Direct interactions with influenza promote bacterial adherence during respiratory infections (original) (raw)
. Author manuscript; available in PMC: 2020 Aug 1.
Published in final edited form as: Nat Microbiol. 2019 May 20;4(8):1328–1336. doi: 10.1038/s41564-019-0447-0
Abstract
Epidemiological observations and animal models have long shown synergy between influenza virus infections and bacterial infections. Influenza virus infection leads to an increase in both the susceptibility to secondary bacterial infections and the severity of the bacterial infections, primarily pneumonias caused by Streptococcus pneumoniae or Staphylococcus aureus. We show that, in addition to the widely described immune modulation and tissue-remodelling mechanisms of bacterial–viral synergy, the virus interacts directly with the bacterial surface. Similar to the recent observation of direct interactions between enteric bacteria and enteric viruses, we observed a direct interaction between influenza virus on the surface of Gram-positive, S. pneumoniae and S. aureus, and Gram-negative, Moraxella catarrhalis and non-typeable Haemophilus influenzae, bacterial colonizers and pathogens in the respiratory tract. Pre-incubation of influenza virus with bacteria, followed by the removal of unbound virus, increased bacterial adherence to respiratory epithelial cells in culture. This result was recapitulated in vivo, with higher bacterial burdens in murine tissues when infected with pneumococci pre-incubated with influenza virus versus control bacteria without virus. These observations support an additional mechanism of bacteria–influenza virus synergy at the earliest steps of pathogenesis.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
It is becoming increasingly apparent that interkingdom interactions between bacteria and viruses play critical roles at the host–pathogen interface. Many of the described interactions derive from studies of the intestinal microbiome. The enteric bacterial microbiome functions in the efficient replication and pathogenesis of enteric viruses1. The enteric virus poliovirus binds directly to multiple bacterial species, which in turn facilitates a more robust viral infection of host cells and mediates more efficient viral recombination2. The direct binding of bacterial surface polysaccharides can enhance the stability of enteric virions and increase viral attachment to host receptors3. In addition, reovirus interactions with bacteria enhance the thermal stability of the virus4. These interactions are complex and involve intricate interactions among the resident bacterial flora, enteric viruses and the immune responses of the mammalian host to the various microbial components5,6. A number of other enteric viruses have evolved strategies to utilize products of the host microbiota to promote viral transmission or infection7. These strategies underscore the mechanisms developed by both viruses and bacteria to exploit cross-kingdom interactions to their mutual benefit. Understanding the mechanisms underlying these interactions is becoming increasingly important not only for enteric pathogens but also for those of other host niches, such as the respiratory tract.
Viral coinfection is a well-established risk factor for subsequent bacterial pneumonia. Streptococcus pneumoniae and human influenza viruses show similar synergy in terms of exacerbating the morbidity and mortality of pneumonia. This has been observed both clinically and in murine models of infection8–10. The underlying mechanisms of this synergy are complex and multifactorial, with bacterial, viral and host immune factors enhancing susceptibility to infection11. One feature of this lethal combination is the temporal nature of the interaction leading to bacterial superinfection. The maximal increase in susceptibility to bacterial infection typically occurs days after viral infection and coincides with the depletion of pulmonary macrophages12. Even with attenuated viruses, multiple days of viral coinfection are usually required for bacterial translocation beyond colonization13. These data clearly establish the viral kinetics that drive invasive bacterial infection and that previous influenza infection is a primary driver of secondary bacterial pneumonia.
Although studies in this area remain somewhat limited, there are multiple examples of direct binding of respiratory viruses to the bacterial surface to mediate enhanced binding and pathogenesis. For S. pneumoniae, the best examples are the interactions of this bacterium with respiratory syncytial virus (RSV). Direct binding of RSV to the pneumococcal surface via penicillin-binding protein 1a enhances bacterial adherence to the epithelial surface and mediates disease with increased severity in murine models of infection14,15. This synergism is most pronounced following simultaneous administration, indicating that RSV is a direct coupling partner in the enhanced binding to respiratory cells15. These interactions with RSV most probably extend to additional bacterial pathogens. Expression of the RSV glycoprotein enhances epithelial binding by both S. pneumoniae and Haemophilus influenzae, indicating that this may be a generalizable strategy for respiratory pathogens16. RSV also mediates the binding of Pseudomonas aeruginosa to epithelial cells from healthy controls and patients with cystic fibrosis17. These studies indicate that pathogenic respiratory viruses can directly interact with multiple, diverse bacterial pathogens to confer fitness benefits during interactions with the host epithelium.
More limited data are available for respiratory viruses, other than RSV, that function by directly binding to the bacterial surface. For example, swine influenza viruses recognize the α2,6-linked sialic acid in the capsular polysaccharide of Streptococcus suis and mediate more robust bacterial adherence18,19. Thus, the interkingdom interplay between respiratory pathogens represents a potentially underappreciated mechanism underlying viral–bacterial synergy during respiratory infection. Here we describe the capacity of influenza viruses to directly bind to S. pneumoniae and other respiratory pathogens, thereby mediating an enhancement of bacterial interaction with host respiratory cells in vitro and enhancing pneumococcal colonization and invasive disease in vivo. These data indicate that direct interactions between influenza and bacterial respiratory pathogens may engender a fitness benefit during transmission and colonization of the mammalian respiratory tract.
Results
Influenza virus binds directly to S. pneumoniae.
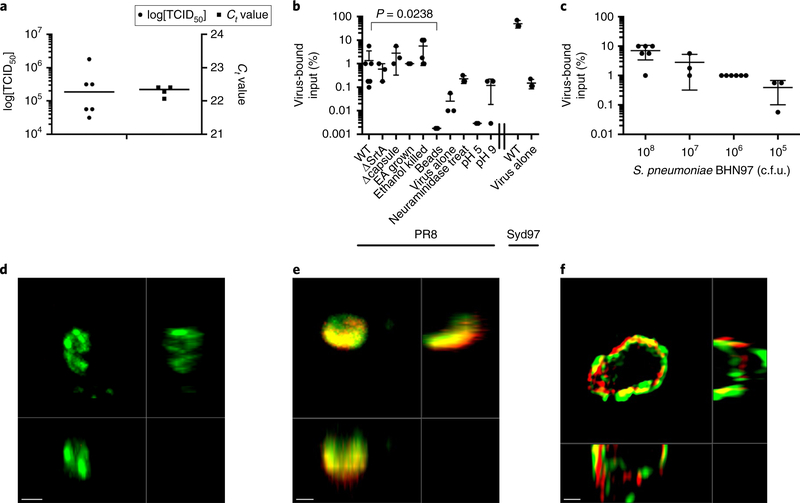
Although it is becoming increasingly understood that enteric viruses interact directly with multiple enteric bacterial species, how these findings apply to viral-bacterial interactions in other host niches remains unknown. Based on extensive research showing synergy between the influenza virus and S. pneumoniae, we initially sought to determine whether these two pathogens interact directly. Bacterial cultures were allowed to mix with purified influenza virus for 30 min followed by bacterial recovery by low-speed centrifugation to harvest bacterial cells and any bound virus. Viable influenza virus co-sedimented with S. pneumoniae was measured by 50% tissue culture infectious dose (TCID50) and the presence of viral RNA was measured by PCR with reverse transcription (RT–PCR; Fig. 1a). Approximately one percent of the input infectious viral particles co-sedimented following mixing with 108 colony-forming units (c.f.u.) S. pneumoniae (Fig. 1b). Viral adhesion was independent of several bacterial factors, including capsule, sortase-anchored adhesins and choline-binding proteins (Fig. 1b). Furthermore, this interaction was independent of bacterial viability, as ethanol-fixed bacterial cells retained the capacity to co-sediment with influenza virus (Fig. 1b). In addition, influenza binding was independent of the glycosylation status of the virus, as deglycosylated virus co-sedimented at similar levels to the wild-type virus as measured by quantitative RT–PCR (Supplementary Fig. 1). Sialation of the pneumococci was not necessary for interaction with influenza A virus (IAV), as pneumococci treated with neuraminiadase also co-sedimented viable virus (Fig. 1b). Co-sedimentation was optimal at physiological pH, as reactions carried out at pH 5 and pH 9 co-sedimented less viable virus (Fig. 1b). These data indicate that IAV may directly bind to S. pneumoniae. Co-sedimentation was specific, as virus incubated without bacteria and virus pre-incubated with stainless steel beads of a similar size (~1 μm) did not efficiently co-sediment IAV (Fig. 1b). This interaction was not restricted to the A/Puerto Rico/8/1934 (PR8) H1N1 strain, as A/Sydney/05/1997 (H3N2) also associated based on in vitro co-sedimentation experiments (Fig. 1b). As we reduced the amount of bacteria in the reaction while maintaining the viral concentration constant, we saw a dose-dependent decrease in the amount of viable co-sedimented IAV (Fig. 1c). These data indicate that influenza virus may interact directly with S. pneumoniae.
Fig. 1 |. Influenza co-sediments with S. pneumoniae and is bound to the bacterial surface.
a, The amount of the influenza virus strain PR8 that co-sedimented with wild-type pneumococcus strain BHN97 (serotype 19F) was quantified by qRT–PCR and TCID50. b, Percentage of influenza virus strains PR8 and A/Sydney/5/1997 (Syd97) that co-sedimented with S. pneumoniae wild-type (WT) and mutant (ΔStrA and Δcapsule) BHN97 strains (BHN97 grown in CY supplemented with ethanolamine (EA) instead of choline to remove choline binding proteins) killed pneumococci, pneumococci treated with neuraminidase or when bound at different pHs. Mann-Whitney tests were used for statistical comparisons; the P value comparing binding of PR8 to WT with binding to inert beads is shown. c, Percentage of virus that co-sedimented with varying amounts of added BHN97. The data represent the mean ± s.d. from 3–6 biological replicates. d-f, Representative images of S. pneumoniae without virus (d; confocal microscopy), the _S. pneumoniae_-IAV complex (e; confocal microscopy) and pneumococcus (green) with influenza virus (red) bound to the bacterial cell surface (f; structured illumination microscopy). The images are each representative of at least five independent fields of view from at least two biological replicates. Scale bars, 1 μm (d,e) and 0.5 μm (f).
We next sought to determine whether this putative association between influenza virus and pneumococci could be confirmed by visualization of influenza virus on the bacterial surface using both confocal and super-resolution microscopy. To visualize influenza viruses, we utilized mRuby2–HA–PR8 H1N1 virus20, which expresses mRuby2 fluorescent protein on the viral envelope, allowing the direct visualization of virions by fluorescence microcopy. The virus was incubated with cultures of S. pneumoniae, and the cells were subsequently harvested by centrifugation, counterstained and fixed. The bacterial surface was enriched in influenza viral particles, with the virus adhering to multiple sites on the pneumococcal surface (Fig. 1e,f and Supplementary Video 1). These data confirm the initial observation that influenza virus may bind directly to the surface of S. pneumoniae.
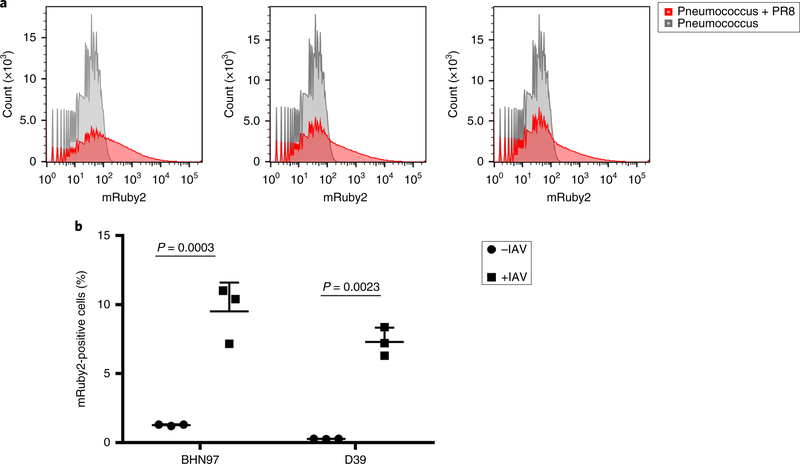
We then sought to obtain a quantitative measure of the magnitude of influenza binding to the pneumococcal surface. As our microscopy images indicated that multiple virions were localized on single bacterial cells, whereas other bacterial cells had no associated virus particles, we examined the population by flow cytometry, again using PR8 expressing mRuby2–HA. Following incubation with influenza virus, the bacterial cells demonstrated a significant shift in fluorescence (Fig. 2a). When these populations were quantitated, we observed approximately 6–10% of the bacterial cells stained positive for virus for both the BHN97 and D39 strains of S. pneumoniae (Fig. 2b). These data further support our hypothesis that influenza can directly bind to the surface of the pneumococcus.
Fig. 2 |. Quatification of binding by flow cytometry.
a, Representative flow plots from three biological replicates of pneumococci alone or mixed with mRuby2–HA–PR8. b, Percentage of the BHN97 and D39 strains associated with mRuby2–HA–PR8. The data represent the mean ± s.d. from three biological replicates. The P values were determined by unpaired Student’s _t_-tests comparing the same strain with or without virus. See Supplementary Fig. 3 for gating strategy.
Surface-bound influenza enhances the adherence of S. pneumoniae.
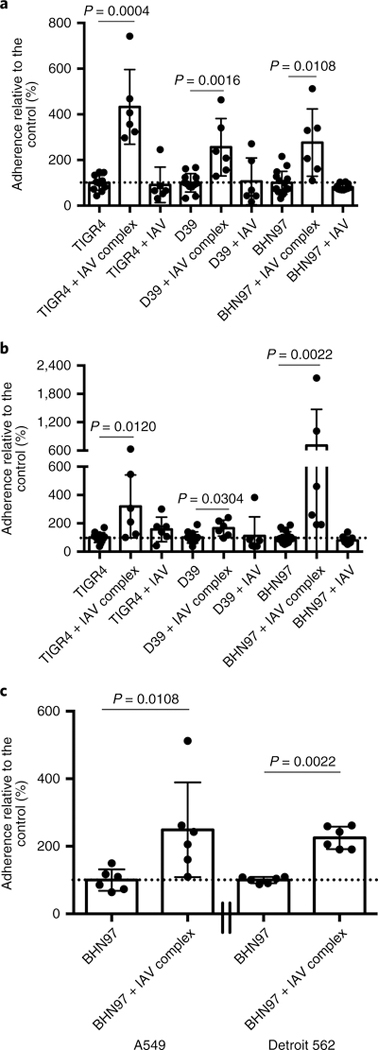
We next sought to determine the biological consequences of influenza bound to the surface of S. pneumoniae. Influenza virus and S. pneumoniae share many tissue tropisms, including the upper and lower respiratory tract, where both pathogens require intimate adherence to host cells to mediate infection. These pathogens use distinct, non-overlapping host receptors for adherence21; thus, we hypothesized that surface-bound influenza would enhance bacterial adherence to host cells. The adherence of three different strains of pneumococci from different serotypes and genetic backgrounds was significantly enhanced in A549 lung epithelial cells (Fig. 3a) and Detroit 562 nasopharyngeal cells (Fig. 3b) when influenza A/California/4/2009 was bound to the bacterial surface. This result indicated that the enhanced adherence was not specific to the pneumococcal genetic background or serotype. Binding of D39 was more enhanced by influenza virus bound to A549 cells and BHN97 was more enhanced by influenza virus bound to Detroit 562 cells, perhaps reflecting the predominance of pneumonia caused in murine models by D39 and colonization/sinusitis caused by BHN97 in murine models. Cultures with equivalent doses of bacteria and IAV as in the complexes, but without pre-incubation, showed no significant alterations in pneumococcal adherence, indicating the virus–bacteria interaction facilitates this phenotype (Fig. 3a,b). The increased adherence was not specific to the viral subtype, as the PR8 virus also mediated enhanced pneumococcal adherence to both lung and nasopharyngeal cell lines (Fig. 3c). These data indicate that influenza virus bound to the pneumococcal surface enhances bacterial adherence to respiratory epithelial tissues.
Fig. 3 |. Surface-bound influenza enhances the adherence of S. pneumoniae.
a,b, Relative pneumococcal adherence by the strains TIGR4 (serotype 4), D39 (serotype 2) and BHN97 (serotype 19F) to A549 (a) and Detroit 562 (b) cells with S. pneumoniae alone, S. pneumoniae pre-incubated with A/California/4/2009 influenza virus (+ IAV complex) and S. pneumoniae simultaneously administered with influenza virus (+ IAV). c, Relative pneumococcal adherence by strain BHN97 (serotype 19F) plus PR8 to A549 or Detroit 562 cells. Dotted lines indicate 100% adherence. The data represent the mean ± s.d. from at least three independent experiments with triplicate technical replicates in each experiment. The P values were determined by Mann-Whitney tests with pairwise comparisons to the control/no virus samples.
Influenza binds to the surface of multiple bacterial respiratory pathogens.
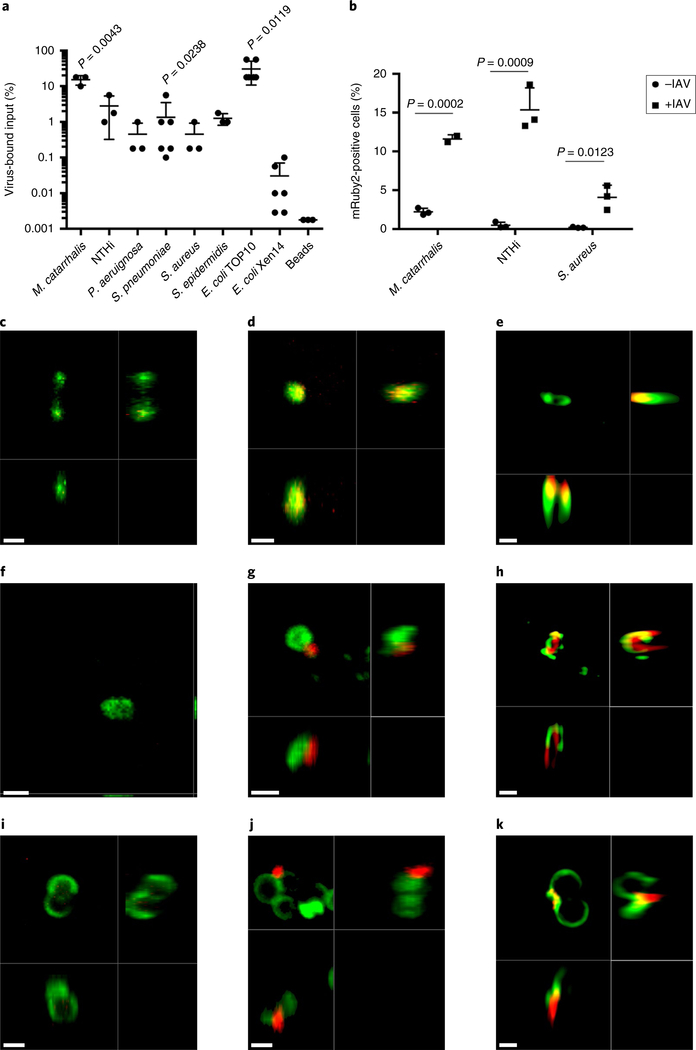
Influenza and other respiratory viruses enhance the pathogenesis of multiple bacterial species, including non-typeable H. influenzae (NTHi), Moraxella catarrhalis and Staphylococcus aureus, during coinfection11,22. These pathogens reside in the same environmental niche and often co-colonize the same individual23. To determine the species specificity of the influenza–bacterial adherence, we tested the capacity of a panel of bacterial pathogens to bind infectious virus particles by TCID50 (Fig. 4a). Influenza virus bound to the Gram-positive species S. aureus and Staphylococcus epidermidis with similar efficiencies. The relative quantities of influenza that bound to Gram-negative species was considerably more variable, with M. catarrhalis displaying the highest level of influenza binding, NTHi demonstrating intermediate binding and P. aeruginosa displaying a lower level of binding. No binding was observed to Escherichia coli Xen14, a bioluminescent version of strain WS2572, but binding to E. coli TOP10 was observed. No significant viral binding to inert steel beads was observed and no detectable virus was recovered from centrifugation alone, indicating that the interaction between the respective pathogens and IAV was specific. These data indicate that influenza can co-sediment with multiple bacterial respiratory pathogens to varying degrees.
Fig. 4 |. Influenza binds to the surface of multiple Gram-positive and Gram-negative bacterial respiratory pathogens.
a,b, Amount of IAV strain PR8 that co-sedimented with representative Gram-positive and Gram-negative bacterial species of the respiratory tract measured by TCID50 (a) or flow cytometry (b). The data represent the mean ± s.d. from 3–6 biological replicates. The P values were determined by Mann–Whitney test comparisons to binding to inert beads in a and unpaired Student’s _t_-tests comparing the same strain with and without virus in b. c-k, Representative confocal (c,d,f,g,i,j) and structured illumination (e,h,k) microscopy images of bacteria (green) with influenza virus (red) localized on the bacterial surface of M. catarrhalis (c–e), NTHi (f–h) and S. aureus (i–k). No virus was added to c,f,i. For the NTHi images, the red channel was cut off at median intensity of unstained NTHi to account for autofluorescence and MitoDeepRed was falsely coloured as green to match the WGA-488 staining of other species. The images are each representative of at least five independent fields of view from at least two biological replicates. Scale bars, 1 μm (c,d,f,g,i,j) and 0.5 μm (e,h,k).
We next examined the proportion of NTHi, S. aureus and M. catarrhalis that had bound IAV. We utilized PR8 expressing mRuby2–HA and flow cytometric analysis to demonstrate the varying binding capacities of these species, ranging from 5 to 15% bacteria positive for virus (Fig. 4b). To corroborate these findings, we undertook confocal and super-resolution microscopy to visualize whether influenza localized on the surface of the bacterial pathogens in a similar manner to what was observed with S. pneumoniae. Influenza virus was observed on the bacterial surface of all of the pathogens investigated (Fig. 4c–k and Supplementary Videos 2–4), similar to S. pneumoniae. These data indicate that influenza virus can bind to multiple bacterial pathogens that frequently colonize the human respiratory tract.
Surface-bound influenza virus enhances adherence of respiratory bacterial pathogens.
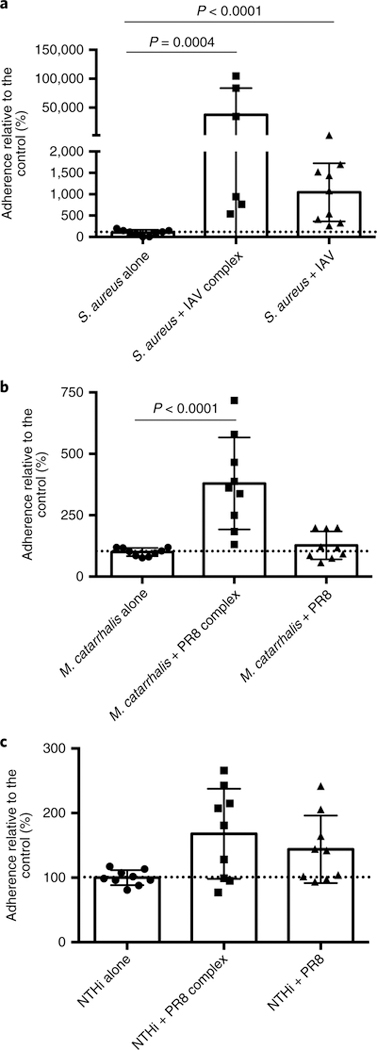
We next sought to determine whether adherent influenza virus would facilitate increased adhesion of these pathogens to human respiratory cells in a manner reminiscent of what was observed with S. pneumoniae. Influenza A/California/4/2009 virus bound to the surface of S. aureus dramatically enhanced the level of adhesion compared with that of the controls (Fig. 5a). Co-administration of influenza virus with S. aureus also increased adherence to Detroit 562 cells but to a lesser extent compared with the surface-bound influenza virus. A similar pattern was observed with M. catarrhalis, with significantly enhanced bacterial binding in the influenza-surface-bound groups compared with either the influenza-negative or coinfected controls (Fig. 5b). No significant enhancement was observed with NTHi (Fig. 5c), although the data trended towards enhanced adherence when influenza virus was bound to the surface or used in the context of coinfection. These data indicate that influenza-bound bacteria demonstrate various degrees of enhanced adherence to respiratory cells in a species-specific manner.
Fig. 5 |. Surface-bound influenza enhances the adherence of multiple bacterial species to mammalian respiratory cells.
a–c, Relative adherence of the bacteria S. aureus (a), M. catarrhalis (b) and NTHi (c)—alone, pre-incubated with A/California/4/2009 influenza virus (strain + IAV complex) or simultaneously administered with influenza (strain + IAV)—to Detroit 562 cells. Dotted lines indicate 100% adherence. The data represent the mean ± s.d. from at least three independent experiments with triplicate technical replicates in each experiment. The P values were determined by Mann–Whitney pairwise comparisons to the control with no virus added.
Surface-bound influenza enhances the initial in vivo fitness of S. pneumoniae.
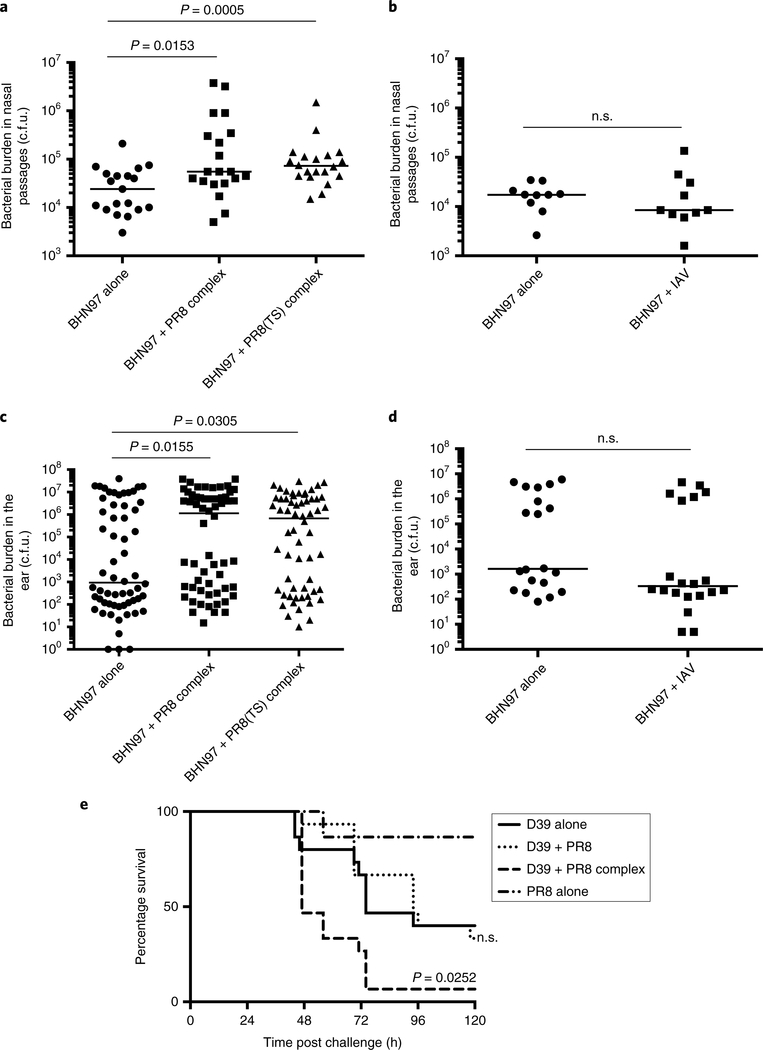
As the in vitro data indicated that influenza virus bound to the surface of S. pneumoniae facilitates a more robust bacterial adherence, we next sought to ascertain whether this enhancement would be conferred during in vivo infection. The pneumococcal strain BHN97 (serotype 19F) was pre-incubated with either wild-type or temperature-sensitive PR8 influenza virus24, and bacteria and adherent virus were instilled into the nasal passages of mice. The mice were euthanized at 24 h post challenge, and the bacterial burdens of the nasal passages and middle ears were determined. The bacterial burden was significantly enhanced in the nasal passages when BHN97 was pre-incubated with either strain of the PR8 virus (Fig. 6a). This enhancement was not observed when equivalent doses of BHN97 and PR8 were instilled into the nasal passages without pre-mixing (Fig. 6b), suggesting the enhancement comes from the bacteria–virus complex and not merely due to the simultaneous infection of two pathogens. In traditional coinfection experiments where the virus is administered before bacterial infection, no significant enhancement of disease was noted at these early time points but instead at multiple days after viral infection12. Influenza-bound S. pneumoniae also significantly enhanced the bacterial burden in the middle ear, indicating that the influenza–pneumococcus interaction may facilitate translocation to the ear, resulting in acute otitis media (Fig. 6c). Enhancement of dissemination was not seen when mice were coinfected with equivalent doses of BHN97 and PR8 without pre-mixing (Fig. 6d), again suggesting dissemination enhancement results from the bacteria–virus complex and not a combination of each pathogen. The bacterial burdens of the ear were highly distributed, as is typical for this model13,25, with some mice having more severe otitis in one ear and some with high burdens in both ears. These data indicate the fitness benefit conferred by influenza bound to the surface of S. pneumoniae extends to the initial adherence during nasal colonization and acute otitis media. Similar results were seen with wild-type and temperature-sensitive PR8-virus infection in both tissues. The viral burdens in the ears and nasal passages were not enriched 24 h post infection when the mice were infected with bacteria-virus complexes versus IAV alone (Supplementary Fig. 2).
Fig. 6 |. Surface-bound influenza enhances the initial colonization of nasal passages and translocation into the middle ear, and lethal disease.
a–d, Bacterial titres of the pneumococcal strain BHN97 (serotype 19F) in the nasal passages (a,b) or ears (c,d) of mice 24 h post-infection with bacteria alone, bacteria co-incubated with wild-type or temperature-sensitive (TS) PR8 (BHN97 + PR8 complex), and bacteria with PR8 without pre-mixing (BHN97 + PR8). Each point represents one sample and the bar is the median (n = 19 or 20 nasal passages per group for a, 10 nasal passages per group for b, 30 animals (60 ears) per group in c and 10 animals (20 ears) per group in d). The P values were determined by Mann–Whitney comparisons to bacteria alone. e, Survival following lethal challenge with D39, D39 premixed with PR8 (D39 + PR8 complex), D39 with PR8 without pre-mixing (D39 + PR8) and PR8 alone (n = 15 animals per group). The P values were determined by Mantel–Cox comparisons to D39 alone. n.s., not significant.
To explore the role of the pneumococcus–IAV complex in invasive disease, we infected mice with S. pneumoniae strain D39, D39 pre-incubated with PR8, D39 and PR8 together without pre-incubation and PR8 alone. The mice were monitored daily for symptoms of disease and blood titres were taken by tail clip at 24 and 48 h post infection to monitor bacterial dissemination into the blood. The mice infected with the D39–IAV complex exhibited significantly higher mortality than mice infected with bacteria alone or with bacteria and virus that was not pre-complexed (Fig. 6e). These mice did not exhibit higher bacterial burdens in the blood at 24 or 48 h post infection, suggesting that the enhancement in disease is localized to the lungs or is inflammatory mediated. This differs from pneumococci–IAV coinfection models where an enhancement of bacteraemia is clinically observed in coinfected animals10,26–28. Together, these data support the hypothesis that the pneumococcus–IAV complex significantly enhances the virulence of S. pneumoniae in multiple host niches.
Discussion
We observed various degrees of influenza virus binding to a number of prominent respiratory pathogens, including Gram-negative and Gram-positive bacteria. This interaction enhanced the adherence properties of the bacterial species, indicating that the bound virus provided a fitness benefit during the initial interaction of the bacteria with the host respiratory cells. This result suggests that having virus bound to the bacterial surface enables the bacterium to exploit host receptors specific for both the virus and the bacterium during cell adhesion. Furthermore, these data suggest that as the influenza virus is released from host cells during replication, it may serve as an important ligand to mediate enhanced bacterial adherence to the infected epithelial tissues. This has been previously observed in vitro for multiple bacterial pathogens, including S. pneumoniae, NTHi, M. catarrhalis and S. aureus29. This mechanism is not likely to extend to all bacterial species as bacterial species from the gastrointestinal tract may destabilize influenza virus30. As such, we suspect the interactions between influenza and bacterial species from the respiratory or gastrointestinal tract are likely to be distinct.
Attachment of influenza virus to the bacterial surface may also facilitate bacterial adherence that is more effective through the proximity of infectious viral particles with active neuraminidase activity. The neuraminidase activity of influenza virus facilitates the cleavage of host sialic acids, thereby providing a nutrient source for S. pneumoniae to facilitate bacterial outgrowth31. In addition, viral neuraminidase activity may degrade mucus and other host glycans, increasing bacterial access to the epithelial surface and availability of receptors for bacterial binding. This may be beneficial for some bacterial species but detrimental for others, as the viral neuraminidase could degrade host glycoproteins that serve as receptors for the bacteria. This may partially explain the species-specific nature of the increased bacterial adherence when influenza virus was bound at the bacterial surface.
The bacterial species we observed binding to influenza are typically considered opportunistic pathogens, existing mostly as nasal commensals but having the capacity to cause disease in various host niches. An additional consequence of the direct interactions could come when an individual colonized with one of these species becomes infected with influenza virus. In this case the incoming virus particles could associate with the colonizing bacteria and facilitate bacterial dissemination to the middle ears or lungs. This could partially explain both clinical and experimental-model observations describing the roles of respiratory viruses in mediating bacterial translocation to the middle ear and other host tissues32–34. The degree to which such bacteria may facilitate viral translocation into the middle ear is unknown, although colonization with S. pneumoniae followed by IAV challenge results in enhanced inflammatory infiltrate in the middle ear compared with either pathogen alone35. As such, there is a possibility that IAV-bacteria complexes with otopathogens may facilitate viral translocation into the middle ear. Reduced binding of IAV to bacteria at a low pH might be relevant, as the pH of the external auditory canal is 5.4 in healthy ears but increases during acute otitis externa36, suggesting that IAV would not infect healthy ear tissues but could raise the pH in the context of bacterial infection.
This intimate association of influenza virus with various respiratory bacterial species could potentially benefit both the bacteria and the virus. For one, essentially ‘hitching a ride’ during transmission could prove to be mutually beneficial in terms of extracellular environmental stability and mediating effective transmission by increasing initial adherence at the epithelium of the new host. This is reflected in multiple reports that influenza coinfection enhances the transmission of S. pneumoniae37–39. These interactions may also play an important role in host immune responses when coinfecting organisms are present. Direct interactions of influenza and these respiratory bacterial pathogens may also have important consequences with regard to immune recognition and responses if a single antigen-presenting cell can express antigens from both pathogens to enhance stimulation of T-cell responses. These data underscore the importance of studying pathogenesis in the context of complex systems to learn how such intraspecies interactions facilitate disease progression.
Methods
Ethics statement.
All experiments involving animals were performed with the approval of and in accordance with the guidelines of the St. Jude Animal Care and Use Committee. The St. Jude laboratory animal facilities have been fully accredited by the American Association for Accreditation of Laboratory Animal Care. Laboratory animals were maintained in accordance with the applicable portions of the Animal Welfare Act and the guidelines prescribed in the DHHS publication Guide for the Care and Use of Laboratory Animals.
Bacterial and viral strains and growth conditions.
The S. pneumoniae strains BHN97 (serotype 19F), D39 (serotype 2) and TIGR4 (serotype 4) were inoculated onto tryptic soy agar (TSA) plates supplemented with 3% sheep blood and 20 μg ml−1 neomycin and then grown overnight at 37 °C in a 5% CO2 humidified incubator. The strains were then inoculated directly into Todd Hewitt broth supplemented with 0.2% yeast extract (ThyB) or CY media and grown to the log phase for use in experiments. A sortase mutant was made from BHN97 genomic DNA, with splicing by overlap-extension PCR to make an erythromycin replacement using the following primers: BHN97 SP1218 up F, GGCCTCCTCCGATAAAGTTTCC; BHN97 SP1218 up erm R, GAGTCGCTTTTGTAAATTTGGTCAATCAAC CATATAAACAATTTTATTAATACAAATCA; BHN97 SP1218 down erm F, GTTTGCTTCTAAGTCTTATTTCCTTATGCTTCACCTTCTGTTTCGTTTTC; BHN97 SP1218 down R, CAAAGACGAATTGGATGAAGTTAAACG; Erm F, GGAAATAAGACTTAGAAGCAAAC and Erm R, CCAAATTTACAAAA GCGACTC. A capsule mutant was generated by transforming SPNY001 genomic DNA containing a Sweet Janus cassette that replaces the capsule locus40 into strain BHN97 and confirmed by the lack of agglutination of either pool B or P (Statens Serum Institute) for the loss of the 19F capsule. Ethanolamine-adapted BHN97 bacteria were grown in CY media supplemented with ethanolamine instead of choline. The NTHi 86–028NP strain, originally isolated from a patient with chronic otitis media, was grown on chocolate agar supplemented with 11,000 U l−1 bacitracin and then directly inoculated into brain heart infusion broth supplemented with 0.2% yeast extract, 10 μg ml−1 hemin and 10 μg ml−1 NAD and grown with aeration to the mid-log phase41. S. aureus strain USA400, S. epidermidis strain M23864:W2 (ATCC), P. aeruginosa Xen41 (PerkinElmer) and M. catarrhalis42 were grown on unsupplemented TSA plates, directly inoculated in brain heart infusion broth supplemented with 0.2% yeast extract and grown with aeration to the mid-log phase for use in experiments. The E. coli strains TOP10 (Invitrogen One Shot) and Xen14 (PerkinElmer) were grown in unsupplemented LB medium with aeration until the mid-log phase for use in experiments.
The influenza A virus PR8 and a temperature-sensitive derivative24 were grown in Madin-Darby canine kidney (MDCK) cells. The A/California/4/2009, A/Sydney/5/1997 (H3N2) and mRuby2–HA PR8 (ref. 20) viruses were grown embryonated chicken eggs on embryonic day 10 or 11.
PR8 was deglycosylated with 500 U ml−1 PNGaseF for 3 h at 37 °C with end-over-end rotation.
Bacteria–virus co-sedimentation.
Mid-log bacterial cultures were washed and normalized to 108 c.f.u. ml−1 in PBS. Influenza virus PR8 (3 × 107 TCID50) or A/Sydney/5/1997 (105 TCID50) was then added. Ethanol-fixed pneumococci were prepared by resuspending 108 c.f.u. BHN97 in 1 ml ice-cold 70% ethanol for 5 min on ice. The cells were pelleted, the supernatant was removed and the pellets were dried at 55 °C for 5 min to remove residual ethanol. Death was confirmed by plating on TSA–blood agar. Control samples without bacteria had only virus added and the protocol was followed as above. Stainless-steel beads of a 1 μm average diameter (Cospheric SSMS-7.8) were resuspended in PBS at a concentration of 10% w/v and 5 μl was added to each reaction to correspond to an equal surface area of 108 c.f.u. pneumococci. PBS was adjusted to pH 5 or pH 9 with HCl or NaOH, respectively. Neuraminidase pre-treatment of bacteria was done with 1 μl Vibrio cholerae neuraminidase (Sigma, N7885–2UN) per reaction in PBS adjusted to pH 6 with HCl. The bacterial–viral mixtures were incubated at 37 °C with end-over-end rotation for 30 min. The samples were then centrifuged for 3 min at 10,000_g_ in a microcentrifuge to pellet bacteria and adherent virus. The pellets were washed in PBS and resuspended in PBS for further analysis. For viral quantification, the pellets were instead resuspended in 100 μl 1 × Pen/Strep and frozen at −80 °C. Viral titres were determined by TCID50 on MDCK cells43. Briefly, MDCK cells were infected with 100 μl tenfold serial dilutions of the sample and incubated at 37 °C for 72 h. Following incubation, the viral titres were determined by a haemagglutination assay using 0.5% turkey red blood cells and analysed using the method described by Reed and Munch44.
Flow cytometric analysis.
Samples were prepared as per the co-sedimentation assay, except mRuby2–HA–PR8 virus20 was used. After the final wash, the samples were resuspended in 100 μl PBS and ultraviolet-light inactivated (3 × 1,000 μJ). Data were acquired using an LSRFortessa X-20 (BD Biosciences) and analysed using FlowJo Version 10 (Tree Star).
Confocal and super-resolution microscopy.
Samples were prepared as per the co-sedimentation assay, except mRuby2–HA–PR8 virus20 virus was used, and following the pelleting of bacteria and adherent virus, the pellets were counterstained with 1 μg ml−1 WGA-488 for S. pneumoniae, S. aureus and M. catarrhalis or 1:10,000 MitoTracker DeepRed for NTHi in PBS for 30 min at room temperature with end-over-end rotation. The samples were pelleted, washed twice with PBS, fixed for 15 min on ice in 4% paraformaldehyde, washed twice in PBS and then mounted in ProLong Diamond mountant. Mounting-media–cured samples were then imaged on a Zeiss 780 confocal or Zeiss Elyra structured illumination microscope. Three-dimensional renderings and videos were made with IMARIS.
Tissue culture adherence assays.
A549 cells were maintained in F12K media supplemented with 10% fetal bovine serum. Detroit 562 cells were maintained in modified eagle media supplemented with 15% fetal bovine serum and 0.002% lactoalbumin hydrolate. For the adherence assays, 2 × 105 cells were seeded in each well of a six-well plate 24 h before infection. The bacterial samples were prepared as for the co-sedimentation assays using influenza virus strains PR8 or A/California/4/2009, and 2 × 106 c.f.u. were added to each well in triplicate (multiplicity of infection = 10) for pneumococcus and 2 × 105 c.f.u. (multiplicity of infection = 1) were added for S. aureus, M. catarrhalis and NTHi. Bacteria-only controls were prepared as above, except no virus was added. For non-adherent controls, the wells were simultaneously infected with the bacteria and 105 TCID50 influenza virus. Cells were infected for 1 h at 37 °C and 5% CO2, washed three times with PBS, lifted with 1 ml 1 × Trypsin–EDTA and enumerated on agar plates.
Mouse challenges.
All of the mice were maintained in BSL2, specific-pathogen–free facilities. All of the experimental inoculation procedures were conducted under general anaesthesia with inhaled isofluorane at 2.5%. Pneumococcal strain BHN97 was grown and prepared as per the co-sedimentation studies, and pre-incubated with wild-type PR8 virus, temperature-sensitive PR8 virus or no virus. Following the removal of non-associated virus, bacteria were resuspended in PBS and normalized to 106 c.f.u. ml−1. Bacteria were introduced into eight-week-old female Balb/c mice (Jackson Laboratory) by intranasal administration of 105 c.f.u. bacteria in 100 μl PBS. For the infection of bacteria and virus without pre-incubation, 3.5 log TCID50 ml−1 wild-type influenza virus PR8 was added to the inoculum just before infection for a final viral dose of 2.5 log TCID50 per mouse, approximately equivalent to the dose in the bacteria–virus complex. At 24 h post challenge, the mice were euthanized by CO2 asphyxiation, decapitated, and their entire nasal passages and ears were isolated as previously described25. Tissues were homogenized in 500 μl PBS and plated on TSA–blood–Neo. The bacterial titres were compared using non-parametric Mann–Whitney tests in Prism 6.
Pneumococcal strain D39 was used for survival assays and prepared as per the co-sedimentation studies. Following the removal of non-associated virus, bacteria were resuspended in PBS at a concentration of approximately 3.3 × 108 CFU ml−1. For the infection of bacteria and virus without pre-incubation, 5.8 log TCID50 ml−1 wild-type influenza virus strain PR8 was added to the inoculum just before infection, for a final dose of 4.3 log TCID50 per mouse. Additional mice were also infected with 4.3 log TCID50 in 30 μl PBS for virus-only controls. Bacteria were introduced into eight-week-old female Balb/C mice (Jackson Laboratory) via intranasal administration of 107 c.f.u. bacteria in 30 μl PBS. The mice were monitored three times per day for signs of morbidity and euthanized when moribund. The experiment was terminated 5 d post challenge when mice infected with PR8 alone began to become moribund and death could be attributed to viral infection.
RNA isolation and viral quantification.
Tissues were processed as above and 100 μl homogenate was stored at −80 °C. The homogenate was thawed and processed with KingFisher using the MagMAX-96 AI/ND viral RNA isolation kit (Ambion, AM1835) according to the manufacturer’s instructions.
The RNA was quantified using Taqman Fast Virus according to the manufacturer’s instructions and primers (Inf A forward, 5′-GACCRATCCTGTCACCTCTGAC-3′ and Inf A reverse, 5′-AGGGCATTYTGGACAAAKCGTCTA-3′) and probe (Inf A probe (FAM, BHQ1), reverse 5′-TGCAGTCCTCGCTCACTGGGCACG-3′) to detect the influenza A M gene.
Supplementary Material
Supplemental Video 2
Supplemental Video 3
Supplemental Video 4
Supplemental Video 1
Supplementary meterial
Acknowledgements
J.W.R. is supported by the National Institutes of Allergic and Infectious Diseases (NIAID) (grant nos. 1U01AI124302 and 1RO1AI110618). S.S.-C. is supported by the NIAID (grant no. HHSN272201400006C). J.W.R. and S.S.-C. are funded by the ALSAC. Images were acquired in the Cell and Tissue Imaging Center, which is supported by St. Jude and NCI P30 CA021765.
Footnotes
Data availability
The data that support these findings are available from J. Rosch on request.
Competing interests
The authors declare no competing interests.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kuss SK et al. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 334, 249–252 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson AK et al. Bacteria facilitate enteric virus co-infection of mammalian cells and promote genetic recombination. Cell Host Microbe 23, 77–88 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson CM, Jesudhasan PR & Pfeiffer JK Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe 15, 36–46 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger AK, Yi H, Kearns DB & Mainou BA Bacteria and bacterial envelope components enhance mammalian reovirus thermostability. PLoS Pathog. 13, e1006768 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeiffer JK & Virgin HW Viral immunity. Transkingdom control of viral infection and immunity in the mammalian intestine. Science 351, aad5872 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karst SM The influence of commensal bacteria on infection with enteric viruses. Nat. Rev. Microbiol. 14, 197–204 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger AK & Mainou BA Interactions between enteric bacteria and eukaryotic viruses impact the outcome of infection. Viruses 10, 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falsey AR et al. Bacterial complications of respiratory tract viral illness: a comprehensive evaluation. J. Infect. Dis. 208, 432–441 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCullers JA Do specific virus-bacteria pairings drive clinical outcomes of pneumonia? Clin. Microbiol. Infect. 19, 113–118 (2013). [DOI] [PubMed] [Google Scholar]
- 10.McCullers JA & Rehg JE Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis. 186, 341–350 (2002). [DOI] [PubMed] [Google Scholar]
- 11.McCullers JA The co-pathogenesis of influenza viruses with bacteria in the lung. Nat. Rev. Microbiol. 12, 252–262 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Ghoneim HE, Thomas PG & McCullers JA Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J. Immunol. 191, 1250–1259 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mina MJ, Klugman KP, Rosch JW & McCullers JA Live attenuated influenza virus increases pneumococcal translocation and persistence within the middle ear. J. Infect. Dis. 212, 195–201 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith CM et al. Respiratory syncytial virus increases the virulence of Streptococcus pneumoniae by binding to penicillin binding protein 1a. A new paradigm in respiratory infection. Am. J. Respir. Crit. Care Med. 190, 196–207 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hament JM et al. Direct binding of respiratory syncytial virus to pneumococci: a phenomenon that enhances both pneumococcal adherence to human epithelial cells and pneumococcal invasiveness in a murine model. Pediatr. Res. 58, 1198–1203 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Avadhanula V, Wang Y, Portner A. & Adderson E. Nontypeable Haemophilus influenzae and Streptococcus pneumoniae bind respiratory syncytial virus glycoprotein. J. Med. Microbiol. 56, 1133–1137 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Van Ewijk BE et al. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr. Res. 61, 398–403 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Wu NH, Meng F, Seitz M, Valentin-Weigand P. & Herrler G. Sialic acid-dependent interactions between influenza viruses and Streptococcus suis affect the infection of porcine tracheal cells. J. Gen. Virol. 96, 2557–2568 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Wang Y. et al. Capsular sialic acid of Streptococcus suis serotype 2 binds to swine influenza virus and enhances bacterial interactions with virus-infected tracheal epithelial cells. Infect. Immun. 81, 4498–4508 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harding AT, Heaton BE, Dumm RE & Heaton NS Rationally designed influenza virus vaccines that are antigenically stable during growth in eggs. mBio 8, e00669–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudd JM, Ashar HK, Chow VT & Teluguakula N. Lethal Synergism between Influenza and Streptococcus pneumoniae. J. Infect. Pulm. Dis. 10.16966/2470-3176.114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edouard S. et al. The nasopharyngeal microbiota in patients with viral respiratory tract infections is enriched in bacterial pathogens. Eur. J. Clin. Microbiol. Infect. Dis 37, 1725–1733 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Navne JE et al. Nasopharyngeal bacterial carriage in young children in Greenland: a population at high risk of respiratory infections. Epidemiol. Infect. 144, 3226–3236 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber VC, Thomas PG & McCullers JA A multi-valent vaccine approach that elicits broad immunity within an influenza subtype. Vaccine 27, 1192–1200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosch JW et al. A live-attenuated pneumococcal vaccine elicits CD4+ T-cell dependent class switching and provides serotype independent protection against acute otitis media. EMBO Mol. Med. 6, 141–154 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kash JC et al. Lethal synergism of 2009 pandemic H1N1 influenza virus and Streptococcus pneumoniae coinfection is associated with loss of murine lung repair responses. mBio 2, e00172-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speshock JL, Doyon-Reale N, Rabah R, Neely MN & Roberts PC Filamentous influenza A virus infection predisposes mice to fatal septicemia following superinfection with Streptococcus pneumoniae serotype 3. Infect. Immun. 75, 3102–3111 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolter N. et al. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J. Infect. Dis. 210, 1649–1657 (2014). [DOI] [PubMed] [Google Scholar]
- 29.El Ahmer OR, Raza MW, Ogilvie MM, Weir DM & Blackwell CC Binding of bacteria to HEp-2 cells infected with influenza A virus. FEMS Immunol. Med. Microbiol. 23, 331–341 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Bandoro C. & Runstadler JA Bacterial lipopolysaccharide destabilizes influenza viruses. mSphere 2, e00267–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King SJ, Hippe KR & Weiser JN Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol. Microbiol. 59, 961–974 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Heikkinen T. & Chonmaitree T. Importance of respiratory viruses in acute otitis media. Clin. Microbiol. Rev. 16, 230–241 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chonmaitree T. et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin. Infect. Dis. 46, 815–823 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wadowsky RM, Mietzner SM, Skoner DP, Doyle WJ & Fireman P. Effect of experimental influenza A virus infection on isolation of Streptococcus pneumoniae and other aerobic bacteria from the oropharynges of allergic and nonallergic adult subjects. Infect. Immun. 63, 1153–1157 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Short KR et al. Influenza virus induces bacterial and nonbacterial otitis media. J. Infect. Dis. 204, 1857–1865 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JK & Cho JH Change of external auditory canal pH in acute otitis externa. Ann. Otol. Rhinol. Laryngol. 118, 769–772 (2009). [PubMed] [Google Scholar]
- 37.Weiser JN, Ferreira DM & Paton JC Streptococcus pneumoniae: transmission, colonization and invasion. Nat. Rev. Microbiol. 16, 355–367 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zafar MA, Wang Y, Hamaguchi S. & Weiser JN Host-to-host transmission of Streptococcus pneumoniae is driven by its inflammatory toxin, pneumolysin. Cell Host Microbe 21, 73–83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zafar MA, Kono M, Wang Y, Zangari T. & Weiser JN Infant mouse model for the study of shedding and transmission during Streptococcus pneumoniae monoinfection. Infect. Immun. 84, 2714–2722 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grijalva CG et al. The role of influenza and parainfluenza infections in nasopharyngeal pneumococcal acquisition among young children. Clin. Infect. Dis. 58, 1369–1376 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison A. et al. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J. Bacteriol. 187, 4627–4636 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helminen ME et al. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J. Infect. Dis. 170, 867–872 (1994). [DOI] [PubMed] [Google Scholar]
- 43.Cline TD et al. Increased pathogenicity of a reassortant 2009 pandemic H1N1 influenza virus containing an H5N1 hemagglutinin. J. Virol. 85, 12262–12270 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed LJ & Meunch H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27, 493–497 (1938). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 2
Supplemental Video 3
Supplemental Video 4
Supplemental Video 1
Supplementary meterial