Nonalcoholic Fatty Liver Disease and Hypercholesterolemia: Roles of Thyroid Hormones, Metabolites, and Agonists (original) (raw)
. Author manuscript; available in PMC: 2020 Mar 1.
Published in final edited form as: Thyroid. 2019 Sep 1;29(9):1173–1191. doi: 10.1089/thy.2018.0664
Abstract
Background
Thyroid hormones (THs) exert a strong influence on mammalian lipid metabolism at the systemic and hepatic levels by virtue of their roles in regulating circulating lipoprotein, triglyceride (TAG), and cholesterol levels, as well as hepatic TAG storage and metabolism. These effects are mediated by intricate sensing and feedback systems that function at the physiological, metabolic, molecular, and transcriptional levels in the liver. Dysfunction in the pathways involved in lipid metabolism disrupts hepatic lipid homeostasis and contributes to the pathogenesis of metabolic diseases, such as nonalcoholic fatty liver disease (NAFLD) and hypercholesterolemia. There has been strong interest in understanding and employing THs, TH metabolites, and TH mimetics as lipid-modifying drugs.
Summary
THs regulate many processes involved in hepatic TAG and cholesterol metabolism to decrease serum cholesterol and intrahepatic lipid content. TH receptor β analogs designed to have less side effects than the natural hormone are currently being tested in phase II clinical studies for NAFLD and hypercholesterolemia. The TH metabolites, 3,5-diiodo-l-thyronine (T2) and T1AM (3-iodothyronamine), have different beneficial effects on lipid metabolism compared with triiodothyronine (T3), although their clinical application is still under investigation. Also, prodrugs and glucagon/T3 conjugates have been developed that direct TH to the liver.
Conclusions
TH-based therapies show clinical promise for the treatment of NAFLD and hypercholesterolemia. Strategies for limiting side effects of TH are being developed and may enable TH metabolites and analogs to have specific effects in the liver for treatments of these conditions. These liver-specific effects and potential suppression of the hypothalamic/pituitary/thyroid axis raise the issue of monitoring liver-specific markers of TH action to assess clinical efficacy and dosing of these compounds.
Keywords: thyroid hormone, metabolites, analogs, NAFLD, cholesterol
Introduction
The liver is the hub for regulating serum cholesterol and triglycerides (TAGs) by virtue of its regulation of their biosynthesis and metabolism, packaging and export within lipoproteins, and reuptake via surface receptors for very-low-density lipoprotein (VLDL), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) (1). The intrahepatic lipid content is maintained by a balanced level of lipid anabolic and catabolic processes (1). Thus, derangements in hepatic TAG and cholesterol metabolism can lead to metabolic disorders, such as nonalcoholic fatty liver disease (NAFLD) (2) and hypercholesterolemia (3).
Thyroid hormone (TH) effects on lipid metabolism have been known for over a century (4,5). In the clinical setting, hypothyroidism is associated with weight gain as well as higher serum TAG and LDL cholesterol (LDL-C) levels, whereas hyperthyroidism has opposite effects (6). Recent studies have demonstrated that TH regulation of hepatic autophagy and mitochondrial metabolism are key processes involved in hepatic TAG metabolism (7,8). TH decreases serum cholesterol by its effects on cholesterol synthesis, LDL clearance, and reverse cholesterol transport (RCT). Besides the major biologically active THs, thyroxine (T4) and triiodothyronine (T3), many TH metabolites exist in human serum and tissues at different concentrations. These metabolites are formed by deiodination, decarboxylation, deamination, N-acetylation, sulfation, and glucuronidation and may also have biological activity (9,10) (Table 1). Additionally, TH analogs are being developed that can specifically activate the TH receptor β (THR_β_) isoform, the most abundant THR isoform in the liver to minimize TH-associated side effects that occur in the heart and bone that express TH receptor α (THR_α_) as the predominant isoform (11). Pharmacological strategies are also being developed to preferentially induce TH or TH analog uptake in the liver to minimize any potential off-target effects of THR_β_ agonist in the central nervous system, hypothalamic/pituitary/thyroid (HPT) axis, and other target tissues (12). In this review, we will provide an overview of the major mechanisms employed by TH to regulate hepatic TAG and cholesterol metabolism, and examine the current status of THs, TH metabolites, and TH analogs as potential therapies for hepatic lipid-associated metabolic diseases.
Table 1. Thyroid Hormone Metabolites.
| TH metabolites | Required reaction | Example |
|---|---|---|
| Less iodized forms | Deiodination | T2 |
| Thyronamines | Decarboxylation; deiodination | 3,5-T2AM; T1AM |
| Thyroacetic and thyropropionic acids | Oxidative deamination; deiodination | Tetrac, Triac, DITPA |
| Conjugated TH metabolites | Sulfation, glucuronidation | S-T4, G-T4 |
| DIT | Ether link cleavage | DIT |
TH Regulation of Hepatic TAG and Cholesterol Metabolism
Thyroid hormones
The thyroid gland synthesizes and secretes THs that are essential for the regulation of many metabolic processes throughout the body. The thyroid gland is composed of thyroid follicles, which are the basic units for the concentration of iodide (I−) and the synthesis of the major THs, thyroxine (T4) and T3. In circulation, most T4 and T3 are reversibly bound to serum carrier proteins such as thyroxine-binding globulin (TBG), transthyretin, and albumin (13,14). T4 and T3 then enter cells via plasma membrane transporters that belong to the monocarboxylate transporter 8 (MCT8), organic-anion-transporting polypeptide 1 (OATP1), and the L-type amino acid transporter (LAT) families (15,16). Once inside the cell, THs are activated or inactivated by a family of enzymes known as deiodinases (DIO1, 2, and 3), which control the concentration of T3 within the cell (17). DIO2 deiodinates T4 to generate T3, whereas DIO3 inactivates both T4 and T3 (17). DIO1 converts serum T4 to T3 to act as an intrathyroidal regulator of T3 concentration and the major extrathyroidal source of circulating T3 (17,18). T3 is the major active form of TH and exerts its action by binding to two nuclear hormone receptor isoforms, THR_α_ and THR_β_, which act as a ligand-inducible transcription factors that interact with TH response elements (TREs) in the regulatory regions within the promoters as well as enhancers and intronic region of target genes (13,19). THR_α_ is the major isoform in the heart and bone, whereas THR_β_ is the major isoform in the liver as it may represent >90% of the THRs in the latter tissue.
TH regulation of hepatic TAG synthesis
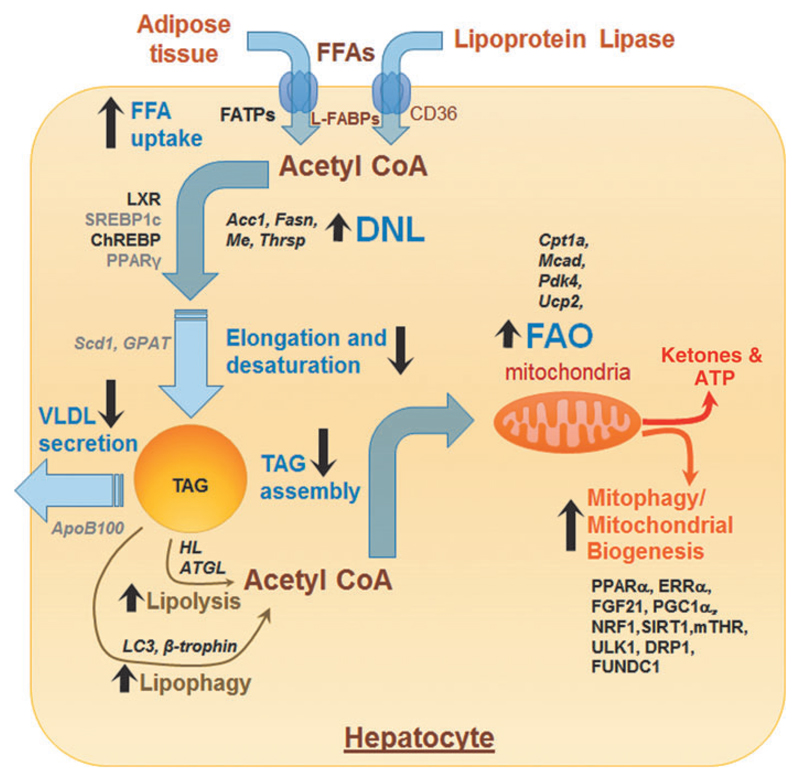
TH critically regulates various steps involved in hepatic non-esterified fatty acid (NEFA) uptake, de novo lipogenesis, and TAG assembly (Fig. 1). TH positively regulates the expression of fatty acid translocase (FAT/CD36) and fatty acid binding protein (FABP) expression in the liver resulting in increased influx of NEFA into the hepatocytes (20–22). TH also increases the intracellular pool of NEFA by increasing the transcription and expression of enzymes involved in de novo lipogenesis (Fig. 1). These TH-regulated genes include fatty acid synthase (Fasn) (23), acetyl-CoA carboxy-lase alpha (Acc1; also known as Acaca) (24), malic enzyme (Me) (25), and TH-responsive Spot14 homologue (Thrsp; also known as Spot14) (26). TH can also indirectly control the transcriptional regulation of hepatic lipogenic gene expression by regulating the expression of other transcription factors, such as sterol regulatory element-binding protein 1C (SREBP1C), liver X receptors (LXRs), carbohydrate-responsive element-binding protein (ChREBP), and peroxisome proliferator-activated receptor gamma (PPAR_γ_), which all have crucial roles in hepatic lipogenesis (27). TH stimulation of LXR and ChREBP mediate oxysterol and carbohydrate-driven lipogenesis in the liver (28,29). In contrast, TH negatively regulates the expression of pro-lipogenic transcription factors, such as SREBP1c and PPAR_γ_ (30,31). However, despite these effects by TH on genes involved in de novo lipogenesis, chronic TH administration does not cause a net increase in hepatic TAG content in rodents (32). It is noteworthy that TH downregulates stearoyl-CoA desaturase 1 (SCD1) and glycerol-3-phosphate acyl-transferase (GPAT) expression and activities to limit the amount of synthesized fatty acids that can be stored as TAG in the liver (33,34).
Fig. 1. TH and hepatic TAG metabolism.
TH regulates several processes involved in TAG anabolism, catabolism, and export. Genes and proteins positively regulated by TH are shown in bold black and those negatively regulated by TH are shown as gray color fonts. Transcription factors and other proteins are shown as running font, whereas genes are italicized. Black arrows represent processes/pathways that are upregulated (upward arrow) or downregulated (downward arrow) by TH. DNL, de novo lipogenesis; FAO, fatty acid oxidation; FFAs, free fatty acids; TAG, triacylglycerol; TH, thyroid hormone; VLDL, very-low-density lipoproteins.
TH regulation of fatty acid _β_-oxidation
Contrary to the sometimes variable and conflicting reports for TH effects on lipogenesis, TH has a strong effect on increasing hepatic lipid catabolism. The catabolic actions of TH on hepatic lipids are primarily mediated by the mobilization of free fatty acids (FFAs) from stored TAGs and their subsequent _β_-oxidation (Fig. 1). In this regard, TH increases TAG hydrolysis by stimulating the transcription and activities of both adipose triglyceride lipase (ATGL) and hepatic lipase (35,36). Additionally, TH induces the expression of zinc-_α_2-glycoprotein in hepatic cells, a protein that may contribute to the lipolytic action of TH (37). Besides its induction of hepatic lipases, TH also stimulates autophagy-lysosomal mediated lipolysis in hepatic cells (8). TH previously was shown to increase the lysosomal activity and lysosomal acid lipases expression (38,39), but the precise mechanism is not well understood. Subsequently, we showed that TH is a potent stimulator of hepatic lipophagy and is required for intracellular TAG hydrolysis and delivery of fatty acids to mitochondria for _β_-oxidation (8). In support of this notion, we showed that small interfering RNA (siRNA) knockdown of the autophagy gene, ATG5, significantly impairs the induction of hepatic _β_-oxidation of fatty acids and ketogenesis by TH in mice (Fig. 1) (8). Indeed, it is likely that lipophagy is the predominant mechanism for mobilizing fatty acids from fat droplets acutely, and lipases may contribute only after chronic exposure to TH. In this regard, induction of ATGL and hepatic lipase gene expression increase only after 10 days of TH treatment. Finally, a recent study revealed a critical role for _β_-trophin (C19orf80; also known as ANGPTL8) in TH-mediated induction of hepatic lipophagy and TAG hydrolysis (40).
Mitochondria are classical targets for TH action in the liver and the major sites for fatty acid catabolism (41). Thus, TH not only regulates the intracellular generation of fatty acids but also their entry into the mitochondria and the expression of mitochondrial enzymes involved in β_-oxidation (Fig. 1) (41). Carnitine palmitoyltransferase I_α (CPT1_α_), a carrier protein that is required for the delivery of long-chain fatty acids (LCFAs) into the mitochondria, is a direct transcriptional target of TH in the liver (42). TH can also regulate the expression of CPT1_α_ indirectly via its stimulation of intrahepatic peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1_α_), estrogen-related receptor alpha (ERR_α_) (43), peroxisome proliferator-activated receptor alpha (PPAR_α_), and fibroblast growth factor 21 (FGF21) levels (44). TH also increases the expression of other mitochondrial enzymes involved in _β_-oxidation and oxidative phosphorylation, such as medium-chain acyl-CoA dehydrogenase (MCAD) (45), pyruvate dehydrogenase kinase isoform 4 (PDK4) (46), and mitochondrial uncoupling protein 2 (UCP2) (47).
TH also regulates mitochondrial β_-oxidation by inducing mitochondria synthesis and maintaining mitochondria quality control (Fig. 1) (48). TH stimulates mitochondria biogenesis by inducing PGC1α gene expression, which in turn stimulates transcription of nuclear respiratory factor 1 (NRF1) and mitochondrial transcription factor A (mtTFA) (48). Furthermore, TH activates SIRT1 to deacetylate PGC1_α and increase its ability to bind the regulatory regions of target genes that are involved in mitochondrial synthesis and function (49). In addition to the stimulation of the PGC1_α_-NRF1-mtTFA axis by TH, we recently showed that TH upregulates a PGC1_α_-ERR_α_ pathway for mitochondrial biogenesis. ERR_α_ is an orphan nuclear receptor that is induced by PGC1_α_ and stimulates the expression of genes involved in mitochondrial biogenesis and lipid oxidation (43). Indeed, our studies suggest that TH stimulation of PGC1_α_-ERR_α_ may be the predominant mechanism for stimulating mitochondrial activity since siR-NA knockdown of ERR_α_ completely abrogates TH-mediated stimulation of oxidative phosphorylation.
Truncated THRs have been reported within the mitochondria and may regulate transcription from the mitochondrial genome (50). Mitochondrial THRs bind to the D-loop of the mitochondrial DNA and increase its transcription and replication, although the mechanism is not well understood (50). Mitochondrial THRs also interact with the mitochondrial trifunctional protein (MTP), which catalyzes the last three reactions of mitochondrial fatty acid oxidation (hydration, dehydrogenation, and cleavage) on LCFAs (51). Although mitochondrial biogenesis is needed to stimulate hepatic _β_-oxidation by TH, other mitochondrial homeostatic processes such as mitochondrial fission, fusion, and mitophagy are needed to sustain it (52). In this regard, we earlier showed that TH stimulates protective autophagy of mitochondria (mitophagy) to reduce cellular injury by reactive oxygen species (ROS) (53). We showed that mitophagy itself is induced by excess ROS generation by mitochondria, which then causes intracellular Ca2+ release, activation of calcium/calmodulin-dependent protein kinase kinase 2 (CAMMK2),5′ AMP-activated protein kinase (AMPK) phosphorylation, and the activation and mitochondrial translocation of Unc-51-like autophagy activating kinase (ULK1) after phosphorylation by AMPK (53). Although excessive ROS production is pathological in severe thyrotoxicosis and can lead to cell death (54), its physiological production may be benefical in hepatic cellular metabolism via a process known as cellular hormesis. Indeed, we found that there is a dose- and time-dependent increase in ROS production that is linked to TH-induced oxidative phosphorylation in hepatic cells (53). This increase in ROS is important for induction of mitophagy to maintain mitochondrial quality for _β_-oxidation of fatty acids and oxidative phosphorylation (53). This ROS-induced mitophagy also ensures that damaged mitochondria are swiftly removed from the cell to avoid further oxidative damage and cell death (53).
Besides stimulating _β_-oxidation of fatty acids, TH also increases lipogenesis, at least acutely. The effects of TH on these two opposing metabolic pathways appear to be paradoxical and are not well understood. It is possible that TH regulation of genes involved in lipogenesis may be temporally regulated (with fatty acid synthesis predominating early and _β_-oxidation of fatty acids later) and/or dependent on underlying nutrient/energy status/concentration/periportal versus pericanalicular location, and species. However, even with the induction of lipogenesis, it appears that _β_-oxidation predominates in vivo as _β_-hydroxybutyrate is detected in serum as early as 3 days after TH administration (8).
TH regulation of hepatic sphingolipid and phospholipid metabolism
One week administration of either T3 or 3,5-diiodo-l-thyronine (T2) prevented upregulation of several hepatic sphingolipid species, including ceramides, in rats that were fed high-fat diet (HFD) (55). Ceramides contribute to the cellular damage caused by inflammation from insulin resistance, mitochondrial dysfunction, and oxidative stress in NAFLD (56). Since saturated fats are the precursors of ceramide biosynthesis, the increased fatty acid oxidation in the mitochondria induced by T2 and T3, and the subsequent decrease in ceramide and sphingomyelin synthesis likely explain their protection against NAFLD in rats fed HFD. Additionally, TH reduces the intracellular concentrations of several phospholipid species such as phosphatidylcholine, phosphatidylserine, and cardiolipin in rodent liver (57).
TH regulation of cholesterol metabolism
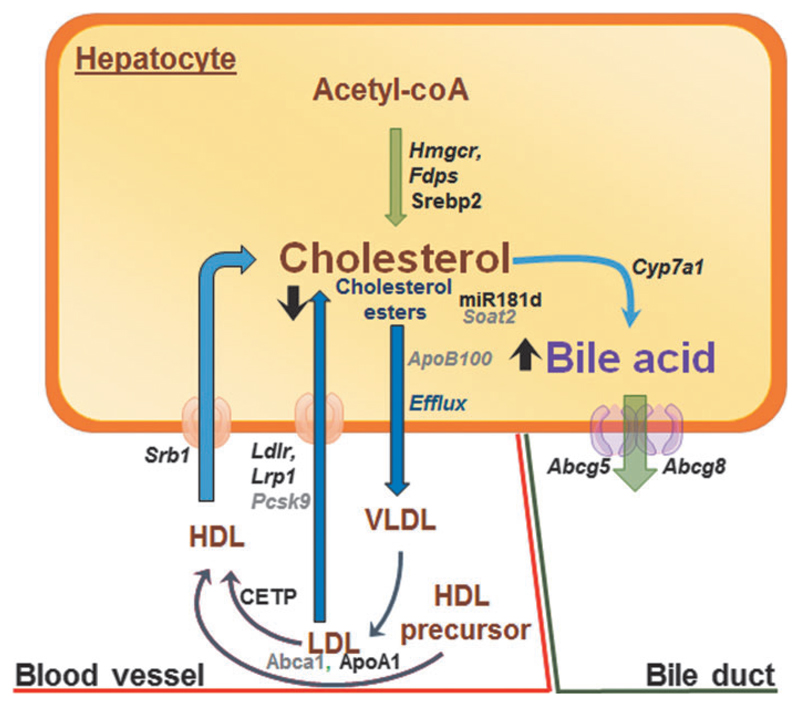
TH regulates hepatic cholesterol metabolism by multiple mechanisms (58,59) (Fig. 2). In rats, TH increases the expression of hydroxymethylglutaryl-CoA reductase (Hmgcr) and farnesyl pyrophosphate synthetase (Fdps) to promote cholesterol biosynthesis in the liver (60). However, TH also negatively regulates hepatic cholesterol secretion by decreasing the expression of both sterol O-acyltransferase 2 (SOAT2) and apolipoprotein B100 (ApoB100), which are required for re-esterification and packaging cholesterol into VLDL and LDL (61–64). TH decreases SOAT2 by inducing the expression of human miR181d, which decreases the expression of caudal-type homeobox protein 2 (CDX2), a transcription factor that positively regulates SOAT2 gene expression (62).
Fig. 2. TH and hepatic cholesterol metabolism.
TH regulates several processes involved in hepatic cholesterol biosynthesis, export, and degradation. Genes and proteins positively regulated by TH are shown in bold black and those negatively regulated by TH are shown as gray color fonts. Transcription factors and other proteins are shown as running font, whereas genes are italicized. Black arrows represent processes/pathways that are upregulated (upward arrow) or downregulated (downward arrow) by TH. HDL, high-density lipoprotein; LDL, low-density lipoprotein.
TH increases the expression/activity of cholesteryl ester transfer protein (CETP) (65) and expression of hepatic LDL receptor (LDLR) (66) to increase serum cholesterol clearance. LDLR is regulated by SREBP2, which is induced transcriptionally by TH in rodents and humans (67,68). TH also increases the transcription of both mouse and human LDLR-related protein 1 (LRP1), a lipoprotein involved in the removal of TAGs from chylomicron remnants and VLDL. Interestingly, TH can decrease circulating pro-protein convertase subtilisin/kexin type 9 (PCSK9) levels, which may contribute to lower plasma LCL-C levels by enhancing LDLR recycling (69).
TH also has major effects on RCT. TH induces the gene and protein expression of ApoA1 and scavenger receptor class B member 1 (SRB1) to increase cholesterol efflux from peripheral tissues to HDL (70,71). TH also stimulates hepatic lipase to decrease HDL particle size resulting in higher relative levels of lipid-poor ApoA-I in HDL that facilitates transfer of cholesterol from peripheral tissues via the ATP-binding cassette transporter (ABCA1) (60,72). Within the liver, TH increases the expression of rat and human cholesterol 7 alpha-hydroxylase (CYP7A1), the rate-limiting enzyme that converts cholesterol into bile acids in the RCT pathway (60,73). In addition, TH increases the efflux of bile acids in both the liver and intestines, which are the last steps of the RCT pathway, by stimulating ATP-binding cassette subfamily G member (Abcg5/Abcg8) gene transcription (74).
Lessons from THR transgenic models
Knock-in mouse models harboring mutant THR_α_/β receptors have provided insights on the role of THRs in hepatic lipid metabolism and enlarged our understanding of their systemic and hepatic functions in vivo. A mouse model that expresses a dominant negative mutation in THR_β_ (THR_β_PV/PV) mimics the resistance to thyroid hormone (RTH) phenotype in humans and exhibits enlarged livers with hepatosteatosis by 4–5 months of age (30,75). Liver TAGs and expression of several key enzymes involved in lipogenesis (e.g., Scd1, Fasn) also were increased in THR_β_−/− mice (76). In contrast, mice expressing a similar dominant negative mutation in the THR_α_ gene locus, THR_α_1PV/PV, as well as THR_α_-null mice, showed reduced liver weights and decreased lipid accumulation (30,75,77). It is thought that increased lipogenic enzyme expression and decreased fatty acid β_-oxidation activity due to PPAR_γ expression contributes to the intrahepatic lipid accumulation in THR_β_PV/PV mice, whereas decreased expression of lipogenic genes reduced liver mass and intrahepatic lipid content in THR_α_1PV/PV mice (30,75). These observations suggest that the regulation of lipid metabolism in the liver is likely to be THR isoform-dependent; however, it is not known whether these effects are mediated by THR-mediated transcription or by alternative mechanisms that do not necessarily require THR binding to DNA. In this connection, it recently was shown that TH effects on heart rate, body temperature, and lipid metabolism were lost in mice in which classical THR DNA binding ability was lost (76).
TH metabolites in lipid metabolism
The less iodinated version of TH, T2, is present in the picomolar range in human serum and has received attention owing to its bioactive role in animals (78). T2 is thought to modulate cellular function by noncanonical pathways, although THR-mediated signaling has not been totally excluded (79). One of the most profound biological effects of T2 is its regulation of lipid metabolism and insulin sensitivity in hepatic cells (79). T2 directly activated SirT1 to increase the PGC1_α_ activity and increase the expression of CPT1_α_ and genes involved in mitochondrial biogenesis in hepatocytes (80). Proteomic pathway analysis performed in the livers from animals fed a HFD showed that T2 altered pathways involved in fatty acid _β_-oxidation and amino acid metabolism as well as respiratory chain activity and ROS (81). Besides its induction of mitochondrial pathways, T2 upregulated the levels of ApoB100, the major protein component of VLDL (82), suggesting that T2 stimulated lipoprotein secretion to reduce intrahepatic fat content. Of note, T2 also increased the activity of ATGL in hepatocytes (83). Contrary to T3, T2 suppressed hepatic lipid accumulation by downregulating the expression of lipogenic proteins (84,85).
Thyronamines (TAMs) are endogenous biogenic amines generated from iodinated TH by deiodination and decarboxylation. However, their precise physiological concentrations and functions in the body are still controversial. Scanlan et al. (86) detected endogenous T1AM (3-iodothyronamine) and T0AM at subpicomolar concentrations in rat brain, although it is noteworthy that another group did not identify significant amounts of endogenous TAMs in rat brain and serum, or in human serum and thyroid tissue (87). This issue notwithstanding, TAMs can bind to distinct receptors such as the G protein-coupled receptor TAAR1 at pharmacological doses in the nanomolar range (86). They were found to have rapid and systemic effects such as hypothermia and bradycardia in mice (86) that were opposite to the effects of T3 administration. Therefore, TAMs may be bona fide hormones if produced in sufficient quantities locally; however, the factors that control their synthesis, transport, action, and catabolism are still not known (10). Previous studies indicated that TAMs are able to switch metabolism from carbohydrate to lipid utilization (88,89). T1AM also decreases serum cholesterol and increase serum TAGs in a dose-dependent manner (90). In liver metabolome analysis, T1AM increased fatty acid oxidation and decreased the expression of SIR T4, a negative regulator of fatty acid oxidation, and Ldlrap1, involved in the efficient endocytosis of the LDLR (88,90). Although TAMs previously were shown to exhibit a wide array of effects on thermoregulation, food intake, heart, and tissue metabolism, as well as alter both TH and corticosterone levels, it is not known whether T1AM has a direct in vivo effect on liver lipid metabolism (10). However, in favor of this notion, in vitro and liver perfusion studies have shown uptake of T1AM and stimulation of fatty acid catabolism in hepatocytes (91).
The thyroacetic acid, 3,3′,5,5′-tetraiodothyroacetic acid (Tetrac), circulates in low nanomolar concentrations. The deiodinated form of Tetrac, Triac (3,5,3′,-triiodothyroacetic acid), has a higher affinity for THR than T3 and binds with higher affinity to THR_β_ than THR_α_ (92). In humans, Triac has a half-life of about 6 hours. Due to its higher affinity for THR and use of different transporters than THs, Triac has been used clinically for patients with RTH syndrome and currently is being tested for patients with MCT8 deficiency (93). When administered to rodents, Triac levels are increased in the liver and induce the expression of T3-regulated target genes (94). To date, there have been no published studies investigating the differences between Triac versus T3 on hepatic lipogenesis, _β_-oxidation, and VLDL secretion, although it appears to have similar effects as T3 in other tissues (93,94). Therefore, current evidence shows that although Triac and T3 may function differently pharmacokinetically, they share common signaling pathways. Additionally, it should be noted that Triac reduces endogenous TH production by suppressing the HPT axis (reduced thyrotropin [TSH], T4, and T3).
Sulfated and glucuronidated THs have increased water solubility to facilitate their excretion through the bile and gut. However, it is thought that both sulfated and glucuronidated THs can act as a reservoir for total TH within the body and can be converted back to T4 and T3 when necessary (9). Sulfated and glucuronidated TH are thought to be inactive metabolites, and to the best of our knowledge, no bioactive role(s) for the sulfated and glucuronidated TH on lipid metabolism has been described. Similarly, diiodotyrosine (DIT), which is formed by breakage of the thyronine backbone at its ether bond, is involved in disposal of TH but does not have any known direct effects on lipid metabolism (9,95).
TSH and lipid metabolism
TSH is produced by the pituitary gland and stimulates TH production by the thyroid gland. Serum TSH and cholesterol levels have been shown to be positively associated, and TSH may have a direct effect on liver lipid metabolism. Zhang et al. demonstrated the presence of TSH receptor (TSHR) messenger RNA (mRNA) expression in human and rat liver tissue that was identical to that found in thyroid tissue and a thyroid cell line (96). The TSHR was located at the cell membrane of hepatocytes, and bovine TSH and IgG from patients with Graves’ disease induced cAMP in hepatic cells in a TSHR-dependent manner (96,97). Recombinant TSH, in the micromolar range, directly upregulated mRNA expression and activity of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), an enzyme involved in cholesterol biosynthesis as well as cholesterol production in human liver cells (93). In thyroidectomized rats, when T4 was supplemented together with increasing concentrations of TSH, there was an increase in HMGCR protein levels and serum cholesterol levels with increasing TSH concentrations (97). Another study from the same group also showed that both thyroidectomized and TSHR−/− mice had repression of bile acid synthesis via SREBP2 induction when supplemented with T4 and bovine TSH. Repression of bile acid synthesis can cause higher intrahepatic and serum cholesterol levels since bile acids are the major means to eliminate excess cholesterol from hepatic cells (97,98).
Recently, a direct relationship between serum TSH and PCSK9 expression and an inverse relationship between serum TSH and LDLR expression were demonstrated (99). PCSK9 inhibitors are effective in lowering serum cholesterol levels via recruitment of LDL-C from circulation by restoring LDLR expression on the cell surface of hepatocytes. Recombinant human TSH alpha/beta heterodimer protein increases PCSK9 mRNA expression and protein levels and impairs LDL-C uptake in human liver cells (99,100). TSHR−/− mice supplemented with T4 also have lower intrahepatic TAG levels both after chow and HFD and lower expression of lipogenic genes than control mice under the same conditions, suggesting that TSH may also regulate intrahepatic lipid metabolism (100). In this context, TSH directly increased lipogenesis by activating SREBP1c via a cAMP/PKA/PPAR_α_ signaling pathway associated with AMPK in hepatic cells (101). Recently, it was shown that the administration of recombinant human TSH increases serum apoB, Lp(a), non-HDL cholesterol, and TAGs in patients after thyroidectomy on a stable dose of LT4 (102). After TSH administration, T3 levels were reduced and changes in lipids were inversely associated with the T3 levels, suggesting that TSH may have had an indirect effect on lipid levels (102), perhaps due to effects on the deiodinase activity. Taken together, these foregoing studies suggest that TSH may worsen hypercholesterolemia by increasing cholesterol biosynthesis, reducing cholesterol clearance, lowering LDLR, and worsen NAFLD by increasing lipogenesis, respectively. However, it should be pointed out that studying the direct role of TSH in vivo is challenging in animal models and humans since it will undoubtedly cause alterations in serum T3 and T4 levels (102,103). Even in studies in which thyroidectomized animals or patients are studied, it is likely that restored serum levels of T3/T4 may not fully recapitulate normal serum or tissue levels of TH due to the absorption/pharmacokinetic characteristics of supplemented hormones versus the natural production/removal of endogenous THs.
THs, TH Analogs, and TH Metabolites in NAFLD
Current evidence on TH, TH analogs, and TH metabolites in NAFLD and hypercholesterolemia is summarized for animal models (Table 2) and humans (Table 3).
Table 2. Effects of Thyroid Hormone, Thyroid Hormone Analogues, and Thyroid Hormone Metabolites in Animal Models of Nonalcoholic Fatty Liver Disease and Hypercholesterolemia on Serum Lipids, Steatosis, Liver Function, Body Weight, Hypothalamic/Pituitary/Thyroid Axis, and Other Side Effects.
| Compound | Study | Animal model (dose) | Serum lipids | Steatosis | Liver function | BW | HPT axis | Other side effects |
|---|---|---|---|---|---|---|---|---|
| TH | ||||||||
| T3 | Grover et al. (141) | Sprague-Dawley rats HCD (1 week, 1.54–154 nmol/kg/day by gavage) | TC ↓ TAG = (ED50 20.6) | TSH ↓ (ED30 9.8) | Heart rate ↑ (ED15 30.8) | |||
| Johansson et al. (70) | C57BL6 mice HCD (8 days, 97 nmol/kg/day intraperitoneal) | TC ↓ TAG ↑ | Liver TAG ↑ | |||||
| Perra et al. (128) | Fischer rats MCD diet (1 week, 6 _μ_mol/kg/diet) | TG ↓ | Liver TAG ↓ | AST, ALT ↓ | BW ↓ | |||
| Cable et al. (135) | ob/ob Mice (9 weeks, 100 nmol/kg/day subcutaneous) | TC ↓ TAG ↑ | Liver TAG = | AST, ALT ↓ | BW ↓ | Heart weight ↑ | ||
| Kannisto et al. (160) | C57BL/6 ApoE-knockout mice HFD (1–10 weeks, 184 nmol/kg/diet) | TC ↓ TAG = | ||||||
| Jonas et al. (146) | C57BL6 mice HFD (4 weeks, 46 nmol/kg/day intraperitoneal) | TC ↓ TAG = | BW = | T4 ↓ T3 = rT3 ↓ T2 = | ||||
| Finan et al. (130) | C57BL6j mice HFHCD (2 weeks, 100 nmol/kg/day subcutaneous) | TC ↓ TAG = | BW = | T4 ↓ T3 ↑ TSH = | Heart rate ↑ heart weight ↑ | |||
| Iannucci et al. (55) | Wistar rats HFD (1 week, 38 nmol/kg/day intraperitoneal) | Liver TAG ↓ | BW = | T4 = T3 ↑ | ||||
| Glucagon-T3 conjugate | Finan et al. (130) | C57BL6j mice HFHCD (2 weeks, 100 nmol/kg/day subcutaneous) | TC ↓ TAG ↓ | BW ↓ | T4 ↓ T3 = TSH = | Heart volume = bone volume = | ||
| C57BL6j mice CD-HFD (3 weeks, 100 nmol/kg/day subcutaneous) | TC ↓ TAG = | Liver TAG ↓ | ALT ↓ | BW ↓ | ||||
| THR_β_ receptor agonist | ||||||||
| GC-1 (sobetirome) | Grover et al. (141) | Sprague-Dawley rats HCD (1 week, 46.2–27,700 nmol/kg/day by gavage) | TC ↓ TAG = (ED50 190) | TSH ↓ (ED30 190) | Heart rate ↑ (ED15 5451) | |||
| Johansson et al. (70) | C57BL6 mice HCD (8 days, 97 nmol/kg/day intraperitoneal) | TC ↓ TAG = | Liver TAG = | |||||
| Perra et al. (128) | Fischer rats MCD diet (2 weeks, 15 _μ_mol/kg/diet) | TAG ↓ | Liver TAG ↓ | ALT, AST ↓ | BW ↓ | |||
| Vatner et al. (133) | Sprague-Dawley rats HFD (10 days, 499 nmol/kg/day intraperitoneal) | Liver TAG ↓ | BW = | T4 ↓ TSH ↓ | ||||
| Martagon et al. (134) | ob/ob Mice (24 days, 1 _μ_mol/kg/diet) | Liver TAG ↓ | BW ↓ | |||||
| KB2115 (eprotirome) | Vatner et al. (133) | Sprague-Dawley rats HFD (10 days, 205 nmol/kg/day intraperitoneal) | Liver TAG ↓ | BW = | T4 ↓ TSH ↓ | |||
| Martagon et al. (134) | ob/ob Mice (24 days, 6 μ/kg/diet) | Liver TAG ↓ | BW ↓ | |||||
| Prodrug THR_β_ receptor agonist | ||||||||
| MB07811 (prodrug MB07344) | Erion et al. (139) | C57BL/6 mice HFD (2 weeks, 6 _μ_mol/kg/day) | TC ↓ TAG ↓ | Liver TAG ↓ | BW = | T4 ↓ T3 ↓ | Heart weight = | |
| Cable et al. (135) | Zucker diabetic fatty rats (28 days, 2–97 _μ_mol/kg/day by gavage) | TC ↓ TAG = | Liver TAG ↓ | ALT/AST = | BW = | Heart rate = | ||
| C57BL6 mice HFD (2 weeks, 6–60 _μ_mol/kg/day by gavage) | TC ↓ TAG = | Liver TAG ↓ | ALT/AST = | BW = | Heart rate, heart weight = | |||
| ob/ob Mice (9 weeks, 19–58 _μ_mol/kg/day by gavage) | TC ↓ TAG = | Liver TAG ↓ | AST, ALT ↓ | BW ↓ | Heart weight = | |||
| Ito et al. (164) | Rabbits HCD (5 weeks, 19 _μ_mol/kg orally in diet) | TC ↓ | ||||||
| TH metabolite | ||||||||
| T2 | Mollica et al. (145) | Wistar rats HFD (4 weeks, 476 nmol/kg/day intraperitoneal) | TC ↓ TAG ↓ | Liver TAG ↓ | ALT ↓ | BW ↓ | TSH = | |
| Goldberg et al. (64) | C57BL6 mice WD (1 week, 2–20 _μ_mol/kg/day by gavage) | TC ↓ TAG ↑ | BW = | T4 ↓ | ||||
| Jonas et al. (146) | C57BL6 mice HFD (4 weeks, 4761 nmol/kg/day) | TC ↓ TAG = | Liver TAG ↓ | BW = | T4 ↓a T3 ↓a rT3 ↓ T2 ↑a | Heart weight ↑ | ||
| Iannucci et al. (55) | Wistar rats HFD (1 week, 476 nmol/kg/day intraperitoneal) | Liver TAG ↓ | BW = | T4 = T3 = | ||||
| T1AM | Assadi-Porter et al. (90) | CD-1 mice (1 week, 70 _μ_mol/kg/day) | TC ↓ TAG ↑ | BW ↓ | ||||
| TRC150094 (T2 mimetic) | Zambad et al. (150) | Obese ZSF1 rats (24 weeks, 18–37 _μ_mol/kg/day orally) | TC = TAG = | Liver fat (MRS) ↓ | Bilirubin ↓ | BW ↓/ = | Heart rate =, cardiac output = | |
| Silvestri et al. (149) | Wister rats HFD (4 weeks, 23 _μ_mol/kg/day intraperitoneal) | TC = TAG = | BW = | fT4 = fT3 = TSH = | Heart weight = | |||
| Triac | Cable et al. (135) | Sprague-Dawley rats normal chow diet (7 days, 1 _μ_mol/kg/day via minipump) | Liver TAG ↓ | BW = | Heart weight ↑ |
Table 3. Effects of Thyroid Hormone, Thyroid Hormone Analogues, and Thyroid Hormone Metabolites in Humans with Nonalcoholic Fatty Liver Disease or Hypercholesterolemia on Serum Lipids, Steatosis, Nonalcoholic Steatohepatitis, Body Weight, Hypothalamic/Pituitary/Thyroid Axis, and Other Side Effects.
| Compound | Study | Human study | Serum lipids | Steatosis | Liver function | BW | HPT axis | Other side effects |
|---|---|---|---|---|---|---|---|---|
| TH | ||||||||
| LT4 | Bruinstroop et al. (127) | 20 Male patients with type 2 diabetes and steatosis (4 months titrated to a TSH 0.34–1.70 IU/L, median dose 18.75 _μ_g/day [24 nmol]) | TC = TAG = | Liver fat (1H-MRS) ↓ | AST, ALT = | BW ↓ | fT4 =, TSH ↓ | Heart rate = |
| TR_β_ receptor agonist | ||||||||
| MGL-3196 | Taub et al. (167) | 48 Subjects with mildly elevated LDL-C and BMI 24.9–27.6 kg/m2 (2 weeks, 50–200 mg/day, [115–460 _μ_mol]) | TC ↓ TAG ↓ | fT4 ↓ (100/200 mg/dose), TSH = | Heart rate =, blood pressure = | |||
| Harrison et al. meeting abstract (136) | 125 Patients with biopsy-proven NASH (9 months, 80 mg/day [184 _μ_mol]) | TC ↓ TAG ↓ | Liver fat (1H-MRS) ↓ | AST, ALT ↓ | Only no change reported | Heart rate = | ||
| KB2115 (eprotirome) | Ladenson et al. (163) | 184 Patients on statin therapy with continued LDL-C ≥ 3 mmol/L (3 months, 25–100 _μ_g/day [51–205 nmol]) | TC ↓ TAG ↓ | BW = | fT4/TT4 ↓, TT3 =, TSH = | Heart rate =, bone turnover = | ||
| Sjouke et al. (166) | 69 Patients with familial hypercholesterolemia (6 weeks, 50–100 _μ_g/day [103–205 nmol]) | TC ↓ TAG ↓ | AST, ALT, _γ_GT, bilirubin ↑ | fT4 ↓, fT3 =, TSH = | Study terminated before initial 1-year endpoint due to reported cartilage damage in dogs and hepatotoxicity | |||
| Prodrug THR_β_ receptor agonist | ||||||||
| MB07811 (prodrug MB07344) | Loomba et al. meeting abstract (137) | 24 Patients with NAFLD and increased LDL-C (3 months, 10 mg every other day or per day [19 _μ_mol]) | LDL-C ↓ | Liver fat (1H-MRS) ↓ | AST, ALT ↓ | Only no change reported | ||
| TH metabolite | ||||||||
| TRC150094 (T2 mimetic) | van der Valk et al. (151) | 40 Patients with the metabolic syndrome (4 weeks, 50 mg/day [152 _μ_mol]) | TC = TAG = | Liver fat (1H-MRS) = | AST, ALT = | BW = | fT4 ↑, fT3 =, TSH = | Pulse =, blood pressure = |
| DITPA | Ladenson et al. (171) | 68 Patients with congestive heart failure NYHA II–IV (8 weeks titrated to TSH <0.02 mU/L, dose 90–360 mg/day [176–706 _μ_mol]) | TC ↓ TAG ↓ | BW ↓ | TT4 ↓, TT3 ↓, TSH ↓ | Bone turnover markers ↑, HR ↑, drug poorly tolerated | ||
| Triac | Sherman et al. (11) | 24 Patients after thyroidectomy including 8 patients with hypercholesterolemia (2 months after titration to TSH <0.1 mU/L compared to LT4, average dose 3.9 mg/day [6 _μ_mol]) | TC ↓ TAG = | fT4 ↓, TSH ↓ | Enhanced bone turnover compared to LT4 |
Nonalcoholic fatty liver disease
NAFLD is a hepatic lipid-related metabolic disorder that is highly associated with diabetes and obesity. The prevalence of NAFLD is rapidly increasing worldwide and currently is thought to afflict 30–40% of all adults in both developed and developing countries (104). NAFLD also increases the risk for developing diabetic complications and cardiovascular diseases later in life (104). NAFLD represents a spectrum of liver derangements starting from benign steatosis that progresses to nonalcoholic steatohepatitis (NASH) characterized by insulin resistance, and liver inflammation and fibrosis (105). Significantly, NASH can progress to cirrhosis and hepatocellular carcinoma in a subset of patients. Although the mechanisms for the pathogenesis and progression of NAFLD are not fully understood, impaired hepatic lipid metabolism appears to have a central role (2).
In early NAFLD, there is an increased uptake of NEFAs inside hepatocytes and de novo lipogenesis followed by intracellular TAG accumulation (106). The major cause for increased NEFA influx in NAFLD is the induction of hormone-sensitive lipase activity in white adipose tissue due to insulin resistance (106). Interestingly, loss of fatty acid transporters such as FATP and CD36 can prevent the development of NAFLD in murine models (107). Besides the increased uptake of NEFAs in early NAFLD, de novo lipogenesis in response to high-carbohydrate feeding leads to the accumulation of TAGs within the liver (108). Furthermore, both TAG lipolysis and mitochondrial _β_-oxidation of fatty acids are impaired in NAFLD (109,110). The defect in mitochondrial fat oxidation likely leads to increased esterification of FFAs into TAGs as well as synthesis of reactive lipid species such as DAG and ceramides (111) that have been associated with insulin resistance in NAFLD (112). Ceramides are also mediators of mitochondrial damage in hepatocytes and cause lipotoxicity, inflammation, and fibrosis (113). Additionally, TAG export in VLDL is increased during hepatosteatosis but is impaired later in NASH, perhaps due to detrimental changes in phospholipid levels (114).
Cholesterol metabolism is also altered in NAFLD (115). Comparative hepatic and serum lipidomic analysis of normal and NAFLD samples revealed increased hepatic free cholesterol (FC) in NASH (116). The role of dietary cholesterol has been investigated in murine models of NAFLD, and a cholate-deficient and cholesterol-enriched, high-fat atherogenic diet promoted both oxidative stress and experimental NASH (117). Furthermore, increased hepatic FC level was observed in association with the transition from hepatosteatosis to NASH in obese hyperinsulinemic mice (118). Both genetic and nutritional models of hepatosteatosis have implicated mitochondrial FC loading as predisposing animals to tumor necrosis factor- and Fas-induced steatohepatitis and activation of hepatic stellate cells to cause fibrosis (119). So far, there is no approved drug or hormone therapy for NAFLD, and lifestyle management with diet and exercise are the current therapeutic cornerstones for limiting the progression of NAFLD (120).
Effects of TH on NAFLD
Several epidemiological studies conducted across the globe have shown an inverse relationship between hypothyroidism and the incidence of NAFLD (121). Even within the reference range, higher TSH and lower TH levels are associated with a higher prevalence of NAFLD in a dose-dependent manner (122,123). Recent studies in rodents and patients further support this inverse relationship between physiological thyroid status and NAFLD. In mice, mild hypothyroidism increases the prevalence of NAFLD possibly due to both intrahepatic and extrahepatic mechanisms (124). Transcriptome analyses of the liver samples from patients who underwent bariatric surgery for NAFLD showed that several of the major genes that had altered expression are regulated by TH (125). Also, evidence of decreased intrahepatic TH levels in NAFLD was shown in rats and humans (126,127). Other studies also suggested a possible direct role of TSH in stimulating hepatosteatosis since TSHR-knockout mice were protected from steatosis when fed HFD (101).
T3 administration significantly decreases hepatosteatosis and inflammation (128), and it restores mitochondrial function in NASH (7,129). In a recent study, T3 administration in the nanomole per kilogram range to rats fed HFD not only reduced hepatic TAG accumulation but also prevented the generation of toxic lipid species, such as ceramides (55). More recently, a compound formed by chemical hybridization of glucagon and TH showed promising results to directly deliver T3 to the liver (130). This glucagon/T3 conjugate was composed of the amine of T3 covalently linked to the 40-mer glucagon analog through a succinate spacer at the terminal lysine. Glucagon/T3 elicited the transcriptional activity of a TRE reporter in cells expressing the glucagon receptor presumably through hydrolysis of the gGlu spacer to release T3. When the molecule was fluorescently labeled, it was shown to be preferentially accumulated by the liver and to have low uptake in fat, in the heart and pancreas. This uptake correlates with the hepatic expression of glucagon receptor as well as increased intrahepatic T3 levels. Glucagon/T3 treatment improved dyslipidemia, atherosclerosis, body weight, and steatosis in several dietary models of obesity (130). The cardiac side effects of T3 on the heart, in terms of cardiac hypertrophy and reduced ejection fraction, were not observed with treatment with glucagon/T3. Whereas T3 monotherapy at 100 nmol/kg significantly reduces bone volume, glucagon/T3 at equimolar dosage had no such effects. Glucagon/T3 also did not cause any change in serum T3 levels but slightly decreased serum T4. Plasma TSH and hypothalamic thyrotropin-releasing hormone mRNA were normal both with T3 monotherapy and glucagon/T3. Although the side effect profile seemed favorable for this conjugate over T3 monotherapy in mice, it remains to be seen whether this side effect profile and its potential effects on peripheral TH metabolism will enable it to be used clinically in patients. Similarly, chemical conjugation of T3 with antisense oligonucleotide (ASO) targeting nicotinamide N-methyltransferase (NNMT)-ASO or apolipoprotein B (ApoB)-ASO also provides protection from diet-induced obesity and intrahepatic lipogenesis, thereby highlighting hormone/drug conjugation as a novel strategy for countering NAFLD, obesity, and hyperlipidemia (131).
Similar to T3, there have been limited studies on the role of T4 therapy in NAFLD. It has been reported that T4 supplementation reduced the prevalence of NAFLD in patients with subclinical hypothyroidism (132). More recently, we investigated the effects of T4 supplementation in euthyroid male subjects with type 2 diabetes and NAFLD using 1H-magnetic resonance spectroscopy (MRS). Low-dose T4 supplementation (average dose 18.75 _μ_g/day) aimed to result in a low–normal TSH between 0.30 and 1.70 mIU/L for 4 months decreased intrahepatic lipid content as measured by MRS (1H-MRS) (127). This reduction in liver fat content also correlated with an improvement in glycemic control after treatment.
TH analogs as therapies for NAFLD
Several TH analogs/mimetics have been developed to treat hypercholesterolemia, obesity, and/or diabetes. These compounds have THR_β_ isoform selectivity and/or tissue-specific uptake (e.g., liver) to target their therapeutic effects while mitigating potential side effects. Several studies in rodent NAFLD models have demonstrated consistently the efficacy of TH analogs/mimetics in reducing both hepatosteatosis and liver injury. The THR_β_-selective compound GC-1 [sobetirome, 2-(4-((4-Hydroxy-3-(1-methylethyl)phenyl)methyl)-3,5-dimethylphenoxy)acetic acid] was one of the first TH analogs examined as a potential therapy for NAFLD. Perra et al. demonstrated that GC-1 prevented the development of hepatosteatosis and lipo-peroxidation in rodents by feeding a methionine- and choline-deficient diet supplemented with 15 _μ_mol GC-1 per kg diet (128). Similarly, GC-1 also reduced hepatosteatosis in other animal models of obesity such as ob/ob mice and rats fed a HFD (133,134). In addition to GC-1, the THR_β_-selective analog KB2115 [eprotirome, 3-((3,5-dibromo-4-(4-hydroxy-3-(1-methylethyl)-phenoxy)-phenyl)-amino)-3-oxopropanoic acid] and the liver-specific analog MB07811 [(2R,4S)-4-(3-chlorophenyl)-2-[(3,5-dimethyl-4-(4-hydroxy-3′-isopropylbenzyl)phenoxy)methyl]-2-oxido-[1,3,2]-dioxaphosphonane] decreased hepatosteatosis in both HFD and ob/ob mice models of NAFLD while also reducing serum TAG levels (133,134). MB07811 is a liver-specific prodrug that undergoes first-pass hepatic extraction and is converted intrahepatically to a negatively charged THR agonist [3,5-dimethyl-4-(4′-hydroxy-3′-isopropylbenzyl(phenoxy)methylphosphonic acid] (MB07344) that has a short biological half-life and, hence, little peripheral side effects. Cable et al. used several experimental models of NAFLD such as Zucker diabetic fatty (ZDF) rats, ob/ob mice, and diet-induced obese mice to demonstrate that MB07811 at micromolar doses markedly reduces hepatosteatosis, as well as plasma FFAs and TAGs (135). This anti-steatotic activity of MB07811 was associated with an increased rate of mitochondrial β_-oxidation and stimulation of CPT1_α expression (135).
Previously, there were no clinical trials investigating TH analogs for the treatment of NAFLD in humans. Recently, a phase II clinical study for a THR_β_-selective analog, MGL-3196, was conducted in patients with biopsy-proven NASH. These patients had a statistically significant reduction in hepatosteatosis after 12 and 36 weeks of treatment as measured by 1H-MRS (136). Also, the first results of a phase II study on the effects of Viking 2809 (MB07811) on NAFLD and hypercholesterolemia showed decreased hepatic lipid content and serum LDL-C (137). Definitive results from these studies are awaited to fully assess the effects of these compounds on the HPT axis and other potential side effects in humans.
In general, it appears that TH analogs can have suppressive effects on the axis, although the effects may depend on the specific compound and the dose used. In terms of axis suppression, GC-1 and KB2115 suppress serum TSH and T4 levels (133,138). MB07811 and another THR_β_-selective thyromimetic KB141 also suppress serum T4, although MB07811 did not have any significant effects on serum TSH at a lower concentration (3 mg/kg) due to its conversion to a short-lived active metabolite in the liver (139). In contrast to T3, GC-1 (64 days, 15 _μ_g/kg) did not reduce bone mineral density (140) and had little effect on heart rate in monkeys (141). Since serum TSH, T3, and T4 levels are not reliable markers for thyrotoxicity in patients who take such compounds, clinical assessment of patients before and during therapy is imperative. Patients with cardiac disease or osteoporosis will likely need to be disqualified beforehand. Patients who are started on such drugs will also need to be monitored for tachycardia and atrial arrhythmias, bone loss (e.g., bone densitometry, markers for bone resorption such as urine hydroxyproline and deoxypyridinoline, and serum osteopontin and bone sialoprotein), and hepatic damage (liver enzymes such as alanine transaminase [ALT] and aspartate transaminase [AST]). Efficacy of treatment can be measured by changes in fat content (measured by MRS, magnetic resonance imaging-proton density fat fraction [MRI-PDFF], or liver biopsy), liver function tests (ALT/AST), serum fibrosis markers (Pro-C3), and histological changes of inflammation and fibrosis in liver biopsy. It may also be necessary to titrate and optimize the dose to serum markers known to be regulated by TH such as sex hormone binding globulin (SHBG), ferritin and cholesterol levels, with SHBG likely having the highest specificity (142). It is also possible that serum metabolomic markers such as serum acylcarnitines or branched chain amino acids may prove to be useful markers for TH action (143).
TH metabolites as therapy for NAFLD
Recently, the TH metabolite, T2, received attention as a potential therapy for NAFLD owing to its bioactive role in animals (78). To mimic hepatosteatosis in vitro, primary cultures of rat hepatocytes were exposed to an oleate/palmitate mixture in lipid-load hepatocytes. After lipid loading hepatocytes in vitro, the addition of T2 caused a significant reduction in hepatocyte lipid content and lipid droplet diameter, as well as reduced activity of acyl-CoA oxidase (ACOX), a rate-limiting enzyme of peroxisomal _β_-oxidation (144). Furthermore, T2 reversed hepatic fat accumulation in a rodent model of NAFLD and it prevented hepatosteatosis at a dose of 476 nmol/kg (80,145). Recently, using a targeted metabolomics approach, we compared the effects of T2 and T3 on the early metabolic adaptation in the livers of rats fed HFD (55). We found that both T2 and T3 strongly induce autophagy and intrahepatic acylcarnitine flux, while preventing the generation of sphingolipid/ceramides in rats fed HFD (55). Interestingly, although both T2 and T3 decrease hepatic fat content, only T2 rescues the impairment in Akt and mitogen-activated protein kinase (MAPK/ERK) signaling pathways caused by HFD (55). Interestingly, T2 suppresses hepatic lipid accumulation by downregulating the expression of lipogenic proteins (85). T2 also seems to have a favorable safety profile compared with T3, although it suppresses the axis by decreasing serum TSH and T3/T4 in rodents in a dose-dependent manner (64,146,147). However, no change in the HPT axis was observed in two patients given T2 for 3 weeks, thus, it is not known whether this suppression also occurs in humans (148). Finally, a synthetic T2 mimetic, TRC150094, reduced hepatic steatosis and increased mitochondrial respiration in obese Zucker rats and rats fed HFD and did not cause any changes in the axis (149,150). However, a phase II clinical study with TRC150094 in obese men with mild steatosis failed to show a beneficial effect on intrahepatic lipid content measured by 1H-MRS after 4 weeks of treatment (151). In this study, there was a small but significant increase in serum free thyroxine (fT4), but no changes in serum TSH or free triiodothyronine (fT3) were observed. Taken together, the foregoing studies suggest that T2 improves NAFLD in animal models; however, it remains to be seen whether these beneficial effects are able to be translated to humans and whether there may be some additional side effects that need to be further considered.
Finally, there has been only limited study of the TH metabolite Triac in NAFLD. Cable et al. showed that the administration of Triac was equally effective as T3 as well as the THR_β_ receptor agonists GC-1, and MB03744 for lowering intrahepatic TAGs in rats (135). No effects on the HPT axis were discussed in this study.
TH, TH Analogs, and TH Metabolites in Hypercholesterolemia
Hypercholesterolemia
Hypercholesterolemia is a key clinical manifestation of metabolic syndrome that occurs with high prevalence in developed countries (World Health Organization 2008 global prevalence of raised total cholesterol). Clinical studies have shown that inappropriately increased cholesterol biosynthesis and/or clearance are major contributing factors for hypercholesterolemia. Notably, hypercholesterolemia increases the risk for atherosclerosis and ischemic heart disease (152).
Atherosclerosis is due to the deposition of oxidized LDL-C that damages the endothelial cell wall to induce inflammation, recruitment of macrophages (to remove oxidized cholesterol), and smooth muscle cell proliferation (152). Over time, this process leads to atherosclerotic plaque formation and causes narrowing of the affected blood vessel lumen (152). Statins currently are the major class of drugs used to treat hypercholesterolemia and reduce serum cholesterol levels by inhibiting the activity of HMGCR, the key rate-limiting enzyme in cholesterol biosynthesis. This leads to a decrease in intrahepatic cholesterol concentration and stimulation of LDLR expression by the SREBP2/INSIG pathway, leading to lower serum cholesterol levels (153). Despite their efficacy and safety, some patients still experience side effects or resistance to statins (154); thus, there is strong clinical and research interest in developing novel drugs that can decrease cholesterol biosynthesis and/or increase clearance in the liver.
Effects of TH on hypercholesterolemia
Clinical studies have shown that hyperthyroid patients have decreased serum cholesterol levels, and hypothyroid patients have elevated levels (155). With the discovery of the inverse relationship between TH status and serum LDL-C in the early 1950s, early intervention studies using L-T4 and D-T4 significantly lowered LDL-C in humans (156,157). However, it soon became apparent that clinical use of these TH compounds to treat hypercholesterolemia was not feasible due to their serious adverse effects on the heart, bone, and muscle (158). Recently, a TH and glucagon conjugate induced a greater decrease in serum cholesterol when compared with equimolar doses of GC-1 and KB-2115 (130). As mentioned previously, side effects of this compound appeared to be less than T3 monotherapy due to relative selectivity for the liver.
TH analogs for hypercholesterolemia
Despite these considerations, the promising results of TH led to the development of other liver-selective compounds (e.g., L-94901, CGH-509A, CGS-23425, and T-0681) that showed efficacy in lowering serum levels of LDL-C in animal studies (138,159). GC-1 (sobetirome) is the first-generation THR_β_ agonist that lowers serum cholesterol and TAG levels in animal models of obesity, and it reduces aortic artery plaque formation in apolipoprotein E (APOE)-deficient mice and acts in an LDLR-independent manner (70,160,161). Other THR_β_-selective thyromimetics, KB-141 (162) and KB2115 (163) (eprotirome), decrease plasma levels of cholesterol in both rodents and primates, primarily through its stimulation of RCT. The liver-selective prodrug, MB07811, is effective in reducing serum levels of LDL-C and total cholesterol in rabbits, dogs, and monkeys (139,164).
In humans, a phase I clinical study with GC-1 (sobetirome) reduced serum levels of LDL-C by more than 40% in healthy participants (165). Also, KB2115 (eprotirome) further decreased serum levels of LDL-C, TAGs, and lipoprotein(s) in patients with familial hypercholesterolemia, but its clinical development was discontinued due to induction of liver enzymes and cartilage/bone side effects in dogs (163,166). In both studies, there were decreases in serum fT4, but there were no changes in TSH levels. Currently, both MB07811 and MGL-3196 are undergoing phase II trials for the treatment of hypercholesterolemia (167,168).
TH metabolites as therapy for hypercholesterolemia
The TH metabolite, T2, can reduce serum cholesterol in rodents (80), possibly via non-LDLR-mediated pathways (64). There has been only one small pilot study of T2 in two human subjects, and it showed that T2 reduced serum cholesterol (148). Although cholesterol-lowering effects of Triac have not been observed in animals, human studies have shown reduction in serum cholesterol levels in euthyroid and hypothyroid patients and reduction in serum TAG levels in hypothyroid subjects (11,169). Even though the cholesterol-lowering effects of Triac may be more potent than T3, the use of Triac in hypercholesterolemia has been limited by its more pronounced side effects on bone and a short half-life requiring dosing multiple times per day.
The TH metabolite, 2,5-diiodothyropropionic acid (DITPA), was originally developed as a potential therapy for congestive heart failure (CHF) due to its inotropic effects. In human studies, decreased serum levels of total cholesterol, LDL-C, and TAGs in patients with CHF treated with DITPA were observed (170). However, this drug has not been further pursued clinically due to intolerable side effects (especially bone), suppression of TSH, and questionable clinical benefit (171). T1AM significantly decreases body weight and serum cholesterol but increases serum TAG in spontaneously overweight female (CD-1) mice that were treated for 7 days (90). In a model of polycystic ovary syndrome liver, TAG and cholesterol were decreased by T1AM, an effect that should be further investigated in an established NAFLD model (172).
In conclusion, TH regulates hepatic and systemic cholesterol and lipid homeostasis through its effects on their metabolism, circulating lipoprotein levels, and intrahepatic TAG and cholesterol concentration. TH also regulates the expression and activities of key enzymes involved in hepatic lipid anabolic and catabolic pathways. Interestingly, defects in intrahepatic deiodinase expression and lower intrahepatic TH concentration may be hallmarks of NASH and suggest that treatment with TH, TH metabolites, or TH mimetics as lipid-modifying drugs may reduce hepatosteatosis, inflammation, and fibrosis in NAFLD. TH also lowers serum cholesterol by virtue of its effects on cholesterol synthesis, LDLR expression, and RCT. From a clinical point of view, it can be argued that patients with hepatosteatosis or hypercholesterolemia in the context of subclinical hypothyroidism may benefit from levothyroxine supplementation to ameliorate these abnormalities. Careful clinical studies will need to be performed to determine whether these interventions will indeed improve the clinical outcomes of such patients. Additionally, there is a need for liver-specific markers of TH status to assess intrahepatic TH action, which may be a better determinant of proper dosing for NAFLD than serum TH and TSH levels. It is also possible that the expression of these markers may change during the course of the disease. Identifying the appropriate liver serum markers for TH action as well as inflammation and fibrosis could be useful in determining the type of patients and stage of NAFLD for which TH or TH agonist treatment would be most appropriate.
Concerning strategies to treat NAFLD and cholesterol with TH, TH metabolites, or TH analogs, the biggest challenge is limiting their side effects while achieving maximal beneficial effect on the liver. Strategies designed to achieve this aim include tissue- or cell-specific delivery of such compounds to the liver, cell-specific activation of prodrugs, preferential uptake by the liver, or isoform-specific activation of THR_β_ expressed in the liver. TH metabolites or mimetics that have one or more of these properties will likely limit the extrahepatic side effects of the drug, particularly in tissues such as the bone and heart that express primarily THRα and are prone to the side effects of hyperthyroidism. However, it should be noted that specific compounds might specifically address noncanonical pathways mediated by THRs. It is also important to point out that TH analogs and metabolites may have variable effects in suppressing the axis and that careful consideration of clinical parameters, for example, tachycardia, weight loss, heat intolerance, and tremor, as well as serum markers of hepatic TH action such as SHBG should be considered in clinical studies and may be useful in assessing the thyroid status once patients are taking such drugs. Another important clinical consideration is when to treat patients, particularly given the wide range of effects of TH on lipid metabolism, autophagy, mitochondrial turnover, ceramide metabolism, and even fibrosis (156). Clinical studies for NAFLD are needed to examine the ability of TH metabolites or mimetics to prevent the progression of NASH as well as their abilities to reverse established NASH. Likewise, it remains to be seen whether TH-like compounds can be used as monotherapy for hypercholesterolemia or should be given in combination with statins. In summary, given the important actions of TH in maintaining metabolic homeostasis within the liver and systemically, selective hepatic targeting with TH metabolites and analogs may represent a novel and exciting therapy for the treatment of liver-associated metabolic diseases, such as NAFLD and hypercholesterolemia.
Funding Information
The authors are thankful to the funding agencies (Ministry of Health, Ministry of Education, and Ministry of Trade, Singapore, and Agency for Science, Technology and Research [A*StaR]) for the grants CIRG/1340/2012, National Medical Research Council (NMRC)/Clinician Scientist Award (CSA) Grant/MH95:03/1–8 (awarded to P.M.Y.), Khoo Postdoctoral Fellowship Award (awarded to E.B.), NMRC/OFYIRG/0002/2016 (awarded to B.K.S.), and Wellcome Trust/DBT India Alliance-Intermediate Fellowship IA/I/16/2/502691 (awarded to R.A.S.).
Footnotes
Author Disclosure Statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
References
- 1.Nguyen P, Leray V, Diez M, Serisier S, Le Bloc’h J, Siliart B, Dumon H. Liver lipid metabolism. J Anim Physiol Anim Nutr (Berl) 2008;92:272–283. doi: 10.1111/j.1439-0396.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- 2.Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci. 2018;75:3313–3327. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ott DB, Lachance PA. Biochemical controls of liver cholesterol biosynthesis. Am J Clin Nutr. 1981;34:2295–2306. doi: 10.1093/ajcn/34.10.2295. [DOI] [PubMed] [Google Scholar]
- 4.Magnus-Levy A. Über den respiratorischen Gas-wechsel unter dem Einfluss der Thyroidea sowie unter verschiedenen pathologischen Zuständen. Berl Klin Wochenschr. 1895;32:650–652. [Google Scholar]
- 5.Mason RL, Hunt HM, Hurxthal L. Blood cholesterol values in hyperthyroidism and hypothyroidism—their significance. N Engl J Med. 1930;203:1273–1278. [Google Scholar]
- 6.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12:287–293. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- 7.Sinha RA, Singh BK, Yen PM. Reciprocal crosstalk between autophagic and endocrine signaling in metabolic homeostasis. Endocr Rev. 2017;38:69–102. doi: 10.1210/er.2016-1103. [DOI] [PubMed] [Google Scholar]
- 8.Sinha RA, You SH, Zhou J, Siddique MM, Bay BH, Zhu X, Privalsky ML, Cheng SY, Stevens RD, Summers SA, Newgard CB, et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J Clin Invest. 2012;122:2428–2438. doi: 10.1172/JCI60580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Spek AH, Fliers E, Boelen A. The classic pathways of thyroid hormone metabolism. Mol Cell Endocrinol. 2017;458:29–38. doi: 10.1016/j.mce.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Hoefig CS, Zucchi R, Kohrle J. Thyronamines and derivatives: physiological relevance, pharmacological actions, and future research directions. Thyroid. 2016;26:1656–1673. doi: 10.1089/thy.2016.0178. [DOI] [PubMed] [Google Scholar]
- 11.Sherman SI, Ringel MD, Smith MJ, Kopelen HA, Zoghbi WA, Ladenson PW. Augmented hepatic and skeletal thyromimetic effects of tiratricol in comparison with levothyroxine. J Clin Endocrinol Metab. 1997;82:2153–2158. doi: 10.1210/jcem.82.7.4054. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara SJ, Bourdette D, Scanlan TS. Hypothalamic-pituitary-thyroid axis perturbations in male mice by CNS-penetrating thyromimetics. Endocrinology. 2018;159:2733–2740. doi: 10.1210/en.2018-00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001;81:1097–1142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]
- 14.Refetoff S. Thyroid hormone serum transport proteins. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, et al., editors. Endotext. South Dartmouth, MA: 2000. [Google Scholar]
- 15.Braun D, Schweizer U. Thyroid hormone transport and transporters. Vitam Horm. 2018;106:19–44. doi: 10.1016/bs.vh.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Visser TJ. Cellular uptake of thyroid hormones. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, et al., editors. Endotext. South Dartmouth, MA: 2000. [Google Scholar]
- 17.Peeters RP, Visser TJ. Metabolism of thyroid hormone. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, et al., editors. Endotext. South Dartmouth, MA: 2000. Vol. [Google Scholar]
- 18.Schweizer U, Steegborn C. New insights into the structure and mechanism of iodothyronine deiodinases. J Mol Endocrinol. 2015;55:R37–R52. doi: 10.1530/JME-15-0156. [DOI] [PubMed] [Google Scholar]
- 19.Sinha R, Yen PM. Cellular action of thyroid hormone. In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, et al., editors. Endotext. South Dartmouth, MA: 2000. Vol. [Google Scholar]
- 20.Klieverik LP, Coomans CP, Endert E, Sauerwein HP, Havekes LM, Voshol PJ, Rensen PC, Romijn JA, Kalsbeek A, Fliers E. Thyroid hormone effects on whole-body energy homeostasis and tissue-specific fatty acid uptake in vivo. Endocrinology. 2009;150:5639–5648. doi: 10.1210/en.2009-0297. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa S, Kawashima Y, Hirose A, Kozuka H. Regulation of hepatic level of fatty-acid-binding protein by hormones and clofibric acid in the rat. Biochem J. 1994;297(Pt 3):581–584. doi: 10.1042/bj2970581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santana-Farre R, Mirecki-Garrido M, Bocos C, Henriquez-Hernandez LA, Kahlon N, Herrera E, Norstedt G, Parini P, Flores-Morales A, Fernandez-Perez L. Influence of neonatal hypothyroidism on hepatic gene expression and lipid metabolism in adulthood. PLoS One. 2012;7:e37386. doi: 10.1371/journal.pone.0037386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radenne A, Akpa M, Martel C, Sawadogo S, Mauvoisin D, Mounier C. Hepatic regulation of fatty acid synthase by insulin and T3: evidence for T3 genomic and nongenomic actions. Am J Physiol Endocrinol Metab. 2008;295:E884–E894. doi: 10.1152/ajpendo.90438.2008. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Yin L, Hillgartner FB. Thyroid hormone stimulates acetyl-coA carboxylase-alpha transcription in hepatocytes by modulating the composition of nuclear receptor complexes bound to a thyroid hormone response element. J Biol Chem. 2001;276:974–983. doi: 10.1074/jbc.M005894200. [DOI] [PubMed] [Google Scholar]
- 25.Desvergne B, Petty KJ, Nikodem VM. Functional characterization and receptor binding studies of the malic enzyme thyroid hormone response element. J Biol Chem. 1991;266:1008–1013. [PubMed] [Google Scholar]
- 26.Campbell MC, Anderson GW, Mariash CN. Human spot 14 glucose and thyroid hormone response: characterization and thyroid hormone response element identification. Endocrinology. 2003;144:5242–5248. doi: 10.1210/en.2002-0008. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol. 2015;16:678–689. doi: 10.1038/nrm4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto K, Ishida E, Matsumoto S, Okada S, Yamada M, Satoh T, Monden T, Mori M. Carbohydrate response element binding protein gene expression is positively regulated by thyroid hormone. Endocrinology. 2009;150:3417–3424. doi: 10.1210/en.2009-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto K, Matsumoto S, Yamada M, Satoh T, Mori M. Liver X receptor-alpha gene expression is positively regulated by thyroid hormone. Endocrinology. 2007;148:4667–4675. doi: 10.1210/en.2007-0150. [DOI] [PubMed] [Google Scholar]
- 30.Araki O, Ying H, Zhu XG, Willingham MC, Cheng SY. Distinct dysregulation of lipid metabolism by unliganded thyroid hormone receptor isoforms. Mol Endocrinol. 2009;23:308–315. doi: 10.1210/me.2008-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto K, Yamada M, Matsumoto S, Monden T, Satoh T, Mori M. Mouse sterol response element binding protein-1c gene expression is negatively regulated by thyroid hormone. Endocrinology. 2006;147:4292–4302. doi: 10.1210/en.2006-0116. [DOI] [PubMed] [Google Scholar]
- 32.Yao X, Hou S, Zhang D, Xia H, Wang YC, Jiang J, Yin H, Ying H. Regulation of fatty acid composition and lipid storage by thyroid hormone in mouse liver. Cell Biosci. 2014;4:38. doi: 10.1186/2045-3701-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto K, Ishida E, Miura A, Ozawa A, Shibusawa N, Satoh T, Okada S, Yamada M, Mori M. Human stearoyl-CoA desaturase 1 (SCD-1) gene expression is negatively regulated by thyroid hormone without direct binding of thyroid hormone receptor to the gene promoter. Endocrinology. 2013;154:537–549. doi: 10.1210/en.2012-1559. [DOI] [PubMed] [Google Scholar]
- 34.Dang AQ, Faas FH, Carter WJ. Influence of hypo- and hyperthyroidism on rat liver glycerophospholipid metabolism. Lipids. 1985;20:897–902. doi: 10.1007/BF02534774. [DOI] [PubMed] [Google Scholar]
- 35.Grasselli E, Voci A, Demori I, Vecchione G, Compalati AD, Gallo G, Goglia F, De Matteis R, Silvestri E, Vergani L. Triglyceride mobilization from lipid droplets sustains the anti-steatotic action of iodothyronines in cultured rat hepatocytes. Front Physiol. 2015;6:418. doi: 10.3389/fphys.2015.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kihara S, Wolle J, Ehnholm C, Chan L, Oka K. Regulation of hepatic triglyceride lipase by thyroid hormone in HepG2 cells. J Lipid Res. 1993;34:961–970. [PubMed] [Google Scholar]
- 37.Simo R, Hernandez C, Saez-Lopez C, Soldevila B, Puig-Domingo M, Selva DM. Thyroid hormone upregulates zinc-alpha2-glycoprotein production in the liver but not in adipose tissue. PLoS One. 2014;9:e85753. doi: 10.1371/journal.pone.0085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coates PM, Hoffman GM, Finegold DN. Effect of thyroid hormones on human mononuclear leukocyte lysosomal acid lipase activity. J Clin Endocrinol Metab. 1982;54:559–562. doi: 10.1210/jcem-54-3-559. [DOI] [PubMed] [Google Scholar]
- 39.DeMartino GN, Goldberg AL. Thyroid hormones control lysosomal enzyme activities in liver and skeletal muscle. Proc Natl Acad Sci U S A. 1978;75:1369–1373. doi: 10.1073/pnas.75.3.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tseng YH, Ke PY, Liao CJ, Wu SM, Chi HC, Tsai CY, Chen CY, Lin YH, Lin KH. Chromosome 19 open reading frame 80 is upregulated by thyroid hormone and modulates autophagy and lipid metabolism. Autophagy. 2014;10:20–31. doi: 10.4161/auto.26126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cioffi F, Lanni A, Goglia F. Thyroid hormones, mitochondrial bioenergetics and lipid handling. Curr Opin Endocrinol Diabetes Obes. 2010;17:402–407. doi: 10.1097/MED.0b013e32833cf354. [DOI] [PubMed] [Google Scholar]
- 42.Jackson-Hayes L, Song S, Lavrentyev EN, Jansen MS, Hillgartner FB, Tian L, Wood PA, Cook GA, Park EA. A thyroid hormone response unit formed between the promoter and first intron of the carnitine palmitoyltransferaseI-alpha gene mediates the liver-specific induction by thyroid hormone. J Biol Chem. 2003;278:7964–7972. doi: 10.1074/jbc.M211062200. [DOI] [PubMed] [Google Scholar]
- 43.Singh BK, Sinha RA, Tripathi M, Mendoza A, Ohba K, Sy JAC, Xie SY, Zhou J, Ho JP, Chang CY, Wu Y, et al. Thyroid hormone receptor and ERRalpha coordinately regulate mitochondrial fission, mitophagy, biogenesis, and function. Sci Signal. 2018;11(536):eaam5855. doi: 10.1126/scisignal.aam5855. [DOI] [PubMed] [Google Scholar]
- 44.Adams AC, Astapova I, Fisher FM, Badman MK, Kurgansky KE, Flier JS, Hollenberg AN, Maratos-Flier E. Thyroid hormone regulates hepatic expression of fibroblast growth factor 21 in a PPARalpha-dependent manner. J Biol Chem. 2010;285:14078–14082. doi: 10.1074/jbc.C110.107375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Djouadi F, Riveau B, Merlet-Benichou C, Bastin J. Tissue-specific regulation of medium-chain acyl-CoA dehydrogenase gene by thyroid hormones in the developing rat. Biochem J. 1997;324(Pt 1):289–294. doi: 10.1042/bj3240289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holness MJ, Bulmer K, Smith ND, Sugden MC. Investigation of potential mechanisms regulating protein expression of hepatic pyruvate dehydrogenase kinase isoforms 2 and 4 by fatty acids and thyroid hormone. Biochem J. 2003;369:687–695. doi: 10.1042/BJ20021509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jekabsons MB, Gregoire FM, Schonfeld-Warden NA, Warden CH, Horwitz BA. T(3) stimulates resting metabolism and UCP-2 and UCP-3 mRNA but not non-phosphorylating mitochondrial respiration in mice. Am J Physiol. 1999;277:E380–E389. doi: 10.1152/ajpendo.1999.277.2.E380. [DOI] [PubMed] [Google Scholar]
- 48.Weitzel JM, Iwen KA. Coordination of mitochondrial biogenesis by thyroid hormone. Mol Cell Endocrinol. 2011;342:1–7. doi: 10.1016/j.mce.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 49.Thakran S, Sharma P, Attia RR, Hori RT, Deng X, Elam MB, Park EA. Role of sirtuin 1 in the regulation of hepatic gene expression by thyroid hormone. J Biol Chem. 2013;288:807–818. doi: 10.1074/jbc.M112.437970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wrutniak-Cabello C, Casas F, Cabello G. Mitochondrial T3 receptor and targets. Mol Cell Endocrinol. 2017;458:112–120. doi: 10.1016/j.mce.2017.01.054. [DOI] [PubMed] [Google Scholar]
- 51.Chocron ES, Sayre NL, Holstein D, Saelim N, Ibdah JA, Dong LQ, Zhu X, Cheng SY, Lechleiter JD. The trifunctional protein mediates thyroid hormone receptor-dependent stimulation of mitochondria metabolism. Mol Endocrinol. 2012;26:1117–1128. doi: 10.1210/me.2011-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mishra P, Chan DC. Metabolic regulation of mitochondrial dynamics. J Cell Biol. 2016;212:379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinha RA, Singh BK, Zhou J, Wu Y, Farah BL, Ohba K, Lesmana R, Gooding J, Bay BH, Yen PM. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy. 2015;11:1341–1357. doi: 10.1080/15548627.2015.1061849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernardes SS, Guarnier FA, Marinello PC, Armani A, Simao AN, Cecchini R, Cecchini AL. Reactive oxygen species play a role in muscle wasting during thyrotoxicosis. Cell Tissue Res. 2014;357:803–814. doi: 10.1007/s00441-014-1881-1. [DOI] [PubMed] [Google Scholar]
- 55.Iannucci LF, Cioffi F, Senese R, Goglia F, Lanni A, Yen PM, Sinha RA. Metabolomic analysis shows differential hepatic effects of T2 and T3 in rats after short-term feeding with high fat diet. Sci Rep. 2017;7 doi: 10.1038/s41598-017-02205-1. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 57.Bucki R, Gorska M, Zendzian-Piotrowska M, Gorski J. Effect of triiodothyronine on the content of phospholipids in the rat liver nuclei. J Physiol Pharmacol. 2000;51:535–540. [PubMed] [Google Scholar]
- 58.Sinha RA, Singh BK, Yen PM. Thyroid hormone regulation of hepatic lipid and carbohydrate metabolism. Trends Endocrinol Metab. 2014;25:538–545. doi: 10.1016/j.tem.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 59.Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol. 2018;14:259–269. doi: 10.1038/nrendo.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ness GC, Pendleton LC, Li YC, Chiang JY. Effect of thyroid hormone on hepatic cholesterol 7 alpha hydroxylase, LDL receptor, HMG-CoA reductase, farnesyl pyrophosphate synthetase and apolipoprotein A-I mRNA levels in hypophysectomized rats. Biochem Biophys Res Commun. 1990;172:1150–1156. doi: 10.1016/0006-291x(90)91568-d. [DOI] [PubMed] [Google Scholar]
- 61.Davidson NO, Powell LM, Wallis SC, Scott J. Thyroid hormone modulates the introduction of a stop codon in rat liver apolipoprotein B messenger RNA. J Biol Chem. 1988;263:13482–13485. [PubMed] [Google Scholar]
- 62.Yap CS, Sinha RA, Ota S, Katsuki M, Yen PM. Thyroid hormone negatively regulates CDX2 and SOAT2 mRNA expression via induction of miRNA-181d in hepatic cells. Biochem Biophys Res Commun. 2013;440:635–639. doi: 10.1016/j.bbrc.2013.09.116. [DOI] [PubMed] [Google Scholar]
- 63.Abrams JJ, Grundy SM, Ginsberg H. Metabolism of plasma triglycerides in hypothyroidism and hyperthyroidism in man. J Lipid Res. 1981;22:307–322. [PubMed] [Google Scholar]
- 64.Goldberg IJ, Huang LS, Huggins LA, Yu S, Nagareddy PR, Scanlan TS, Ehrenkranz JR. Thyroid hormone reduces cholesterol via a non-LDL receptor-mediated pathway. Endocrinology. 2012;153:5143–5149. doi: 10.1210/en.2012-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan KC, Shiu SW, Kung AW. Plasma cholesteryl ester transfer protein activity in hyper- and hypothyroidism. J Clin Endocrinol Metab. 1998;83:140–143. doi: 10.1210/jcem.83.1.4491. [DOI] [PubMed] [Google Scholar]
- 66.Lopez D, Abisambra Socarras JF, Bedi M, Ness GC. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochim Biophys Acta. 2007;1771:1216–1225. doi: 10.1016/j.bbalip.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 67.Shin DJ, Osborne TF. Thyroid hormone regulation and cholesterol metabolism are connected through sterol regulatory element-binding protein-2 (SREBP-2) J Biol Chem. 2003;278:34114–34118. doi: 10.1074/jbc.M305417200. [DOI] [PubMed] [Google Scholar]
- 68.Moon JH, Kim HJ, Kim HM, Choi SH, Lim S, Park YJ, Jang HC, Cha BS. Decreased expression of hepatic low-density lipoprotein receptor-related protein 1 in hypothyroidism: a novel mechanism of atherogenic dyslipidemia in hypothyroidism. Thyroid. 2013;23:1057–1065. doi: 10.1089/thy.2012.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonde Y, Breuer O, Lutjohann D, Sjoberg S, Angelin B, Rudling M. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J Lipid Res. 2014;55:2408–2415. doi: 10.1194/jlr.M051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johansson L, Rudling M, Scanlan TS, Lundasen T, Webb P, Baxter J, Angelin B, Parini P. Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc Natl Acad Sci U S A. 2005;102:10297–10302. doi: 10.1073/pnas.0504379102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mooradian AD, Wong NC, Shah GN. Age-related changes in the responsiveness of apolipoprotein A1 to thyroid hormone. Am J Physiol. 1996;271:R1602–R1607. doi: 10.1152/ajpregu.1996.271.6.R1602. [DOI] [PubMed] [Google Scholar]
- 72.Tan KC, Shiu SW, Kung AW. Effect of thyroid dysfunction on high-density lipoprotein subfraction metabolism: roles of hepatic lipase and cholesteryl ester transfer protein. J Clin Endocrinol Metab. 1998;83:2921–2924. doi: 10.1210/jcem.83.8.4938. [DOI] [PubMed] [Google Scholar]
- 73.Lammel Lindemann JA, Angajala A, Engler DA, Webb P, Ayers SD. Thyroid hormone induction of human cholesterol 7 alpha-hydroxylase (Cyp7a1) in vitro. Mol Cell Endocrinol. 2014;388:32–40. doi: 10.1016/j.mce.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bonde Y, Plosch T, Kuipers F, Angelin B, Rudling M. Stimulation of murine biliary cholesterol secretion by thyroid hormone is dependent on a functional ABCG5/G8 complex. Hepatology. 2012;56:1828–1837. doi: 10.1002/hep.25861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu X, Cheng SY. New insights into regulation of lipid metabolism by thyroid hormone. Curr Opin Endocrinol Diabetes Obes. 2010;17:408–413. doi: 10.1097/MED.0b013e32833d6d46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hones GS, Rakov H, Logan J, Liao XH, Werbenko E, Pollard AS, Praestholm SM, Siersbaek MS, Rijntjes E, Gassen J, Latteyer S, et al. Noncanonical thyroid hormone signaling mediates cardiometabolic effects in vivo. Proc Natl Acad Sci U S A. 2017;114:E11323–E11332. doi: 10.1073/pnas.1706801115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jornayvaz FR, Lee HY, Jurczak MJ, Alves TC, Guebre-Egziabher F, Guigni BA, Zhang D, Samuel VT, Silva JE, Shulman GI. Thyroid hormone receptor-alpha gene knockout mice are protected from diet-induced hepatic insulin resistance. Endocrinology. 2012;153:583–591. doi: 10.1210/en.2011-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kowalik MA, Columbano A, Perra A. Thyroid hormones, thyromimetics and their metabolites in the treatment of liver disease. Front Endocrinol (Lausanne) 2018;9:382. doi: 10.3389/fendo.2018.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Senese R, Cioffi F, de Lange P, Goglia F, Lanni A. Thyroid: biological actions of ‘‘nonclassical’’ thyroid hormones. J Endocrinol. 2014;221:R1–R12. doi: 10.1530/JOE-13-0573. [DOI] [PubMed] [Google Scholar]
- 80.de Lange P, Cioffi F, Senese R, Moreno M, Lombardi A, Silvestri E, De Matteis R, Lionetti L, Mollica MP, Goglia F, Lanni A. Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-l-thyronine in rats. Diabetes. 2011;60:2730–2739. doi: 10.2337/db11-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Silvestri E, Cioffi F, Glinni D, Ceccarelli M, Lombardi A, de Lange P, Chambery A, Severino V, Lanni A, Goglia F, Moreno M. Pathways affected by 3,5-diiodo-l-thyronine in liver of high fat-fed rats: evidence from two-dimensional electrophoresis, blue-native PAGE, and mass spectrometry. Mol Biosyst. 2010;6:2256–2271. doi: 10.1039/c0mb00040j. [DOI] [PubMed] [Google Scholar]
- 82.Grasselli E, Voci A, Demori I, Canesi L, De Matteis R, Goglia F, Lanni A, Gallo G, Vergani L. 3,5-Diiodo-l-thyronine modulates the expression of genes of lipid metabolism in a rat model of fatty liver. J Endocrinol. 2012;212:149–158. doi: 10.1530/JOE-11-0288. [DOI] [PubMed] [Google Scholar]
- 83.Grasselli E, Voci A, Canesi L, Salis A, Damonte G, Compalati AD, Goglia F, Gallo G, Vergani L. 3,5-Diiodo-l-thyronine modifies the lipid droplet composition in a model of hepatosteatosis. Cell Physio Biochem. 2014;33:344–356. doi: 10.1159/000356674. [DOI] [PubMed] [Google Scholar]
- 84.Damiano F, Rochira A, Gnoni A, Siculella L. Action of thyroid hormones, T3 and T2, on hepatic fatty acids: differences in metabolic effects and molecular mechanisms. Int J Mol Sci. 2017;18(4):E744. doi: 10.3390/ijms18040744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rochira A, Damiano F, Marsigliante S, Gnoni GV, Siculella L. 3,5-Diiodo-l-thyronine induces SREBP-1 proteolytic cleavage block and apoptosis in human hepatoma (Hepg2) cells. Biochim Biophys Acta. 2013;1831:1679–1689. doi: 10.1016/j.bbalip.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 86.Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, Frascarelli S, Crossley DA, Bunzow JR, Ronca-Testoni S, Lin ET, et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med. 2004;10:638–642. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- 87.Ackermans MT, Klieverik LP, Ringeling P, Endert E, Kalsbeek A, Fliers E. An online solid-phase extraction-liquid chromatography-tandem mass spectrometry method to study the presence of thyronamines in plasma and tissue and their putative conversion from 13C6-thyroxine. J Endocrinol. 2010;206:327–334. doi: 10.1677/JOE-10-0060. [DOI] [PubMed] [Google Scholar]
- 88.Mariotti V, Melissari E, Iofrida C, Righi M, Di Russo M, Donzelli R, Saba A, Frascarelli S, Chiellini G, Zucchi R, Pellegrini S. Modulation of gene expression by 3-iodothyronamine: genetic evidence for a lipolytic pattern. PLoS One. 2014;9:e106923. doi: 10.1371/journal.pone.0106923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haviland JA, Reiland H, Butz DE, Tonelli M, Porter WP, Zucchi R, Scanlan TS, Chiellini G, Assadi-Porter FM. NMR-based metabolomics and breath studies show lipid and protein catabolism during low dose chronic T(1)AM treatment. Obesity (Silver Spring) 2013;21:2538–2544. doi: 10.1002/oby.20391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Assadi-Porter FM, Reiland H, Sabatini M, Lorenzini L, Carnicelli V, Rogowski M, Selen Alpergin ES, Tonelli M, Ghelardoni S, Saba A, Zucchi R, et al. Metabolic reprogramming by 3-iodothyronamine (T1AM): a new perspective to reverse obesity through co-regulation of Sirtuin 4 and 6 expression. Int J Mol Sci. 2018;19(5):E1535. doi: 10.3390/ijms19051535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ghelardoni S, Chiellini G, Frascarelli S, Saba A, Zucchi R. Uptake and metabolic effects of 3-iodothyronamine in hepatocytes. J Endocrinol. 2014;221:101–110. doi: 10.1530/JOE-13-0311. [DOI] [PubMed] [Google Scholar]
- 92.Martinez L, Nascimento AS, Nunes FM, Phillips K, Aparicio R, Dias SM, Figueira AC, Lin JH, Nguyen P, Apriletti JW, Neves FA, et al. Gaining ligand selectivity in thyroid hormone receptors via entropy. Proc Natl Acad Sci U S A. 2009;106:20717–20722. doi: 10.1073/pnas.0911024106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Groeneweg S, Peeters RP, Visser TJ, Visser WE. Triiodothyroacetic acid in health and disease. J Endocrinol. 2017;234:R99–R121. doi: 10.1530/JOE-17-0113. [DOI] [PubMed] [Google Scholar]
- 94.Medina-Gomez G, Calvo RM, Obregon MJ. Thermogenic effect of triiodothyroacetic acid at low doses in rat adipose tissue without adverse side effects in the thyroid axis. Am J Physiol Endocrinol Metab. 2008;294:E688–E697. doi: 10.1152/ajpendo.00417.2007. [DOI] [PubMed] [Google Scholar]
- 95.Balsam A, Sexton F, Borges M, Ingbar SH. Formation of diiodotyrosine from thyroxine. Ether-link cleavage, an alternate pathway of thyroxine metabolism. J Clin Invest. 1983;72:1234–1245. doi: 10.1172/JCI111079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang W, Tian LM, Han Y, Ma HY, Wang LC, Guo J, Gao L, Zhao JJ. Presence of thyrotropin receptor in hepatocytes: not a case of illegitimate transcription. J Cell Mol Med. 2009;13:4636–4642. doi: 10.1111/j.1582-4934.2008.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tian L, Song Y, Xing M, Zhang W, Ning G, Li X, Yu C, Qin C, Liu J, Tian X, Sun X, et al. A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology. 2010;52:1401–1409. doi: 10.1002/hep.23800. [DOI] [PubMed] [Google Scholar]
- 98.Song Y, Xu C, Shao S, Liu J, Xing W, Xu J, Qin C, Li C, Hu B, Yi S, Xia X, et al. Thyroid-stimulating hormone regulates hepatic bile acid homeostasis via SREBP-2/HNF-4alpha/CYP7A1 axis. J Hepatol. 2015;62:1171–1179. doi: 10.1016/j.jhep.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 99.Gong Y, Ma Y, Ye Z, Fu Z, Yang P, Gao B, Guo W, Hu D, Ye J, Ma S, Zhang F, et al. Thyroid stimulating hormone exhibits the impact on LDLR/LDL-c via up-regulating hepatic PCSK9 expression. Metabolism. 2017;76:32–41. doi: 10.1016/j.metabol.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 100.Min HK, Kapoor A, Fuchs M, Mirshahi F, Zhou H, Maher J, Kellum J, Warnick R, Contos MJ, Sanyal AJ. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012;15:665–674. doi: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yan F, Wang Q, Lu M, Chen W, Song Y, Jing F, Guan Y, Wang L, Lin Y, Bo T, Zhang J, et al. Thyrotropin increases hepatic triglyceride content through upregulation of SREBP-1c activity. J Hepatol. 2014;61:1358–1364. doi: 10.1016/j.jhep.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 102.Beukhof CM, Massolt ET, Visser TJ, Korevaar TIM, Medici M, de Herder WW, Roeters van Lennep JE, Mulder MT, de Rijke YB, Reiners C, Verburg FA, et al. Effects of thyrotropin on peripheral thyroid hormone metabolism and serum lipids. Thyroid. 2018;28:168–174. doi: 10.1089/thy.2017.0330. [DOI] [PubMed] [Google Scholar]
- 103.Bianco AC, Anderson G, Forrest D, Galton VA, Gereben B, Kim BW, Kopp PA, Liao XH, Obregon MJ, Peeters RP, Refetoff S, et al. American Thyroid Association Guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid. 2014;24:88–168. doi: 10.1089/thy.2013.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One. 2015;10:e0140908. doi: 10.1371/journal.pone.0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 106.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doege H, Grimm D, Falcon A, Tsang B, Storm TA, Xu H, Ortegon AM, Kazantzis M, Kay MA, Stahl A. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J Biol Chem. 2008;283:22186–22192. doi: 10.1074/jbc.M803510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghosh M, Niyogi S, Bhattacharyya M, Adak M, Nayak DK, Chakrabarti S, Chakrabarti P. Ubiquitin ligase COP1 controls hepatic fat metabolism by targeting ATGL for degradation. Diabetes. 2016;65:3561–3572. doi: 10.2337/db16-0506. [DOI] [PubMed] [Google Scholar]
- 110.Koliaki C, Szendroedi J, Kaul K, Jelenik T, Nowotny P, Jankowiak F, Herder C, Carstensen M, Krausch M, Knoefel WT, Schlensak M, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 111.Sanyal AJ, Pacana T. A Lipidomic readout of disease progression in a diet-induced mouse model of nonalcoholic fatty liver disease. Trans Am Clin Climatol Assoc. 2015;126:271–288. [PMC free article] [PubMed] [Google Scholar]
- 112.Petersen MC, Shulman GI. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol Sci. 2017;38:649–665. doi: 10.1016/j.tips.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bikman BT, Summers SA. Sphingolipids and hepatic steatosis. Adv Exp Med Biol. 2011;721:87–97. doi: 10.1007/978-1-4614-0650-1_6. [DOI] [PubMed] [Google Scholar]
- 114.Cano A, Ciaffoni F, Safwat GM, Aspichueta P, Ochoa B, Bravo E, Botham KM. Hepatic VLDL assembly is disturbed in a rat model of nonalcoholic fatty liver disease: is there a role for dietary coenzyme Q? J Appl Physiol (1985) 2009;107:707–717. doi: 10.1152/japplphysiol.00297.2009. [DOI] [PubMed] [Google Scholar]
- 115.Ioannou GN. The role of cholesterol in the pathogenesis of NASH. Trends Endocrinol Metab. 2016;27:84–95. doi: 10.1016/j.tem.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 116.Kerr TA, Davidson NO. Cholesterol and nonalcoholic fatty liver disease: renewed focus on an old villain. Hepatology. 2012;56:1995–1998. doi: 10.1002/hep.26088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, Yokoyama M, Honda M, Zen Y, Nakanuma Y, Miyamoto K, et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–1403. doi: 10.1002/hep.21874. [DOI] [PubMed] [Google Scholar]
- 118.Van Rooyen DM, Larter CZ, Haigh WG, Yeh MM, Ioannou G, Kuver R, Lee SP, Teoh NC, Farrell GC. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology. 2011;141:1393–1403. doi: 10.1053/j.gastro.2011.06.040. 1403.e1–1403.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, Garcia-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4:185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 120.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 121.Mantovani A, Nascimbeni F, Lonardo A, Zoppini G, Bonora E, Mantzoros CS, Targher G. Association between primary hypothyroidism and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Thyroid. 2018;28:1270–1284. doi: 10.1089/thy.2018.0257. [DOI] [PubMed] [Google Scholar]
- 122.Xu C, Xu L, Yu C, Miao M, Li Y. Association between thyroid function and nonalcoholic fatty liver disease in euthyroid elderly Chinese. Clin Endocrinol. 2011;75:240–246. doi: 10.1111/j.1365-2265.2011.04016.x. [DOI] [PubMed] [Google Scholar]
- 123.Ludwig U, Holzner D, Denzer C, Greinert A, Haenle MM, Oeztuerk S, Koenig W, Boehm BO, Mason RA, Kratzer W, Graeter T, et al. Subclinical and clinical hypothyroidism and non-alcoholic fatty liver disease: a cross-sectional study of a random population sample aged 18 to 65 years. BMC Endocr Disord. 2015;15:41. doi: 10.1186/s12902-015-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ferrandino G, Kaspari RR, Spadaro O, Reyna-Neyra A, Perry RJ, Cardone R, Kibbey RG, Shulman GI, Dixit VD, Carrasco N. Pathogenesis of hypothyroidism-induced NAFLD is driven by intra- and extrahepatic mechanisms. Proc Natl Acad Sci U S A. 2017;114:E9172–E9180. doi: 10.1073/pnas.1707797114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pihlajamaki J, Boes T, Kim EY, Dearie F, Kim BW, Schroeder J, Mun E, Nasser I, Park PJ, Bianco AC, Goldfine AB, et al. Thyroid hormone-related regulation of gene expression in human fatty liver. J Clin Endocrinol Metab. 2009;94:3521–3529. doi: 10.1210/jc.2009-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bohinc BN, Michelotti G, Xie G, Pang H, Suzuki A, Guy CD, Piercy D, Kruger L, Swiderska-Syn M, Machado M, Pereira T, et al. Repair-related activation of hedgehog signaling in stromal cells promotes intrahepatic hypothyroidism. Endocrinology. 2014;155:4591–4601. doi: 10.1210/en.2014-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bruinstroop E, Dalan R, Cao Y, Bee YM, Chandran K, Cho LW, Soh SB, Teo EK, Toh SA, Leow MKS, Sinha RA, et al. Low-dose levothyroxine reduces intrahepatic lipid content in patients with type 2 diabetes mellitus and NAFLD. J Clin Endocrinol Metab. 2018;103:2698–2706. doi: 10.1210/jc.2018-00475. [DOI] [PubMed] [Google Scholar]
- 128.Perra A, Simbula G, Simbula M, Pibiri M, Kowalik MA, Sulas P, Cocco MT, Ledda-Columbano GM, Columbano A. Thyroid hormone (T3) and TRbeta agonist GC-1 inhibit/reverse nonalcoholic fatty liver in rats. FASEB J. 2008;22:2981–2989. doi: 10.1096/fj.08-108464. [DOI] [PubMed] [Google Scholar]
- 129.Sinha RA, Yen PM. Thyroid hormone-mediated autophagy and mitochondrial turnover in NAFLD. Cell Biosci. 2016;6:46. doi: 10.1186/s13578-016-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Finan B, Clemmensen C, Zhu Z, Stemmer K, Gauthier K, Muller L, De Angelis M, Moreth K, Neff F, Perez-Tilve D, Fischer K, et al. Chemical hybridization of glucagon and thyroid hormone optimizes therapeutic impact for metabolic disease. Cell. 2016;167:843.e14–857.e14. doi: 10.1016/j.cell.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 131.Cao Y, Matsubara T, Zhao C, Gao W, Peng L, Shan J, Liu Z, Yuan F, Tang L, Li P, Guan Z, et al. Antisense oligonucleotide and thyroid hormone conjugates for obesity treatment. Sci Rep. 2017;7 doi: 10.1038/s41598-017-09598-z. 9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu L, Yu Y, Zhao M, Zheng D, Zhang X, Guan Q, Xu C, Gao L, Zhao J, Zhang H. Benefits of levothyroxine replacement therapy on nonalcoholic fatty liver disease in subclinical hypothyroidism patients. Int J Endocrinol. 2017;2017 doi: 10.1155/2017/5753039. 5753039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vatner DF, Weismann D, Beddow SA, Kumashiro N, Erion DM, Liao XH, Grover GJ, Webb P, Phillips KJ, Weiss RE, Bogan JS, et al. Thyroid hormone receptor-beta agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. Am J Physiol Endocrinol Metab. 2013;305:E89–E100. doi: 10.1152/ajpendo.00573.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martagon AJ, Lin JZ, Cimini SL, Webb P, Phillips KJ. The amelioration of hepatic steatosis by thyroid hormone receptor agonists is insufficient to restore insulin sensitivity in ob/ob mice. PLoS One. 2015;10:e0122987. doi: 10.1371/journal.pone.0122987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cable EE, Finn PD, Stebbins JW, Hou J, Ito BR, van Poelje PD, Linemeyer DL, Erion MD. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology. 2009;49:407–417. doi: 10.1002/hep.22572. [DOI] [PubMed] [Google Scholar]
- 136.Harrison S, Moussa S, Bashir M, Alkhouri N, Frias J, Baum S, Tetri B, Bansal M, Taub R. MGL-3196, a selective thyroid hormone receptor-beta agonist significantly decreases hepatic fat in NASH patients at 12 weeks, the primary endpoint in a 36 week serial liver biopsy study. J Hepatol. 2018;68:S38. [Google Scholar]
- 137.Loomba R, Neutel J, Bernard D, Severance R, Mohseni R, Dao M, Saini S, Margaritescu C, Homer K, Tran B, Mancini M, et al. VK2809, a novel liver-directed thyroid receptor beta agonist, significantly reduces liver fat in patients with non-alcoholic fatty liver disease: a phase 2 randomized, placebo-controlled trial AASLD. J Hepatol. 2018;70:e150–e151. [Google Scholar]
- 138.Taylor AH, Stephan ZF, Steele RE, Wong NC. Beneficial effects of a novel thyromimetic on lipoprotein metabolism. Mol Pharmacol. 1997;52:542–547. doi: 10.1124/mol.52.3.542. [DOI] [PubMed] [Google Scholar]
- 139.Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, Zhang BH, Hou J, Boyer SH, van Poelje PD, Linemeyer DL. Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci U S A. 2007;104:15490–15495. doi: 10.1073/pnas.0702759104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Freitas FR, Moriscot AS, Jorgetti V, Soares AG, Passarelli M, Scanlan TS, Brent GA, Bianco AC, Gouveia CH. Spared bone mass in rats treated with thyroid hormone receptor TR beta-selective compound GC-1. Am J Physiol Endocrinol Metab. 2003;285:E1135–E1141. doi: 10.1152/ajpendo.00506.2002. [DOI] [PubMed] [Google Scholar]
- 141.Grover GJ, Egan DM, Sleph PG, Beehler BC, Chiellini G, Nguyen NH, Baxter JD, Scanlan TS. Effects of the thyroid hormone receptor agonist GC-1 on metabolic rate and cholesterol in rats and primates: selective actions relative to 3,5,3′-triiodo-l-thyronine. Endocrinology. 2004;145:1656–1661. doi: 10.1210/en.2003-0973. [DOI] [PubMed] [Google Scholar]
- 142.Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14:348–399. doi: 10.1210/edrv-14-3-348. [DOI] [PubMed] [Google Scholar]
- 143.Chng CL, Lim AY, Tan HC, Kovalik JP, Tham KW, Bee YM, Lim W, Acharyya S, Lai OF, Chong MF, Yen PM. Physiological and metabolic changes during the transition from hyperthyroidism to euthyroidism in Graves’ disease. Thyroid. 2016;26:1422–1430. doi: 10.1089/thy.2015.0602. [DOI] [PubMed] [Google Scholar]
- 144.Grasselli E, Voci A, Canesi L, De Matteis R, Goglia F, Cioffi F, Fugassa E, Gallo G, Vergani L. Direct effects of iodothyronines on excess fat storage in rat hepatocytes. J Hepatol. 2011;54:1230–1236. doi: 10.1016/j.jhep.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 145.Mollica MP, Lionetti L, Moreno M, Lombardi A, De Lange P, Antonelli A, Lanni A, Cavaliere G, Barletta A, Goglia F. 3,5-Diiodo-l-thyronine, by modulating mitochondrial functions, reverses hepatic fat accumulation in rats fed a high-fat diet. J Hepatol. 2009;51:363–370. doi: 10.1016/j.jhep.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 146.Jonas W, Lietzow J, Wohlgemuth F, Hoefig CS, Wiedmer P, Schweizer U, Kohrle J, Schurmann A. 3,5-Diiodol-l-thyronine (3,5-t2) exerts thyromimetic effects on hypothalamus-pituitary-thyroid axis, body composition, and energy metabolism in male diet-induced obese mice. Endocrinology. 2015;156:389–399. doi: 10.1210/en.2014-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Padron AS, Neto RA, Pantaleao TU, de Souza dos Santos MC, Araujo RL, de Andrade BM, da Silva Leandro M, de Castro JP, Ferreira AC, de Carvalho DP. Administration of 3,5-diiodothyronine (3,5-T2) causes central hypothyroidism and stimulates thyroid-sensitive tissues. J Endocrinol. 2014;221:415–427. doi: 10.1530/JOE-13-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Antonelli A, Fallahi P, Ferrari SM, Di Domenicantonio A, Moreno M, Lanni A, Goglia F. 3,5-Diiodo-l-thyronine increases resting metabolic rate and reduces body weight without undesirable side effects. J Biol Regul Homeost Agents. 2011;25:655–660. [PubMed] [Google Scholar]
- 149.Silvestri E, Glinni D, Cioffi F, Moreno M, Lombardi A, de Lange P, Senese R, Ceccarelli M, Salzano AM, Scaloni A, Lanni A, et al. Metabolic effects of the iodothyronine functional analogue TRC150094 on the liver and skeletal muscle of high-fat diet fed overweight rats: an integrated proteomic study. Mol Biosyst. 2012;8:1987–2000. doi: 10.1039/c2mb25055a. [DOI] [PubMed] [Google Scholar]
- 150.Zambad SP, Munshi S, Dubey A, Gupta R, Busiello RA, Lanni A, Goglia F, Gupta RC, Chauthaiwale V, Dutt C. TRC150094 attenuates progression of nontraditional cardiovascular risk factors associated with obesity and type 2 diabetes in obese ZSF1 rats. Diabetes Metab Syndr Obes. 2011;4:5–16. doi: 10.2147/DMSOTT.S15323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.van der Valk F, Hassing C, Visser M, Thakkar P, Mohanan A, Pathak K, Dutt C, Chauthaiwale V, Ackermans M, Nederveen A, Serlie M, et al. The effect of a diiodothyronine mimetic on insulin sensitivity in male cardiometabolic patients: a double-blind randomized controlled trial. PLoS One. 2014;9:e86890. doi: 10.1371/journal.pone.0086890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Goldstein JL, Brown MS. A century of cholesterol and coronaries: from plaques to genes to statins. Cell. 2015;161:161–172. doi: 10.1016/j.cell.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 154.Reiner Z. Resistance and intolerance to statins. Nutr Metab Cardiovasc Dis. 2014;24:1057–1066. doi: 10.1016/j.numecd.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 155.Rugge JB, Bougatsos C, Chou R. Screening for and Treatment of Thyroid Dysfunction: An Evidence Review for the U.S. Preventive Services Task Force. Agency for Healthcare Research and Quality (US); Rockville, MD: 2014. Oct, Report No.: 15-05217-EF-1. [PubMed] [Google Scholar]
- 156.Cappola AR, Ladenson PW. Hypothyroidism and atherosclerosis. J Clin Endocrinol Metab. 2003;88:2438–2444. doi: 10.1210/jc.2003-030398. [DOI] [PubMed] [Google Scholar]
- 157.Galioni EF, Gofman JW, Guzvich P, Pouteau J, Rubinger JH, Strisower B. Long-term effect of dried thyroid on serum-lipoprotein and serum-cholesterol levels. Lancet. 1957;272:120–123. doi: 10.1016/s0140-6736(57)90199-x. [DOI] [PubMed] [Google Scholar]
- 158.The coronary drug project. Findings leading to further modifications of its protocol with respect to dextrothyroxine. The coronary drug project research group. JAMA. 1972;220:996–1008. [PubMed] [Google Scholar]
- 159.Tancevski I, Demetz E, Eller P, Duwensee K, Hoefer J, Heim C, Stanzl U, Wehinger A, Auer K, Karer R, Huber J, et al. The liver-selective thyromimetic T-0681 influences reverse cholesterol transport and atherosclerosis development in mice. PLoS One. 2010;5:e8722. doi: 10.1371/journal.pone.0008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kannisto K, Rehnmark S, Slatis K, Webb P, Larsson L, Gafvels M, Eggertsen G, Parini P. The thyroid receptor beta modulator GC-1 reduces atherosclerosis in ApoE deficient mice. Atherosclerosis. 2014;237:544–554. doi: 10.1016/j.atherosclerosis.2014.09.035. [DOI] [PubMed] [Google Scholar]
- 161.Lin JZ, Martagon AJ, Hsueh WA, Baxter JD, Gustafsson JA, Webb P, Phillips KJ. Thyroid hormone receptor agonists reduce serum cholesterol independent of the LDL receptor. Endocrinology. 2012;153:6136–6144. doi: 10.1210/en.2011-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grover GJ, Mellstrom K, Malm J. Development of the thyroid hormone receptor beta-subtype agonist KB-141: a strategy for body weight reduction and lipid lowering with minimal cardiac side effects. Cardiovasc Drug Rev. 2005;23:133–148. doi: 10.1111/j.1527-3466.2005.tb00161.x. [DOI] [PubMed] [Google Scholar]
- 163.Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, Klein I, Baxter JD, Angelin B. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med. 2010;362:906–916. doi: 10.1056/NEJMoa0905633. [DOI] [PubMed] [Google Scholar]
- 164.Ito BR, Zhang BH, Cable EE, Song X, Fujitaki JM, MacKenna DA, Wilker CE, Chi B, van Poelje PD, Linemeyer DL, Erion MD. Thyroid hormone beta receptor activation has additive cholesterol lowering activity in combination with atorvastatin in rabbits, dogs and monkeys. Br J Pharmacol. 2009;156:454–465. doi: 10.1111/j.1750-3639.2009.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tancevski I, Demetz E, Eller P. Sobetirome: a selective thyromimetic for the treatment of dyslipidemia. Recent Pat Cardiovasc Drug Discov. 2011;6:16–19. doi: 10.2174/157489011794578473. [DOI] [PubMed] [Google Scholar]
- 166.Sjouke B, Langslet G, Ceska R, Nicholls SJ, Nissen SE, Ohlander M, Ladenson PW, Olsson AG, Hovingh GK, Kastelein JJ. Eprotirome in patients with familial hypercholesterolaemia (the AKKA trial): a randomised, double-blind, placebo-controlled phase 3 study. Lancet Diabetes Endocrinol. 2014;2:455–463. doi: 10.1016/S2213-8587(14)70006-3. [DOI] [PubMed] [Google Scholar]
- 167.Taub R, Chiang E, Chabot-Blanchet M, Kelly MJ, Reeves RA, Guertin MC, Tardif JC. Lipid lowering in healthy volunteers treated with multiple doses of MGL-3196, a liver-targeted thyroid hormone receptor-beta agonist. Atherosclerosis. 2013;230:373–380. doi: 10.1016/j.atherosclerosis.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 168.Lian B, Hanley R, Schoenfeld S. A phase 1 randomized, double-blind, placebo-controlled, multiple ascending dose study to evaluate safety, tolerability and pharmacokinetics of the liver-selective TR-beta agonist VK2809 (MB07811) in hypercholesterolemic subjects. J Am Coll Cardiol. 2016;67:1932. [Google Scholar]
- 169.Brenta G, Schnitman M, Fretes O, Facco E, Gurfinkel M, Damilano S, Pacenza N, Blanco A, Gonzalez E, Pisarev MA. Comparative efficacy and side effects of the treatment of euthyroid goiter with levo-thyroxine or triiodothyroacetic acid. J Clin Endocrinol Metab. 2003;88:5287–5292. doi: 10.1210/jc.2003-030095. [DOI] [PubMed] [Google Scholar]
- 170.Goldman S, McCarren M, Morkin E, Ladenson PW, Edson R, Warren S, Ohm J, Thai H, Churby L, Barnhill J, O’Brien T, et al. DITPA (3,5-diiodothyropropionic acid), a thyroid hormone analog to treat heart failure: phase II trial veterans affairs cooperative study. Circulation. 2009;119:3093–3100. doi: 10.1161/CIRCULATIONAHA.108.834424. [DOI] [PubMed] [Google Scholar]
- 171.Ladenson PW, McCarren M, Morkin E, Edson RG, Shih MC, Warren SR, Barnhill JG, Churby L, Thai H, O’Brien T, Anand I, et al. Effects of the thyromimetic agent diiodothyropropionic acid on body weight, body mass index, and serum lipoproteins: a pilot prospective, randomized, controlled study. J Clin Endocrinol Metab. 2010;95:1349–1354. doi: 10.1210/jc.2009-1209. [DOI] [PubMed] [Google Scholar]
- 172.Selen Alpergin ES, Bolandnazar Z, Sabatini M, Rogowski M, Chiellini G, Zucchi R, Assadi-Porter FM. Metabolic profiling reveals reprogramming of lipid metabolic pathways in treatment of polycystic ovary syndrome with 3-iodothyronamine. Physiol Rep. 2017;5(1):e13097. doi: 10.14814/phy2.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]