IκBα/IκBε deficiency reveals that a critical NF-κB dosage is required for lymphocyte survival (original) (raw)
Abstract
In most cells, the NF-κB transcription factor is sequestered in the cytoplasm by interaction with inhibitory proteins, the IκBs. Here, we show that combined IκBα/IκBε deficiency in mice leads to neonatal death, elevated κB binding activity, overexpression of NF-κB target genes, and disruption of lymphocyte production. In IκBα/IκBε-deficient fetuses, B220+IgM+ B cells and single-positive T cells die by apoptosis. In adults, IκBα-/-IκBε-/- reconstituted chimeras exhibit a nearly complete absence of T and B cells that is not rescued by cotransfer with wild-type bone marrow. These findings demonstrate that IκBs tightly control NF-κB activity in vivo and that increased NF-κB activity intrinsically impairs lymphocyte survival. Because reduction or rise of NF-κB activity leads to similar dysfunction, they also reveal that only a narrow window of NF-κB activity is tolerated by lymphocytes.
Keywords: transcription factor NF-κB/Rel, apoptosis, chimera, mice, IκB
The NF-κB/Rel family of transcription factors plays a prominent role in the control of immune response, inflammation, cell proliferation, and apoptosis (1-3). It comprises five members in mammals, p50, p52, p65/RelA, c-Rel, and RelB, which can homo- or heterodimerize and are identified by an N-terminal Rel homology domain. P50 and p52 are produced by proteolytic processing from inactive precursors, p105 and p100, respectively. In most cells, NF-κB/Rel dimers are sequestered in the cytoplasm by interaction with inhibitory molecules, called IκBs. In response to a wide variety of stimuli, an IκB kinase (IKK) complex is activated that phosphorylates specific serines within the IκBs, leading to their ubiquitination and degradation through the proteasome pathway. Free NF-κB dimers then migrate to the nucleus to regulate the transcription of a large number of genes.
IκBs form another evolutionary conserved multigenic family, composed in mammals of IκBα, IκBβ, IκBε, p100, and p105 (4). IκBα, IκBβ, and IκBε harbor in their N terminus two phosphorylation-inducible serines and one or two proximal ubiquitination sites, which make them the primary inhibitors of inducible NF-κB activity (4). Therefore, these three inhibitors represent an important level of NF-κB regulation. The activity of the various NF-κB dimers that coexist within a given cell type may be controlled by different IκBs, although no precise dependence of specific NF-κB complexes on one particular IκB could be definitely established. It remains also to be determined whether each IκB has specific or redundant functions in vivo. To address such issues, mice lacking individual IκB members were generated (5-8). IκBα knockout mice died by 7-8 days after birth and exhibited extensive granulocytosis, acute runting, and inflammatory dermitis (5, 6). The absence of IκBα rendered mouse embryonic fibroblasts unable to stop NF-κB activation after tumor necrosis factor (TNF) treatment (5), demonstrating the essential role of IκBα in postinduction repression. In contrast, IκBε knockout mice developed normally and displayed a mild phenotype, with altered basal and antigen-specific Ig production (7). Different cytokine genes were overexpressed in IκBα-null (5, 6) and IκBε-null (7) mice, indicating that in vivo distinct IκBs control the expression of separate cytokine genes.
The up-regulation of IκBε in IκBα-nullizygous mouse embryonic fibroblasts (9), together with that of IκBα in several organs of IκBε-deficient mice (7), suggested that compensatory mechanisms might take place in these mutant animals. To investigate the redundant functions of IκBα and IκBε, mice carrying null alleles in both _I_κ_B_α and _I_κ_B_ε genes were generated. They died at birth from respiratory distress and displayed increased NF-κB activity. We used IκBα-/-IκBε-/- embryonic day (E)18.5 fetuses and reconstituted chimeras to study the effect of elevated NF-κB activity on hematopoietic cell development in fetuses and adults. Our findings emphasize the importance of a strict control of NF-κB activity for lymphocyte survival and demonstrate that only a narrow window of NF-κB activity is tolerated by lymphocytes.
Materials and Methods
Mice. IκBα+/- (5) and IκBε+/- mice (7) were interbred to generate IκBα-/-/IκBε-/- mice. The two independent IκBε-null mouse lines generated (7) were used indiscriminately and led to identical results. The IκBα and IκBε mutant mice are on a mixed background (Sv129xC57Bl/6 and Sv129xC57Bl/6xDBA/2, respectively). Double-mutant mice therefore express the H-2b and H-2d antigens. Mice were kept in a specific-pathogen-free animal house facility and genotyped by PCR. The morning of the vaginal plug was designated as day 0.5. All experiments were performed in accordance with institutional guidelines.
Western Blot Analysis. Whole-cell extracts were analyzed as described (10). Antibodies to IκBα (C-21) or IκBε (M-121) were purchased from Santa Cruz Biotechnology.
Electrophoretic Mobility-Shift Assay. Bandshift assays were performed as reported (11).
Flow Cytometric Analysis and Apoptosis Assay. Single-cell suspensions from thymus, spleen, bone marrow, or fetal liver (FL) were surface-stained either with monoclonal antibodies coupled to fluorescein, phycoerythrin, or allophycocyanin or with biotinylated monoclonal antibodies (Pharmingen, Caltag, South San Francisco, CA) followed by tricolor-streptavidin (Caltag), allophycocyaninavidin, or phycoerythrin-avidin (Pharmingen) and then analyzed by using a FACSCalibur flow cytometer and CELLQUEST 3.1 software (BD Immunocytometry Systems) according to standard protocols. The following Abs were used for fluorescence-activated cell sorting (FACS) staining: anti-IgM-biotin (R33-24, home-made); anti-mouse IgM (Mu chain spf) from Jackson ImmunoResearch; and anti-H2Kb (AF6-88.5), anti-H2Kd (SF1-1.1), anti-H2Kk (36-7-5), anti-I-Ab (AF6-120.1), anti-I-Ad (2G9), anti-CD3 (145-2C11 and 17A2), anti-CD4 (L3T4 and RM4-5), anti-CD8 (Ly-2/53-6.7), anti-CD11b/Mac1 (M1/70), anti-T cell antigen receptor β (TCRβ) (H57-597), anti-CD43 (S7), anti-CD45R/B220 (RA3-6B2), anti-CD19 (1D3), anti-GR1 (Ly-6G/RB6-8C5), and anti-Ly-5.2 (104) from Pharmingen. For identification of apoptotic cells by FACS, lymphocytes were first stained with the appropriate monoclonal antibodies and then washed once before additional labeling with 3,3′-diexyloxacarbocyanine iodide (DiOC6) (Molecular Probes) and analyzed by flow cytometry.
Detection of Ig Heavy and Light Chain Rearrangements. Assays were performed as described (12) with the following modifications. Splenic cells from reconstituted mice were directly lysed in PCR lysis buffer, whereas FL lymphocytes were first enriched by sedimentation/elimination of hepatocytes (800 rpm for 1 min) from liver cell suspensions. Two microliters of lysate were used in 50-μl PCR reactions containing 5′ and 3′ primers as described for Ig heavy chain gene and Ig light chain gene PCR (13, 14). For TCRα gene PCR, denaturated samples were subjected to 30 cycles of amplification in a thermal cycler with 5′ (GAATCCTCCTGCTGAAAGTAGCC) and 3′ (GGCTCTGTCAGTCTTGCAGACC) primers (15 s at 94°C, 1 min at 65°C, and 1 min at 72°C) (Roche Diagnostics). PCR reactions were then separated on a 2.4% agarose gel, transferred onto positive nylon membrane, which retains small DNA fragments (Oncor), and hybridized with a32P-labeled oligonucleotide (15). The probes were as follows: Jκ1, 5′-CCAGCTTGGTGCCTCCACCGAACGTCCACCACAG-3′; JH4, 5′-AATAGTGATGGTGGTAGCACCTAT-3′; and TCR, 5′-CCAAGTCACATCAAGGCTCACACAGC-3′.
In Vitro Culture of FL Cells and FTOC. E15.5 FL single-cell suspensions were cultured on irradiated S17 feeder cells supplemented with IL-7, SCF, or FlT3-l for 7-13 days and analyzed by flow cytometry. For fetal thymic organ culture (FTOC), E18.5 thymic lobes were put on top of an 0.8-μm isopore membrane filter (Millipore) floating on optiMEM medium containing 10% FCS and left at 37°C in 5% CO2 for 2.5 days before analysis by flow cytometry.
Adoptive Transfer of FL Cells. E13.5/E14.5 FL were harvested and placed in 1 ml of RPMI medium 1640 supplemented with 100 μM gentamicin at 4°C. After genotyping, FL suspensions were obtained by passage of the tissue through a 23-gauge needle. RAG2/γc host mice on the H-2Kk background (16) were irradiated with 0.6 Gy from a137Cs source, and 4-6 h later they were injected retroorbitally with 106 FL cells. To distinguish between donor fetal-derived and host RAG2/γc-derived cells in the reconstituted chimeras, H-2Kk-positive host cells were excluded from all subsequent fluorescence-activated cell sorting analysis. For competition experiments, RAG2/γc host mice were on a Ly-5.2 background, and wild-type bone marrow was on a Ly-5.1 background. Fetal donor cells and wild type were mixed at 1:1 ratio.
Statistical Analysis. Statistical comparisons were carried out with Microsoft EXCEL software, applying the two-tailed Student's t test.
Results
Mice Nullizygous for Both IκBα and IκBε Exhibit Increased NF-κB Activity. To gain insight, the redundant functions of the two NF-κB inhibitors, IκBα and IκBε, mice deficient in both _I_κ_B_α and _I_κ_B_ε genes, were generated. Immunoblotting analysis of total thymic extracts showed the absence of IκBε together with a strong increase of IκBα protein levels in IκBε-nullizygous mice (Fig. 1_A_,lanes3-5), the lack of IκBα accompanied by a slight rise in IκBε levels in IκBα-deficient mice (Fig. 1_A_, lanes 6 and 7), and no detectable IκBα and IκBε in compound homozygous mice, confirming the inactivation of both _I_κ_B_α and _I_κ_B_ε genes (Fig. 1_A_, lanes 8 and 9). IκBα/IκBε-nullizygous mice developed until birth with no obvious morphological abnormalities, and Mendelian ratios of IκBα-/- IκBε-/- fetuses were observed shortly before parturition, at E18.5. Close monitoring of E18.5 fetuses after caesarian section and of litters after birth revealed that the double-knockout pups were born alive. They could open their mouth but remained cyanotic, were less active than the rest of the litter, and died invariably of anoxia within 10-30 min. None survived this very early stage. Thus, ex vivo analyses were all carried out just before birth at E18.5.
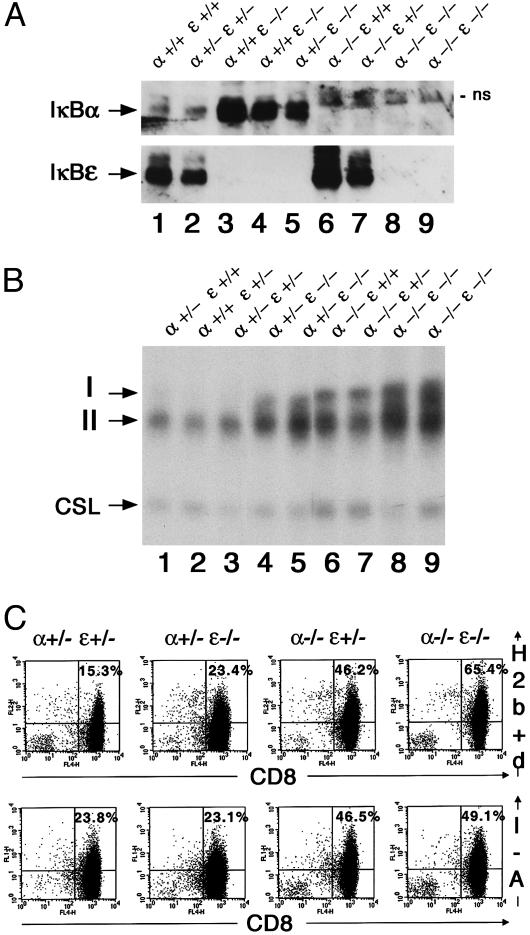
Fig. 1.
Constitutive up-regulation of NF-κB DNA binding activity and overexpression of NF-κB target genes in IκBα/IκBε-nullizygous mice. (A)IκBα/IκBε status in total thymic protein extracts (120 μg) from E18.5 fetuses analyzed by Western blot. Genotypes are indicated; ns, nonspecific band. (B) Nuclear extracts (0.5 μg) from thymus of E18.5 fetuses analyzed by electrophoretic mobility-shift assay for binding to a canonical κB site. Arrows indicate the complexes visualized: I and II for the different Rel/NF-κB dimers; CSL was used as an internal control. (C) Expression of H-2Kb+d and I-A antigens on CD8+ thymocytes was assayed by two-color flow cytometry. An identical number of CD8+ cells was found in all genotypes. Percentages of positive events in the corresponding quadrant thus reflect the levels of expression of MHC antigens.
The double deficiency in IκBα and IκBε of E18.5 fetuses resulted in the thymus in a dramatic increase in constitutive κB binding activity, represented by two distinct complexes I and II, compared with the one observed for other genotypes, which gradually rose from wild type to IκBα+/-IκBε-/- and IκBα-/-IκBε+/+ (Fig. 1_B_). This augmentation was specific to the transcription factor NF-κB, because the binding activity of another transcription factor CSL, a component of the Notch signaling pathway that recognizes a half-κB site (17), was stable regardless of the genotype (Fig. 1_B_). Specific antisera were used to identify the components of the binding complexes (data not shown), demonstrating that complex I mainly consists of the p50/p65 heterodimer with contributions of p50/RelB and c-Rel/p65 species, and that complex II is composed of p50/p50 homodimers only.
The augmented NF-κB binding activity in the thymus was reflected by an overexpression of two NF-κB target genes, the MHC Class I and II antigens (Fig. 1_C_). For the thymic CD8+ population, which at this age represents mostly double-positive thymocytes, there was a constant increase in the level of expression of MHC class I from wild type to IκBε or IκBα single mutant and to IκBα-/-IκBε-/- fetuses. For the class II antigen, a similar rise in expression was seen in IκBα single mutant and IκBα-/-IκBε-/- animals compared with wild-type and IκBε-deficient fetuses.
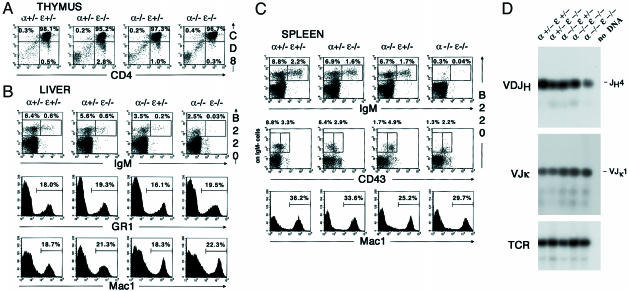
Impaired B Cell Development in IκBα-/-IκBε-/- Fetuses. To establish whether hematopoietic cell development could be affected by the loss of both IκBα and IκBε, we performed an extensive analysis of hepatic, thymic, and splenic cell populations of E18.5 fetuses by flow cytometry. At that stage, few cells are present in the bone marrow, whereas liver still exhibit a hematopoietic function. This latter organ was therefore preferred for the analysis of primary lymphoid cells. Identical data were obtained with single mutants on a wild-type or a heterozygous background, indicating an absence of IκB gene dosage effect on the generation of hematopoietic subpopulations. Only data from heterozygous background or double-mutant cells are presented in Fig. 2. By E18.5, regardless of the genotype, thymocytes had progressed to the double-positive stage (DP) and were just beginning to undergo positive selection and lineage commitment to the mature single-positive stage (SP) (Fig. 2_A_). No significant difference in the proportion of DP thymocyte was observed, suggesting that the CD4-CD8-CD3- triple-negative stage to DP transition occurred normally in IκBα-/-IκBε-/- fetuses. No variation in the expression of the macrophage (Mac1) or granulocyte (GR1) markers in the liver or the spleen could be detected (Fig. 2 B and C), attesting normal granulopoiesis and myelopoiesis in IκBα-/-IκBε-/- fetuses. However, very low numbers of immature B cells (B220+IgM+) as well as a decrease of B cell precursors (B220+IgM-) were observed in the liver and the spleen of IκBα-/-IκBε-/- fetuses compared to controls (Fig. 2 B and C). Characterization of B cell progenitors after exclusion of the IgM+ cell population with the B220 and CD43 cell-surface markers revealed a clear reduction of B220+CD43- splenic pre-B cell subset compared with wild-type or single mutants, whereas the B220+CD43+ pro-B cell pool was barely affected (Fig. 2_C_). We further examined the rearrangement status of the heavy and light chain loci in lymphoid cells isolated from E18.5 FL by genomic DNA PCR (Fig. 2_D_). DJH4 and VJk1 rearrangements occurred in the double-mutant cells in a similar fashion as in controls, suggesting that the lymphocytic lineage commitment was occurring normally. We conclude that B cell differentiation in IκBα/IκBε-nullizygous fetuses is arrested after the pre-B stage at the time of IgM expression.
Fig. 2.
Impaired B cell development in IκBα/IκBε-nullizygous fetuses. Single-cell suspensions from thymus (A), liver (B), and spleen (C) of E18.5 fetuses genotyped as indicated were stained for CD4, CD8, IgM, B220, CD43, GR1, or Mac1 and then analyzed by flow cytometry. Gates are shown by boxes, and percentages of gated cells within the plot are specified above or inside the quadrant. For single-color staining, percentages of positive events are indicated in each graph. (D) Analysis of Ig heavy (D-JH) and light (V-Jκ) chain rearrangement status in E18.5 FL lymphocytes genotyped as indicated. No DNA corresponds to the analysis done in the absence of DNA as a negative control. TCR gene amplification was done in parallel on the same DNA preparations as an internal control.
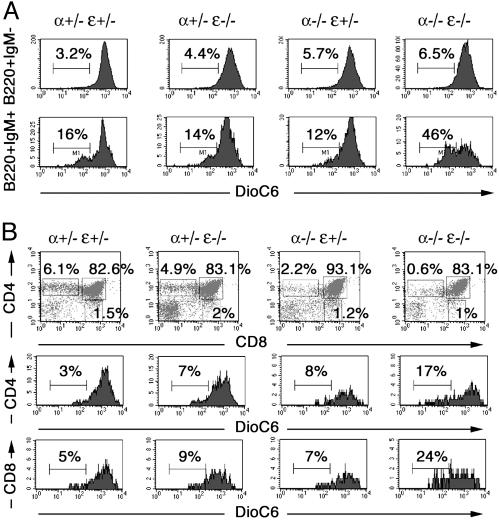
Apoptosis Is Responsible for the B Cell Developmental Defect of IκBα/IκBε-Nullizygous Fetuses and Also Impedes Generation of SP Thymocytes. To determine whether the B cell deficiency in E18.5 IκBα-/-IκBε-/- fetuses results from programmed cell death, E18.5 liver lymphocytes obtained from double-heterozygous matings were labeled with DioC6, a lipophilic fluorochrome whose uptake is decreased by the alterations of the mitochondrial membrane in early apoptotic cells (18, 19). Fig. 3_A_ displays a similar labeling for B220+IgM- lymphocytes, regardless of the genotype. However, when focusing on the B220+IgM+ cell population, the proportion of apoptotic cells tripled for the IκBα-/-IκBε-/- cells compared with wild-type or single-mutant cells. The B cell developmental defect in double-knockout fetuses is thus the consequence of increased apoptosis of B cells, in particular the B220+IgM+ cell subset.
Fig. 3.
In IκBα/IκBε-nullizygous fetuses, programmed cell death is a feature of immature B cells and SP thymocytes. (A) Spleen lymphocytes from E18.5 fetuses genotyped as indicated were stained for B220 and IgM, assayed for apoptosis by DioC6 staining, and analyzed by flow cytometry. Percentages of apoptotic cells within the B220+IgM- and B220+IgM+ populations are indicated in the histograms. Results are representative of at least six separate experiments. (B) Thymocytes from 2.5-day FTOC of E18.5 thymi were stained for CD4 and CD8 and analyzed by flow cytometry (Top). Gates are shown by boxes, and percentages of gated cells within the plot are specified above or inside the boxes. Cells gated as SP CD4+ (Middle) or CD8+ (Bottom) were further assayed for apoptosis by DioC6 staining. Percentages indicate the apoptotic cells. Results are representative of at least four separate experiments.
To examine the effect of IκBα/IκBε deletion on further maturation of thymocytes, E18.5 thymi were cultured in vitro, and thymic cell subpopulations were analyzed by flow cytometry after 2.5 days (Fig. 3_B_). Transition from DP to SP cells occurred in a similar fashion in wild-type or single IκBε-/- mutant FTOC, whereas IκBα-/- mutant FTOC produced fewer CD4+ cells (Fig. 3_B_ Top). In the double-mutant thymic culture, a clear defect in generation of CD8+ and especially CD4+ SP T cells was observed. This reduced number of SP T cells was accompanied by normal expression of TCRβ and TCRγδ (data not shown). It correlated with apoptosis of the SP CD4+ or CD8+ double-mutant cells (Fig. 3_B_ Middle and Bottom). We conclude that despite normal capacity to mature to the DP stage, double-mutant fetal thymocytes die by apoptosis at the SP stage.
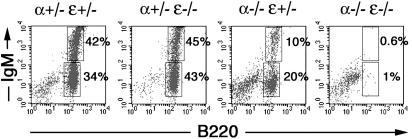
IκBα/IκBε-Nullizygous FL Cells Are Unable to Differentiate in Vitro. In an attempt to decipher the mechanism responsible for impaired B lymphocyte generation in IκBα/IκBε-nullizygous mice, we used an in vitro culture system. Normal B cell development with production of B220+IgM+ immature B cells was observed for wild-type or single mutant fetal cells, although IκBα-deficient cells showed a reduced differentiation potential (Fig. 4). However, B220+ cells were almost totally absent in the culture from double-deficient mice (Fig. 4). This defect, which is more severe than the one observed for E18.5 fetuses, could not be rescued by treatment with a general caspase inhibitor (Boc-D-FMK) (20) or by retroviral infection of a prosurvival gene such as Bcl-2 or by treatment with neutralizing anti-TNFα antibodies (data not shown). These data provide evidence that IκBα/IκBε-nullizygous lymphoid precursors are unable to differentiate in vitro and undergo passive cell death in culture in a TNF-independent fashion. Survival of very early B cell progenitors in vitro is thus impaired by the combined absence of IκBα and IκBε.
Fig. 4.
Impaired B cell survival of IκBα/IκBε-nullizygous FL cultures in vitro. Flow cytometry analysis of E15.5 FL cells cultured for 7 days and stained for B220 and IgM. Gates are shown by boxes, and percentages of gated cells of total cells within the plot are specified at the right of the quadrant. Data shown are examples of three independent experiments.
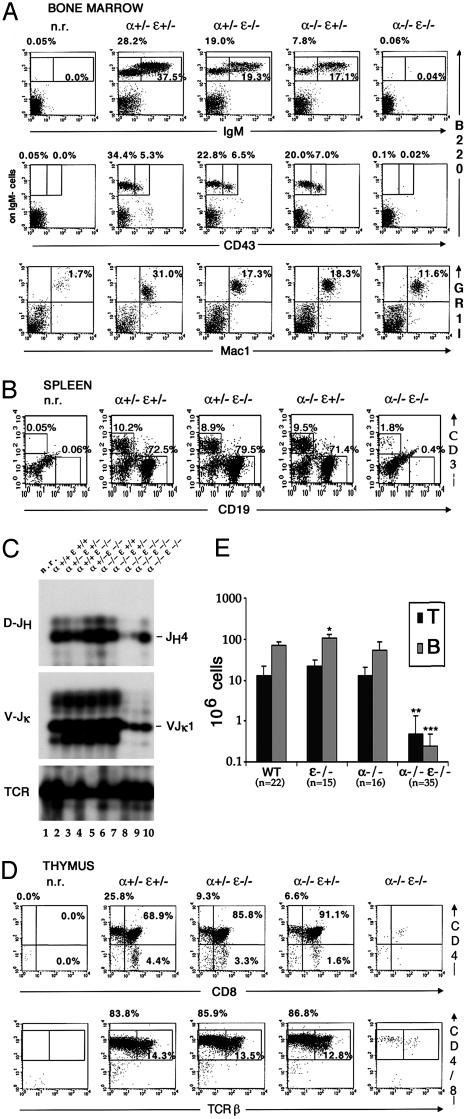
Defective Lymphopoiesis of Mice Reconstituted with IκBα/IκBε-Nullizygous FL Cells. To examine hematopoietic development in adult mice, E13.5 FL cells from wild type, single-IκB mutant, or double IκB mutant were isolated and transplanted into sublethally irradiated alymphoid RAG2/γc hosts (21). Analysis by flow cytometry of the myeloid and lymphocyte cell populations of the grafted mice 9 weeks after transplantation is shown in Fig. 5. Wild-type fetal cell reconstitution led to normal bone marrow macrophage/granulocyte ratios, whereas adoptive transfer with single-IκB or double-IκBα/IκBε mutant fetal cells was less efficient (Fig. 5_A_), revealing a subnormal myelopoiesis/granulopoiesis and/or a lower reconstitution potential in IκBα/IκBε nullizygous chimeras. Adoptive transfer with wild-type or single-mutant fetal cells produced normal B cell development, the single mutant chimeras harboring small decreases of each cell subset in the bone marrow. In the spleen, similar numbers of B cells were observed for wild-type or IκBα chimeras, whereas IκBε-/- reconstituted mice displayed significantly enhanced percentages of CD19+ B cells. In contrast, an almost complete absence of B220+IgM+ immature B cells, as well as IgM-B220+CD43+ and IgM-B220+CD43- B cell precursors, was observed in the bone marrow of doubly deficient chimeras. In the spleen, compared to the host, some CD19+ B cells were detected in mice reconstituted with doubly deficient fetal cells (Fig. 5_B_). Absolute splenic B cell numbers in these chimeras were 300-fold lower than values obtained for wild-type chimeras (Fig. 5_E_). When assessing the rearrangement status of the Ig loci by genomic PCR (Fig. 5_C_), it seems that whereas DJH4 and VJκ1 rearrangements are absent in nonreconstituted RAG2/γc hosts, they were detectable in the double-mutant chimeras even if it was at much lower levels than in wild-type and single-mutant chimeras (Fig. 5_C_). We conclude that the very few B cells present in IκBα/IκBε-deficient chimeras have retained the capacity to mature to the late pre-B stage. However, survival of B cell progenitors in IκBα/IκBε-nullizygous chimeras is severely impaired.
Fig. 5.
Extended lymphopenia in IκBα/IκBε-nullizygous chimeras. Single-cell suspensions from bone marrow (A), spleen (B), and thymus (D) isolated from RAG2/γc mice nonreconstituted (n.r.) or reconstituted with FL cells genotyped as indicated were stained for IgM, B220, CD43, GR1, Mac1, CD3, CD19, CD4, CD8, or TCRβ and analyzed by flow cytometry. Gates are shown by boxes, and percentages of gated cells within the plot are specified above or inside the quadrant. (C) Analysis of Ig heavy (D-JH) and light (V-Jκ) chain rearrangement status in nonreconstituted (n.r., lane 1), wild-type (lanes 2 and 3), IκBε (lanes 4 and 5), IκBα (lanes 6 and 7), and IκBα/IκBε-nullizygous (lanes 8-10) chimeric splenocytes. TCR gene amplification was done in parallel on the same DNA preparations as an internal control. Note the absence of Ig heavy and light chain gene rearrangement in the nonreconstituted host despite normal TCR gene amplification. (E) T and B cell absolute numbers in the spleen of RAG2/γc chimeras.*, P < 0.00023; **, P < 4 × 10-7; ***, P < 4 × 10-17.
Wild-type and single-mutant FL cells reconstituted the thymus of RAG2/γc hosts similarly to wild type, the IκBα chimeras harboring decreased numbers of the SP cell subsets (Fig. 5_D_). These thymocytes expressed normal levels of TCRα/β. In contrast, IκBα-/- IκBε-/- RAG2/γc chimeras displayed a considerable thymic atrophy, the remnant containing a few CD4+CD8+TCR+ thymocytes (Fig. 5_D_), which are absent in the RAG2/γc host mice. A clear reconstitution of T cell development in the periphery was observed for the wild-type or single-mutant chimeras, whereas double-IκBα/IκBε mutant chimeras exhibited scarce splenic T lymphocytes (25-fold less than wild type) that are missing in the nonreconstituted host (Fig. 5 B and E). The disappearance of most thymocytes in both primary and secondary lymphoid organs in IκBα-/-IκBε-/- chimeras is reminiscent of the B cell situation, underscoring a more pronounced defect than in IκBα/IκBε double-mutant fetuses. These grafting experiments suggest that in adult life, elevated NF-κB is detrimental to lymphocyte survival from primitive stages of T and B lymphopoiesis.
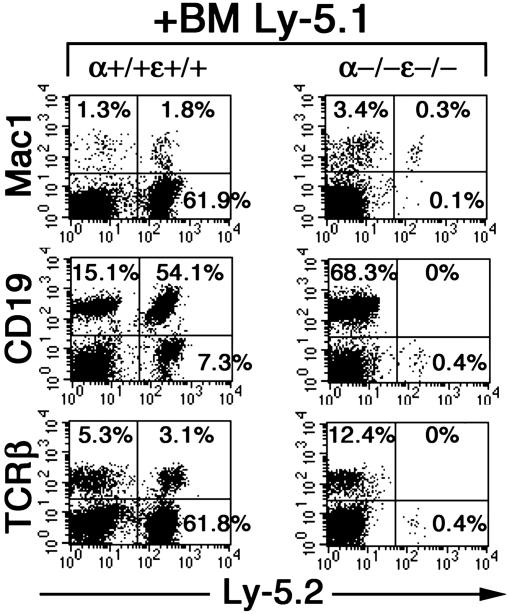
The Lymphopoiesis Defect of IκBα/IκBε-Nullizygous Chimeras Is Cell Autonomous. To determine the contribution of the hematopoietic environment to the lymphopoiesis failure of IκBα-/-IκBε-/- chimeras, we investigated whether this defect could be rescued by cotransplantation of FL cells (Ly-5.2) with bone marrow cells from wild-type mice congenic to the RAG2/γc host (Ly-5.1) and performed a mixing experiment as described (22). Eight weeks after transfer, chimeras were analyzed by flow cytometry. Successful engraftment of wild-type or IκBα/IκBε-nullizygous fetal donor cells was assessed by the presence of splenic Ly-5.2 macrophages (Fig. 6 Top). Whereas B (CD19+Ly-5.2+) and T (TCRβ+Ly-5.2+) cell development from wild-type fetal-derived donor cells occurred normally, no fetal-derived IκBα/IκBε-deficient T or B cells could be detected in the spleen of co-reconstituted mice (Fig. 6 Middle and Bottom) or in their bone marrow or thymus (data not shown). These results are consistent with the impaired B cell survival of double mutant FL progenitors observed in vitro and suggest that the lymphopoiesis defect of IκBα-/-IκBε-/- chimeras is intrinsic to the T and B cell lineages.
Fig. 6.
T and B lymphopoiesis defect of IκBα/IκBε-nullizygous RAG2/γc reconstituted chimeras is cell intrinsic. Immunostaining of splenocytes from FL wild-type- or IκBα/IκBε-nullizygous- and Ly-5.1-coinjected mice. Cells were stained for specific markers of donor (Ly-5.2) macrophages (Mac1), B lymphocytes (CD19), and T lymphocytes (TCRβ) and analyzed by fluorescence-activated cell sorting. The percentage of gated cells of total cells within the plot are specified inside the corresponding quadrant. Genotypes above plots refer to that of donor Ly-5.2 FL cells.
Discussion
In this study, the roles of IκBα and IκBε were examined in vivo. Compound homozygous mice carrying null alleles of both _I_κBα and _I_κBε genes died soon after birth, i.e., earlier than single IκBα-nullizygous mice, and exhibited a more drastic phenotype than each mutant mouse. These results confirm the hypothesized compensatory role of IκBα accounting for the subtle phenotype of IκBε-nullizygous mice. They also indicate that IκBε has specific roles that cannot be taken over by IκBα.IκBα and IκBε have overlapping but distinct functions in vivo, and their mutual absence is not essential for embryonic development. The demise at birth of the double mutants from respiratory failure moots a putative role of IκB-controlled genes in lung development and function and/or brain breathing control. The simultaneous absence of IκBα and IκBε caused a dramatic increase in the constitutive nuclear NF-κB activity in the thymus of E18.5 fetuses compared to wild-type or single mutants. This increase is evidenced by the overexpression of two NF-κB target genes, encoding the MHC class I and II antigens. The level of NF-κB activity is directly correlated with the presence or absence of the IκBs, and its increase in IκB-deficient mice reflects the portion of NF-κB complexes commonly associated with one or the other inhibitors.
Analysis of hematopoietic cell populations of IκBα-/-IκBε-/- E18.5 fetuses reveals normal erythropoiesis, thrombopoiesis, myelopoiesis, and granulopoiesis. In the lymphoid lineage, IκBα/IκBε-deficient fetal cells failed to generate normal numbers of IgM+ immature B cells as well as SP T cells, because of enhanced apoptosis. Persistent activation of NF-κB at high levels in the lymphocyte population in IκBα/IκBε-nullizygous fetuses has a direct lethal effect at a late stage of development at the time of positive and negative selection of lymphocytes. A strict control of NF-κB dimers seems essential at these regulatory checkpoints in the lymphoid lineage. Because activation of NF-κB has been shown to promote negative selection of immature thymocytes by endogenous MHC ligands in vivo (23), overactivation of NF-κB could result in apoptosis through a pseudonegative mechanism. Consistent with this hypothesis, high NF-κB nuclear levels have been shown recently to generate massive apoptosis of T cells at the DP stage in Id1 and Tal1 transgenic mice that can be partially overcome by the super repressor IκBα (24). However, moderate NF-κB activation promotes survival of CD44-CD25+ and DN CD4-CD25- T cells (25). Depending on the level of NF-κB activation, moderate versus high, the biological outcome, apoptosis or survival, may vary. Alternatively, lymphocytes at different developmental stages may react differently to NF-κB activation. Previous studies have revealed that NF-κB plays an important role in the regulation of T cell apoptosis and behaves as a cell death promoting or protecting factor, depending on the context (23, 26-32). For B cell development, our data are consistent with the apoptosis observed after overexpression of p65 in a pro-B cell line (33). Multiple studies with various mutant mice, p65(RelA)-/-c-Rel-/- (34), IKKβ-/- (35, 36), and Nemo-/- (36, 37), have also underscored a prominent role of NF-κB in B cell survival.
When analyzing hematopoiesis in adults, extensive lymphopenia with a near absence of B and T cells was observed in adoptive transfers of FL cells. It remains striking that the lymphoid defect of IκBα/IκBε-null chimeras is more severe than that of IκBα/IκBε-deficient fetuses, where T and B cell development proceeds normally to a late stage. A different hematopoietic microenvironment, which consists of stromal cells from hematopoietic and nonhematopoietic origin (38, 39) in fetuses and adults, might account for such discrepancies and protect from cell death the fetal cells in vivo, because IκBα/IκBε-deficient fetal liver stem cells are incapable of differentiating in vitro. The lymphoid phenotype of p50/RelA doubly deficient or IKKβ-null chimeras, both characterized by a deficit of T and B cells, is remarkably reminiscent of that of IκBα/IκBε-nullizygous reconstituted chimeras (22, 31). Similarly, reduced viability of Nemo-/- IgM+ cells has been described after coculture of ES cells and OP9 bone marrow cells (37), whereas we have shown that the same population in IκBα/IκBε-deficient fetuses die from apoptosis. These data suggest that a critical NF-κB dosage is required for lymphocyte survival and that its reduction or rise produces a similar dysfunction. However, distinct mechanisms lead to cell death depending on the lack or excess of NF-κB activity; for both p50/p65- and IKKβ-deficient chimeras (22, 31), cotransplantation of mutant FL cells with wild-type bone marrow rescues lymphopoiesis, as does elimination of TNFR1 in IKKβ-null mice. In contrast, cotransplantation of IκBα/IκBε-deficient FL with wild-type bone marrow cells has no effect, indicating that survival of early T and B cell progenitors is intrinsically impaired in IκBα/IκBε-deficient chimeras. The inability of IκBα/IκBε-deficient FL cells to differentiate into B cells in vitro is not rescued either by treatment with a general caspase inhibitor or neutralizing antibodies against TNF-α, suggesting that unlike IKKβ-null mice, caspase activation and TNF-α may not be involved in the death of IκBα/IκBε-deficient B cell progenitors.
These findings demonstrate that NF-κB activity is tightly controlled by IκBs and should fit in a narrow window. Altogether, our observations indicate that IκBα and IκBε proteins play a critical role in the intrinsic survival of B and T cells by keeping NF-κB latent in the cytoplasm and that this control of NF-κB activity is necessary for completion of lymphocyte development and maintenance of lymphocyte homeostasis.
Acknowledgments
We thank C. Cimper for helpful histological assistance, L. Fiette for preliminary histopathological analysis, R. Kelly for critical reading of the manuscript, and M. Goodhardt, J. P. Kolb, and N. Israël for providing reagents. S.M. is particularly indebted to the service of Chirurgie 3 d'Auxerre and Dr. I. Aupérin. This work was supported by grants from Institut National de la Santé et de la Recherche Médicale, Ligue Nationale Française contre le Cancer, and Agence Nationale de Recherches sur le SIDA.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: E_n_, embryonic day n; IKK, IκB kinase; DP, double-positive stage; SP, single-positive stage; TNF, tumor necrosis factor; FL, fetal liver; FTOC, fetal thymic organ culture; TCR, T cell antigen receptor.
References
- 1.Gerondakis, S., Grumont, R., Rourke, I. & Grossmann, M. (1998) Curr. Opin. Immunol. 10**,** 353-359. [DOI] [PubMed] [Google Scholar]
- 2.Karin, M. & Lin, A. (2002) Nat. Immunol. 3**,** 221-227. [DOI] [PubMed] [Google Scholar]
- 3.Li, Q. & Verma, I. M. (2002) Nat. Rev. Immunol. 2**,** 725-734. [DOI] [PubMed] [Google Scholar]
- 4.Whiteside, S. T. & Israel, A. (1997) Semin. Cancer Biol. 8**,** 75-82. [DOI] [PubMed] [Google Scholar]
- 5.Beg, A. A., Sha, W. C., Bronson, R. T. & Baltimore, D. (1995) Genes Dev. 9**,** 2736-2746. [DOI] [PubMed] [Google Scholar]
- 6.Klement, J. F., Rice, N. R., Car, B. D., Abbondanzo, S. J., Powers, G. D., Bhatt, H., Chen, C. H., Rosen, C. A. & Stewart, C. L. (1996) Mol. Cell. Biol. 16**,** 2341-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mémet, S., Laouini, D., Epinat, J.-C., Whiteside, S. T., Goudeau, B., Philpott, D., Kayal, S., Sansonetti, P. J., Berche, P., Kanellopoulos, J. & Israël, A. (1999) J. Immunol. 163**,** 5994-6005. [PubMed] [Google Scholar]
- 8.Chen, C. L., Singh, N., Yull, F. E., Strayhorn, D., Van Kaer, L. & Kerr, L. D. (2000) J. Immunol. 165**,** 5418-5427. [DOI] [PubMed] [Google Scholar]
- 9.Whiteside, S. T., Epinat, J. C., Rice, N. R. & Israel, A. (1997) EMBO J. 16**,** 1413-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt-Ullrich, R., Mémet, S., Lilienbaum, A., Feuillard, J., Raphael, M. & Israël, A. (1996) Development (Cambridge, U.K.) 122**,** 2117-2128. [DOI] [PubMed] [Google Scholar]
- 11.Feuillard, J., Mémet, S., Goudeau, B., Lilienbaum, A., Schmidt-Ullrich, R., Raphaël, M. & Israël, A. (2000) Int. Immunol. 12**,** 613-621. [DOI] [PubMed] [Google Scholar]
- 12.Schlissel, M. S. & Baltimore, D. (1989) Cell 58**,** 1001-1007. [DOI] [PubMed] [Google Scholar]
- 13.Marshall, A. J., Wu, G. E. & Paige, G. J. (1996) J. Immunol. 156**,** 2077-2084. [PubMed] [Google Scholar]
- 14.Goodhardt, M., Cavelier, P., Doyen, N., Kallenbach, S., Babinet, C. & Rougeon, F. (1993) Eur. J. Immunol. 23**,** 1789-1795. [DOI] [PubMed] [Google Scholar]
- 15.Church, G. M. & Gilbert, W. (1984) Proc. Natl. Acad. Sci. USA 81**,** 1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colucci, F., Samson, S. I., DeKoter, R. P., Lantz, O., Singh, H. & Di Santo, J. P. (2001) Blood 97**,** 2600-2608. [DOI] [PubMed] [Google Scholar]
- 17.Jarriault, S., Brou, C., Logeat, F., Schroeter, E. H., Kopan, R. & Israel, A. (1995) Nature 377**,** 355-358. [DOI] [PubMed] [Google Scholar]
- 18.Petit, P. X., Lecoeur, H., Zorn, E., Dauguet, C., Mignotte, B. & Gougeon, M. L. (1995) J. Cell Biol. 130**,** 157-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamzami, N., Marchetti, P., Castedo, M., Zanin, C., Vayssiere, J. L., Petit, P. X. & Kroemer, G. (1995) J. Exp. Med. 181**,** 1661-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Mello, S., Aglieco, F., Roberts, M. R., Borodezt, K. & Haycock, J. W. (1998) J. Neurochem. 70**,** 1809-1818. [DOI] [PubMed] [Google Scholar]
- 21.Colucci, F., Soudais, C., Rosmaraki, E., Vanes, L., Tybulewicz, V. L. & Di Santo, J. P. (1999) J. Immunol. 162**,** 2761-2765. [PubMed] [Google Scholar]
- 22.Horwitz, B. H., Scott, M. L., Cherry, S. R., Bronson, R. T. & Baltimore, D. (1997) Immunity 6**,** 765-772. [DOI] [PubMed] [Google Scholar]
- 23.Mora, A. L., Stanley, S., Armistead, W., Chan, A. C. & Boothby, M. (2001) J. Immunol. 167**,** 5628-5635. [DOI] [PubMed] [Google Scholar]
- 24.Kim, D., Xu, M., Nie, L., Peng, X. C., Jimi, E., Voll, R. E., Nguyen, T., Ghosh, S. & Sun, X. H. (2002) Immunity 16**,** 9-21. [DOI] [PubMed] [Google Scholar]
- 25.Voll, R. E., Jimi, E., Phillips, R. J., Barber, D. F., Rincon, M., Hayday, A. C., Flavell, R. A. & Ghosh, S. (2000) Immunity 13**,** 677-689. [DOI] [PubMed] [Google Scholar]
- 26.Gerondakis, S. & Strasser, A. (2001) Nat. Immunol. 2**,** 377-379. [DOI] [PubMed] [Google Scholar]
- 27.Hettmann, T., DiDonato, J., Karin, M. & Leiden, J. M. (1999) J. Exp. Med. 189**,** 145-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boothby, M. R., Mora, A. L., Scherer, D. C., Brockman, J. A. & Ballard, D. W. (1997) J. Exp. Med. 185**,** 1897-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dudley, E., Hornung, F., Zheng, L. X., Scherer, D., Ballard, D. & Lenardo, M. (1999) Eur. J. Immunol. 29**,** 878-886. [DOI] [PubMed] [Google Scholar]
- 30.Zheng, Y., Vig, M., Lyons, J., Van Parijs, L. & Beg, A. A. (2003) J. Exp. Med. 197**,** 861-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senftleben, U., Li, Z. W., Baud, V. & Karin, M. (2001) Immunity 14**,** 217-230. [DOI] [PubMed] [Google Scholar]
- 32.Guerin, S., Baron, M. L., Valero, R., Herrant, M., Auberger, P. & Naquet, P. (2002) Eur. J. Immunol. 32**,** 1-9. [DOI] [PubMed] [Google Scholar]
- 33.Sheehy, A. M. & Schlissel, M. S. (1999) J. Biol. Chem. 274**,** 8708-8716. [DOI] [PubMed] [Google Scholar]
- 34.Grossmann, M., O'Reilly, L. A., Gugasyan, R., Strasser, A., Adams, J. M. & Gerondakis, S. (2000) EMBO J. 19**,** 6351-6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, Z. W., Omori, S. A., Labuda, T., Karin, M. & Rickert, R. C. (2003) J. Immunol. 170**,** 4630-4637. [DOI] [PubMed] [Google Scholar]
- 36.Pasparakis, M., Schmidt-Supprian, M. & Rajewsky, K. (2002) J. Exp. Med. 196**,** 743-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim, S., La Motte-Mohs, R. N., Rudolph, D., Zuniga-Pflucker, J. C. & Mak, T. W. (2003) Proc. Natl. Acad. Sci. USA 100**,** 1203-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carsetti, R. (2000) J. Exp. Med. 191**,** 23-32.10620602 [Google Scholar]
- 39.Melchers, F., Rolink, A. G. & Schaniel, C. (1999) Cell 99**,** 351-354. [DOI] [PubMed] [Google Scholar]