Tumor heterogeneity 2016 (original) (raw)
. Author manuscript; available in PMC: 2017 Jan 3.
Abstract
Heterogeneity is commonplace in all cancer types and at several levels – intrinsic (genetic), epigenetic, positional and at the population level. The different subpopulations with a tumor mass communicate with each other and influence the behavior of other tumor cells both locally and at a distance. These properties have profound implications regarding the understanding of tumor behavior and how therapies are (or should be) administered. This brief commentary highlights the insightful review of Gloria Heppner and how it has influenced cancer research even three decades after it was published.
In this time of easy access to papers via electronic means, too often some older literature is overlooked or, even worse, ignored because the plethora of newer papers relegate key older literature to an extended session of scrolling through pages on PubMed. In the 75th Anniversary of Cancer Research, we take a look back at 48 papers that have had high impact on cancer research. In 1984, Gloria Heppner published Tumor Heterogeneity (1), a review in which the longstanding recognition that cancers are comprised of multiple subpopulations was analyzed. This paper was highlighted by more editors, AACR Fellows and cancer researchers than any other. Why do I think that is the case? I am struck in re-reading this paper by the clarity of thought, objective presentation and interpretation of the data and, as importantly, by the insights that have withstood the tests of time.
All tumors (not necessarily neoplasms) are heterogeneous (2, 3). Yet, surprisingly, recent deep sequencing reports seem to hail the existence of as a new concept. Hardly --- literature dates back to the 1800’s. Virtually every phenotype in cancer is heterogeneous. And, yes, while some of the recent deep sequencing papers elegantly verify that there is genetic heterogeneity at the single cell level (4–7), the findings parallel what was described decades ago in other systems.
What still remains 30 years hence is a still-unclear understanding of the origins of tumor heterogeneity. Some might argue that the cancer ‘stem cell’ theory explains the origins of heterogeneity (8–11); others might argue that genomic/genetic instability drives diversification of cancers (2, 6, 9, 12–14). In truth, both are likely drivers (Figure 1).
Figure 1.
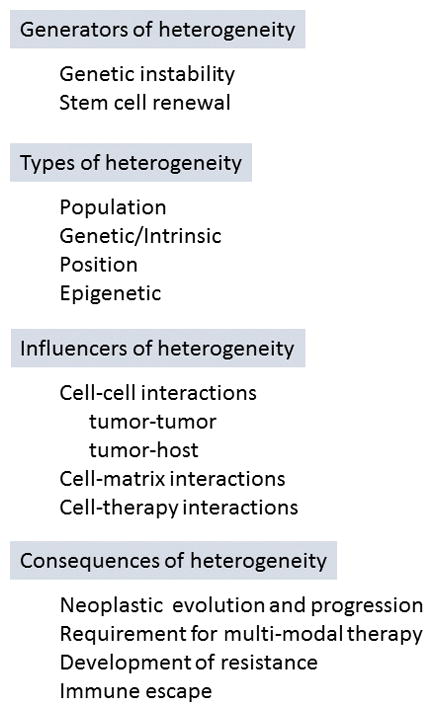
Discussion of the heterogeneity in tumors is too often limited to discussions of clonal differences. But there are, in fact, three other types of tumor heterogeneity – population, positional and temporal (Figure 1). Population heterogeneity refers to the uniqueness of tumors arising from the same cell type in different patients. That is, each patient’s tumor is unique, albeit sharing some properties. Underlying all of the oncogenic and genetic instability drivers are quantitative trait loci that control cellular behaviors (15, 16) that contribute to inter-patient variability.
Likewise, patient-specific selective pressures modulate the progression of individual cancers. Such are the parameters (e.g., hormonal or immune status, stress, nutrition, therapies) that contribute to temporal heterogeneity. A biopsy is merely a snapshot of a small subset of neoplastic cells at a given moment in time. Tumor progression is an evolutionary process in which tumor cells with a selective advantage begin to appear as clonally dominant, while disadvantaged cells are selected against (17–19). Thus, at any given time, tumor composition is unique.
Finally, cellular phenotypes are influenced by the signals they receive, e.g., pO2, growth factor concentration, matrix biophysical properties, signals from other tumor cells. For example, disseminating cells that have been hypoxic are more likely to successfully form macroscopic metastases than cells which have been adequately oxygenated (20–22). Similarly, responses to therapy can be influenced by proximity to vessels, e.g., cells nearer blood vessels are more oxygenated and therefore sensitive to ionizing radiation; or, cells distant from vessels may receive lower drug concentrations because of diffusion distance (23).
These considerations were eloquently addressed by Dr. Heppner in her review. She ultimately showed the similarities between tumor progression, heterogeneity development and evolutionary theory. Those parallels profoundly shaped a generation of researchers’ and clinicians’ thinking about how to address heterogeneity in experimental design and treatment. Tumor cell societies, in which the distinct subpopulations of cancer cells influence the behavior of other cells in the mass (or even at distant sites in the body) (Figure 1), is now well appreciated, but was first introduced in this paper.
While the above insights have proven helpful. Deeper understanding about heterogeneity is needed, especially in light of new research and treatment strategies. In recent years, some have argued that cell lines are not as heterogeneous as in situ tumor implants or xenografts. However, subcloning argues against that point (reviewed in (2)). Certainly, the vast majority of tumor cells are genetically unstable both in vitro and in vivo (2, 13). Some argue that patient-derived xenografts (PDX) are more reflective of their state in patients; however, selective pressures associated with placing them into non-human tissues (with non-human vessels providing nutrients) and continued evolution and genetic instability still exist (24). So, while there are certainly some advantages to PDX models, there will be artificialities associated with their use, just as there are with all experimental models. Those limitations will emerge as the models are more widely utilized. Some assume that clones remain homogeneous, but the data overwhelmingly argue against that assumption (2, 13).
So, while new ways of defining the heterogeneity that exists within a tumor mass have been added since 1984, the principles outlined in Dr. Heppner’s seminal review have withstood the tests of time. Reflecting upon the insights she presented continues to provide a context upon which cancer researchers should interpret their data. They further provide cautionary notes to the notion that monotherapies will rarely be effective against tumors comprised of mixed populations and strong recognition that tumors are continually evolving ways to circumvent attempts to limit them. Oncologists fight a wily enemy.
Tumor heterogeneity exists; it is a hallmark of cancer cells; cancer cells do not exist in isolation and the communication between tumor cells, tumor cells and host cells and selection pressure imposed upon the tumor influence the evolution of the tumor; and, cancer researchers and oncologists ignore heterogeneity at their own peril.
Acknowledgments
I am thankful for the generous support from the National Cancer Institute, National Foundation for Cancer Research, Susan G. Komen for the Cure and many other agencies over the years. I apologize to the many authors whose work was not cited due to space limitations.
References
- 1.Heppner GH. Tumor heterogeneity. Cancer Res. 1984;44:2259–65. [PubMed] [Google Scholar]
- 2.Welch DR, Tomasovic SP. Implications of tumor progression on clinical oncology. Clin Exptl Metastasis. 1985;3:151–88. doi: 10.1007/BF01786761. [DOI] [PubMed] [Google Scholar]
- 3.Welch DR. Factors involved in the development and maintenance of tumor heterogeneity. In: Abraham S, editor. Carcinogenesis and Dietary Fat. Boston: Kluwer Academic Publishers; 1989. pp. 279–301. [Google Scholar]
- 4.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Fujimoto J, Zhang J, Wedge DC, Song X, Zhang J, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science. 2014;346:256–9. doi: 10.1126/science.1256930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein CA. Selection and adaptation during metastatic cancer progression. Nature. 2013;501:365–72. doi: 10.1038/nature12628. [DOI] [PubMed] [Google Scholar]
- 7.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–37. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skibinski A, Kuperwasser C. The origin of breast tumor heterogeneity. Oncogene. 2015;34:5309–16. doi: 10.1038/onc.2014.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang S. Genetic and non-genetic instability in tumor progression: link between the fitness landscape and the epigenetic landscape of cancer cells. Cancer Metastasis Rev. 2013;32:423–48. doi: 10.1007/s10555-013-9435-7. [DOI] [PubMed] [Google Scholar]
- 10.Valent P, Bonnet D, Wohrer S, Andreeff M, Copland M, Chomienne C, et al. Heterogeneity of neoplastic stem cells: Theoretical, functional, and clinical implications. Cancer Res. 2013;73:1037–45. doi: 10.1158/0008-5472.CAN-12-3678. [DOI] [PubMed] [Google Scholar]
- 11.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–96. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox EJ, Prindle MJ, Loeb LA. Do mutator mutations fuel tumorigenesis? Cancer Metastasis Rev. 2013;32:353–61. doi: 10.1007/s10555-013-9426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loeb LA. Human cancers express mutator phenotypes: origin, consequences and targeting. Nature Rev Cancer. 2011;11:450–7. doi: 10.1038/nrc3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loeb LA, Bielas JH, Beckman RA. Cancers exhibit a mutator phenotype: Clinical implications. Cancer Res. 2008;68:3551–7. doi: 10.1158/0008-5472.CAN-07-5835. [DOI] [PubMed] [Google Scholar]
- 15.Feeley KP, Bray AW, Westbrook DG, Johnson LW, Kesterson RA, Ballinger SW, et al. Mitochondrial Genetics Regulate Breast Cancer Tumorigenicity and Metastatic Potential. Cancer Res. 2015;75:4429–36. doi: 10.1158/0008-5472.CAN-15-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter KW. Mouse models of cancer: does the strain matter? Nature Rev Cancer. 2012;12:144–9. doi: 10.1038/nrc3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swanton C. Intratumor heterogeneity: evolution through space and time. Cancer Res. 2012;72:4875–82. doi: 10.1158/0008-5472.CAN-12-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price JE, Bell C, Frost P. The use of a genotypic marker to demonstrate clonal dominance during the growth and metastasis of a human breast carcinoma in nude mice. Int J Cancer. 1990;45:968–71. doi: 10.1002/ijc.2910450532. [DOI] [PubMed] [Google Scholar]
- 19.Waghorne C, Thomas M, Lagarde AE, Kerbel RS, Breitman ML. Genetic evidence for progressive selection and overgrowth of primary tumors by metastatic cell subpopulations. Cancer Res. 1988;48:6109–14. [PubMed] [Google Scholar]
- 20.Lunt SJ, Chaudary N, Hill RP. The tumor microenvironment and metastatic disease. Clin Exptl Metastasis. 2009;26:19–34. doi: 10.1007/s10585-008-9182-2. [DOI] [PubMed] [Google Scholar]
- 21.De Jaeger K, Kavanagh MC, Hill RP. Relationship of hypoxia to metastatic ability in rodent tumours. Br J Cancer. 2001;84:1280–5. doi: 10.1054/bjoc.2001.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–9. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nature Rev Cancer. 2002;2:727–39. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 24.Eirew P, Steif A, Khattra J, Ha G, Yap D, Farahani H, et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature. 2015;518:422–6. doi: 10.1038/nature13952. [DOI] [PMC free article] [PubMed] [Google Scholar]