TRANCE, a Tumor Necrosis Factor Family Member Critical for CD40 Ligand–independent T Helper Cell Activation (original) (raw)
Abstract
CD40 ligand (CD40L), a tumor necrosis factor (TNF) family member, plays a critical role in antigen-specific T cell responses in vivo. CD40L expressed on activated CD4+ T cells stimulates antigen-presenting cells such as dendritic cells, resulting in the upregulation of costimulatory molecules and the production of various inflammatory cytokines required for CD4+ T cell priming in vivo. However, CD40L- or CD40-deficient mice challenged with viruses mount protective CD4+ T cell responses that produce normal levels of interferon γ, suggesting a CD40L/CD40-independent mechanism of CD4+ T cell priming that to date has not been elucidated. Here we show that CD4+ T cell responses to viral infection were greatly diminished in CD40-deficient mice by administration of a soluble form of TNF-related activation-induced cytokine receptor (TRANCE-R) to inhibit the function of another TNF family member, TRANCE. Thus, the TRANCE/TRANCE-R interaction provides costimulation required for efficient CD4+ T cell priming during viral infection in the absence of CD40L/CD40. These results also indicate that not even the potent inflammatory microenvironment induced by viral infections is sufficient to elicit efficient CD4+ T cell priming without proper costimulation provided by the TNF family (CD40L or TRANCE). Moreover, the data suggest that TRANCE/TRANCE-R may be a novel and important target for immune intervention.
Keywords: TRANCE, CD40 ligand, T cell, dendritic cell, virus
ATNF family member, CD40L, has been shown to be critical for the generation of antigen-specific T cell responses in vivo (1–8). CD40L expressed on activated T cells triggers CD40 on macrophages and dendritic cells (DCs),1 resulting in the upregulation of costimulatory molecules and the induction of IL-12 in these APCs (9–13). These costimulatory molecules and IL-12 then potentiate CD4+ T cell responses in vivo (1–3, 9, 10, 14–18). However, CD40L- or CD40-deficient mice challenged with viral infections such as lymphocytic choriomeningitis virus (LCMV) are able to mount protective CD4+ T cell responses that produce normal levels of IFN-γ (19–21). In addition, a majority of CD40L-deficient patients with hyper-IgM syndrome (HIGM) do not show increased susceptibility to various infections associated with defective CD4+ T cell immune responses (22), suggesting that some pathogens are able to activate CD4+ T cells via a CD40L/CD40-independent pathway. The mechanism determining CD40/ CD40L independence of CD4+ T cell responses during intracellular infections is not understood. It has been speculated that destruction of infected cells and the production of various inflammatory cytokines (e.g., INF-γ) in response to, for example, viral infections constitute a sufficiently powerful adjuvant effect to allow the activation of T cells in the absence of costimulation provided by CD40L/CD40 interaction.
In this study, the cellular immune responses to viral infections were examined in order to elucidate the factor(s) responsible for CD40L/CD40-independent CD4+ T cell priming. We show here that CD40L/CD40-independent activation of CD4+ T cells during viral infection requires TRANCE/TRANCE-R interaction (23–25). Thus, this study suggests that CD4+ T cell priming in general is likely to be regulated by one of two TNF family members (CD40L or TRANCE).
Materials and Methods
Mice, Viruses, Cells, Abs, and Recombinant Proteins.
CD40-deficient mice have been described and were originally provided by Dr. H. Kikutani (Osaka University, Osaka, Japan [26]). As control mice, CD40+/− or C57BL/6 mice were used, giving similar results. LCMV (WE strain) was grown on L cells at a low multiplicity of infection. LCMV WE was originally provided by Dr. R. Zinkernagel (University of Zürich, Zürich, Switzerland). Influenza virus (strain PR8) was originally provided by Dr. J. Pavlovic (University of Zürich) and grown in day 10–fertilized chicken eggs. Mature bone marrow–derived DCs were generated as described (24). Anti–IL-12 p35 (C18.2) and p40 (C15.1) mAbs were provided by G. Trinchieri (Wistar Institute, Philadelphia, PA [27]). For activation of T cells in vitro, T cells were purified and stimulated with anti-CD3 and anti-CD28 Abs as described previously (24). Soluble TRANCE produced from recombinant baculoviruses has been described previously (24). TRANCE-R–Fc (TR-Fc), a recombinant protein of the extracellular domain of TRANCE-R fused to the constant region of human IgG1, was produced in a similar way using a baculovirus system and purified on protein A–Sepharose beads (Amersham Pharmacia Biotech).
Infection and Treatment with TR-Fc.
For LCMV-specific CD4+ T cell proliferation, mice were infected intravenously or into one hind footpad with 200 PFU of LCMV WE. Spleen cells were isolated 13 or 30 d later, and proliferation and cytokine production were measured as described (19). To assess cytotoxicity, mice were infected intravenously with 200 PFU of LCMV, and spleen cells were isolated 8 d later. For influenza virus–specific proliferation, mice were infected intranasally with virus (0.1 hemagglutination U/mouse). Spleen cells were isolated 8 d later. Mice were injected three times, on days 0, 2, and 5 after infection, with 100 μg of either TR-Fc or control hIgG1.
CTL and B Cell Responses.
EL-4 target cells were pulsed with peptide p33 (KAVYNFATM) at a concentration of 10−7 M for 90 min at 37°C in the presence of [51Cr]sodium chromate in IMDM supplemented with 10% FCS. Cells were washed three times, and 104 cells were transferred to a well of a round-bottomed 96-well plate. Stimulated or ex vivo–isolated spleen cell suspensions were serially diluted and mixed with peptide-pulsed target cells. Plates were centrifuged and incubated for various time spans at 37°C. At the end of the assays, 70 μl of supernatant was counted in a γ-counter. Spontaneous release was determined by adding medium instead of effector cells, and total release was determined by adding 2 M HCl instead of effector cells. Percent specific release was calculated as 100 × (experimental release − spontaneous release)/(total release − spontaneous release). For assessment of B cell responses, LCMV-specific IgG Abs were determined as described on plates coated with LCMV nucleoprotein produced by recombinant baculoviruses (19). PNA staining was performed on acetone-fixed frozen sections as described (28).
In Vitro Proliferation and Production of IFN-γ.
For LCMV-specific CD4+ T cell proliferation, spleen cells were isolated 13 or 30 d after infection and CD4+ T cells were purified by MACS® according to the instructions of the supplier (Miltenyi Biotech). Purity was >95%. 105 CD4+ T cells were stimulated with 105 irradiated LCMV (highest concentration = multiplicity of infection = 0.3) or peptide 13 (GLNGTDIYKGVYQFKSVEFD; highest concentration = 3 μg/ml)–pulsed splenic APCs, and proliferation was assessed 3 d later by [3H]thymidine incorporation. Production of IFN-γ was assessed in the wells with the highest antigen concentration by ELISA (19). For influenza virus–specific CD4+ T cell responses, spleen cells were isolated 8 d after infection, and purified CD4+ T cells (2 × 105 cells/well) were restimulated with irradiated spleen cells (105 cells/well) in the presence of various concentrations of UV light–inactivated, purified influenza virus. Proliferation and IFN-γ production were measured as described above.
Results and Discussion
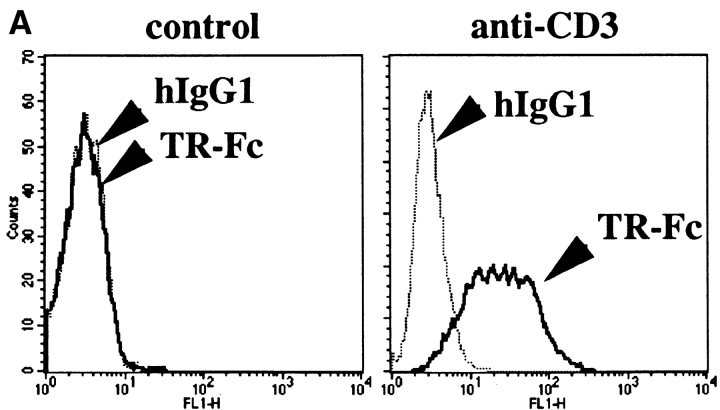
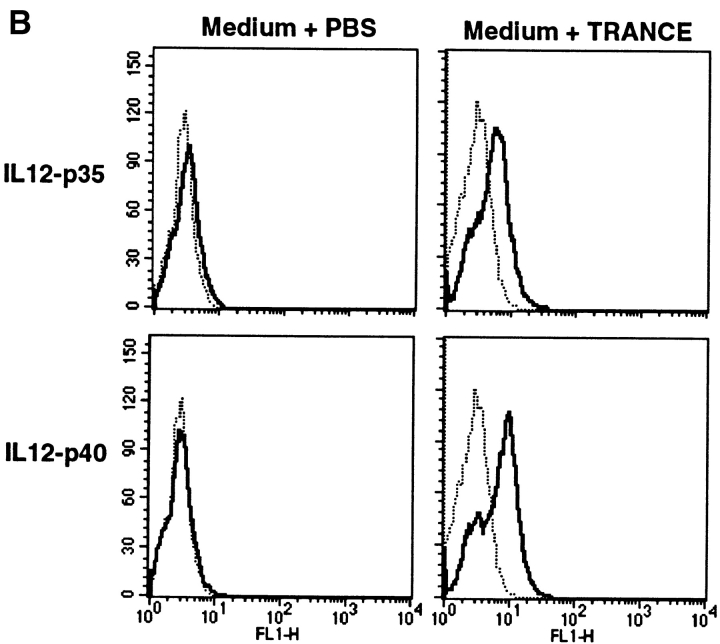
A recently identified member of the TNF receptor family, TRANCE-R (also called RANK), has been shown to be expressed at high levels on mature DCs (23–25). Moreover, TRANCE treatment enhanced the survival of mature DCs, indicating that TRANCE-R may exhibit a similar function as CD40 on these cells (24). To test whether TRANCE/ TRANCE-R interaction may play a role in T cell activation, surface expression of TRANCE was analyzed on activated T cells. Similar to CD40L, surface TRANCE expression was highly upregulated on T cells upon stimulation through antigen receptors (Fig. 1 A). Moreover, when mature DCs were treated with soluble TRANCE, the expression of IL-12 (Fig. 1 B) and other inflammatory cytokines (e.g., IL-1 or IL-6; data not shown) was induced in mature DCs, a property also shared by CD40L (11–13). Together, these results suggested that TRANCE and CD40L may share some similar functions in vivo during T cell activation and that TRANCE may be responsible for CD40L-independent CD4+ T cell responses, as observed in some murine model systems such as during viral infections (19–21).
Figure 1.
(A) TRANCE expression is upregulated after T cell activation. Purified T cells were stimulated with anti-CD3 plus anti-CD28 and stained with TR-Fc or control hIgG1, followed by FITC-conjugated goat anti–human IgG (Fc-specific) F(ab′)2 fragment (Jackson ImmunoResearch Laboratories). (B) TRANCE induces IL-12 production in mature DCs. Mature bone marrow–derived DCs were cultured for 18 h in the presence or absence of soluble TRANCE (1 μg/ml), then fixed in 2% PFA. After incubation in 0.5% saponin, the cells were stained with anti– IL-12 p35 (C18.2), anti–IL-12 p40 (C15.1) (solid line), or control rat IgG (dotted line) followed by anti–rat IgG-PE (Jackson ImmunoResearch Laboratories), and analyzed by FACS®. In parallel experiments, soluble CD40L also induced IL-12 p35 and IL-12 p40, at levels quantitatively similar to those induced by soluble TRANCE (data not shown).
To test this hypothesis, we chose to study the immune response to LCMV infection as a murine model since it has been extensively characterized and also because the activation of CD4+ T cells during LCMV infection was shown not to be affected in CD40L- or CD40-deficient mice (19).
To analyze whether TRANCE is upregulated in vivo during the course of an immune response after viral infection, mice were infected with LCMV, and spleen cells were analyzed for TRANCE expression 8 d later. Indeed, the proportion of TRANCE-expressing T cells increased after infection (∼6% of CD4+ T cells and ∼7% of CD8+ T cells became TRANCE-positive, whereas 0% of T cells expressed TRANCE in uninfected control mice).
To determine whether TRANCE plays a role during immune responses in vivo, and if so, whether it exhibits a compensatory role for CD40L during viral infections, we tested the consequences of blocking the TRANCE/TRANCE-R interaction by injection of TR-Fc on antigen-specific B, CD8+, and CD4+ T cell responses induced by LCMV infection in control (C57BL/6 or CD40+/−) and CD40-deficient mice (26).
The most prominent role of CD40L is to promote isotype switching in activated B cells and to allow the formation of germinal centers (GCs; 1–3). Indeed, CD40-deficient mice failed to produce high titers of LCMV-specific IgG Abs and produced no GCs (Fig. 2, A, D, and E). In contrast, TR-Fc–treated C57BL/6 mice mounted LCMV-specific IgG responses comparable to those of control mice treated with hIgG1 (Fig. 2 A) and generated similar numbers of GCs of normal architecture (Fig. 2, B and C). These results suggest that the TRANCE/TRANCE-R interaction does not play a critical role in T–B cell collaboration, despite the low level of TRANCE-R that can be detected on activated B cells (data not shown).
Figure 2.
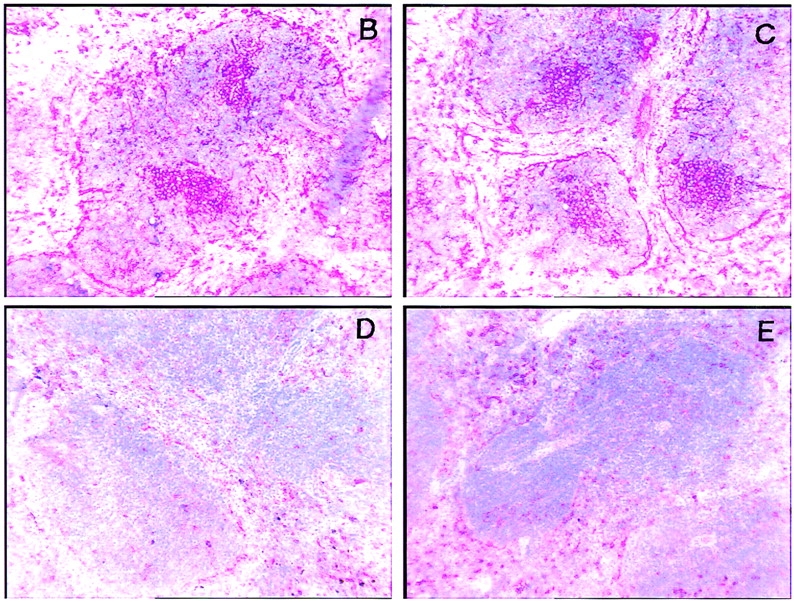
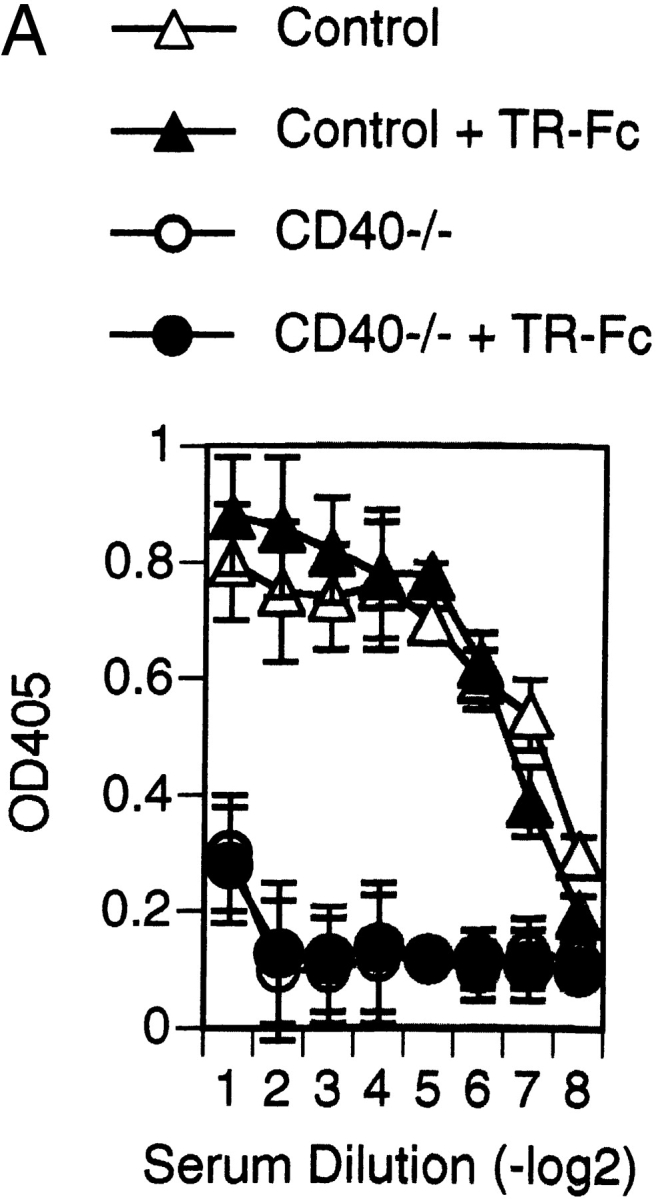
(A) Blocking TRANCE does not affect isotype switching after LCMV infection. C57BL/6 mice (triangles) or CD40-deficient mice (circles) were infected with LCMV and treated with TR-Fc (filled symbols) or control hIgG1 (open symbols). LCMV-specific IgG Abs were assessed 14 d later by ELISA. One representative experiment of two is shown. (B–E) Blocking TRANCE does not affect GC formation after LCMV infection. C57BL/6 mice (B and C) and CD40-deficient mice (D and E) were infected with LCMV and treated with either TR-Fc (B and D) or control hIgG1 (C and E). The presence of GCs was assessed 14 d later in spleens by PNA staining. One representative experiment of two is shown.
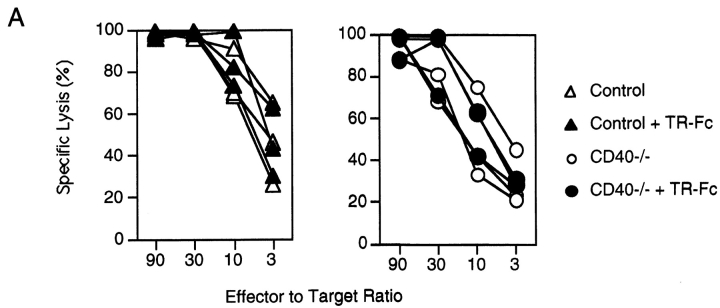
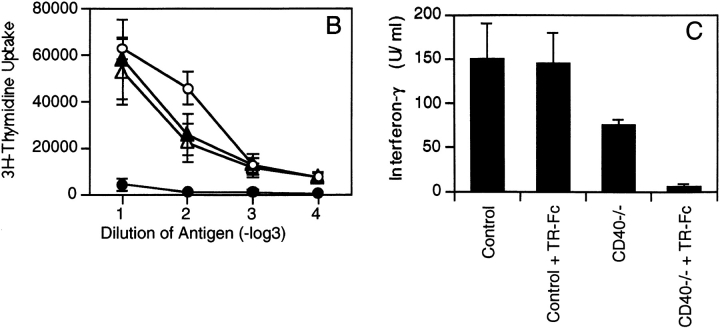
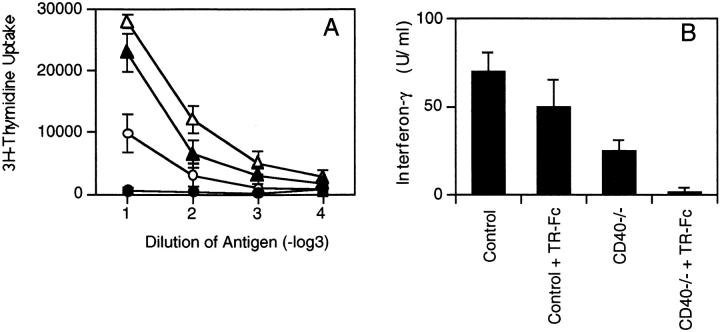
We next analyzed the ability of TR-Fc–treated control and CD40-deficient mice to mount LCMV-specific T cell responses. Mice were injected with LCMV, and CD8+ T cell–mediated responses were analyzed in a 51Cr-release assay 8 d later (Fig. 3 A). In keeping with previous reports (20, 21), the CD40L/CD40 interaction was not required for efficient primary CTL responses against LCMV (Fig. 3 A). In addition, inhibition of the TRANCE/TRANCE-R interaction did not affect the LCMV-specific acute CTL responses (Fig. 3 A). Moreover, inhibition of both the TRANCE/ TRANCE-R and CD40L/CD40 interactions did not affect acute CTL responses (Fig. 3 A). These results suggest that primary LCMV-specific CTL responses are largely independent of CD40L and TRANCE on activated T cells.
Figure 3.
Blocking TRANCE does not interfere with the induction of cytotoxic T cells but plays a role in the LCMV-specific CD4+ T cell responses. C57BL/6 mice (triangles) or CD40-deficient mice (circles) were infected with LCMV and treated with TR-Fc (filled symbols) or control hIgG1 (open symbols). (A) The presence of LCMV-specific cytotoxic T cells was assessed 8 d after infection using peptide p33– pulsed EL-4 cells as target cells. (B and C) Spleen cells were isolated 13 d later, and CD4+ T cells were purified and stimulated in vitro with LCMV-infected splenic APCs. (B) Proliferation was assessed 3 d later by [3H]thymidine incorporation. Results are shown as mean ± SEM for three mice per group. (C) Secretion of IFN-γ was assessed from culture supernatants by ELISA. Results are shown as mean ± SEM from three mice per group. Identical results were obtained with the LCMV-derived class II binding peptide 13 (data not shown). One representative experiment of two is shown.
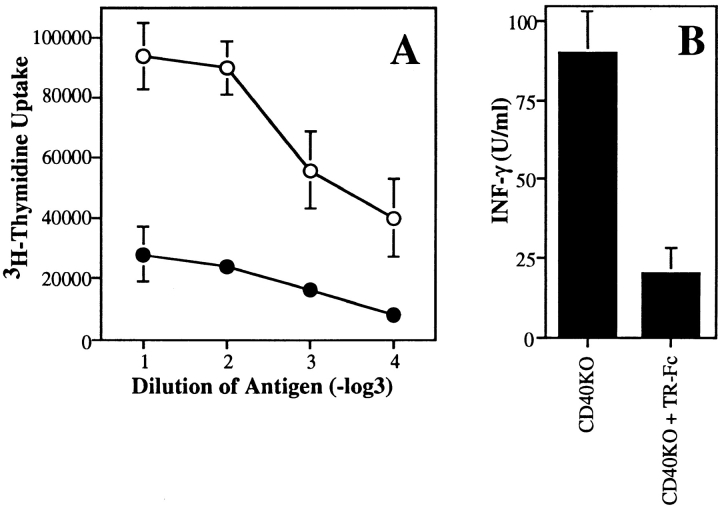
LCMV-specific CD4+ T cell responses were then examined early after infection (day 13) in TR-Fc–treated control and CD40-deficient mice by measuring in vitro recall proliferative responses. As reported previously (19), LCMV-specific CD4+ T cells produced a Th1 cytokine pattern, since large amounts of IFN-γ (Fig. 3 C) but not IL-4 (data not shown) were detected in culture supernatants. Purified CD4+ T cells from CD40-deficient mice proliferated normally and produced, although at reduced levels, IFN-γ after stimulation with LCMV-derived antigens (Fig. 3, B and C), indicating that LCMV can prime antigen-specific CD4+ T cells in a CD40L/CD40-independent manner. TR-Fc–treated control mice also mounted normal CD4+ T cell responses (Fig. 3, B and C). In marked contrast, the proliferative response of CD4+ T cells in TR-Fc–treated CD40-deficient mice was nearly completely blocked (Fig. 3 B). In addition, the production of IFN-γ was also completely abrogated in these mice (Fig. 3 C). This was not due to immune deviation, since blocking the TRANCE/TRANCE-R interaction in control or CD40-deficient mice did not upregulate IL-4 production (data not shown). To determine whether the lack of LCMV-specific CD4+ T cell responses was due to a delay in T cell priming in the absence of both CD40L/ CD40 and TRANCE/TRANCE-R interactions, CD4+ T cells were purified and restimulated with viral antigens 1 mo after infection. Even 30 d after infection, no significant LCMV-specific CD4+ T cell responses were detected in the absence of both CD40L/CD40 and TRANCE/ TRANCE-R interactions (Fig. 4). Therefore, the results indicate that either the CD40L/CD40 or the TRANCE/ TRANCE-R interaction is required for induction of CD4+ T cell responses by LCMV.
Figure 4.
LCMV-specific CD4+ T cell responses at a later time point. C57BL/6 mice (triangles) or CD40-deficient mice (circles) were infected with LCMV and treated with either TR-Fc (filled symbols) or control hIgG1 (open symbols). Spleen cells were isolated 30 d later, and CD4+ T cells were purified and stimulated in vitro with LCMV-infected splenic APCs. (A) Proliferation was assessed 3 d later by [3H]thymidine incorporation. Results are shown as mean ± SEM of triplicate values from pooled spleen cells of three mice per group. (B) Secretion of IFN-γ was assessed from culture supernatants by ELISA. Results are shown as mean ± SEM of triplicate values from pooled spleen cells of three mice per group. Identical results were obtained with the LCMV-derived class II binding peptide 13 (data not shown). One representative experiment of two is shown.
To analyze whether TRANCE can also mediate CD40L/ CD40-independent CD4+ T cell responses in other viral systems, CD40-deficient mice were infected with influenza virus, and virus-specific CD4+ T cell responses were analyzed (Fig. 5). As observed for LCMV, influenza virus can prime antigen-specific CD4+ T cells in a CD40L/CD40-independent manner, and the induction of virus-specific CD4+ T cell responses was greatly inhibited in the TR-Fc–treated CD40-deficient mice (Fig. 5). Thus, TRANCE/TRANCE-R provides a major costimulatory stimulus in the absence of CD40L/CD40 for CD4+ T cell responses to influenza viruses.
Figure 5.
TRANCE plays a role in influenza virus–specific CD4+ T cell responses. CD40-deficient mice were infected with influenza virus and treated with either TR-Fc (filled circles) or control hIgG1 (open circles). Spleen cells were isolated 8 d later, and CD4+ T cells were purified and restimulated in vitro with UV light–inactivated influenza viruses. (A) Proliferation was assessed 3 d later by [3H]thymidine incorporation. Results are shown as mean ± SEM from triplicate values from pooled spleen cells of three mice per group. Background proliferation is subtracted. (B) Secretion of IFN-γ was assessed from culture supernatants by ELISA. Results are shown as mean ± SEM from three mice per group. Background is <2 U/ml. One representative experiment of three is shown.
In summary, this study establishes the TRANCE/ TRANCE-R interaction as an important player in CD4+ T cell responses in vivo. Moreover, we also show that the TRANCE/TRANCE-R interaction compensates for a lack of CD40L/CD40 interaction to allow efficient CD4+ T cell responses during viral infection. This explains why viruses can induce CD4+ T cell immune responses in CD40L- or CD40-deficient mice. In addition, this study also shows that despite the destruction of infected cells and the production of various inflammatory cytokines in response to viral infection, efficient CD4+ T cell priming still requires costimulation predominantly by TNF family members (i.e., either TRANCE or CD40L), which is analogous to CD4+ T cell priming induced by purified proteins administered with CFA (in this case, costimulation provided by CD40L). Therefore, it is possible that CD4+ T cell priming in general may require costimulation by at least one TNF family member (e.g., CD40L or TRANCE).
CD40L-mediated CD4+ T cell activation occurs indirectly via activation of the APCs (1–3, 9–13). Specifically, in vitro stimulation of CD40 on DCs stimulates a maturation process culminating in the upregulation of costimulatory molecules and the capacity to produce IL-12, a cytokine important for production of IFN-γ by CD4+ T cells (1–3, 9–13). Although stimulation of TRANCE-R on mature DCs fails to upregulate costimulatory molecules on these cells (24), we showed that, similar to CD40L, TRANCE treatment triggered generation of IL-12 and other proinflammatory cytokines by mature DCs. In addition, when stimulated in vitro by anti-CD3, purified T cells proliferated and produced normal levels of cytokines in the presence of TR-Fc (data not shown), suggesting that, similar to the CD40L/CD40 interaction, there is no direct role for the TRANCE/TRANCE-R interaction in T cells. Therefore, the TRANCE/TRANCE-R and CD40L/CD40 interactions between CD4+ T cells and APCs may have functional consequences primarily for the APCs, e.g., promoting DC viability and cytokine production (1–3, 9–13, 24). It is presently not known why some antigens (e.g., proteins in adjuvants) use predominantly the CD40L-dependent pathway (1–3) while others (e.g., viruses [this study]) use both TRANCE- and CD40L-dependent pathways of CD4+ T cell stimulation. It is possible that certain viruses directly upregulate TRANCE-R during DC differentiation. Alternatively, there may be different requirements for induction of TRANCE and CD40L on T cells. It is also possible, although not yet determined, that some pathogens might use predominantly the TRANCE-dependent pathway to elicit efficient CD4+ T cell responses. The CD40L/CD40 interaction is an important site for manipulating the immune response in order to facilitate organ transplantation and to reduce atherosclerosis (1–3, 29), and our in vivo findings now suggest that the interaction of TRANCE and its receptor may be an additional target for immunotherapy.
Acknowledgments
We thank Manfred Kopf and Marco Colonna for comments and critical discussion. We also thank Angela Santana, Hong-Li Li, and Barbara Ecabert for excellent technical assistance.
Abbreviations used in this paper
DC
dendritic cell
GC
germinal center
LCMV
lymphocytic choriomeningitis virus
TRANCE
TNF-related activation-induced cytokine
Footnotes
The Basel Institute for Immunology was founded and is supported by F. Hoffmann-La Roche, Basel, Switzerland. This work was supported in part by National Institutes of Health Medical Scientist Training Program grant GM-07739 (to B.R. Wong), and National Institutes of Health grants AI-44264 (to Y. Choi), AI-13013 (to R.M. Steinman), and AI-39672 (to R.M. Steinman). R. Josien is supported by a fellowship from the Revson Foundation. Y. Choi is an investigator of the Howard Hughes Medical Institute.
References
- 1.Foy TM, Aruffo A, Bajorah J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 2.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–136. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 3.Van Kooten C, Banchereau J. CD40-CD40 ligand: a multifunctional receptor-ligand pair. Adv Immunol. 1996;61:1–77. doi: 10.1016/s0065-2776(08)60865-2. [DOI] [PubMed] [Google Scholar]
- 4.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 5.van Essen D, Kikutani H, Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature. 1995;378:620–623. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]
- 6.Campbell KA, Ovendale PJ, Kennedy MK, Fanslow WC, Reed SG, Maliszewski CR. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. . Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 7.Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania majorinfection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 8.Soong L, Xu J-C, Grewal IS, Kima P, Sun J, Longley BJ, Jr, Ruddle NH, McMahon-Pratt D, Flavell RA. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensisinfection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 9.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 10.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 11.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 12.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kämpgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cella M, Scheider D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grewal IS, Foellmer HG, Grewal KD, Xu J, Hardardottir F, Baron JL, Janeway CA, Jr, Flavell RA. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 1996;273:1864–1867. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 15.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker BC, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 16.Ridge JP, DiRosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 17.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 18.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 19.Oxenius A, Campbell KA, Maliszewski CR, Kishimoto T, Kikutani H, Hengartner H, Zinkernagel RM, Bachmann MF. CD40–CD40 ligand interactions are critical in T–B cooperation but not for other anti-viral CD4+T cell functions. J Exp Med. 1996;183:2209–2218. doi: 10.1084/jem.183.5.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borrow P, Tishon A, Lee S, Xu J, Grewal IS, Oldstone MB, Flavell RA. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+CTL response. J Exp Med. 1996;183:2129–2142. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitmire JK, Slifka MK, Grewal IS, Flavell RA, Ahmed R. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J Virol. 1996;70:8375–8381. doi: 10.1128/jvi.70.12.8375-8381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Notarangelo LD, Duse M, Ugazio AG. Immunodeficiency with hyper-IgM (HIM) Immunodefic Rev. 1992;3:101–121. [PubMed] [Google Scholar]
- 23.Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS, III, Frankel WN, Lee SY, Choi Y. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinases in T cells. J Biol Chem. 1997;272:25910–25914. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 24.Wong BR, Josien R, Lee SY, Sauter B, Li H-L, Steinman RM, Choi Y. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell–specific survival factor. J Exp Med. 1997;186:2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 26.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu N, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 27.Wysocka M, Kubin M, Vieira LQ, Ozmen L, Garotta G, Scott P, Trinchieri G. Interleukin-12 is required for interferon-gamma production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–676. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 28.Bachmann MF, Odermatt B, Hengartner H, Zinkernagel RM. Induction of long-lived germinal centers associated with persisting antigen after viral infection. J Exp Med. 1996;183:2259–2269. doi: 10.1084/jem.183.5.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mach F, Schonbeck U, Sukhova GK, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]