Inhibition of TATA-Binding Protein Function by SAGA Subunits Spt3 and Spt8 at Gcn4-Activated Promoters (original) (raw)
Abstract
SAGA is a 1.8-MDa yeast protein complex that is composed of several distinct classes of transcription-related factors, including the adaptor/acetyltransferase Gcn5, Spt proteins, and a subset of TBP-associated factors. Our results indicate that mutations that completely disrupt SAGA (deletions of SPT7 or SPT20) strongly reduce transcriptional activation at the HIS3 and TRP3 genes and that Gcn5 is required for normal HIS3 transcriptional start site selection. Surprisingly, mutations in Spt proteins involved in the SAGA-TBP interaction (Spt3 and Spt8) cause derepression of HIS3 and TRP3 transcription in the uninduced state. Consistent with this finding, wild-type SAGA inhibits TBP binding to the HIS3 promoter in vitro, while SAGA lacking Spt3 or Spt8 is not inhibitory. We detected two distinct forms of SAGA in cell extracts and, strikingly, one lacks Spt8. Conditions that induce HIS3 and TRP3 transcription result in an altered balance between these complexes strongly in favor of the form without Spt8. These results suggest that the composition of SAGA may be dynamic in vivo and may be regulated through dissociable inhibitory subunits.
The process of transcriptional activation can be carried out by numerous mechanisms, including chromatin modification and recruitment of general transcription factors, such as transcription factor IID (TFIID) (58), to promoter DNA via sequence-specific binding of activators. Models for these mechanisms have become increasingly unified in the last several years with the discovery that certain proteins in coactivator complexes have intrinsic histone acetyltransferase (HAT) activity and thus have the potential to promote nucleosome remodeling (63). The first of these was Gcn5 (7), originally characterized as a transcriptional adaptor in yeast (20, 40). Other transcriptionally relevant HATs identified since then include human p300/CBP (2, 44), PCAF (69), and TAFII250, as well as yeast homolog TAFII145/130 (42).
SAGA, a Gcn5-containing protein complex in Saccharomyces cerevisiae, incorporates multiple transcription-related functions, displaying both HAT activity and interactions with activators and the TATA-binding protein (TBP) of TFIID (24). The 1.8-MDa SAGA complex was originally purified on the basis of its ability to acetylate nucleosomal substrates in vitro (22), but it has subsequently been found to contain several different groups of proteins involved in transcription: adaptors (Ada proteins), Spts, and TAFIIs (TBP-associated factors of TFIID), as well as the ATM and DNA-dependent protein kinase-related Tra1 protein (54).
Along with Gcn5, the adaptors present in SAGA include Ada1 (29), Ada2 (4), Ada3 (6, 46), and Ada5 (39, 50). First identified in a genetic screen as mutants that suppress the toxicity of overexpressed chimeric activator Gal4-VP16 (4), the Ada proteins were later shown to interact physically and functionally in vitro and in vivo with one another (10, 29, 40), with TBP (3), and with acidic activation domains, such as those of VP16 and Gcn4 (3, 12, 57). Functional aspects of the adaptors were further defined with the characterization of Gcn5 as a HAT (7). Subsequent studies with HAT domain substitution mutants (25, 34, 64) showed that the level of nucleosome acetylation activity of Gcn5 within SAGA correlates well with growth, transcription, histone acetylation, and chromatin remodeling in vivo.
Another major group of proteins identified in SAGA is a subset of the Spt proteins: Spt3 (67), Spt7 (19), Spt8 (16), and Spt20 (39, 50). Discovered as suppressors of defects caused by transposable element insertions into the promoter regions of marker genes, the Spts were classified into groups based on shared phenotypes (reviewed in reference 65). One class was described as functionally related to TBP, since one of its members, Spt15, is in fact yeast TBP (17, 26). These Spts (except TBP itself) are stable components of SAGA (22). An in vivo relationship among TBP, Spt3, and Spt8 was demonstrated by allele-specific suppression between their genes (15, 16). In addition, there have been several indications that SAGA associates with TBP in vivo. TBP has been recovered from yeast extracts via Spt3 coimmunoprecipitation and glutathione _S_-transferase (GST)–Spt20 pulldown assays (15, 49) and has been immunoprecipitated as part of a large complex containing Ada3 (53). SAGA also has recently been shown to bind to GST-TBP in vitro, and this interaction is greatly reduced in the absence of the Spt8 subunit (59). Spt3 also displays genetic interactions with Mot1, Not1, or TFIIA (13, 38). The involvement of these latter proteins in the regulation of TBP function further suggests that the Spts interact with TBP.
Deletions of the SAGA components described above result in three major subsets of growth phenotypes in vivo (24, 30, 49): Ada−-related moderate (Ada2/Ada3/Gcn5), Spt−-related moderate (Spt3/Spt8), and Ada− Spt− severe (Ada1/Spt7/Spt20). The number and severity of these phenotypes correlate with the effect of the mutations on complex integrity, since neither of the moderate groups disrupts the complex, but mutations in the severe group result in its apparent loss. Interestingly, the double mutants _gcn5_Δ _spt3_Δ and _gcn5_Δ _spt8_Δ display severe phenotypes but have intact SAGA (59). Taken together, these data suggest that at least two discrete biochemical functions possessed by SAGA (nucleosome acetylation and TBP interaction) contribute to the regulation of transcriptional activation. Activator interaction is a third function of SAGA demonstrated in a recent study (61).
Immunochemical and peptide analyses of purified SAGA have revealed that a subset of the TAFIIs is present in the complex (23) and that these TAFIIs interact with Adas in vivo (14). These include TAFII90, TAFII68/61, TAFII60, TAFII25/23, and TAFII20/17. Interestingly, human and Drosophila homologs of three of these (TAFII68, TAFII60, and TAFII20) are histone related (28, 68), and homologs of all five have been shown to interact directly with TBP in vitro (8). Notably, TAFII145/130, the yeast TAF protein known to have both TBP binding ability (47) and HAT function (42), is not contained within SAGA. TAFII68 was shown to be required for SAGA nucleosome acetylation activity and for SAGA-mediated transcriptional activation from a nucleosomal template. Furthermore, SAGA lacking this TAF had reduced Spt3 levels yet was able to interact with TBP (23), consistent with findings mentioned above that another subunit, Spt8, is critical for the TBP interaction in vitro (59). Finally, recently characterized human PCAF and GCN5 complexes (43, 62) contain the analogous TAFs as well as Ada, Spt, and Tra1 homologs, indicating that the structure and function of SAGA have been conserved throughout evolution.
Taken together, the genetic and biochemical characterizations of SAGA suggest that it may be targeted to promoters by activator interaction, resulting in acetylation of nucleosomal histones and recruitment of TBP. However, this model for SAGA function has been obtained largely through in vitro studies, and much less is known about mechanisms in vivo. In this study, we have determined how deletions of SAGA subunits affect transcription of the endogenous HIS3 and TRP3 genes. These genes were chosen because they are regulated by the acidic activator Gcn4, which has been shown to interact with components of SAGA (3, 14, 61), and because GCN5 is required for full activation of HIS3 in vivo (20, 34). Our results suggest that SAGA is important for accurate regulation of these genes and that the different components of SAGA have clearly distinct roles. Our findings indicate that SAGA is potentially regulated through dynamic changes in its composition.
MATERIALS AND METHODS
Yeast strains and media.
The S. cerevisiae strains used in this study are listed in Table 1. All FY strains are congenic and were originally derived from the S288C derivative FY2 (66). The spt mutant strains have been described previously (48) or, in the case of L864, were made by similar methods: gene knockouts (52) were made in a diploid strain, and a resulting transformant was sporulated and tetrad dissected (1) to obtain a haploid with the appropriate combination of genotypes. Strain SB325 was produced from FY61 by one-step gene disruption (52) of GCN5 with the _Bgl_II/_Xho_I fragment of _gcn5_Δ::his G-URA3 plasmid pyGCN5.KO (11) followed by selection on 5-fluoro-orotic acid plates to remove the URA3 gene. SB327, a plasmid integrant containing Spt8 with an amino-terminal c-myc epitope tag, was prepared as follows. The 4.5-kb _Bgl_I fragment of plasmid pSR6 (16), containing the tagged Spt8 gene, was ligated with the 2.7-kb _Bgl_I fragment of pRS306. The resulting plasmid was digested with _Stu_I and integrated into the _spt8_Δ strain FY463. To complement the _his4-917_δ mutation, strains FY61, SB325, and SB327 were transformed with plasmid pRBHis as described previously (40).
TABLE 1.
S. cerevisiae strains
| Strain | Genotype |
|---|---|
| FY61 | MATa _his4-917_δ ura3-52 |
| FY631 | MATa _his4-917_δ ura3-52 lys2-173R2 trp1_Δ_63 leu2_Δ_1 |
| FY294 | MATa _spt3_Δ_202 his4-917_δ leu2_Δ_1 lys2-173R2 trp1_Δ_63 ura3-52 |
| FY402 | MATa spt15-21 leu2_Δ_1 lys2-173R2 ura3-52 |
| FY463 | MATα spt8_Δ_302::LEU2 his4-917_δ leu2Δ_1 lys2-173R2 trp1_Δ_63 ura3-52 |
| FY963 | MATa spt7_Δ_402::LEU2 his4-917_δ leu2Δ_1 ura3-52 |
| FY1095 | MATa spt20_Δ_100::URA3 his4-917_δ leu2Δ_1 lys2-173R2 trp1_Δ_63 ura3-52 |
| L805 | _MATα spt15-21 spt3-401 his4-917_δ lys2-173R2 trp1_Δ_63 ura3-52 |
| L861 | _MATα spt3-401 his4-917_δ leu2_Δ_1 lys2-173R2 ura3-52 |
| L864 | MATa _spt3-401 spt8_Δ_302::LEU2 his4-917_δ ura3-52 |
| SB325 | MATa _gcn5_Δ _his4-917_δ ura3-52 |
| SB327 | _MATα URA3::_pRS306-myc-SPT8 spt8_Δ_302::_LEU2 his4-917_δ leu2_Δ_1 lys2-173R2 trp1_Δ_63 |
| SB330 | As FY631 but gcn4::URA3 |
| SB331 | As FY294 but gcn4::URA3 |
| SB332 | As FY463 but gcn4::TRP1 |
| SB333 | As FY402 but gcn4::URA3 |
| SB334 | As SB330 but _LEU2::_pRS405-_GCN4_C |
| SB335 | As SB331 but _LEU2::_pRS405-_GCN4_C |
| SB336 | As SB332 but _URA3::_pRS406-_GCN4_C |
| SB337 | As SB333 but _LEU2::_pRS405-_GCN4_C |
Strains SB330, SB331, and SB333 were produced from FY631, FY294, and FY402, respectively, by one-step gene gene disruption of GCN4 with the _Mlu_I/Bst_EII fragment of gcn4_Δ::URA3 plasmid pM214 (gift from A. Hinnebusch). Strain SB332 was made from FY463 by PCR-based GCN4 gene deletion as described previously (37) with a TRP1 cassette. A constitutive allele of GCN4 (gift from A. Hinnebusch) was first subcloned into integrating vectors pRS405 (LEU2) and pRS406 (URA3). Strains SB334, SB335, SB336, and SB337 were produced from strains SB330 to SB333 by integration of a constitutive allele of GCN4 at either the URA3 locus for SB336 or the LEU2 locus for the other strains.
Rich (YPD), minimal, synthetic complete (SC), 5-fluoro-orotic acid, and sporulation media were prepared as described previously (51). Standard protocols for transformation were used in strain constructions (51).
In vivo RNA analysis.
Total RNA was isolated from yeast cultures grown either in YPD medium or in SC medium to an optical density at 600 nm of 0.8 to 1.2 by the hot phenol method as described previously (32). To derepress HIS3 transcription, 40 mM 3-aminotriazole (3-AT) was added to the SC medium for 2 h prior to RNA isolation. The RNA concentration was quantitated spectrophotometrically at 260 nm. Each RNA sample (50 μg) was hybridized to completion with an excess of 32P-end-labelled HIS3 and tRNAW oligonucleotides and treated with S1 nuclease as described elsewhere (32, 45). HIS3 RNA levels were quantitated on a PhosphorImager (Molecular Dynamics). S1 nuclease assays were performed in duplicate several times, and PhosphorImager quantitation showed less than a 15% error. A tRNAW probe was used as an internal control for equal loading.
DNase I footprinting.
For DNase I footprinting experiments, the HIS3 promoter region was cloned by inserting a PCR product generated with primers 5′-CTGCCAGGTATCTAGAGAACACGGCATTAGTCAGG-3′ and 5′-CTATCGCTAGAATTCCACCCTTTAAAGAGATCGC-3′ into vector pBS+ digested with _Eco_RI and _Xba_I. The HIS3 probe was prepared by filling in the _Eco_RI site with [32P]ATP and followed by _Xba_I digestion of the plasmid to generate a 350-bp fragment. Yeast recombinant TBP and native SAGA complexes were used in binding reactions. The wild-type, _spt3_Δ, and _spt8_Δ SAGA complexes were described by Sterner et al. (59) and had been purified with an additional Superose 6 column after the standard Mono Q chromatographic step (see below). Binding reactions were performed with 12.5 μl of binding buffer (12.5 mM HEPES [pH 7.9], 12.5% glycerol, 5 mM MgCl2, 70 mM KCl, 0.2 mM EDTA, 10 mM β-mercaptoethanol) containing 0.5 mg of bovine serum albumin per ml, 20 to 40 μg of poly(dG-dC) per ml, and ∼6 fmol of DNA probe. Binding was done at 30°C for 60 min. DNase I footprinting was described previously (35).
HAT complex purification and assays.
Nucleosomal HAT complexes were prepared from Spt8-tagged wild-type strain SB327 and from _spt8_Δ strain FY463 by a previously described purification scheme (22): growth in 4 liters of YPD medium to an optical density at 600 nm of 2 to 2.5, cell breakage with glass beads, incubation of extract with Ni2+-agarose, recovery of a 300 mM imidazole eluate, and purification on a Mono Q column with a 100 to 500 mM NaCl gradient. For study of complexes under _HIS3_-derepressing conditions, an additional SB327 preparation was made as described above, except that the starting material was a 6-liter culture of SC medium lacking histidine, and 3-AT was added to 40 mM 2 h before harvesting at an optical density at 600 nm of 0.8 to 1.2.
HA assays were performed by a previously described method (22). Reaction mixtures (30 μl) contained 2 μl of enzyme sample, 1 μg of free histones, and 0.25 μCi of 3H-labeled acetyl coenzyme A in HAT buffer (50 mM Tris-HCl [pH 8.0], 50 mM KCl, 5% glycerol, 0.1 mM EDTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 10 mM sodium butyrate) and were incubated for 30 min at 30°C. Free histones (Sigma) were from calf thymus. Half of each reaction mixture was spotted on P81 filter paper (Whatman) for histone binding, washed, and used for liquid scintillation counting; the other half was heated in sodium dodecyl sulfate (SDS) sample buffer with β-mercaptoethanol and run on an SDS–18% polyacrylamide gel, which was fluorographed with Enhance (Dupont NEN) and dried. Fluorography was performed with Kodak X-Omat film at −70°C with 1.5 days of exposure.
SAGA and SAGAalt (alternate form of SAGA) complexes used in the in vitro transcription assays were purified as described previously (23) with minor modifications.
Antibodies and Western blotting.
For Western blot experiments, samples were boiled in SDS–β-mercaptoethanol sample buffer, electrophoresed on SDS-polyacrylamide (8 or 10%) gels, electroblotted to nitrocellulose, and visualized immunochemically by standard methods (27). Anti-Ada2 and anti-Spt3 antisera and anti-Spt20 affinity-purified antibodies were described by Grant et al. (22); primary antibody dilutions used were 1:4,000, 1:500, and 1:4,000, respectively. Anti-TAFII60, anti-TAFII68, and anti-TAFII145 (23) were used at dilutions of 1:3,000, 1:3,000, and 1:2,000, respectively. Immunodetection was performed with a secondary antibody (goat anti-rabbit immunoglobulin G–horseradish peroxidase conjugate [Bio-Rad]) and an ECL kit (Amersham). Mouse anti-c-myc antibody was purchased from Boehringer Mannheim Biochemicals and used at 5 μg/ml with a Bio-Rad anti-mouse secondary antibody and immunodetection as described above. Some blots were stripped of antibody before being reprobed; this procedure was accomplished with 62.5 mM Tris (pH 6.8)–2% SDS–100 mM β-mercaptoethanol at 50°C for 30 min.
Transcription assays.
Promoter-dependent transcription assays with a G-less cassette template, performed as described previously (18, 56), were done with 250 ng of plasmid template pMLG (containing the adenovirus major late promoter fused to a 400-bp G-less cassette) (55), 3 μl of a crude fraction containing holo-PolII and TFIIF (2 μl of TFIIH), each purified from yeast, and 30 ng of TBP (unless otherwise stated) and 30 ng of TFIIB, each overexpressed in and purified from Escherichia coli. 32P-labeled transcripts resistant to RNase T1 cleavage were precipitated with ethanol, redissolved in formamide sample buffer, resolved by electrophoresis on a 6% polyacrylamide gel containing 8.3 M urea, and visualized by autoradiography of the dried gel.
RESULTS
Subunits of SAGA regulate HIS3 and TRP3 transcription through distinct mechanisms.
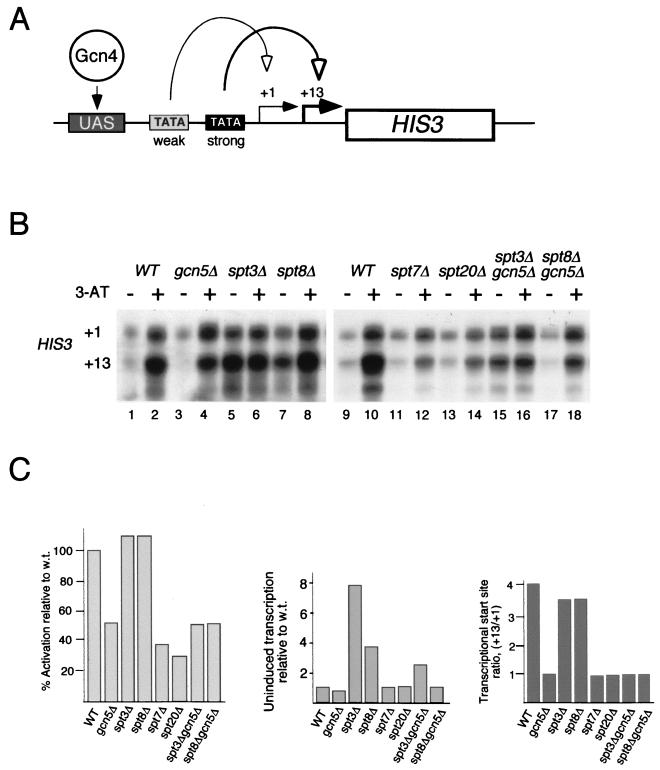
Transcriptional regulation of the HIS3 gene has been extensively studied, and the fine structure of its promoter has been characterized (60). The promoter contains both nonconsensus and consensus TATA boxes, directing transcription from +1 and +13 start sites, respectively (Fig. 1A). These two start sites differ in their strength of response to Gcn4-mediated activation. When yeast cells are grown in noninducing conditions, transcription from both +1 and +13 is low and is approximately equal in wild-type yeast. Under derepressing conditions (in the presence of 3-AT), a competitive inhibitor of His3), transcription from both start sites increases, but the +13 transcript is approximately four times more abundant than the +1 transcript (Fig. 1B, lanes 1 and 2).
FIG. 1.
Effects of SAGA subunit deletions on uninduced and activated transcription of the HIS3 gene. (A) Model of HIS3 gene function. Two transcriptional start sites, +1 and +13, are used. Under noninducing conditions, similar levels of +1 and +13 transcripts are produced. The +13 transcripts are more abundant under activated conditions, as +13 transcription is directed from a strong TATA box influenced by the activator Gcn4 when it is bound to an upstream activating sequence (UAS). +1 transcription is much less Gcn4 dependent and is due to a weaker TATA box located upstream of the stronger one. (B) Detection of HIS3 transcripts in wild-type (WT) and various SAGA mutant strains. Gene expression was analyzed by an S1 nuclease protection assay. tRNAW levels were used as a control for intact RNA (see Fig. 2A). RNA was prepared from yeast cells grown in SC medium with (+) or without (−) 3-AT added. (C) PhosphorImager analyses of S1 nuclease assays presented as activated transcription relative to that of the wild type (w.t.) (left), uninduced transcription relative to that of the w.t. (middle), and the +13/+1 transcriptional start site ratio (right). Experiments were repeated at least three times, and PhosphorImager quantitation showed less than 20% error.
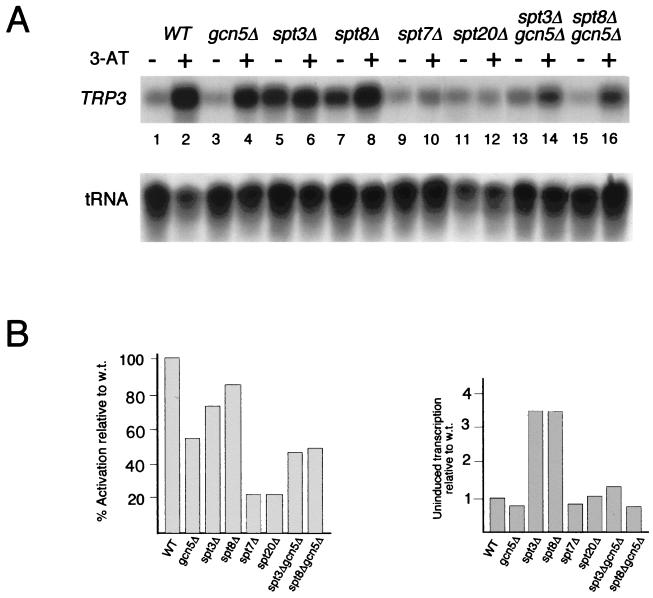
Deletion of different SAGA components caused three clear changes in transcription patterns. First, as previously reported (59), disruption of GCN5 resulted in an altered HIS3 start site preference, where +1 and +13 were transcribed at similar levels in the activated state (Fig. 1B, compare lanes 2 and 4, and Fig. 1C, right). Second, transcriptional activation of HIS3 was greatly affected by mutations that disrupt SAGA, i.e., _spt7_Δ and _spt20_Δ (Fig. 1B, lanes 11 to 14, and Fig. 1C, left), but was not greatly affected by either ada/gcn5 or spt mutations that do not disrupt SAGA, i.e., _spt3_Δ or _spt8_Δ (Fig. 1B and C). A similar loss of transcriptional activation of _spt7_Δ and _spt20_Δ was detected for the TRP3 gene (Fig. 2A and B, left). Third, deletion of either SPT3 or SPT8 resulted in a striking elevation of the uninduced levels of both HIS3 (Fig. 1B, compare lane 1 with lanes 5 and 7, and Fig. 1C, middle) and TRP3 (Fig. 2A, lanes 1, 5, and 7, and Fig. 2B, right) transcription. These data suggest an inhibitory role for Spt3 and Spt8 proteins in noninducing conditions at Gcn4-dependent promoters. The elevation in the uninduced levels of HIS3 and TRP3 transcription required Gcn5 function, since the levels of transcription were significantly reduced in the double mutants spt3 gcn5 and spt8 gcn5 (Fig. 1B and 2A, last four lanes). The loss of both Spt3 and Gcn5 does not completely abolish the high level observed in the _spt3_Δ strain, because Gcn5 is not solely responsible for activated HIS3 transcription (as shown in Fig. 1B, lane 4). In contrast, the activated HIS3 and TRP3 RNA levels in these double-mutant strains were comparable to the level in the _gcn5_Δ strain, further evidence that Spt3 and Spt8 are not required for the activation of these genes.
FIG. 2.
Effects of SAGA mutations on uninduced and activated transcription of the TRP3 gene. (A) Detection of TRP3 transcripts in wild-type (WT) and various SAGA mutant strains. Gene expression was analyzed by an S1 nuclease protection assay. tRNAW levels were used as a control for intact RNA. Growth conditions were the same as those described in the legend to Fig. 1. (B) PhosphorImager quantitation of S1 nuclease assays presented as described in the legend to Fig. 1C. Experiments were repeated at least three times, and PhosphorImager quantitation showed less than 20% error.
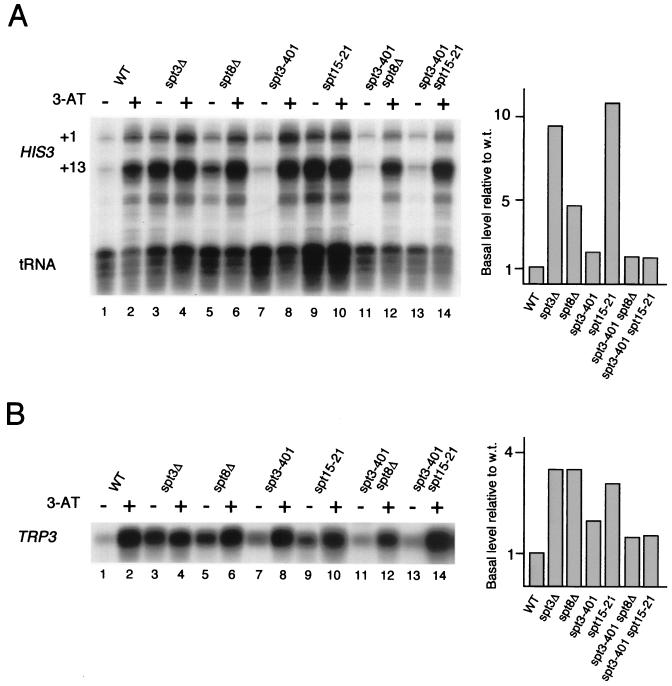
We examined the apparent inhibitory effect of Spt3 and Spt8 to begin to understand the underlying mechanism. Winston and colleagues have observed functional interactions between Spt3 or Spt8 and TBP (15, 16). Specifically, deletions of these SPT genes exhibit phenotypes similar to those resulting from certain mutant alleles in the gene encoding TBP (SPT15) (17). Therefore, we analyzed the effect of the spt15-21 mutation on HIS3 and TRP3 transcription. Similar to the effect of _spt8_Δ and, especially, _spt3_Δ, the uninduced levels of HIS3 and TRP3 transcripts were strongly elevated in the spt15-21 strain (Fig. 3A and B, lanes 3, 5, and 9). Thus, mutations in SPT3, SPT8, and SPT15 (TBP) all result in high levels of uninduced transcription, suggesting that the normal low level of uninduced transcription might be caused by interactions between Spt3 or Spt8 and TBP.
FIG. 3.
Functional interaction of Spt3 with Spt8 and TBP in HIS3 and TRP3 transcription. (A) S1 nuclease analysis of HIS3 expression in wild-type (WT) cells, SPT3 and SPT8 deletion mutants, specific SPT3 (spt3-401) and TBP (spt15-21) point mutants, and combinations thereof. The bar graph at right presents PhosphorImager quantitation of the S1 nuclease assays as uninduced transcription (without 3-AT) relative to the wild-type (w.t.) level. tRNAW levels were used as a control for intact RNA. (B) Equivalent set of assays for TRP3. RNA was isolated from yeast cells grown in SC medium with (+) or without (−) 3-AT added.
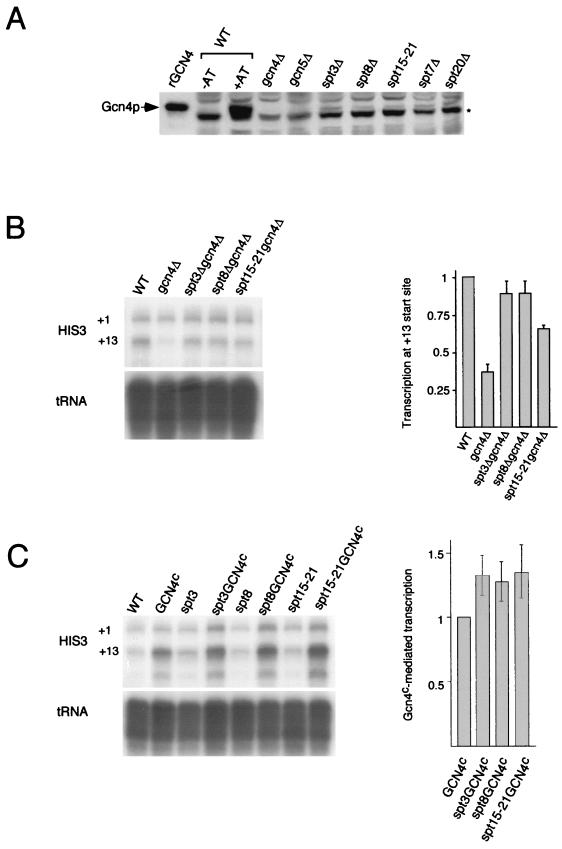
It was possible that the increase in the uninduced levels of transcription of HIS3 and TRP3 in the strains bearing _spt3_Δ, _spt8_Δ, and spt15-21 mutations was due to an indirect effect of the elevated expression of the activator Gcn4, rather than to a direct effect of a SAGA interaction with TBP. We determined the level of Gcn4 protein and found only a very slight increase in synthetic media in both the wild type and all the spt mutants, but in each case the amount of Gcn4 was far below that found in the wild-type strain during normal induction with 3-AT (Fig. 4A). Since the deletion of either SPT7 or SPT20 had no effect on the uninduced level of HIS3 RNA but, instead, resulted in a significant decrease in activation (Fig. 1 and 2), there is no strict correlation between the increase in Gcn4 protein levels and the increase in uninduced levels of HIS3 transcription. Thus, it is highly unlikely that the increased levels of HIS3 and TRP3 transcription in the spt mutant strains are caused exclusively by the increased expression of Gcn4.
FIG. 4.
Analysis of the role of the activator Gcn4 in the high levels of uninduced transcription detected in spt3, spt8, and TBP mutant strains. (A) Western blot analysis of the Gcn4 protein level in the wild-type (WT) strain under noninducing (SC medium) and inducing (SC medium plus 3-AT) conditions and in spt mutant strains. The levels of Gcn4 protein were similar in all strains under noninducing conditions (−AT) and were much lower than those under inducing (+AT) conditions. The asterisk indicates a nonspecific background band which was present in all samples and which exhibited slightly faster mobility than Gcn4, as is clear from the _gcn4_Δ lane. Bacterially expressed recombinant Gcn4 (rGCN4) is also shown. (B) S1 nuclease analysis of the effect of _spt3_Δ, _spt8_Δ, and spt15-21 mutations on HIS3 transcription in the absence of Gcn4, i.e., in a _gcn4_Δ background. Transcription from the +13 start site was greatly reduced in the _gcn4_Δ mutant but not in the _spt3_Δ _gcn4_Δ, _spt8_Δ _gcn4_Δ, and _spt15-21 gcn4_Δ double mutants, as shown in the quantitative bar graph. RNA was isolated from yeast cells grown in SC medium. (C) S1 nuclease analysis of the effect of high levels of constitutive (superscript c) expression of the Gcn4 activator on HIS3 transcription under rich-medium (YPD) conditions. Overexpression of the Gcn4 protein resulted in increased HIS3 transcription, which was increased further in either _spt3_Δ, _spt8_Δ, or spt15-21 strains, as shown in the quantitative bar graph. Error bars in panels B and C show standard deviations. tRNAW levels were used as a control for intact RNA (panels B and C).
The requirement for the activator Gcn4 was tested in two additional experiments. We examined whether SPT3, SPT8, or SPT15 mutations affect HIS3 transcription in the absence of Gcn4 or during constitutive expression of Gcn4. Deletion of GCN4 caused a severe reduction of transcription initiating at the +13 start site (Fig. 4B, lanes 1 and 2), as expected for the initiation site regulated mainly by the activator Gcn4. However, in double mutants (_gcn4_Δ _spt3_Δ, _gcn4_Δ _spt8_Δ, or _gcn4_Δ spt15-21), the +13 start site was transcribed at nearly normal levels (Fig. 4B, lanes 3 to 5, and Fig. 4B, right), and the overall HIS3 expression pattern in these mutants was similar to that in wild-type yeast. This change in transcription pattern may be caused by direct binding of SAGA to DNA, possibly through its TAFII subunits. Thus, even in the absence of Gcn4, transcription at the +13 site is restored to normal uninduced levels by mutations in Spt3, Spt8, or TBP. It should be noted that this increase is still far below the level of HIS3 transcription achieved in the presence of the activator Gcn4 (compare Fig. 4B, lanes 3 to 5, to Fig. 1, lanes 5 and 7, and Fig. 3A, lane 9), indicating that Gcn4 is absolutely required for high-level HIS3 expression. Furthermore, as shown in Fig. 4C, constitutive high levels of Gcn4 expression in YPD medium (which yields the lowest level of Gcn4-dependent transcription) resulted in strong activation of HIS3 transcription, which was further slightly increased (an average of 1.3-fold) by _spt3_Δ, _spt8_Δ, or spt15-21 mutations. Taken together, the results in Fig. 4 suggest that SAGA modulates HIS3 transcription both in a Gcn4-dependent manner and also through an additional mechanism, suggested by these observations to be related to the regulation of TBP function.
Spt3 and Spt8 function in SAGA to inhibit TBP from binding to the HIS3 TATA box.
The above genetic data show that mutations in either SPT3, SPT8, or SPT15 (TBP) result in high levels of uninduced transcription, suggesting that Spt3 and Spt8 negatively regulate TBP. We next investigated whether SAGA has a direct effect on TBP function in vitro. At many genes (including HIS3), TBP binds to TATA sequences to regulate transcription; therefore, we tested whether Spt3 or Spt8 affects TBP binding to the consensus TATA box present in the HIS3 promoter. Our previous observations indicate that, although Spt3 and Spt8 are within SAGA, TBP is not a stable subunit of the complex throughout conventional chromatography (23).
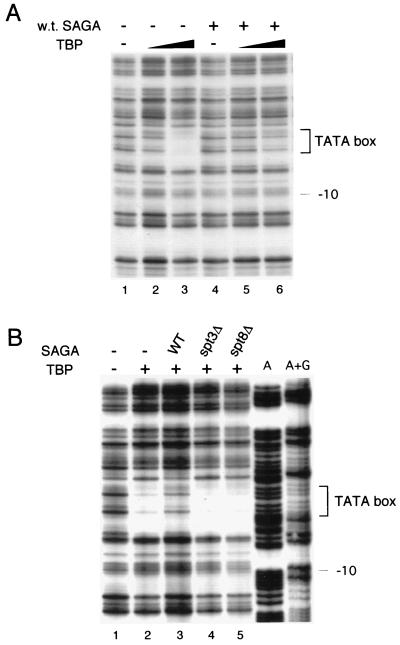
DNase I footprinting analysis was used to examine TATA box protection by TBP in the presence or absence of SAGA. The binding reaction mixture contained DNA bearing the endogenous HIS3 promoter, recombinant yeast TBP, and native SAGA complex obtained from wild-type yeast. As expected, increasing amounts of recombinant yeast TBP protected the HIS3 TATA box from DNase I digestion (Fig. 5A, lanes 1 to 3). The addition of wild-type SAGA, however, actually reduced TBP protection of the TATA sequences (Fig. 5A, lanes 4 to 6).
FIG. 5.
DNase I footprinting analysis of the effect of SAGA on TBP binding to the HIS3 TATA box in vitro. (A) DNase I protection of the consensus TATA box region (brackets) was assayed in the presence and absence (−) of recombinant TBP and the native SAGA complex purified from wild-type (w.t.) cells. TBP concentrations were 6 ng in lanes 2 and 5 and 18 ng in lanes 3 and 6. (B) Comparison of TBP footprints developed in the presence of either wild-type (WT) SAGA or SAGA isolated from _spt3_Δ or _spt8_Δ yeast strains. The amounts of SAGA were normalized by Western analysis. Binding reaction mixtures contained 18 ng of TBP. Lanes labelled A and A+G indicate sequencing reactions used to determine the footprint locations.
We then tested whether the loss of Spt3 or Spt8 affected SAGA inhibition of TBP binding to the TATA box. First, the amounts of mutant SAGA complexes were normalized to wild-type SAGA by Western blot analysis to quantitate Ada2. In contrast to the negative effect of wild-type SAGA, complexes obtained from either _spt3_Δ or _spt8_Δ strains did not possess this inhibitory function (Fig. 5B). Thus, the results indicated that SAGA negatively affects TBP binding to the HIS3 promoter and that the Spt3 and Spt8 proteins may mediate this inhibition. Together with the genetic effects of Spt3, Spt8, and TBP mutations on HIS3 and TRP3 transcription, these findings suggest that certain components of SAGA (Spt3 and Spt8), under noninducing conditions, inhibit its ability to activate transcription.
To further test the idea of an inhibitory interaction between Spt3 or Spt8 and TBP, we examined the effect of certain SPT3 mutations on HIS3 and TRP3 transcription. Previous work showed that the Spt− phenotypes caused by either spt15-21 or _spt8_Δ mutations were reversed by suppressing alleles of SPT3, such as spt3-401, leading to the hypothesis that Spt3 and Spt8 functionally interact with TBP (15, 16). We therefore tested whether the high levels of uninduced HIS3 and TRP3 transcription caused by spt15-21 or _spt8_Δ could be suppressed by spt3-401. The spt3-401 mutation did have this effect, suppressing the high levels of uninduced HIS3 and TRP3 transcription nearly to wild-type levels for both spt15-21 and _spt8_Δ (Fig. 3A and B, lanes 11 and 13); the suppression is shown clearly in the quantitation depicted in the bar graphs in Fig. 3. On its own, the spt3-401 mutation caused only a very weak induction of uninduced HIS3 or TRP3 transcription (Fig. 3A and B, lanes 7), also in agreement with the previous characterization of the spt3-401 mutation as possessing a weak Spt− phenotype (16).
The similar transcriptional patterns in the _spt3_Δ, _spt8_Δ, and spt15-21 strains are consistent with previous evidence that Spt3 and Spt8 are components of SAGA involved in the regulation of TBP function. Based on the data described above, the elevated transcription in these mutant strains may be caused by negative regulation of TBP by Spt3 and Spt8 at Gcn4-regulated promoters, since (i) uninduced transcription is increased in mutant cells, (ii) the high uninduced levels of transcription in spt15-21 and _spt8_Δ strains are suppressed by the spt3-401 allele, and (iii) the SAGA complex is inhibitory to TBP binding to TATA, while SAGAΔSpt3 and SAGAΔSpt8 (the SAGA complexes existing in these mutant strains) do not have this inhibitory effect.
Identification of a novel form of SAGA lacking the inhibitory Spt8 subunit.
Taken together, our findings suggest that the SAGA complex is composed of proteins playing distinct regulatory roles in transcription. The observation that inhibitory components are present in the complex suggests that, upon induction of HIS3 transcription, these negative regulators within SAGA could be either modified or removed. To test the hypothesis that the SAGA complex is altered during gene induction, we determined whether the function or protein composition of SAGA is changed under conditions inducing HIS3.
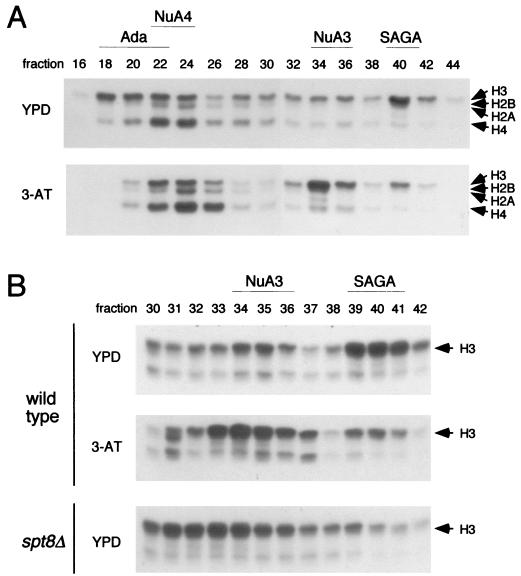
SAGA was analyzed by a chromatographic fractionation procedure that separates SAGA from three other nucleosomal acetylation complexes in yeast extracts (22). First, we compared HAT activity and SAGA structure in extracts from cells grown under conditions noninducing or inducing for HIS3. Cell extracts were prepared from wild-type yeast cells grown in rich medium or in synthetic complete medium lacking histidine and containing 3-AT. The extracts were fractionated through Ni2+-agarose and Mono Q ion-exchange resins to separate four previously identified HAT complexes. These complexes are, from earlier- to later-eluting fractions, Ada, NuA4, NuA3, and SAGA (Fig. 6A, upper panel). In extracts from cells grown under noninducing conditions, a peak of histone H3 HAT activity was clearly evident in fraction 40 (Fig. 6A, upper panel), the fraction containing SAGA. In contrast, extracts from 3-AT-induced cells had far less activity in fraction 40 and contained unusually strong activity in fraction 34 (Fig. 6A, lower panel); the latter far exceeded the normal histone H3 acetylation activity due to NuA3 under noninducing conditions (Fig. 6A, upper panel). This pattern of HAT activity was reminiscent of the alteration of the migration of SAGA upon disruption of SPT8 that we had observed previously (59), where no activity was present in fraction 40 but strong histone H3 HAT activity was found in fractions 32 through 34. Thus, the HAT profiles of the wild-type strain grown under noninducing and inducing conditions were directly compared to the profile of the _spt8_Δ mutant grown under noninducing conditions. In this experiment, we focused on the Mono Q fractions between 30 and 43 and examined the HAT activity of each fraction. The NuA3 (fractions 34 to 36) and SAGA (fractions 39 to 41) peaks were evident in the profile of wild-type cells grown under noninducing conditions (Fig. 6B, upper panel). The decrease in the SAGA level and the increase in HAT activity in fractions 33 to 35 in the wild-type strain grown under inducing conditions were in fact similar to the position of SAGA in the _spt8_Δ strain under noninducing conditions (fractions 31 to 34) (Fig. 6B, compare middle and lower panels).
FIG. 6.
Comparison of HAT activity profiles under conditions inducing and noninducing for HIS3 and TRP3. HAT complexes were prepared by the previously described two-step fractionation of yeast extracts over Ni2+-agarose and Mono Q columns (22). (A) Fluorograph of free histone acetylation assays with even-numbered Mono Q fractions derived from wild-type cells grown under conditions repressive (YPD) or inducing (3-AT) for HIS3 transcription. Arrows denote relative positions of the core histones. Indicated at the top are the fractions containing the four distinct HAT complexes identified previously from wild-type yeast by the established purification procedure (see the text). (B) Pattern of free HAT activity in Mono Q fractions 30 to 42 from the fractionation described in panel A and from an _spt8_Δ strain grown in YPD medium.
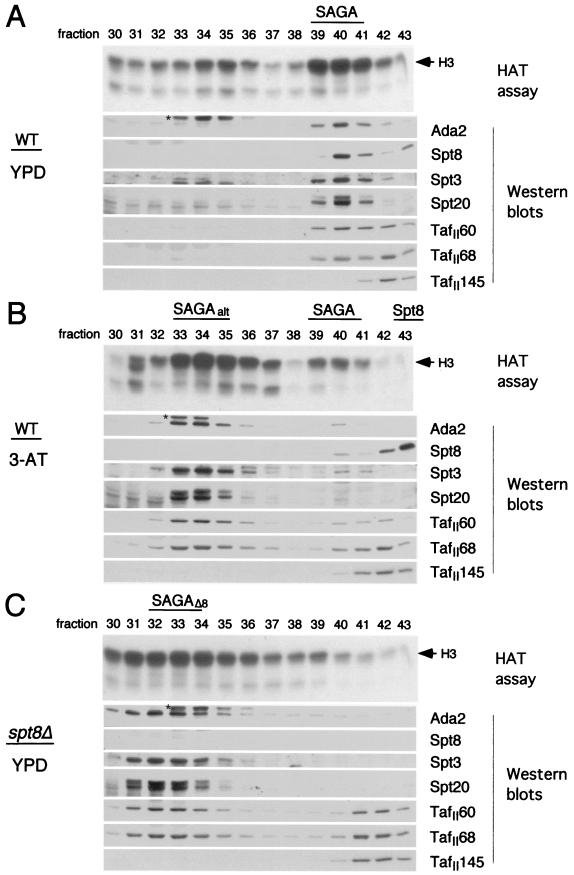
We then determined whether the new activity profile identified in the induced wild-type strain was due to altered SAGA, and if so, how its protein composition might have changed. Western blot analysis was performed on fractions from all three Mono Q profiles by use of a series of antibodies to detect proteins in each subclass of SAGA. These included antibodies specific for Ada2, c-myc (to detect epitope-tagged Spt8), Spt3, Spt20, TAFII60, and TAFII68. As expected, in the wild-type strain grown under noninducing conditions, each of the antibodies detected proteins in fraction 40 (Fig. 7A), the position of SAGA, as previously identified (22). In addition, antisera to TAFII60 and TAFII68 detected a second peak, in fraction 42, which also included TAFII145, previously shown to be absent from SAGA (23). Although this latter peak is apparently related to TFIID, it did not include TBP (P. A. Grant, unpublished observation). The identity of these peaks is made clearer by inspection of the protein composition within the Mono Q profile of an _spt8_Δ strain grown under noninducing conditions (compare Fig. 7C to Fig. 7A). In Fig. 7C it is clear from the Western analysis that SAGA has shifted to fractions 31 to 34 (SAGAΔ8). In addition, the SAGA-independent TFIID-related peak (containing TAFII68, TAFII60, and TAFII145) is now quite evident in fractions 41 and 42.
FIG. 7.
Subunit analysis of SAGA under conditions of HIS3 and TRP3 repression or induction or in the presence of _spt8_Δ. Mono Q fractions used (30 to 43) are from the purifications described in the legend to Fig. 6; the HAT profiles, as determined in Fig. 6B, are presented at the top of each section. (A) Western blot analysis of wild-type (WT) SAGA from a _HIS3_-repressed culture (YPD medium) of strain SB327 containing c-myc epitope-tagged Spt8. Antibodies used were specific for c-myc (for Spt8 visualization), for the other SAGA subunits indicated, or for TAFII145 (not a component of SAGA). On the anti-Ada2 blot, an asterisk indicates an artifactual cross-reactive species. (B) Western analysis of SAGA from the same wild-type strain under _HIS3_-inducing conditions (3-AT). Designated above the panels are the predominant form of SAGA (SAGAalt), wild-type SAGA, and a novel species containing the majority of Spt8. (C) Western analysis of SAGA from _spt8_Δ strain FY463. The complex, chromatographically shifted relative to wild-type SAGA, is designated SAGAΔ8. The blot labelled Spt8 was visualized with anti-c-myc antibodies as a control for panels A and B. In all panels, a TAF-containing, TFIID-related peak independent of SAGA is present in fractions 41 and 42.
We then analyzed the wild-type strain grown under inducing conditions and found that there was a small amount of protein reacting to all SAGA-specific proteins, peaking at the normal SAGA position in fraction 40 (Fig. 7B). However, the vast majority of SAGA was detected in fractions 33 and 34 (SAGAalt). Thus, the quantity of protein in the SAGAalt fractions, compared to that in fraction 40, reflected the relative HAT activities in these two peaks. Most interestingly, all of the antibodies that detect components of SAGA reacted against proteins of the appropriate size in SAGAalt, with the notable exception of the anti-c-myc antibody: c-myc–Spt8 was absent from fractions 33 and 34. However, a novel peak of c-myc–Spt8 was found in fraction 43, and no other SAGA components that were tested were found to coelute in this fraction in stoichiometric amounts (Fig. 7B). It is also interesting to note that a small amount of c-myc–Spt8 is present in fraction 43 even in wild-type extracts grown under noninducing conditions; moreover, a small amount of SAGA lacking c-myc–Spt8 is present in fraction 33 (Fig. 7A). Superose 6 size fractionation of the Spt8 moiety indicates that it is ∼200 kDa, suggesting that it is associated with other proteins (data not shown).
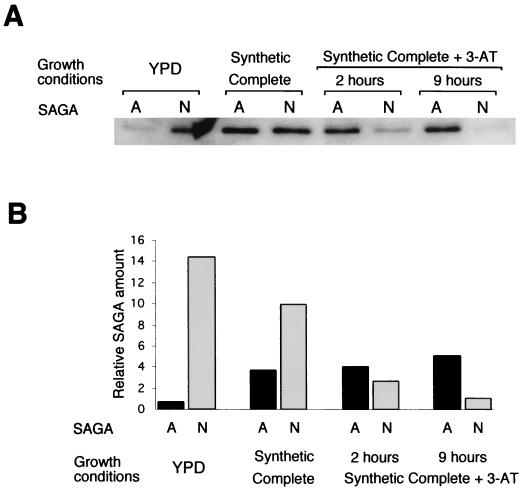
In the experiment described above, we examined SAGA in YPD medium or in SC medium containing 3-AT to strongly induce HIS3 transcription. It was important to determine whether the altered SAGA complex appears only upon amino acid starvation or whether it is also present as amino acids become limiting. We thus compared SAGA complexes fractionated from cells grown in YPD medium, SC medium, or SC medium with 3-AT added for either 2 or 9 h (Fig. 8). A progressive shift was detected in the ratio between SAGA and SAGAalt as the cells became starved for amino acids. The greatest change in the ratio between the complexes occurred in the shift from YPD medium to SC medium; however, in SC medium, where HIS3 transcription was still low, the amount of SAGA was still much larger than the amount of SAGAalt. Only with the addition of 3-AT did the amount of SAGAalt exceed that of SAGA; this factor may be critical for gene induction.
FIG. 8.
Analysis of SAGA and SAGAalt under different growth conditions. (A) Quantitative Western analysis of the peak SAGA (N) and SAGAalt (A) fractions from fractionation of yeasts grown in YPD medium, SC medium, and SC medium with 3-AT added for either 2 or 9 h. Anti-Ada2 antibody and 125I-protein A were used for immunoblot detection. (B) Relative amounts of complexes determined by normalizing the 125I-Ada2-specific signal from panel A through quantitation on a PhosphorImager to the protein concentration of the corresponding fraction.
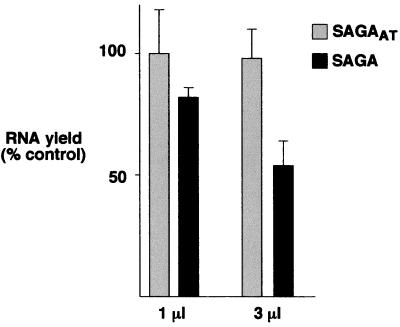
To begin to characterize the function of SAGA and SAGAalt, each complex was fractionated through six chromatographic steps. These nearly homogeneous samples were normalized for equivalent protein amounts and HAT activity and then tested for their effects on basal transcription in vitro by use of a highly defined system combining recombinant and purified general transcription factors. SAGA was inhibitory to basal transcription in a dose-dependent manner; i.e., 1 μl of the complex inhibited transcription by 20%, while 3 μl did so by almost 50%. In contrast, SAGAalt was not inhibitory at either concentration (Fig. 9). These data are consistent with SAGA containing negative regulatory subunits that are absent or altered in SAGAalt.
FIG. 9.
Effects of SAGA and SAGAalt on basal transcription in vitro. Two forms of the SAGA complex (SAGA and SAGAalt [SAGAAT]) were purified as described by Grant et al. (23) to near homogeneity, and their effects were tested in in vitro transcription assays containing plasmid template pMLG (adenovirus major late promoter fused to a 400-bp G-less cassette) and either native (holo-PolII, TFIIF, and TFIIH) or recombinant (TBP and TFIIB) yeast general transcription factors. The results presented summarize six independent experiments. Error bars indicate standard deviations.
Thus, there are two forms of SAGA. Under optimal growth conditions, most of SAGA is in a form containing potential negative regulators, with only a small amount in an altered form lacking Spt8. In contrast, upon a switch to amino acid starvation conditions, most of SAGA is in the altered form.
DISCUSSION
This study demonstrates that the SAGA complex functions in vivo in the general amino acid control pathway to regulate HIS3 and TRP3 gene expression. At the promoters for these genes, it is evident that the previously described subunits within SAGA play distinct roles in transcriptional regulation, both stimulatory and inhibitory. The most striking findings are that first, under conditions repressing for HIS3 and TRP3 expression, SAGA inhibits transcription through interactions of Spt3 and Spt8 with TBP. Second, SAGA exists in two forms: one contains the Spt8 subunit, and the other, which lacks Spt8, predominates during induction of the pathway resulting in HIS3 and TRP3 transcription.
SAGA functions at HIS3 and TRP3 promoters.
Several Gcn5-dependent acetylation complexes exist in yeast, including the 800-kDa Ada complex and the 1.8-MDa SAGA complex, as well as a smaller complex (6, 22). The existence of multiple complexes raises the question of whether they have unique roles, perhaps functioning in activator- and promoter-specific contexts. Based on several lines of evidence, it appears that SAGA itself functions at the HIS3 and TRP3 promoters. First, the Spt proteins mentioned have been found only in SAGA (22, 59), and disruption of the SPT genes results in transcriptional defects at these promoters; Spt proteins that maintain the integrity of the SAGA structure (Spt7 and Spt20) are required for full transcriptional activation at both promoters, and the loss of Spt3 and Spt8 results in elevated levels of uninduced transcription. Second, the _spt7_Δ and _spt20_Δ mutants, in which SAGA is disrupted, exhibit a _gcn5_− phenotype, showing altered start sites at HIS3, much like _gcn5_Δ. Taken together, these data suggest that it is indeed SAGA and not the concurrent action of two separate complexes, such as the Ada complex and a yet-unknown Spt complex, that functions at HIS3 and TRP3. Furthermore, the data also indicate that the role of SAGA in transcriptional activation results from the combined action of a number of separate activities, including Gcn5 (whose HAT activity is required to position normal initiation sites [59]), the Spt7-Spt20 class (required for transcriptional activation), and other distinct biochemical functions, such as TBP interactions (15, 59; this study) and activator interactions (3, 61).
Spt3 and Spt8 are both required for the repression of uninduced HIS3 and TRP3 transcription.
Previous genetic and biochemical data have suggested that Spt3 and Spt8 are both involved in functional interactions with TBP (15, 16). The increase in uninduced HIS3 and TRP3 transcription exhibited in _spt3_Δ, _spt8_Δ, and spt15-21 strains supports the idea that Spt3, Spt8, and a region of TBP have a common function. Importantly, this common phenotype indicates an inhibitory role for Spt3 and Spt8. This finding was unexpected, since in previous analyses, Spt proteins were deduced to exert positive transcriptional regulation (16, 19, 38). In our experiments with Gcn4-regulated genes, however, the high levels of uninduced transcription in mutants and the suppression of this high level by spt3-401 in _spt8_Δ and spt15-21 strains suggest negative regulation of TBP function by the Spt3 and Spt8 proteins. We hypothesize that both Spt3 and Spt8 regulate the TBP-SAGA interaction to inhibit TBP function under noninducing conditions for these genes. In this view, deletion of either SPT3 or SPT8 or the spt15-21 mutation results in less stable TBP-SAGA contact at the inhibitory site, leading to more efficient transcription initiation by SAGA.
One important question raised by these data is the role of the activator Gcn4 in the high levels of uninduced HIS3 and TRP3 transcription achieved in the _spt3_Δ, _spt8_Δ, and spt15-21 mutants. Specifically, under noninducing conditions, even in the complete absence of Gcn4 (i.e., in a _gcn4_Δ background), deletion of SPT3 or SPT8 or mutation of TBP caused an increase in HIS3 RNA levels, nearly to wild-type levels (Fig. 4B). Since these levels are well below full activation (for example, Fig. 1B, lane 2), our results imply a dual mechanism in which induced levels of Gcn4 combined with SAGAalt result in high levels of transcription. This dual mechanism was also evident in the experiment in which high constitutive levels of Gcn4 protein under noninducing conditions caused increased HIS3 transcription which was further elevated by deletion of SPT3 or SPT8 or mutation of TBP (Fig. 4C).
Our recent experiments (59) examining the in vitro interaction between GST-TBP and SAGA complexes derived from wild-type, _spt3_Δ, and _spt8_Δ strains demonstrated that both wild-type and Spt3− complexes bound to GST-TBP. However, in the absence of Spt8, very little SAGA was bound, and the only component detected by Western blotting was a small amount of Spt3 (59). This result suggests that both Spt3 and Spt8 are involved in contacts between SAGA and TBP but that Spt8 makes the stronger interactions. In this view, the ability of Spt3-401 to compensate for the loss of Spt8 suggests that the Spt3-401 protein may make stronger contact with TBP under these conditions. On the other hand, the restoration of the wild-type phenotype (i.e., low uninduced HIS3 and TRP3 transcription levels) in the spt3-401 spt15-21 double mutant may result from mutual alterations in the charge and/or conformation of mutant Spt3 and TBP that restore their normal affinity.
Biochemical evidence further supports the proposal that Spt3 and Spt8 inhibit TBP function, since wild-type SAGA negatively affected TBP binding to the HIS3 TATA box in vitro, while SAGA complexes prepared from either _spt3_Δ or _spt8_Δ strains no longer inhibited TBP binding to the TATA box. These two proteins apparently act somewhat separately, since in the absence of either, the other protein persists in SAGA (59). However, the similarity of many of their phenotypes and their biochemical activities strongly indicates that they are functionally linked. The precise mechanism of repression of TBP function by Spt3 and Spt8 is not fully understood. One hypothesis is that wild-type SAGA interacts with TBP through Spt3 or Spt8 and, through some unknown mechanism, reduces the ability of TBP to bind to DNA. In this view, in the absence of Spt3 or Spt8, SAGA binds to TBP through other components, such as TAFIIs, and this interaction results in the productive binding of TBP to DNA. In light of this notion, striking similarities have been noted between the SAGA complex and the TFIID complex (62), including shared subunits (the histone-fold TAFIIs) as well as functional similarities, such as activator interaction, acetyltransferase activity, and ability to bind TBP. One additional property of the TFIID complex is to negatively regulate the function of TBP, including binding to the TATA box (33). Within TFIID, this action is accomplished by the largest TAF (yeast TAFII145, human TAFII250, and Drosophila TAFII230), which is absent from SAGA (23, 43); however, within SAGA, the Spt3 and Spt8 components apparently serve this role. Thus, negative regulation of TBP may be an additional conserved function between the coactivator complexes SAGA and TFIID.
Certain characteristics of Spt3 and Spt8 may be relevant to the mechanism of TBP inhibition. Spt3 has recently been shown to possess histone-fold motifs (5). Interestingly, the TBP-inhibitory protein NC2/Dr1 also possesses histone folds (31, 41), which are required for inhibition of TBP function (21). Spt8 is a very acidic protein, as is the region of TAFII145 (the amino terminus) which inhibits TBP binding to the TATA box (9, 36). Thus, while requiring further investigation, it is possible that these properties of Spt3 and Spt8 are important for the inhibition of TBP.
Detection of a novel SAGA-related complex under conditions inducing HIS3 transcription.
As discussed above, SAGA is required both for transcriptional repression and for full activation of the HIS3 and TRP3 genes. The presence of inhibitory subunits suggested that SAGA might be altered in composition under conditions that induce HIS3 and TRP3 expression. Indeed, the chromatographic properties and subunit composition of the complex were altered when cells were grown in 3-AT. Western blot analyses revealed that, under inducing conditions, two types of SAGA complex were present in the cell. While a very small amount of intact SAGA containing both inhibitory Spt3 and Spt8 subunits was detected, the vast majority of SAGA (SAGAalt) contained no detectable Spt8. However, most of the Spt8 was found as a separate moiety and might have been associated with additional proteins, based on the size of the small Spt8-containing complex (∼200 kDa versus 66 kDa for monomeric Spt8). In addition, a very small amount of SAGA lacking Spt8 was found in wild-type cells under noninducing conditions, and the separate Spt8 moiety was found as well. Further analysis indicated that SAGAalt is present in yeast cells grown in SC medium, where the level of HIS3 transcription is increased only approximately twofold over that in YPD. However, in SC medium, the amount of SAGA containing the inhibitory Spt8 subunit far exceeds that of SAGAalt. It is not until the addition of 3-AT, which is strongly inducing for HIS3 transcription, that the amount of SAGAalt is larger than that of SAGA. Thus, it appears that it may be critical that the amount of SAGAalt is larger than that of SAGA for the activation of Gcn4-regulated promoters.
These results suggest that the complex may be dynamic and that the altered form lacking Spt8 may be derived from the previously characterized SAGA. Spt3 was still associated with SAGAalt, indicating that Spt3 and Spt8 are structurally independent of one another in the complex, agreeing with our previous characterization of the composition of SAGAΔSpt3 and SAGAΔSpt8 (59). It is also possible that Spt8 or some other component of SAGA is modified in SAGAalt and that this modification causes the release of Spt8 during preparation and fractionation of the extract. Interestingly, we have detected an electrophoretic mobility alteration of Spt7 in SAGAalt compared to SAGA; this alteration may also be mechanistically related to altered activity and composition (R. Belotserkovskaya, unpublished data). In an alternative model for the relationship between the complexes, both forms coexist in the cell and altered growth conditions change the balance between them, such that the SAGA concentration is decreased relative to that of SAGAalt. Our current investigations are directed toward both fully defining the components of SAGAalt and exploring the nature of the relationship between SAGA and SAGAalt. We want to determine whether there is an actual conversion or whether there is an altered balance in distinct stable complexes.
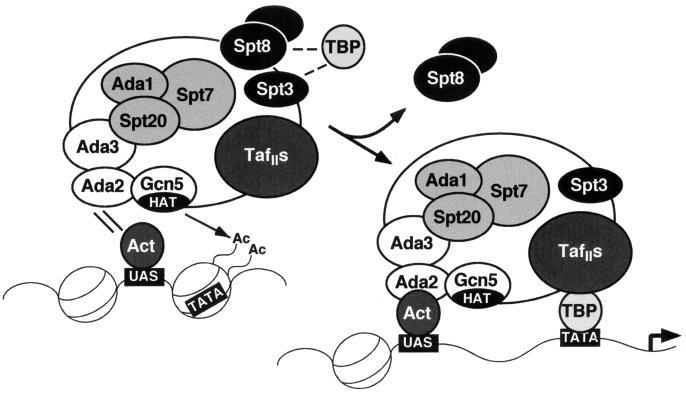
Hence, SAGA appears to exert negative regulation, with regard to at least one pathway in which it functions, the general amino acid control pathway. Based on these data and previous analyses of SAGA (22, 59), a model can be envisaged for the function of SAGA. When there is a plentiful source of amino acids for growth, TBP function is inhibited at certain SAGA-dependent promoters (Fig. 10, left), and Spt3 and Spt8 are involved in this inhibition. When amino acids are lacking, the SAGA complex loses inhibitory subunits, including Spt8. Finally, an activated promoter (Fig. 10, right) consists of a strong interaction of SAGA with transcriptional activators positioned at the upstream activation sequence, acetylated nucleosomes around the TATA box, and stabilization of TBP binding at the TATA box, perhaps by the TAF subgroup.
FIG. 10.
Model of SAGA complex function during transcriptional activation. (Left) Under noninducing conditions, specific components of SAGA, Spt3 and Spt8, down-regulate TBP function. Under inducing conditions, the SAGA complex is targeted to promoters of certain genes (e.g., HIS3 or TRP3) through interaction of its Ada2 subunit with an acidic activator (Act), such as Gcn4, bound to an upstream activation sequence (UAS). The HAT activity of Gcn5 acetylates (Ac) the histone tails of a nucleosome, destabilizing it and perhaps making a TATA box available for binding. (Right) In full activation, Spt8 is dissociated from the SAGA complex, and TBP-TATA binding is assisted by TAFIIs within SAGA.
Two novel and significant interpretations of this study are that Spt3 and Spt8 act as negative transcriptional regulators within SAGA and that Spt8 is part of a stable module that may dissociate from SAGA to activate the transcription of HIS3 and TRP3 under inducing conditions. Might changes in SAGA composition occur under other conditions? Spt3 also behaves as an inhibitor of SAGA function, although it is not lost from the complex during 3-AT induction. It is conceivable that Spt3 responds to other inducers of transcription to allow SAGA to function in transcriptional activation. In this regard, it is interesting that the SAGA-homologous human PCAF (p300-CBP-associated factor, a human Gcn5 homologue) complex possesses an Spt3 homolog (43), indicating that the human complex, too, may be negatively regulated. Also, most generally, the negative regulation of TBP may be a conserved function of different promoter-specific coactivator complexes, such as SAGA and TFIID.
ACKNOWLEDGMENTS
We thank F. Winston and L. Pacella heartily for valuable discussions and for generosity in providing the many SPT strains, plasmids, and antibodies used in this study. We also thank J. Workman, P. Grant, and members of the Workman laboratory as well as Thanos Halazonetis for advice and help in the fractionation of SAGA and SAGAalt. We thank J. Reese and M. Green for TAFII antibodies and A. Hinnebusch for Gcn4 antibodies and the kind gift of plasmids for GCN4 gene deletion and constitutive Gcn4 expression. We thank G. Moore, J. Workman, and members of the Berger laboratory for helpful discussions and critical comments on the manuscript.
D.E.S. was supported by a postdoctoral fellowship from the National Institutes of Health and by an NIH Cancer Core training grant to The Wistar Institute. This research was supported by grants from the National Institute of General Medical Sciences and the National Science Foundation to S.L.B. and the National Institute of General Medical Sciences to P.M.L.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 2.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 3.Barlev N A, Candau R, Wang L, Darpino P, Silverman N, Berger S L. Characterization of physical interactions of the putative transcriptional adaptor, ADA2, with acidic activation domains and TATA-binding protein. J Biol Chem. 1995;270:19337–19344. doi: 10.1074/jbc.270.33.19337. [DOI] [PubMed] [Google Scholar]
- 4.Berger S L, Piña B, Silverman N, Marcus G A, Agapite J, Regier J L, Triezenberg S J, Guarente L. Genetic isolation of ADA2: a potential transcriptional adaptor required for function of certain acidic activation domains. Cell. 1992;70:251–265. doi: 10.1016/0092-8674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- 5.Birck C, Poch O, Romier C, Ruff M, Mengus G, Lavigne A-C, Davidson I, Moras D. Human TAFII28 and TAFII18 interact through a canonical histone fold encoded by atypical evolutionary conserved sequence motifs also found in the SPT3 TAFII family. Cell. 1998;94:239–249. doi: 10.1016/s0092-8674(00)81423-3. [DOI] [PubMed] [Google Scholar]
- 6.Brandl C, Furlanetto A, Martens J, Hamilton K. Characterization of NGG1, a novel yeast gene required for glucose repression of GAL4p-regulated transcription. EMBO J. 1993;12:5255–5265. doi: 10.1002/j.1460-2075.1993.tb06221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brownell J E, Zhou J, Ranalli T, Kobayashi R, Edmondson D G, Roth S Y, Allis C D. Tetrahymena histone acetyltransferase A: a transcriptional co-activator linking gene expression to histone acetylation. Cell. 1996;84:843–851. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 8.Burley S K, Roeder R G. Biochemistry and structural biology of transcription factor IID (TFIID) Annu Rev Biochem. 1996;65:769–799. doi: 10.1146/annurev.bi.65.070196.004005. [DOI] [PubMed] [Google Scholar]
- 9.Burley S K, Roeder R G. TATA box mimicry by TFIID: autoinhibition of PolII transcription. Cell. 1998;94:551–553. doi: 10.1016/s0092-8674(00)81596-2. [DOI] [PubMed] [Google Scholar]
- 10.Candau R, Berger S L. Structural and functional analysis of yeast putative adaptors: evidence for an adaptor complex in vivo. J Biol Chem. 1996;271:5237–5245. doi: 10.1074/jbc.271.9.5237. [DOI] [PubMed] [Google Scholar]
- 11.Candau R, Moore P A, Wang L, Barlev N, Ying C Y, Rosen C A, Berger S L. Identification of functionally conserved human homologues of the yeast adaptors ADA2 and GCN5. Mol Cell Biol. 1996;16:593–602. doi: 10.1128/mcb.16.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang Y C, Komarnitsky P, Chase D, Denis C L. ADR1 activation domains contact the histone acetyltransferase GCN5 and the core transcriptional factor TFIIB. J Biol Chem. 1996;271:32359–32365. doi: 10.1074/jbc.271.50.32359. [DOI] [PubMed] [Google Scholar]
- 13.Collart M A. The NOT, SPT3, and MOT1 genes functionally interact to regulate transcription at core promoters. Mol Cell Biol. 1996;16:6668–6676. doi: 10.1128/mcb.16.12.6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drysdale C M, Jackson B M, McVeigh R, Klebanow E R, Bai Y, Kokubo T, Swanson M, Nakatani Y, Weil P A, Hinnebusch A G. The Gcn4p activation domain interacts specifically in vitro with RNA polymerase II holoenzyme, TFIID, and the Adap-Gcn5p coactivator complex. Mol Cell Biol. 1998;18:1711–1724. doi: 10.1128/mcb.18.3.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenmann D M, Arndt K M, Ricupero S L, Rooney J W, Winston F. SPT3 interacts with TFIID to allow normal transcription in Saccharomyces cerevisiae. Genes Dev. 1992;6:1319–1331. doi: 10.1101/gad.6.7.1319. [DOI] [PubMed] [Google Scholar]
- 16.Eisenmann D M, Chapon C, Roberts S M, Dollard C, Winston F. The Saccharomyces cerevisiae SPT8 gene encodes a very acidic protein that is functionally related to SPT3 and TATA-binding protein. Genetics. 1994;137:647–657. doi: 10.1093/genetics/137.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eisenmann D M, Dollard C, Winston F. SPT15, the gene encoding the yeast TATA binding factor TFIID, is required for normal transcription initiation in vivo. Cell. 1989;58:1183–1191. doi: 10.1016/0092-8674(89)90516-3. [DOI] [PubMed] [Google Scholar]
- 18.Feaver W J, Henry N L, Bushnell D A, Sayre M H, Brickner J H, Gileadi O, Kornberg R D. Yeast TFIIE: cloning, expression and homology to vertebrate proteins. J Biol Chem. 1994;269:27549–27553. [PubMed] [Google Scholar]
- 19.Gansheroff L J, Dollard C, Tan P, Winston F. The Saccharomyces cerevisiae SPT7 gene encodes a very acidic protein important for transcription in vivo. Genetics. 1995;139:523–536. doi: 10.1093/genetics/139.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georgakopoulos T, Thireos G. Two distinct yeast transcriptional activators require the function of the GCN5 protein to promote normal levels of transcription. EMBO J. 1992;11:4145–4152. doi: 10.1002/j.1460-2075.1992.tb05507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goppelt A, Stelzer G, Lottspeich F, Meisterernst M. A mechanism for repression of class II gene transcription through specific binding of NC2 to TPB-promoter complexes via heterodimeric histone fold domains. EMBO J. 1996;15:3105–3116. [PMC free article] [PubMed] [Google Scholar]
- 22.Grant P A, Duggan L, Côté J, Roberts S M, Brownell J E, Candau R, Ohba R, Owen-Hughes T, Allis C D, Winston F, Berger S L, Workman J L. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 1997;11:1640–1650. doi: 10.1101/gad.11.13.1640. [DOI] [PubMed] [Google Scholar]
- 23.Grant P A, Schieltz D, Pray-Grant M G, Steger D J, Reese J C, Yates III J R, Workman J L. A subset of TAFIIs are integral components of the SAGA complex required for nucleosome acetylation and transcription stimulation. Cell. 1998;94:45–53. doi: 10.1016/s0092-8674(00)81220-9. [DOI] [PubMed] [Google Scholar]
- 24.Grant P A, Sterner D E, Duggan L J, Workman J L, Berger S L. The SAGA unfolds: convergence of transcription regulators in chromatin-modifying complexes. Trends Cell Biol. 1998;8:193–197. doi: 10.1016/s0962-8924(98)01263-x. [DOI] [PubMed] [Google Scholar]
- 25.Gregory P D, Schmid A, Zavari M, Liu L, Berger S L, Hörz W. Absence of Gcn5 HAT activity defines a novel state in the opening of chromatin at the PHO5 promoter in yeast. Mol Cell. 1998;1:495–505. doi: 10.1016/s1097-2765(00)80050-7. [DOI] [PubMed] [Google Scholar]
- 26.Hahn S, Buratowski S, Sharp P A, Guarente L. Isolation of the gene encoding the yeast TATA binding protein TFIID: a gene identical to the SPT15 suppressor of Ty element insertions. Cell. 1989;58:1173–1181. doi: 10.1016/0092-8674(89)90515-1. [DOI] [PubMed] [Google Scholar]
- 27.Harlow E, Lane I. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 28.Hoffmann A, Chiang C-M, Oelgeschläger T, Xie X, Burley S K, Nakatani Y, Roeder R G. A histone octamer-like structure within TFIID. Nature. 1996;380:356–359. doi: 10.1038/380356a0. [DOI] [PubMed] [Google Scholar]
- 29.Horiuchi J, Silverman N, Marcus G A, Guarente L. ADA3, a putative transcriptional adaptor, consists of two separable domains and interacts with ADA2 and GCN5 in a trimeric complex. Mol Cell Biol. 1995;15:1203–1209. doi: 10.1128/mcb.15.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horiuchi J, Silverman N, Piña B, Marcus G A, Guarente L. ADA1, a novel component of the ADA/GCN5 complex, has broader effects than GCN5, ADA2, or ADA3. Mol Cell Biol. 1997;17:3220–3228. doi: 10.1128/mcb.17.6.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inostroza J A, Mermelstein F H, Ha I, Lane W S, Reinberg D. Dr1, a TATA-binding protein–associated phosphoprotein and inhibitor of class II gene transcription. Cell. 1992;70:477–489. doi: 10.1016/0092-8674(92)90172-9. [DOI] [PubMed] [Google Scholar]
- 32.Iyer V, Struhl K. Absolute mRNA levels and transcriptional initiation rates in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5208–5212. doi: 10.1073/pnas.93.11.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kokubo T, Yamashita S, Horikoshi M, Roeder R G, Nakatani Y. Interaction between the N-terminal domain of the 230-kDa subunit and the TATA box-binding subunit of TFIID negatively regulates TATA-box binding. Proc Natl Acad Sci USA. 1994;91:3520–3524. doi: 10.1073/pnas.91.9.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo M-H, Zhou J, Jambeck P, Churchill M E A, Allis C D. Histone acetyltransferase activity of yeast Gcn5p is required for the activation of target genes in vivo. Genes Dev. 1998;12:627–639. doi: 10.1101/gad.12.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieberman P M, Berk A J. The Zta trans-activator protein stabilizes TFIID association with promoter DNA by direct protein-protein interaction. Genes Dev. 1991;5:2441–2454. doi: 10.1101/gad.5.12b.2441. [DOI] [PubMed] [Google Scholar]
- 36.Liu D, Ishima R, Tong K I, Bagby S, Kokubo T, Muhandiram D R, Kay L E, Nakatani Y, Ikura M. Solution structure of a TBP-TAF(II)230 complex: protein mimicry of the minor groove surface of the TATA box unwound by TBP. Cell. 1998;94:573–583. doi: 10.1016/s0092-8674(00)81599-8. [DOI] [PubMed] [Google Scholar]
- 37.Longtine M S, McKenzie III A, Demarini D J, Shah N G, Wach A, Brachat A, Philippsen P, Pringle J R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 38.Madison J M, Winston F. Evidence that Spt3 functionally interacts with Mot1, TFIIA, and TATA-binding protein to confer promoter-specific transcriptional control in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:287–295. doi: 10.1128/mcb.17.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcus G A, Horiuchi J, Silverman N, Guarente L. ADA5/SPT20 links the ADA and SPT genes, which are involved in yeast transcription. Mol Cell Biol. 1996;16:3197–3205. doi: 10.1128/mcb.16.6.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcus G A, Silverman N, Berger S L, Horiuchi J, Guarente L. Functional similarity and physical association between GCN5 and ADA2: putative transcriptional adaptors. EMBO J. 1994;13:4807–4815. doi: 10.1002/j.1460-2075.1994.tb06806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meisterernst M, Roeder R G. Family of proteins that interact with TFIID and regulate promoter activity. Cell. 1991;67:557–567. doi: 10.1016/0092-8674(91)90530-c. [DOI] [PubMed] [Google Scholar]
- 42.Mizzen C A, Yang X-J, Kokubo T, Brownell J E, Bannister A J, Owen-Hughes T, Workman J, Wang L, Berger S L, Kouzarides T, Nakatani Y, Allis C D. The TAF(II)250 subunit of TFIID has histone acetyltransferase activity. Cell. 1996;87:1261–1270. doi: 10.1016/s0092-8674(00)81821-8. [DOI] [PubMed] [Google Scholar]
- 43.Ogryzko V V, Kotani T, Zhang X, Schiltz R L, Howard T, Yang X-J, Howard B H, Qin J, Nakatani Y. Histone-like TAFs within the PCAF histone acetylase complex. Cell. 1998;94:35–44. doi: 10.1016/s0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- 44.Ogryzko V V, Schlitz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 45.Ozer J, Lezina L E, Ewing J, Audi S, Lieberman P M. Association of transcription factor IIA with TATA binding protein is required for transcriptional activation of a subset of promoters and cell cycle progression in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:2559–2570. doi: 10.1128/mcb.18.5.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piña B, Berger S, Marcus G A, Silverman N, Agapite J, Guarente L. ADA3: a gene, identified by resistance to GAL4-VP16, with properties similar to and different from those of ADA2. Mol Cell Biol. 1993;13:5981–5989. doi: 10.1128/mcb.13.10.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reese J C, Apone L, Walker S S, Griffin L A, Green M R. Yeast TAFIIS in a multisubunit complex required for activated transcription. Nature. 1994;371:523–527. doi: 10.1038/371523a0. [DOI] [PubMed] [Google Scholar]
- 48.Roberts S G, Choy B, Walker S S, Lin Y S, Green M R. A role for activator-mediated TFIIB recruitment in diverse aspects of transcriptional regulation. Curr Biol. 1995;5:508–516. doi: 10.1016/s0960-9822(95)00103-5. [DOI] [PubMed] [Google Scholar]
- 49.Roberts S M, Winston F. Essential functional interactions of SAGA, a Saccharomyces cerevisiae complex of Spt, Ada, and Gcn5 proteins, with the Snf/Swi and Srb/mediator complexes. Genetics. 1997;147:451–465. doi: 10.1093/genetics/147.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts S M, Winston F. SPT20/ADA5 encodes a novel protein functionally related to the TATA-binding protein and important for transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:3206–3213. doi: 10.1128/mcb.16.6.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rose M D, Winston F, Hieter P. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 52.Rothstein R J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- 53.Saleh A, Lang V, Cook R, Brandl C J. Identification of native complexes containing the yeast coactivator/repressor proteins NGG1/ADA3 and ADA2. J Biol Chem. 1997;272:5571–5578. doi: 10.1074/jbc.272.9.5571. [DOI] [PubMed] [Google Scholar]
- 54.Saleh A, Schieltz D, Ting N, McMahon S B, Litchfield D W, Yates III J R, Lees-Miller S P, Cole M D, Brandl C J. Tra1p is a component of the yeast Ada.Spt transcriptional regulatory complexes. J Biol Chem. 1998;273:26559–26565. doi: 10.1074/jbc.273.41.26559. [DOI] [PubMed] [Google Scholar]
- 55.Sawadogo M, Roeder R. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985;43:165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- 56.Sayre M H, Tschochner H, Kornberg R D. Reconstitution of transcription with five purified initiation factors and RNA polymerase II from Saccharomyces cerevisiae. J Biol Chem. 1992;267:23376–23382. [PubMed] [Google Scholar]
- 57.Silverman N, Agapite J, Guarente L. Yeast ADA2 protein binds to the VP16 protein activation domain and activates transcription. Proc Natl Acad Sci USA. 1994;91:11665–11668. doi: 10.1073/pnas.91.24.11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stargell L A, Struhl K. Mechanisms of transcriptional activation in vivo: two steps forward. Trends Genet. 1996;12:311–315. doi: 10.1016/0168-9525(96)10028-7. [DOI] [PubMed] [Google Scholar]
- 59.Sterner D E, Grant P A, Roberts S M, Duggan L J, Belotserkovskaya R, Pacella L A, Winston F, Workman J L, Berger S L. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TBP binding. Mol Cell Biol. 1999;19:86–98. doi: 10.1128/mcb.19.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Struhl K. Constitutive and inducible Saccharomyces cerevisiae promoters: evidence for two distinct molecular mechanisms. Mol Cell Biol. 1986;6:3847–3853. doi: 10.1128/mcb.6.11.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Utley R T, Ikeda K, Grant P A, Côté J, Steger D J, Eberharter A, John S, Workman J L. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature. 1998;394:498–502. doi: 10.1038/28886. [DOI] [PubMed] [Google Scholar]
- 62.Vassilev A, Yamauchi J, Kotani T, Prives C, Avantaggiati M L, Qin J, Nakatani Y. The 400 kDa subunit of the PCAF histone acetylase complex belongs to the ATM superfamily. Mol Cell. 1998;2:869–875. doi: 10.1016/s1097-2765(00)80301-9. [DOI] [PubMed] [Google Scholar]
- 63.Wade P A, Wolffe A P. Histone acetyltransferases in control. Curr Biol. 1997;7:82–84. doi: 10.1016/s0960-9822(06)00042-x. [DOI] [PubMed] [Google Scholar]
- 64.Wang L, Liu L, Berger S L. Critical residues for histone acetylation by GCN5, functioning in Ada and SAGA complexes, are also required for transcriptional function in vivo. Genes Dev. 1998;12:640–653. doi: 10.1101/gad.12.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winston F. Analysis of SPT genes: a genetic approach toward analysis of TFIID, histones, and other transcription factors of yeast. In: McKnight S L, Yamamoto K R, editors. Transcriptional regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 1271–1293. [Google Scholar]
- 66.Winston F, Dollard C, Ricupero-Hovasse S L. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast. 1995;11:53–55. doi: 10.1002/yea.320110107. [DOI] [PubMed] [Google Scholar]
- 67.Winston F, Durbin K J, Fink G R. The SPT3 gene is required for normal transcription of Ty elements in S. cerevisiae. Cell. 1984;39:675–682. doi: 10.1016/0092-8674(84)90474-4. [DOI] [PubMed] [Google Scholar]
- 68.Xie X, Kokubo T, Cohen S L, Mirza U A, Hoffmann A, Chait B T, Roeder R G, Nakatani Y, Burley S K. Structural similarity between TAFs and the heterotetrameric core of the histone octamer. Nature. 1996;380:316–322. doi: 10.1038/380316a0. [DOI] [PubMed] [Google Scholar]
- 69.Yang X-J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral E1A oncoprotein. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]