Brassinosteroid-Insensitive Dwarf Mutants of Arabidopsis Accumulate Brassinosteroids (original) (raw)
Abstract
Seven dwarf mutants resembling brassinosteroid (BR)-biosynthetic dwarfs were isolated that did not respond significantly to the application of exogenous BRs. Genetic and molecular analyses revealed that these were novel alleles of BRI1 (Brassinosteroid-Insensitive 1), which encodes a receptor kinase that may act as a receptor for BRs or be involved in downstream signaling. The results of morphological and molecular analyses indicated that these represent a range of alleles from weak to null. The endogenous BRs were examined from 5-week-old plants of a null allele (bri1-4) and two weak alleles (bri1-5 and bri1-6). Previous analysis of endogenous BRs in several BR-biosynthetic dwarf mutants revealed that active BRs are deficient in these mutants. However, bri1-4 plants accumulated very high levels of brassinolide, castasterone, and typhasterol (57-, 128-, and 33-fold higher, respectively, than those of wild-type plants). Weaker alleles (bri1-5 and bri1-6) also accumulated considerable levels of brassinolide, castasterone, and typhasterol, but less than the null allele (bri1-4). The levels of 6-deoxoBRs in bri1 mutants were comparable to that of wild type. The accumulation of biologically active BRs may result from the inability to utilize these active BRs, the inability to regulate BR biosynthesis in bri1 mutants, or both. Therefore, BRI1 is required for the homeostasis of endogenous BR levels.
Based on their wide distribution in the plant kingdom, their diverse physiological effects at nanomolar levels, and the discovery of mutants deficient in their biosynthesis, it is now widely accepted that brassinosteroids (BRs) are important hormones that regulate growth and development (Fujioka and Sakurai, 1997a, 1997b; Sakurai and Fujioka, 1997; Yokota, 1997; Altmann, 1998; Clouse and Sasse, 1998). During a relatively short time, a number of BR mutants were isolated from Arabidopsis, pea, and tomato (for review, see Clouse and Feldmann, 1999). Many of the BR mutants have been well characterized by genetic, molecular, and biochemical studies. All of the BR mutants are dwarfs in that they exhibit a short, robust stature and dark-green, round leaves. In addition, most of the mutants have reduced fertility, a prolonged lifespan, and display abnormal skotomorphogenesis when grown in the dark. These BR dwarf mutants are divided into two classes based on their phenotypic response to exogenously supplied BRs (Clouse and Feldmann, 1999).
One class of BR dwarf mutants is impaired in BR biosynthesis. The phenotype of this class of BR mutants can be rescued by exogenous application of BRs, but not by treatment with any other plant hormone. At present, six BR-biosynthetic mutants in Arabidopsis have been characterized (det2: Chory et al., 1991; Li et al., 1996, 1997; Fujioka et al., 1997; Noguchi et al., 1999; cpd: Szekeres et al., 1996; Mathur et al., 1998; dwf4: Azpiroz et al., 1998; Choe et al., 1998; dwf1: Feldmann et al., 1989; Takahashi et al., 1995; Klahre et al., 1998; Choe et al., 1999a; dwf7/ste1: Choe et al., 1999b; and sax1: Ephritikhine et al., 1999). In addition, two BR-deficient mutants have been isolated and characterized from pea (lkb: Nomura et al., 1997, 1999; lk: Yokota et al., 1997) and two from tomato (dwarf: Bishop et al., 1996, 1999; dpy: Koka et al., 1999).
A second class of BR dwarf mutants resembles the biosynthetic mutants in morphology, but cannot be rescued by BR feeding. This class of mutants is predicted to be blocked in the perception of BRs or in essential components of BR signaling downstream of perception. BR-insensitive mutants have been identified for Arabidopsis (bri1: Clouse et al., 1996; Kauschmann et al., 1996; Li and Chory, 1997), pea (lka: Nomura et al., 1997, 1999), and tomato (cu-3: Koka et al., 1999). Clouse et al. (1996) first identified a BR-insensitive mutant in Arabidopsis. This mutant, bri1-1 (brassinosteroid-insensitive 1-1), did not respond to BRs in root-inhibition assays, but did retain sensitivity to other plant hormones, including auxin and GA. Genetic analysis showed that bri1-1 was caused by a recessive mutation in a single gene. Kauschmann et al. (1996) also identified a BR-insensitive mutant they called cbb2 (cabbage 2), which was allelic to bri1-1.
Li and Chory (1997) identified 18 additional alleles of bri1, and isolated the BRI1 gene by mapping, isolation of DNA near BRI1, and the identification of a mutation in one allele that resulted in a detectable RFLP. The BRI1 gene was predicted to encode a membrane-bound Leu-rich repeat (LRR) receptor kinase (RK), which appeared to be constitutively expressed throughout the plant, both in the light and in the dark. The predicted protein showed striking similarities to other plant LRR-RK gene products, such as those of CLAVATA1 (Clark et al., 1997) and Xa21 (Song et al., 1995), which are involved in developmental signaling pathways and in pathogen response, respectively. Although physiological and molecular data raise the possibility that BRI1 is a receptor for BRs, direct biochemical evidence has not yet been described.
The natural occurrence of BRs in Arabidopsis was first demonstrated by Fujioka et al. (1996). Castasterone, 6-deoxocastasterone, typhasterol, and 6-deoxotyphasterol were identified from extracts made from Arabidopsis shoots. A subsequent study expanded the BR profiles in Arabidopsis. From fully expanded siliques of Arabidopsis, six BRs, including brassinolide, castasterone, typhasterol, 6-deoxocastasterone, 6-deoxotyphasterol, and 6-deoxoteasterone, were identified (Fujioka et al., 1998). All BRs identified in Arabidopsis are important components of either the early or late C6-oxidation pathways. These pathways were previously established with studies using cultured cells of Catharanthus roseus (Fujioka and Sakurai, 1997b; Sakurai and Fujioka, 1997). The studies in Arabidopsis suggested that both the early and late C6-oxidation pathways were functional in this species as well.
The analysis of BR levels in the BR-biosynthetic mutants have thus far substantiated the predicted BR pathways in Arabidopsis. The accumulation of precursor molecules in the BR biosynthetic pathway has been observed for several of these mutants. In addition, the BR-biosynthetic mutants of Arabidopsis have been shown to be deficient in endogenous BRs or sterols downstream of the blocked step (det2: Fujioka et al., 1997; dwf1: Klahre et al., 1998; Choe et al., 1999a; dwf7/ste1: Choe et al., 1999b). However, no information about endogenous BRs in BR-insensitive mutants of Arabidopsis is available. In GA-insensitive mutants such as Dwarf-8 in maize (Fujioka et al., 1988), gai in Arabidopsis (Talon et al., 1990), and Rht3 in wheat (Appleford and Lenton, 1991), the accumulation of bioactive C19-GAs such as GA1 and GA20 has been reported. Furthermore, transcription of one GA-biosynthetic gene is increased in the gai mutant (Peng et al., 1997). The accumulation of GAs and the increased level of GA-biosynthetic gene transcription have led to the conclusion that GAI, which may encode a transcription factor, is also required for proper feedback regulation of the GA-biosynthetic pathway (Peng et al., 1997; Harberd et al., 1998).
There is little known about how BR biosynthesis and signaling are coordinated. BRs are synthesized using sterols as precursors, but much less is understood about how sterols are funneled into BR biosynthesis. Within the BR-specific pathway, the 22α-hydroxylase supplied by DWF4 appears to be rate limiting (Choe et al., 1998). However, there is currently nothing known about how DWF4 transcription is regulated or spatially controlled. Analysis of CPD, a Cyt P450 (CYP) 90A one step downstream of DWF4 in the current BR-biosynthetic pathway, indicated that CPD mRNA levels are decreased by BR treatment (Mathur et al., 1998). This suggests that BR biosynthesis is regulated by a negative-feedback loop. Although the RK encoded by BRI1 is likely a key component of BR signal transduction, little is known about the regulation of BRI1 or about the nature of additional proteins that function downstream of BRI1. In animals, for both nuclear steroid hormone receptors and for some membrane-spanning ligands and receptors, feedback regulation occurs as a result of receptor activation (Wilkinson et al., 1994; Blumberg et al., 1998). We studied the BR profile of bri1 mutants to understand the regulation of BRs in Arabidopsis.
In this study, we describe the isolation of seven bri1 alleles and present morphometric analysis of plants homozygous for one severe allele and two weak alleles. We have also identified mutations within the BRI1 gene for all seven alleles, and provide data suggesting that the severe alleles represent the null phenotype of BRI1. We also determined the levels of endogenous BRs in three bri1 alleles with gas chromatography-selective ion monitoring (GC-SIM) analysis using 2H-labeled internal standards. We show here that mutations in BRI1 cause very large accumulations of 6-oxoBRs such as brassinolide, castasterone, and typhasterol, and that the level of BRs is positively correlated with allele and phenotypic severity.
MATERIALS AND METHODS
Isolation and Mapping of bri1 Alleles
bri1-3 (dwf2-32) and bri1-4 (dwf2-2074) were found in a screen for dwarf mutants from a population of plants of the Wassilewskija-2 (Ws-2) ecotype transformed with the T-DNA plasmid of Agrobacterium tumefaciens using the seed transformation method (Feldmann and Marks, 1987; Feldmann and Azpiroz, 1994). However, the dwarf phenotype did not cosegregate with kanamycin resistance, and therefore bri1-3 and bri1-4 represent untagged alleles (data not shown). bri1-5(dwf2-W41), bri1-7, bri1-8, and bri1-9 were isolated after ethyl methanesulfonate (EMS) mutagenesis of Ws-2 seeds, and bri1-6(dwf2-399) was identified among the dwarf mutants obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus) (CS399, http://aims.cps.msu.edu/aims/). CS399 had been isolated previously using an unknown mutagen. All seven alleles were classified as insensitive to BRs based on their lack of a significant response in feeding studies performed on inflorescences and seedlings (data not shown, see Choe et al., 1998, 1999a). Three of these mutations were initially designated as Ws-2 EMS (WM) mutants (see Table I) but have been renamed as bri1 alleles.
Table I.
bri1 mutant alleles
| Allele | Former Designation | Mutagen | DNA Sequence Change | Predicted Change in BRI1 Proteina |
|---|---|---|---|---|
| bri1-3 | dwf2-32 | T-DNA | bp 2,745, 4-bp deletion | 44 Amino acids, then stop codon after amino acid 914 |
| bri1-4 | dwf2-2074 | T-DNA | bp 459, 10-bp deletion | 13 Amino acids, then stop codon after amino acid 153 |
| bri1-5 | dwf2-w41 | EMS | TGC-TAC, bp 206 | C69-Y69 |
| bri1-6 | dwf2-399 | EMS | GGT-GAT, bp 1,931 | G644-D644 |
| bri1-7 | dwf2-WM3-2 | EMS | GGT-AGT, bp 1,838 | G613-S613 |
| bri1-8 | dwf2-WM6-2 | EMS | CGG-CAG, bp 2,948 | R983-N983 |
| bri1-9 | dwf2-WMB19 | EMS | CTT-TTT, bp 1,985 | S662-F662 |
The original bri1 allele, bri1-1, showed tight linkage to a marker on the bottom of chromosome IV (1/126 chromosomes recombinant for the CAPS marker DHS1, Clouse et al., 1996). DHS1 is tightly linked to SSLP marker nga1107, and BRI1 is physically located between these two markers (Li and Chory, 1997). We established mapping populations by crossing bri1 alleles to wild-type plants of the Columbia ecotype and selecting dwarf plants among the F2 progeny. DNA was isolated from single leaves or flowers as described previously (Dellaporta et al., 1983; Krysan et al., 1996). PCR reactions were prepared as described by Bell and Ecker (1994).
Since five of these seven BR-insensitive dwarf mutants mapped to the same general location as bri1-1, we performed crosses between all seven of these mutants to determine if they were alleles of the same gene. All combinations tested generated dwarf plants in the F1, and thus these represent alleles of the same gene (data not shown).
Morphometric Analysis
Approximately 20 seeds were planted in round pots (10 cm in diameter) with soil (Metromix 350, Grace Sierra, Miltipas, CA) presoaked in water. Flats containing the pots were covered in plastic wrap and cold treated for 3 to 4 d before transfer to a growth chamber (16 h of light [240 μmol m−2 s−1] at 22°C and 8 h of dark at 21°C, with 75%–90% humidity). The plastic wrap was removed after the seedlings were established (5–7 d), and the seedlings were thinned so that there were four or five well-spaced seedlings per pot. The pots were subirrigated with water or Hoagland's nutrient solution as necessary. When the plants were 5 weeks of age, the morphological traits listed in Table II were measured. Plant height was measured to the nearest millimeter, and the length of siliques and the length and width of leaves were measured to the nearest half-millimeter using a ruler. The length of siliques and the distance between siliques on the main inflorescence were measured to the nearest 10th of a millimeter using a ruler in the ocular of a dissecting microscope.
Table II.
Morphometric analysis of Ws-2 and bri1 mutants at 5 weeks of age
| Parameter | Ws-2 | bri1-4 | bri1-5 | bri1-7 |
|---|---|---|---|---|
| Height (cm) | 29.7 ± 2.2 | 1.26 ± 0.43 | 4.02 ± 1.12 | 3.18 ± 1.15 |
| Distance between siliques on main inflorescencea (cm) | 0.86 ± 0.37 | 0.05 ± 0.03 | 0.14 ± 0.08 | 0.18 ± 0.10 |
| Length of leaf bladeb (cm) | 1.29 ± 0.26 | 0.22 ± 0.06 | 1.02 ± 0.14 | 0.58 ± 0.12 |
| Width of leaf bladeb (cm) | 0.91 ± 0.12 | 0.30 ± 0.08 | 1.09 ± 0.11 | 0.69 ± 0.11 |
| Length of siliquesc (cm) | 1.38 ± 0.06 | 0.20 ± 0.02 | 0.62 ± 0.14 | 0.32 ± 0.14 |
| No. of seedsc | 50.9 ± 4.6 | 0.13 ± 0.35 | 22.9 ± 14.3 | 3.73 ± 6.6 |
Determination of the DNA Sequence of bri1 Alleles
The specific mutations in these seven bri1 alleles were identified by first designing primers based on the genomic sequence (Li and Chory, 1997; GenBank accession no. AF017056). Using oligonucleotides synthesized by Genosys Biotechnologies (The Woodlands, TX), a fragment of DNA corresponding to the coding region of BRI1 was amplified using XTaq (Panvera, Madison, WI), the F1 and R1 primers (F1 [79] agagataggtggttgggggtaaaatgtat, R1 [4366] aaaaatagacccaaggaaaatcggactga), and DNA isolated from a single leaf or flower from each mutant (Krysan et al., 1996). The bracketed numbers refer to the nucleotide position (Li and Chory, GenBank accession no. AF017056). DNA fragments were purified (Prep-A-Gene DNA purification system, Bio-Rad, Hercules, CA) and sequenced at the Arizona Research Laboratory (University of Arizona, Tucson).
For each mutant, the entire fragment was sequenced in one strand using the following additional oligonucleotides: F2 (431) taatcagaagaagaggtaac, F3 (896) tgcagagacgacaaagttac, F4 (1368) tttctcgatgtttcctccaa, F5 (1836) ccggaatctctgacgaatct, F6 (2298) agaatctcgctatcctcaag, F7 (2741) caatttgggtcataacgata, F8 (3236) gaagctgactggtgtgaaag, F9 (3702) catatcatccacagagacat, and F10 (4208) aatagaggatggagggttca. Putative mutations were confirmed by sequencing the second strand using the following primers: R2 (1437) ttcccggagatgtcaagatg, R3 (3503) caagaagaggcacaagattt, and R4 (2862) tccgtaagcatagtaagagc, and repeating the sequencing on fragments isolated from independent PCR reactions.
Quantitative Analysis of Endogenous BRs
Plants were grown for 5 weeks on soil. The aerial portions (rosette leaves, inflorescences, flowers, and siliques) were harvested and frozen. The tissue was lyophilized (Lyphlick 12, LabConco, Kansas City, MO) and ground to a fine powder using a mortar and pestle.
Lyophilized plant material (40 g fresh weight equivalent) was extracted with 400 mL of MeOH-CHCl3 (4:1) twice, and [2H6]brassinolide, [2H6]castasterone, [2H6]typhasterol, [2H6]teasterone, [2H6]6-deoxocastasterone, [2H6]6-deoxotyphasterol, and [2H6]6-deoxoteasterone (100 ng each) were added to the extract as internal standards. After evaporation of the solvent in vacuo, the extract was partitioned between CHCl3 and water three times. The CHCl3-soluble fraction was subjected to silica gel chromatography (Sep-Pak Vac Silica, 35 mL, Waters, Milford, MA). The column was subsequently eluted with 100 mL of CHCl3, 2% (v/v) MeOH in CHCl3, and 7% (v/v) MeOH in CHCl3. The 2% (v/v) MeOH and 7% (v/v) MeOH fractions were purified by Sephadex LH-20 column chromatography (column volume of 200 mL).
The column was eluted with MeOH-CHCl3 (4:1). The effluents of elution volume to total column volume 0.6 to 0.8 were collected as the BR-containing fraction. After purification with an ODS cartridge (Sep-Pak Plus C18, Waters) with 20 mL of MeOH, eluates were subjected to ODS-HPLC (Senshu Pak Pegasil ODS, 10 × 30 mm + Senshu Pak Pegasil ODS, 20 × 250 mm; Senshu Scientific, Tokyo) at a flow rate of 8 mL min−1. Ninety percent acetonitrile was used as a solvent for the eluate derived from the 2% (v/v) MeOH fraction, and 70% (v/v) acetonitrile was used for the eluate derived from the 7% MeOH fraction. HPLC purification from the 7% MeOH fraction yielded a brassinolide fraction (retention time [Rt] from 8–10 min), a castasterone fraction (Rt from 12–14 min), a teasterone fraction (Rt from 17–20 min), a typhasterol fraction (Rt from 26–32 min), and a 6-deoxocastasterone fraction (Rt from 38–44 min). HPLC purification from the 2% (v/v) MeOH fraction yielded a 6-deoxoteasterone fraction (Rt from 32–36 min) and a 6-deoxotyphasterol fraction (Rt from 48–52 min). Each fraction was analyzed by GC-SIM after derivatization.
Quantitative Analysis of Endogenous Sterols
For sterol analysis, lyophilized plant material (2 g fresh weight equivalent) from wild-type and bri1 mutant alleles was used. Plant material was extracted with 50 mL of MeOH-CHCl3 (4:1) twice, and [2H7]24-methylenecholesterol (3 μg/g fresh weight), [2H6]campesterol (30 μg/g fresh weight), and [2H6]campestanol (1 μg/g fresh weight) were added to the extract as internal standards. After evaporation of the solvent in vacuo, the extract was partitioned between CHCl3 and water three times. The CHCl3-soluble fraction was purified with a silica gel cartridge column (Sep-Pak Vac Silica, 12 mL, Waters), which was eluted with 20 mL of CHCl3. The eluate was purified with an ODS cartridge (Sep-Pak Plus C18, Waters), which was eluted with 20 mL of MeOH. The eluent was subjected to ODS-HPLC (Senshu Pak ODS 4150-N; 10 × 150 mm, Senshu Scientific) at a flow rate of 2 mL min−1 with MeOH. Fractions were collected every 0.5 min (Rt between 10 and 20 min). The main fractions of each sterol were as follows: 24-methylenecholesterol (Rt of 13 to 13.5 min), campesterol (Rt of 15.5 to 16 min), and campestanol (Rt of 16.5 to 17 min). Each fraction was analyzed by full-scan gas chromatography-mass spectrometry (GC-MS) after derivatization.
2H Standards
[2H6]Campesterol was kindly supplied by Tama Biochemical (Tokyo). [2H6]Brassinolide, [2H6]castasterone, [2H6]typhasterol, [2H6]teasterone (Takatsuto and Ikekawa, 1986), [2H6]6-deoxocastasterone (Choi et al., 1996), [2H6]6-deoxotyphasterol, [2H6]6-deoxoteasterone (Choi et al., 1997), [2H7]24-methylenecholesterol (Takatsuto et al., 1998), and [2H6]campestanol (Noguchi et al., 1999) were chemically synthesized.
GC-MS Analysis
GC-MS analysis was carried out under the following conditions: a mass spectrometer (Automass JMS-AM150, JEOL, Tokyo) was connected to a gas chromatograph (model 5890A-II, Hewlett-Packard, Wilmington, DE), electron ionization (70 eV) with a source temperature of 210°C, a DB-5 column (J&W Scientific, Folsom, CA; 15-m × 0.25-mm, 0.25-μm film thickness), and an injection temperature of 250°C. The column temperature program was: 80°C for 1 min, raised to 320°C at a rate of 30°C min−1, and held at this temperature for 5 min. The interface temperature was 250°C and the carrier gas was He at a flow rate of 1 mL min−1 with splitless injection. BR fractions were analyzed by GC-SIM after derivatization as below. Fractions containing brassinolide, castasterone, and 6-deoxocastasterone were derivatized to bis-methaneboronate, and fractions of teasterone, typhasterol, 6-deoxoteasterone, and 6-deoxotyphasterol were derivatized to methaneboronate-trimethylsilyl ether.
Monitored ions in the analysis of each BR were as follows: brassinolide, m/z 534, 528, 338, 332, 161, and 155; castasterone, m/z 518, 512, 287, 161, and 155; typhasterol and teasterone, m/z 550, 544, 535, and 529; 6-deoxocastasterone, m/z 504, 498, 489, and 483; and 6-deoxotyphasterol and 6-deoxoteasterone, m/z 536, 530, 521, 515, and 215. The endogenous levels of BRs, except for brassinolide, were determined as the ratio of the peak area of molecular ions for the internal standard to that of the endogenous steroid. The endogenous levels of brassinolide were determined as the ratio of the peak areas of fragment ions of m/z 338 and m/z 332. Sterols were analyzed by full-scan GC-MS after derivatization to the trimethylsilyl ether, and the endogenous levels were determined as the ratio of the peak areas of molecular ions for the internal standard to that of the endogenous sterol. Molecular ions of the internal standard and the endogenous sterol were as follows: 24-methylenecholesterol, m/z 477 and 470; campesterol, m/z 478 and 472; and campestanol, m/z 480 and 474.
RESULTS
Identification and Morphological Characterization of Seven bri1 Mutants
We have screened plants mutagenized by T-DNA insertional mutagenesis and EMS for dwarf mutants displaying the typical characteristics of BR biosynthetic mutants. These characteristics include short stature, small, dark green leaves, and reduced fertility. These mutants were divided into two categories based on the responses of the inflorescences to exogenous application of brassinolide. Nine mutants with no response or a reduced response in these feeding experiments were mapped to see if they were linked to bri1-1, a previously identified brassinolide-insensitive mutant. For bri1-3, zero of 44 recombinants were detected with nga1107; for bri1-5, two of 88 recombinant chromosomes were detected with nga1107 and zero of 88 with DHS1; and for bri1-6, two of 108 recombinants were detected with nga1107. For bri1-7 and bri1-9, no recombinants for nga1107 were detected out of 32 chromosomes for each allele. bri1-4 and bri1-5 were also crossed to bri1-1, and the resulting F1 generation were dwarf, indicating that these mutants were alleles of bri1. The results of the mapping and complementation tests were supported by the finding of a unique DNA sequence alteration in each of our seven bri1 alleles within the BRI1 coding region (Table I). The two other mutants did not map to the same location as bri1 and will be described elsewhere (S. Choe, F.E. Tax, and K.A. Feldmann, unpublished data).
These seven bri1 mutants can be divided into severe, intermediate, and weak alleles based on their morphological characteristics. bri1-3 and bri1-4 represent severe alleles. bri1-4 plants, as shown in Figure 1 and Table II, are extremely small, with all major above-ground organs reduced in size and dark-green in color, and rarely produce seeds. Plants of the intermediate allele bri1-8 are slightly larger than bri1-3 and bri1-4 plants, and are more fertile (data not shown). Four alleles comprise the weak class of bri1 alleles; bri1-5 and bri1-6 shown in Figure 1 are typical of this class. Plants from the four weak alleles were between 3 and 6 cm in height at 5 weeks of age, resemble the wild type in color, and are reasonably fertile, although not as fertile as the wild type (Table II). There are some interesting differences among these weak alleles. For example, bri1-5 plants have very short internodes along the inflorescence, develop leaves that are wider than those of the wild type (data for the third and fourth leaves are shown in Table II), and are the most fertile of these bri1 mutants. In contrast, bri1-9 plants have the longest internode length of these four weak alleles, yet have the smallest rosette leaves (data not shown).
Figure 1.
Wild type and four representative alleles of bri1 at 5 weeks of age. A, Wild type; B, bri1-3; C, bri1-4; D, bri1-5; and E, bri1-6.
DNA Sequence Analysis of bri1 Mutants
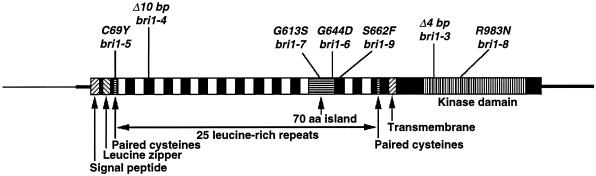
To determine the specific DNA sequence alterations responsible for the phenotypes of these bri1 alleles, we amplified the coding region of BRI1 from these mutants using PCR and performed DNA sequence analysis on each allele. For each allele, a single alteration was identified within the sequenced region. bri1-3 and bri1-4, the most severe alleles, both contain small deletions that are predicted to alter the BRI1 ORF, resulting in a premature stop codon. Deletions of this size are common in untagged mutations resulting from T-DNA mutagenesis (S. Choe and K.A. Feldmann, unpublished results). The location of the deletions in these two severe alleles may indicate that these represent null alleles of bri1 (see Fig. 2).
Figure 2.
Schematic of the BRI1 locus including the positions of the bri1 mutations. aa, Amino acid.
The intermediate and weak alleles each contained a single base pair change resulting in an amino acid substitution. These mutations were distributed throughout the BRI1 gene: four mutations were located in the extracellular domains of BRI1, and one (bri1-8) altered a conserved residue in the intracellular kinase domain. Two mutations (bri1-6 and bri1-7) were changes of Gly in a region located between two LRR domains. This island has been postulated to be a ligand-binding domain (Li and Chory, 1997), and these two mutations result in weak alleles. The remaining two mutations include a change of a Cys near the amino terminus and a mutation in a LRR just carboxy-terminal of the island. Interestingly, all of the missense mutations we have identified in the extracellular domain are weak alleles.
Quantitative Analysis of Endogenous Sterols and BRs
Quantitative analysis was performed to determine the BR and sterol levels in several bri1 alleles. 2H-labeled BRs and sterols were used as internal standards to determine the endogenous levels of BRs and sterols. Plant materials were from 5-week-old plants of the null allele bri1-4, the two weaker alleles bri1-5 and bri1-6, and corresponding wild-type plants. The bri1-4 and bri1-5 mutations are in the Ws-2 background and the bri1-6 mutation is in the Enkheim-2 (En-2) background.
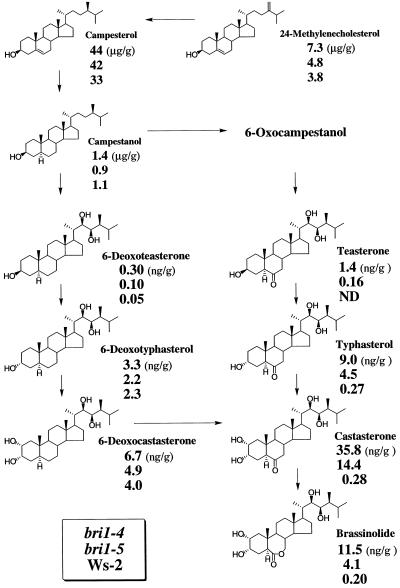
First, bri1-4 and bri1-5 (Ws-2 background) were examined, and the results are shown in Figure 3. The endogenous levels of sterols such as 24-methylenecholesterol, campesterol, and campestanol in both alleles were comparable to those of wild type (Ws-2). The levels of 6-deoxo-BRs such as 6-deoxotyphasterol and 6-deoxocastasterone in bri1-4 were only slightly higher than those of the wild type. In bri1-5, the levels of 6-deoxoBRs were comparable to those of the wild type. However, striking differences were observed in the levels of 6-oxoBRs. The levels of brassinolide, castasterone, and typhasterol in bri1-4 were 57-, 128-, and 33-fold higher, respectively, than those of wild-type (Ws-2) plants (Fig. 3). The level of teasterone in bri1-4 was significantly higher than that of wild type. In addition, the weaker allele, bri1-5, accumulated considerable levels of brassinolide, castasterone, and typhasterol (22-, 51-, and 17-fold, respectively).
Figure 3.
The proposed brassinolide biosynthetic pathway and the quantification of endogenous sterols and BRs from bri1-4 (a null allele), bri1-5 (a weaker allele), and wild type (Ws-2). Values in top, middle, and bottom represent endogenous levels (per gram fresh weight) in bri1-4, bri1-5, and the wild type, respectively. Most of the data for the wild type have been already published (Choe et al., 1999b). Data quantifying teasterone were not available in our previous study because of low recovery. In this study, we repeated the analysis using the same plant materials. Endogenous teasterone was not detected, while recovery of the internal standard ([2H6]teasterone) was very good.
To further confirm our findings, endogenous sterols and BRs were examined from 5-week-old plants of another weak allele, bri1-6, which is in a different background from bri1-5. Both bri1-5 and bri1-6 show similar phenotypes, although they are in different backgrounds (see Fig. 1). There was no significant difference in sterol and BR levels in Ws-2 and En-2 (see Fig. 3; Table III). bri1-6 contained the same pattern of sterols and BRs as bri1-5. bri1-6 also accumulated brassinolide, castasterone, and typhasterol (26-, 70-, and 10-fold higher than those of wild-type [En-2] plants, respectively). The accumulation of brassinolide and castasterone appears to be related to BRI1 gene dosage. However, the levels of 6-deoxoBRs such as 6-deoxocastasterone, 6-deoxotyphasterol, and 6-deoxoteasterone in bri1-6 were comparable to wild type. Our findings in bri1-4, bri1-5, and Ws-2 were confirmed in an allele isolated from a different ecotypic background.
Table III.
Endogenous levels of sterols and BRs in bri1-6 and wild-type plants (En-2)
| Sterol/BR | En-2 | bri1-6 |
|---|---|---|
| μg/g fresh wt | ||
| Sterol | ||
| 24-Methylenecholesterol | 3.5 | 2.3 |
| Campesterol | 40 | 31 |
| Campestanol | 0.63 | 0.52 |
| ng/g fresh wt | ||
| BR | ||
| 6-Deoxoteasterone | 0.08 | 0.05 |
| 6-Deoxotyphasterol | 3.0 | 1.7 |
| 6-Deoxocastasterone | 2.1 | 2.7 |
| Teasterone | 0.07 | 0.08 |
| Typhasterol | 0.26 | 2.5 |
| Castasterone | 0.23 | 16.1 |
| Brassinolide | 0.10 | 2.6 |
DISCUSSION
Theoretically, hormone-insensitive mutants can be predicted to show the same phenotype as hormone-deficient mutants. In fact, bri1 mutants are dwarfed and the phenotypes are similar to BR-deficient mutants such as cpd, det2, dwf1, and dwf7/ste1. The one major difference is that the dwarfism and other growth characteristics of bri1 mutants are not rescued by BRs. The bri1-1 phenotype is the result of a recessive mutation in a gene located on chromosome IV (Clouse et al., 1996). The gene affected in BR-insensitive mutants was isolated and shown to encode a putative membrane-bound LRR-RK (Li and Chory, 1997). From these molecular and genetic studies, BRI1 has been suggested to encode a BR receptor or an essential component involved in BR signaling. We have described the isolation of seven additional bri1 alleles ranging from nulls to weak alleles, and report that BRI1 plays a role in the homeostasis of BRs.
Isolation and Characterization of a Broad Spectrum of bri1 Alleles
The bri1 alleles described in this study possess phenotypes that range from severe dwarf mutants resembling the original bri1-1 dwarf mutant (Clouse et al., 1996) to semi-dwarfs. Molecular analysis indicated that the two severe alleles, bri1-3 and bri1-4, contain small deletions predicted to cause a frame shift, and introduce a premature stop codon into BRI1. The positions of these deletions, in the fourth LRR and in the second of 11 conserved kinase subdomains, predict that these two severe alleles should produce truncated forms of BRI1. The two severe bri1 alleles are similar to cpd mutants in their overall size and morphology. cpd and bri1 mutants are the smallest dwarfs of the eight BR dwarf loci isolated thus far (Clouse and Feldmann, 1999). Based on the description of the 18 mutants isolated by Li and Chory (1997) and the observation that the infertility of bri1 mutants is positively correlated with their severely reduced stature (see Table II), 17 of these are likely also severe mutants, but little morphological description of these mutants has been presented.
The intermediate and weak bri1 alleles we have isolated have at least a 7-fold reduction in plant height compared with their respective wild type, and thus are still classified as dwarf mutants. These resemble loss-of-function alleles of dwf1 or dwf7/ste1 in their overall morphology (Feldmann et al., 1989; Choe et al., 1999). Most morphological parameters of the plant are altered proportionally in these mutants (see Fig. 1; Table II), but there are some exceptions. Leaves from bri1-5 mutants are wider than the wild type, and the internode distance in bri1-5 mutants is short compared with other bri1 weak alleles such as bri1-7 (see Fig. 1; Table II), even though bri1-5 plants are taller than bri1-7 plants. bri1-9 mutants, which have small, rosette leaves, have longer inflorescences and longer internodes than would be predicted from the size of the leaves (data not shown).
The five intermediate and weak mutants generated by EMS mutagenesis each had a single base change leading to an amino acid substitution within the BRI1 coding region. These mutations are dispersed throughout the BRI1 protein, both within the kinase domain and in different domains of the extracellular domain. The intermediate allele, bri1-8, is caused by a change of a conserved Arg in subdomain VIa that is present in many RKs identified to date (Walker 1993; Li and Chory, 1997). However, the phenotype of bri1-8 is not as strong as would be expected for a change in such a conserved residue.
The extracellular region of BRI1 is composed of several different domains, including a putative leucine zipper, two sets of paired Cys residues, 25 LRRs, and a domain nestled within the LRRs called the island domain. Two weak alleles (bri1-6 and bri1-7) contain mutations that change different Gly residues within this island domain. A mutant with a change of a different Gly within this island domain was reported by Li and Chory (1997); however, their mutant was not fertile and therefore was probably a severe allele. Two other mutations were identified in the extracellular domain: a change in a Cys to a Tyr in the paired Cys domains located in the amino terminus of the extracellular domain (bri1-5; see Fig. 2), and a change in a Ser to a Phe in the first LRR after the island domain (bri1-9). These two weak alleles are the first mutations reported in the extracellular regions of BRI1 not in the island domain.
Quantitative Analysis of BRs in bri1 Mutants
At the biochemical level, several dwarf mutants of Arabidopsis have been shown to be blocked in specific steps of the BR-biosynthetic pathway. In addition, the endogenous BRs present in each mutant have been shown to be dependent on the positions of the genetic blocks in the pathway (det2: Fujioka et al., 1997; Noguchi et al., 1999; dwf1: Klahre et al., 1998; Choe et al., 1999a; dwf7/ste1: Choe et al., 1999b). bri1 mutants have phenotypes that mimic those of BR biosynthetic mutants, but bri1 mutants do not respond significantly to BRs. This raises the possibility that bri1 is either a BR-receptor mutant or a mutant acting in a downstream step. If so, bri1 mutants should contain the same endogenous BRs found in wild type, but they may accumulate BRs, especially castasterone and brassinolide. Our data show that both of the above predictions are correct. All BRs found in the wild type are present in bri1. Brassinolide, the presumptive terminal biologically active BR for the growth of Arabidopsis, accumulates in bri1 mutants. The ratio of brassinolide for a null allele (bri1-4), a weaker allele (bri1-5), and wild type (Ws-2) was 57:22:1. Thus, the accumulation of brassinolide is related to the amount of functional BRI1. A similar trend was observed for typhasterol and castasterone, the biosynthetic precursors of brassinolide. The ratios were 33:17:1 and 128:51:1, respectively. The pattern and accumulation of BRs suggests that the BRI1 gene controls either a step associated with the binding of brassinolide to a receptor or a subsequent downstream step.
The presence of abnormally high levels of brassinolide and castasterone in BR-insensitive dwarf mutants indicates a link between BR biosynthesis and BR action. In mutants with impaired response to BR, BR biosynthesis might be normal or activated. Our preliminary study suggested that the abundance of transcripts of biosynthesis enzymes might be increased in bri1 mutants (S. Choe and K.A. Feldmann, unpublished data). Detailed northern analyses and metabolic studies will answer the question of whether BR biosynthesis in bri1 mutants is activated or not.
Models for the Regulation of BR Synthesis and Signaling
End-product feedback regulation of biosynthetic genes is common among plant hormones. For example, treatment of plants by exogenous ethylene inhibits further ethylene production (Yang and Hoffman, 1984). For GA and BRs, there is also evidence for negative regulation after application of hormone, and a mechanism involving regulation at the transcriptional level has also been demonstrated (Phillips et al., 1995; Mathur et al., 1998). For example, treatment with specific BR intermediates or end products such as brassinolide and castasterone caused a reduction in the mRNA levels of the CPD gene, a gene required for necessary structural modification of brassinolide. Interestingly, this repression is inhibited by cycloheximide, indicating that at least one factor needs to be newly synthesized for this repression to occur (Mathur et al., 1998).
A second theme in these hormone biosynthetic and signaling pathways is that mutants deficient in receptors or other downstream components fail to regulate hormone levels. For example, the etr1-1 mutant in Arabidopsis lacks the ability to suppress ethylene synthesis after treatment with exogenous ethylene, although normal amounts of ethylene are made in untreated plants (Bleecker et al., 1988). This suggests that feedback regulation operates through a functional signaling pathway. Accumulation of bioactive GAs has also been observed in several mutants that are insensitive to GA treatment (Fujioka et al., 1988; Talon et al., 1990; Appleford and Lenton, 1991). Of these three GA insensitive genes in various plant species, only the molecular nature of the GAI gene is known, and it encodes a product related to transcription factors (Peng et al., 1997). In the gai mutant, there is also an increase in the transcription of GA5, a key GA-biosynthetic gene, indicating, as with BRs, that there is feedback regulation at the transcriptional level.
The results presented here indicate that the BRI1 gene is required for feedback regulation of BR biosynthesis. There is substantial accumulation of brassinolide and other intermediates in both a null allele and two weak alleles of bri1. One possibility is that BRI1, which encodes a predicted transmembrane RK, phosphorylates cellular proteins that directly or indirectly regulate the activity or transcription of BR-biosynthetic proteins such as CPD. Alternatively, BRI1 phosphorylation could result in the transcription and/or translation of a repressor of BR biosynthesis, as has been proposed for the regulation of CPD (Mathur et al., 1998). Interestingly, the lka mutant from pea, which is BR insensitive, also accumulates bioactive BRs (Nomura et al., 1997, 1999). However, the degree of the accumulation is not so high in the lka mutant. Presumably, the role of LKA in BR signaling pathway could be different from that of BRI1, or lka may be a weak allele of a pea BRI1 homolog. Molecular characterization of the LKA gene will provide important information about its role in the BR signaling pathway.
ACKNOWLEDGMENTS
We thank Steve Clouse for sending seeds of bri1-1 and the Arabidopsis Biological Resource Center at Ohio State University for supplying CS399 (bri1-6) seeds. We thank Alice Traut, Amanda Ross, and Brian Gregory for technical assistance with the mutant isolation and mapping.
Footnotes
1
This work was supported by a Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Science, Sports and Culture of Japan (grant no. 10460050 to S.F.), by the National Science Foundation (grant no. 9604439 to K.A.F.), and by the U.S. Department of Agriculture (grant no. 97–353044708 to F.E.T.).
LITERATURE CITED
- Altmann T. Recent advances in brassinosteroid molecular genetics. Curr Opin Plant Biol. 1998;1:378–383. doi: 10.1016/s1369-5266(98)80259-8. [DOI] [PubMed] [Google Scholar]
- Appleford NEJ, Lenton JR. Gibberellins and leaf expansion in near-isogenic wheat lines containing Rht1 and Rht3 dwarfing alleles. Planta. 1991;183:229–236. doi: 10.1007/BF00197793. [DOI] [PubMed] [Google Scholar]
- Azpiroz R, Wu Y, LoCascio JC, Feldmann KA. An Arabidopsis brassinosteorid-dependent mutant is blocked in cell elongation. Plant Cell. 1998;10:219–230. doi: 10.1105/tpc.10.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CJ, Ecker JR. Assignment of thirty microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- Bishop GJ, Harrison K, Jones JDG. The tomato DWARF gene isolated by heterologous transposon tagging encodes the first member of a new cytochrome P450 family. Plant Cell. 1996;8:959–969. doi: 10.1105/tpc.8.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GJ, Nomura T, Yokota T, Harrison K, Noguchi T, Fujioka S, Takatsuto S, Jones JDG, Kamiya Y. The tomato DWARF enzyme catalyzes C-6 oxidation in brassinosteroid biosynthesis. Proc Natl Acad Sci USA. 1999;96:1761–1766. doi: 10.1073/pnas.96.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. Insensitivity of ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science. 1988;241:1086–1089. doi: 10.1126/science.241.4869.1086. [DOI] [PubMed] [Google Scholar]
- Blumerg B, Sabbagh W, Juguilon H, Bolado J, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, Feldmann KA. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22α-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell. 1998;10:231–243. doi: 10.1105/tpc.10.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Gregory BD, Ross AS, Yuan H, Noguchi T, Fujioka S, Takatsuto S, Tanaka A, Yoshida S, Tax FE, Feldmann KA. Arabidopsis dwarf1 is defective in the conversion of 24-methylenecholestrol to campesterol in brassinosteroid biosynthesis. Plant Physiol. 1999a;119:897–907. doi: 10.1104/pp.119.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Noguchi T, Fujioka S, Takatsuto S, Tissier CP, Gregory BD, Ross AS, Tanaka A, Yoshida S, Tax FE, Feldmann KA. Arabidopsis dwf7/ste1 is defective in the Δ7 sterol C-5 desaturation step leading to brassinosteroid biosynthesis. Plant Cell. 1999b;11:207–221. [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Fujioka S, Harada A, Yokota T, Takatsuto S, Sakurai A. A brassinolide biosynthetic pathway of 6-deoxocastasterone. Phytochemistry. 1996;43:593–596. [Google Scholar]
- Choi YH, Fujioka S, Nomura T, Harada A, Yokota T, Takatsuto S, Sakurai A. An alternative brassinolide biosynthetic pathway via late C-6 oxidation. Phytochemistry. 1997;44:609–613. [Google Scholar]
- Chory J, Nagpal P, Peto CA. Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell. 1991;3:445–459. doi: 10.1105/tpc.3.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE, Williams RW, Meyerowitz EM. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell. 1997;89:575–585. doi: 10.1016/s0092-8674(00)80239-1. [DOI] [PubMed] [Google Scholar]
- Clouse SD, Feldmann KA. Molecular genetics of brassinosteroid action. In: Sakurai A, Yokota T, Clouse SD, editors. Brassinosteroids: Steroidal Plant Hormones. Tokyo: Springer-Verlag; 1999. pp. 163–190. [Google Scholar]
- Clouse SD, Langford M, McMorris TC. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–678. doi: 10.1104/pp.111.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Sasse JM. Brassinosteroids: essential regulators of plant growth and development. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:427–451. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Rep. 1983;1:19–21. [Google Scholar]
- Ephritikhine G, Pagant S, Fujioka S, Takatsuto S, Lapous D, Caboche M, Kendrick RE, Barbier-Brygoo H. The sax1 mutation defines a new locus involved in the brassinosteroid biosynthesis pathway in Arabidopsis thaliana. Plant J. 1999;18:315–320. doi: 10.1046/j.1365-313x.1999.00455.x. [DOI] [PubMed] [Google Scholar]
- Feldmann KA, Azpiroz R. Primary dwarfs. In: Bowman J, editor. Arabidopsis: An Atlas of Morphology and Development. New York: Springer-Verlag; 1994. pp. 82–85. [Google Scholar]
- Feldmann KA, Marks MD. Agrobacterium-mediated transformation of germinating seeds of Arabidopsis thaliana: a non-tissue culture approach. Mol Gen Genet. 1987;208:1–9. [Google Scholar]
- Feldmann KA, Marks MD, Christianson ML, Quatrano RS. A dwarf mutant of Arabidopsis generated by T-DNA insertion mutagenesis. Science. 1989;243:1351–1354. doi: 10.1126/science.243.4896.1351. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Choi YH, Takatsuto S, Yokota T, Li J, Chory J, Sakurai A. Identification of castasterone, 6-deoxocastasterone, typhasterol and 6-deoxotyphasterol from the shoots of Arabidopsis thaliana. Plant Cell Physiol. 1996;37:1201–1203. doi: 10.1093/oxfordjournals.pcp.a029074. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Li J, Choi YH, Seto H, Takatsuto S, Noguchi T, Watanabe T, Kuriyama H, Yokota T, Chory J, Sakurai A. The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell. 1997;9:1951–1962. doi: 10.1105/tpc.9.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Noguchi T, Yokota T, Takatsuto S, Yoshida S. Brassinosteroids in Arabidopsis thaliana. Phytochemistry. 1998;48:595–599. doi: 10.1016/s0031-9422(98)00065-x. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Sakurai A. Brassinosteroids. Nat Prod Rep. 1997a;14:1–10. doi: 10.1039/np9971400001. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Sakurai A. Biosynthesis and metabolism of brassinosteroids. Physiol Plant. 1997b;100:710–715. [Google Scholar]
- Fujioka S, Yamane H, Spray CR, Katsumi M, Phinney BO, Gaskin P, MacMillan J, Takahashi N. The dominant non-gibberellin-responding dwarf mutant (D8) of maize accumulates native gibberellins. Proc Natl Acad Sci USA. 1988;85:9031–9035. doi: 10.1073/pnas.85.23.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harberd NP, King KE, Carol P, Cowling RJ, Peng J, Richards DE. Gibberellin: inhibitor of an inhibitor of… ? BioEssays. 1998;20:1001–1008. doi: 10.1002/(SICI)1521-1878(199812)20:12<1001::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Kauschmann A, Jessop A, Koncz C, Szekeres M, Willmitzer L, Altmann T. Genetic evidence for an essential role of brassinosteroids in plant development. Plant J. 1996;9:701–713. [Google Scholar]
- Klahre U, Noguchi T, Fujioka S, Takatsuto S, Yokota T, Nomura T, Yoshida S, Chua NH. The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis. Plant Cell. 1998;10:1677–1690. doi: 10.1105/tpc.10.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka CV, Cerny RE, Gardner RG, Naguchi T, Fujioka S, Takatsuto S, Yoshida S, Clouse SD (1999) A putative role for the tomato genes DUMPY and CURL-3 in brassinosteroid biosynthesis and response. Plant Physiol (in press) [DOI] [PMC free article] [PubMed]
- Krysan PJ, Young JC, Tax F, Sussman MR. Identification of transferred DNA insertions within Arabidopsis genes involved in signal transduction and ion transport. Proc Natl Acad Sci USA. 1996;93:8145–8150. doi: 10.1073/pnas.93.15.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Biswas M, Chao A, Russel D, Chory J. Conservation of function between mammalian and plant steroid 5α-reductase. Proc Natl Acad Sci USA. 1997;94:3554–3559. doi: 10.1073/pnas.94.8.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- Mathur J, Molnar G, Fujioka S, Takatsuto S, Sakurai A, Yokota T, Adam G, Voigt B, Nagy F, Maas C, Schell J, Koncz C, Szekeres M. Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroid. Plant J. 1998;14:593–602. doi: 10.1046/j.1365-313x.1998.00158.x. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Takatsuto S, Sakurai A, Yoshida S, Li J, Chory J. Arabidopsis det2 is defective in the conversion of (24R)-24-methylcholest-4-en-3-one to (24R)-24-methyl-5α-cholestan-3-one in brassinosteroid biosynthesis. Plant Physiol. 1999;120:833–839. doi: 10.1104/pp.120.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Kitasaka Y, Takatsuto S, Reid JB, Fukami M, Yokota T. Brassinosteroid/sterol synthesis and plant growth as affected by lka and lkb mutations of pea. Plant Physiol. 1999;119:1517–1526. doi: 10.1104/pp.119.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Nakayama M, Reid JB, Takeuchi Y, Yokota T. Blockage of brassinosteroid biosynthesis and sensitivity cause dwarfism in garden pea. Plant Physiol. 1997;113:31–37. doi: 10.1104/pp.113.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Carol P, Richards DE, King KE, Cowling RJ, Murphey GP, Harberd NP. The Arabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes Dev. 1997;11:3194–3205. doi: 10.1101/gad.11.23.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Ward DA, Uknes S, Appleford NEJ, Lange T, Huttly AK, Gaskin P, Graebe JE, Hedden P. Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol. 1995;108:1049–1057. doi: 10.1104/pp.108.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A, Fujioka S. Studies on biosynthesis of brassinosteroids. Biosci Biotechnol Biochem. 1997;61:757–762. doi: 10.1271/bbb.61.757. [DOI] [PubMed] [Google Scholar]
- Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T, Gardner J, Wang B, Zhai WX, Zhu LH, Fauquet C, Ronald P. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science. 1995;270:1804–1806. doi: 10.1126/science.270.5243.1804. [DOI] [PubMed] [Google Scholar]
- Szekeres M, Nemeth K, Koncz-Kalman Z, Mathur J, Kauschmann A, Altmann T, Redei GP, Nagy F, Schell J, Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Gasch A, Nishizawa N, Chua NH. The DIMINUTO gene of Arabidopsis is involved in regulating cell elongation. Genes Dev. 1995;9:97–107. doi: 10.1101/gad.9.1.97. [DOI] [PubMed] [Google Scholar]
- Takatsuto S, Gotoh C, Noguchi T, Nomura T, Fujioka S, Yokota T (1998) Synthesis of deuterio-labelled 24-methylenecholesterol and related steroids. J Chem Research (S) 206–207
- Takatsuto S, Ikekawa N. Synthesis of deuterio-labelled brassinosteroids, [26,28-2H6]brassinolide, and [26,28-2H6]castasterone, [26,28-2H6]typhasterol, and [26,28-2H6]teasterone. Chem Pharm Bull. 1986;34:4045–4049. [Google Scholar]
- Talon M, Koornneef M, Zeevaart JAD. Accumulation of C19-gibberellins in the gibberellin-insensitive dwarf mutant gai of Arabidopsis thaliana (L.) Heynh. Planta. 1990;182:501–505. doi: 10.1007/BF02341024. [DOI] [PubMed] [Google Scholar]
- Walker JC. Receptor-like protein kinase genes of Arabidopsis thaliana. Plant J. 1993;3:451–456. doi: 10.1111/j.1365-313x.1993.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson HA, Fitzgerald K, Greenwald I. Reciprocal changes in expression of the receptor lin-12 and its ligand lag-2 prior to commitment in a C. elegans cell fate decision. Cell. 1994;79:1187–1198. doi: 10.1016/0092-8674(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]
- Yokota T. The structure, biosynthesis and function of brassinosteroids. Trends Plant Sci. 1997;2:137–143. [Google Scholar]
- Yokota T, Nomura T, Kitasaka Y, Takatsuto S, Reid JB. Biosynthetic lesions in brassinosteroid-deficient pea mutants. In: Latimer JG, editor. The 24th Proceedings of Plant Growth Regulation Society of America, Atlanta, Georgia, August, 1997. LaGrange: The Plant Growth Regulation Society of America; 1997. , GA, p 94. [Google Scholar]