Plasma neuronal exosomal levels of Alzheimer's disease biomarkers in normal aging (original) (raw)
Abstract
Plasma neuronal exosomal levels of pathogenic Alzheimer's disease (AD) proteins, cellular survival factors, and lysosomal proteins distinguish AD patients from control subjects, but changes in these exosomal proteins associated with normal aging have not been described for cognitively intact subjects. Plasma neuronal exosomal levels of P‐T181‐tau, P‐S396‐tau, A_β_ 1‐42, cathepsin D, repressor element 1‐silencing transcription factor, and neurogranin were quantified longitudinally in cognitively intact older adults using two samples collected at 3‐ to 11‐year intervals. Except for P‐S396‐tau, exosomal protein levels changed significantly with aging, but were largely outside the range observed in AD patients.
Background
Blood‐based biomarkers of Alzheimer's disease (AD) are needed given high costs and invasiveness of most available biomarker technologies, including neuroimaging and quantification of analytes in cerebrospinal fluid (CSF).1 Exosomes are released into the blood from a variety of cells, including neurons.2 Exosomes have been shown to play a role in neuronal development and regeneration,3 and they have been implicated in the transport of pathogenic proteins in the brain and in the progression of neurodegenerative diseases.4
Recent studies showed that neuronally derived plasma exosomal protein levels of β_‐amyloid1‐42 (A_β 1‐42) and tau proteins (P‐T181‐tau and P‐S396‐tau) were increased both in AD patients and in cognitively intact subjects (CIS) who transitioned to AD 2–10 years later compared to controls.5 Similarly, neuronally protective repressor element 1‐silencing transcription factor (REST) was decreased in both AD patients and in CIS who eventually transitioned to AD relative to controls.6 Autolysosomal proteins, including cathepsin D, were higher both in AD patients and in CIS who eventually transitioned to AD relative to controls.7
Longitudinal studies of these neuronally derived plasma exosome proteins in CIS are needed as an appropriate framework for interpretation of elevated values. Additionally, neurogranin, a postsynaptic protein associated with synapse loss, is reduced in the brains of AD patients,8 but is increased in CSF9, 10 of AD patients compared to controls. Here, we examine levels of A_β_ 1‐42, P‐T181‐tau, P‐S396‐tau, REST, cathepsin D, and, for the first time, neurogranin, in CIS followed at the University of Kentucky Alzheimer's Disease Center (UK‐ADC) in order to assess aging effects on the levels of these neuronally derived exosomal proteins.
Methods
Participant characterization, study design, and blood sampling technique
We studied plasma and CSF samples from 20 volunteers from the UK‐ADC longitudinal cohort.11 All volunteers were at least age 60 at UK‐ADC enrollment. Volunteers were followed annually with in‐person examinations, including neurocognitive assessment, physical exam, medical history, and neurological exam.
CIS provided blood at annual visits. Blood was centrifuged to yield platelet‐free plasma and stored in 2.0‐mL self‐standing polypropylene tubes at −80°C. Plasma samples were collected from November 2011 to August 2013 (“second draw”) from participants in a biomarker study, and each was paired with a sample collected from the same participant at least 3 years earlier (“first draw”) in order to assess longitudinal protein changes in CIS. The CIS in this study also provided CSF at the second draw. Two participants had no available earlier first draw. The UK Institutional Review Board approved all research procedures; participants provided written informed consent.
CSF collection and measurement
Lumbar CSF was drawn using a 20‐gauge needle the morning after fasting since midnight and was maintained in single use 0.5‐mL aliquots in polypropylene storage tubes in a −80°C freezer. Briefly, CSF was collected into 15‐mL sterile polypropylene collection tubes and transferred into storage tubes without any centrifugation step. CSF was batch shipped overnight to the Biomarker Research laboratory at the University of Pennsylvania Medical Center on dry ice. Upon receipt the samples were transferred to a −80°C freezer and maintained at this temperature until the day of analysis. A_β_ 1‐42, total tau, and P‐T181‐tau were measured using the multiplex xMAP Luminex platform (Luminex Corp, Austin, TX) with Fujirebio (INNO‐BIA AlzBio3; Ghent, Belgium; for research use–only reagents) immunoassay kit–based reagents. The capture and detection antibodies for A_β_ 1‐42 were 4D7A3 and 3D6, respectively.12 CSF analytes were assayed without dilution.
Isolation of exosomes from plasma for extraction and ELISA quantification of exosome proteins
Samples were relabeled prior to analysis to blind the laboratory. One‐fourth ml of plasma was incubated with thromboplastin‐D (Fisher Scientific, Inc., Hanover Park, IL) followed by addition of calcium‐ and magnesium‐free Dulbecco's balanced salt solution with protease inhibitor cocktail (Roche Applied Sciences, Inc., Indianapolis, IN) and phosphatase inhibitor cocktail (Pierce Halt, Thermo Scientific, Inc., Rockford, IL) as described earlier.5, 6 After centrifugation at 3000_g_ for 20 min at room temperature, supernatants were incubated with ExoQuick exosome precipitation solution (System Biosciences, Inc., Mountainview, CA), and the resultant suspensions were centrifuged for 30 min at 1500_g_ at 4°C. Each pellet was resuspended in 300 _μ_L of distilled water with inhibitor cocktails for immunochemical enrichment of exosomes from neuronal sources.7
Exosome suspensions were incubated with 2 _μ_g of mouse anti‐human CD171 (L1CAM neural adhesion protein) biotinylated antibody (clone 5G3, eBioscience, San Diego, CA) in 50 _μ_L of 3% BSA for 60 min at 20°C followed by addition of 10 _μ_L of Streptavidin‐Plus UltraLink resin (Pierce‐Thermo Scientific, Inc.) in 40 μ_L of 3% BSA.7 After incubation for 30 min at 20°C, centrifugation at 400_g for 5 min at 4°C, and removal of supernatants, pellets were resuspended in 50 μ_L of 0.05 mol/L glycine‐HCl (pH 3.0), incubated 10 min at 4°C, and recentrifuged for 10 min at 4000_g at 4°C. Each supernatant in a new Eppendorf tube received 5 _μ_L of 1 mol/L Tris‐HCl (pH 8.0) and 45 _μ_L of 3% BSA followed by 0.40 mL of mammalian protein extraction reagent (Thermo Scientific, Inc.) containing protease and phosphatase inhibitors, mixed and stored at−80°C.
Exosome proteins were quantified by human‐specific ELISAs for A_β_ 1‐42, P‐S396‐tau (Life Technologies/Invitrogen, Camarillo, CA), P‐T181‐tau (Innogenetics Division of Fujirebio US, Inc., Alpharetta, GA), REST (Cusabio, American Research Products, Inc., Waltham, MA), neurogranin (Cloud‐Clone, Inc., American Research Products, Inc.), cathepsin D (EMD Millipore Corp., Billerica, MA), and tetraspanin exosome marker human CD81 (American Research Products‐Cusabio), according to the suppliers' directions. One in ten representative preparations of exosomes was counted and CD63 levels were determined along with CD81. The mean value for all determinations of CD81 in each assay group was set at 1.00 and the relative values for each sample used to normalize their recovery. Units for all analytes are pg/neuronal‐derived exosomes in 1 mL of plasma (pg/ml). A_β_ 1‐42 and P‐S396‐tau in exosome extracts were quantified at a 1:2 (v:v) dilution, whereas CD81, P‐T181‐tau, REST, and cathepsin D were quantified at a 1:4 (v:v) dilution. Evidence for enrichment of exosomes from neuronal sources has been provided previously.7, 13
Statistical analyses
We used paired t tests to estimate mean change in protein levels over time, and we used linear regression to estimate mean P‐T181‐tau, P‐S‐396‐tau, A_β_ 1‐42, cathepsin D, REST, and neurogranin levels at the first draw using age at draw as the predictor. Next, we regressed the change score on age at first draw and time between draws (years). Last, we compared protein levels at the second draw to distributions obtained from 10 age‐ and sex‐matched AD patients for overlap. Plasma from AD patients was obtained from the National Institute on Aging plasma bank; AD diagnosis was made by neurological exam, psychometric testing, CSF protein analytes, and MRI using standard criteria.13 All analyses were conducted with SAS 9.4® (SAS Institute, Inc.; Cary, NC).
Results
Median time between draws was 8.7 years (range 3.3–11.4), and all but four pairs occurred at least 7 years apart. Mini‐Mental State Examination14 scores decreased slightly over the interval (P = 0.049; Table 1). Paired t tests showed that P‐T181‐tau (P = 0.0047), A_β_ 1‐42 (P = 0.014), cathepsin D (P = 0.0022), and REST (P = 0.0078) increased over time (Table 2). Neurogranin decreased (P < 0.0001) and P‐S396‐tau (P = 0.57) did not change. Regression revealed significant association between P‐S396‐tau change and age at first draw (P = 0.024), with older participants experiencing larger increases. However, significance did not persist (P = 0.31) after excluding the oldest participant (age 92), who also had the largest increase in P‐S396‐tau level. This participant was diagnosed with probable AD 2 years following the second blood draw. No other change scores were associated significantly with age at first draw or time between draws. Analyses were repeated excluding participants with draws less than 7 years apart. Results were similar and conclusions did not change.
Table 1.
Sample characteristics of cognitively intact University of Kentucky Alzheimer's Disease Center research volunteersa
| Characteristic | First draw (n = 18) | Second draw (n = 20) |
|---|---|---|
| Age, y | 69.3 (5.6) | 77.6 (6.1)b |
| Education, y | 16.3 (2.5) | 16.3 (2.5) |
| Female (%) | 55.6 | 50.0 |
| White race (%) | 100 | 100 |
| Mini‐mental state examination | 29.7 (0.5) | 29.4 (0.8)b |
| APOE‐_ε_4 carriers (n) | 3 | 3 |
| CSF A_β_ 1‐42 (pg/mL) | n/a | 300.1 (76.9) |
| CSF P‐T181‐tau (pg/mL) | n/a | 23.9 (8.4) |
| CSF total tau (pg/mL) | n/a | 57.3 (17.3) |
Table 2.
Mean normalized protein levels (pg/mL) in plasma neuronal‐derived exosomes.a
| First draw (n = 18) | Second draw (n = 20) | |
|---|---|---|
| P‐T181 tau | 73.4 (25.3) | 88.2 (22.7)c |
| P‐S396 tau | 10.9 (5.1)b | 11.4 (4.3) |
| A_β_1‐42 | 2.9 (1.3) | 3.8 (1.9)c |
| Cathepsin D | 1972.4 (1382.0) | 4074.6 (2117.4)c |
| REST | 906.4 (503.0) | 1437.0 (720.0)c |
| Neurogranin | 285.1 (76.8) | 224.8 (61.0)c |
Cathepsin D, REST, and neurogranin levels in CIS were distinct from those of AD patients (Fig. 1). There was frequent overlap between CIS and AD patients for A_β_ 1‐42 levels (6/20) and less frequent for P‐T181‐tau (1/20) and P‐S396‐tau (2/20) (Fig. 1). In comparison, some participants also had CSF protein levels in the AD range15: CSF A_β_ 1‐42 (2/20; <192 pg/mL), total tau (1/20; >93 pg/mL), and P‐T181‐tau (10/20; >23 pg/mL).
Figure 1.
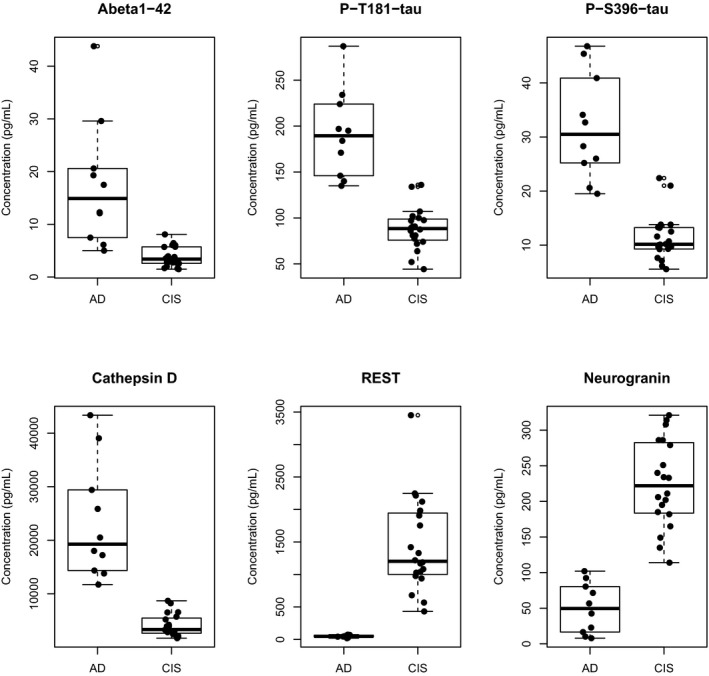
Levels of plasma exosomal proteins in age‐ and sex‐matched cognitively intact subjects (CIS) and patients with Alzheimer's disease(AD).
Discussion
This study provides evidence that neuronally derived exosome proteins A_β_ 1‐42, P‐T181‐tau, REST, and cathepsin D in older CIS increase over 3–11 years, whereas neurogranin decreases and P‐S396‐tau changes little over the same interval. Despite these changes, levels of REST, cathepsin D, and neurogranin were distinct from the ranges associated with AD. Importantly, we showed that levels of the neuroprotection factor REST, measured in plasma neuronal exosomes, increased with normal aging, similar to what has been shown in human brain tissue.16 We also reported results of the first analyses of neurogranin in plasma neuronal exosomes; results were similar to the decreased levels seen in human brain tissue7 in contrast to increases in CSF concentrations with aging and dementia.9, 10 This study further establishes the distinctive natures of the CSF and exosomal pathways for exportation from CNS neurons of proteins relevant to the pathogenesis of dementias.
Changes in established AD biomarkers, A_β_ 1‐42, P‐T181‐tau, and P‐S396‐tau, moved some levels for CIS into the range of measurements observed in AD patients, as has been observed with CSF analytes. Overlap may represent variability in the distribution of values but may also identify participants at increased risk for future AD diagnosis.17 Indeed, for the CIS who transitioned to Probable AD 2 years after the second draw, protein levels were in the AD range for both A_β_ 1‐42 (6.4 pg/mL) and P‐S396‐tau (22.4 pg/mL). Basic conclusions, except for the significance of P‐S396‐tau age associations, were not altered as a result of repeating statistical analyses excluding this participant.
Our data demonstrated that normal aging is associated with increases in certain AD biomarkers. Based on our prior work, preclinical AD cases may show mean protein levels similar to CIS on measures like A_β_ 1‐42, P‐S396‐tau, and P‐T181‐tau, although the upper range of measurement tends to be lower in CIS.5 In comparison, REST and cathepsin D levels may be completely distinct in preclinical AD and CIS.6, 7 Future determination of thresholds for transition from normal aging to AD is critically important for interpretation of such biomarkers including A_β_ 1‐42, P‐T181‐tau, REST, cathepsin D, and neurogranin. In contrast, P‐S396‐tau appears to exhibit distinctive specificity for the diagnosis of imminent or fulminant AD. This is consistent with our previous work demonstrating a lack of P‐S396‐tau elevations in FTD, and nonoverlapping P‐S396‐tau levels in controls who did not progress to AD over a 10‐year period compared to those with AD and those who eventually developed AD.5 The specificity of the antemortem CSF level of P‐S396‐tau epitope for fulminant AD or AD in situ with imminent clinical presentation has been published previously and parallels our findings in exosomes,18 which represent a less invasive, peripheral blood‐derived biomarker. While the utility of exosomal P‐S396‐tau for detection of fulminant AD appears consistent across studies, we note that this biomarker may lack diagnostic utility prior to the imminent development of symptomatic AD. Further research should address the temporal course of exosomal epitope changes heralding the onset of AD and the establishment of diagnostic thresholds for exosomal markers of preclinical AD.
Our findings add important information to existing literature on neuronally derived exosome‐based biomarkers, but there are limitations in interpretation. Although the participants are well‐characterized clinically, sample size is limited and may not be representative of the larger population of CIS. Additional participants may transition to AD in the future. Replication studies are needed to confirm our results.
Author contributions
E. L. A. performed the statistical analysis and drafted and revised the manuscript; G. A. J. evaluated participants and revised the manuscript; L. M. S. performed CSF analyses and revised the manuscript; J. Q. T. performed CSF analyses and revised the manuscript; E. J. G. developed the plasma analytical methodology, performed laboratory bench work, and drafted and revised the manuscript.
Conflict of Interest
EJG reports grants from NanoSomix, Inc., during the conduct of the study and grants from NanoSomix, Inc., outside the submitted work. In addition, Dr. Goetzl has a patent USA provisional regarding assay methods pending. LMS reports consulting fees from Eli Lilly and Janssen Research & Development regarding biomarker analyses and interpretation, and personal fees from Novartis, outside the submitted work. ELA, GAJ, and JQT report no conflicts of interest.
Acknowledgments
The authors are grateful to the UK‐ADC participants for their time and generosity and to Ms. Sonya Anderson (UK) for maintaining and distributing biospecimens.
References
- 1.O'Bryant SE, Gupta V, Henriksen K, et al. Guidelines for the standardization of preanalytic variables for blood‐based biomarker studies in Alzheimer's disease research. Alzheimers Dement 2015;11:549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo I, Bubacco L, Greggio E. Exosomes‐associated neurodegeneration and progression of Parkinson's disease. Am J Neurodegener Dis 2012;1:217–225. [PMC free article] [PubMed] [Google Scholar]
- 3.Marzesco AM, Janich P, Wilsch‐Brauninger M, et al. Release of extracellular membrane particles carrying the stem cell marker prominin‐1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci 2005;118:2849–2858. [DOI] [PubMed] [Google Scholar]
- 4.Bellingham SA, Guo BB, Coleman BM, et al. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases? Front Physiol 2012;3:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiandaca MS, Kapogiannis D, Mapstone M, et al. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case‐control study. Alzheimers Dement 2015;11:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goetzl EJ, Boxer A, Schwartz JB, et al. Low neural exosomal levels of cellular survival factors in Alzheimer's disease. Ann Clin Transl Neurol 2015;2:769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goetzl EJ, Boxer A, Schwartz JB, et al. Altered lysosomal proteins in neural‐derived plasma exosomes in preclinical Alzheimer disease. Neurology 2015;85:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidsson P, Blennow K. Neurochemical dissection of synaptic pathology in Alzheimer's disease. Int Psychogeriatr 1998;10:11–23. [DOI] [PubMed] [Google Scholar]
- 9.Portelius E, Zetterberg H, Skillbäck T, et al. Cerebrospinal fluid neurogranin: relation to cognition and neurodegeneration in Alzheimer's disease. Brain 2015;138:3373–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kester MI, Teunissen CE, Crimmins DL, et al. Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol 2015;72:1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt FA, Nelson PT, Abner E, et al. University of Kentucky Sanders‐Brown Healthy Brain Aging Volunteers: donor characteristics, procedures, and neuropathology. Curr Alzheimer Res 2012;9:724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw LM, Vanderstichele H, Knapik‐Czajka M, et al. Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta Neuropathol 2011;121:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kapogiannis D, Boxer A, Schwartz JB, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural‐derived blood exosomes of preclinical Alzheimer's disease. FASEB J 2015;29:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folstein M, Folstein SE, McHugh PR. “Mini‐Mental State”: a Practical Method for Grading the Cognitive State of Patients for the Clinician. J Psych Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 15.Shaw LM, Vanderstichele H, Knapik‐Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's Disease Neuroimaging Initiative Subjects. Ann Neurol 2009;65:430–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu T, Aron L, Zullo J, et al. REST and stress resistance in ageing and Alzheimer's disease. Nature 2014;507:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toledo JB, Zetterberg H, van Harten AC, et al. Alzheimer's disease cerebrospinal fluid biomarker in cognitively normal subjects. Brain 2015;138:2701–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu YY, He SS, Wang X, et al. Levels of nonphosphorylated and phosphorylated tau in cerebrospinal fluid of Alzheimer's disease patients: an ultrasensitive bienzyme‐substrate‐recycle enzyme‐linked immunosorbent assay. Am J Pathol 2002;160:1269–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]