CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli (original) (raw)
Abstract
The transcription factor cAMP response element (CRE)-binding protein (CREB) has been shown to regulate neural plasticity. Drugs of abuse activate CREB in the nucleus accumbens, an important part of the brain's reward pathways, and local manipulations of CREB activity have been shown to affect cocaine reward, suggesting an active role of CREB in adaptive processes that follow exposure to drugs of abuse. Using CRE-LacZ reporter mice, we show that not only rewarding stimuli such as morphine, but also aversive stimuli such as stress, activate CRE-mediated transcription in the nucleus accumbens shell. Using viral-mediated gene transfer to locally alter the activity of CREB, we show that this manipulation affects morphine reward, as well as the preference for sucrose, a more natural reward. We then show that local changes in CREB activity induce a more general syndrome, by altering reactions to anxiogenic, aversive, and nociceptive stimuli as well. Increased CREB activity in the nucleus accumbens shell decreases an animal's responses to each of these stimuli, whereas decreased CREB activity induces an opposite phenotype. These results show that environmental stimuli regulate CRE-mediated transcription within the nucleus accumbens shell, and that changes in CREB activity within this brain area subsequently alter gating between emotional stimuli and their behavioral responses. This control appears to be independent of the intrinsic appetitive or aversive value of the stimulus. The potential relevance of these data to addiction and mood disorders is discussed.
Transcription factors, by regulating protein expression, participate in neural plasticity and adaptation. Stimuli that change transcriptional activity in a brain structure may alter over time the way information is processed by that structure. At more integrated levels, this plasticity can lead to changes in the interaction between an individual and its environment. Examples include learning processes, and changes in perception, interpretation, and behavioral responses to environmental stimuli. The cAMP response element (CRE)-binding protein, CREB, is a constitutively expressed transcription factor activated by phosphorylation through the cAMP pathway and other intracellular signaling cascades (1). Within the central nervous system, CREB has been associated with learning and memory (2–6), as well as with molecular and behavioral changes induced by antidepressants (7, 8) and drugs of abuse (9–15). In these latter cases, changes in second messenger pathways activating CREB (7, 9), changes in CREB levels (12), and changes in CRE-mediated transcription (8, 15) have been observed in several discrete brain areas.
The nucleus accumbens, a forebrain structure critical for reward and motivation (16–23), has a key role in reinforcing properties of drugs of abuse (12, 17–21). Chronic exposure to cocaine or to several other drugs of abuse increases cAMP levels and cAMP-dependent protein kinase (PKA) activity in the nucleus accumbens (9, 12). These adaptations cause sustained activation of CREB. Stimulation of PKA (24), or CREB (13, 14) overexpression, in the nucleus accumbens reduces the rewarding effects of cocaine, suggesting that activation of this pathway might counteract positive feedback adaptations that tend to intensify drug reward (25, 26).
In the present study, we used viral-mediated gene transfer to manipulate CREB within the nucleus accumbens. Our aim was to understand the behavioral consequences of sustained local changes in CREB activity. Microinjections of a herpes simplex virus (HSV) vector (13, 14) allowed us to overexpress either CREB itself or the dominant negative mutant mCREB, in which mutation of Ser-133 to alanine prevents its own activation and renders it an inhibitor of endogenous CREB (27). Using these molecular tools, we show that CREB overexpression in the nucleus accumbens shell reduces the rewarding actions of morphine and sucrose, whereas mCREB expression has the opposite effect. We also found, using CRE-LacZ reporter mice, that CRE-mediated transcription in the nucleus accumbens is activated not only by drugs of abuse, but also by aversive stimuli. Based on this observation, we studied a possible role for CREB in regulating responses to several types of aversive conditions, including anxiogenic and nociceptive stimuli. Our data show that CREB overexpression reduces sensitivity to these stimuli, whereas mCREB expression increases it.
We conclude that levels of CREB activity in the nucleus accumbens shell can be regulated by environmental stimuli, and are a key regulator of behavioral responses to emotional stimuli. This control appears to be independent of the intrinsic appetitive or aversive value of the stimulus.
Materials and Methods
Animals.
Two lines of CRE-LacZ mice with a reporter gene expressing the β-galactosidase (β-gal) under the control of CREs were used. One line, previously described (3), has low basal expression of the transgene; the second line has higher basal expression. The construct of this second line, which contains seven CRE-consensus sequences in tandem upstream of a minimum promoter and the β-gal gene, is flanked by insulator sequences from the chicken β-globulin gene. Male Sprague–Dawley rats (Charles River Breeding Laboratories), initially weighing 250–275 g, were acclimatized to housing conditions for 1 week before starting the experiments. Procedures were approved by local animal care and use committees.
Stereotaxic Surgery.
Surgery was performed as described (13). Bilateral injections (1.5 μl) of HSV vectors were delivered over 7.5 min into the nucleus accumbens shell (relative to bregma: rat, AP = +1.9, Lat = +2.4, DV = −6.7 mm below dura, with a 10° lateral angle; mouse, AP = +1.7, Lat = +2.3, DV = −4.7 mm below skull, with a 20° lateral angle). Injection placements were checked at the end of the experiments. The injected viruses were: HSV-LacZ, coding for the control protein β-gal; HSV-CREB; HSV-mCREB; or HSV-CreGFP, coding a bacteriophage Cre recombinase-GFP fusion protein.
CRE-Mediated Transcription.
As described (15), 25-mg morphine pellets were implanted on day 1 and day 3 in mice from the CRE-LacZ line with low basal expression (3). Mice were perfused on day 6. Mice from the same line were subjected to different stress conditions and perfused 4 h after the start of the stress. The unpredictable foot-shock procedure consisted of 120 shocks (0.3 mA, 5 s duration), delivered through a metallic rod floor at random intervals over 1 h. The restraint stress lasted 1 h, during which mice were placed in a cylinder (2.8 cm diameter, 11 cm long, 0.6 cm hole at the end). The social stress involved placing the mice for 4 h in a cage of 5 C57BL/6J mice that had been housed together for 2 weeks. A group of mice was also subjected to 5 days of repeated unpredictable stress similar to that described elsewhere (28); they were perfused 4 h after the start of the last stress (a 1-hr restraint procedure). To test the influence of HSV vectors on CRE-mediated transcription, we used the CRE-LacZ line with higher basal expression. Those mice were subjected to the foot-shock procedure before perfusion. Cells overexpressing CREB or expressing mCREB were detected using an antibody recognizing both proteins (see below), and double staining was used to quantify the percentage of infected cells that also expressed high levels of β-gal.
Immunostaining.
Perfusion of the animals, cutting of the brains (40-μm sections), and immunostaining were done using standard procedures (15). CREB (1:200, Upstate Biotechnology, Lake Placid, NY) and β-gal (1:500, 5 Prime → 3 Prime) immunostaining was assessed with rabbit antisera; DARPP-32 (1:10,000, gift from P. Greengard, Rockefeller University, New York) and GFAP (glial fibrillary acidic protein, 1:1,000, Chemicon) immunostaining was assessed with mouse antibodies. We used a goat anti-β-gal antibody (1:5,000, Biogenesis, Brentwood, NH) for CREB/β-gal double staining. Because the CREB antibody was not directed against the part of the protein that is mutated in mCREB, it was also used to detect cells expressing high levels of mCREB. Secondary antibodies were Cy2 or Cy3 conjugated (1:200, Jackson ImmunoResearch). One section every 200 μm was processed.
Time Course of the Transgene Expression.
Rats were perfused at different time points after HSV-LacZ injection (3, 6, or 12 h; 1, 2, 3, 4, 5, or 6 days). β-gal expressing cells were assessed by X-Gal assay and counted bilaterally on 40-μm sections. One section was counted every 200 μm over the entire extent of the nucleus accumbens.
Place Conditioning.
Conditioned place preference to morphine sulfate (concentrations expressed as base) and place aversion to naloxone-HCl were conducted in independent experiments, using a previously described procedure (13). Before viral injections, rats freely explored the apparatus for 30 min. On days 3 and 4 postinjection, they first received saline (1 ml/kg, s.c.) and were confined to one side compartment of the apparatus for 1 h; 3 h later, they received the drug (s.c.) and were confined to the other side compartment for 1 h. On day 5, they explored the entire apparatus for 30 min, and the time spent in each compartment was recorded. Place conditioning was calculated as the difference in time spent on the drug-paired side vs. the saline-paired side.
Sucrose Preference.
Rats were habituated to drink a 1% sucrose solution for 3 days, then went through a two bottles choice procedure. Four days later, the HSV vectors were injected and 3 days thereafter the rats were tested. The experiments started at 7 p.m., when lights turned off in the animal room, and were conducted under red light. Two hours before each test, the rats were individually housed with access to food. At 7 p.m., they were given access to the two bottles, and their fluid intake was measured over 30 min.
Anxiety-Related Behaviors.
In the first experiment, 3 days after viral injection, we tested for 5 min the time spent in the open and closed arms of an elevated plus-maze (1 m from the floor, 12 cm × 50 cm arms). In two other independent experiments, 4 days after viral injection, we tested the locomotor activity as well as the time in the center (20 cm square) and in the borders of a 75-cm square open-field, using a video-tracking system (Ethovision, Noldus Information Technology, Wageningen, The Netherlands). One experiment was conducted under dim light (3 lx), and the second under brighter light (250 lx). Under 250 lx, HSV-mCREB rats showed an initial freezing when placed in the center of the open-field. To avoid confounding data related to this initial freezing, the analysis of the behavior started 1 min after placing the rat in the center. The behavior was studied for the next 10 min.
Response to Nociceptive Stimuli.
The threshold sensitivity to foot shocks and the paw licking latency on a hot plate were determined in independent experiments. In the first procedure, after 2 min of habituation the rats received a foot shock every 30 s starting at 0.05 mA with a 0.05-mA increment between each shock. The first appearance of a flinch, an audible vocalization, and a jump were recorded. In the second experiment, we measured the paw licking latency on a 52°C hot plate. A 20-s cut-off time was used to prevent tissue damage.
Statistics.
Analysis of variance was used to study differences between the groups (independent variables), and to compare pretest to test data (dependent variables). The Duncan test was used for post hoc comparisons. A t test was used for all other analysis implicating only a two-groups comparison.
Results
Response to Rewarding Stimuli.
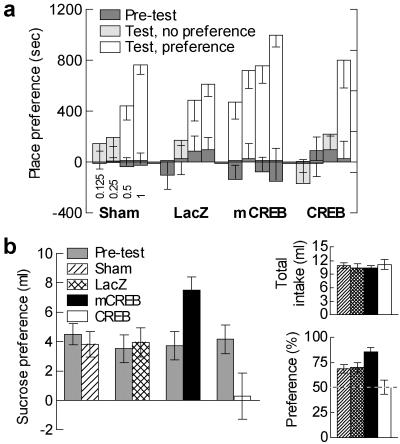
CREB manipulations within the nucleus accumbens shell alter morphine reward in a place-conditioning protocol (_F_3,155 = 9.16, P < 0.0001; Fig. 1a). In control groups (sham-operated and HSV-LacZ rats), morphine place preference first occurred for 0.5 mg/kg (P < 0.02 in both cases), suggesting that viral infection per se did not alter morphine place-conditioning. Local CREB overexpression reduced the sensitivity to morphine (P < 0.01 preference for 1 mg/kg; no preference at lower doses), whereas mCREB expression increased it (P < 0.015 preference for all doses).
Fig 1.
Response to rewarding stimuli. (a) Morphine place preference after sham surgery (n = 10–18), or expression of LacZ (n = 7–10), mCREB (n = 5–11), or CREB (n = 6–13). Four morphine doses were tested (indicated in mg/ml under the Sham bar graph). CREB overexpression reduced the sensitivity to morphine, whereas mCREB expression increased it. (b) Sucrose preference. Sham surgery (n = 14) or β-gal expression (n = 15) did not affect the sucrose preference; CREB overexpression (n = 16) reduced it, whereas mCREB expression (n = 12) increased it. Data are presented as difference in liquid intake between the two bottles (sucrose vs. water), or as sucrose intake in percentage of the total fluid intake (Lower Right). CREB manipulation did not affect the total fluid intake (Upper Right).
To generalize these findings to a natural reward, and to a paradigm free of associative memory, we studied sucrose preference (Fig. 1b). CREB manipulations did not affect the total amount of liquid the rats drank (_F_3,53 = 0.21, P>0.88), but did affect their choice between water and sucrose (_F_3,53 = 6.99, P < 0.001). Control groups showed similar sucrose preference; CREB overexpression decreased this preference (P < 0.025 against controls), whereas mCREB expression increased it (P < 0.05 against control and HSV-CREB groups).
Validation of the Approach.
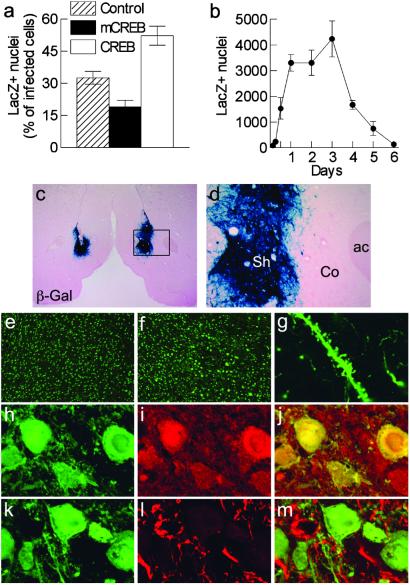
Using mice from the high-expression CRE-LacZ line, we show that viral-mediated CREB overexpression increases CRE-mediated transcription in the nucleus accumbens shell, whereas mCREB expression decreases it (_F_2,11 = 24.4, P < 0.001; controls differ from other groups at _P_ < 0.025), confirming the expected functional effects of these viral vectors on CREB activity (Fig. 2_a_). The behavioral experiments were performed within the time-window of viral-mediated gene expression, as shown using HSV-LacZ (Fig. 2_b_). Transgene expression was apparent within hours postinjection and largely dissipated by day 6. Consistent with this time course, CREB manipulation had no effect on sucrose preference when animals were studied 18 days after viral injection (_F_3,29 = 0.13, _P_ > 0.9; data not shown), at which time transgene expression is no longer detectable. We also confirmed that HSV vectors are neurotropic (Fig. 2 g_–_m). A large majority of infected cells in nucleus accumbens shell expressed DARPP-32 (dopamine- and cAMP-regulated phosphoprotein of 32 kDa), a marker for medium spiny neurons that predominate in this brain area. In contrast, no infected cell stained positively for GFAP (glial fibrillary acidic protein), a marker for astroglia.
Fig 2.
Viral-mediated gene transfer. (a) In CRE-LacZ reporter mice, the proportion of infected cells expressing β-gal is increased with CREB overexpression (n = 5) and decreased by mCREB expression (n = 5), as compared with cells infected by a control virus expressing a GFP fusion protein (n = 4). (b) Time course of transgene expression determined in rats by using HSV-LacZ (n = 5–8 accumbens). (c) Bilateral injection of HSV-LacZ in the nucleus accumbens shell revealed by X-Gal assay. (d) Higher magnification showing that the infection is restricted to the shell and does not diffuse to the core (ac, anterior commissure; Co, core; Sh, shell). (e and f) CREB immunoreactivity in the nucleus accumbens shell in a control (e) or in an HSV-CREB (f) injected side. (g_–_m) The majority of the infected cells are medium spiny neurons, as shown by β-gal positive processes of HSV-LacZ infected cells (g), and by the colocalization of β-gal (h) with DARPP-32 (i) (merged confocal image in j). No colocalization of β-gal (k) and GFAP (l) was observed (merged confocal image in m).
CRE-Mediated Transcription.
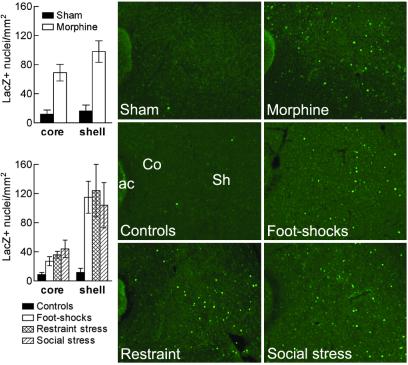
Both rewards (17–22, 29–31) and stress (30–32) stimulate dopamine transmission, which is upstream of CREB, in the nucleus accumbens. In this brain area, c-Fos, an identified target gene of CREB (1, 10), is also induced in response to both rewards (30) and stress (30). We show here that chronic morphine increased CRE-mediated transcription in the nucleus accumbens (shell: P < 0.003; core: _P_ < 0.005; Fig. 3). Stress also increased CRE activity in the nucleus accumbens shell and to a lesser extent in the core (Fig. 3). This increase was observed after intermittent inescapable foot shocks (shell: _P_ < 0.002; core: _P_ < 0.03; shell > core, _F_1,6 = 37.17, P < 0.001). The increase was also observed after restraint stress (shell: _P_ < 0.008; core: _P_ < 0.003; shell > core, _F_1,6 = 12.83, P < 0.02), as well as after a more natural stress—namely, the social stress of being introduced into an unfamiliar group of animals (shell: _P_ < 0.009; core: _P_ < 0.02; shell > core, _F_1,6 = 14.14, P < 0.01). No significant induction of CRE activity was observed in dorsal striatum (data not shown). A course of repeated unpredictable stress also increased CRE activity (shell: P < 0.007; core: P < 0.002; data not shown).
Fig 3.
CRE-mediated transcription. In CRE-LacZ mice, chronic morphine (n = 3, Top Right) increases the density of β-gal positive neurons in the nucleus accumbens as compared with sham-operated mice (n = 4, Top Left). Foot shocks (n = 3, Middle Right), restraint stress (n = 3, Bottom Left), and social stress (n = 3, Bottom Right) also increase CRE-mediated transcription as compared with controls (n = 5, Middle Left). ac, anterior commissure; Co, core; Sh, shell.
Anxiety-Related Behaviors.
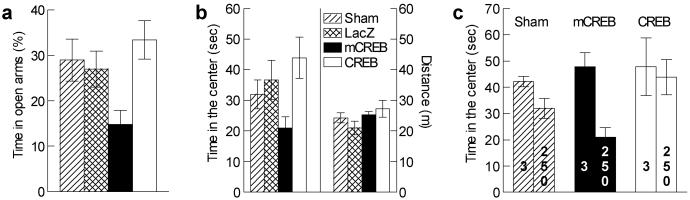
Increased CRE-mediated transcription by stress suggested that CREB activity within the nucleus accumbens might have broader influence than simply modulating reward sensitivity. To evaluate this possibility, we first tested whether CREB manipulation influences behavior in anxiogenic situations. mCREB expression increased anxiety-related behaviors in the elevated plus-maze (Fig. 4a), HSV-mCREB rats spending less time in the open arms of the apparatus (_F_3,28 = 3.87, P < 0.02; mCREB different from other groups at _P_ < 0.05). This finding was confirmed by measuring the time spent in the center of an open-field under bright light (_F_3,37 = 3.84, _P_ < 0.02; Fig. 4_b Left_). A comparison between bright and dim light conditions showed that brighter light did not affect the time HSV-CREB rats spent in the center (_P_ > 0.75), but reduced it for both controls (P < 0.05) and HSV-mCREB rats (_P_ < 0.001) (Fig. 4_c_), this effect being stronger with HSV-mCREB than in controls (_F_1,35 = 4.64, _P_ < 0.04). Lastly, manipulations of CREB activity had no influence on the time spent in the center of the test at 3 lx (_F_2,19 = 0.25, _P_ > 0.75; Fig. 4c) or on the total locomotor activity under either 3 lx (_F_2,19 = 1.71, P > 0.2; data not shown) or 250 lx (_F_3,37 = 1.84, P > 0.15) (Fig. 4b Right).
Fig 4.
Anxiety-related behaviors. (a) In the elevated plus-maze, local mCREB expression reduces the time spent in the open-arms (n = 7–8). (b) In the open-field (250 lx), similar results were obtained with the time spent in the center of the test (Left; n = 8–12). No influence of CREB was observed on the locomotor activity of the same rats (Right). (c) Comparison between the 250-lx illumination and a less anxiogenic condition (3 lx; n = 6–9) shows that higher illumination reduces the time spent in the center of the test. This anxiogenic effect of light intensity disappears with CREB overexpression, and is enhanced with mCREB expression.
Aversive and Nociceptive Responses.
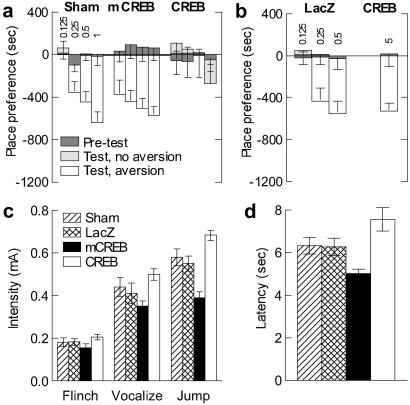
Our findings thus far show that CREB overexpression in the nucleus accumbens shell decreases an animal's responses both to rewarding stimuli and to certain anxiogenic situations, whereas mCREB expression has the opposite effects. This suggested that local CREB activity could control the stimulus intensity necessary to elicit a behavioral response independent of the emotional valence (reward vs. aversion) of the stimulus. To test this hypothesis, we studied the influence of CREB on the sensitivity to an aversive stimulus, naloxone (Fig. 5 a and b). We first confirmed that a place aversion for naloxone in morphine-naive rats could readily be observed (data not shown). Then, we found that CREB overexpression reduced the sensitivity to naloxone, whereas mCREB expression increased it. In control groups, naloxone aversion appeared at 0.25 mg/kg (P < 0.05 in both cases). The only dose inducing significant aversion after CREB overexpression was 5 mg/kg (P < 0.01), whereas HSV-mCREB rats showed an aversion at all of the doses tested (P < 0.05 for all doses).
Fig 5.
Response to aversive and nociceptive stimuli. (a) Naloxone place aversion after sham surgery (n = 12–18), or expression of mCREB (n = 6–10) or CREB (n = 5–8) in the nucleus accumbens shell. Four naloxone doses were tested (indicated in mg/ml above the Sham bar graph). CREB overexpression reduced naloxone aversion, whereas mCREB expression increased it. (b) HSV-LacZ group (n = 6–7) had the same aversion threshold as the Sham group. Rats overexpressing CREB (n = 6) show naloxone aversion only when exposed to a high dose (5 mg/kg). (c) HSV-mCREB rats (n = 9) vocalized and jumped in response to lower foot-shock intensities than HSV-CREB rats (n = 9); intermediate jumping threshold was seen in control groups (n = 6–8). (d) The paw licking latency on a hot plate is shorter in HSV-mCREB rats (n = 11) than in HSV-CREB rats (n = 12). Control groups showed intermediate latencies (n = 9–14).
We next tested the influence of CREB on the unconditioned behavioral responses to nociceptive stimuli. Local manipulation of CREB changed the threshold foot-shock intensities required to elicit vocalization (_F_3,28 = 3.09, P < 0.05) or jumping (_F_3,28 = 15.18, _P_ < 0.001), without significantly affecting the threshold intensity eliciting a flinch reaction (_F_3,28 = 1.45, _P_ > 0.24; Fig. 5c). Rats expressing mCREB vocalized and jumped at lower intensities than rats overexpressing CREB (P < 0.02 and P < 0.001, respectively); intermediate threshold intensities for jumping were seen in control groups (P < 0.04 in each case). We confirmed the influence of CREB activity on nociception with another test, which measures paw licking latency on a hot plate (_F_3,42 = 5.95, P < 0.002) (Fig. 5d). This latency was shorter in HSV-mCREB rats than in HSV-CREB rats (P < 0.001), with the control groups showing intermediate latencies (P ≤ 0.05 in each case).
Discussion
We show that localized changes in a transcription factor can have broad functional consequences, affecting interactions between an individual and its environment. By overexpressing CREB in the nucleus accumbens shell, we decreased responses to appetitive and aversive stimuli, and we obtained opposite effects with the dominant negative mutant mCREB.
Recent (15) and present data show that chronic morphine stimulates CRE-mediated transcription in the nucleus accumbens. Because overexpression of CREB in this brain area decreases morphine reward, this suggests that sustained CREB activation in the nucleus accumbens could be a mechanism by which drugs of abuse produce tolerance to their rewarding effects. A similar phenomenon was observed with cocaine (9, 12–14). We also show that the influence of CREB can be extended to natural rewards, such as sucrose. Interestingly, a loss of interest in natural rewards, to the gain of drug-directed behaviors, is one of the clinical symptoms defining addiction (33). Our data suggest that elevated CREB activity in the nucleus accumbens could be one of the molecular mechanisms underlying this reduction in the rewarding value of natural stimuli after drug treatment. It should however be emphasized that chronic drug intake has been associated not only with tolerance mechanisms, but also with sensitization, in particular of drug-seeking behaviors (26, 34). Our data show that CREB activity in the nucleus accumbens is unlikely to be the molecular switch of the sensitization mechanisms, which might involve other transcription factors (35, 36) or other brain areas.
Mutant mice partly deficient in CREB show enhanced cocaine preference, consistent with our findings, but show reduced morphine preference, which is not consistent (37). However, CREB is knocked down in the entire brain and other tissues of these mice, beginning at the earliest developmental periods. An advantage of the approach used in the present study is the temporal and anatomical specificity allowed by viral vectors. Our data indicate that the morphine phenotype of these mice may be related either to brain areas other than the nucleus accumbens or to developmental adaptations.
We also show here that stress, like drugs of abuse, activates CRE-mediated transcription in the nucleus accumbens. Thus, this transcriptional response appears to be independent of the valence of the stimulus. The functional significance of nucleus accumbens regulation by aversive stimuli is less understood than its regulation by rewards (17–23). Dopamine transmission in the nucleus accumbens has been proposed to control motivational rather than hedonic responses (26, 38); it might also be involved in the development of emotional memory to both appetitive and aversive stimuli (39, 40). Based on this literature, we chose to further analyze CREB's influence in this brain region, by considering not only how it affects reward, but also how it affects responses to anxiogenic, aversive, and nociceptive stimuli. The data show that the behavioral consequences of CREB manipulation in the nucleus accumbens, like the activation of CREB seen in this region, are independent of the valence of the stimulus.
Recent attention has been given to the influence of the nucleus accumbens in nociceptive responses (41–43). We show here that CREB activity in the nucleus accumbens shell participates in setting the nociceptive threshold. The neuroanatomical basis for this influence is not yet fully understood, but the nucleus accumbens does receive direct afferents from spinal cord neurons (44). It also receives inputs from or sends projections to several structures (45) that are involved in nociceptive responses, including periaqueductal gray, VTA, habenula, lateral hypothalamus, and amygdala. We show here that local CREB activity affects an animal's sensitivity to environmental changes, such as brighter light, which make a situation more anxiogenic, an effect that could be related to inputs from the bed nucleus of the stria terminalis (45), which is proposed to control anxiety (46). Lastly, CREB manipulation produced a robust change in sensitivity to the aversive properties of naloxone. CREB overexpression decreased naloxone aversion, just as it decreased cocaine (13, 14), morphine, and sucrose reward. This indicates that the nucleus accumbens shell could play a critical role in the interaction between endogenous opioid tone, revealed by naloxone, and the hedonic state of the animal. It should be noted that CREB manipulation in the nucleus accumbens did not affect locomotor activity, similar to previous observations (14). This suggests that CREB's influence on behavioral responses may be restricted to stimuli with a strong emotional component. The striatal complex is thought to be a key structure controlling sensorimotor gating. This interface function of the dorsal striatum has been extended to the more limbic nucleus accumbens, which is proposed to serve as an interface between motivation and action (16). Our data, showing a general influence of the nucleus accumbens on behavioral responses to emotional stimuli, independent of their valence, support such an interface role and extend it to a wide range of situations. We propose that one of the functions of the nucleus accumbens shell might be to gate behavioral responses to emotional stimuli by controlling the stimulus intensity necessary to produce the appropriate behavioral expression of the emotion, a function that CREB activity can locally influence.
A possible role for the nucleus accumbens in the symptomatology of mood disorders is not surprising considering its role in motivation and responses to hedonic stimuli (16–23), both of which are severely affected in mood disorders (33, 47). Indeed, abnormalities in the activity and morphology of nucleus accumbens neurons have been observed in depressed patients (48). We show here that inescapable foot shocks, following a protocol which induces “learned helplessness,” as well as other forms of stress, activate CRE-mediated transcription in the nucleus accumbens. The forced swim test, a procedure used to screen antidepressants (49), was also previously shown to increase CREB phosphorylation in the same brain area (14). The decreased rewarding effects of cocaine after CREB overexpression in the nucleus accumbens led to our earlier proposal that CREB hyperactivity in this region might induce an anhedonia-like state, or even dysphoria (13, 14). The results shown here with morphine and sucrose are consistent with this hypothesis. However, when studying an aversive stimulus, such as naloxone, we observed that HSV-CREB decreased the aversion, instead of increasing it, as would have been expected from a simple dysphoric state. Moreover, when studying anxiogenic situations, we found that local expression of mCREB, not CREB, is associated with increased anxiety-like behavior. While our new data confirm CREB's influence in reducing responses to rewarding stimuli, they also suggest a more complex behavioral effect of CREB activity in the nucleus accumbens than we had previously hypothesized. Thus, we have reconsidered our data in light of the gating function attributed to the striatal complex and nucleus accumbens (16), and suggest that CREB hyperactivity might locally inhibit this function. This would explain why responses to both rewarding and aversive stimuli are similarly reduced on CREB overexpression in this brain region. This hypothesis is consistent with recent data obtained in the forced swim test (14), where we showed that CREB overexpression in the nucleus accumbens decreases struggling behavior in response to swim stress. Together, these findings raise the possibility that increased CREB activity in the nucleus accumbens shell may contribute to certain symptoms associated with depression and other mood disorders (33, 47), such as anhedonia and decreased emotional reactivity. For example, severe (melancholic) depression is characterized by reduced emotional reactivity, and lower sensitivity to exogenous nociceptive stimuli has been observed in depressed patients (50). Also, a general deficit in responses to emotional stimuli has been observed in posttraumatic stress disorder (33, 51). However, we must emphasize that a localized molecular change, such as activation of CREB in the nucleus accumbens, cannot by itself mimic or explain complex syndromes like depression or any other mood disorder. Many additional brain areas and molecular mechanisms are certainly involved.
Results of the present study also raise the question of the molecular changes, downstream of CREB, responsible for the behavioral adaptations observed. As a transcription factor, CREB itself is unlikely to be the molecule acutely gating information; the time frame of its action is far too slow. Rather, CREB function is more likely adaptive: modification of CREB activity induces protein expression changes that then alter neuronal responses to subsequent stimuli. Dynorphin is one of CREB's target genes in the nucleus accumbens (13). Previous work showed that dynorphin, released from nucleus accumbens neurons, locally inhibits dopamine transmission. Moreover, dynorphin induction can account for some of the behavioral changes seen on CREB manipulation (13, 14). However, it is likely that other neurotransmitter systems and the expression of many other genes are also affected by changes in CREB function. In vitro studies and analysis of promoter sequences have led to a list of potential target genes (1), including other neuropeptides, receptors, and signal transduction molecules, although their actual regulation by CREB in the nucleus accumbens remains to be proved. The search for target genes is further complicated by the fact that some of them, such as c-Fos (1, 10), are themselves transcription factors that also affect gene expression. A genome-wide analysis would be useful to more fully understand the molecular changes that occur in the nucleus accumbens when CREB activity is locally affected.
Acknowledgments
We thank Faustina Donkor, Deanna Wallace-Black, and Phillip Williams for their technical assistance. M.B. was supported by a long-term fellowship from the Human Frontier Science Program organization. This work was supported by grants from the National Institute on Drug Abuse and the National Institute of Mental Health (to E.J.N.), the National Institute of Neurological Disorders and Stroke (to D.R.S.), and the National Alliance for Research on Schizophrenia and Depression (to M.B.).
Abbreviations
- β-gal, β-galactosidase
- CRE, cAMP response element
- CREB, CRE-binding protein
- HSV, herpes simplex virus
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Mayr B. & Montminy, M. (2001) Nat. Rev. Mol. Cell Biol. 2**,** 599-609. [DOI] [PubMed] [Google Scholar]
- 2.Yin J. C. & Tully, T. (1996) Curr. Opin. Neurobiol. 6**,** 264-268. [DOI] [PubMed] [Google Scholar]
- 3.Impey S., Smith, D. M., Obrietan, K., Donahue, R., Wade, C. & Storm, D. R. (1998) Nat. Neurosci. 1**,** 595-601. [DOI] [PubMed] [Google Scholar]
- 4.Mayford M. & Kandel, E. R. (1999) Trends Genet. 15**,** 463-470. [DOI] [PubMed] [Google Scholar]
- 5.Silva A. J. & Murphy, G. G. (1999) Brain Res. Bull. 50**,** 441-442. [DOI] [PubMed] [Google Scholar]
- 6.Josselyn S. A., Shi, C., Carlezon, W. A., Jr., Neve, R. L., Nestler, E. J. & Davis, M. (2001) J. Neurosci. 21**,** 2404-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nibuya M., Nestler, E. J. & Duman, R. S. (1996) J. Neurosci. 16**,** 2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thome J., Sakai, N., Shin, K., Steffen, C., Zhang, Y. J., Impey, S., Storm, D. & Duman, R. S. (2000) J. Neurosci. 20**,** 4030-4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terwilliger R. Z., Beitner-Johnson, D., Sevarino, K. A., Crain, S. M. & Nestler, E. J. (1991) Brain Res. 548**,** 100-110. [DOI] [PubMed] [Google Scholar]
- 10.Konradi C., Cole, R. L., Heckers, S. & Hyman, S. E. (1994) J. Neurosci. 14**,** 5623-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turgeon S. M., Pollack, A. E. & Fink, J. S. (1997) Brain Res. 749**,** 120-126. [DOI] [PubMed] [Google Scholar]
- 12.Nestler E. J. (2001) Nat. Rev. Neurosci. 2**,** 119-128. [DOI] [PubMed] [Google Scholar]
- 13.Carlezon W. A., Thome, J., Olson, V. G., Lane-Ladd, S. B., Brodkin, E. S., Hiroi, N., Duman, R. S., Neve, R. L. & Nestler, E. J. (1998) Science 282**,** 2272-2275. [DOI] [PubMed] [Google Scholar]
- 14.Pliakas A. M., Carlson, R. R., Neve, R. L., Konradi, C., Nestler, E. J. & Carlezon, W. A., Jr. (2001) J. Neurosci. 21**,** 7397-7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw-Lutchman T. Z., Barrot, M., Wallace, T., Gilden, L., Zachariou, V., Impey, S., Duman, R. S., Storm, D. & Nestler, E. J. (2002) J. Neurosci. 22**,** 3663-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mogenson G. J., Jones, D. L. & Yim, C. Y. (1980) Prog. Neurobiol. 14**,** 69-97. [DOI] [PubMed] [Google Scholar]
- 17.Le Moal M. & Simon, H. (1991) Physiol. Rev. 71**,** 155-234. [DOI] [PubMed] [Google Scholar]
- 18.Robbins T. W. & Everitt, B. J. (1996) Curr. Opin. Neurobiol. 6**,** 228-236. [DOI] [PubMed] [Google Scholar]
- 19.Wise R. A. (1998) Drug Alcohol Depend. 51**,** 13-22. [DOI] [PubMed] [Google Scholar]
- 20.Berke J. D. & Hyman, S. E. (2000) Neuron 25**,** 515-532. [DOI] [PubMed] [Google Scholar]
- 21.Koob G. F. & Le Moal, M. (2001) Neuropsychopharmacology 24**,** 97-129. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell J. B. & Gratton, A. (1994) Rev. Neurosci. 5**,** 317-329. [DOI] [PubMed] [Google Scholar]
- 23.Kelley A. E. (1999) Ann. N.Y. Acad. Sci. 877**,** 71-90. [DOI] [PubMed] [Google Scholar]
- 24.Self D. W., Genova, L. M., Hope, B. T., Barnhart, W. J., Spencer, J. J. & Nestler, E. J. (1998) J. Neurosci. 18**,** 1848-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lett B. T. (1989) Psychopharmacology 98**,** 357-362. [DOI] [PubMed] [Google Scholar]
- 26.Robinson T. E. & Berridge, K. C. (1993) Brain Res. Rev. 18**,** 247-291. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez G. A. & Montminy, M. R. (1989) Cell 59**,** 675-680. [DOI] [PubMed] [Google Scholar]
- 28.Ortiz J., Fitzgerald, L. W., Lane, S., Terwilliger, R. & Nestler, E. J. (1996) Neuropsychopharmacology 14**,** 443-452. [DOI] [PubMed] [Google Scholar]
- 29.Pontieri F. E., Tanda, G. & Di Chiara, G. (1995) Proc. Natl. Acad. Sci. USA 92**,** 12304-12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barrot M., Marinelli, M., Abrous, D. N., Rouge-Pont, F., Le Moal, M. & Piazza, P. V. (1999) Eur. J. Neurosci. 11**,** 1155-1166. [DOI] [PubMed] [Google Scholar]
- 31.Barrot M., Marinelli, M., Abrous, D. N., Rougé-Pont, F., Le Moal, M. & Piazza, P. V. (2000) Eur. J. Neurosci. 12**,** 973-979. [DOI] [PubMed] [Google Scholar]
- 32.Kalivas P. W. & Duffy, P. (1995) Brain Res. 675**,** 325-328. [DOI] [PubMed] [Google Scholar]
- 33.American Psychiatric Association, (1994) Diagnostic and Statistical Manual of Mental Disorders (Am. Psychiatric Assoc., Washington, DC).
- 34.Deroche V., Le Moal, M. & Piazza, P. V. (1999) Eur. J. Neurosci. 11**,** 2731-2736. [DOI] [PubMed] [Google Scholar]
- 35.Kelz M. B., Chen, J., Carlezon, W. A., Jr., Whisler, K., Gilden, L., Beckmann, A. M., Steffen, C., Zhang, Y. J., Marotti, L., Self, D. W., et al. (1999) Nature (London) 401**,** 272-276. [DOI] [PubMed] [Google Scholar]
- 36.Nestler E. J., Barrot, M. & Self, D. W. (2001) Proc. Natl. Acad. Sci. USA 98**,** 11042-11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walters C. L. & Blendy, J. A. (2001) J. Neurosci. 21**,** 9438-9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berridge K. C. & Robinson, T. E. (1998) Brain Res. Rev. 28**,** 309-369. [DOI] [PubMed] [Google Scholar]
- 39.Di Chiara G. (1995) Drug Alcohol Depend. 38**,** 95-137. [DOI] [PubMed] [Google Scholar]
- 40.Fenu S., Bassareo, V. & Di Chiara, G. (2001) J. Neurosci. 21**,** 6897-6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gear R. W., Aley, K. O. & Levine, J. D. (1999) J. Neurosci. 19**,** 7175-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altier N. & Stewart, J. (1999) Life Sci. 65**,** 2269-2287. [DOI] [PubMed] [Google Scholar]
- 43.Becerra L., Breiter, H. C., Wise, R., Gonzales, R. G. & Borsook, D. (2001) Neuron 32**,** 927-946. [DOI] [PubMed] [Google Scholar]
- 44.Cliffer K. D., Burstein, R. & Giesler, G. J., Jr. (1991) J. Neurosci. 11**,** 852-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brog J. S., Salyapongse, A., Deutch, A. Y. & Zahm, D. S. (1993) J. Comp. Neurol. 338**,** 255-278. [DOI] [PubMed] [Google Scholar]
- 46.Davis M. (1998) Biol. Psychiatry 44**,** 1239-1247. [DOI] [PubMed] [Google Scholar]
- 47.Nestler E. J., Barrot, M., DiLeone, R. J., Eisch, A. J., Gold, S. J. & Monteggia, L. (2002) Neuron 34**,** 13-25. [DOI] [PubMed] [Google Scholar]
- 48.Manji H. K., Drevets, W. C. & Charney, D. S. (2001) Nat. Med. 7**,** 541-547. [DOI] [PubMed] [Google Scholar]
- 49.Porsolt R. D., Le Pichon, M. & Jalfre, M. (1977) Nature (London) 266**,** 730-732. [DOI] [PubMed] [Google Scholar]
- 50.Dworkin R. H., Clark, W. C. & Lipsitz, J. D. (1995) Psychiatry Res. 56**,** 173-181. [DOI] [PubMed] [Google Scholar]
- 51.Feeny N. C., Zoellner, L. A., Fitzgibbons, L. A. & Foa, E. B. (2000) J. Trauma. Stress 13**,** 489-498. [DOI] [PubMed] [Google Scholar]