Predicting therapeutic nanoparticle efficacy using a companion MR imaging nanoparticle (original) (raw)
. Author manuscript; available in PMC: 2017 Jun 7.
Published in final edited form as: Sci Transl Med. 2015 Nov 18;7(314):314ra183. doi: 10.1126/scitranslmed.aac6522
Abstract
Therapeutic nanoparticles (TNPs) have shown heterogeneous responses in human clinical trials, raising the question of whether imaging should be used to identify patients with a higher likelihood of nanoparticle accumulation, and thus therapeutic response. Despite extensive debate about the enhanced permeability and retention (EPR) effect in tumors, it is increasingly clear that EPR is extremely variable yet little experimental data exists to predict its clinical utility. Based on the hypothesis that an FDA-approved 30-nm magnetic nanoparticle (MNP) could predict co-localization of therapeutic nanoparticles by MRI, we performed single-cell resolution imaging of fluorescently labeled MNPs and TNPs and studied their intratumoral distribution. We visualized MNPs circulating in tumor microvasculature and found sustained uptake into cells of the tumor microenvironment within minutes. MNPs could predictably demonstrate areas of co-localization for a model TNP [poly(D,L-lactic-co-glycolic acid)-_b_-polyethylene glycol; PLGA-_b_-PEG] within the tumor microenvironment (> 85% accuracy) and circulating within the microvasculature (>95% accuracy) despite their markedly different sizes and compositions. Computational analysis of NP transport enabled predictive modeling of TNP distribution based on imaging data, and identified key parameters governing intratumoral NP accumulation and macrophage uptake. Finally, MRI imaging accurately predicted initial treatment response and drug accumulation in a therapeutic study testing for the efficacy of paclitaxel-encapsulated nanoparticle. These approaches yield valuable insight into the in vivo kinetics of NP distribution and suggest that clinically-relevant imaging can be used to select patients with high EPR for treatment with TNPs.
Introduction
Nanoscale platforms have been developed to improve drug delivery, particularly in oncology, where controlled drug release can mitigate chemotherapeutic toxicities and where structural properties of solid tumors are thought to enhance nanomedicine accumulation (1). Multiple factors including aberrant vascular architecture and basement membrane disruption contribute to the enhanced permeability and retention (EPR) effect, thought to facilitate accumulation of therapeutic nanoparticles (TNPs) (2, 3). Several nanotherapeutics have been clinically approved for treatment of various solid cancers, including liposomal doxorubicin (Myocet), PEGylated liposomal doxorubicin (Doxil and Caelyx), nanoparticle albumin-paclitaxel (nab-paclitaxel, Abraxane) and poly(styrene-_co_-maleic acid) conjugated neocarzinostatin (SMANCS) while others are undergoing clinical trials (4, 5). Many such TNPs have the potential to increase efficacy by enhancing plasma and target tissue drug exposure (AUC), and/or reduce toxicities by mitigating adverse effects associated with harmful solvents and high peak drug concentrations (Cmax) of conventional intravenous (i.v.) drug formulations (5).
There is a lack of conclusive data establishing superior clinical impact of TNPs compared with standard treatments (6), and it is hypothesized that this is largely due to substantial variation in EPR from patient to patient, and even across sites within individual patients (3). For instance, modest correlation has been observed between tumor microvasculature (TMV) and highly variable tumoral accumulation of Caelyx in patients (7), suggesting EPR factors may substantially contribute to clinical efficacy. Consequently, several treatment strategies aim to therapeutically augment EPR effects within patients for improving nanotherapeutic efficacy, for example by stimulating vasodilation via heat, nitric oxide induction, and prostaglandins; by stimulating hypertension via angiotensin II; or by degrading extracellular matrix via collagenase (6). While these approaches may potentiate EPR effects, they may also complicate the clinical development of nanotherapeutics. More recently, targeted nanoparticles have entered human clinical trials for siRNA delivery (8) and for small molecule drug delivery (9). It is expected that these targeted TNPs may improve clinical outcome, in part by directing NP uptake more specifically to tumor cells once reaching the tumor microenvironment (10). Nonetheless, EPR variability continues to be a potential barrier for maximal clinical impact. Thus, one key translational challenge has been to better match patients to novel TNP therapies based on physiological determinants of the EPR effect.
The FDA has approved the carboxymethyl dextran-coated magnetic nanoparticle (MNP) ferumoxytol (Feraheme) for treatment of iron deficiency. Ferumoxytol and other related MNPs have been used with magnetic resonance imaging (MRI) to visualize and estimate vascular permeability, NP retention, and phagocyte infiltration in both cancer (11, 12) and inflammation (13). Consequently, ferumoxytol has potential as a quantifier of EPR and thus a means of patient stratification. Despite clinical introduction several years ago and several studies for different indications (11, 14), relatively little is known regarding how these MNPs distribute in different tumor compartments and cell types, how distribution is related to EPR effects, how MNP distribution correlates with TNP distribution, and whether MNP imaging can be used to stratify patients according to preferable tumor uptake of TNPs.
The goal of this study was to understand TNP distribution in vivo, and determine whether MNPs can be used as companion particles for predicting therapeutic efficacy. We used high-resolution microscopic imaging in live tumor-bearing mice, which allows single-cell quantification of nanoparticle uptake in specific cell populations (tumor vs. host) at a resolution superior to MRI (15, 16). Results from these in vivo imaging studies as well as prospective MRI demonstrate the feasibility of using MNPs as surrogate markers of intratumoral nanomedicine transport, particularly by labeling NP circulation in the TMV and accumulation in macrophages within the tumor mass. We validated these findings in various orthotopic and syngeneic cancer models, and further provide a computational framework to parse measurements for predictive modeling of TNP transport and single-cell uptake in vivo.
RESULTS
Intratumoral pharmacokinetics of magnetic and polymeric therapeutic nanoparticles
Using intravital imaging, we first studied the intratumoral pharmacokinetics (PK) of ferumoxytol to see if this MNP behaved similarly to a model TNP (9, 17, 18). Poly(D,L-lactic-_co_-glycolic acid)-b-poly(ethylene glycol) (PLGA-b-PEG) polymeric TNPs are an attractive drug-delivery platform for several reasons, including controlled drug release, tunable physical properties, extended plasma half-lives, safety, and biodegradability. A fluorescent version of a model PLGA-PEG polymeric NP (λex = 488 nm) (fig. S1) was co-injected with the MNP ferumoxytol-VT680XL (λex = 630 nm) and both NPs were simultaneously tracked in subcutaneous HT1080 human fibrosarcoma xenografts in nude mice (Fig. 1A–B; fig. S2). MNPs distributed throughout the entire tumor microcirculation with an initial _t_1/2 plasma of 70 min (Fig. 1C). This is consistent with additional ear-imaging measurements in non-tumor-bearing mice (initial **t**1/2 plasma = 71 min; fig. S3) and previous studies in non-tumor-bearing rats (_t_1/2 plasma = 67 min) (19), and which would scale to a terminal _t_1/2 plasma of 10–14 h in humans by allometric predictions (11). The TNPs exhibited similar initial plasma half-lives in both the TMV (Fig. 1B–C; _t_1/2 plasma, tumor: 55 min) and ear vasculature in non-tumor-bearing animals (fig. S3; _t_1/2 plasma, ear: 56 min), with initial kinetics approximately in the range of other clinically relevant polymeric and liposomal formulations (9, 17) such as PEGylated liposomal Doxorubicin (initial _t_1/2 plasma of 5.2 h in humans for Doxil at 20 mg/m2 (20)).
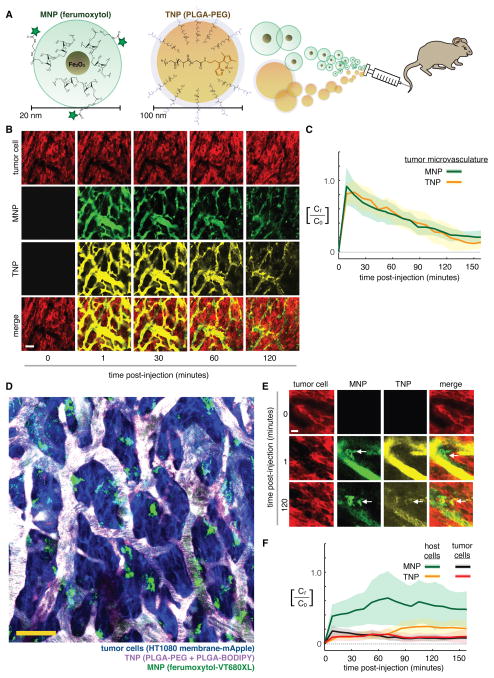
Figure 1. High-resolution intravital imaging of ferumoxytol and polymeric NPs show similar intratumoral behavior.
(A) Fluorescently labeled ferumoxytol (MNP) and PLGA-PEG (TNP) were co-injected i.v. into mice for real-time imaging. (B) Time-course measurement of intratumoral MNP and TNP distribution within a live xenograft mouse model of fibrosarcoma transgenically expresses membrane-localized RFP/mApple (HT1080-membrane-mApple). Scale bar = 50 μm. (C) Pharmacokinetics and tumor tissue uptake were quantified for MNPs and TNPs, normalized to concentration (Ct) as a fraction of initial vascular concentration (C0). Data are means (thick lines) ± SD (shading; n≥7 tumor areas across n=3 animals). (D) In the same tumor model as (B–C), contrast-enhanced images show perivascular host cells (green) 10 min after NP injection, distinguishable by cellular morphology, perivascular location, lack of tumor-specific mApple, and MNP accumulation but lack of TNP uptake at early time-points (scale bar = 50 μm). (E) Zoomed-in MNP/TNP distribution within a perivascular host cell (arrows). Note the accumulation kinetics within minutes. Scale bar = 14 μm. (F) Perivascular host cells uptake MNP more rapidly than TNP. Data are means (thick lines) ± SD (shading; n=3 tumors; n>50 cells).
Pixel-by-pixel correlation showed co-localization between MNPs and TNPs, particularly at early time-points when the NPs were mostly confined to circulating in the TMV, with MNPs successfully labeling >95% of the TMV accessible to TNPs (Fig. 1B). Single-injection control experiments for each NP confirmed no fluorescence bleed-through (fig. S2C–D) and demonstrated that MNP injection at the imaging dose does not impact tumor accumulation of subsequently injected TNPs (fig. S3C–D).
Once reaching the TMV, MNPs accumulated rapidly (within minutes) in perivascular host cells that closely neighbor or even extend cytoplasmic processes to tumor capillaries (Fig. 1D–E). Phagocytic perivascular macrophages influence vessel permeability and cancer intravasation during metastasis; to further study perivascular host cells in the context of EPR effects, we imaged several metastases of ovarian cancer following NP administration. MNPs again accumulated within minutes in the metastases (fig. S4), and polymeric TNPs were also taken up by perivascular host cells within the tumor, albeit at a lower level and more slowly (Fig. 1E–F; fig. S4).
To better visualize NP uptake within tumor-associated host cells, we also imaged MNP distribution in subcutaneous tumor xenografts using fractalkine _Cx3cr1_GFP/+ reporter mice, which have GFP+ macrophages. MNPs accumulated exclusively within these GFP+ host leukocytes (fig. S5A–B). Global expression of membrane-targeted tdTomato in all host cells of the Cx3cr1GFP/+ mice enabled simultaneous visualization of endothelium near tumor xenografts, revealing MNP uptake especially in host leukocytes adjacent to microvasculature (fig. S5C–D).
Although initial plasma kinetic timescales are roughly 1 h for both MNPs and TNPs, extended circulating nanoparticle half-life and the EPR effect drove gradual accumulation in tumor tissue over the course of 24 h (fig. S6A–C). In fact, TNP accumulation in tumor cells increased by nearly 20-fold from 3 to 24 h after administration (fig. S6C), despite the fact that plasma levels had significantly declined by this time (Fig. 1C), and increases were also seen in host cells (fig. S6C). These results underscore that intratumoral NP accumulation via EPR effects continues over the course of 24 h, despite a rapid initial phase of plasma clearance.
NPs co-localize with tumor cells at a macroscopic but not single-cell level
Low-magnification tumor imaging showed substantial accumulation and co-localization between NPs and the bulk tumor mass 24 h after treatment (Fig. 2A, left), which likely explains MRI observations of enhanced MNP accumulation in various cancers (2, 6, 14). We next determined the cellular basis of the overall NP accumulation using high-resolution (40x) images (Fig. 2A, right). Although host cells within the tumor microenvironment substantially accumulated both NPs (Fig. 1), tumor cell uptake was considerably lower and slower (Fig. 1F; fig. S6C): 3 h post-injection, >90% of both NPs were associated with host cells rather than tumor cells (fig. S6C). Although MNPs underestimate the amount of TNPs taken up by tumor cells themselves, they label host cells, such as leukocytes, that accumulate TNPs with >85% accuracy (fig. S6C). An orthotopic model of disseminated metastatic ovarian cancer showed similar patterns of co-localization and predominant accumulation in host rather than tumor cells (fig. S6D–E).
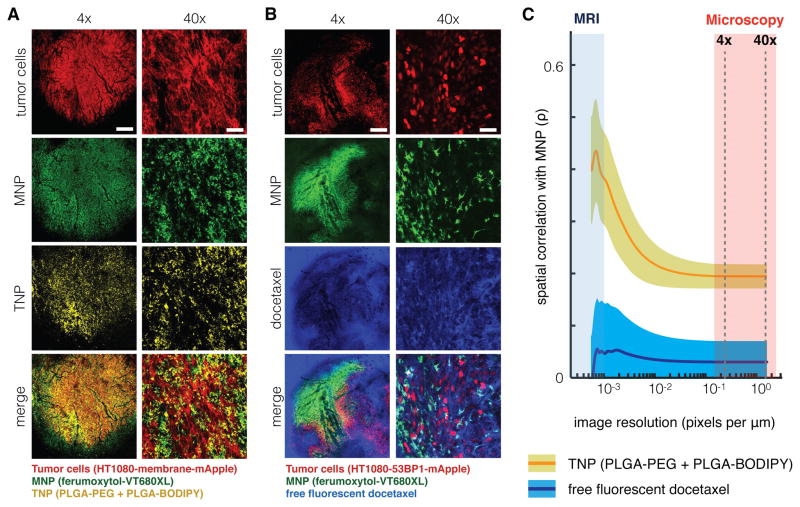
Figure 2. Multi-scale spatial co-localization between MNP and TNP.
(A–B) NP tumor uptake in a live xenograft model, 24 h post-injection with MNP. For low (4x) and high (40x) magnification, scale bar denotes 500 μm and 50 μm, respectively. i.v. co-injection with either (A) TNP or (B) a free, un-encapsulated fluorescent derivative of docetaxel were imaged following vascular clearance. (C) MNP and TNP co-localization improves at lower spatial resolution, but MNP and docetaxel co-localization do not. Microscopy images (A–B) were computationally down-sampled to reduce spatial resolution, and pixel-by-pixel Pearson’s correlation (ρ) between MNP/TNP or MNP/docetaxel intensities were calculated across a range of pixel resolutions. Data are means (thick lines) ± SE (n=3 animals and >400 images).
We also examined co-localization between MNPs and a model liposomal formulation similar to those used clinically for chemotherapeutics, such as Myocet and DaunoXome. As with the polymeric TNPs, liposomes strongly co-localized with MNPs in tumor-associated host cells (fig. S7A). To assess the potential impact of dye-labeling on co-localization, we performed several experiments with various combinations of five alternative fluorophores and found no significant difference in spatial co-localization according to pixel-by-pixel correlation (fig. S7B). In sum, EPR effects, largely influenced by NP uptake in host cells, contribute to selective NP accumulation within the bulk tumor mass.
We next analyzed how these high-resolution microscopy results apply to more clinically relevant imaging modalities, such as MRI, which have substantially lower spatial resolution. We decreased microscopy resolution by computationally downsampling, and then calculated correlation between MNP and TNP fluorescence intensities as they varied from pixel to pixel. This analysis revealed that, although MNPs and TNPs exhibit modest high-resolution correlation (ρ = 0.2) at 24 h post-injection, correlation increases nearly 3-fold (ρ = 0.55) at spatial resolutions typical of clinical MRI (Fig. 2C). Notably, this trend was not evident when comparing MNPs to the spatial distribution of a free model chemotherapeutic docetaxel that was not encapsulated in a nanoparticle, demonstrating that increased MNP/TNP co-localization at lower spatial resolution is not simply an artifact of all injected compounds (Fig. 2B–C). Overall, this provides further evidence that MNPs and TNPs accumulate and co-localize within the bulk tumor mass, and suggests that imaging MNP at lower MRI resolution may be a valuable predictor of TNP accumulation.
MNPs and TNPs are primarily taken up by tumor-associated macrophages
We next used flow cytometry and histology to quantitatively map NP distribution to immunologically defined cell populations within the bulk tumor mass. To better study tumor interactions with the immune system, we used a syngeneic immunocompetent model of non-small cell lung cancer (NSCLC) based on the subcutaneous implantation of Kras mutant _p53_−/− (KP) cells derived from autochthonous lung tumors in a genetically engineered mouse model (21). Leukocytes (CD45+ cells) comprised roughly a third of all cells within the tumor (Fig. 3A). Similar to our observations in xenograft models of fibrosarcoma (Fig. 1; fig. S6B–C) and ovarian cancer (fig. S6D), host phagocytes (macrophages and neutrophils) accumulated more MNPs and TNPs than did tumor cells (Fig. 3B–C). Immunostaining confirmed co-localization between F4/80+ host phagocytes and accumulation of both NPs (Fig. 3D).
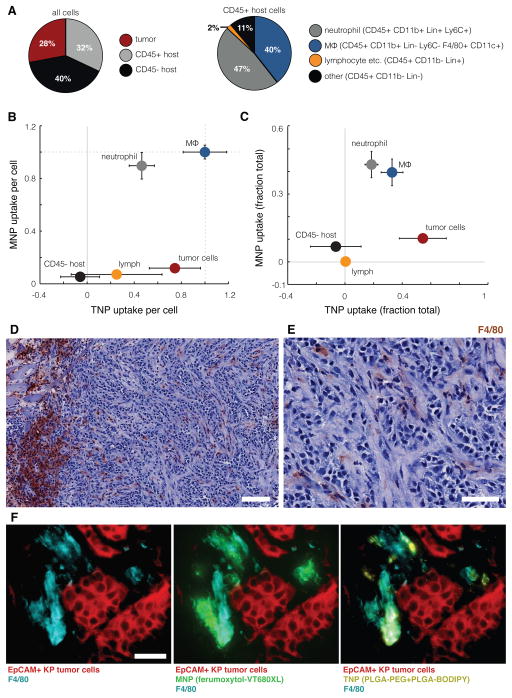
Figure 3. MNP and TNP co-localize to tumor-associated macrophages in a syngeneic cancer model.
(A–B) Flow cytometry analysis of intratumoral cellular composition (A) and single-cell NP distribution (B) in KP subcutaneous xenografts co-treated with TNP and MNP for 24 h. Cellular NP uptake was quantified by fluorescence intensity after subtracting autofluorescence of each population and normalized to the highest average NP uptake (macrophage). (C) Cumulative NP uptake across total cell populations within the bulk tumor mass, normalized such that NP uptake across all cell populations, weighted by their relative frequency, sums to 1. Data are means ± SEM (n=12). (D–E) KP xenografts were excised 24 h after MNP and TNP co-treatment, stained with hematoxylin and F4/80 (brown), and imaged at 10x (D; scale-bar = 100 μm) and 40x (E; scale-bar = 50 μm). (F) Adjacent tumor sections were immunostained for EpCAM to label tumor cells and for F4/80 to label macrophages. Note the high uptake of both NPs in F4/80+ phagocytes.
Tumor cells accumulated significantly more TNPs than MNPs, while CD45+ host cells accumulated significantly more MNPs than TNPs (Fig. 3B). CD45− host cell populations, which include endothelial cells and tumor-associated fibroblasts, did not accumulate substantial levels of MNPs or TNPs compared to tumor cells or leukocytes (Fig. 3B), thus reflecting previous reports that fibroblasts limit, rather than enhance, intratumoral NP accumulation (22). When weighted against overall cell fractions within the bulk tumor, our data likewise indicate MNPs distribute primarily to host phagocytes, while TNPs distribute within a mix of phagocytes and tumor cells (Fig. 3C), in agreement with HT1080 and OVCA xenograft results (fig. S6). Phagocytosis of tumor cells by host macrophages may complicate both flow cytometry and imaging analyses; however, imaging data suggest that this population represents < 5% of all cells analyzed and therefore has minimal impact on the median-based statistics calculated here.
Computational modeling quantifies EPR effects for nanoparticles and enables spatiotemporal mapping
Computational modeling was used to quantify the observed kinetic processes involved in intratumoral NP accumulation and retention, to compare differences among NPs, to predict in vivo behavior in different tumor models, and to rank the relative contributions of individual parameters, such as vessel permeability, MF content, cellular uptake rates, and interstitial diffusion. We used an approach based on finite-element analysis that incorporated spatial NP diffusion and heterogeneous NP uptake at the single-cell level (Fig. 4A). Reaction/diffusion parameters were computationally inferred for each type of NP (table S1), and comparison of these parameter sets for each NP allowed for the derivation of a quantitative normalization factor that corrected for differences in their kinetics.
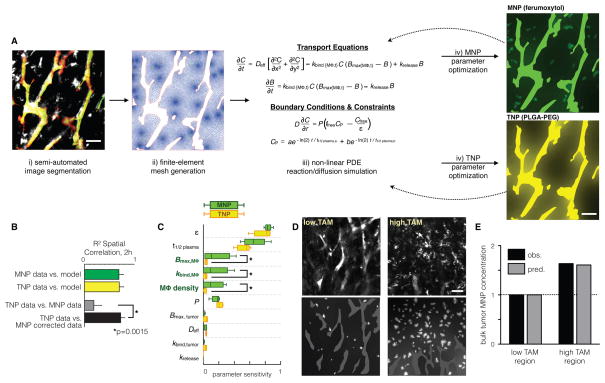
Figure 4. Quantitative finite element analysis describes single-cell reaction-diffusion processes of the EPR effect.
(A) Overview of computational modeling and optimization. i) In HT1080 xenografts, automated morphological criteria identify vessels (green/red masking), and early MNP accumulation (white) identifies macrophage (as in Fig. 1D), which are computationally segmented with manual optimization. ii) The finite-element mesh is generated based on image segmentation. iii) Change in concentration over time of free NP (dC/dt) and bound NP (dB/dt), along with boundary conditions describing NP flux across vessel walls (D(dC/dr)) and vessel NP concentrations over time (_C_P) are integrated across the finite-element mesh. iv) Parameters are iteratively optimized by fitting model results to time-lapse imaging data. (B) Model-fitting validation (green and yellow bars) and spatial correlation between MNPs and TNPs, with and without non-linear PK correction based on finite element modeling (grey and black bars). Correlation data are means ± SEM (n > 200 regions; P value determined by permutation test). (C) Parametric sensitivity analysis showing modeling parameters that most sensitively influence total NP accumulation within the bulk tumor at 2 h post-injection. ε: extracellular volume fraction; t1/2 plasma: NP plasma half-life; _B_max: max NP cellular uptake; _k_bind: NP uptake rate; P: vessel permeability. Data are medians ± IQR (n =5; *p=0.01, pooled two-tailed t-test). (D) Example images and corresponding modeling show heterogenous MNP accumulation in tumor regions with few (n=18; left) and many (n=98; right) phagocytes. All scale bars, 50 μm. (E) Finite element modeling accurately predicts increased MNP accumulation in the “high TAM” tumor region (D), measured as average MNP concentration within tumor tissue outside of vessels.
Non-linear PK correction, when applied to MNP images, improved spatial correlation between MNPs and TNPs by ~300% and thus greatly increased the accuracy of MNPs in predicting intratumoral TNP levels (Fig. 4B). This enhanced accuracy matched the model’s validation accuracy in fitting imaging data to modeling data of the same NP (Fig. 4B, green and yellow bars), which marks the upper accuracy limit for the modeling framework. Thus although MNP and TNP kinetics differ, they overlap in spatial distribution, particularly among host phagocytes.
To assess the relative importance of different EPR factors in intratumoral NP accumulation, we performed a parametric sensitivity analysis for each NP type (Fig. 4C). We locally adjusted individual modeling parameters (± 25%), simulated NP behavior with the new parameter sets, and recorded the resulting impact on bulk tumor NP accumulation (including tumor cells and host phagocytes, but excluding vasculature) at 2 h post-injection. This analysis revealed that extracellular volume fraction in the tissue, ε, and systemic plasma half-life of the NPs, _t_1/2 plasma, were the two most important factors governing tumor uptake 2 h after injection, suggesting that cellular uptake was fairly limited at this early time-point.
Because MNPs and TNPs have multiple distinct parameters that interact with each other, local changes in reaction rates can affect accumulation of each type of NP differently. MNPs were highly sensitive to macrophage uptake capacity (_B_max,MΦ), kinetics (_k_bind,MΦ), and density (macrophages per tumor tissue area), whereas TNPs were not, largely because MNP uptake far outstripped TNP uptake at the early time-point of 2 h (Fig. 4C). Macrophage density has been previously reported as varying widely across tumor types and patients, often correlating with clinical outcome (23). There was heterogeneity in macrophage density even within different regions of single tumors (Fig. 4D, top images; CV = 100% across n=6 tumors). We independently tested the computational model on such heterogeneous regions and accurately captured the significant effects of variable macrophage density on NP accumulation (Fig. 4D–E).
Pre-treatment MRI predicts extent of tumor-cell DNA damage after TNP administration
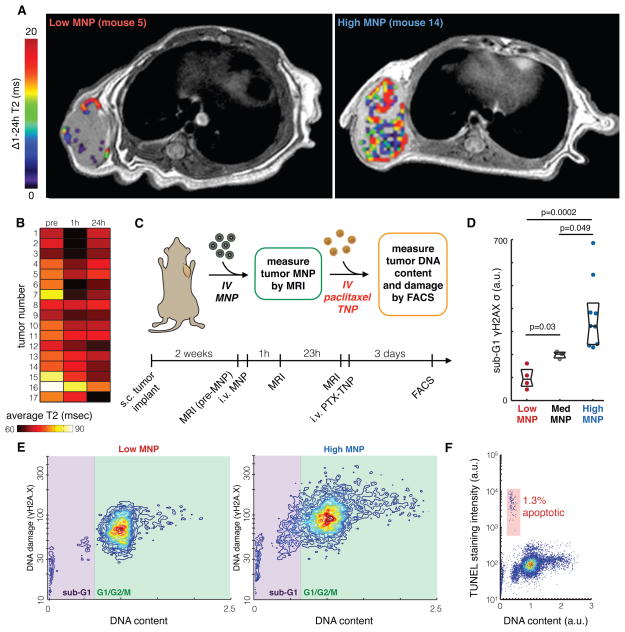
To test whether MNP-MRI would help to select animals for preclinical trials, we performed several prospective translational experiments to mimic a clinical scenario. A cohort of mice bearing subcutaneous HT1080 tumors was imaged by MRI before and after i.v. administration of ferumoxytol MNP to measure total tumor accumulation. MRI 1 h after ferumoxytol was used to label tumor microvascularization accessible to circulating TNPs, and MRI at 24 h labeled cellular uptake within the bulk tumor mass (Fig. 5A). Change in average T2 mapping of the tumor region (ΔT2) was used to quantify MNP levels. The difference in MNP accumulation between 1 and 24 h was highly heterogeneous across the cohort (CV = 750%). We used this metric to stratify mice into “low,” “medium,” and “high” MNP categories (Fig. 5, A and B). Paclitaxel-loaded TNPs (fig. S8A–C) were then administered at a dose of 3 mg/kg paclitaxel and tumors were analyzed for subsequent treatment responses (Fig. 5C).
Figure 5. MRI quantifies heterogeneous MNP accumulation and predicts initial TNP response.
(A) Example cross-sectional T2 images of HT1080 tumors accumulating low and high intratumoral MNP, with pseudo-color overlays indicating ΔT2 within the tumor region. (B) Heat-map shows T2 mapping averaged over the entire area of each tumor, which stratified a subset of tumors as “low MNP,” “med MNP,” or “high MNP.” (C) Experimental design for using MNP-MRI to predict paclitaxel-loaded TNP response in HT1080 xenografts. (D) FACS analysis shows drug response in tumors exhibiting either low, medium, or high MNP uptake, as determined by MNP-MRI. Tumors with high MNP showed greater populations with abnormally low DNA content (sub-G1 cells) and elevated heterogeneous levels of DNA damage response, determined by deviation (σ) in γH2A.X staining across the population. Data are medians ± IQR (two-tailed t-test). (E) Representative FACS data showing DNA content (using a DNA-intercalating dye) and DNA damage response (using γH2A.X immunostaining) in “low MNP” and “high MNP” tumors. (F) Representative FACS data showing DNA content (using a DNA-intercalating dye) and apoptosis (determined by terminal deoxynucleotidyl transferase dUTP nick end labeling, TUNEL) in tumor cells from a “high MNP” tumor. Contour lines and colors (E–F) denote single-cell distribution density.
Our data indicate that the different imaging groups also had vastly different therapeutic responses, measured by cell-cycle distribution and DNA damage (Fig. 5D–F). Compared to tumors with “medium” MNP uptake, we found that “high MNP” tumors contained substantially more cells with abnormally low DNA content (“sub-G1” population) and elevated DNA damage response, as measured by γH2A.X staining (Fig. 5D–E). These sub-G1 cells are mostly viable, with only 2–10% (IQR over all tumors) of the sub-G1 cells being apoptotic (Fig. 5F), and have been implicated as a key feature in paclitaxel action (24); no significant difference in apoptosis between “low MNP” and “high MNP” tumors was observed (p=0.3, one-way ANOVA). In contrast, “low MNP” tumors exhibited substantially lower levels of DNA damage response compared to the “medium MNP” tumors (Fig. 5D–E). These data indicate that MRI can identify tumors that are either more or less responsive to TNP treatment.
MNP distribution predicts disease progression and TNP payload accumulation
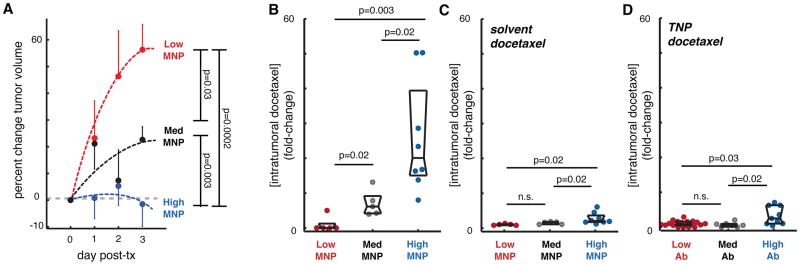
MNP-MRI could be used to predict longitudinal disease progression following TNP treatment. A total of 33 subcutaneous HT1080 tumors were imaged before and after ferumoxytol administration to quantify MNP uptake. To induce greater heterogeneity of tumoral MNP concentration (similar to what is observed clinically), half the cohort was pre-treated with systemic liposomal clodronate to reduce TAM prior to NP administration and MR imaging. As done in the previous experiment (Fig. 5), MR imaging of MNP uptake was again quantified by calculating ΔT2 between 1 h and 24 h post-administration of MNP. Tumors were stratified into “low”, “medium”, and “high” MNP groups, paclitaxel-encapsulated TNPs (3 mg/kg) were administered i.v. immediately following MNP-MRI, and tumor sizes were measured daily until excised for further analysis. MNP accumulation predicted tumor growth following TNP treatment. The “low MNP” tumors grew 2-fold faster than the “medium MNP” tumors, while “high MNP” tumors did not increase in size at all (Fig. 6A).
Figure 6. MNP predicts longitudinal TNP response and accumulation of TNP payload.
(A) Tumor progression in HT1080 tumors ranked according to low, medium, and high MNP as measured by MNP-MRI. Data are means ± SEM (total n=33). (B) In orthotopic 4T1 breast cancer tumors, TNP-encapsulated docetaxel accumulates 25-fold higher in “high MNP” compared to “low MNP” tumors, as determined by fluorescence of excised tumors 1 day after MNP/TNP injection. (C) Using same tumor model as in (B), MNP prediction of un-encapsulated solvent-docetaxel accumulation is significant but relatively modest, only 2.8-fold higher in “high MNP” compared to “low MNP” tumors, as determined by fluorometry. (D) Using same tumor model as in (B), TNP-encapsulated docetaxel was co-injected with fluorescent tumor-targeting (α-EGFR) antibody, which stratified tumors into either “low-,” “med-,” and “high-Ab” groups. Tumor-Ab only modestly predicted drug accumulation, with “high-Ab” tumors showing 3.3-fold higher TNP payload accumulation (*two-tailed t-tests for all; C–E, data are medians ± IQR).
We next investigated the degree to which MNPs predict accumulation of the TNP chemotherapeutic payload itself. For these experiments we used a syngeneic, orthotopic model of invasive breast cancer consisting of 4T1 mouse mammary carcinoma cells implanted into the mammary fat pads of immunocompetent BALB/c mice. To quantitatively and sensitively detect drug accumulation within tumors, we loaded TNPs with a fluorescent docetaxel derivative (fig. S8D). MNPs and docetaxel-encapsulated TNPs were administered i.v., and 20 h later tumors were excised and analyzed for TNP accumulation. Tumor MNP levels significantly correlated with accumulation of the TNP therapeutic payload, such that “high MNP” tumors exhibited roughly 25-fold higher levels of docetaxel compared to “low MNP” tumors (Fig. 6B).
Using the same 4T1 tumor model, we also performed a control experiment to determine how effectively MNPs predict tumoral accumulation of free docetaxel. For this control, we used a silicon-rhodamine fluorophore to generate a spectrally distinguishable fluorescent docetaxel derivative (fig. S8E) that exhibits similar PK properties as the BODIPY labeled drug (fig. S8F). Following MNP and un-encapsulated solvent-based docetaxel injection, tumors were excised and analyzed for drug and MNP uptake. Although MNPs somewhat predicted the accumulation of un-encapsulated solvent-based docetaxel, the difference between “low MNP” and “high MNP” was much more modest (2.8-fold; Fig. 6C) compared to when the docetaxel was encapsulated in TNPs (25-fold) (Fig. 6B).
We also compared the stratifying ability of MNPs versus an antibody targeting epidermal growth factor receptor (EGFR), which has been used for imaging tumor burden, selecting treatments, and monitoring response in patients with EGFR-overexpressing cancers, including those receiving docetaxel and other chemotherapeutics (25). With the EGFR-expressing 4T1 tumor model (26), we stratified excised tumors into “low,” “medium,” and “high” antibody groups. Co-injected TNP-encapsulated docetaxel was then quantified. Although the anti-EGFR could predict TNP accumulation for high versus low and medium groups, the difference between “low” and “high” was modest (3.3-fold; Fig. 6D) compared to the difference seen between “low MNP” and “high MNP” groups (25-fold; Fig. 6B). The order of magnitude greater effect for correlation between MNPs and TNPs suggest nanoparticle-specific EPR factors play a dominant role in governing heterogeneous TNP tumor accumulation. Given the ability of MNP to predict therapeutic efficacy as in Fig. 6A, the poor correlation between antibody labeling and TNP accumulation imply that the anti-EGFR antibody is a poor predictor of tumor response to TNP treatment. Taken together, these results demonstrate that MNPs are effective predictors of TNP payload accumulation within tumors and, in turn, therapeutic efficacy.
Discussion
TNPs distribute differently across tumors in different patients. A central question in nanomedicine is whether imaging could be used to identify patients with higher predisposition to TNP accumulation and, in turn, efficacy (5, 9). Answering this question could aid in the decision of whether to actively target TNPs or to let them accumulate “passively” within a tumor (3). These issues are at the core of understanding how to best exploit EPR effects (6) for clinical applications, how to design better TNPs, and how to alter key physiologic parameters to maximize distributions to and within tumors.
We hypothesized that an FDA-approved carboxymethyl dextran-coated magnetic nanoparticle (ferumoxytol) can be used to predict TNP behavior by measuring intratumoral distribution and kinetics across different tumor compartments. To define these compartments, we relied upon intravital imaging capable of resolving intracellular details (15). Our study uncovered several interesting findings, paving the way for companion particles in predictive nanomedicine. MNPs and TNPs, despite being of different sizes and composition, co-localized to a high degree, especially in the circulating vascular phase, at the macroscopic level (i.e. resolutions used in MRI), and in phagocytic host cells. MNP accumulation within the bulk tumor was significantly influenced by host phagocyte content and surprisingly rapid peritumoral host cell uptake within minutes. For both NPs, tumor cell uptake was much slower than expected, but was greater for TNPs than MNPs despite TNPs’ larger size. Importantly, for translation to a therapeutic setting, the heterogeneity of intratumoral TNP accumulation could be predicted and measured by MRI and correlated with responsiveness to TNP treatment.
Understanding the magnitude of individual EPR factors is essential for predicting and eventually tailoring NP behavior. EPR effects of vascular permeability and NP extravasation have been studied extensively in the past (2, 3, 22, 27); however, these studies have largely been limited by spatiotemporal resolution and consequently do not sufficiently account for heterogeneous NP transport and uptake at the single-cell level, particularly in TAM. We studied not only the multiple EPR effect components, but also used high-resolution time-lapse microscopy to reveal their highly dynamic character. Vascularization and permeability drove PK at early time points (t < _t_1/2 plasma) for both NPs, and rapid cellular uptake contributed to early EPR effects for MNPs, particularly in perivascular regions where capillary-associated phagocytes accumulated MNPs within minutes. TNP uptake was minimal at this early stage. In contrast, after 24 h (t ≫ _t_1/2 plasma) EPR effects were dominated by cellular NP uptake, particularly in TAM.
TNPs are typically engineered to release chemotherapeutic payloads at prescribed and tunable rates. From a therapeutic standpoint, the dynamic EPR effect critically influences the target tissue PK of both the TNP vehicle and its payload. The relative therapeutic importance of tumor vascularity, vessel permeability, interstitial fluid content, extracellular matrix composition, fluid pressure gradients, and phagocyte infiltration will ultimately depend on TNP physicochemical properties including size, shape, rigidity, the time scales of payload release from the TNP vehicle, and subsequent transport properties of the drug. When keeping the TNP physicochemical properties constant as done in our study, both slow and rapid payload release will influence intratumoral distribution: cellular NP uptake will dominate in the former, while vascular permeability and extracellular volume fraction will dominate in the latter. Overall, single-cell analysis of dynamic EPR effects highlights how cellular uptake, particularly by TAM, can dramatically influence both the kinetics and distribution of intratumoral NP delivery. Although we did not extensively investigate different TNP formulations, our study lays the groundwork for understanding how heterogeneous cell populations within the tumor microenvironment impact drug delivery, and demonstrates that MNPs may be used to predict the behavior of NPs with differing physicochemical properties, especially when explicitly accounting for their quantitative differences in transport parameters.
We used computational modeling to provide a framework for quantifying and comparing physiological effects that govern tumoral NP accumulation. This work builds on previous modeling of intratumoral drug transport (15) and extends it to nanotherapeutics, which required the explicit modeling of heterogeneous NP uptake activity at single-cell resolution. In fact, the largest inferred differences in NP behavior all related to heterogeneous cellular NP uptake. For macrophages, the MNP uptake rate was roughly sixfold faster than for TNP, and maximum uptake levels were threefold higher, which is not surprising considering the differences in surface modifications between MNPs and TNPs. Ultimately, direct modeling of heterogeneous NP uptake activity across multiple cell populations was critical to enabling accurate prediction of TNP levels based on MNP measurements. Future studies should extend this approach to better capture 3D behavior, link NP kinetics with drug payload release and cellular response, more closely examine differences in tumoral penetration between MNPs and TNPs, and address additional heterogeneity in parameters related to interstitial pressure, convection, diffusion through fibrotic tissue, and pH. Several of our findings have direct implications for the effective design of nanotherapeutic clinical trials. Heterogeneous tumor vascularization is a recognized clinical feature that can be detected using various angiography modalities and that can affect both drug delivery (27) and overall survival (28). Immediate MNP MRI showed the tumor vasculature accessible to TNPs, which will be especially important in the context of therapeutics that affect vascular structure, such as targeted anti-angiogenics, vascular dilators, and hyperthermic induction. MNP imaging after 24 h revealed peritumoral cell populations that accumulated high levels of TNPs. Macrophage content represents a critical and yet highly variable component of the overall EPR effect. Recent work has highlighted the extensive heterogeneity of phagocyte populations within tumors and its significant effects on drug response and clinical outcome (23). Moreover, several new therapeutics directly target TAM themselves, for instance by blocking colony-stimulating-factor 1 receptor; therefore, assessing phagocyte content will be especially important for selecting patients to receive these drugs and to monitor their response. MNP imaging offers an effective and non-invasive means to longitudinally monitor such phagocyte populations.
To progress clinically, the principals described here should be applied in greater detail to more anatomically representative disease models such as patient-derived xenografts, genetically-engineered autochthonous mouse models, and larger animals; to study EPR effects in metastatic lesions; to examine correlation between MNP uptake and long term efficacy and overall survival; and to use MNPs for screening multiple in vivo models to identify which general cancer types may be more responsive to TNP treatment. Ultimately, we provide a high-resolution description of dynamic EPR effects governing MNP and TNP transport and demonstrate the potential of MNP to predict TNP efficacy in the clinic—a major stepping stone toward translation of new nanomedicine and for eventually selecting patients for nanotherapeutic trials.
METHODS
Study design
The hypothesis was that MNP would predict accumulation and efficacy of TNP in tumors. Imaging studies and drug accumulation measurements were designed to measure kinetics and co-localization between MNP and TNP within tumor tissue; tumor caliper measurements, along with flow cytometry measurement of DNA damage, cell cycle, and apoptosis were designed to assess correlation between MNP accumulation and drug response efficacy. All experiments were performed with at least two independent replicates (specified in figure legends). Data collection methods were pre-determined for all experiments, and animals were assigned randomly to treatment groups. No outliers were excluded. Cohort size in the experiments using the 4T1 model (Fig. 6) were informed by a power analysis based on MRI data in the HT1080 model (Fig. 5), using measured intragroup heterogeneity in MNP uptake (CV=60%), intergroup difference of 80% between low and high MNP groups, and objective power (1-β) of 0.95 calculated with available software (29). Researchers were blinded to groups for MNP and anti-EGFR Ab uptake during non-imaging experiments in Fig. 5–6, including caliper measurement, as pre-specified stratification procedures were not performed until after all data had been collected.
TNP synthesis
All polymeric TNPs were synthesized by nanoprecipitation, were characterized by size and surface charge using dynamic light scattering (DLS; Malvern Zetasizer), and were freshly prepared before each experiment. Synthesis details are in Supplementary Methods.
Animal and cell models
All animal research was performed in accordance with guidelines from the Institutional Subcommittee on Research Animal Care. All experiments were performed using female mice that were 5–7 weeks old at the start of the experiment. For experiments with HT1080 tumors, 2 million cells were subcutaneously implanted into nu/nu mice; roughly 2–3 weeks later (once tumors reached approximately 8 mm diameter), imaging experiments were initiated. For 4T1 tumors, 0.5 million cells were implanted into the mammary fat pads of BALB/c mice; roughly 10 days later, imaging and therapeutic agents were i.v. injected and tumors were excised for analysis the following day. For ovarian cancer imaging, 10 million A2780CP cells were intraperitoneally injected into nu/nu mice, and experiments were performed roughly 6 weeks later with evident ascites or tumor masses. For imaging experiments using KP1.9 cells, 1 million cells were subcutaneously implanted into C57Bl/6 background animals (all JAX), including _Cx3cr1_GFP/+ and _Cx3cr1_GFP/+ _R26_mT-mG/+ dual-reporter mice, both containing GFP+ monocytes, macrophages, and dendritic cells. Mouse and human cell lines HT1080, A2780CP, 4T1, and KP1.9 are described in Supplementary Methods.
Intravital microscopic imaging
Intravital microscopy was performed on an Olympus FV1000 confocal-multiphoton imaging system using a XLUMPLFLN 20x water immersion objective (NA 1.0; Olympus America) with 2x digital zoom. Images were scanned sequentially using 405-nm, 473-nm, 559-nm, and 635-nm diode lasers with a DM405/473/559/635-nm dichroic beam splitter; emitted light was collected using combinations of beam splitters (SDM473, SDM560, and/or SDM 640) and emission filters BA430-455, BA490-540, BA575-620, and BA655-755 (all Olympus America).
Dorsal window chamber imaging was performed following previously described procedures (16); briefly, 2 million HT1080-membrane-mApple cells in 50 μl PBS were injected under the fascia of nu/nu mice (Cox7, MGH) 30 min after surgical chamber implantation and imaged two weeks later.
MR imaging
Mice were anesthetized by isoflurane inhalation and placed in a birdcage radio-frequency coil with an inner diameter of 38 mm. Using a 4.7-T MRI system (PharmaScan; Bruker BioSpin), mice were scanned for a baseline T2 value before MNP injection. Without removal of the mouse from the coil, mice were scanned after i.v. MNP injection (20 mg Fe/kg), and a third scan was performed 24 h later. Using Osirix software, T2 values were calculated by fitting of a standard exponential relaxation model to the data averaged over the tumor region of interest on each slice. The quantitative probe accumulation was calculated as follows: [ln(T21h post MNP/T224h post MNP)], where ‘T21h post MNP’ indicates T2 value after MNP injection (vascular phase) and ‘T224h post-MNP’ indicates T2 value 24h after MNP injection (cell accumulation phase).
Computational image analysis
Intravital microscopy images were analyzed using either Matlab (Mathworks) or ImageJ and were pre-processed using background subtraction based on data acquired immediately prior to NP injection. Vascular half-life calculations, finite-element analysis, and other details are described in Supplementary Methods.
MNP prediction of drug uptake, response, and progression
Three-week old subcutaneous HT1080 tumors were imaged before, 1 h after, and 24 h after i.v. ferumoxytol injection, once tumors reached an average diameter of 8 mm. To generate heterogeneity in EPR effects (Fig. 6A), tumors were evenly split into two groups of equally distributed tumor sizes, receiving either 5 mg/ml clodronate liposomes (clod-lip) or PBS liposomes as a vehicle control, 150 μl intraperitoneally 3 days prior to imaging, and again 100 μl i.v. administered 24 h prior to imaging (ClodLip BV). Tumoral MNP uptake was quantified as [T21h post MNP - T224h post MNP], and weighted by MNP plasma-half life for each treatment group to control for residual MNP in circulation (30). To measure tumor volume (V = 4/3 π r3), caliper measurements were performed by two blinded researchers. Results were categorized into three groups defined by boundaries that maximized the statistical significance in differential drug response and tumor progression as measured by DNA damage response or tumor volume, respectively. MR images (Fig. 5A) were selected based on their representativeness for each group and also for tumors being of roughly equal anatomical position and size.
For correlation between tumor uptake of MNPs, TNP, free drug, and EGFR-targeting antibody, we used the orthotopic 4T1 breast cancer syngeneic mouse model, as described in Supplementary Methods.
Statistical analysis
Statistical analyses were performed using GraphPad Prism, Matlab, and Microsoft Excel. Measurement uncertainties throughout are denoted by error-bars and shading as indicated in figure legends. All statistical tests were two-tailed with testing level thresholds of α=0.05. As a pre-defined procedure, stratifications by MNP or Ab uptake (Fig. 5–6) were performed using thresholds that maximized the statistical difference, according to t-test, in drug response, tumor progression, or docetaxel uptake.
Supplementary Material
Supplemental materials
Figure S1. Fluorescent NP synthesis and characterization.
Figure S2. Imaging single-cell kinetics of MNP distribution with no TNP/MNP fluorescence spectral bleed-through.
Figure S3. MNP match TNP plasma kinetics and do not influence TNP uptake.
Figure S4. MNP & TNP uptake by perivascular host cells in ovarian cancer.
Figure S5. MNP uptake in tumor-associated Cx3cr1+ host cells.
Figure S6. Imaging cytometry analysis of single-cell NP distribution kinetics.
Figure S7. MNP co-localizes with a clinically relevant liposome formulation in tumor-associated cells.
Figure S8. Characterization of paclitaxel-loaded TNP and fluorescent derivatives.
Table S1. Optimized FEM modeling parameters and reference values.
Acknowledgments
The authors thank A. Zaltsman, D. Pirovich, M. Sebas, O. Kister, N. Sergeyev, K. King, and Y. Iwamoto for technical assistance, and S. Lippard, and Y. Zheng for helpful discussions.
Funding: This work was supported in part by the U.S. National Institutes of Health (NIH) (R01CA164448, U54-CA151884, 5P50CA086355, and HL084312). MAM was supported by T32 CA 79443. BWH investigators acknowledge the the David Koch–Prostate Cancer Foundation Award in Nanotherapeutics; C.P. is in part supported by Deutsche Forschungsgemeinschaft (DFG) PF809/1-1.
Footnotes
Competing interests: In compliance with institutional guidelines, O.C.F. discloses his financial interest in BIND Therapeutics, Selecta Biosciences and Blend Therapeutics, which develop nanoparticle medical technologies but did not support this study. Other authors declare no conflicts of interest.
Author contributions: M.A.M., S.G., O.C.F., and R.W. developed the concept; M.A.M., S.G., C.P., M.P., O.C.F., and R.W. designed the experiments; M.A.M., S.G., N.K., and S.B. synthesized and characterized PLGA-PEG NPs; M.A.M. synthesized and characterized MNP, docetaxel-TNP, and Ab-dye conjugate; M.A.M., C.P., and C.E. performed FACS experiments; M.A.M. and R.H.K. performed intravital imaging experiments; M.A.M., G.W., O.C.F. and R.W. designed or performed therapeutic MRI experiments; M.S. designed and synthesized docetaxel-dye conjugates; M.A.M., A.L., and K.S.Y. generated the cell lines; M.A.M., S.G., O.C.F., and R.W. wrote the paper; all authors analyzed results, and edited the manuscript.
Data and materials availability: All cell lines were obtained through MTAs. Requests for collaboration involving materials used in the research will be fulfilled provided that a written agreement is executed in advance between BWH or MGH and the requesting parties.
References AND NOTES
- 1.Chow EK, Ho D. Cancer nanomedicine: from drug delivery to imaging. Sci Transl Med. 2013;5:216rv4. doi: 10.1126/scitranslmed.3005872. [DOI] [PubMed] [Google Scholar]
- 2.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraft JC, Freeling JP, Wang Z, Ho RJ. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J Pharm Sci. 2014;103:29. doi: 10.1002/jps.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem Soc Rev. 2012;41:2971. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prabhakar U, Maeda H, Jain RK, Sevick-Muraca EM, Zamboni W, Farokhzad OC, Barry ST, Gabizon A, Grodzinski P, Blakey DC. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koukourakis MI, Koukouraki S, Giatromanolaki A, Archimandritis SC, Skarlatos J, Beroukas K, Bizakis JG, Retalis G, Karkavitsas N, Helidonis ES. Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J Clin Oncol. 1999;17:3512. doi: 10.1200/JCO.1999.17.11.3512. [DOI] [PubMed] [Google Scholar]
- 8.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M, Horhota A, Low S, McDonnell K, Peeke E, Retnarajan B, Sabnis A, Schnipper E, Song JJ, Song YH, Summa J, Tompsett D, Troiano G, Van Geen Hoven T, Wright J, LoRusso P, Kantoff PW, Bander NH, Sweeney C, Farokhzad OC, Langer R, Zale S. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4:128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 10.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006;66:6732. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 11.Harisinghani M, Ross RW, Guimaraes AR, Weissleder R. Utility of a new bolus-injectable nanoparticle for clinical cancer staging. Neoplasia. 2007;9:1160. doi: 10.1593/neo.07940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Y, Kim M, Carrasco D, Kung AL, Chin L, Weissleder R. In vivo assessment of RAS-dependent maintenance of tumor angiogenesis by real-time magnetic resonance imaging. Cancer Res. 2005;65:8324. doi: 10.1158/0008-5472.CAN-05-0027. [DOI] [PubMed] [Google Scholar]
- 13.Weissleder R, Nahrendorf M, Pittet MJ. Imaging macrophages with nanoparticles. Nature materials. 2014;13:125. doi: 10.1038/nmat3780. [DOI] [PubMed] [Google Scholar]
- 14.Bashir MR, Bhatti L, Marin D, Nelson RC. Emerging applications for ferumoxytol as a contrast agent in MRI. J Magn Reson Imaging. 2015;41:884. doi: 10.1002/jmri.24691. [DOI] [PubMed] [Google Scholar]
- 15.Thurber GM, Yang KS, Reiner T, Kohler RH, Sorger P, Mitchison T, Weissleder R. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat Commun. 2013;4:1504. doi: 10.1038/ncomms2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller MA, Askevold B, Yang KS, Kohler RH, Weissleder R. Platinum compounds for high-resolution in vivo cancer imaging. ChemMedChem. 2014;9:1131. doi: 10.1002/cmdc.201300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci U S A. 2011;108:1850. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci U S A. 2006;103:6315. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon GH, von Vopelius-Feldt J, Fu Y, Schlegel J, Pinotek G, Wendland MF, Chen MH, Daldrup-Link HE. Ultrasmall supraparamagnetic iron oxide-enhanced magnetic resonance imaging of antigen-induced arthritis: a comparative study between SHU 555 C, ferumoxtran-10, and ferumoxytol. Invest Radiol. 2006;41:45. doi: 10.1097/01.rli.0000191367.61306.83. [DOI] [PubMed] [Google Scholar]
- 20.Doxil (package insert) Horsham, PA: Janssen Products, LP; 2015. [Google Scholar]
- 21.DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc. 2009;4:1064. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 23.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demidenko ZN, Kalurupalle S, Hanko C, Lim CU, Broude E, Blagosklonny MV. Mechanism of G1-like arrest by low concentrations of paclitaxel: next cell cycle p53-dependent arrest with sub G1 DNA content mediated by prolonged mitosis. Oncogene. 2008;27:4402. doi: 10.1038/onc.2008.82. [DOI] [PubMed] [Google Scholar]
- 25.Tolmachev V, Stone-Elander S, Orlova A. Radiolabelled receptor-tyrosine-kinase targeting drugs for patient stratification and monitoring of therapy response: prospects and pitfalls. Lancet Oncol. 2010;11:992. doi: 10.1016/S1470-2045(10)70088-7. [DOI] [PubMed] [Google Scholar]
- 26.Rao S, Larroque-Lombard AL, Peyrard L, Thauvin C, Rachid Z, Williams C, Jean-Claude BJ. Target modulation by a kinase inhibitor engineered to induce a tandem blockade of the epidermal growth factor receptor (EGFR) and c-Src: the concept of type III combi-targeting. PLoS One. 2015;10:e0117215. doi: 10.1371/journal.pone.0117215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS, Bawendi MG, Fukumura D, Jain RK. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7:383. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goscinski MA, Nesland JM, Giercksky KE, Dhakal HP. Primary tumor vascularity in esophagus cancer. CD34 and HIF1-alpha expression correlate with tumor progression. Histol Histopathol. 2013;28:1361. doi: 10.14670/HH-28.1361. [DOI] [PubMed] [Google Scholar]
- 29.Lenth RV. Statistical power calculations. J Anim Sci. 2007;85:E24. doi: 10.2527/jas.2006-449. [DOI] [PubMed] [Google Scholar]
- 30.Jones SW, Roberts RA, Robbins GR, Perry JL, Kai MP, Chen K, Bo T, Napier ME, Ting JP, Desimone JM, Bear JE. Nanoparticle clearance is governed by Th1/Th2 immunity and strain background. J Clin Invest. 2013;123:3061. doi: 10.1172/JCI66895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukinavičius G, Reymond L, D’Este E, Masharina A, Göttfert F, Ta H, Güther A, Fournier M, Rizzo S, Waldmann H, Blaukopf C, Sommer C, Gerlich DW, Arndt HD, Hell SW, Johnsson K. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat Methods. 2014;11:731. doi: 10.1038/nmeth.2972. [DOI] [PubMed] [Google Scholar]
- 32.Courtis AM, Santos SA, Guan Y, Hendricks JA, Ghosh B, Szantai-Kis DM, Reis SA, Shah JV, Mazitschek R. Monoalkoxy BODIPYs--a fluorophore class for bioimaging. Bioconjug Chem. 2014;25:1043. doi: 10.1021/bc400575w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Pol E, Coumans FA, Grootemaat AE, Gardiner C, Sargent IL, Harrison P, Sturk A, van Leeuwen TG, Nieuwland R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost. 2014;12:1182. doi: 10.1111/jth.12602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental materials
Figure S1. Fluorescent NP synthesis and characterization.
Figure S2. Imaging single-cell kinetics of MNP distribution with no TNP/MNP fluorescence spectral bleed-through.
Figure S3. MNP match TNP plasma kinetics and do not influence TNP uptake.
Figure S4. MNP & TNP uptake by perivascular host cells in ovarian cancer.
Figure S5. MNP uptake in tumor-associated Cx3cr1+ host cells.
Figure S6. Imaging cytometry analysis of single-cell NP distribution kinetics.
Figure S7. MNP co-localizes with a clinically relevant liposome formulation in tumor-associated cells.
Figure S8. Characterization of paclitaxel-loaded TNP and fluorescent derivatives.
Table S1. Optimized FEM modeling parameters and reference values.