch DH. Beta-catenin function is required for cerebellar morphogenesis. Brain Res. 2007;1140:161–169. doi: 10.1016/j.brainres.2006.05.105. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton SR, Liu B, Parsons RE, et al. The molecular basis of Turcot’s syndrome. N Engl J Med. 1995;332:839–847. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- 49.Zurawel RH, Chiappa SA, Allen C, et al. Sporadic medulloblastomas contain oncogenic beta-catenin mutations. Cancer Res. 1998;58:896–899. [PubMed] [Google Scholar]
- 50.Huang H, Mahler-Araujo BM, Sankila A, et al. APC mutations in sporadic medulloblastomas. Am J Pathol. 2000;156:433–437. doi: 10.1016/S0002-9440(10)64747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eberhart CG, Tihan T, Burger PC. Nuclear localization and mutation of beta-catenin in medulloblastomas. J Neuropathol Exp Neurol. 2000;59:333–337. doi: 10.1093/jnen/59.4.333. [DOI] [PubMed] [Google Scholar]
- 52.Koch A, Hrychyk A, Hartmann W, et al. Mutations of the Wnt antagonist AXIN2 (Conductin) result in TCF-dependent transcription in medulloblastomas. Int J Cancer. 2007;121:284–291. doi: 10.1002/ijc.22675. [DOI] [PubMed] [Google Scholar]
- 53.Ellison DW, Onilude OE, Lindsey JC, et al. Beta-catenin status predicts a favorable outcome in childhood medulloblastoma: The United Kingdom Children’s Cancer Study Group Brain Tumour Committee. J Clin Oncol. 2005;23:7951–7957. doi: 10.1200/JCO.2005.01.5479. [DOI] [PubMed] [Google Scholar]
- 54.Clifford SC, Lusher ME, Lindsey JC, et al. Wnt/Wingless pathway activation and chromosome 6 loss characterize a distinct molecular sub-group of medulloblastomas associated with a favorable prognosis. Cell Cycle. 2006;5:2666–2670. doi: 10.4161/cc.5.22.3446. [DOI] [PubMed] [Google Scholar]
- 55.Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- 56.Machold RP, Kittell DJ, Fishell GJ. Antagonism between Notch and bone morphogenetic protein receptor signaling regulates neurogenesis in the cerebellar rhombic lip. Neural Develop. 2007;2:5. doi: 10.1186/1749-8104-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Givogri MI, de Planell M, Galbiati F, et al. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev Neurosci. 2006;28:81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- 58.Solecki DJ, Liu XL, Tomoda T, et al. Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron. 2001;31:557–568. doi: 10.1016/s0896-6273(01)00395-6. [DOI] [PubMed] [Google Scholar]
- 59.Fan X, Mikolaenko I, Elhassan I, et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 60.Dakubo GD, Mazerolle CJ, Wallace VA. Expression of Notch and Wnt pathway components and activation of Notch signaling in medulloblastomas from heterozygous patched mice. J Neurooncol. 2006;79:221–227. doi: 10.1007/s11060-006-9132-2. [DOI] [PubMed] [Google Scholar]
- 61.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gustafsson MV, Zheng X, Pereira T, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 63.Di Marcotullio L, Ferretti E, De Smaele E, et al. REN(KCTD11) is a suppressor of Hedgehog signaling and is deleted in human medulloblastoma. Proc Natl Acad Sci U S A. 2004;101:10833–10838. doi: 10.1073/pnas.0400690101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Argenti B, Gallo R, Di Marcotullio L, et al. Hedgehog antagonist REN(KCTD11) regulates proliferation and apoptosis of developing granule cell progenitors. J Neurosci. 2005;25:8338–8346. doi: 10.1523/JNEUROSCI.2438-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- 66.Hatton BA, Knoepfler PS, Kenney AM, et al. N-myc is an essential downstream effector of Shh signaling during both normal and neoplastic cerebellar growth. Cancer Res. 2006;66:8655–8661. doi: 10.1158/0008-5472.CAN-06-1621. [DOI] [PubMed] [Google Scholar]
- 67.Oliver TG, Grasfeder LL, Carroll AL, et al. Transcriptional profiling of the Sonic hedgehog response: A critical role for N-myc in proliferation of neuronal precursors. Proc Natl Acad Sci U S A. 2003;100:7331–7336. doi: 10.1073/pnas.0832317100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Browd SR, Kenney AM, Gottfried ON, et al. N-myc can substitute for insulin-like growth factor signaling in a mouse model of sonic hedgehog-induced medulloblastoma. Cancer Res. 2006;66:2666–2672. doi: 10.1158/0008-5472.CAN-05-2198. [DOI] [PubMed] [Google Scholar]
- 69.Grotzer MA, Hogarty MD, Janss AJ, et al. MYC messenger RNA expression predicts survival outcome in childhood primitive neuroectodermal tumor/medulloblastoma. Clin Cancer Res. 2001;7:2425–2433. [PubMed] [Google Scholar]
- 70.Stearns D, Chaudhry A, Abel TW, et al. C-myc overexpression causes anaplasia in medulloblastoma. Cancer Res. 2006;66:673–681. doi: 10.1158/0008-5472.CAN-05-1580. [DOI] [PubMed] [Google Scholar]
- 71.von Bueren AO, Shalaby T, Rajtarova J, et al. Anti-proliferative activity of the quassinoid NBT-272 in childhood medulloblastoma cells. BMC Cancer. 2007;7:19. doi: 10.1186/1471-2407-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su X, Gopalakrishnan V, Stearns D, et al. Abnormal expression of REST/NRSF and Myc in neural stem/progenitor cells causes cerebellar tumors by blocking neuronal differentiation. Mol Cell Biol. 2006;26:1666–1678. doi: 10.1128/MCB.26.5.1666-1678.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lawinger P, Venugopal R, Guo ZS, et al. The neuronal repressor REST/NRSF is an essential regulator in medulloblastoma cells. Nat Med. 2000;6:826–831. doi: 10.1038/77565. [DOI] [PubMed] [Google Scholar]
- 74.Boon K, Eberhart CG, Riggins GJ. Genomic amplification of orthodenticle homologue 2 in medulloblastomas. Cancer Res. 2005;65:703–707. [PubMed] [Google Scholar]
- 75.Di C, Liao S, Adamson DC, et al. Identification of OTX2 as a medulloblastoma oncogene whose product can be targeted by all-trans retinoic acid. Cancer Res. 2005;65:919–924. [PubMed] [Google Scholar]
- 76.Leung C, Lingbeek M, Shakhova O, et al. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004;428:337–341. doi: 10.1038/nature02385. [DOI] [PubMed] [Google Scholar]
- 77.Wiederschain D, Chen L, Johnson B, et al. Contribution of polycomb homologues Bmi-1 and Mel-18 to medulloblastoma pathogenesis. Mol Cell Biol. 2007;27:4968–4979. doi: 10.1128/MCB.02244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang JC, Dick JE. Cancer stem cells: Lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 79.Tan BT, Park CY, Ailles LE, et al. The cancer stem cell hypothesis: A work in progress. Lab Invest. 2006;86:1203–1207. doi: 10.1038/labinvest.3700488. [DOI] [PubMed] [Google Scholar]
- 80.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 81.Ehrbrecht A, Muller U, Wolter M, et al. Comprehensive genomic analysis of desmoplastic medulloblastomas: Identification of novel amplified genes and separate evaluation of the different histological components. J Pathol. 2006;208:554–563. doi: 10.1002/path.1925. [DOI] [PubMed] [Google Scholar]
- 82.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Galli R, Binda E, Orfanelli U, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 84.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 85.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 86.Eberhart CG. In search of the medulloblast: Neural stem cells and embryonal brain tumors. Neurosurg Clin N Am. 2007;18:59–69. doi: 10.1016/j.nec.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 87.Beier D, Hau P, Proescholdt M, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67:4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 88.Wechsler-Reya R, Read TA. Medulloblastomas from patched mutant mice are propagated by a CD15+ CD133− neural progenitor. Neuro-Oncology. 2007;9:559. [Google Scholar]
- 89.Kvinlaug BT, Huntly BJ. Targeting cancer stem cells. Expert Opin Ther Targets. 2007;11:915–927. doi: 10.1517/14728222.11.7.915. [DOI] [PubMed] [Google Scholar]
- 90.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 91.Blazek ER, Foutch JL, Maki G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133− cells, and the CD133+ sector is enlarged by hypoxia. Int J Radiat Oncol Biol Phys. 2007;67:1–5. doi: 10.1016/j.ijrobp.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 92.Hirschmann-Jax C, Foster AE, Wulf GG, et al. A distinct “side population” of cells in human tumor cells: Implications for tumor biology and therapy. Cell Cycle. 2005;4:203–205. [PubMed] [Google Scholar]
- 93.Hirschmann-Jax C, Foster AE, Wulf GG, et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci U S A. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bar EE, Chaudhry A, Farah MH, et al. Hedgehog signaling promotes medulloblastoma survival via Bc/II. Am J Pathol. 2007;170:347–355. doi: 10.2353/ajpath.2007.060066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanchez P, Ruiz IAA. In vivo inhibition of endogenous brain tumors through systemic interference of Hedgehog signaling in mice. Mech Dev. 2005;122:223–230. doi: 10.1016/j.mod.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 96.Berman DM, Karhadkar SS, Hallahan AR, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 97.Romer JT, Kimura H, Magdaleno S, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 98.Bar EE, Chaudhry A, Lin A, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fan X, Matsui W, Khaki L, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment of embryonal brain tumors. Cancer Res. 2006;66:7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 100.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: Stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 101.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 102.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 103.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]