(+) RNA virus replication compartments: a safe home for (most) viral replication (original) (raw)
Highlights
- •
(+) RNA virus replication compartments form two structural classes. - •
Both classes of replication compartments use cellular membrane curvature proteins. - •
Both classes of replication compartments manipulate de novo lipid synthesis. - •
Some double membrane vesicles use cellular lipid kinases and transfer proteins. - •
Limited transient replication may occur before replication compartment formation.
Abstract
This review describes recent advances in our understanding of the mechanisms by which (+) RNA viruses establish their replication niche.
Current Opinion in Microbiology 2016, 32:82–88
This review comes from a themed issue on Host-microbe interactions: viruses
Edited by Jonathan C Kagan
For a complete overview see the Issue and the Editorial
Available online 30th May 2016
http://dx.doi.org/10.1016/j.mib.2016.05.003
1369-5274/© 2016 Elsevier Ltd. All rights reserved.
Introduction
A hallmark of all (+) RNA viruses is their ability to sequester host intracellular membranes to generate replication compartments (RCs). These RCs contain viral RNA and proteins as well as several recruited host proteins and lipids that create a favorable environment for RNA replication. RCs may serve as platforms to concentrate viral RNA, proteins, and nucleotides, creating an appropriate replicase topology. Additionally, RCs form a barrier between viral RNA replication and the cytosol, which contains innate immune sensors and RNA degradation machinery. In this paper, we review in mechanistic detail the formation of viral RCs and highlight recent findings that have advanced our understanding of (+) RNA virus replication.
Replication compartment morphology
RCs are derived from different sources, depending on the virus, including the endoplasmic reticulum (ER), Golgi, peroxisomes, endosomes, mitochondria and plasma membrane [1]. For some viruses, the source of membranes is unimportant. Flock house virus (FHV) replication is unperturbed by targeting RCs to a different subcellular location [2]. However, other (+) RNA viruses such as hepatitis C virus (HCV) have distinct but spatially linked sites of replication and virion assembly that likely require specific localization of RC formation [3, 4•]. Multiple lines of evidence support a role for RCs as the site of viral replication, as opposed to a cellular response to viral infection. Immuno electron microscopy (EM) detects BrUTP incorporation into FHV viral RNA localized inside RCs [5], while long (>40 nucleotide) dsRNA, indicative of the RNA replication intermediate, is detected in RCs for several (+) RNA viruses [6, 7, 8, 9, 10, 11, 12•].
Although viral RCs have been described using standard EM for a long time, we have only begun to appreciate their fine structural details in the last decade. Advances in EM tomography (EMT) have enabled a high-resolution description of viral RCs for multiple viruses [3, 5, 7, 11, 12•, 13, 14, 15, 16, 17, 18, 19, 20]. Despite the evolutionary distance between these (+) strand RNA viruses, they appear to induce two general classes of membrane modifications: invaginations/spherules or double membrane vesicles (DMVs), suggesting conserved mechanisms behind their formation [21].
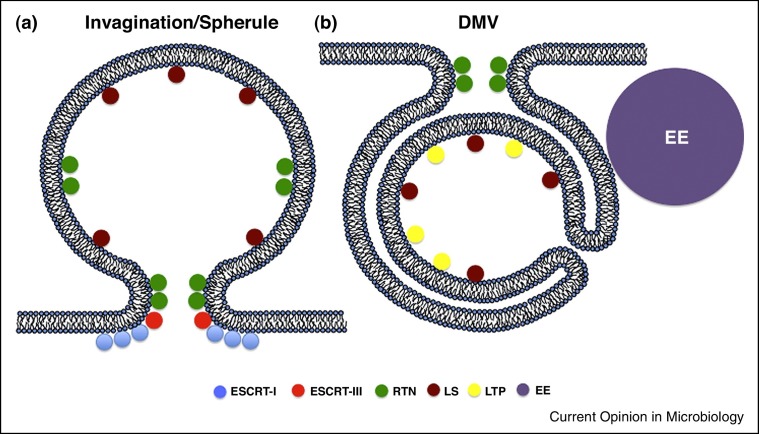
Invagination RCs were first visualized by EMT for FHV, which induces them at the outer mitochondrial membrane [5]. Invaginations entail the induction of negative membrane curvature (bending away from the cytosol) on an intracellular membrane to yield a vesicle budded into the luminal space (Figure 1a). These invaginations have portions of positive membrane curvature (bending towards the cytosol) at their neck that keeps the vesicle attached to the organelle from which it originated [22].
Figure 1.
Host cell pathways involved in replication compartment formation of (+) RNA viruses. (a) Schematic illustration of an invagination replication compartment with overall negative membrane curvature. (b) Schematic illustration of a DMV replication compartment with overall positive membrane curvature. RTN: reticulon; LS: lipid synthesis; LTP: lipid transfer protein; ESCRT: endosomal sorting complexes required for transport; EE: early endosome.
Capsids have not been observed in RCs, leading to a model wherein viral RNA is replicated inside RCs and then (+) RNAs are transported outside to sites of virion assembly. EMT of dengue virus (DENV)-infected cells revealed invaginations into the ER lumen that are connected to the cytoplasm via a neck-like opening [7, 19]. On the cytosolic side of the neck are nucleocapsids, suggesting that viral (+) RNA is transported through the neck for capsid assembly immediately adjacent to the RC [7]. ER invaginations that retain a pore to the cytosol have also been shown for other flaviviruses, including tick borne encephalitis virus [18], West Nile virus (WNV) [13] and Langat virus [17].
Other viruses that induce invaginations include brome mosaic virus (BMV), tombusvirus and the togaviruses (Table 1). Togavirus infection leads to formation of cytopathic vacuoles, which are modified endosomes, lysosomes and internalized plasma membrane fragments of around 600–2000 nm that accommodate invaginations to create replication compartments [14, 23, 24].
Table 1.
(+) RNA viruses and their replication compartments
| Family | Bromoviridae | Flaviviridae | Flaviviridae | Nodaviridae | Togaviridae | Togaviridae | Tombusviridae |
|---|---|---|---|---|---|---|---|
| Genus | Bromovirus | Flavivirus | Flavivirus | Alphanodavirus | Alphavirus | Rubivirus | Tombusvirus |
| Species | BMV | DENV | WNV | FHV | SFV | RUBV | TBSV |
| Replication compartment | Invaginations | Invaginations | Invaginations | Invaginations | Invaginations | Invaginations | Invaginations |
| Membrane source | ER | ER | ER | Mitochondria | PM/Endosomes | Endosomes/Lysosomes | Perixosomes |
| Viral proteins | 1a [32] | NS4A, NS4B [65, 66, 67] | NS4A, NS4B [68, 69] | Protein A [70] | P123 [71] | P150, P90 [72] | p33 [73] |
| Host Pathways | RTN, ESCRT, LS | LS | LS | LS | LS | LS | ESCRT, LS |
| Family | Arteriviridae | Coronaviridae | Dicistroviridae | Flaviviridae | Picornaviridae | Picornaviridae |
|---|---|---|---|---|---|---|
| Genus | Arterivirus | Coronavirus | Cripavirus | Hepacivirus | Enterovirus | Cardiovirus |
| Species | EAV | SARS-CoV | Drosophila C | HCV | PV, CBV3, EV71 | EMCV |
| Replication compartment | DMV | DMV | DMV | DMV | DMV | DMV |
| Membrane source | ER | ER | Golgi | ER | Golgi/ER | Golgi/ER |
| Viral proteins | nsp2, nsp3 [74, 75] | nsp3, nsp4, nsp6 [76] | — | NS4B, NS5A [77, 78] | 2B, 2C, 3A [79, 80] | 2B, 2C, 3A [81] |
| Host pathways | ERAD | ERAD | LS | LS, LTP | LS, LTP, EE, RTN | LS, LTP |
The other class of RCs is DMVs, which are more complicated structurally and frequently accompanied by other membrane rearrangements (Figure 1b). Prototype viruses of the DMV RC class include the coronaviruses, picornaviruses, and hepatitis C virus (HCV). HCV DMVs are ER-derived vesicles that appear sealed from the cytoplasm [3]. Since there is not an obvious neck, as with the invagination RCs, it is unclear how HCV RNA could traffic from the RC to the site of virion assembly, the lipid droplet. It has been proposed that components of the nuclear pore complex are recruited to HCV RCs to regulate traffic into and out of the RC [25, 26•]. HCV also induces single-membrane and multi-membrane vesicles that are not associated with replicating viral RNA, but may represent endosomes and/or autophagosomes, which are sometimes observed in the proximity of RCs [10, 27, 28, 29].
For enteroviruses, the replication compartments are a mix of single and double membrane vesicles that may originate from multiple cellular membrane sources. EMT analysis revealed that early in infection (∼2 hpi) RCs consist of mainly single-membrane tubules that transform into larger DMVs and subsequently into multilamellar structures as infection progresses [15]. Formation of DMVs is observed during coronavirus and arterivirus replication as well [16, 30]. Coronavirus DMVs range in size from 150 to 300 nm and appear as a network of membranes continuous with the rough-ER [11, 31]. The DMV inner membrane appears as a closed compartment and it is not clear how import of substrates or export of RNA is achieved.
Mechanisms of replication compartment formation
Induction of membrane curvature via viral and cellular proteins
Multiple processes that curve cellular membranes have been described, such as insertion of proteins or irregularly shaped lipids [1]. Many viral proteins have been implicated in the formation of RCs (Table 1). These may alter membrane shape directly, by associating with membranes and inducing curvature; or indirectly, by recruiting cellular factors to alter membrane morphology. A direct role for viral proteins altering membrane curvature has been challenging to prove because RC formation has not been reconstituted in vitro for most viruses. Many of the viral proteins implicated in RC formation have properties that may alter membrane morphology, such as multiple transmembrane domains and/or amphipathic helices, in addition to protein oligomerization.
Two examples of recruiting cellular machinery to modify membrane curvature have been defined thus far: reticulons and the endosomal sorting complexes required for transport (ESCRT) proteins. BMV 1A protein, which lines the interior of the spherule and is postulated to induce negative membrane curvature, recruits reticulons to invaginations [32]. Reticulons induce positive membrane curvature, which may promote two functions: (i) counter-balance the 1a negative membrane curvature to enable spherule expansion or (ii) promote positive membrane curvature at the neck of the spherule (Figure 1a). Reticulons may also be involved in DMV RC formation since multiple enteroviruses encode 2C proteins that interact with reticulon 3 within its RC to promote replication [33].
It has been noted that there are structural, and perhaps mechanistic commonalities between invaginations and retroviral budding [34]. Given these similarities, the role of the ESCRT machinery, which is required for retroviral budding, has been investigated in two plant viruses that form invaginations: tomato bushy shunt virus (TBSV) and BMV. TBSV p33 recruits cellular ESCRT-I proteins to induce formation of its RC [35, 36], while ESCRT-III proteins are required for proper RC morphology [36, 37]. This suggests a role for ESCRT-I in initiating invagination, with ESCRT-III required to produce the correct RC morphology, possibly closing the neck of the invagination via positive membrane curvature with incomplete membrane scission. Thus, there are similarities between TBSV RC formation and the steps of retroviral budding before membrane scission. BMV also co-opts ESCRT-III factors for proper BMV spherule formation [38•]. Unlike TBSV and HIV, BMV does not require components of ESCRT-I. Presumably, its 1a protein can initiate RC invagination without the need for ESCRT-I.
De novo lipid synthesis
A second strategy for modulating membrane curvature is to modify its lipid composition, either via lipid transfer proteins (LTPs) or de novo lipid synthesis. This can involve the insertion of lipids that (i) increase membrane fluidity (cholesterol, sphingomyelin, or unsaturated phospholipids), (ii) induce positive membrane curvature (ceramide), or (iii) induce negative membrane curvature (lysophosphatidylcholine). Modifying membrane lipid composition is likely crucial for RC formation, virion envelopment (and possibly assembly), and virion infectivity. A key node for viral manipulation of lipid synthesis is fatty acid synthase (FASN), which generates palmitate (C16:0). Palmitate is either post-translationally linked to proteins or further modified by lipid synthetic enzymes to produce the bulk of membrane lipids. Replication of multiple (+) RNA viruses requires FASN [39, 40, 41, 42, 43, 44]. HCV increases the expression of FASN, although FASN does not appear to localize to RCs [43]. FASN is redistributed to RCs during DENV [39, 45] and WNV infection [42]. DENV NS3 binds FASN, recruits it to RCs stimulates its activity and de novo lipid synthesis [39]. This is required for DENV replication and lipid alterations at the RC, including increases in sphingomyelin and ceramide upon DENV infection of mosquito cells [41].
Lipids downstream of FASN, including phosphatidylcholine (PC) and phosphatidylethanolamine (PE), play essential roles in the replication of multiple viruses. Picornaviruses stimulate the activity of the fatty acid modifying enzyme, long chain acyl CoA synthetase 3 (Acsl3), which results in increased PC accumulation at RCs [46]. Although PC and PE are increased during HCV infection [47], only PC accumulates in HCV RCs [48•]. In the case of BMV RC formation, the BMV 1a protein interacts with and recruits choline requiring 2 (Cho2p), a cellular enzyme involved in PC synthesis, to sites of viral replication, which is essential for BMV replication [48•]. Alternatively, TBSV RC formation relies on PE. TBSV p33 promotes cellular redistribution of PE and in vitro reconstitution of TBSV RCs from liposomes revealed an exclusive role for PE in maintaining TBSV replication. [49•]. Modulation of lipid synthesis is thus a general strategy for formation of both classes of RCs.
Phospholipid kinases and lipid transfer
The picornaviruses and HCV, both of which form DMVs, convergently evolved strategies that coopt phosphatidylinositol (PI)-4 kinases to stimulate PI(4)P production at RCs. PI(4)P recruits LTPs to RCs leading to alterations in the lipid composition of the RC. HCV NS5A binds and activates the ER resident kinase, PI4K-IIIαat RCs, which is essential for HCV replication and appropriate RC morphology [50, 51, 52, 53, 54]. Two LTPs that bind PI(4)P are implicated in HCV replication: four-phosphate adaptor protein 2 (FAPP2) and oxysterol-binding protein (OSBP). FAPP2 is recruited to viral RCs where it might supply glycosphingolipids [55]. OSBP interacts with NS5A and its silencing leads to inhibition of both HCV replication and particle secretion [56, 57, 58]. OSBP is recruited to HCV replication compartments in a PI(4)P-dependent manner and mediates transport of cholesterol to RCs [56]. PI4K-IIIα has two other functions in HCV replication. It modulates NS5A phosphorylation and the downstream product of PI(4)P, PI(4,5)P2, also accumulates at RCs and is bound by NS5A, which may be important for appropriate replicase topology [59, 60].
Picornaviruses require distinct PI4 kinases, which differ based on their subcellular location. Enteroviruses and human rhinoviruses manipulate the Golgi-localized PI4K-IIIβ [61, 62], while encephalomyocarditis virus (EMCV) requires PI4K-IIIα for RC formation [63•]. Similarly to HCV infection, a primary role for these kinases is to recruit OSBP to promote cholesterol accumulation [62, 63•].
Picornaviruses modulate two other lipid transport pathways to promote RC formation. Long chain fatty acids that are imported in the infected cell are diverted from storage as triglycerides in lipid droplets, and instead are transported to the viral RCs where they are substrates for PC synthesis [46]. A second source of cholesterol for RCs, in addition to OSBP, is retrograde trafficking from the plasma membrane to RCs via early endosomes during enterovirus infection [64]. This may also occur in HCV infection, since HCV RCs are rich in cholesterol and early endosomes localize in proximity of RCs [9, 27, 28, 29].
Transient replication before replication compartment formation?
A spatiotemporal analysis of HCV replication using single molecule RNA detection of viral (+) and (−) RNA uncovered a surprise. Low levels of HCV RNA replication occur soon after infection, which are then shutoff before the beginning of detectable RC formation [4•]. The interpretation was that a few ‘backup copies’ of HCV RNA are made to lessen the reliance on the integrity of the initially infecting genomic RNA. RC formation is then required for robust RNA replication. If this interpretation is correct, that leaves interesting unanswered questions as to the subcellular location of these RNAs and how they are protected from innate immune sensors and/or RNA degradation machinery.
Conclusions
Much progress has been made in defining the structural composition of (+) RNA virus RCs and mechanisms associated with their formation. In particular, our understanding of the formation of invaginations in model plant (+) RNA virus infection is becoming quite advanced. The development of a cell free system for TBSV invaginations will allow biochemical confirmation of much of these proposed mechanisms. However, numerous questions remain, particularly in the formation of DMVs. How is DMV structure achieved? Additionally, the role of PI4 kinases in DMV infection is not entirely clear. Enteroviruses can easily mutate to replicate in their absence, and they appear to have other roles in HCV infection beyond LTP recruitment. Finally, can the success of targeting proteins involved in RC formation as antiviral strategies in cell culture be extended to patient therapeutic strategies?
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
- • of special interest
Acknowledgements
We thank Yasmine Baktash and Tristan Jordan for critical reading of the manuscript. G.R. is supported by the National Institutes of Health (AI080703 and DK102883) and American Cancer Society (118676-RSG-10-059-01-MPC). A.S. is supported by the American Cancer Society (PF-12-210-01-MPC).
References
- 1.Miller S., Krijnse-Locker J. Modification of intracellular membrane structures for virus replication. Nat Rev Microbiol. 2008;6:363–374. doi: 10.1038/nrmicro1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller D.J., Schwartz M.D., Dye B.T., Ahlquist P. Engineered retargeting of viral RNA replication complexes to an alternative intracellular membrane. J Virol. 2003;77:12193–12202. doi: 10.1128/JVI.77.22.12193-12202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero-Brey I., Merz A., Chiramel A., Lee J.Y., Chlanda P., Haselman U., Santarella-Mellwig R., Habermann A., Hoppe S., Kallis S. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLoS Pathog. 2012;8:e1003056. doi: 10.1371/journal.ppat.1003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Shulla A., Randall G. Spatiotemporal analysis of hepatitis C virus infection. PLoS Pathog. 2015;11:e1004758. doi: 10.1371/journal.ppat.1004758. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors use single molecule detection of (+) and (−) HCV RNAs to determine their spatiotemporal regulation during infection and reveal low levels of dsRNA intermediates before replication compartment formation.
- 5.Kopek B.G., Perkins G., Miller D.J., Ellisman M.H., Ahlquist P. Three-dimensional analysis of a viral RNA replication complex reveals a virus-induced mini-organelle. PLoS Biol. 2007;5:e220. doi: 10.1371/journal.pbio.0050220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westaway E.G., Mackenzie J.M., Kenney M.T., Jones M.K., Khromykh A.A. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J Virol. 1997;71:6650–6661. doi: 10.1128/jvi.71.9.6650-6661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsch S., Miller S., Romero-Brey I., Merz A., Bleck C.K., Walther P., Fuller S.D., Antony C., Krijnse-Locker J., Bartenschlager R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe. 2009;5:365–375. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uchida L., Espada-Murao L.A., Takamatsu Y., Okamoto K., Hayasaka D., Yu F., Nabeshima T., Buerano C.C., Morita K. The dengue virus conceals double-stranded RNA in the intracellular membrane to escape from an interferon response. Sci Rep. 2014;4:7395. doi: 10.1038/srep07395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul D., Hoppe S., Saher G., Krijnse-Locker J., Bartenschlager R. Morphological and biochemical characterization of the membranous hepatitis C virus replication compartment. J Virol. 2013;87:10612–10627. doi: 10.1128/JVI.01370-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferraris P., Blanchard E., Roingeard P. Ultrastructural and biochemical analyses of hepatitis C virus-associated host cell membranes. J Gen Virol. 2010;91:2230–2237. doi: 10.1099/vir.0.022186-0. [DOI] [PubMed] [Google Scholar]
- 11.Knoops K., Kikkert M., Worm S.H., Zevenhoven-Dobbe J.C., van der Meer Y., Koster A.J., Mommaas A.M., Snijder E.J. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6:e226. doi: 10.1371/journal.pbio.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.de Castro I.F., Fernandez J.J., Barajas D., Nagy P.D., Risco C. Three-dimensional imaging of the intracellular assembly of a functional viral RNA replicase complex. J Cell Sci. 2016 doi: 10.1242/jcs.181586. [DOI] [PubMed] [Google Scholar]; The authors of this study use metal-tagging TEM to visualize viral replicase molecules assemble within the replication compartment.
- 13.Gillespie L.K., Hoenen A., Morgan G., Mackenzie J.M. The endoplasmic reticulum provides the membrane platform for biogenesis of the flavivirus replication complex. J Virol. 2010;84:10438–10447. doi: 10.1128/JVI.00986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontana J., Lopez-Iglesias C., Tzeng W.P., Frey T.K., Fernandez J.J., Risco C. Three-dimensional structure of Rubella virus factories. Virology. 2010;405:579–591. doi: 10.1016/j.virol.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Limpens R.W., van der Schaar H.M., Kumar D., Koster A.J., Snijder E.J., van Kuppeveld F.J., Barcena M. The transformation of enterovirus replication structures: a three-dimensional study of single- and double-membrane compartments. MBio. 2011:2. doi: 10.1128/mBio.00166-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knoops K., Barcena M., Limpens R.W., Koster A.J., Mommaas A.M., Snijder E.J. Ultrastructural characterization of arterivirus replication structures: reshaping the endoplasmic reticulum to accommodate viral RNA synthesis. J Virol. 2012;86:2474–2487. doi: 10.1128/JVI.06677-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Offerdahl D.K., Dorward D.W., Hansen B.T., Bloom M.E. A three-dimensional comparison of tick-borne flavivirus infection in mammalian and tick cell lines. PLoS One. 2012;7:e47912. doi: 10.1371/journal.pone.0047912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miorin L., Romero-Brey I., Maiuri P., Hoppe S., Krijnse-Locker J., Bartenschlager R., Marcello A. Three-dimensional architecture of tick-borne encephalitis virus replication sites and trafficking of the replicated RNA. J Virol. 2013;87:6469–6481. doi: 10.1128/JVI.03456-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junjhon J., Pennington J.G., Edwards T.J., Perera R., Lanman J., Kuhn R.J. Ultrastructural characterization and three-dimensional architecture of replication sites in dengue virus-infected mosquito cells. J Virol. 2014;88:4687–4697. doi: 10.1128/JVI.00118-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao X., Jin X., Zhang X., Li Y., Wang C., Wang X., Hong J., Wang X., Li D., Zhang Y. Morphogenesis of endoplasmic reticulum membrane-invaginated vesicles during beet black scorch virus infection: role of auxiliary replication protein and new implications of three-dimensional architecture. J Virol. 2015;89:6184–6195. doi: 10.1128/JVI.00401-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul D., Bartenschlager R. Architecture and biogenesis of plus-strand RNA virus replication factories. World J Virol. 2013;2:32–48. doi: 10.5501/wjv.v2.i2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.den Boon J.A., Ahlquist P. Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu Rev Microbiol. 2010;64:241–256. doi: 10.1146/annurev.micro.112408.134012. [DOI] [PubMed] [Google Scholar]
- 23.Kujala P., Ikaheimonen A., Ehsani N., Vihinen H., Auvinen P., Kaariainen L. Biogenesis of the Semliki Forest virus RNA replication complex. J Virol. 2001;75:3873–3884. doi: 10.1128/JVI.75.8.3873-3884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frolova E.I., Gorchakov R., Pereboeva L., Atasheva S., Frolov I. Functional Sindbis virus replicative complexes are formed at the plasma membrane. J Virol. 2010;84:11679–11695. doi: 10.1128/JVI.01441-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neufeldt C.J., Joyce M.A., Levin A., Steenbergen R.H., Pang D., Shields J., Tyrrell D.L., Wozniak R.W. Hepatitis C virus-induced cytoplasmic organelles use the nuclear transport machinery to establish an environment conducive to virus replication. PLoS Pathog. 2013;9:e1003744. doi: 10.1371/journal.ppat.1003744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Neufeldt C.J., Joyce M.A., Van Buuren N., Levin A., Kirkegaard K., Gale M., Jr., Tyrrell D.L., Wozniak R.W. The hepatitis C virus-induced membranous web and associated nuclear transport machinery limit access of pattern recognition receptors to viral replication sites. PLoS Pathog. 2016;12:e1005428. doi: 10.1371/journal.ppat.1005428. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides further evidence of nuclear pore complexes localizing to HCV replication sites and serving as a pore for RNA to exit these sites.
- 27.Stone M., Jia S., Heo W.D., Meyer T., Konan K.V. Participation of rab5, an early endosome protein, in hepatitis C virus RNA replication machinery. J Virol. 2007;81:4551–4563. doi: 10.1128/JVI.01366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coller K.E., Berger K.L., Heaton N.S., Cooper J.D., Yoon R., Randall G. RNA interference and single particle tracking analysis of hepatitis C virus endocytosis. PLoS Pathog. 2009;5:e1000702. doi: 10.1371/journal.ppat.1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiches G.N., Eyre N.S., Aloia A.L., Van Der Hoek K., Betz-Stablein B., Luciani F., Chopra A., Beard M.R. HCV RNA traffic and association with NS5A in living cells. Virology. 2016;493:60–74. doi: 10.1016/j.virol.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 30.Hagemeijer M.C., Rottier P.J., de Haan C.A. Biogenesis and dynamics of the coronavirus replicative structures. Viruses. 2012;4:3245–3269. doi: 10.3390/v4113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snijder E.J., van der Meer Y., Zevenhoven-Dobbe J., Onderwater J.J., van der Meulen J., Koerten H.K., Mommaas A.M. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J Virol. 2006;80:5927–5940. doi: 10.1128/JVI.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz A., Wang X., Ahlquist P. Membrane-shaping host reticulon proteins play crucial roles in viral RNA replication compartment formation and function. Proc Natl Acad Sci U S A. 2010;107:16291–16296. doi: 10.1073/pnas.1011105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang W.F., Yang S.Y., Wu B.W., Jheng J.R., Chen Y.L., Shih C.H., Lin K.H., Lai H.C., Tang P., Horng J.T. Reticulon 3 binds the 2C protein of enterovirus 71 and is required for viral replication. J Biol Chem. 2007;282:5888–5898. doi: 10.1074/jbc.M611145200. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz M., Chen J., Janda M., Sullivan M., den Boon J., Ahlquist P. A positive-strand RNA virus replication complex parallels form and function of retrovirus capsids. Mol Cell. 2002;9:505–514. doi: 10.1016/s1097-2765(02)00474-4. [DOI] [PubMed] [Google Scholar]
- 35.Barajas D., Jiang Y., Nagy P.D. A unique role for the host ESCRT proteins in replication of Tomato bushy stunt virus. PLoS Pathog. 2009;5:e1000705. doi: 10.1371/journal.ppat.1000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovalev N., de Castro Martin I.F., Pogany J., Barajas D., Pathak K., Risco C., Nagy P.D. Role of viral RNA and co-opted cellular ESCRT-I and ESCRT-III factors in formation of tombusvirus spherules harboring the tombusvirus replicase. J Virol. 2016;90:3611–3626. doi: 10.1128/JVI.02775-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barajas D., Martin I.F., Pogany J., Risco C., Nagy P.D. Noncanonical role for the host Vps4 AAA+ ATPase ESCRT protein in the formation of Tomato bushy stunt virus replicase. PLoS Pathog. 2014;10:e1004087. doi: 10.1371/journal.ppat.1004087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Diaz A., Zhang J., Ollwerther A., Wang X., Ahlquist P. Host ESCRT proteins are required for bromovirus RNA replication compartment assembly and function. PLoS Pathog. 2015;11:e1004742. doi: 10.1371/journal.ppat.1004742. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides further evidence of the involvement of host ESCRT proteins in BMV replication and especially ESCRT-III in spherule formation.
- 39.Heaton N.S., Perera R., Berger K.L., Khadka S., Lacount D.J., Kuhn R.J., Randall G. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc Natl Acad Sci U S A. 2010;107:17345–17350. doi: 10.1073/pnas.1010811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fogg M.H., Teterina N.L., Ehrenfeld E. Membrane requirements for uridylylation of the poliovirus VPg protein and viral RNA synthesis in vitro. J Virol. 2003;77:11408–11416. doi: 10.1128/JVI.77.21.11408-11416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perera R., Riley C., Isaac G., Hopf-Jannasch A.S., Moore R.J., Weitz K.W., Pasa-Tolic L., Metz T.O., Adamec J., Kuhn R.J. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog. 2012;8:e1002584. doi: 10.1371/journal.ppat.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin-Acebes M.A., Blazquez A.B., Jimenez de Oya N., Escribano-Romero E., Saiz J.C. West Nile virus replication requires fatty acid synthesis but is independent on phosphatidylinositol-4-phosphate lipids. PLoS One. 2011;6:e24970. doi: 10.1371/journal.pone.0024970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nasheri N., Joyce M., Rouleau Y., Yang P., Yao S., Tyrrell D.L., Pezacki J.P. Modulation of fatty acid synthase enzyme activity and expression during hepatitis C virus replication. Chem Biol. 2013;20:570–582. doi: 10.1016/j.chembiol.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Cherry S., Kunte A., Wang H., Coyne C., Rawson R.B., Perrimon N. COPI activity coupled with fatty acid biosynthesis is required for viral replication. PLoS Pathog. 2006;2:e102. doi: 10.1371/journal.ppat.0020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang W.C., Lin R.J., Liao C.L., Lin Y.L. Rab18 facilitates dengue virus infection by targeting fatty acid synthase to sites of viral replication. J Virol. 2014;88:6793–6804. doi: 10.1128/JVI.00045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nchoutmboube J.A., Viktorova E.G., Scott A.J., Ford L.A., Pei Z., Watkins P.A., Ernst R.K., Belov G.A. Increased long chain acyl-Coa synthetase activity and fatty acid import is linked to membrane synthesis for development of picornavirus replication organelles. PLoS Pathog. 2013;9:e1003401. doi: 10.1371/journal.ppat.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diamond D.L., Syder A.J., Jacobs J.M., Sorensen C.M., Walters K.A., Proll S.C., McDermott J.E., Gritsenko M.A., Zhang Q., Zhao R. Temporal proteome and lipidome profiles reveal hepatitis C virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog. 2010;6:e1000719. doi: 10.1371/journal.ppat.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Zhang J., Zhang Z., Chukkapalli V., Nchoutmboube J.A., Li J., Randall G., Belov G.A., Wang X. Positive-strand RNA viruses stimulate host phosphatidylcholine synthesis at viral replication sites. Proc Natl Acad Sci U S A. 2016;113:E1064–E1073. doi: 10.1073/pnas.1519730113. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals that phosphatidylcholine is enriched at replication sites of BMV, HCV and polivirus. The authors also elucidate the mechanism by which BMV 1a protein recruits the lipid synthesis enzyme, Cho2p, to viral replication sites.
- 49•.Xu K., Nagy P.D. RNA virus replication depends on enrichment of phosphatidylethanolamine at replication sites in subcellular membranes. Proc Natl Acad Sci U S A. 2015;112:E1782–E1791. doi: 10.1073/pnas.1418971112. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study determines that TBSV, unlike BMV, HCV and poliovirus, requires enrichment of phosphatidylethanolamine at replication sites and the p33 protein is responsible for redistribution of phosphatidylethanolamine to viral replication sites.
- 50.Berger K.L., Cooper J.D., Heaton N.S., Yoon R., Oakland T.E., Jordan T.X., Mateu G., Grakoui A., Randall G. Roles for endocytic trafficking and phosphatidylinositol 4-kinase III alpha in hepatitis C virus replication. Proc Natl Acad Sci U S A. 2009;106:7577–7582. doi: 10.1073/pnas.0902693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tai A.W., Benita Y., Peng L.F., Kim S.S., Sakamoto N., Xavier R.J., Chung R.T. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe. 2009;5:298–307. doi: 10.1016/j.chom.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reiss S., Rebhan I., Backes P., Romero-Brey I., Erfle H., Matula P., Kaderali L., Poenisch M., Blankenburg H., Hiet M.S. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe. 2011;9:32–45. doi: 10.1016/j.chom.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berger K.L., Kelly S.M., Jordan T.X., Tartell M.A., Randall G. Hepatitis C virus stimulates the phosphatidylinositol 4-kinase III alpha-dependent phosphatidylinositol 4-phosphate production that is essential for its replication. J Virol. 2011;85:8870–8883. doi: 10.1128/JVI.00059-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tai A.W., Salloum S. The role of the phosphatidylinositol 4-kinase PI4KA in hepatitis C virus-induced host membrane rearrangement. PLoS One. 2011;6:e26300. doi: 10.1371/journal.pone.0026300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan I., Katikaneni D.S., Han Q., Sanchez-Felipe L., Hanada K., Ambrose R.L., Mackenzie J.M., Konan K.V. Modulation of hepatitis C virus genome replication by glycosphingolipids and four-phosphate adaptor protein 2. J Virol. 2014;88:12276–12295. doi: 10.1128/JVI.00970-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H., Perry J.W., Lauring A.S., Neddermann P., De Francesco R., Tai A.W. Oxysterol-binding protein is a phosphatidylinositol 4-kinase effector required for HCV replication membrane integrity and cholesterol trafficking. Gastroenterology. 2014;146:1373–1385. doi: 10.1053/j.gastro.2014.02.002. e1371-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amako Y., Sarkeshik A., Hotta H., Yates J., 3rd, Siddiqui A. Role of oxysterol binding protein in hepatitis C virus infection. J Virol. 2009;83:9237–9246. doi: 10.1128/JVI.00958-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amako Y., Syed G.H., Siddiqui A. Protein kinase D negatively regulates hepatitis C virus secretion through phosphorylation of oxysterol-binding protein and ceramide transfer protein. J Biol Chem. 2011;286:11265–11274. doi: 10.1074/jbc.M110.182097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harak C., Radujkovic D., Taveneau C., Reiss S., Klein R., Bressanelli S., Lohmann V. Mapping of functional domains of the lipid kinase phosphatidylinositol 4-kinase type III alpha involved in enzymatic activity and hepatitis C virus replication. J Virol. 2014;88:9909–9926. doi: 10.1128/JVI.01063-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho N.J., Lee C., Pang P.S., Pham E.A., Fram B., Nguyen K., Xiong A., Sklan E.H., Elazar M., Koytak E.S. Phosphatidylinositol 4,5-bisphosphate is an HCV NS5A ligand and mediates replication of the viral genome. Gastroenterology. 2015;148:616–625. doi: 10.1053/j.gastro.2014.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsu N.Y., Ilnytska O., Belov G., Santiana M., Chen Y.H., Takvorian P.M., Pau C., van der Schaar H., Kaushik-Basu N., Balla T. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 2010;141:799–811. doi: 10.1016/j.cell.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roulin P.S., Lotzerich M., Torta F., Tanner L.B., van Kuppeveld F.J., Wenk M.R., Greber U.F. Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host Microbe. 2014;16:677–690. doi: 10.1016/j.chom.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 63•.Dorobantu C.M., Albulescu L., Harak C., Feng Q., van Kampen M., Strating J.R., Gorbalenya A.E., Lohmann V., van der Schaar H.M., van Kuppeveld F.J. Modulation of the host lipid landscape to promote RNA virus replication: the picornavirus encephalomyocarditis virus converges on the pathway used by hepatitis C virus. PLoS Pathog. 2015;11:e1005185. doi: 10.1371/journal.ppat.1005185. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides evidence that EMCV, unlike the related poliovirus, requires the enzymatic activity of the ER-resident PI4K-IIIa for its replication.
- 64.Ilnytska O., Santiana M., Hsu N.Y., Du W.L., Chen Y.H., Viktorova E.G., Belov G., Brinker A., Storch J., Moore C. Enteroviruses harness the cellular endocytic machinery to remodel the host cell cholesterol landscape for effective viral replication. Cell Host Microbe. 2013;14:281–293. doi: 10.1016/j.chom.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stern O., Hung Y.F., Valdau O., Yaffe Y., Harris E., Hoffmann S., Willbold D., Sklan E.H. An N-terminal amphipathic helix in dengue virus nonstructural protein 4A mediates oligomerization and is essential for replication. J Virol. 2013;87:4080–4085. doi: 10.1128/JVI.01900-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller S., Kastner S., Krijnse-Locker J., Buhler S., Bartenschlager R. The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. J Biol Chem. 2007;282:8873–8882. doi: 10.1074/jbc.M609919200. [DOI] [PubMed] [Google Scholar]
- 67.Zou J., Xie X., Wang Q.Y., Dong H., Lee M.Y., Kang C., Yuan Z., Shi P.Y. Characterization of dengue virus NS4A and NS4B protein interaction. J Virol. 2015;89:3455–3470. doi: 10.1128/JVI.03453-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roosendaal J., Westaway E.G., Khromykh A., Mackenzie J.M. Regulated cleavages at the West Nile virus NS4A-2K-NS4B junctions play a major role in rearranging cytoplasmic membranes and Golgi trafficking of the NS4A protein. J Virol. 2006;80:4623–4632. doi: 10.1128/JVI.80.9.4623-4632.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaufusi P.H., Kelley J.F., Yanagihara R., Nerurkar V.R. Induction of endoplasmic reticulum-derived replication-competent membrane structures by West Nile virus non-structural protein 4B. PLoS One. 2014;9:e84040. doi: 10.1371/journal.pone.0084040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller D.J., Ahlquist P. Flock house virus RNA polymerase is a transmembrane protein with amino-terminal sequences sufficient for mitochondrial localization and membrane insertion. J Virol. 2002;76:9856–9867. doi: 10.1128/JVI.76.19.9856-9867.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salonen A., Vasiljeva L., Merits A., Magden J., Jokitalo E., Kaariainen L. Properly folded nonstructural polyprotein directs the semliki forest virus replication complex to the endosomal compartment. J Virol. 2003;77:1691–1702. doi: 10.1128/JVI.77.3.1691-1702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fontana J., Tzeng W.P., Calderita G., Fraile-Ramos A., Frey T.K., Risco C. Novel replication complex architecture in rubella replicon-transfected cells. Cell Microbiol. 2007;9:875–890. doi: 10.1111/j.1462-5822.2006.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagy P.D., Barajas D., Pogany J. Host factors with regulatory roles in tombusvirus replication. Curr Opin Virol. 2012;2:691–698. doi: 10.1016/j.coviro.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Posthuma C.C., Pedersen K.W., Lu Z., Joosten R.G., Roos N., Zevenhoven-Dobbe J.C., Snijder E.J. Formation of the arterivirus replication/transcription complex: a key role for nonstructural protein 3 in the remodeling of intracellular membranes. J Virol. 2008;82:4480–4491. doi: 10.1128/JVI.02756-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snijder E.J., van Tol H., Roos N., Pedersen K.W. Non-structural proteins 2 and 3 interact to modify host cell membranes during the formation of the arterivirus replication complex. J Gen Virol. 2001;82:985–994. doi: 10.1099/0022-1317-82-5-985. [DOI] [PubMed] [Google Scholar]
- 76.Angelini M.M., Akhlaghpour M., Neuman B.W., Buchmeier M.J. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. MBio. 2013:4. doi: 10.1128/mBio.00524-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Egger D., Wolk B., Gosert R., Bianchi L., Blum H.E., Moradpour D., Bienz K. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J Virol. 2002;76:5974–5984. doi: 10.1128/JVI.76.12.5974-5984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Romero-Brey I., Berger C., Kallis S., Kolovou A., Paul D., Lohmann V., Bartenschlager R. NS5A domain 1 and polyprotein cleavage kinetics are critical for induction of double-membrane vesicles associated with hepatitis C virus replication. MBio. 2015;6:e00759. doi: 10.1128/mBio.00759-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho M.W., Teterina N., Egger D., Bienz K., Ehrenfeld E. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells. Virology. 1994;202:129–145. doi: 10.1006/viro.1994.1329. [DOI] [PubMed] [Google Scholar]
- 80.Suhy D.A., Giddings T.H., Jr., Kirkegaard K. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles. J Virol. 2000;74:8953–8965. doi: 10.1128/jvi.74.19.8953-8965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin J.Y., Chen T.C., Weng K.F., Chang S.C., Chen L.L., Shih S.R. Viral and host proteins involved in picornavirus life cycle. J Biomed Sci. 2009;16:103. doi: 10.1186/1423-0127-16-103. [DOI] [PMC free article] [PubMed] [Google Scholar]