Human lupus autoantibody–DNA complexes activate DCs through cooperation of CD32 and TLR9 (original) (raw)
Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by pathogenic autoantibodies against nucleoproteins and DNA. Here we show that DNA-containing immune complexes (ICs) within lupus serum (SLE-ICs), but not protein-containing ICs from other autoimmune rheumatic diseases, stimulates plasmacytoid DCs (PDCs) to produce cytokines and chemokines via a cooperative interaction between Toll-like receptor 9 (TLR9) and FcγRIIa (CD32). SLE-ICs transiently colocalized to a subcellular compartment containing CD32 and TLR9, and CD32+, but not CD32–, PDCs internalized and responded to SLE-ICs. Our findings demonstrate a novel functional interaction between Fc receptors and TLRs, defining a pathway in which CD32 delivers SLE-ICs to intracellular lysosomes containing TLR9, inducing a signaling cascade leading to PDC activation. These data demonstrate that endogenous DNA-containing autoantibody complexes found in the serum of patients with SLE activate the innate immune system and suggest a novel mechanism whereby these ICs contribute to the pathogenesis of this autoimmune disease.
Introduction
Systemic lupus erythematosus (SLE), the second most common autoimmune disorder, after rheumatoid arthritis and the prototypic immune complex (IC) disease, is characterized by autoantibody production and IC formation, which results in immunologically mediated tissue injury (1, 2). The serological hallmark of SLE is the presence of high levels of autoantibodies against nuclear antigens, in particular autoantibodies against DNA (3). These autoantibodies are thought to contribute directly to the pathogenesis of SLE, as they precede the development of clinical disease in most patients, and levels of anti-DNA antibodies correlate with disease activity (4, 5). Deposition of autoantibody–nuclear antigen ICs in tissue is thought to induce local activation of the innate immune system. Mutations in C1q and Fc receptor genes have been associated with SLE (6–8), which suggests that immune cell activation by complement peptides and/or Fc-receptor cross-linking may participate in SLE pathogenesis. Recent evidence, however, has suggested that a mechanism of immune cell activation, in which the involvement of the DNA component of SLE-ICs is critical, may also contribute to the development of SLE pathology (9–14).
Recent evidence from both human SLE patients and mouse SLE models has implicated IFN-α in the development and/or progression of this disease (12, 13). Many SLE patients have extremely high levels of serum IFN-α, and levels of this cytokine have been found to correlate with disease severity (15). In addition, crossbreeding of SLE-prone NZB mice to mice genetically deficient for the α chain of the IFN-α/β receptor mitigates the SLE phenotype of the NZB mice, reducing both the levels of anti-DNA antibodies and the severity of glomerulonephritis (16). Anti-DNA antibodies isolated from SLE serum, when combined with DNA from apoptotic cells to form ICs, have been shown to induce IFN-α production in PBMCs (10). Plasmacytoid DCs (PDCs) were subsequently found to be the major source of IFN-α within the PBMC population stimulated with these ICs (13, 17). In human blood, 2 types of DCs are present: CD11c+ myeloid DCs and CD11c– plasma cell–like PDCs. PDCs are present in the blood at a relatively low frequency (approximately 0.1% of PBMCs), but upon activation they produce high levels of IFN-α. Taken together, these data suggest an important role for the production of IFN-α by PDCs in the pathogenesis of SLE (9, 17, 18).
A possible role for the DNA component of SLE-ICs in immune cell activation was suggested after the DNA contained in these complexes was characterized. The DNA in DNA:anti-DNA autoantibody complexes purified from the blood of SLE patients has an average length of 180 bp, a size consistent with apoptotic cleavage of chromatin (19). Interestingly, the CpG dinucleotide frequency of the DNA found in SLE-ICs is 5–6 times higher than the expected frequency in the genome (19). In vertebrate DNA, CpG dinucleotide sequences are generally suppressed; however, unmethylated CpG DNA sequences are found in clusters near the 5′ coding region of genes called CpG islands, and this DNA can serve as a stimulatory ligand (20–22). Synthetic oligonucleotides based on the DNA sequences cloned from SLE-ICs stimulated the expression of IL-12 and IFN-γ in human PBMCs (23). These data suggest that CpG-containing human DNA fragments in autoantibody complexes can induce direct cellular activation.
The discovery of CpG motifs in the DNA of SLE-ICs suggests that Toll-like receptors (TLRs) are involved in the activation of immune cells by these complexes. TLRs are pattern-recognition receptors that serve the important function of detecting pathogens and activating innate immune and inflammatory responses (24–28). While originally thought of as a receptor that recognized bacterial DNA, TLR9 has been demonstrated to directly mediate recognition of unmethylated CpG DNA sequences regardless of origin (29, 30). TLR9 has been shown to be solely responsible for the recognition of CpG DNA motifs, as cells from TLR9-deficient mice are unresponsive to the immuno-stimulatory activity of CpG DNA (31).
In PDCs as well as in macrophages and B cells, TLR9 is localized to the endoplasmic reticulum (32). In order for CpG DNA to activate PDCs, it must therefore be internalized to TLR9-containing vesicles. How PDCs internalize CpG DNA is unknown, and this is one of the central questions we sought to address in this study. Synthetic ICs of mouse IgG and CpG-containing DNA have been demonstrated to activate mouse transgenic B cells that express a B cell receptor (BCR) specific for self IgG, suggesting that the BCR is involved in the presentation of CpG DNA to TLR9 in B cells (14, 33). While these studies are intriguing, their relevance to human autoimmune disease and to the mechanism by which ICs containing CpG DNA activate B cells other than those specific for IgG remains unclear.
Recent data from several groups suggest that SLE serum activates human PBMCs (9–12, 17, 18, 34, 35). In this report, we extend these findings by demonstrating that serum from patients with active SLE, as well as DNA-containing ICs (SLE-IC) purified from the serum of these patients, stimulates PDCs in a TLR9-dependent manner. In contrast, serum or ICs isolated from patients with other rheumatic autoimmune diseases do not stimulate PDCs. SLE-ICs stimulate PDC expression of IFN-α as well as PDC expression of many other cytokines and chemokines that may play important roles in the pathogenesis of lupus. Further, we found that CD32 expression on PDCs was necessary for these cells to internalize and to be activated by SLE-ICs. Finally, using confocal microscopy, we demonstrate that all 3 components of this pathway — SLE-ICs, TLR9, and CD32 — associate intracellularly.
Results
Anti-DNA antibody–containing ICs in SLE serum stimulate cellular activation.
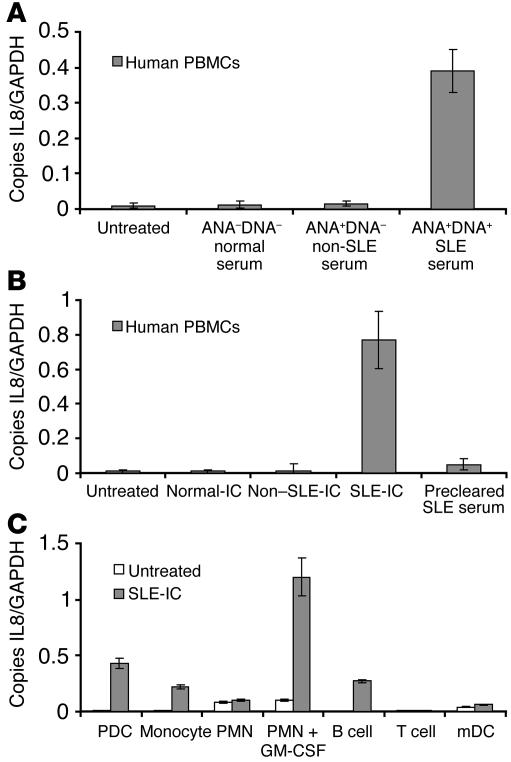
Human PBMCs were stimulated with 20% serum from antinuclear antibody/anti-DNA antibody–negative (ANA–DNA–) normal donors, ANA+DNA– Sjogren syndrome or rheumatoid arthritis patients, or ANA+DNA+ SLE patients (Figure 1A). Only SLE serum containing anti-DNA autoantibodies induced IL-8 expression in human PBMCs. Next we compared the stimulatory ability of ANA+DNA+ ICs purified from SLE patients to ANA+DNA– ICs purified from Sjogren syndrome or rheumatoid arthritis patients. IC formation in our purified preparations was verified by the relative ability of ICs to bind C1q, as ICs bind more avidly than monomeric IgG (see Supplemental Table 1; supplemental material available online with this article; doi:10.1172/JCI200523025DS1). Both ANA+DNA+ and ANA+DNA– IC preparations bound more strongly to C1q than did uncomplexed IgG (data not shown). We also verified IC formation in these purified samples by anti–double-stranded DNA (anti-dsDNA) ELISA (Supplemental Table 1). We found that only ICs containing anti-DNA antibodies (SLE-IC) stimulated IL-8 expression (Figure 1B). Moreover, SLE serum cleared of ICs lost its stimulatory ability (Figure 1B). These results demonstrate that ICs containing DNA and anti-DNA antibodies are responsible for the stimulatory activity of SLE serum. To determine which cell types in the PBMC population were responding to SLE-ICs, we stimulated purified human PDCs, monocytes, neutrophils (PMNs), B cells, T cells, and monocyte-derived DCs (mDCs) for 3 hours. We found that PDCs, monocytes, and B cells responded to SLE-IC stimulation (Figure 1C). In addition, we found that PMNs pretreated with 50 ng/ml GM-CSF for 90 minutes responded robustly to SLE-ICs. This finding is consistent with our recent demonstration that TLR9 function on PMNs requires GM-CSF pretreatment (36). Finally, SLE-IC stimulation of IL-8 expression correlated with TLR9 expression, which was found at high levels in human PDCs, monocytes, B cells, and GM-CSF–treated PMNs, but not T cells or mDCs (data not shown).
Figure 1.
Purified anti-DNA–containing ICs in SLE serum induce cellular activation. (A) Normal human PBMCs were isolated and stimulated with 20% serum isolated from normal patients (ANA–DNA–), non-SLE patients (ANA+DNA–, 4 Sjogren syndrome and 4 rheumatoid arthritis patients), or 5 SLE patients (ANA+DNA+). After 8 hours of stimulation, the cells were harvested for RNA. (B) PBMCs were stimulated for 8 hours with 100 ng/ml of ICs purified from the serum samples in A. Some PBMCs were stimulated with a 20% serum equivalent of protein G precleared SLE serum. (C) Purified subsets of white blood cells were stimulated with 100 ng/ml SLE-ICs for 8 hours. Expression of IL-8 was determined by QPCR and depicted as the number of copies of mRNA per copies of the control mRNA GAPDH. Error bars indicate standard deviation of triplicate measurements. Data are representative of 4 similar experiments.
SLE-ICs, but not non–SLE-ICs, induce cytokine and chemokine production from PDCs.
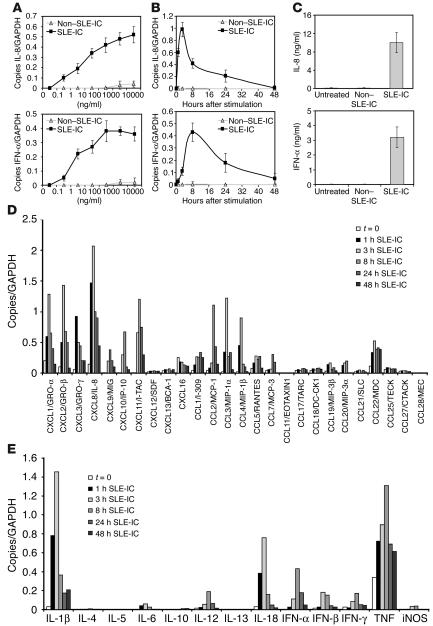
Several groups have demonstrated that sera isolated from SLE patients induces cellular activation in vitro (23, 37). In addition, Ronnblom and coworkers have demonstrated that a combination of SLE-IgG and apoptotic U937 cells induces IFN-α production in PDCs (10, 11, 35). In order to determine the dose response of SLE-IC–induced chemokine and cytokine activation in PDCs, we stimulated PDCs with ICs from SLE and non-SLE patients for 8 hours, then measured IFN-α and IL-8 mRNA expression by quantitative PCR (QPCR). Stimulation of PDCs induced a robust, dose-responsive IFN-α and IL-8 mRNA accumulation (Figure 2A). In order to determine the kinetics of this response, PDCs were stimulated for 1, 3, 8, 24, or 48 hours with 100 ng/ml non–SLE-ICs or 100 ng/ml SLE-ICs. We detected maximal IL-8 mRNA after 3 hours of stimulation, while IFN-α mRNA expression peaked at 8 hours (Figure 2B). Based on this data, 100 ng/ml SLE-ICs were used for the remaining experiments in this study. To demonstrate that increases in IL-8 and IFN-α mRNA levels were accompanied by protein production, ELISA was performed on the supernatants of PDCs stimulated with SLE-ICs for 24 hours. The results were consistent with the RNA data (Figure 2C). Finally, while IL-8 expression was induced by SLE-ICs in TLR9-positive cell types (B cells, PDCs, monocytes, GM-CSF–treated PMNs), IFN-α expression was only induced by SLE-ICs in PDCs and monocytes (data not shown).
Figure 2.
ICs in SLE serum activate cytokine and chemokine production in human PDCs. (A) Normal human PDCs were isolated and stimulated at the indicated doses of purified non–SLE-ICs or SLE-ICs. After 8 hours of stimulation, the cells were harvested for RNA. (B) Normal human PDCs were stimulated with 100 ng/ml of non–SLE-ICs or SLE-ICs. Cells were harvested at the indicated time points for RNA. Expression of IL-8 and IFN-α was determined by QPCR and depicted as the number of copies of mRNA per copies of the control mRNA GAPDH. (C) Supernatants of the stimulated cells were collected after the cells were removed by centrifugation. ELISA was performed to detect human IL-8 and IFN-α at the 24-hour time point. Error bars indicate standard deviation of triplicate measurements. Expression of chemokines (D) and cytokines (E) was quantified by QPCR using total RNA isolated from PDCs (1 × 105 cells) stimulated with 100 ng/ml SLE-ICs for 1, 3, 8, 24, or 48 hours. Data are representative of 4 similar experiments conducted using 4 different donors.
SLE-IC–induced chemokine and cytokine profile of PDCs.
Since other cytokines and chemokines besides IFN-α and IL-8 have been implicated in the pathogenesis of SLE, we examined the full spectrum of cytokines and chemokines induced by SLE-ICs in PDCs. PDCs were stimulated with SLE-ICs for 1, 3, 8, 24, or 48 hours, and 13 cytokines and 26 chemokines were measured by QPCR (Figure 2, D and E). These data demonstrate that SLE-ICs induce chemokines active on PMNs (IL-8, CXCL1/GRO-α, CXCL2/GRO-β, CXCL3/GRO-γ), on NK cells (CCL5/RANTES), on immature DCs (CCL3/MIP-1α, CCL4/MIP-1β), and on effector T cells (CXCL9/MIG, CXCL10/IP-10, CXCL11/I-TAC, CCL22/MDC, CCL1/I-309). We found no induction of chemokines active on B cells (CXCL13/BCA-1) or eosinophils (CCL11/EOTAXIN1) (Figure 2D). In addition, SLE-ICs stimulated the expression of the proinflammatory cytokines IL-1β, IL-6, TNF, IL-18, and the Th1 cytokines (IFN-γ, IL-12p40) but not the Th2 cytokines (IL-4, IL-5, IL-10, IL-13) (Figure 2E). Interestingly, many of these cytokines and chemokines have potent immunoregulatory functions that have been implicated in autoimmunity. Furthermore, ICs purified from non-SLE patients or uncomplexed anti-DNA antibodies alone did not induce chemokine/cytokine production in PDCs (data not shown). These data demonstrate that FcγR cross-linking alone does not induce chemokine/cytokine production by PDCs and also suggest that low levels of free DNA, which might be released into the tissue culture supernatant, do not induce PDC activation. Finally, the chemokine and cytokine pattern induced by SLE-ICs in PDCs correlates well with the pattern found in PDCs stimulated with synthetic CpG-A DNA (data not shown).
The DNA and antibody components of ICs contribute to cellular activity.
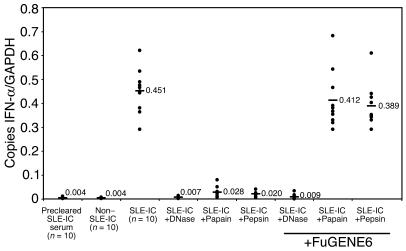
In order to test our hypothesis that the DNA component of ICs isolated from lupus serum is necessary for inducing cellular activation, we stimulated PDCs for 8 hours with non–SLE-ICs, SLE-ICs, or DNase-treated SLE-ICs. We found that DNase treatment of SLE-ICs neutralized 90–100% of their cellular activity (Figure 3). This demonstrates that the DNA component of ICs is required for its immunostimulatory activity. Interestingly, proteinase K treatment also neutralized SLE-IC–induced activation, demonstrating a role for the protein IgG portion of the complex (data not shown). To determine whether the contribution of the IgG occurs solely through its ability to cross-link and form complexes or the Fc portion delivers a signal to PDCs, the SLE-ICs were treated with pepsin or papain, proteases that cleave human IgG antibodies into Fc/Fab and F(ab′)2 fragments, respectively. We found that these enzymes abrogated 85–95% of the SLE-ICs’ cellular activity (Figure 3). These data suggest that both the DNA and the antibody components of ICs contained in lupus serum are important for inducing maximal cellular activity and that the Fc portion of the antibody is required. These data are also consistent with data by Bave et al., which demonstrated that the stimulatory capability of lupus IgG combined with apoptotic DNA was neutralized by papain and pepsin treatment (35). To determine whether the Fc portion of the antibody is involved in direct signaling or is solely required for internalization of DNA, we added FuGENE6 to papain-, pepsin-, and DNase-treated SLE-ICs. FuGENE6 is a liposomal carrier used to transfect DNA into cells. We found that the liposomal carrier rescued the cellular activity of the papain- and pepsin-treated SLE-ICs to a level similar to that of untreated SLE-ICs but had no effect on DNase-treated ICs (Figure 3). In control experiments, we found that FuGENE6 treatment alone failed to induce chemokine or cytokine production in PDCs. In addition, experiments using heat-aggregated IgG further demonstrate that FcγR cross-linking alone fails to induce robust chemokine or cytokine production in PDCs (data not shown). These results demonstrate that the IgG component of the SLE-IC is used primarily to facilitate uptake of the DNA component, which is necessary for cell activation.
Figure 3.
Both the DNA and antibody components of SLE-ICs are necessary for activation of PDCs. Total RNA was isolated from normal human PDCs (1 × 105 cells) stimulated with 100 ng/ml of non–SLE-ICs, SLE-ICs, or SLE-ICs pretreated with immobilized DNase, papain, or pepsin. Some ICs were pretreated with 5 μl of FuGENE6 for 15 minutes prior to stimulation. Expression of IFN-α was determined by QPCR and is depicted as the number of copies of mRNA per copies of the control mRNA GAPDH.
TLR9 expression confers SLE-IC–induced cellular activation.
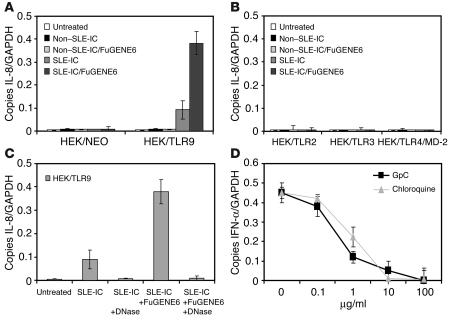
Since the DNA isolated from SLE patients has been shown to be enriched in CpG motifs, we hypothesized that it stimulated cells through TLR9. To test this hypothesis, human embryonic kidney (HEK) cells, which are normally unresponsive to ICs, were stably transfected with TLR9, TLR2, TLR3, or TLR4/MD-2. As expected, HEK cells expressing TLR9, but not those expressing TLR2, TLR3, or TLR4/MD-2, were responsive to SLE-ICs (Figure 4, A and B). We recently demonstrated that TLR9 is localized intracellularly in the endoplasmic reticulum of PDCs and macrophages (32). In addition, we demonstrated that as CpG DNA is internalized, TLR9 redistributes from the endoplasmic reticulum to CpG-containing lysosomes where signaling occurs. To facilitate SLE-IC uptake by HEK cells, we combined SLE-ICs with the liposomal carrier FuGENE6. SLE-ICs pretreated with FuGENE6 induced a 5-fold increase of IL-8 mRNA over that in cells treated with SLE-ICs alone. Furthermore, DNase treatment of the SLE-ICs completely abrogated its TLR9-dependent activation (Figure 4C). These data suggest that internalization of SLE-ICs is a limiting factor in the response of HEK/TLR9 cells. To demonstrate that TLR9 plays a role in the response of PDCs to SLE-ICs, we stimulated PDCs in the presence of increasing doses of chloroquine or a synthetic GpC-DNA oligonucleotide. Several groups have shown that CpG DNA signaling can be inhibited by synthetic inhibitory oligonucleotides or by administration of chloroquine (14, 30, 38), which blocks endosome acidification and maturation (39). Both chloroquine and GpC oligonucleotides blocked SLE-IC–induced IFN-α production in a dose-dependent manner in PDCs (Figure 4D). In control experiments, we found that chloroquine and GpC oligonucleotides were unable to block lipopeptide-induced IL-8 production in PDCs (data not shown). These results demonstrate that TLR9 expression plays a critical role in recognizing ICs in SLE serum. Moreover, these data suggest that the DNA component of ICs is required for cellular activation. Chloroquine inhibition is also noteworthy because it is used as a treatment for autoimmune diseases, including SLE (40, 41).
Figure 4.
TLR9 confers responsiveness to ICs in SLE serum. (A and B) Total RNA was isolated from HEK cells stably transfected with TLR9, TLR2, TLR3, or TLR4/MD-2 (1 × 106 cells) stimulated with 100 ng/ml non–SLE-ICs or SLE-ICs in the presence or absence of 10 μl of FuGENE6 for 3 hours. NEO, neomycin. (C) Total RNA was isolated from HEK/TLR9 cells stimulated with 100 ng/ml SLE-ICs in the presence or absence of 10 μl FuGENE6 and/or pretreated with DNase. Expression of IL-8 was determined by QPCR and is depicted as the number of copies of mRNA per copies of the control mRNA GAPDH. (D) Human PDCs were stimulated with 100 ng/ml SLE-ICs in the presence or absence of increasing doses of chloroquine or inhibitory synthetic GpC oligonucleotide.
CD32 expression is required for SLE-IC internalization and activation by PDCs.
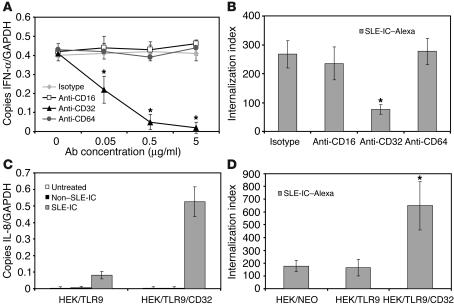
The above data using papain and pepsin demonstrated that the Fc portion of SLE-ICs is necessary for inducing maximal cellular activation. To test whether FcγRs are involved in mediating SLE-IC–induced cell activation, we stimulated PDCs in the presence or absence of neutralizing antibodies against CD16 (FcγRIII), CD32 (FcγRII), or CD64 (FcγRI). We found that neutralizing antibodies against CD32, but not against CD16 or CD64, blocked SLE-IC–induced IFN-α production by PDCs (Figure 5A). This result is consistent with recent reports demonstrating that anti-CD32 antibodies neutralized lupus serum and lupus IgG combined with apoptotic DNA activation of PBMCs (34, 35). To determine whether CD32 is involved in internalization of SLE-ICs, we stimulated PDCs in the presence or absence of neutralizing antibodies against CD16, CD32, or CD64 and measured internalization of fluorescently conjugated SLE-ICs. We found that antibodies specific for CD32, but not for CD16 or CD64, blocked PDC uptake of Alexa Fluor 633–conjugated SLE-ICs (Figure 5B). Since HEK cells do not express CD32, we made stable cell lines expressing TLR9 and CD32 (HEK/TLR9/CD32). We found that expression of human CD32 (isoform A) on HEK/TLR9 cells increased their sensitivity to SLE-ICs to a level similar to that of cells stimulated with SLE-ICs plus FuGENE6 (Figures 5C and 4A). CD32 expression in HEK/TLR9 cells also enhanced internalization of ICs (Figure 5D). These results demonstrate a mechanism whereby CD32 facilitates the uptake of SLE-ICs and delivers it to intracellularly expressed TLR9.
Figure 5.
Human CD32 is required for PDC activation and internalization of SLE-ICs. (A) Total RNA was isolated from normal human PDCs stimulated with 100 ng/ml SLE-ICs for 8 hours in the absence or presence of increasing concentrations (0.05, 0.5, 5 μg/ml) of antibodies against CD16, CD32, or CD64. Expression of IFN-α was determined by QPCR and depicted as the number of copies of mRNA per copies of the control mRNA GAPDH. (B and D) Alexa Fluor 633–conjugated SLE-ICs (SLE-IC–Alexa) were incubated with normal human PDCs, HEK/TLR9 cells, or HEK/TLR9/CD32 cells at a ratio of 10:1 for 15 minutes at 37–C; and in the presence or absence of 5 μg/ml of neutralizing antibodies against CD16, CD32, or CD64. Internalization was measured by fluorescent microscopy. Data are presented as the number of internalized complexes per 100 cells × 100. *P < 0.01 versus isotype-treated or control, Student’s t test. Error bars indicate standard deviation of triplicate samples. (C) Total RNA was isolated from HEK/TLR9 or HEK/TLR9/CD32 cells stimulated with 100 ng/ml non–SLE-ICs or SLE-ICs for 3 hours. Expression of IL-8 was determined by QPCR.
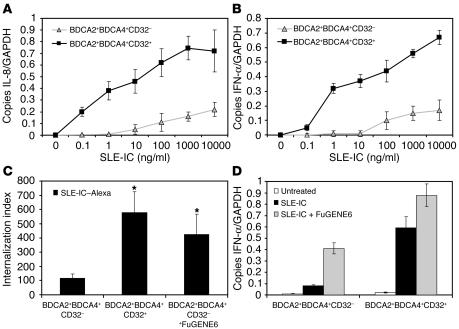
To rule out possible nonspecific effects of the neutralizing antibodies, we tested whether CD32 expression on PDCs was necessary for SLE-IC internalization and activation. Previous experiments have demonstrated that approximately 50% of the BDCA2+ PDC population is CD32+ (Supplemental Figure 1; ref. 35). For our experiments, we sorted bulk BDCA4+ PDC population into BDCA2+CD32+ and BDCA2+CD32– subsets and stimulated these cells with increasing doses of SLE-ICs. We found that SLE-IC induced robust expression of IFN-α and IL-8 in CD32+ PDCs, but not CD32– PDCs (Figure 6A). We also found that CD32+ PDCs, but not CD32– PDCs, efficiently internalized SLE-ICs (Figure 6B). Together these data demonstrate that the BDCA4+BDCA2+CD32+ PDC subset internalizes and responds to SLE-ICs. Since both CD32– and CD32+ PDCs expressed similar levels of intracellular TLR9 (data not shown), we determined whether the addition of FuGENE6 with SLE-ICs could stimulate CD32– PDCs. We found that FuGENE6 enhanced SLE-IC uptake and restored IFN-α production in CD32– PDCs (Figure 6, C and D). These data suggest that the CD32+ PDC cell population is particularly sensitive to SLE-IC stimulation and also suggest a mechanism whereby CD32 shuttles the SLE-IC to the intracellular TLR9 compartment.
Figure 6.
CD32+ PDCs internalize and respond to SLE-ICs. (A and B) PDCs from normal donors were isolated on a MoFlo cell sorter using fluorescently labeled antibodies against BDCA2, BDCA4, and CD32. The CD32– and CD32+ PDC subsets were stimulated with the indicated concentrations of SLE-ICs. Cells were harvested at 3 and 8 hours, and expression of IL-8 (A) and IFN-α (B) was determined by QPCR. (C) Alexa Fluor 633–conjugated SLE-ICs in the presence or absence of 10 μl FuGENE6 were incubated with CD32+ and CD32– PDCs at a ratio of 10:1 for 15 minutes at 37–C. Internalization was measured by fluorescent microscopy. Data are presented as the number of internalized complexes per 100 cells × 100. *P < 0.01 versus control, Student’s t test. Error bars indicate standard deviation of triplicate samples. (D) Total RNA was isolated from PDCs stimulated with 100 ng/ml SLE-ICs in the presence or absence of 10 μl of FuGENE6. Expression of IFN-α was determined by QPCR and is depicted as the number of copies of mRNA per copies of the control mRNA GAPDH.
SLE-IC colocalization with CD32 and TLR9.
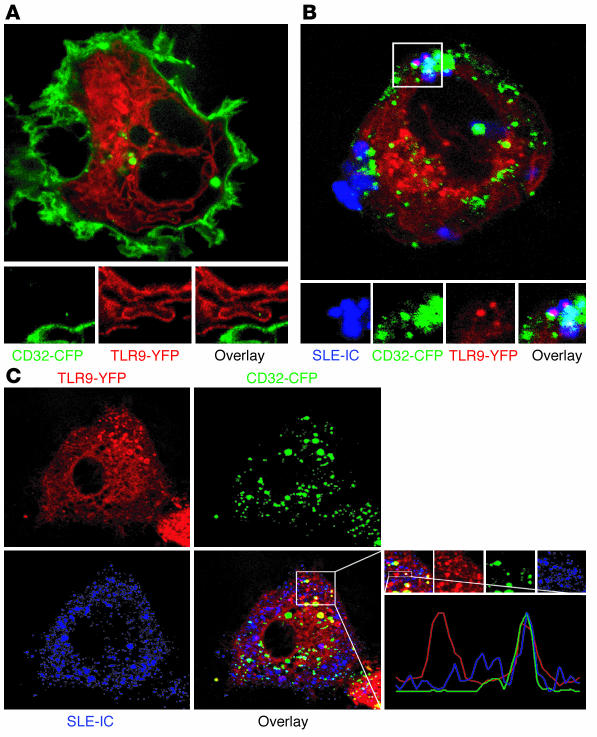
To determine whether trafficking of SLE-ICs into cells was related to TLR9 signal transduction, we performed confocal microscopy studies of yellow fluorescent protein–tagged (YFP-tagged) TLR9-expressing U373 cells exposed to Alexa Fluor 633–conjugated SLE-ICs. We found that TLR9 was actively recruited to SLE-IC–containing vesicles (Supplemental Figure 2). This interaction occurred rapidly, but transiently, in as little as 5 minutes, with no interactions found after 30 minutes. In addition, we found no interaction of non–SLE-ICs with TLR9 at any time point (data not shown). These results demonstrate that vesicles in which TLR9/SLE-ICs colocalize might be the intracellular location of ligand receptor interaction.
Since our earlier experiments suggested a role for CD32 in the internalization of SLE-ICs, we also generated U373 cells stably expressing CD32–cyan fluorescent protein (CD32-CFP) and TLR9-YFP. We found that CFP-tagged CD32 (green) is located on the plasma membrane and that YFP-tagged TLR9 (red) is located in the endoplasmic reticulum of quiescent U373 cells (Figure 7A). Following treatment with Alexa Fluor 633–conjugated SLE-IC (blue), we found that SLE-ICs colocalized with CD32 and TLR9 (Figure 7, B and C).
Figure 7.
SLE-ICs associate with TLR9 and CD32. U373 cells coexpressing YFP-tagged TLR9 (red) and CFP-tagged CD32 (green) were left untreated (A) or incubated with Alexa Fluor 633–conjugated SLE-ICs (blue) for 5 minutes (B and C), and living cells were imaged by confocal microscopy.
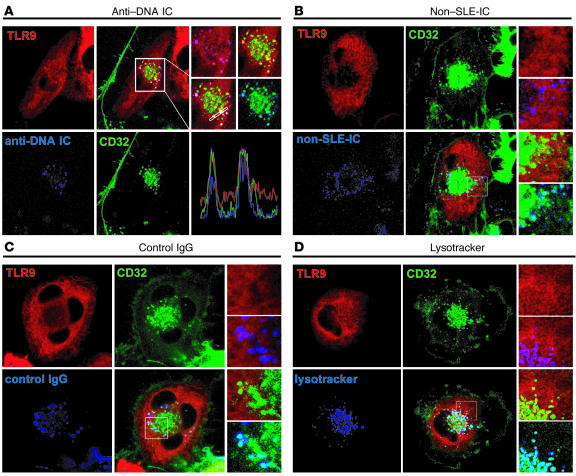
In the above experiments, we found limited and focal colocalization of CD32 and TLR9 with ICs purified from SLE patients (SLE-IC, ANA+DNA+), but not from non-SLE patients (non–SLE-IC, ANA+DNA–). These experiments suggest that DNA-containing ICs present in SLE-ICs were responsible for TLR9/CD32 colocalization and that the noncolocalizing ICs observed in these experiments were most likely non-DNA–containing ICs, since the SLE-IC also contains many non-DNA ICs (Figure 7, B and C). To ensure that anti-DNA ICs in SLE serum were responsible for this observed TLR9/CD32 colocalization, we isolated anti-DNA ICs from SLE serum using a DNA affinity column. Following treatment with purified anti-DNA ICs (blue), we found that nearly all of the anti-DNA ICs colocalized with CD32 and TLR9 (Figure 8A). Meanwhile, non–SLE-ICs and free IgG colocalized with CD32, but not TLR9 (Figure 8, B and C). While anti-DNA ICs, non–SLE-ICs, and free IgG induced a redistribution of CD32 toward the center of cells, only anti-DNA ICs induced TLR9 redistribution. These data support our hypothesis that CD32 delivers DNA-containing ICs contained in SLE serum to intracellular pools of TLR9.
Figure 8.
CD32 delivers anti-DNA ICs to TLR9-containing lysosomes. U373 cells coexpressing YFP-tagged TLR9 (red) and CFP-tagged CD32 (green) were incubated with Alexa Fluor 633–conjugated (blue) anti-DNA ICs (A), Alexa Fluor 633–conjugated non–SLE-ICs (B), or Alexa Fluor 633–conjugated IgG (C) for 5 minutes, and living cells were imaged by confocal microscopy. (D) Cells expressing TLR9-CFP (red) and CD32-CFP (green) were incubated with lysotracker (blue).
We previously demonstrated that after CpG DNA stimulation, TLR9 translocates from the ER to acidic lysosomes (32). To investigate whether CD32 redistributes to the same compartment in which TLR9 is expressed, we incubated cells with lysotracker, which identifies acidic lysosomes. We found that CD32 redistributes from the plasma membrane (Figure 7A) to small vesicular structures toward the center of the cells following incubation with ICs (Figure 8, A–D). Colocalization studies of cells incubated with lysotracker indicated that these structures are acidic lysosomes (Figure 8D). Thus, CD32 sequentially binds, internalizes, and delivers anti-DNA ICs to lysosomes where it interacts with TLR9.
Discussion
In this study, we have demonstrated that DNA-containing ICs purified from the serum of patients with active SLE stimulate specific classes of leukocytes in a TLR9-dependent manner. This stimulation required the expression of CD32, which facilitated the internalization of SLE-ICs into subcellular lysosomes containing TLR9, where binding and signal transduction can be initiated. Our findings demonstrate a novel functional interaction between Fc receptors and TLRs and raise the possibility that ICs containing ligands for other TLRs, such as RNA, may activate immune cells through similar pathways (i.e., FcR/TLR3).
We found that leukocyte subsets that have been shown to express both TLR9 and CD32, including human PDCs, monocytes, B cells, and PMNs pretreated with GM-CSF, responded to SLE-IC stimulation (35, 36, 42–44). In contrast, leukocytes not expressing both components of the TLR9/CD32 pathway, including T cells, mDCs, and untreated PMNs, did not respond to SLE-IC stimulation. Of note, there appear to be species-specific differences in TLR9 expression. TLR9 is functionally expressed on mouse but not human mDCs, a difference which might render mouse models of SLE less directly relevant to human SLE (45).
Human CD32 is a low-affinity receptor for monomeric IgG but binds avidly to IgG present in ICs. CD32 has been previously demonstrated to be required for IFN-α expression induced in PDCs by ICs that were produced by combining autoantibody-containing IgG from SLE patients with apoptotic U937 cells (35). Our study extends this finding, demonstrating that the presence of CD32 is critical for PDC IFN-α expression induced both by the serum of patients with active SLE and by ICs purified from this serum. As noted, our study also identifies the mechanism through which CD32 participates in the stimulation of PDCs by SLE-IC: CD32 is responsible for delivering SLE-ICs to intracellular lysosomes containing TLR9, enabling TLR9 to bind CpG DNA contained in these ICs, thereby initiating TLR9-signaling pathways. In our experiments, we found that CD32– PDCs were able to respond to hypomethylated CpG DNA if we bypassed the CD32 internalization pathway, delivering this DNA to intracellular TLR9 in CD32– PDCs by transfection. In future experiments, it will be interesting to determine whether CD32– PDCs express an internalization receptor for CpG DNA other than CD32, which may recognize this DNA when it is present in contexts other than ICs, such as in bacteria or viruses (46, 47).
The dependence of SLE-IC–induced TLR9 activation on internalization of these complexes through the CD32 pathway is consistent with the concept that TLRs are not phagocytic receptors but rather are dependent on other receptors for internalization of their microbial ligands. Surface immunoglobulin, i.e., the BCR, has been demonstrated to be able to deliver CpG DNA to TLR9 in B cells (14, 33). In these experiments, synthetic ICs of mouse IgG and CpG-containing DNA have been demonstrated to activate mouse transgenic B cells that express a BCR specific for self IgG. Pretreatment of these ICs with DNase and of these B cells with TLR9 inhibitors both prevented these ICs from inducing B cell proliferation (14). Further, mouse transgenic B cells that express a BCR recognizing specific DNA sequences may be directly activated by CpG DNA (33). Together, these data suggest a pathway in which the BCR is able to deliver CpG DNA to TLR9 in murine B cells. Our data now extend these findings by demonstrating that DNA-containing complexes isolated from SLE patients can activate human leukocytes through a non-BCR pathway. Human B cells also express CD32 (44); however, we found that SLE-IC internalization and activation by B cells was only modestly (10–15%) decreased in the presence of CD32 (AT10) blocking antibodies (data not shown). Together these results suggest that the TLR9/CD32 pathway is necessary for delivery of SLE-ICs in PDCs while the BCR/TLR9 pathway is sufficient in B cells.
We found that stimulation of PDCs by SLE-ICs through the TLR9/CD32 pathway induced robust PDC expression of IFN-α. As noted, correlation between disease severity and IFN-α levels in SLE patients (15) and mitigation of disease in a mouse model of SLE by disruption of IFN-α signaling (16) have implicated IFN-α in SLE pathogenesis. High levels of IFN-α in the serum of SLE patients have been postulated to play a role in the loss of tolerance to autoantigens in SLE. It has been demonstrated that IFN-α in the serum of SLE patients drives the maturation of monocytes into DCs (48). The ability of these mDCs to engulf apoptotic cells and activate autoreactive T cells and B cells has been hypothesized to drive the autoimmune response in SLE (48). Our study suggests that activation of PDCs by SLE-ICs through the TLR9/CD32 pathway may be a major source of IFN-α in this disease.
We found that SLE-IC stimulation of PDCs also induced the production of many other cytokines and chemokines in addition to IFN-α. In SLE, deposition of ICs in tissues leads to the production of multiple proinflammatory cytokines and chemokines. Local production of the chemokines IL-8, MCP-1, and RANTES has been noted in inflamed joints of SLE patients (49, 50), and serum levels of MCP-1 and IP-10 are higher in SLE patients than in controls. The importance of chemokines in the pathogenesis of lupus nephritis has been suggested by experiments in a spontaneous mouse model of SLE. Crossbreeding of mice that are prone to develop nephritis to mice that are genetically deficient for MCP-1 reduced macrophage and T cell recruitment to the kidney, protecting offspring from kidney disease and prolonging their survival (51). Our data suggest that the TLR9/CD32 pathway can mediate SLE-IC–induced production of multiple cytokines and chemokines that play important roles in the pathogenesis of SLE.
Our data extend the recent findings by Boule et al., which demonstrated that artificial chromatin-containing, but not purely protein-containing, IgG ICs activated murine bone marrow–derived DCs purified from wild-type mice (52). Induction of TNF by chromatin-ICs was shown to be significantly reduced in DCs purified from MyD88 or TLR9-deficient mice. Interestingly, not all of the chromatin-IC activity was abolished in MyD88/TLR9-deficient DCs, suggesting the existence of another MyD88/TLR9-independent pathway. This study also evaluated the role of FcRs in DC activation by chromatin ICs and found that stimulation of DCs from FcγRIII-deficient mice did not produce TNF in response to chromatin ICs, while DCs from FcγRII-deficient mice did (52). This contrasts with our findings using human PDCs and human SLE sera, which demonstrate that FcγRIIa is required for human PDC activation by SLE-IC. These differences likely reflect the heterogeneity in FcR and TLR9 expression and function on human and murine DC subsets. Thus, FcγRIII is required for murine bone marrow–derived DC activation by chromatin ICs while FcγRIIa is required for human PDC activation by SLE-ICs. These data emphasize the limitations of mouse models of SLE and the need for further study in humans.
In this report we provide evidence that a novel functional interaction between CD32 and TLR9 mediates the activation of PDCs by SLE-ICs. This TLR9/CD32 pathway is distinct from the BCR/TLR9 pathway previously described in B cells, in which the BCR is required for delivery of CpG DNA to TLR9. These findings suggest that the TLR9/CD32 pathway we have now shown to be operative in PDCs may prove to be a novel target for future SLE therapies.
Methods
Cells, plasmids, and reagents.
HEK cells stably expressing TLR9, TLR2, TLR3, or TLR4/MD-2 were described previously (32, 53). The cDNA for human CD32A was a gift from Jeffrey Ravetch (Rockefeller University, New York, New York, USA). The CD32A cDNA was recloned into pECFP-N1 (BD Biosciences — Clontech) vector and is expressed as a fusion protein containing a fluorescent C-terminal ECFP tag. Retroviral constructs were made by recloning YFP-tagged TLR9 and CFP-tagged CD32 into peak12mmp. U373 cells stably expressing YFP-tagged TLR9 and CFP-tagged CD32 were generated following retroviral infection. Immobilized papain and pepsin were obtained from Pierce. Immobilized DNase I was constructed by coupling 100 mg of DNase I (Invitrogen Corp.) to 20 ml of Affigel-10 (Bio-Rad Laboratories). FuGENE6 was purchased from Roche Diagnostics. Human GM-CSF was purchased from PeproTech Inc. Lysotracker Red was purchased from Molecular Probes Inc. All other reagents were purchased from Sigma-Aldrich unless stated otherwise.
Cell purification.
Buffy coats were obtained from healthy volunteers and fractionated over Histopaque-1077. The PBMC layer was recovered and erythrocyte depleted by incubation in red blood cell lysis buffer for 5 minutes at room temperature. Primary human PDCs were purified from the PBMCs by immunomagnetic-positive selection using BDCA-4 microbeads (Miltenyi Biotec). In some experiments, PDCs were cell sorted on a MoFlo (Cytomation Inc.) using fluorescently labeled antibodies against BDCA2, BDCA4, and CD32. The isolated PDCs were consistently 95%–98% BDCA4+BDCA2+, as measured by flow cytometry. Human T and B cells were purified using indirect magnetic labeling systems (Miltenyi Biotec) for the isolation of untouched T and B cells from human blood. The isolated T and B cells were more than 97% CD3+ and CD19+, respectively, as assessed by flow cytometry. Human mDCs and PMNs were generated as previously described (54, 55). Purity of PMNs was more than 95% as measured by differential count following Diff-Quik staining. All cells were cultured in complete medium (RPMI 1640, 1% L-glutamine, 1% penicillin/streptomycin, and 10% low endotoxin FCS) and maintained at 37°C and 5% CO2.
QPCR.
Total RNA was extracted using the RNeasy Kit according to the manufacturer’s protocol (QIAGEN). After DNase I (Invitrogen Corp.) treatment, 1 μg of total RNA from each sample was used as a template for the reverse transcription reaction. 100 μl of cDNA was synthesized using Oligo(dT)15, random hexamers, and MultiScribe Reverse Transcriptase (Applied Biosystems). All samples were reverse transcribed under the same conditions (25°C for 10 minutes, 48°C for 30 minutes) and from the same reverse transcription master mix in order to minimize differences in reverse transcription efficiency. The 25 μl QPCR reaction contains 2 μl of cDNA, 12.5 μl of 2× SYBR Green Master Mix (Stratagene), and 250 nmol of sense and antisense primer. The reaction conditions were as follows: 50°C for 2 minutes, 95°C for 10 minutes, then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Emitted fluorescence for each reaction was measured during the annealing/extension phase, and amplification plots were analyzed using MX4000 software, version 3.0 (Stratagene). A series of standards was prepared by performing 10-fold serial dilutions of full-length cDNAs in the range of 20 million copies to 2 copies per QPCR reaction. Quantity values (i.e., copies) for gene expression were generated by comparison of the fluorescence generated by each sample with standard curves of known quantities. Next the calculated number of copies was divided by the number of copies of the housekeeping gene GAPDH. In addition, we saw no significant changes in the QPCR results when the data were normalized using another constitutively active gene, β2-microglobulin.
Quantitation of cytokines by ELISA.
Human CXCL8/IL-8 (BioSource International Inc.) and IFN-α protein levels in the PDC and HEK culture supernatants were measured by sandwich ELISA according to the manufacturer’s protocol (R&D Systems).
IgG subclass detection.
To determine the IgG subclass distribution of autoantibodies against dsDNA in our purified SLE-ICs, ELISAs were performed using antibodies specific for each IgG subclass (see Supplemental Table 1). Briefly, purified SLE-ICs were added to microtiter plates that were precoated with DNA for 3 hours at 25°C. Plates were washed and mouse anti-human monoclonal antibodies (anti-IgG1, 2, 3, 4; Research Diagnostics Inc.) were added to separate wells for 1 hour at 25°C. Following incubation, the plate was washed, and substrate solution reactive with HRP was added to each well. The reaction was terminated by the addition of acid, and the absorbance was measured at 450 nm on a Molecular Devices plate reader.
Purification of ICs.
ICs were prepared from the serum of SLE, rheumatoid arthritis, and Sjogren syndrome patients during a flare or with active disease (i.e., arthritis, rash, renal disease, high ANA/DNA titers) based on clinical classification criteria (ref. 56; see Supplemental Table 1). Patient serum was screened using HEp-2 ANA test and anti-DNA Crithidia luciliae test (Clinical Immunology Laboratory, Massachusetts General Hospital; ref. 57). Only serum samples with true positive ANA (greater than 1:640) and/or anti-DNA (greater than 1:80) titers were selected. These studies were approved by the Institutional Review Board of Massachusetts General Hospital. The serum was passed through a 0.45 μm filter and applied to an antibody-purification spin column (Prosep-G, Millipore Corp.). The flow-through (precleared SLE serum) was saved and used in some experiments (Figure 1B). Next, the eluted ICs were desalted and concentrated using an Amicon centrifugal filter device with a 300,000 nominal molecular weight limit (NMWL) cutoff. The stability of ICs greater than 300,000 NMWL following purification was verified by C1q and anti-DNA ELISA assays (Alpha Diagnostics International). ICs (IgG:nuclear proteins) from non-SLE patients were also purified by the above method. Some purified ICs were labeled with Alexa Fluor 633, using a protein-labeling kit (Molecular Probes Inc.). In control experiments, we found that purification and labeling of SLE-ICs did not affect their stimulatory activity (data not shown).
Purification of anti-DNA ICs.
To purify anti-DNA ICs we incubated SLE-ICs with DNA-coated Miltenyi microbeads (Miltenyi Biotec). The magnetically labeled anti-DNA ICs were bound to a separator column placed in a magnetic field while other proteins were washed away. Next, the separator column was removed from the magnetic field, and the anti-DNA ICs were eluted with PBS. Finally, the purified ICs were concentrated using an Amicon centrifugal filter device with a 300,000 NMWL cutoff and labeled with Alexa Fluor 633 using a protein labeling kit (Molecular Probes Inc.). In control experiments, we found that purification and labeling of anti-DNA ICs did not affect their stimulatory activity (data not shown).
Antibodies and inhibitors.
Antibodies specific for CD16 (clone 3G8), CD32 (clone AT10), and CD64 (clone 10.1) were used (0.05, 0.5, 5 μg/ml). These were obtained from Research Diagnostics Inc. The stimulatory CpG oligonucleotide (ODN) KJ-6, with the sequence 5′-TTTTCAATTCGAAGATGAAT-3′, which was derived from DNA purified from the serum of a patient with active SLE as previously described was used as a positive control in some experiments (23). GpC-ODN KJ-6 (0.1–100 μg/ml), with the sequence 5′-TTTTCAATTGCAAGATGAAT-3′, was used as a negative control, and an inhibitor in several assays was synthesized by Invitrogen Corp. Chloroquine (0.1–100 μg/ml) was used as an inhibitor and was purchased from Sigma-Aldrich.
Confocal microscopy.
Confocal microscopy was performed using a Leica SP2 AOBS laser scanning microscope. Cells were cultured on glass-bottom 35 mm tissue-culture dishes (MatTek Corp.). Dual- or triple-color images were acquired by consecutive scanning with only 1 laser line active per scan to avoid cross-excitation.
IC uptake assays.
PDCs were incubated with 1–100 ng/ml Alexa Fluor 633–labeled ICs in growth medium for various time periods. Cells were washed in prewarmed culture medium and imaged immediately by confocal microscopy at 37°C. After washing, some samples were fixed with 10% formalin and viewed with a Nikon fluorescent microscope or analyzed by flow cytometry. The data are expressed as the number of internalized complexes per 100 PDCs × 100 (internalization index).
Supplementary Material
Supplemental data
Acknowledgments
We thank the clinical immunology laboratory at Massachusetts General Hospital for collection and testing of patient serum and A. Schoenemeyer (University of Massachusetts Medical School) for preparing retroviruses of TLR9 and CD32. The authors would like to thank A. Tager for critical reading of the manuscript. Research support was provided by NIH grants P01-DK50305, R01-CA69212 (to A.D. Luster), and 5T32AR07258 (to F. Hayashi and T.K. Means), and by The Irvington Institute for Immunological Research and the Dana Foundation (to A.D. Luster and T.K. Means).
Footnotes
Nonstandard abbreviations used: ANA, antinuclear antibody; BCR, B cell receptor; CFP, cyan fluorescent protein; HEK, human embryonic kidney; IC, immune complex; mDC, monocyte-derived DC; PDC, plasmacytoid DC; PMN, neutrophil; QPCR, quantitative PCR; SLE, systemic lupus erythematosus; TLR, Toll-like receptor; YFP, yellow fluorescent protein.
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J. Clin. Pathol. 2003;56:481–490. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence RC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Bootsma H, et al. Anti-double stranded DNA antibodies in systemic lupus erythematosus: detection and clinical relevance of IgM-class antibodies. Scand. J. Rheumatol. 1996;25:352–359. doi: 10.3109/03009749609065646. [DOI] [PubMed] [Google Scholar]
- 4.Arbuckle MR, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 5.ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CG. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 1990;33:634–643. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]
- 6.Botto M, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 7.Morgan BP, Walport MJ. Complement deficiency and disease. Immunol. Today. 1991;12:301–306. doi: 10.1016/0167-5699(91)90003-C. [DOI] [PubMed] [Google Scholar]
- 8.Ravetch JV, Clynes RA. Divergent roles for Fc receptors and complement in vivo. Annu. Rev. Immunol. 1998;16:421–432. doi: 10.1146/annurev.immunol.16.1.421. [DOI] [PubMed] [Google Scholar]
- 9.Ronnblom L, Alm GV. Systemic lupus erythematosus and the type I interferon system. Arthritis Res. Ther. 2003;5:68–75. doi: 10.1186/ar625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bave U, Alm GV, Ronnblom L. The combination of apoptotic U937 cells and lupus IgG is a potent IFN-alpha inducer. J. Immunol. 2000;165:3519–3526. doi: 10.4049/jimmunol.165.6.3519. [DOI] [PubMed] [Google Scholar]
- 11.Bave U, Vallin H, Alm GV, Ronnblom L. Activation of natural interferon-alpha producing cells by apoptotic U937 cells combined with lupus IgG and its regulation by cytokines. J. Autoimmun. 2001;17:71–80. doi: 10.1006/jaut.2001.0519. [DOI] [PubMed] [Google Scholar]
- 12.Vallin H, Perers A, Alm GV, Ronnblom L. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J. Immunol. 1999;163:6306–6313. [PubMed] [Google Scholar]
- 13.Vallin H, Blomberg S, Alm GV, Cederblad B, Ronnblom L. Patients with systemic lupus erythematosus (SLE) have a circulating inducer of interferon-alpha (IFN-alpha) production acting on leucocytes resembling immature dendritic cells. Clin. Exp. Immunol. 1999;115:196–202. doi: 10.1046/j.1365-2249.1999.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leadbetter EA, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 15.Ronnblom LE, Alm GV, Oberg KE. Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. J. Intern. Med. 1990;227:207–210. doi: 10.1111/j.1365-2796.1990.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 16.Santiago-Raber ML, et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J. Exp. Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronnblom L, Alm GV. The natural interferon-alpha producing cells in systemic lupus erythematosus. Hum. Immunol. 2002;63:1181–1193. doi: 10.1016/s0198-8859(02)00757-7. [DOI] [PubMed] [Google Scholar]
- 18.Ronnblom L, Alm GV. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J. Exp. Med. 2001;194:F59–F63. doi: 10.1084/jem.194.12.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sano H, et al. Binding properties of human anti-DNA antibodies to cloned human DNA fragments. Scand. J. Immunol. 1989;30:51–63. doi: 10.1111/j.1365-3083.1989.tb01188.x. [DOI] [PubMed] [Google Scholar]
- 20.Antequera F, Bird A. Number of CpG islands and genes in human and mouse. Proc. Natl. Acad. Sci. U. S. A. 1993;90:11995–11999. doi: 10.1073/pnas.90.24.11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 22.Bird AP, Taggart MH, Nicholls RD, Higgs DR. Non-methylated CpG-rich islands at the human alpha-globin locus: implications for evolution of the alpha-globin pseudogene. EMBO J. 1987;6:999–1004. doi: 10.1002/j.1460-2075.1987.tb04851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato Y, Miyata M, Nishimaki T, Kochi H, Kasukawa R. CpG motif-containing DNA fragments from sera of patients with systemic lupus erythematosus proliferate mononuclear cells in vitro. J. Rheumatol. 1999;26:294–301. [PubMed] [Google Scholar]
- 24.Means TK, Golenbock DT, Fenton MJ. Structure and function of Toll-like receptor proteins. Life Sci. 2000;68:241–258. doi: 10.1016/s0024-3205(00)00939-5. [DOI] [PubMed] [Google Scholar]
- 25.Underhill DM, Ozinsky A. Toll-like receptors: key mediators of microbe detection. Curr. Opin. Immunol. 2002;14:103–110. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 26.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 27.Sieling PA, Modlin RL. Toll-like receptors: mammalian “taste receptors” for a smorgasbord of microbial invaders. Curr. Opin. Microbiol. 2002;5:70–75. doi: 10.1016/s1369-5274(02)00288-6. [DOI] [PubMed] [Google Scholar]
- 28.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 29.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 30.Bauer S, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 32.Latz E, et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 2004;5:190–198. doi: 10.1038/ni1028. [DOI] [PubMed] [Google Scholar]
- 33.Viglianti GA, et al. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 34.Batteux F, Palmer P, Daeron M, Weill B, Lebon P. FCgammaRII (CD32)-dependent induction of interferon-alpha by serum from patients with lupus erythematosus. Eur. Cytokine Netw. 1999;10:509–514. [PubMed] [Google Scholar]
- 35.Bave U, et al. FcgammaRIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J. Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 36.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 37.Ronnelid J, Tejde A, Mathsson L, Nilsson-Ekdahl K, Nilsson B. Immune complexes from SLE sera induce IL10 production from normal peripheral blood mononuclear cells by an FcgammaRII dependent mechanism: implications for a possible vicious cycle maintaining B cell hyperactivity in SLE. Ann. Rheum. Dis. 2003;62:37–42. doi: 10.1136/ard.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenert P, Stunz L, Yi AK, Krieg AM, Ashman RF. CpG stimulation of primary mouse B cells is blocked by inhibitory oligodeoxyribonucleotides at a site proximal to NF-kappaB activation. Antisense Nucleic Acid Drug Dev. 2001;11:247–256. doi: 10.1089/108729001317022241. [DOI] [PubMed] [Google Scholar]
- 39.Hacker H, et al. CpG-DNA–specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230–6240. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Canadian Hydroxychloroquine Study Group. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N. Engl. J. Med. 1991;324:150–154. doi: 10.1056/NEJM199101173240303. [DOI] [PubMed] [Google Scholar]
- 41.Furst DE, et al. Dose-loading with hydroxychloroquine improves the rate of response in early, active rheumatoid arthritis: a randomized, double-blind six-week trial with eighteen-week extension. Arthritis Rheum. 1999;42:357–365. doi: 10.1002/1529-0131(199902)42:2<357::AID-ANR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 42.Hornung V, et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 43.Krug A, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 44.Rabinovitch N, Gelfand EW. Expression of functional activating and inhibitory Fcgamma receptors on human B cells. Int. Arch. Allergy Immunol. 2004;133:285–294. doi: 10.1159/000076836. [DOI] [PubMed] [Google Scholar]
- 45.Kadowaki N, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krug A, et al. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 48.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 49.Kaneko H, et al. Circulating levels of beta-chemokines in systemic lupus erythematosus. J. Rheumatol. 1999;26:568–573. [PubMed] [Google Scholar]
- 50.Narumi S, Takeuchi T, Kobayashi Y, Konishi K. Serum levels of ifn-inducible PROTEIN-10 relating to the activity of systemic lupus erythematosus. Cytokine. 2000;12:1561–1565. doi: 10.1006/cyto.2000.0757. [DOI] [PubMed] [Google Scholar]
- 51.Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR. Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J. Exp. Med. 1999;190:1813–1824. doi: 10.1084/jem.190.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boule MW, et al. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J. Exp. Med. 2004;199:1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Latz E, et al. LPS rapidly traffics to and from the Golgi apparatus with the TLR4/MD-2/CD14 complex in a process that is distinct from the initiation of signal transduction. J. Biol. Chem. 2002;277:47834–47843. doi: 10.1074/jbc.M207873200. [DOI] [PubMed] [Google Scholar]
- 54.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 55.Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 56.Tan EM, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 57.Aarden LA, de Groot ER, Feltkamp TE. Immunology of DNA. III. Crithidia luciliae, a simple substrate for the determination of anti-dsDNA with the immunofluorescence technique. Ann. N. Y. Acad. Sci. 1975;254:505–515. doi: 10.1111/j.1749-6632.1975.tb29197.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental data