Cell-Permeating α-Ketoglutarate Derivatives Alleviate Pseudohypoxia in Succinate Dehydrogenase-Deficient Cells (original) (raw)
Abstract
Succinate dehydrogenase (SDH) and fumarate hydratase (FH) are components of the tricarboxylic acid (TCA) cycle and tumor suppressors. Loss of SDH or FH induces pseudohypoxia, a major tumor-supporting event, which is the activation of hypoxia-inducible factor (HIF) under normoxia. In SDH- or FH-deficient cells, HIF activation is due to HIF1α stabilization by succinate or fumarate, respectively, either of which, when in excess, inhibits HIFα prolyl hydroxylase (PHD). To reactivate PHD, we focused on its substrate, α-ketoglutarate. We designed and synthesized cell-permeating α-ketoglutarate derivatives, which build up rapidly and preferentially in cells with a dysfunctional TCA cycle. This study shows that succinate- or fumarate-mediated inhibition of PHD is competitive and is reversed by pharmacologically elevating intracellular α-ketoglutarate. Introduction of α-ketoglutarate derivatives restores normal PHD activity and HIF1α levels to SDH-suppressed cells, indicating new therapy possibilities for the cancers associated with TCA cycle dysfunction.
Under normal oxygenation conditions (normoxia), hypoxia-inducible factor alpha (HIFα) proteins (HIF1α and/or HIF2α), which are the formation-limiting units of the HIF transcription factor heteromer, are constantly both synthesized and degraded, a process that maintains them in high availability but at a very low steady state (4, 25). The high turnover of HIFα is mediated by HIFα prolyl hydroxylases (PHD1 to -3, also known as EglN1 to -3 and HPH1 to -3). PHDs hydroxylate proline residues on the oxygen-dependent degradation (ODD) domain of HIFα, generating docking sites for pVHL—part of an E3 ubiquitin ligase complex that targets HIFα for degradation (21, 22). To catalyze proline hydroxylation, PHDs convert molecular oxygen and α-ketoglutarate to carbon dioxide and succinate (22). Although PHDs depend on oxygen for activity, their affinity for oxygen is low (Km = 230 to 250 μM), making them good oxygen sensors (11). Under hypoxia, prolyl hydroxylation of HIFα and its consequent interaction with pVHL are prevented, and HIFα proteins are stabilized (21). Stabilized HIFα units bind to the HIFβ unit, setting off HIF transcription activity. Thus, HIF is physiologically activated by hypoxia, and its downstream targets respond accordingly by increasing angiogenesis and glycolysis.
The aberrant stabilization of HIFα under normoxic conditions is termed pseudohypoxia. Pseudohypoxia is probably a major cause of tumors associated with VHL mutations (15). Pseudohypoxia was also shown recently in tumor cells with mutations in fumarate hydratase (FH) or in any of three of the four subunits of succinate dehydrogenase (SDH), SDHB, SDHC, or SDHD (5, 8, 9, 19, 20, 28). Both FH and SDH are mitochondrial proteins; SDH forms complex II of the electron transport chain, and both SDH and FH are enzymes of the tricarboxylic acid (TCA) cycle. In addition, SDH and FH are also bona fide tumor suppressors. Dysfunction of either of these TCA cycle enzymes causes pseudohypoxia, leading to the enhanced neovascularization and glycolysis that support cancer formation (10). Identifying ways to prevent HIFα stabilization under pseudohypoxia could lead to treatments for tumors with SDH or FH dysfunction.
Two models explain HIF1α stabilization due to SDH mutations. The first suggested that reactive oxygen species (ROS) from an impaired complex II of the electron transport chain (18, 30) potentially inhibit PHDs (7). However, although ROS can arise in some SDH-mutated mitochondria (1, 14, 26), there is no evidence that ROS mediate HIF1α stabilization in SDH-deficient cells (24). The second model, established over the last 2 years, showed that succinate and fumarate (substrates of SDH and FH, respectively), which build up from the impaired TCA cycles of SDH- or FH-deficient mitochondria, are intracellular messengers that inhibit PHD activity and so mediate HIFα stabilization (23).
MATERIALS AND METHODS
Plasmids.
The scrambled and the _SDHD_-targeting small interfering RNA short hairpins, Sc and Di3, which were described previously (23), were cloned into pSUPER-GFP-neo.
pRK5/HA-ODD and pEGFP/ODD, used to translate hemagglutinin (HA)-ODD in vitro or to express the green fluorescent protein (GFP)-ODD fusion protein, respectively, were described previously (23). pRC-CMV/HA-pVHL was used to coexpress HA-pVHL in the GFP-ODD-expressing clones and was a gift from W. G. Kaelin, Jr.
Cell culture.
HEK293 human embryonic kidney cells, RCC4 human renal cell carcinoma cells, and ARPE human retinal pigment epithelial cells were grown in Dulbecco's modified Eagle's medium. HCT116 human colon carcinoma cells were grown in McCoy's medium supplemented with 10% fetal bovine serum. GFP-ODD-expressing clones were generated by cotransfecting pEGFP/ODD, pRC-CMV/HA-pVHL, and pBabe-Puro into HEK293 cells. Following selection in puromycin, 96 clones were randomly picked and duplicated in 96-well plates, each incubated with or without CoCl2. Clones that showed a low basal level of GFP fluorescence with a significant induction of fluorescence when treated with CoCl2 were studied further.
Synthesis of α-ketoglutarate derivatives.
The methods for synthesis of α-ketoglutarate esters were adopted from previous work (6, 27). The octyl-α-ketoglutarate ester was prepared using the following method: octyl-chloroformate was added drop by drop to a solution of α-ketoglutaric acid (10 mmol) and triethylamine (1.0 eq) in dichloromethane (50 ml) at ambient temperature. The resulting mixture was stirred at ambient temperature for 16 h and then diluted with dichloromethane (50 ml), washed with 0.5 N aqueous hydrochloric acid (50 ml), and dried (magnesium sulfate), and the solvent was removed under reduced pressure.
For benzyl- or 3-trifluoromethylbenzyl-α-ketoglutarate ester analogues, the corresponding benzyl bromide was added (1.1 eq) to a solution of α-ketoglutaric acid (10 mmol) and dicyclohexylamine (1.2 eq) in dimethyl formamide (50 ml), and the solution was heated at 50°C for 16 h. The mixture was concentrated under reduced pressure, and the residue was resuspended in dichloromethane (100 ml) and washed with 0.5 N aqueous hydrochloric acid (50 ml). The organic layer was dried (magnesium sulfate), and the solvent was removed under reduced pressure. The crude products were purified using normal-phase flash column chromatography (hexane/ethyl acetate).
In vitro PHD activity.
The reaction was carried out as described previously (23) using in vitro-translated HA-ODD as a substrate and HeLa cell extracts as an enzymatic source of PHD (20 mM Tris [pH 7.4], 5 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol supplemented with “complete” protease inhibitor cocktail [Roche], and 100 μM _N_-acetyl-Leu-Leu-Norleu-CHO). The reaction was carried out for 15 min at 37°C in the presence of 5 mM ascorbate, 100 μM FeCl2, and the indicated amounts of α-ketoglutarate and succinate. Reactions were terminated by adding Laemmli sample buffer and immediately boiling them. Following sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), samples were analyzed by Western blotting using an anti-HA antibody.
Protein analyses.
Cells were extracted in Laemmli sample buffer. Following SDS-PAGE, proteins were blotted onto nitrocellulose and analyzed with the following antibodies: anti-HIF1α (BD Biosciences), anti-HIF2α (Novus Biologicals), anti-HA (Roche), anti-GFP (BD Biosciences), and antiactin (Sigma).
Measurement of α-ketoglutarate.
Cells were grown as described above. All of the following operations were performed at 4°C. Cell monolayers were washed with phosphate-buffered saline and lysed with RIPA buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS supplemented with “complete” protease inhibitor cocktail [Roche]). Cell lysates were collected from the plate, vortexed vigorously, and centrifuged for 5 min at 15,000 × g at 4°C. Aliquots of the extracts were analyzed immediately for α-ketoglutarate. The assay solution consisted of 100 mM KH2PO4 (pH 7.2), 10 mM NH4Cl, 5 mM MgCl2, and 0.15 mM NADH. Following equilibration at 37°C with extract, the reaction was started by the addition of 5 units of glutamate dehydrogenase. The absorbance decrease was monitored at 340 nm. The intracellular level of α-ketoglutarate was determined from the absorbance decrease in NADH (extinction coefficient [ɛ] = 6.22 mM−1 cm−1).
Measurement of glycolysis.
ARPE cells were either left untreated or treated with monoethyl-fumarate and/or trifluoromethyl benzyl (TFMB)-α-ketoglutarate. Due to a toxic effect of long-term incubation with monoethyl-fumarate, cells were incubated for 12 hours only, and the medium was replaced at 6 and 9 hours to refurbish α-ketoglutarate levels. The glycolytic rate of the cells was calculated from the rate for converting [5-3H]glucose to 3H2O, as previously described (3), without a glucose starvation step.
RESULTS
α-Ketoglutarate overcomes succinate-mediated inhibition of PHD in vitro.
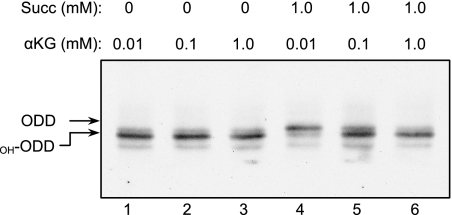
Succinate, the substrate of SDH, is also a product of the α-ketoglutarate-dependent hydroxylases and, when present at sufficiently high levels in SDH-deficient cells, can inhibit PHD activity (23). Therefore, succinate may be a competitive inhibitor of PHD, competing with its substrate, α-ketoglutarate; hence, elevating α-ketoglutarate levels may overcome this inhibition. To test this hypothesis, the effect of α-ketoglutarate on PHD activity was examined by employing an in vitro hydroxylation assay (12): hydroxylated and nonhydroxylated species were resolved using a gel shift migration assay. PHD activity was determined using in vitro-translated HA-tagged ODD as a substrate and HeLa cell extracts as a source of PHD. Incubation of HA-ODD with cell extracts in the presence of Fe2+ and ascorbate and in the absence of succinate, showed that even low concentrations of α-ketoglutarate led to near-maximal hydroxylation of HA-ODD, which was evident from its faster migration on SDS-PAGE (Fig. 1, lanes 1 to 3). The presence of succinate at a constant concentration of 1 mM caused inhibition of PHD activity, as seen by the appearance of the nonhydroxylated, slower-migrating form of HA-ODD (Fig. 1, lane 4). Addition of increasing amounts of α-ketoglutarate, ranging from 0.1 to 1 mM, resulted in concentration-dependent stimulation of PHD activity and a progressive increase in the production of the faster-migrating, hydroxylated form of HA-ODD (Fig. 1, lanes 4 to 6). These results demonstrate that succinate inhibits PHD and that α-ketoglutarate can reverse this PHD inhibition in vitro.
FIG. 1.
Succinate-mediated inhibition of PHD can be overcome by increasing α-ketoglutarate levels in vitro. A hydroxylation reaction of the ODD domain was carried out in vitro with the indicated amounts of succinate and α-ketoglutarate (αKG). Hydroxylation of ODD (OH-ODD) resulted in a faster-migrating band on SDS-PAGE.
Cell-permeating α-ketoglutarate derivatives increase intracellular levels of free α-ketoglutaric acid.
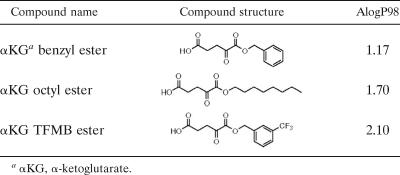
α-Ketoglutarate is hydrophilic and cannot efficiently cross the plasma membrane to achieve sufficiently high intracellular levels (see below). We therefore designed and synthesized three membrane-permeating monoester derivatives of α-ketoglutarate with different hydrophobic indices (Table 1). Once these derivatives enter the cells, the ester is hydrolyzed by cytosolic esterases, increasing the concentration of α-ketoglutarate in the cytosol. With complete plasma membrane permeability and equilibration, [α-ketoglutarate ester]in should equal [α-ketoglutarate ester]out. The conversion of α-ketoglutarate ester to α-ketoglutaric acid by cytosolic esterases both traps α-ketoglutarate in the cell and creates a concentration gradient to drive more α-ketoglutarate ester from the medium into the cytosol. The α-ketoglutarate thus formed and metabolized in the cells would be rapidly replaced by exogenous α-ketoglutarate ester and, in principle, intracellular α-ketoglutarate could exceed the concentration of extracellular α-ketoglutarate ester.
TABLE 1.
Molecular structure and relative hydrophobicity (in terms of AlogP98) of the different ester derivatives of α-ketoglutarate synthesized
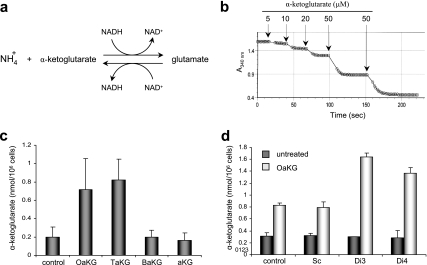
To examine the ability of α-ketoglutarate esters to increase cellular levels of α-ketoglutarate, HEK293 cells were incubated with 1 mM of either underivatized α-ketoglutarate, benzyl-α-ketoglutarate, octyl-α-ketoglutarate, or TFMB-α-ketoglutarate. Cell extracts were prepared and immediately analyzed for free intracellular α-ketoglutarate levels using a spectrophotometric assay that employs glutamate dehydrogenase (GDH) (29) (Fig. 2a). High levels of NH4+ and NADH were used to shift the GDH reaction toward glutamate formation (reductive amination), in which the concentration of α-ketoglutarate is stoichiometric to the amount of NADH oxidized (seen as a decrease in light absorbance at 340 nm) (Fig. 2b). Addition of known amounts of α-ketoglutarate to extracts of untreated cells resulted in recovery of nearly 95% of the exogenously added α-ketoglutarate (data not shown), indicating that α-ketoglutarate is chemically and metabolically stable in the extracts. Importantly, neither of the α-ketoglutarate derivatives (prodrugs) caused a decrease in 340-nm absorbance when added directly to the GDH assay, demonstrating that only free α-ketoglutarate can participate in the GDH reaction, so that residual nonhydrolyzed α-ketoglutarate esters present in cells at the time of extraction do not contribute to the measurement of the free acid (data not shown).
FIG. 2.
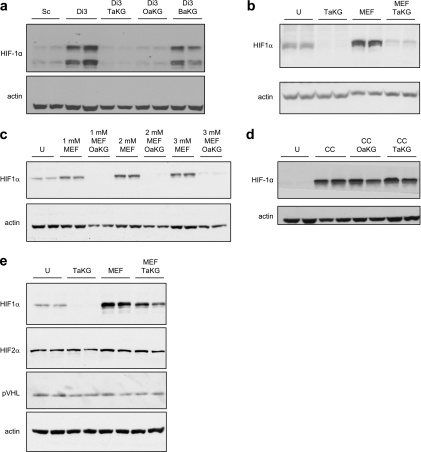
The intracellular α-ketoglutarate level is elevated in cells treated with the α-ketoglutarate ester derivatives. (a) Schematic illustration of the GDH reaction. (b) The decrease in NADH (as measured by light absorbance at 340 nm) is stoichiometric to α-ketoglutarate added to the GDH reaction. (c) HEK293 cells were either left untreated or treated with 1 mM of the indicated α-ketoglutarate derivative or with underivatized α-ketoglutarate. The α-ketoglutarate level in cell extracts was analyzed using the GDH reaction as in panel b. (d) HEK293 cells were transfected with either the control Sc or shRNAs targeting SDHD (Di3 or Di4) and 48 h later cells were either left untreated or treated with 1 mM octyl-α-ketoglutarate. Intracellular levels of α-ketoglutarate were analyzed as in panels b and c. aKG, underivatized α-ketoglutarate; OaKG, TaKG, and BaKG, octyl-, TFMB-, and benzyl-α-ketoglutarate esters, respectively. The error bars indicate standard deviations.
When cells were treated with octyl-α-ketoglutarate or TFMB-α-ketoglutarate for 2 h prior to extraction, intracellular α-ketoglutarate levels rose by approximately fourfold (Fig. 2c). In contrast, cells treated with either underivatized or benzyl-α-ketoglutarate (the least hydrophobic derivative) (Table 1) showed no increase in the level of intracellular α-ketoglutarate above basal levels (Fig. 2c). These results indicate that underivatized α-ketoglutarate does not efficiently enter cells, and this emphasizes that derivatizing the acid, and introducing sufficient hydrophobicity, is crucial for effectiveness. In subsequent experiments, octyl- and TFMB-α-ketoglutarate esters were used to elevate intracellular α-ketoglutarate, while underivatized and benzyl-α-ketoglutarate were used as negative controls.
In the above-mentioned experiments, α-ketoglutarate derivatives were used on cells with a normal TCA cycle. We hypothesized that exogenous α-ketoglutarate would accumulate preferentially in cells where the metabolism of TCA cycle intermediates is impaired. To test this hypothesis, cells were transiently transfected with the Di3 or Di4 short-hairpin RNA (shRNA) targeting the SDHD subunit and known to efficiently suppress SDH activity in cells (23). Scrambled shRNA (Sc) was used as a negative control. Forty-eight hours after transfection, the cells were treated with 1 mM octyl-α-ketoglutarate for 2 h, after which free intracellular α-ketoglutarate levels were analyzed as described above. None of the shRNA constructs had an effect on the level of basal α-ketoglutarate of untreated cells. However, SDH-deficient cells that were treated with the octyl-α-ketoglutarate derivative showed a twofold rise in α-ketoglutarate levels above the rise observed in the scrambled control-transfected cells (Fig. 2d). Interestingly, an increase in α-ketoglutarate levels was also observed in cells in which the TCA cycle was inhibited by α-keto-β-methyl-_n_-valeric acid, an inhibitor of α-ketoglutarate dehydrogenase (data not shown). This indicates that conditions that slow down the TCA cycle prevent α-ketoglutarate oxidation in the mitochondria and thus accelerate the buildup of cytosolic α-ketoglutarate.
Elevated levels of α-ketoglutarate in cells reactivate succinate- or fumarate-inhibited PHD.
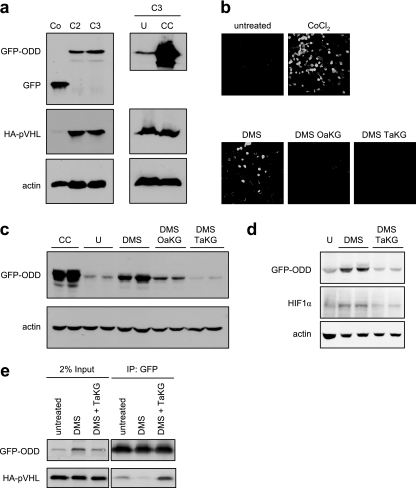
To analyze the effects of α-ketoglutarate derivatives on PHD activity in cells, we generated two HEK293-derived cell lines expressing both a GFP-ODD fusion protein and HA-tagged pVHL (Fig. 3a, left). In these cells, GFP levels are tightly regulated by PHD activity, which constitutively targets the GFP-ODD fusion protein for pVHL-mediated proteasomal degradation. Cells treated with CoCl2, a hypoxia-mimetic compound that inhibits PHD activity, showed an increase in GFP-ODD levels seen both in Western blot (Fig. 3a, right) and confocal microscopy (Fig. 3b, top) analyses, confirming that GFP fluorescence levels in these cells reflect the activity of PHD. To determine whether elevating intracellular α-ketoglutarate overcomes succinate-mediated PHD inhibition, cells were first treated with dimethyl-succinate, and these cells showed an increase in GFP fluorescence 48 hours after treatment (Fig. 3b). Octyl-α-ketoglutarate or TFMB-α-ketoglutarate reduced GFP fluorescence within 12 h of their addition to dimethyl-succinate-treated cells (Fig. 3b), indicating a stimulation of PHD activity. Corresponding Western blots showed that the levels of GFP-ODD, as well as of the endogenous HIF1α, rose in cells treated with dimethyl-succinate and fell back to the basal level after α-ketoglutarate derivatives were added (Fig. 3c and d).
FIG. 3.
The inhibition of PHD activity by succinate in cells is alleviated by the increase in the intracellular α-ketoglutarate level. (a, left) Clones (C2 and C3) coexpressing the GFP-ODD fusion protein and HA-tagged pVHL were analyzed by Western blotting. Cells transiently transfected with a plasmid encoding GFP alone were used as a reference for GFP molecular weight (Co). Actin was used as a loading control. (Right) Clone 3 (C3) cells were either left untreated (U) or treated with the hypoxia-mimetic compound CoCl2 (CC), and GFP-ODD and HA-pVHL protein levels were analyzed by Western blotting. (b) Clone 3 cells were either left untreated or treated with CoCl2 or 25 mM dimethyl-succinate (DMS) for 48 h. Where indicated, 1 mM of the α-ketoglutarate derivatives was added for the final 12 h of the incubation (with dimethyl-succinate) and GFP-ODD levels were visualized microscopically. (c) Clone 3 cells were treated as in panel b, and GFP-ODD levels were detected by Western blotting. CoCl2-treated cells (CC) were used as a positive control for PHD inhibition, and the actin level was used as a loading control. (d) Clone 3 cells were either left untreated (U) or treated with dimethyl-succinate (DMS) with or without TFMB-α-ketoglutarate. GFP-ODD and HIF1α levels were detected by Western blotting. Actin was used as a loading control. (e) Clone 3 cells were treated as in panel d, and protein lysate was immunoprecipitated using anti-GFP (polyclonal) antibody. The lysate before (2% input) or after immunoprecipitation (IP) was analyzed by Western blotting using anti-GFP (monoclonal) antibody and anti-HA antibody. OaKG and TaKG, octyl- and TFMB-α-ketoglutarate esters, respectively.
Since pVHL binding to ODD is dramatically enhanced by ODD prolyl hydroxylation, the degree to which GFP-ODD is associated with HA-VHL is a good indication of the hydroxylation levels of the ODD. Therefore, to determine whether GFP-ODD levels declined in α-ketoglutarate-treated cells due to ODD hydroxylation, the interaction between GFP-ODD and HA-pVHL was analyzed by a coimmunoprecipitation assay. As expected, cells treated with dimethyl-succinate yielded less GFP-ODD-associated HA-pVHL, and the addition of α-ketoglutarate derivatives restored this hydroxylation-dependent protein-protein interaction (Fig. 3e). Moreover, the total levels of HA-pVHL were unaltered by either dimethyl-succinate or α-ketoglutarate (Fig. 3e, left), indicating that the levels of GFP-ODD-associated HA-pVHL are regulated posttranslationally.
In these experiments, to achieve maximal induction of GFP-ODD with dimethyl-succinate (indicative of maximal inhibition of PHD), cells were maintained under conditions of 10% oxygen that did not affect the basal level of GFP-ODD (Fig. 3b, untreated). It is reasonable to suggest that succinate causes maximum PHD inhibition under suboptimal oxygen conditions that would still suffice for PHD activity in its absence. This may help explain the incomplete penetrance and milder phenotype of SDHD mutations in families living at low, compared to high, altitudes (2). However, HEK293 cells transiently transfected with the Di3 shRNA, targeting the SDHD subunit, showed increased HIF1α levels under normoxic conditions (Fig. 4a). This HIF1α induction was reversed by treatment with either 1 mM octyl-α-ketoglutarate or 1 mM TFMB-α-ketoglutarate derivatives (Fig. 4a). This demonstrates that PHD inhibition, when caused by SDH dysfunction and the consequent rise in succinate, can be overcome by excess α-ketoglutarate. Underivatized α-ketoglutarate (not shown) and the least hydrophobic benzyl α-ketoglutarate derivative had no effect on HIF1α levels (Fig. 4a).
FIG. 4.
α-Ketoglutarate esters overcome HIF1α stabilization mediated by succinate or fumarate. (a) HEK293 cells were transfected with either scrambled control Sc or shRNA targeting SDHD (Di3). Where indicated, the α-ketoglutarate derivatives were added 48 h after transfection, followed by a Western blot analysis for HIF1α. The actin level was used as a loading control. (b) HCT116 cells were treated with or without 2 mM monoethyl-fumarate for 24 h and/or 2 mM TFMB-α-ketoglutarate ester for the last 2 h prior to extraction. Western blot analysis was performed as in panel a. (c) ARPE cells were either left untreated or treated with the indicated amounts of monoethyl-fumarate for 3 h, with or without 2 mM octyl-α-ketoglutarate ester. Western blot analysis was performed as in panel a. (d) HEK293 cells were left untreated or treated with 0.5 mM CoCl2 for 2 h with or without 2 mM of the indicated α-ketoglutarate derivatives. Western blot analysis was performed as in panel a. (e) ARPE cells were either untreated or treated with 2 mM monoethyl-fumarate and/or 2 mM TFMB-α-ketoglutarate ester. The endogenous protein levels of HIF1α, HIF2α, pVHL, and actin were detected by Western blotting. U, untreated; CC, CoCl2; MEF, monoethyl-fumarate; OaKG, octyl-α-ketoglutarate; TaKG, TFMB-α-ketoglutarate; BaKG, benzyl-α-ketoglutarate.
Unlike patients with SDH mutations, different carriers of mutated FH do not have different susceptibilities to tumor formation depending on their altitude (oxygen levels). Moreover, it was suggested that, in vitro, fumarate is a better inhibitor of PHD than succinate (13). Therefore, the effects of monoethyl-fumarate on HIF1α protein levels under normoxic conditions were tested in different cell lines. Following treatment with 1 to 3 mM monoethyl-fumarate, an elevated HIF1α protein level was observed in kidney (HEK293) (not shown), colon (HCT116) (Fig. 4b), and retina epithelial (ARPE) (Fig. 4c) cells. In all cases, adding an effective α-ketoglutarate derivative completely blocked this induction (Fig. 4b and c). Importantly, while it reversed fumarate- or succinate-mediated HIF1α induction of monoethyl-fumarate-treated or SDH-knocked down cells, α-ketoglutarate had no effect on CoCl2-induced HIF1α levels, consistent with the fact that CoCl2 inhibits PHD by an independent mechanism that does not involve succinate or α-ketoglutarate (Fig. 4d).
We have shown previously that, in HEK293 cells, changes in HIF2α levels are less sensitive to increased succinate (23). In order to analyze the effects of fumarate and α-ketoglutarate on HIF2α, ARPE cells were treated with monoethyl-fumarate with or without TFMB-α-ketoglutarate. In contrast to HIF1α, HIF2α levels responded less dramatically to both compounds (Fig. 4e). Taken together, these and our previous results indicate that HIF1α and HIF2α may be regulated by different PHDs with different sensitivities to succinate and fumarate. Importantly, no changes in the endogenous levels of pVHL were observed under these conditions (Fig. 4e), further supporting the notion that α-ketoglutarate destabilizes HIF1α without changing pVHL levels.
α-Ketoglutarate targets HIF1α for ubiquitylation and proteasomally mediated degradation.
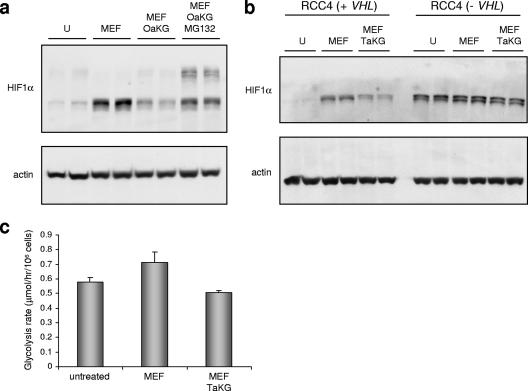
In order to verify that exogenously supplied α-ketoglutarate leads to retargeting of HIFα for proteasomally mediated degradation, the proteasome inhibitor MG132 was added to ARPE cells treated with monoethyl-fumarate, together with the α-ketoglutarate derivatives. MG132 blocked the effect of α-ketoglutarate on HIF1α levels and induced the appearance of higher-molecular-weight forms of HIF1α, which are likely to be polyubiquitylated HIF1α proteins (Fig. 5a). This indicates that the α-ketoglutarate derivatives restored hydroxylation and, potentially, ubiquitylation of HIF1α. In order to confirm that the retargeting of HIF1α for degradation by α-ketoglutarate is mediated by pVHL, the _VHL_-negative RCC4 renal cell carcinoma cells were used. Parental (_VHL_−) or _VHL_-reconstituted cells were treated with monoethyl-fumarate in the presence or absence of TFMB-α-ketoglutarate. As expected, the VHL-expressing RCC4 cells responded to monoethyl-fumarate and TFMB-α-ketoglutarate in a manner comparable to those of HEK293, HCT116, and ARPE cells. HIF1α levels were augmented by monoethyl-fumarate, and this increase was reversed by TFMB-α-ketoglutarate (Fig. 5b). The parental RCC4 cells, which lack pVHL, express higher basal HIF1α levels (Fig. 5b). However, HIF1α levels were not further increased by monoethyl-fumarate and could not be overcome with TFMB-α-ketoglutarate treatment (Fig. 5b). These data show that the targeting of HIF1α for proteasomally mediated degradation by α-ketoglutarate derivatives is mediated by pVHL and therefore most likely involves stimulation of PHD activity.
FIG. 5.
α-Ketoglutarate retargets HIF1α for ubiquitylation and proteasomally mediated degradation. (a) ARPE cells were left untreated or treated with 2 mM monoethyl-fumarate for 24 h with or without 2 mM of octyl-α-ketoglutarate ester added 30 min before cell lysis. Where indicated, MG132, a proteasomal inhibitor, was added to cells 30 min prior to the α-ketoglutarate derivative. HIF1α and actin as loading control were analyzed by Western blotting. (b) Parental RCC4 cells (VHL negative) of _VHL_-transfected RCC4 cells were either left untreated or treated with 2 mM monoethyl-fumarate with or without 2 mM TFMB-α-ketoglutarate as indicated. HIF1α and actin as loading controls were analyzed by Western blotting. (c) ARPE cells were either untreated or treated with 2 mM monoethyl fumarate with or without TFMB-α-ketoglutarate ester for 12 h. The glycolytic rate was analyzed as described in Materials and Methods. U, untreated; MEF, monoethyl-fumarate; OaKG, octyl-α-ketoglutarate; TaKG, TFMB-α-ketoglutarate.
The identification of cancer syndromes in which the primary cause is abrogation of oxidative phosphorylation was the first genetic example of the infamous “Warburg effect” (16). The subsequent observations that elevated HIF activity is a major contributor to the tumorigenic outcome of TCA cycle deficiency further supports a role for accelerated glycolysis in the pathologies of these tumors. To test whether reversing HIF1α levels by reactivating PHDs has an effect on glycolysis, ARPE cells were either left untreated or treated with monoethyl-fumarate with or without TFMB-α-ketoglutarate, and the rate of conversion of [5-3H]glucose to 3H2O was analyzed. This process requires most of the glycolytic enzymes, from hexokinase (step 1) to enolase (step 8). A small but significant increase in the glycolytic rate was observed in cells treated with monoethyl-fumarate. This increase was completely abolished in cells cotreated with monoethyl-fumarate and TFMB-α-ketoglutarate (Fig. 5c), indicating that the alleviation of pseudohypoxia by PHD reactivation prevented the HIF-mediated “Warburg effect”.
DISCUSSION
The present study suggests that in cells in which TCA cycle impairment supports tumorigenesis, tumor promotion can be curbed by exogenous supplementation with cell-permeating derivatives of α-ketoglutarate. We previously showed that SDH dysfunction results in an accumulation of succinate that inhibits hydroxylation of HIF1α. This is achieved by a product inhibition mechanism, given that PHD hydroxylates its targets while utilizing α-ketoglutarate as a substrate and producing succinate (23). The studies described here show that an excess of α-ketoglutarate, provided in a form that allows uptake by cells, overcomes PHD inactivation by succinate. Succinate inhibition of PHD is competitive in nature, and therefore, the ratio, rather than the absolute concentrations, of α-ketoglutarate to succinate in cells should critically affect PHD activity and HIF1α stability. Native α-ketoglutarate does not readily enter cells, so to test whether α-ketoglutarate can indeed counter the PHD-inhibiting effect of succinate, we designed cell-permeating α-ketoglutarate derivatives that are hydrolyzed in the cytosol. These derivatives support PHD activity, thereby lowering HIF1α levels in SDH-deficient cells. We have shown that α-ketoglutarate preferentially accumulates in SDH-deficient cells, probably because TCA cycle metabolism is impaired. This indicates that upon treatment with readily permeating drugs, the concentration of α-ketoglutarate in target cells will rise to a level fully capable of countering the effect of succinate. Our study suggests that well-designed α-ketoglutarate derivatives may have therapeutic potential in the treatment of tumors with functional down-regulation or mutations of SDH, where it could restore normal low levels of HIF1α.
Interestingly, increasing the levels of intracellular α-ketoglutarate had a marked effect on the basal levels of HIF1α protein (Fig. 4b and e). This indicates that α-ketoglutarate may be a limiting factor for PHD activity under normoxia. This possibility has far-reaching consequences, since pseudohypoxia due to mTor-mediated increased translation of HIF1α has been associated with several other tumors (16). It is therefore possible that the increased rate of HIF1α translation in these tumors can be overcome if HIF1α hydroxylation, and therefore degradation, can be accelerated by α-ketoglutarate.
Aberrant HIF activity is a major contributor to tumorigenesis, and it is plausible that restoring normal HIF levels in TCA cycle-impaired tumors will be sufficient for tumor control. However, it is also likely that, in addition to HIFα, other substrates for PHD exist that may contribute to the tumorigenic effect of succinate, for example, a substrate that may activate apoptosis once hydroxylated (17). Moreover, PHDs are not the only α-ketoglutarate-dependent hydroxylases in cells. Therefore, treatment of TCA cycle-impaired tumors with α-ketoglutarate derivatives or analogues may have a broader effect on tumor development.
Acknowledgments
This work was supported by the Association for International Cancer Research and Cancer Research UK.
We thank Ayala King for excellent editorial work.
Footnotes
▿
Published ahead of print on 26 February 2007.
REFERENCES
- 1.Albayrak, T., V. Scherhammer, N. Schoenfeld, E. Braziulis, T. Mund, M. K. Bauer, I. E. Scheffler, and S. Grimm. 2003. The tumor suppressor cybL, a component of the respiratory chain, mediates apoptosis induction. Mol. Biol. Cell. 14**:**3082-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astrom, K., J. E. Cohen, J. E. Willett-Brozick, C. E. Aston, and B. E. Baysal. 2003. Altitude is a phenotypic modifier in hereditary paraganglioma type 1: evidence for an oxygen-sensing defect. Hum. Genet. 113**:**228-237. [DOI] [PubMed] [Google Scholar]
- 3.Bensaad, K., A. Tsuruta, M. A. Selak, M. N. Vidal, K. Nakano, R. Bartrons, E. Gottlieb, and K. H. Vousden. 2006. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126**:**107-120. [DOI] [PubMed] [Google Scholar]
- 4.Covello, K. L., and M. C. Simon. 2004. HIFs, hypoxia, and vascular development. Curr. Top. Dev. Biol. 62**:**37-54. [DOI] [PubMed] [Google Scholar]
- 5.Dahia, P. L. M., K. N. Ross, M. E. Wright, C. Y. Hayashida, S. Santagata, M. Barontini, A. L. Kung, G. Sanso, J. F. Powers, A. S. Tischler, R. Hodin, S. Heitritter, F. J. Moore, R. Dluhy, J. A. Sosa, I. T. Ocal, D. E. Benn, D. J. Marsh, B. G. Robinson, K. Schneider, J. Garber, S. M. Arum, M. Korbonits, A. Grossman, P. Pigny, S. P. A. Toledo, V. Nose, C. Li, and C. D. Stiles. 2005. A HIF1α regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 1**:**e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domagala, J. M. 1980. A mild, rapid and convenient esterification of -keto acids. Tetrahedron Lett. 21**:**4997. [Google Scholar]
- 7.Gerald, D., E. Berra, Y. M. Frapart, D. A. Chan, A. J. Giaccia, D. Mansuy, J. Pouyssegur, M. Yaniv, and F. Mechta-Grigoriou. 2004. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell 118**:**781-794. [DOI] [PubMed] [Google Scholar]
- 8.Gimenez-Roqueplo, A. P., J. Favier, P. Rustin, J. J. Mourad, P. F. Plouin, P. Corvol, A. Rotig, and X. Jeunemaitre. 2001. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am. J. Hum. Genet. 69**:**1186-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimenez-Roqueplo, A. P., J. Favier, P. Rustin, C. Rieubland, V. Kerlan, P. F. Plouin, A. Rotig, and X. Jeunemaitre. 2002. Functional consequences of a SDHB gene mutation in an apparently sporadic pheochromocytoma. J. Clin. Endocrinol. Metab. 87**:**4771-4774. [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb, E., and I. P. Tomlinson. 2005. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat. Rev. Cancer 5**:**857-866. [DOI] [PubMed] [Google Scholar]
- 11.Hirsila, M., P. Koivunen, V. Gunzler, K. I. Kivirikko, and J. Myllyharju. 2003. Characterization of the human prolyl 4-hydroxylases that modify the hypoxia-inducible factor. J. Biol. Chem. 278**:**30772-30780. [DOI] [PubMed] [Google Scholar]
- 12.Huang, J., Q. Zhao, S. M. Mooney, and F. S. Lee. 2002. Sequence determinants in hypoxia-inducible factor-1α for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J. Biol. Chem. 277**:**39792-39800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isaacs, J. S., Y. J. Jung, D. R. Mole, S. Lee, C. Torres-Cabala, Y. L. Chung, M. Merino, J. Trepel, B. Zbar, J. Toro, P. J. Ratcliffe, W. M. Linehan, and L. Neckers. 2005. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8**:**143-153. [DOI] [PubMed] [Google Scholar]
- 14.Ishii, T., K. Yasuda, A. Akatsuka, O. Hino, P. S. Hartman, and N. Ishii. 2005. A mutation in the SDHC gene of complex II increases oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Res 65**:**203-209. [PubMed] [Google Scholar]
- 15.Kim, W. Y., and W. G. Kaelin. 2004. Role of VHL gene mutation in human cancer. J. Clin. Oncol. 22**:**4991-5004. [DOI] [PubMed] [Google Scholar]
- 16.King, A., M. A. Selak, and E. Gottlieb. 2006. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene 25**:**4675-4682. [DOI] [PubMed] [Google Scholar]
- 17.Lee, S., E. Nakamura, H. Yang, W. Wei, M. S. Linggi, M. P. Sajan, R. V. Farese, R. S. Freeman, B. D. Carter, W. G. Kaelin, Jr., and S. Schlisio. 2005. Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell 8**:**155-167. [DOI] [PubMed] [Google Scholar]
- 18.Messner, K. R., and J. A. Imlay. 2002. Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidase. J. Biol. Chem. 277**:**42563-42571. [DOI] [PubMed] [Google Scholar]
- 19.Pollard, P., N. Wortham, E. Barclay, A. Alam, G. Elia, S. Manek, R. Poulsom, and I. Tomlinson. 2005. Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J. Pathol. 205**:**41-49. [DOI] [PubMed] [Google Scholar]
- 20.Pollard, P. J., J. J. Briere, N. A. Alam, J. Barwell, E. Barclay, N. C. Wortham, T. Hunt, M. Mitchell, S. Olpin, S. J. Moat, I. P. Hargreaves, S. J. Heales, Y. L. Chung, J. R. Griffiths, A. Dalgleish, J. A. McGrath, M. J. Gleeson, S. V. Hodgson, R. Poulsom, P. Rustin, and I. P. Tomlinson. 2005. Accumulation of Krebs cycle intermediates and over-expression of HIF1α in tumours which result from germline FH and SDH mutations. Hum. Mol. Genet. 14**:**2231-2239. [DOI] [PubMed] [Google Scholar]
- 21.Safran, M., and W. G. Kaelin, Jr. 2003. HIF hydroxylation and the mammalian oxygen-sensing pathway. J. Clin. Investig. 111**:**779-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schofield, C. J., and P. J. Ratcliffe. 2004. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell. Biol. 5**:**343-354. [DOI] [PubMed] [Google Scholar]
- 23.Selak, M. A., S. M. Armour, E. D. MacKenzie, H. Boulahbel, D. G. Watson, K. D. Mansfield, Y. Pan, M. C. Simon, C. B. Thompson, and E. Gottlieb. 2005. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 7**:**77-85. [DOI] [PubMed] [Google Scholar]
- 24.Selak, M. A., R. V. Duran, and E. Gottlieb. 2006. Redox stress is not essential for the pseudo-hypoxic phenotype of succinate dehydrogenase deficient cells. Biochim. Biophys. Acta 1757**:**567-572. [DOI] [PubMed] [Google Scholar]
- 25.Semenza, G. L. 2002. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol. Med. 8**:**S62-S67. [DOI] [PubMed] [Google Scholar]
- 26.Slane, B. G., N. Aykin-Burns, B. J. Smith, A. L. Kalen, P. C. Goswami, F. E. Domann, and D. R. Spitz. 2006. Mutation of succinate dehydrogenase subunit C results in increased O2−, oxidative stress, and genomic instability. Cancer Res. 66**:**7615-7620. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi, Y., Y. Nagao, K. Toma, Y. Yoshikawa, T. Akiyama, H. Nishioka, H. Abe, T. Harayama, and S. Yamamoto. 1999. Synthesis and siderophore activity of vibrioferrin and its diastereomeric isomer. Chem. Pharm. Bull. 47**:**1284-1287. [Google Scholar]
- 28.Vanharanta, S., P. J. Pollard, H. J. Lehtonen, P. Laiho, J. Sjoberg, A. Leminen, K. Aittomaki, J. Arola, M. Kruhoffer, T. F. Orntoft, I. P. Tomlinson, M. Kiuru, D. Arango, and L. A. Aaltonen. 2006. Distinct expression profile in fumarate-hydratase-deficient uterine fibroids. Hum Mol. Genet. 15**:**97-103. [DOI] [PubMed] [Google Scholar]
- 29.Williamson, J. R., and B. E. Corkey. 1979. Assay of citric acid cycle intermediates and related compounds—update with tissue metabolite levels and intracellular distribution. Methods Enzymol. 55**:**200-222. [DOI] [PubMed] [Google Scholar]
- 30.Yankovskaya, V., R. Horsefield, S. Tornroth, C. Luna-Chavez, H. Miyoshi, C. Leger, B. Byrne, G. Cecchini, and S. Iwata. 2003. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 299**:**700-704. [DOI] [PubMed] [Google Scholar]