Syk signaling is necessary for E-selectin-induced LFA-1-ICAM-1 association and rolling but not arrest (original) (raw)
. Author manuscript; available in PMC: 2008 Dec 11.
Abstract
Engagement of neutrophils by E-selectin results in integrin activation through unknown molecular mechanisms. Here, we investigate primary mouse neutrophils in their native whole blood using intravital microscopy and autoperfused flow chamber approaches. E-selectin-dependent slow rolling on immobilized E-selectin and ICAM-1 required P-selectin glycoprotein ligand (PSGL)-1. Slow rolling was dependent on LFA-1 and required continuous E-selectin engagement. Slow rolling was abolished by blocking spleen tyrosine kinases (Syk) using the inhibitor piceatannol and was absent in Syk-/- bone marrow chimeric mice. Treatment with tumor necrosis factor-α induced further reduction of rolling velocity and CXCL1/CXCR2-dependent leukocyte arrest on E-selectin/ICAM-1. This arrest was dependent on CXCR2 and Gαi and was blocked by an allosteric inhibitor of LFA-1 activation. The physiologic importance of the PSGL-1-Syk pathway is shown by near complete inhibition of neutrophil recruitment into the inflamed peritoneal cavity of PSGL-1-/- mice or Syk-/- bone marrow chimeras treated with pertussis toxin to block Gαi.
Keywords: neutrophil, E-selectin, G-protein coupled receptor, Syk, integrin
Introduction
E-selectin is expressed on inflamed endothelial cells and engages P-selectin Glycoprotein Ligand (PSGL)-1 (Xia et al., 2002), CD44 (Katayama et al., 2005), macrophage antigen (Mac)-1 (αMβ2) (Crutchfield et al., 2000) and other ligands. E-selectin is known to mediate slow leukocyte rolling in vivo (Kunkel and Ley, 1996). Slow rolling requires the presence and function of β2-integrins (Jung et al., 1998). Rolling of isolated human neutrophils on Eselectin induces p38 MAPK-dependent adhesion to inflamed endothelial cells or cells transfected with E-selectin and intracellular adhesion molecule (ICAM)-1 (Simon et al., 2000). However, when neutrophils are rolled over immobilized E-selectin and ICAM-1 in their physiological whole blood environment, rolling is greatly enhanced compared to immobilized E-selectin alone, but no firm adhesion is seen (Chesnutt et al., 2006).
Neutrophils can also activate their β2-integrins and arrest in response to immobilized chemokines (Rainger et al., 1997). The most relevant chemokine receptor responsible for arrest of mouse neutrophils under conditions of inflammation in vivo was recently identified as CXCR2 (Smith et al., 2004a). In reconstituted systems, immobilized chemokines are sufficient to trigger arrest of model leukocytes within less than one second (Campbell et al., 1998; Grabovsky et al., 2000).
During inflammation, endothelial cells express both CXCL1, an effective arrest chemokine that activates CXCR2 (Smith et al., 2005), and E-selectin. The E-selectin-dependent and chemokine-dependent recruitment pathways are partially redundant, because blocking either one alone blocks neutrophil recruitment to the inflamed peritoneal cavity by approximately 50%, but blocking both almost completely eliminates neutrophil recruitment (Smith et al., 2004a). In vivo, neutrophils integrate signals from chemokine and E-selectin engagement to eventually become adherent (Kunkel et al., 2000). The E-selectin ligand on neutrophils responsible for integrin activation and subsequent slow rolling is unknown.
PSGL-1 is a ligand for all selectins and binds L- and P-selectin through its NH2terminal sialyl Lewis x (sLex)-containing O-glycan and a nearby tyrosine sulfate residue (McEver, 2002; McEver and Cummings, 1997). E-selectin binding to PSGL-1 requires sialylated and fucosylated O-glycans but not tyrosine sulfatation (McEver and Cummings, 1997). PSGL-1 deficient mice show a pronounced impairment of P-selectin dependent neutrophil rolling, adhesion, and recruitment, whereas E-selectin dependent rolling and adhesion is only mildly impaired (Xia et al., 2002; Yang et al., 1999). Binding of P-selectin to PSGL-1 on leukocytes generates signals that are thought to be integrated with those from other activators (Zimmerman et al., 1996): The expression of transcription factors, chemokines and cytokines is enhanced when leukocytes receive simultaneous signals via PSGL-1 and G-protein coupled receptors (Galt et al., 2001; Lindemann et al., 2005; Weyrich et al., 1996; Weyrich et al., 1995). Furthermore, engagement of PSGL-1 induces tyrosine phosphorylation and activation of MAP kinases in human neutrophils, as well as activation of β2-integrin binding to ICAM-1 (Hidari et al., 1997; Simon et al., 2000).
Upon PSGL-1 engagement, the cytoplasmatic tail of PSGL-1 becomes associated with the spleen tyrosine kinase (Syk) (Urzainqui et al., 2002). Syk, a nonreceptor tyrosine kinase found in hematopoietic cells, has two SH2 domains, which are involved in the binding to the immunoreceptor tyrosine-based activation motifs (ITAM) of the actin-linking proteins moesin and ezrin in the PSGL-1 complex and other surface receptor complexes (Berton et al., 2005). This leads to recruitment and activation of Syk with subsequent phosphorylation of intracellular substrates. Downstream signals from PSGL-1 engagement are incompletely understood (Abbal et al., 2006). Syk is also involved in integrin outside-in signaling (Mocsai et al., 2002; Schymeinsky et al., 2006).
Neutrophils express lymphocyte function antigen (LFA)-1 (αLβ2), Mac-1, and small amounts of αxβ2 (gp 150,95) (Parkos, 1997). Both LFA-1 and Mac-1 (Dunne et al., 2002) have been found to contribute to neutrophil adhesion in inflamed microvessels in vivo. LFA-1 undergoes a series of conformational changes resulting in partial and full activation (Laudanna, 2005). Allosteric inhibitors of LFA-1 activation were recently shown to stabilize a partially activated form of LFA-1 (Salas et al., 2004; Shimaoka et al., 2003a) that supports rolling on ICAM-1 but not firm adhesion.
The first suggestion that E-selectin engagement may activate β2-integrins came from observations that neutrophils that adhered to TNF-α-stimulated HUVECs showed increased rosetting with C3bi-coated erythrocytes, reflecting activated Mac-1 (Lo et al., 1991). This Mac-1 activation was completely prevented when E-selectin was blocked (Lo et al., 1991). The relevance of E-selectin binding for integrin activation was confirmed in a flow chamber assay using Ficoll-isolated human blood neutrophils on L-cells transfected with E-selectin alone or with ICAM-1 (Simon et al., 2000). Ficoll-isolated neutrophils undergo a number of phenotypic changes during and after the isolation process (Forsyth and Levinsky, 1990; Glasser and Fiederlein, 1990; Kuijpers et al., 1991) and cannot maintain rolling at high wall shear stress levels similar to those in vivo.
We reasoned that it would be worthwhile to revisit E-selectin signaling using neutrophils in their native whole blood, whithout any isolation procedure that might alter their activation status and rolling ability. To this end, we employed a recently developed autoperfused flow chamber (Chesnutt et al., 2006; Smith et al., 2006; Smith et al., 2004b), which allows direct visualization of neutrophil interactions with a molecularly defined substrate. Since E-selectin is not as efficient as P-selectin at inducing leukocyte capture (Smith et al., 2004b), we increased the E-selectin concentration, which resulted in lower rolling velocities than typically seen in vivo, where both E- and P-selectin are expressed. To confirm the physiological relevance of the findings made in the autoperfused flow chamber, we studied neutrophil rolling and adhesion by intravital microscopy and neutrophil recruitment in a thioglycollate-induced model of peritonitis.
Results
PSGL- engagement triggers slow rolling
All experiments were conducted in whole blood in vivo or in an autoperfused flow chamber at 5.94 dyn/cm2 (Chesnutt et al., 2006; Smith et al., 2006). Some flow chambers were perfused with blood for 6 minutes, fixed, and stained for myeloperoxidase to identify neutrophils (data not shown). The rolling velocity of WT neutrophils was 2.2 μm/sec on Eselectin and decreased to 1.0 μm/sec on E-selectin and ICAM-1 (Figure 1A). To test the role of CD44 and PSGL-1 in E-selectin-dependent slow rolling, CD44-/- or PSGL-1-/- mice were cannulated and connected to autoperfused flow chambers. PSGL-1-/- neutrophils in whole blood showed a slightly higher rolling velocity on E-selectin compared to WT neutrophils and a drastically elevated rolling velocity on E-selectin/ICAM-1 that was identical to the rolling velocity on E-selectin (Figure 1B). By contrast, CD44-/- neutrophils rolled normally (Figure 1A). These results show that PSGL-1 on neutrophils is required for slowing down rolling neutrophils upon E-selectin engagement.
Figure 1. PSGL-1, but not CD44, is required for neutrophil slow rolling on ICAM-1 upon E-selectin engagement.
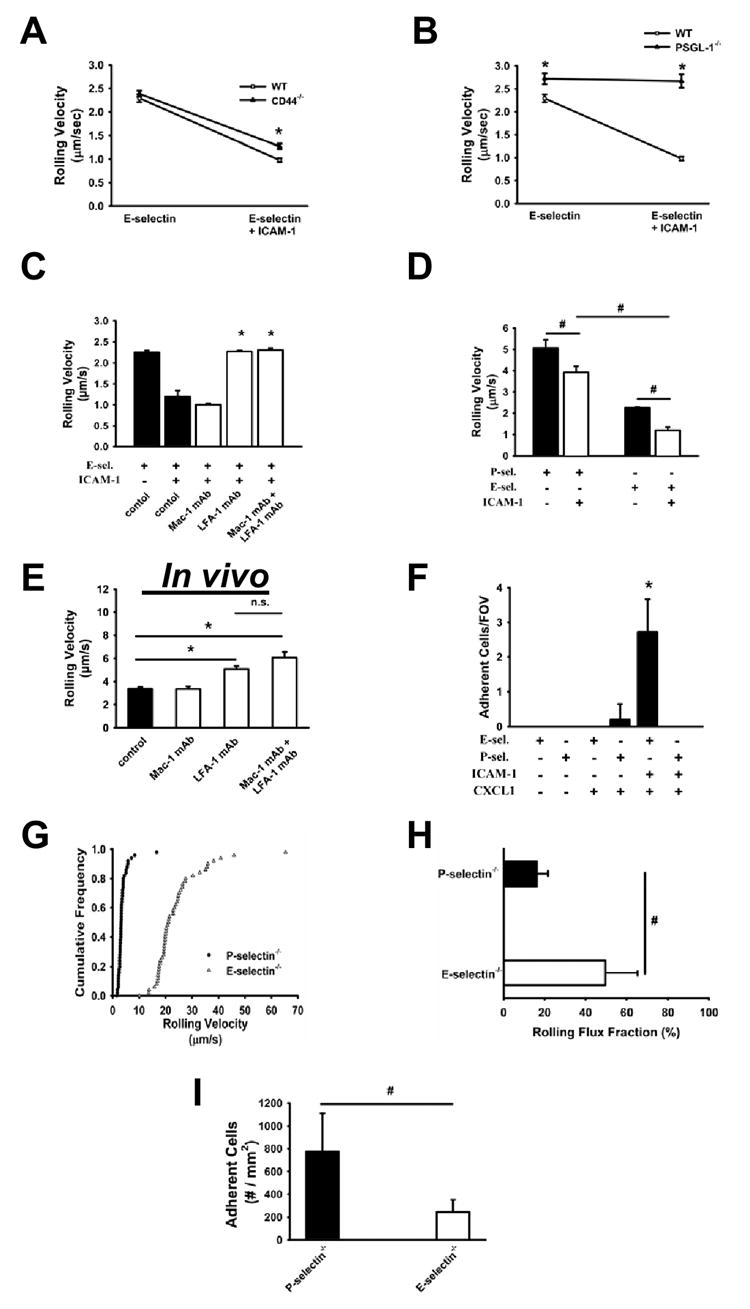
Carotid cannulas were placed in untreated WT and CD44-/- mice and connected to E-selectin and E-selectin+ICAM-1-coated autoperfused flow chambers. Rolling velocity of neutrophils presented as mean ± SEM (A). Rolling velocity of neutrophils from untreated WT and PSGL-1 deficient mice on E-selectin and E-selectin+ICAM presented as mean ± SEM (B). Rolling velocity of untreated neutrophils on E-selectin+ICAM-1 before and after blocking LFA-1 or Mac-1 or both (C). (D) Rolling velocity of neutrophils from untreated mice on P-selectin and P-selectin+ICAM-1. (E) Analysis of the rolling velocity of neutrophils in inflamed cremaster venules of P-selectin-/- mice 2h after TNF-α injection. (F) Neutrophil adhesion in the flow chamber after co-immobilization of CXCL1 (10 µg/ml) with P-selectin+ICAM-1 or E-selectin+ICAM-1. The wall shear stress in all flow chamber experiments was 5.94 dynes/cm2. At least three mice and four flow chambers per group. (G-I) Intravital microscopy of the cremaster muscle from P-selectin deficient mice and E-selectin deficient mice 2h after TNF-α application. Rolling velocity (G), rolling flux fraction (H), and number of adherent cells (I).At least four mice and 20 vessels per group. * P < 0.05.
To examine which β2-integrin is responsible for slow rolling on E-selectin/ICAM-1, we measured the rolling velocity of neutrophils of untreated mice before and after blocking of CD11a (LFA-1) and/or CD11b (Mac-1). In the autoperfused flow chamber, blocking CD11b had no effect on the rolling velocity compared to the control (Figure 1C). However, the inhibition of CD11a (LFA-1) led to an increase of rolling velocity to a level similar to that seen in the absence of ICAM-1, which was not further increased by blocking of both LFA-1 and Mac-1 (Figure 1C). Adding ICAM-1 to P-selectin-coated flow chambers also reduced rolling velocity by a similar amount as on E-selectin (Figure 1D), which is also largely LFA-1-dependent (data not shown).
To investigate which β2-integrin is responsible isolated E-selectin induced slow rolling, we used intravital microscopy of cremaster muscle venules in P-selectin deficient mice. Two hours after TNF-α injection, the hemodynamic parameters were similar in all groups (Table 1) and average rolling velocity was 3.3 ± 0.7 μm/sec (Figure 1E). Blocking of CD11b did not elevate the rolling velocity, whereas blocking of CD11a or both CD11a and CD11b led to a significant increase. These data demonstrate that E-selectin-induced slow rolling is LFA-1, and not Mac-1 dependent in vitro and in vivo.
Table 1.
Diameter, centerline velocity, and wall shear rate are presented as mean ± SEM of all investigated venules
| Antibody Treatment | Venules | Diameter (μm) | Centerline Velocity (mm/s) | Wall shear rate (1000 s-1) |
|---|---|---|---|---|
| Without Ab | 18 | 28 ± 2 | 3.2 ± 0.1 | 2.0 ± 0.2 |
| M1/70 | 15 | 27 ± 2 | 3.1 ± 0.1 | 2.0 ± 0.1 |
| TIB-217 | 14 | 26 ± 2 | 3.1 ± 0.1 | 2.0 ± 0.2 |
| M1/70 + TIB-217 | 16 | 29 ± 2 | 3.1 ± 0.1 | 1.9 ± 0.1 |
In order to investigate the physiological role of slow rolling on E-selectin/ICAM-1 compared to faster rolling on P-selectin/ICAM-1 (Figure 1D), we performed flow chamber experiments with co-immobilized CXCL1 and measured neutrophil adhesion. CXCL1 at 10 μg/ml induced neutrophil arrest on E-selectin/ICAM-1 but not on P-selectin/ICAM-1 in flow chambers perfused by untreated mice (Figure 1F). To confirm these findings in vivo, we conducted intravital microscopy of the cremaster muscle of P-selectin deficient mice and E-selectin deficient mice 2h after TNF-α application and measured rolling velocities (Figure 1G), rolling flux fraction (Figure 1H), and number of adherent cells (Figure 1I). E-selectin deficient mice showed a higher rolling velocity (Figure 1G), increased rolling flux fraction (Figure 1H), and reduced number of adherent cells (Figure 1I) compared to P-selectin deficient mice. These in vivo and in vitro data show that slower rolling velocities induced by E-selectin/ICAM-1 support adhesion.
Continous E-selectin engagement is required to sustain LFA-1-ICAM-1 interactions
We previously showed that neutrophils did not roll in autoperfused flow chambers coated with ICAM-1 alone (Chesnutt et al., 2006; Smith et al., 2006). To determine whether E-selectin engagement “switches on” β2-integrins activation or whether continous engagement is required to keep LFA-1 in an activated conformation, we coated one half of the chambers with E-selectin/ICAM-1 and the other half with ICAM-1. The cells rolled on E-selectin/ICAM-1 and immediately detached when they reached the ICAM-1 only zone (Figure 2A). Neutrophils rolling on E-selectin and ICAM-1 for 6 minutes immediately detached after injecting a monoclonal E-selectin antibody (9A9) (Figure 2B). These data show that continuous E-selectin-induced signaling is required to keep LFA-1 in an activated conformation; there is no apparent “memory” for prior engagement of E-selectin ligands.
Figure 2. Continuous E-selectin engagement is required to keep LFA-1 in an activated conformation.
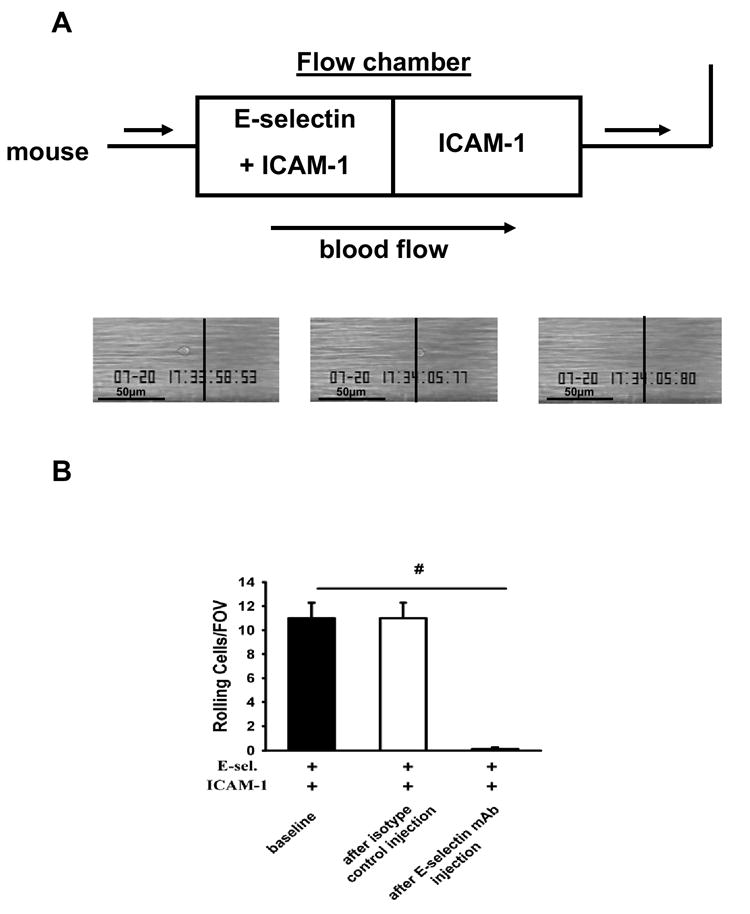
(A) The first half of autoperfused flow chambers was coated with E-selectin+ICAM-1 and the second half with ICAM-1 alone. Cells rolled on E-selectin+ICAM-1 but detached immediately upon reaching ICAM-1 alone (representative of four independent experiments). (B) Number of rolling neutrophils on E-selectin+ICAM-1 coated chambers before and after injection of a blocking E-selectin antibody (9A9). Data presented are the mean ± SEM from at least four mice and four flow chambers (n=4). # P < 0.05.
Syk is required for integrin activation after E-selectin and P-selectin binding to PSGL-1
The tyrosine kinase Syk is associated with the cytoplasmatic tail of PSGL-1 and is also involved in downstream signaling (Urzainqui et al., 2002). Pre-treatment of mice with piceatannol, an inhibitor of Syk, did not influence the rolling velocity of neutrophils on Eselectin or P-selectin, but the decrease of rolling velocity on E-selectin/ICAM-1 and P-selectin/ICAM-1 was almost completely abolished after Syk blockade (Figure 3A and data not shown). To confirm our findings, chimeric mice were used which lack Syk expression in hematopoietic cells. Syk-/- neutrophils showed a similar rolling velocity on E-selectin as WT neutrophils but failed to reduce their rolling velocity on E-selectin/ICAM-1 (Figure 3B). Similarly, blocking mitogen-activated protein (MAP)-kinase p38 did not influence the rolling velocity on E-selectin, but partially elevated the rolling velocity on E-selectin/ICAM-1 (Figure 3C).
Figure 3. Syk is involved in integrin activation after E-selectin or P-selectin binding to PSGL-1.
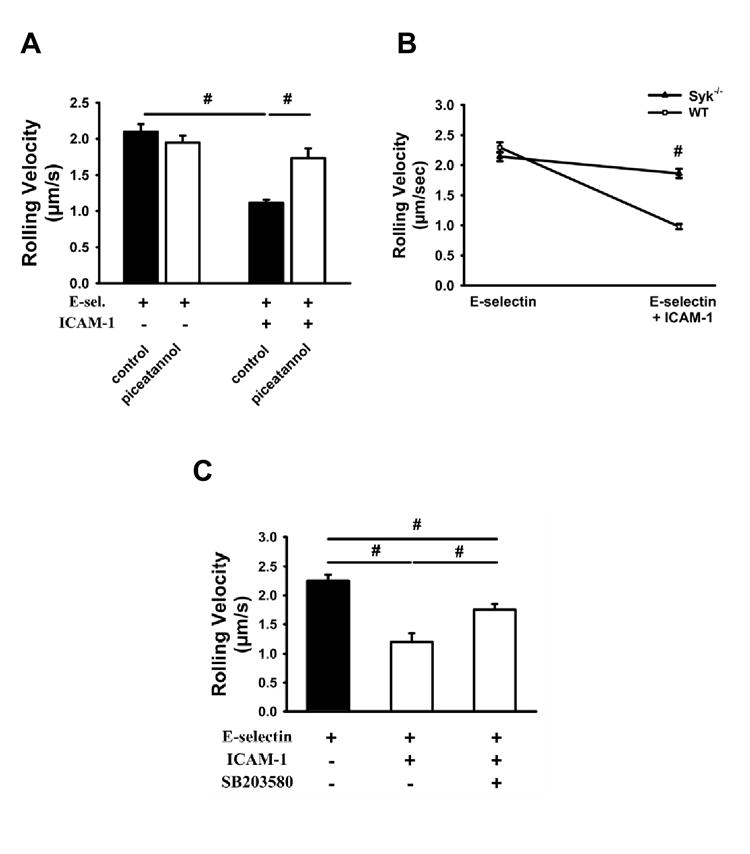
Whole blood was perfused through E-selectin and E-selectin+ICAM-1 coated autoperfused flow chambers at the indicated wall shear stress. The rolling velocity of neutrophils from mice pre-treated with piceatannol (1mg/mouse, A), a specific Syk inhibitor, and Syk-/- chimeric mice (B) were determined after 6 minutes (n=4). (C) Rolling velocity on E-selectin and E-selectin+ICAM-1 was measured after blocking of the mitogen-activated protein (MAP)-kinase p38 by the inhibitor SB203580 (100 μg/mouse i.p.) 1h prior to the experiment (n=3). # P < 0.05.
TNF-α, but not IL-1β, induces neutrophil adhesion under flow
In many in vivo models, inflammation is induced by injecting TNF-α, which causes up-regulation of adhesion molecules on endothelial cells, slow rolling, adhesion and recruitment of leukocytes (Kunkel et al., 2000). Pre-treatment of WT mice with TNF-α did not change the rolling velocity in autoperfused flow chambers coated with E-selectin. After TNF-α, but not IL-1β, the rolling velocity on E-selectin/ICAM-1 significantly dropped significantly (Figure 4A). TNF-α, but not IL-1β, treatment also induced neutrophil adhesion on E-selectin/ICAM-1 (Figure 4B). TNF-α, but not IL-1β, treated blood induced adhesion, showing that factors produced by blood cells are sufficient for this effect (Figure 4C). To test whether chemokine deposition on the surface of E-selectin/ICAM-1 coated flow chambers during the perfusion of whole blood through the chamber was relevant, we perfused E-selectin/ICAM-1 coated flow chambers with plasma from untreated or TNF-α-treated mice for 6 minutes. Then, these same flow chambers were connected to untreated mice. Perfusion of plasma from TNF-α-treated mice did not induce neutrophil adhesion (Supplemental Figure 1).
Figure 4. TNF-α, but not IL-1β, causes neutrophil arrest on E-selectin + ICAM-1.
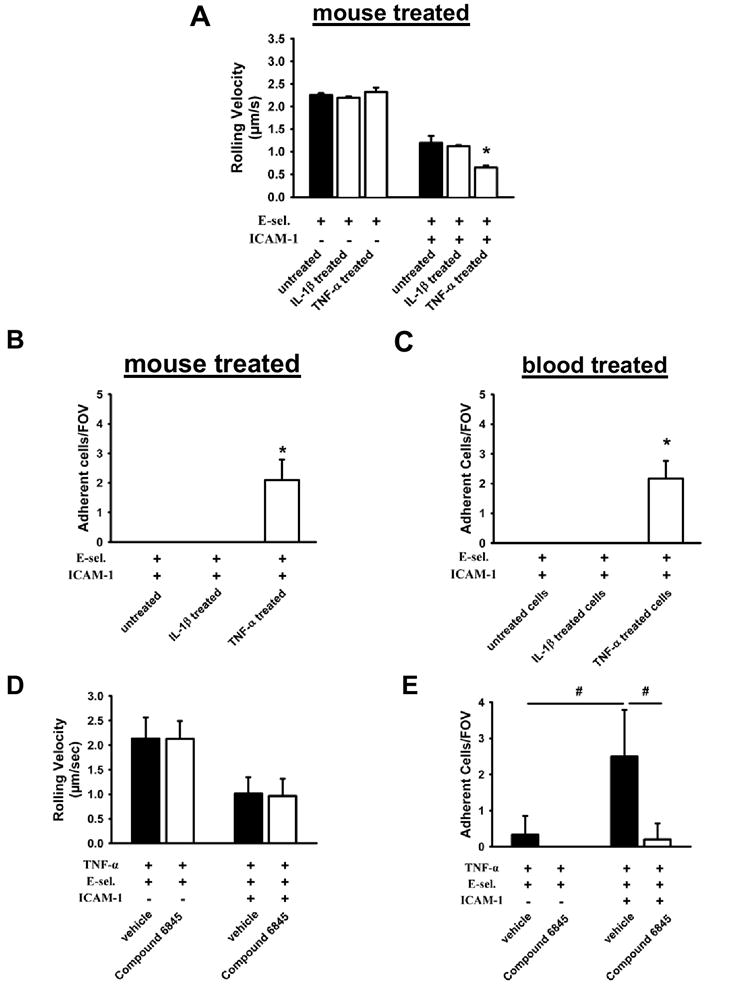
(A) Carotid cannulas of TNF-α or IL-1β pre-treated mice were connected to E-selectin and E-selectin+ICAM-1-coated autoperfused flow chambers. Rolling velocity of neutrophils was determined at a wall shear stress of 5.94 dyn/cm2 (n=4) (B, C) Number of adherent neutrophils on E-selectin+ICAM-1 of TNF-α pre-treated mice or whole blood after 6 minutes. Mice were pre-treated with TNF-α and an allosteric inhibitor (Compound 6845) of LFA-1 or vehicle and rolling velocity (D) and number of adherent neutrophils (E) were determined on E-selectin and E-selectin+ICAM-1. To ensure that only LFA-1-dependent rolling and adhesion were investigated, all mice were treated with a blocking monoclonal antibody to Mac-1 (n=4). * and # P < 0.05.
In order to investigate which conformation of LFA-1 is responsible for slow rolling and adhesion, we used an allosteric inhibitor of LFA-1 (Compound 6845). This inhibitor is a structural analog of the allosteric inhibitor XVA143 (Shimaoka et al., 2003a), which has been shown to target the I-like domain on the β2-subunit and prevent firm adhesion by stabilizing the αLβ2 heterodimer, activating the β2 I-like domain, and inhibiting activation of the α I domain. This inhibitor therefore stabilizes the intermediate affinity conformation of LFA-1. Pre-treatment of mice with the allosteric inhibitor before TNF-α application did not influence the rolling velocity on E-selectin and E-selectin/ICAM-1 compared to vehicle-treated mice (Figure 4D), but abolished leukocyte adhesion in response to TNF-α (Figure 4E). These data show that intermediate affinity LFA-1 is responsible for slow rolling, whereas the fully activated conformation with high affinity of LFA-1 is necessary for leukocyte adhesion to ICAM-1.
CXCR2, but not Syk, controls neutrophil arrest in response to TNF-α
CXCR2 is an important chemokine receptor for neutrophil adhesion (Smith et al., 2004a). In order to investigate whether GPCRs and specifically CXCR2 is involved in the modulation of rolling velocities and to address whether Syk is downstream of CXCR2, we used PTx to inhibit Gαi, piceatannol to inhibit Syk, and Syk-/- chimeric mice. PTx and piceatannol did not influence the rolling velocity on E-selectin (Figure 5A). Syk inhibition by piceatannol completely prevented the drop of rolling velocity on E-selectin/ICAM-1, whereas blocking of Gαi increased the rolling velocity by only 28% (Figure 5A). In contrast, PTx totally abolished neutrophil adhesion, whereas inhibition of Syk by piceatannol had no effect (Figure 5B).
Figure 5. CXCR2 and Gαi, but not Syk, control neutrophil arrest in response to TNF-α.
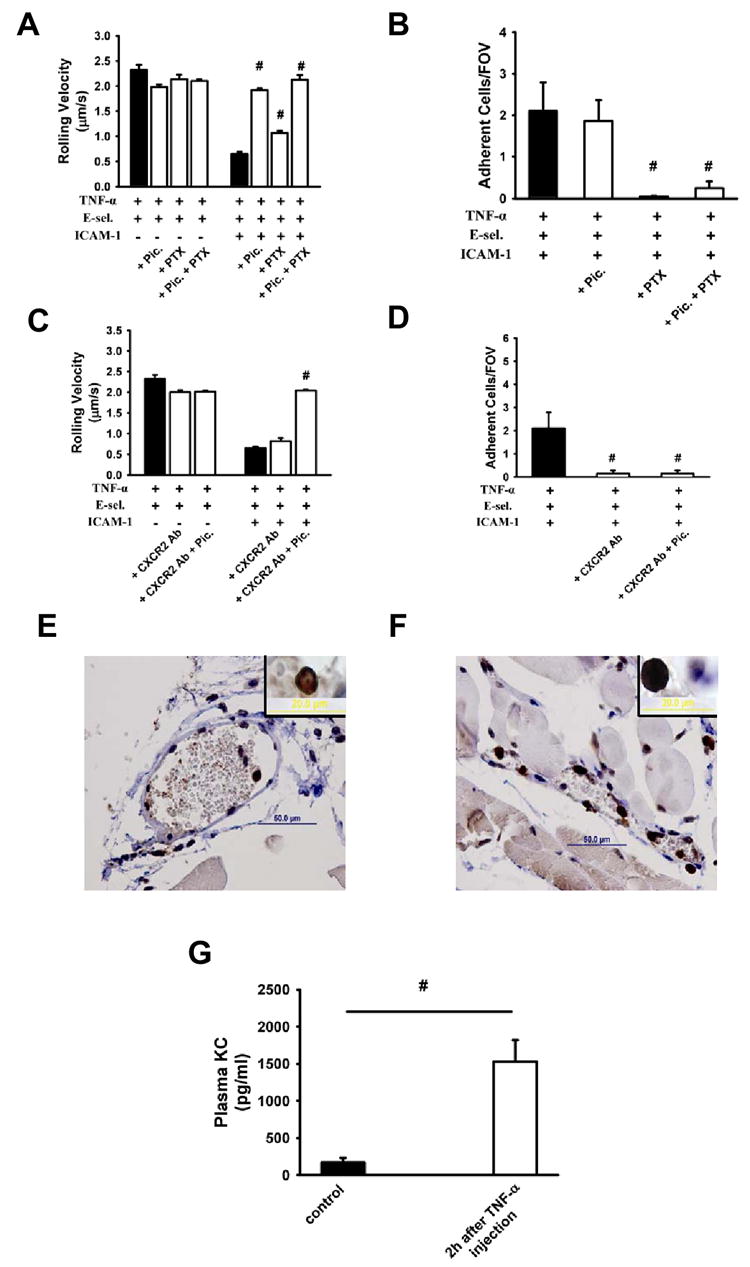
Rolling velocity of neutrophils from TNF-α pre-treated mice on E-selectin and E-selectin+ICAM-1 coated flow chambers after blocking Gαi with PTx or Syk by piceatannol alone or in combination (A). (B) Neutrophil adhesion on E-selectin+ICAM-1 coated flow chambers after blocking Gαi with PTx or Syk by piceatannol alone or in combination of TNFα pre-treated mice. Rolling velocity (C) and neutrophil adhesion (D) on E-selectin+ICAM-1 coated flow chambers after blockade of CXCR2 by antibody or of Syk by piceatannol alone or in combination following TNF-α application. Cremaster muscles of WT mice were stained for CXCL1 without (E) and with (F) TNF-α. TNF-α injection induced an increase in adherent leukocytes in the microcirculation of the cremaster muscle in WT mice compared to untreated mice. Inserts show typical neutrophils stained for CXCL1 (E, F). (G) CXCL1 concentration in plasma before and 2h after TNF-α injection (n=4). # P < 0.05.
In order to identify the GPCR involved in neutrophil adhesion, we used a blocking CXCR2 antibody. Blocking CXCR2 alone or in combination with Syk did not alter the rolling velocity on E-selectin. On E-selectin/ICAM-1, blocking CXCR2 had no effect on rolling velocity, but inhibition of Syk by piceatannol elevated the rolling velocity to the level seen on E-selectin (Figure 5C). TNF-α-induced adhesion was completely blocked by the CXCR2 antibody (Figure 5D). These findings were confirmed by intravital microscopy of mouse cremaster venules (Supplemental Figure 2). Taken together, these results demonstrate that Syk is not involved in CXCR2-dependent arrest.
To investigate the mechanism by which TNF-α triggered CXCR2-dependent neutrophil arrest, we investigated CXCL1 production in neutrophils after TNF-α application in vivo. We stained the cremaster muscle for CXCL1 (Figure 5E and F) and measured the CXCL1 concentration in the plasma in the presence or absence of TNF-α (Figure 5G). Intrascrotal injection of TNF-α 2h before exteriorization induced an increase of adherent leukocytes and CXCL1 expression (Figure 5F) in venules of the cremaster muscle in WT mice compared to untreated mice (Figure 5E) and increased CXCL1 in plasma (Figure 5G). Taken together with the data presented above, we conclude that TNF-α induced CXCL1 activates LFA-1 through CXCR2 and Gαi to promote arrest, whereas ligation of PSGL-1 by E-selectin induces partial LFA-1 activation through Syk to promote slow rolling on E-selectin/ICAM-1 (Figure 6).
Figure 6. Model of LFA-1 activation during neutrophil rolling.
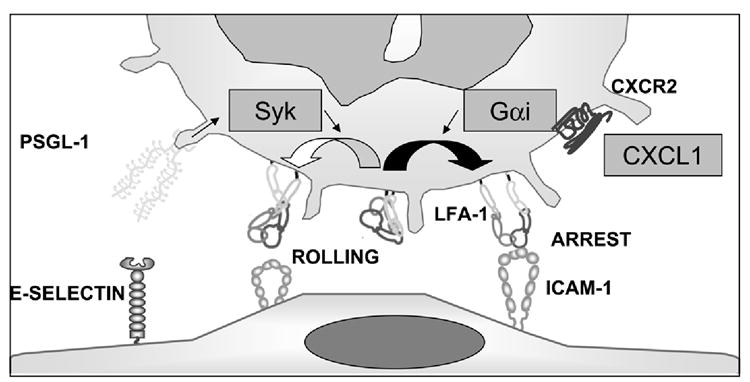
E-selectin binding to PSGL-1 leads, through Syk (arrows), to partial LFA-1 (open curved arrow) activation that supports rolling on ICAM-1 (shown as dimer). CXCR2 engagement by CXCL1 leads, through Gai, to full LFA-1 activation, resulting in arrest on ICAM-1 (filled curved arrow). Straight arrows indicate signaling pathway, but interactions may be indirect. LFA-1 conformations from Takagi et al (Takagi and Springer, 2002).
Inhibitions of Syk- and GPCR-signaling reduce neutrophil recruitment
The inability of neutrophils from PSGL-1 deficient mice to roll on ICAM-1 upon E-selectin engagement and the elimination of adhesion by blocking CXCR2 after TNF-α application suggests that inhibition of both PSGL-1 signaling and chemokine-mediated adhesion may prevent neutrophil influx into inflamed tissues. To compare the physiological importance of the two signaling pathways, neutrophil recruitment into thioglycollate-induced peritonitis was investigated in WT, PSGL-1-/-, and E-selectin-/- mice with or without PTx treatment. Consistent with previous data (Smith et al., 2004a), pre-treatment with PTx reduced neutrophil recruitment to the peritoneum 4 h after thioglycollate injection into WT mice (Figure 7). PSGL-1-/- mice also showed a reduced neutrophil influx after thioglycollate application compared to WT mice (Yang et al., 1999). However, blocking both pathways by combining PTx treatment with the absence of PSGL-1 almost completely eliminated neutrophil recruitment into the peritoneal cavity after thioglycollate injection (Figure 7). In order to show Syk involvement, we used either Syk-/- bone marrow chimeric mice or blocked Syk by the inhibitor piceatannol (Figure 7). Blocking of Syk by piceatannol showed no reduction of neutrophil influx (data not shown), but pharmacological blockade of Gαi in Syk-/- chimeric mice or blockade of both Gαi and Syk in WT mice almost completely abolished thioglycollate-induced neutrophil recruitment to the peritoneal cavity (Figure 7). These data suggest PSGL-1-Syk signaling and chemokine-GPCR signaling together control neutrophil recruitment in this model of thioglycollate-induced peritonitis.
Figure 7. Inhibition of Gαi in PSGL-1-/- mice blocks neutrophil recruitment in vivo.
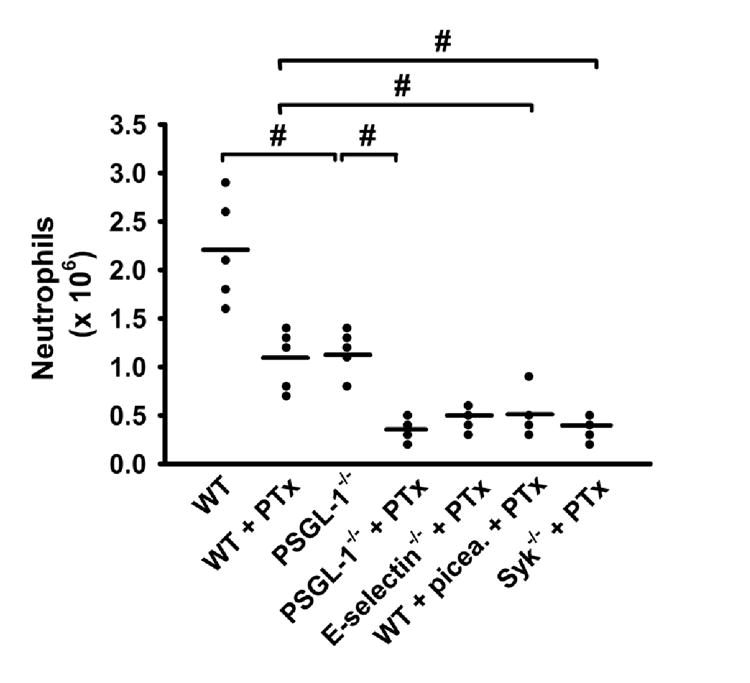
Peritoneal neutrophil influx 4 h after 1 ml injection of 4% thioglycollate into WT mice, WT mice that had received 4 μg PTx before thioglycollate injection, PSGL-1-/- mice, PSGL-1-/- mice plus PTx, E-selectin-/- mice, E-selectin-/- mice plus PTx, WT mice that had received 1mg piceatannol (picea) and 4 μg PTx, and Syk-/- chimeric mice (Syk-/-) plus 4 μg PTx (n=5 each). Data expressed as total numbers of neutrophils in the peritoneal lavage fluid counted using Kimura-stained samples, # P < 0.05.
Discussion
Many studies have demonstrated that E-selectin engagement leads to activation of neutrophils (Chesnutt et al., 2006; Green et al., 2004; Mattila et al., 2005; Simon et al., 2000), but the ligand responsible for signaling was unknown. Here, we demonstrate that PSGL-1 is the signaling molecule responsible for LFA-1-dependent rolling upon E- or P-selectin engagement. This interaction induces Syk-dependent partial LFA-1 activation that causes slow rolling on ICAM-1, but not arrest. In response to TNF-α, neutrophils produce CXCL1, which fully activates LFA-1 through CXCR2 and Gαi in an autocrine/paracrine manner and causes firm adhesion.
Syk has previously been shown to be responsible for signaling events (Berton et al., 2005) secondary to outside-in signaling through integrins (Mocsai et al., 2002; Schymeinsky et al., 2006). Syk was needed for respiratory burst, degranulation, and spreading in response to pro-inflammatory stimuli (Mocsai et al., 2002) but not for G-protein coupled receptor signaling (Mocsai et al., 2003). Although Syk was previously shown to be activated by PSGL-1 (Urzainqui et al., 2002), our experiments with neutrophils in their native whole blood suggest that Syk is involved in the signaling pathway leading to β2-integrin conformational change upon PSGL-1 binding to E-selectin or P-selectin. Syk-deficient neutrophils or Syk blockade increased rolling velocities on E-selectin/ICAM-1 to levels which were comparable with rolling velocities on E-selectin alone. We confirm that Syk is not involved in the signalling downstream of CXCR2 (Mocsai et al., 2003). Inhibition of Syk did not reduce the number of adherent neutrophils upon TNF-α stimulation and Syk chimeric mice showed a similar increase in leukocyte arrest upon CXCL1 application. Also, blocking Syk had no effect on rolling on E-selectin, suggesting that Syk blockade does not introduce gross morphological changes such as flattening of microvilli.
It has long been known that E-selectin induces slow rolling (Kunkel and Ley, 1996; Ley et al., 1998; Norman et al., 2000). This slow rolling also requires CD18 integrins (Dunne et al., 2002; Dunne et al., 2003; Jung et al., 1998; Kunkel et al., 2000), but the mechanism of integrin activation was not understood. Our in vivo and in vitro data show that the β2-integrin LFA-1, and not Mac-1, is responsible for E-selectin induced slow rolling. Depending on the state of activation, integrins can be found in different conformations with different affinities to their ligands (Shimaoka et al., 2003b). The β2-integrin LFA-1 in the intermediate-affinity conformation mediates rolling on ICAM-1, whereas LFA-1 in the high-affinity and extended conformation induces arrest (Salas et al., 2004); (Shimaoka et al., 2003b). An allosteric LFA1 inhibitor that stabilizes the intermediate conformation did not influence slow rolling, suggesting that PSGL-1 binding to E-selectin induces the intermediate-affinity conformation of LFA-1. An alternative interpretation of our data is that some fraction of LFA-1 exists in the partially open, intermediate affinity state, and that Syk is involved in outside-in signaling following ligand engagement, which may stabilize this conformation. By contrast, the chemokine induced adhesion after TNF-α-application was completely blocked by this allosteric inhibitor, suggesting that firm adhesion requires the high affinity conformation of LFA-1.
The discovery of partial LFA-1 activation by PSGL-1 engagement was made possible by using neutrophils in whole blood rather than isolated neutrophils. Isolated neutrophils express increased levels of Mac-1 and show a decreased ability to roll (Forsyth and Levinsky, 1990; Glasser and Fiederlein, 1990; Kuijpers et al., 1991). As a case in point, isolated human neutrophils rolled at similar velocities on L-cells transfected with E-selectin alone or in combination with ICAM-1 (Simon et al., 2000), whereas we show a clear difference in rolling velocity on E-selectin versus E-selectin/ICAM-1. PSGL-1 engagement by E-selectin leads to slow rolling by inducing the partially activated conformation of LFA-1. This was probably missed in earlier studies, because isolated neutrophils are already partially activated.
TNF-α primes neutrophils and induces increased surface expression of CD11b, oxidative burst (Nathan et al., 1989; Richter et al., 1995), and chemokine production (Hachicha et al., 1995). Here we show that TNF-α also triggers neutrophil adhesion on E-selectin/ICAM-1 through CXCR2 and Gαi. Blocking of Gαi-coupled receptors by PTx abolished neutrophil adhesion, and also influenced rolling velocity. Antibody blockade of CXCR2 prevented neutrophil adhesion, but did not influence the rolling velocity. These data demonstrate that CXCR2 is an important receptor for adhesion, and suggest that other Gαi-coupled receptors may be involved in controlling rolling velocity.
After TNF-α application, CXCL1 production in neutrophils and systemic CXCL1 levels increased. The treatment of isolated blood with TNF-α demonstrates that CXCL1 produced in neutrophils (Hachicha et al., 1995) activates CXCR2 in an autocrine/paracrine way and effectively induces arrest. Taken together, our results show that different signaling pathways for slow LFA-1-dependent rolling and arrest exist, but this study does not address where the Gαi and Syk signaling pathways converge. Downstream molecules that have been implicated in integrin activation include p38 MAPK (Simon et al., 2000), ERM proteins (Urzainqui et al., 2002) and talin (Tadokoro et al., 2003).
Previous work has shown reduced neutrophil recruitment to thioglycollate-induced peritonitis in PSGL-1-/- (Yang et al., 1999) and in PTx-treated mice (Smith et al., 2004a). Treating E-selectin-/- mice with PTx almost completely abolished neutrophil recruitment in this model (Smith et al., 2004a). Here, we directly show the relevance of the PSGL-1-/- pathway by demonstrating that PTx treatment of PSGL-1-/- blocks neutrophil recruitment as effectively as PTx treatment of E-selectin-/- mice. These data show that both the PSGL-1-Syk pathway and the CXCR2-Gαi pathway are physiologically relevant in vivo. Therefore, therapeutic antiinflammatory strategies targeting neutrophils must consider both chemokine and E-selectin pathways. Since most lymphocytes bind E-selectin poorly (Ley and Kansas, 2004), novel anti-inflammatory strategies may be able to spare lymphocyte trafficking and thus avoid unwanted immunosuppression.
Materials and Methods
Animals and generation of bone marrow chimeras
We used 8-12 weeks old C57BL76 mice (Jackson Laboratory, Bar Harbor, USA), P-selectin, E-selectin, CD44, and PSGL-1 deficient mice backcrossed to C57BL/6 for at least 10 generations. Mice carrying the Syktm1Tyb mutation (Turner et al., 1995) were maintained as +/− heterozygotes on the C57BL/6 genetic background. The generation of mice with a Syk/- hematopoetic system was performed as described previously (Mocsai et al., 2002). Mice were housed in a barrier facility under specific pathogen-free conditions. The Animal Care and Use Committee of the University of Virginia (Charlottesville) approved all animal experiments.
Antibodies, Cytokines, Recombinant Proteins, and other Reagents
Recombinant murine E-selectin-Fc, P-selectin-Fc, ICAM-1-Fc, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and the blocking CXCR2 mAb (clone 242216) were obtained from R&D Systems (Minneapolis, MN, USA). CXCL1 [keratinocyte-derived chemokine (KC)] was purchased from PeproTech (Rocky Hill, NJ). The Syk-inhibitor piceatannol was purchased from A.G. Scientific (San Diego, CA, USA) and was used in a concentration of 1mg/mouse. The blocking monoclonal antibody (mAb) 9A9 (rat immunoglobulin G1 [IgG1], 30μg/mouse) against murine E-selectin was provided by B. Wolitzky (MitoKor, San Diego, CA, USA). The blocking lymphocyte function antigen (LFA)-1 mAb TIB-217 (rat IgG2a, 30μg/mouse) was purified at the biomolecular facility of the University of Virginia from hybridoma supernatant (ATCC, Rockville, MD, USA). The blocking Mac-1 mAb M1/70 (rat IgG2b, 30μg/mouse) was purchased from Pharmingen (San Diego, CA, USA). The small molecule antagonist, Compound 6845, binds to the β2 subunit I-like domain and was a gift from Dr. Grace Ju (Hoffmann La-Roche, USA). If not stated otherwise, all other reagents were obtained from Sigma-Aldrich (Sigma Chemical Co., St.Louis, MO, USA).
Surgical Preparation and intravital microscopy
Mice were anesthetized with an i.p. injection of ketamine hydrochloride (125 mg/kg, Sanofi Winthrop Pharmaceuticals, New York, NY), atropine sulfate (0.025 mg/kg, Fujisawa USA, Inc., Deerfield, IL) and xylazine (12.5 mg/kg, Tranqui Ved, Phonix Scientific, Inc., St. Joseph, MO) and placed on a heating pad to maintain the body temperature at 37°C. Following tracheal intubation (Polyethylene PE 90 tubing, ID 0.86 mm, OD 1.27 mm), cannulation of the carotid artery (PE 10, ID 0.28 mm, OD 0.61mm) and flushing the cannula with 10 units/ml heparin sodium in saline, the cremaster muscle was prepared for intravital microscopy as previously described (Ley et al., 1995). Microscopic observations were conducted on postcapillary venules ranging from 20–40 μm in diameter using an intravital microscope (Axioskop; Zeiss, Thornwood, NY) with a saline immersion objective (SW 40/0.75 numerical aperture). A CCD camera (model VE-1000CD, Dage-MTI) was used for recording. Surface area, S, was calculated for each vessel using S=π*d*lν, where d is the diameter and lν is the length of the vessel. Rolling flux was measured as the number of cells that roll past a line perpendicular to the vessel axis per minute. Leukocyte rolling flux fraction is defined as the flux of rolling leukocytes in percent of total leukocyte flux (Ley and Gaehtgens, 1991). Leukocyte arrest was determined before and 1 minute after intravenous injection of 600ng CXCL1 as described previously (Smith et al., 2004a). Arrest was defined as leukocyte adhesion longer than 30 seconds and expressed as cells per surface area. Blood flow centerline velocity was measured using dual photodiode and a digital on-line crosscorrelation program (Circusoft Instrumentation, Hockessin, DE). Centerline velocities were converted to mean blood flow velocities by multiplying with an empirical factor of 0.625 (Lipowsky and Zweifach, 1978).
Blood-Perfused Microflow Chamber
In order to investigate the rolling velocity, the number of rolling cells, and adherent cells we used a recently described microflow chamber system (Chesnutt et al., 2006; Smith et al., 2006). 20 × 200 μm rectangular glass capillaries were filled with E-selectin (30 μg/ml) or Pselectin (20 μg/ml) alone or in combination with ICAM-1 (15 μg/ml) and/or CXCL1 (10 μg/ml) for 2 h and blocked for 1h using 10% casein (Pierce Chemicals, Dallas, TX). The chamber was connected at one side to a PE 10 tubing and inserted into the carotid artery. The other side of the chamber was connected to a PE 50 tubing and used to control the wall shear stress, which was calculated as described (Smith et al., 2004b). Microscopy was conducted using a Zeiss Axioskop (Carl Zeiss, Inc., Thornwood, NY) with a saline immersion objective (SW 20/0.5). Images were recorded with a 3CCD color video camera (model DXC-390, SONY Corporation, Japan) connected to a Panasonic S-VHS recorder. The chamber was perfused with blood for 6 min before one representative field of view was recorded for 1 min. In some experiments, blood was collected by cardiac puncture and stimulated with IL-1β (10ng/ml) or TNF-α (10ng/ml) for 30 minutes according to a previously published protocol (Thompson et al., 2001).
Chemokines
CXCL1 (Keratinocyte-derived chemokine, KC) concentration in plasma was measured in triplicates by enzyme-linked immunosorbent assay kits according to manufactures instructions (R&D Systems, Minneapolis, MN, USA) under baseline conditions and 2h after TNF-α injection. Paraffin-embedded cremaster sections (5 μm) were incubated with a goat anti-mouse polyclonal antibody against KC (C-19, Santa Cruz Biotechnology, Santa Cruz, USA). This was followed by a biotinylated secondary antibody (Vector Laboratories, CA, USA) and avidin-biotin-peroxidase (Vector Laboratories, CA, USA). Sections stained without primary antibody served as negative controls.
Pertonitis model
Peritoneal recruitment of leukocytes was induced as previously described (Smith et al., 2004a). Briefly, 1 ml of sterile water containing 4% thioglycollate was injected i.p. 4h before the peritoneal lavage with 10 ml PBS (containing 2 mM EDTA) was performed. Total number of neutrophils was determined using Kimura-staining. Some mice received tail vein injections of 4 μg PTx 2 h before thioglycollate injection.
Statistics
Statistical analysis was performed with SPSS (version 14.0, Chicago, IL) and included one-way analysis of variance, Student-Newman-Keuls test, and t-test where appropriate. All data are presented as mean ± SEM P < 0.05 were considered significant.
Supplementary Material
01
Supplemental Figure 1: Number of adherent cells after perfusion of E-selectin/ICAM-1-coated flow chambers with plasma from TNF-α-treated and untreated mice. Flow chambers were coated with E-selectin/ICAM-1 and perfused with plasma of TNF-α-treated or untreated mice for 6 minutes. Then, flow chambers were connected to untreated mice and the chambers were perfused with whole blood for 6 minutes. After this time, number of adherent cells was measured. n=3.
Supplemental Figure 2: Reduced baseline adhesion in Syk-/- BM chimeras but robust arrest in response to CXCL1. (A) Intravital microscopy of venules in the untreated mouse cremaster muscle. The number of arrested leukocytes (A) and rolling flux (B) in Syk chimeric and control mice before and after CXCL1 injection (600 ng i.v., n=4). # P < 0.05
Acknowledgments
The authors wish to thank Yongmei Hu for generating Syk-/- bone marrow chimeras. We are grateful for Dr. Grace Ju’s (Roche, Nutley, NJ) gift of the allosteric LFA-1 inhibitor. This study was supported by grants from Deutsche Forschungsgemeinschaft to A.Z. (AZ 428/2-1) and from NIH to K.L. (NIH HL 73361).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbal C, Lambelet M, Bertaggia D, Gerbex C, Martinez M, Arcaro A, Schapira M, Spertini O. Lipid raft adhesion receptors and Syk regulate selectin-dependent rolling under flow conditions. Blood. 2006;108:3352–3359. doi: 10.1182/blood-2006-04-013912. [DOI] [PubMed] [Google Scholar]
- Berton G, Mocsai A, Lowell CA. Src and Syk kinases: key regulators of phagocytic cell activation. Trends Immunol. 2005;26:208–214. doi: 10.1016/j.it.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- Chesnutt BC, Smith DF, Raffler NA, Smith ML, White EJ, Ley K. Induction of LFA-1-dependent neutrophil rolling on ICAM-1 by engagement of E-selectin. Microcirculation. 2006;13:99–109. doi: 10.1080/10739680500466376. [DOI] [PubMed] [Google Scholar]
- Crutchfield KL, Shinde Patil VR, Campbell CJ, Parkos CA, Allport JR, Goetz DJ. CD11b/CD18-coated microspheres attach to E-selectin under flow. J Leukoc Biol. 2000;67:196–205. doi: 10.1002/jlb.67.2.196. [DOI] [PubMed] [Google Scholar]
- Dunne JL, Ballantyne CM, Beaudet AL, Ley K. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood. 2002;99:336–341. doi: 10.1182/blood.v99.1.336. [DOI] [PubMed] [Google Scholar]
- Dunne JL, Collins RG, Beaudet AL, Ballantyne CM, Ley K. Mac-1, but not LFA-1, uses intercellular adhesion molecule-1 to mediate slow leukocyte rolling in TNF-alpha-induced inflammation. J Immunol. 2003;171:6105–6111. doi: 10.4049/jimmunol.171.11.6105. [DOI] [PubMed] [Google Scholar]
- Forsyth KD, Levinsky RJ. Preparative procedures of cooling and re-warming increase leukocyte integrin expression and function on neutrophils. J Immunol Methods. 1990;128:159–163. doi: 10.1016/0022-1759(90)90206-b. [DOI] [PubMed] [Google Scholar]
- Galt SW, Lindemann S, Medd D, Allen LL, Kraiss LW, Harris ES, Prescott SM, McIntyre TM, Weyrich AS, Zimmerman GA. Differential regulation of matrix metalloproteinase-9 by monocytes adherent to collagen and platelets. Circ Res. 2001;89:509–516. doi: 10.1161/hh1801.096339. [DOI] [PubMed] [Google Scholar]
- Glasser L, Fiederlein RL. The effect of various cell separation procedures on assays of neutrophil function. A critical appraisal. Am J Clin Pathol. 1990;93:662–669. doi: 10.1093/ajcp/93.5.662. [DOI] [PubMed] [Google Scholar]
- Grabovsky V, Feigelson S, Chen C, Bleijs DA, Peled A, Cinamon G, Baleux F, Arenzana-Seisdedos F, Lapidot T, van Kooyk Y, et al. Subsecond induction of alpha4 integrin clustering by immobilized chemokines stimulates leukocyte tethering and rolling on endothelial vascular cell adhesion molecule 1 under flow conditions. J Exp Med. 2000;192:495–506. doi: 10.1084/jem.192.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CE, Pearson DN, Camphausen RT, Staunton DE, Simon SI. Shear-dependent capping of L-selectin and P-selectin glycoprotein ligand 1 by E-selectin signals activation of high-avidity beta2-integrin on neutrophils. J Immunol. 2004;172:7780–7790. doi: 10.4049/jimmunol.172.12.7780. [DOI] [PubMed] [Google Scholar]
- Hachicha M, Naccache PH, McColl SR. Inflammatory microcrystals differentially regulate the secretion of macrophage inflammatory protein 1 and interleukin 8 by human neutrophils: a possible mechanism of neutrophil recruitment to sites of inflammation in synovitis. J Exp Med. 1995;182:2019–2025. doi: 10.1084/jem.182.6.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidari KI, Weyrich AS, Zimmerman GA, McEver RP. Engagement of P-selectin glycoprotein ligand-1 enhances tyrosine phosphorylation and activates mitogen-activated protein kinases in human neutrophils. J Biol Chem. 1997;272:28750–28756. doi: 10.1074/jbc.272.45.28750. [DOI] [PubMed] [Google Scholar]
- Jung U, Norman KE, Scharffetter-Kochanek K, Beaudet AL, Ley K. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J Clin Invest. 1998;102:1526–1533. doi: 10.1172/JCI119893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y, Hidalgo A, Chang J, Peired A, Frenette PS. CD44 is a physiological E-selectin ligand on neutrophils. J Exp Med. 2005;201:1183–1189. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers TW, Tool AT, van der Schoot CE, Ginsel LA, Onderwater JJ, Roos D, Verhoeven AJ. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 1991;78:1105–1111. [PubMed] [Google Scholar]
- Kunkel EJ, Dunne JL, Ley K. Leukocyte arrest during cytokine-dependent inflammation in vivo. J Immunol. 2000;164:3301–3308. doi: 10.4049/jimmunol.164.6.3301. [DOI] [PubMed] [Google Scholar]
- Kunkel EJ, Ley K. Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ Res. 1996;79:1196–1204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- Laudanna C. Integrin activation under flow: a local affair. Nat Immunol. 2005;6:429–430. doi: 10.1038/ni0505-429. [DOI] [PubMed] [Google Scholar]
- Ley K, Allietta M, Bullard DC, Morgan S. Importance of E-selectin for firm leukocyte adhesion in vivo. Circ Res. 1998;83:287–294. doi: 10.1161/01.res.83.3.287. [DOI] [PubMed] [Google Scholar]
- Ley K, Bullard DC, Arbones ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Gaehtgens P. Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ Res. 1991;69:1034–1041. doi: 10.1161/01.res.69.4.1034. [DOI] [PubMed] [Google Scholar]
- Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat Rev Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- Lindemann SW, Weyrich AS, Zimmerman GA. Signaling to translational control pathways: diversity in gene regulation in inflammatory and vascular cells. Trends Cardiovasc Med. 2005;15:9–17. doi: 10.1016/j.tcm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- Lo SK, Lee S, Ramos RA, Lobb R, Rosa M, Chi-Rosso G, Wright SD. Endothelial-leukocyte adhesion molecule 1 stimulates the adhesive activity of leukocyte integrin CR3 (CD11b/CD18, Mac-1, alpha m beta 2) on human neutrophils. J Exp Med. 1991;173:1493–1500. doi: 10.1084/jem.173.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila PE, Green CE, Schaff U, Simon SI, Walcheck B. Cytoskeletal interactions regulate inducible L-selectin clustering. Am J Physiol Cell Physiol. 2005;289:C323–332. doi: 10.1152/ajpcell.00603.2004. [DOI] [PubMed] [Google Scholar]
- McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:S97–103. [PubMed] [Google Scholar]
- Mocsai A, Zhang H, Jakus Z, Kitaura J, Kawakami T, Lowell CA. G-protein-coupled receptor signaling in Syk-deficient neutrophils and mast cells. Blood. 2003;101:4155–4163. doi: 10.1182/blood-2002-07-2346. [DOI] [PubMed] [Google Scholar]
- Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- Nathan C, Srimal S, Farber C, Sanchez E, Kabbash L, Asch A, Gailit J, Wright SD. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989;109:1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KE, Katopodis AG, Thoma G, Kolbinger F, Hicks AE, Cotter MJ, Pockley AG, Hellewell PG. P-selectin glycoprotein ligand-1 supports rolling on E- and P-selectin in vivo. Blood. 2000;96:3585–3591. [PubMed] [Google Scholar]
- Parkos CA. Cell adhesion and migration. I. Neutrophil adhesive interactions with intestinal epithelium. Am J Physiol. 1997;273:G763–768. doi: 10.1152/ajpgi.1997.273.4.G763. [DOI] [PubMed] [Google Scholar]
- Rainger GE, Fisher AC, Nash GB. Endothelial-borne platelet-activating factor and interleukin-8 rapidly immobilize rolling neutrophils. Am J Physiol. 1997;272:H114–122. doi: 10.1152/ajpheart.1997.272.1.H114. [DOI] [PubMed] [Google Scholar]
- Richter J, Gullberg U, Lantz M. TNF-induced superoxide anion production in adherent human neutrophils involves both the p55 and p75 TNF receptor. J Immunol. 1995;154:4142–4149. [PubMed] [Google Scholar]
- Salas A, Shimaoka M, Kogan AN, Harwood C, von Andrian UH, Springer TA. Rolling adhesion through an extended conformation of integrin alphaLbeta2 and relation to alpha I and beta I-like domain interaction. Immunity. 2004;20:393–406. doi: 10.1016/s1074-7613(04)00082-2. [DOI] [PubMed] [Google Scholar]
- Schymeinsky J, Sindrilaru A, Frommhold D, Sperandio M, Gerstl R, Then C, Mocsai A, Scharffetter-Kochanek K, Walzog B. The Vav binding site of the non-receptor tyrosine kinase Syk at Tyr 348 is critical for {beta}2 integrin (CD11/CD18)-mediated neutrophil migration. Blood. 2006 doi: 10.1182/blood-2005-12-030387. [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Salas A, Yang W, Weitz-Schmidt G, Springer TA. Small molecule integrin antagonists that bind to the beta2 subunit I-like domain and activate signals in one direction and block them in the other. Immunity. 2003a;19:391–402. doi: 10.1016/s1074-7613(03)00238-3. [DOI] [PubMed] [Google Scholar]
- Shimaoka M, Xiao T, Liu JH, Yang Y, Dong Y, Jun CD, McCormack A, Zhang R, Joachimiak A, Takagi J, et al. Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell. 2003b;112:99111. doi: 10.1016/s0092-8674(02)01257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on Eselectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol. 2000;164:4348–4358. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- Smith DF, Deem TL, Bruce AC, Reutershan J, Wu D, Ley K. Leukocyte phosphoinositide-3 kinase {gamma} is required for chemokine-induced, sustained adhesion under flow in vivo. J Leukoc Biol. 2006 doi: 10.1189/jlb.0306227. [DOI] [PubMed] [Google Scholar]
- Smith DF, Galkina E, Ley K, Huo Y. GRO family chemokines are specialized for monocyte arrest from flow. Am J Physiol Heart Circ Physiol. 2005;289:H1976–1984. doi: 10.1152/ajpheart.00153.2005. [DOI] [PubMed] [Google Scholar]
- Smith ML, Olson TS, Ley K. CXCR2- and E-selectin-induced neutrophil arrest during inflammation in vivo. J Exp Med. 2004a;200:935–939. doi: 10.1084/jem.20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Sperandio M, Galkina EV, Ley K. Autoperfused mouse flow chamber reveals synergistic neutrophil accumulation through P-selectin and E-selectin. J Leukoc Biol. 2004b;76:985–993. doi: 10.1189/jlb.1003483. [DOI] [PubMed] [Google Scholar]
- Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–106. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- Takagi J, Springer TA. Integrin activation and structural rearrangement. Immunol Rev. 2002;186:141–163. doi: 10.1034/j.1600-065x.2002.18613.x. [DOI] [PubMed] [Google Scholar]
- Thompson RD, Noble KE, Larbi KY, Dewar A, Duncan GS, Mak TW, Nourshargh S. Platelet-endothelial cell adhesion molecule-1 (PECAM-1)-deficient mice demonstrate a transient and cytokine-specific role for PECAM-1 in leukocyte migration through the perivascular basement membrane. Blood. 2001;97:1854–1860. doi: 10.1182/blood.v97.6.1854. [DOI] [PubMed] [Google Scholar]
- Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- Urzainqui A, Serrador JM, Viedma F, Yanez-Mo M, Rodriguez A, Corbi AL, Alonso-Lebrero JL, Luque A, Deckert M, Vazquez J, Sanchez-Madrid F. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity. 2002;17:401–412. doi: 10.1016/s1074-7613(02)00420-x. [DOI] [PubMed] [Google Scholar]
- Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, Prescott SM, Zimmerman GA. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyrich AS, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J Clin Invest. 1995;95:2297–2303. doi: 10.1172/JCI117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Sperandio M, Yago T, McDaniel JM, Cummings RD, Pearson-White S, Ley K, McEver RP. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109:939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Hirata T, Croce K, Merrill-Skoloff G, Tchernychev B, Williams E, Flaumenhaft R, Furie BC, Furie B. Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration. J Exp Med. 1999;190:1769–1782. doi: 10.1084/jem.190.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman GA, McIntyre TM, Prescott SM. Adhesion and signaling in vascular cell--cell interactions. J Clin Invest. 1996;98:1699–1702. doi: 10.1172/JCI118967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
Supplemental Figure 1: Number of adherent cells after perfusion of E-selectin/ICAM-1-coated flow chambers with plasma from TNF-α-treated and untreated mice. Flow chambers were coated with E-selectin/ICAM-1 and perfused with plasma of TNF-α-treated or untreated mice for 6 minutes. Then, flow chambers were connected to untreated mice and the chambers were perfused with whole blood for 6 minutes. After this time, number of adherent cells was measured. n=3.
Supplemental Figure 2: Reduced baseline adhesion in Syk-/- BM chimeras but robust arrest in response to CXCL1. (A) Intravital microscopy of venules in the untreated mouse cremaster muscle. The number of arrested leukocytes (A) and rolling flux (B) in Syk chimeric and control mice before and after CXCL1 injection (600 ng i.v., n=4). # P < 0.05