Identification of the circadian transcriptome in adult mouse skeletal muscle (original) (raw)
. Author manuscript; available in PMC: 2018 Aug 7.
Abstract
Circadian rhythms are approximate 24-h behavioral and physiological cycles that function to prepare an organism for daily environmental changes. The basic clock mechanism is a network of transcriptional-translational feedback loops that drive rhythmic expression of genes over a 24-h period. The objectives of this study were to identify transcripts with a circadian pattern of expression in adult skeletal muscle and to determine the effect of the Clock mutation on gene expression. Expression profiling on muscle samples collected every 4 h for 48 h was performed. Using COSOPT, we identified a total of 215 transcripts as having a circadian pattern of expression. Real-time PCR results verified the circadian expression of the core clock genes, Bmal1, Per2, and Cry2. Annotation revealed cycling genes were involved in a range of biological processes including transcription, lipid metabolism, protein degradation, ion transport, and vesicular trafficking. The tissue specificity of the skeletal muscle circadian transcriptome was highlighted by the presence of known musclespecific genes such as Myod1, Ucp3, Atrogin1 (Fbxo32), and Myh1 (myosin heavy chain IIX). Expression profiling was also performed on muscle from the Clock mutant mouse and sarcomeric genes such as actin and titin, and many mitochondrial genes were significantly downregulated in the muscle of Clock mutant mice. Defining the circadian transcriptome in adult skeletal muscle and identifying the significant alterations in gene expression that occur in muscle of the Clock mutant mouse provide the basis for understanding the role of circadian rhythms in the daily maintenance of skeletal muscle.
Keywords: circadian rhythms, gene expression, MyoD, Clock mutant
Summary
Expression profiling was used to identify the circadian transcriptome of adult mouse skeletal muscle. Results from this work identified 215 circadian transcripts in skeletal muscle, and, consistent with previous circadian studies, few of the muscle circadian genes were found to cycle in other cell types. The genes were primarily associated with regulation of transcription, protein synthesis and degradation, and lipid metabolism illustrating the fundamental role that the core clock plays in maintaining cellular homeostasis.
Circadian Rhythms are Daily cycles in the behavior and physiology of an organism. The cycles are endogenously generated self-sustaining rhythms but can be influenced, in mammals, by environmental stimuli such as light and feeding (29, 34, 53). The temporal coordination between endogenous cellular rhythms and the environment has been experimentally shown to provide an adaptive advantage by enhancing an organism’s ability to anticipate daily changes in light, temperature, and humidity (68). The molecular mechanism regulating circadian rhythms is described as a network composed of transcriptional translational feedback loops and is referred to as the core clock (36, 51).
The positive loop of the core clock is formed by two members of the PER-ARNT-SIM basic helix-loop-helix (PAS-bHLH) family of transcription factors, Clock (circadian locomotor output control kaput), and Bmal1 (brain muscle arnt-like 1). The CLOCK:BMAL1 heterodimer activates transcription of additional core clock genes Period (Per1, Per2, and Per3) and Cryptochrome (Cry1 and Cry2). The CRY and PER proteins constitute the negative loop of the core clock by forming a multimeric complex that inhibits CLOCK:BMAL1 activity upon translocation to the nucleus. The orphan nuclear receptors Rev-erbα., Rev-erbβ, and Rora (RAR-related orphan receptor alpha) are the remaining core clock genes and are interwoven into the core clock mechanism via their action in repressing and activating Bmal1 gene transcription, respectively (1, 36, 52, 64). Components of the core clock have also been shown to regulate the expression of genes outside the core and these genes are designated as clock-controlled genes (CCGs). CCGs often encode transcription factors or proteins that control rate-limiting steps in metabolic pathways (43).
The components of the molecular clock have been identified in peripheral tissues such as the liver, heart, kidney, and skeletal muscle (2, 28, 39, 43, 60, 71). In addition to the core clock genes, ~4% of a tissue’s transcriptome has a circadian pattern of expression with <10% of the genes shared between any two tissues (2, 43, 60). This remarkable tissue specificity of circadianly expressed genes is thought to reflect the unique physiological demands of each tissue. How this high degree of specificity is achieved remains unknown but is thought to involve regulation by both core-clock components as well as clock-controlled transcription factors.
In the current study, expression profiling was employed to determine the circadian transcriptome of adult mouse skeletal muscle. The skeletal muscle circadian transcriptome was found to include components of the molecular core clock (Bmal1, Cry2, Per2, Rora, and Rev-erbα. and β), known CCGs (Dbp and Tef1), and numerous genes with important muscle-specific functions such as Myod1, Atrogin1, MuRF1, PGC-1β, Pdk4, and Ucp3. The observation that genes like Myod1, Atrogin1, and PGC-1β are under circadian control strongly suggests circadian rhythms will have a central role in the daily regula-tion of skeletal muscle function and phenotype.
METHODS
Experimental design.
The muscle collections, RNA preparations, and microarray chips have been described previously by Miller et al. (40). In brief, 60 male C57BL/6J mice (The Jackson Laboratory), 7–10 wk of age, were housed in a temperature- and humidity-controlled room and entrained to a 12-h light, 12-h dark cycle (LD12:12) for 14 days with food and water ad libitum. Following the entrainment period, mice were placed in constant darkness (DD: 12-h dark, 12-h dark), and after 30 h, corresponding to circadian time 18 (CT18), gastrocnemius muscles from both hindlimbs were collected from five mice every 4 h for two complete circadian cycles (DD30 – DD72 corresponding to ~CT18 –CT62). In addition, at DD34 through DD58, gastrocnemius muscles from age-matched male homozygous Clock mutant mice, backcrossed onto the C57BL/6J background, were collected (66).
RNA isolation.
Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA) according to manufacturer’s directions. RNA integrity was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Each sample for expression profiling was prepared by pooling equivalent amounts of total RNA (10 µg) as follows: total RNA was isolated separately from 10 individual hind-limb muscles at each time point (5 mice/time point × 2 hindlimb muscles/mouse = 10 hindlimb muscles/time point) (24). For each time point, 10 µg of total RNA from each right (or left) leg muscle (n = 5) was pooled providing a total of 50 µg of total RNA/sample (43, 44). In preparation for cRNA synthesis, each pooled sample (50 µg total RNA) was further processed to remove small RNA molecules (<200 bp) and genomic DNA with the RNeasy MinElute Cleanup Kit (Qiagen, Valencia, CA). This strategy resulted in two distinct samples comprising RNA from five different muscles for analysis at each time point for both wild-type and Clock mutant skeletal muscle.
Microarray chip design.
The microarray chip used in this study was a custom Affymetrix gene chip as described by Su et al. (62) and designated GNF1M. Briefly, the GNF1M chip is a whole-transcriptome array representing 36,182 nonredundant mouse sequences drawn from RefSeq (12,029 sequences), Celera (29,331 sequences), and RIKEN (46,299 sequences) databases (26, 42, 46). Repetitive sequences were removed using RepeatMasker and sequence identity between individual sequences determined by pairwise BLAT or BLAST and sim4 analyses (3, 13, 25). Single-linkage clustering insured the highest degree of confidence of computational prediction with a bias toward Inter-pro domain containing sequences and away from noncoding RNAs.
Microarray procedure.
Microarray analysis was performed essentially as described (35). We used 5 µg of the pooled RNA sample to synthesize cDNA that was then used as a template to generate biotinylated cRNA. cRNA was fragmented and hybridized to the GNF1M custom chip, washed, and scanned, and intensity values for each probe set were condensed using the GC-RMA algorithm (18). The GC-RMA (robust multiarray average) algorithm has been shown to be better at reducing noise at low levels of expression while maintaining a greater dynamic range of expression compared with other condensation algorithms (67). A total of 38 chips were processed in this manner: 24 chips (2 replicates/time point × 12 time points) for the wild-type analysis and 14 chips (2 replicates/time point × 7 time points) for the Clock mutant analysis. The condensed data files are available at Gene Expression Omnibus (www.ncbi.nih.gov/geo) accession GSE3746.
Identification of circadian transcripts.
The COSOPT program was used to determine whether the temporal pattern of expression for each transcript fit circadian criteria. (61). COSOPT is a cosine wave-fitting algorithm that employs a multiple measures corrected-β (MMC-β) parameter for determining the goodness of fit of the time course to a circadian pattern of expression with an average periodicity of 24 h. For this analysis a transcript was considered to have a circadian pattern of expression if it had a cosine wave period length between 20 –28 h and a MMC-β value ≤0.15. This is more stringent than the MMC-β value ≤0.2 used by Miller et al. (40) and resulted in a dataset of 215 genes (for MMC-β ≤0.15) in contrast to 383 (for MMC-β ≤0.2).
Analysis of noncircadian transcripts.
A custom-written MATLAB routine (MathWorks, Natick, MA) was used to compare the mean expression level of noncycling transcripts from muscle of wild-type and Clock mutant mice. The data set was analyzed as follows: 1) the 215 circadian probe sets were removed from the 36,182 probe pairs on the array, 2) the replicate samples for each of the seven common time points (DD34 through DD58) for wild-type and Clock mutant were averaged, 3) probe sets exhibiting significant variation (ANOVA, P < 0.05) over the seven time points were removed (n = 1,305 transcripts), 4) the average intensity value for each probe set across the seven time points was calculated, and those probe sets with mean expression levels for both wild-type and Clock mutant muscle < 350 were removed, and 5) Student _t_-tests (P < 0.001) were performed on the remaining 9,002 probe sets to identify transcripts with significantly different expression levels between wild-type and Clock mutant mice. A total of 3,146 transcripts showed a difference in expression between wild-type and Clock mutant; in the Clock mutant, relative to the wild-type, 673 transcripts were upregulated (21%) and 2,473 transcripts were downregulated (79%). The final data set was annotated using GNF SymAtlas (http://symatlas.gnf.org/SymAtlas) and GOToolBox functional cluster analysis (http://crfb.univ-mrs.fr/GOToolBox/index.php) (38, 62). GOToolBox analysis identified Gene Ontology (GO) functional clusters statistically (P < 0.01) enriched or depleted in the data set.
Real-time PCR analysis.
TaqMan real-time RT-PCR assays were performed using the comparative cycle threshold (CT) method with an ABI 7700 Sequence Detector and TaqMan EZ RT-PCR kit reagents (Applied Biosystems, Foster City, CA). Probe and primer sets were designed with Primer Express software (Applied Biosystems). Target gene mRNA levels were measured by determining the cycle number at which amplification detection threshold was reached (CT). In each sample, CT was normalized to GAPDH expression (∆CT). Normalized ∆CT values from each time point were then subtracted from the average ∆CT value of all time points (∆∆CT) to determine the relative abundance values (2–∆∆CT).
RESULTS AND DISCUSSION
Circadian gene expression in adult skeletal muscle.
The COSOPT algorithm has been previously used to identify genes with a circadian pattern of expression in the suprachiasmatic nucleus (SCN), liver, and heart (39, 43, 70). COSOPT employs a MMC-β parameter to assess the statistical probability a transcript has a circadian pattern of expression displaying a cosine wave form of period length 20 –28 h. For this study, the MMC-β cut-off was set at 0.15 based on the ability of COSOPT to successfully identify known circadian genes (known as guide genes) in other expression profiling studies (2, 43, 60). The use of guide genes, such as Per2, has been demonstrated to be a reliable strategy in setting MMC-β by providing a conservative balance of the false positive and false negative identifications by COSOPT (39, 43). The list of “guide” genes and their respective MMC-β values are presented in Table 1. At a MMC-β ≤0.15, >80% of the genes in Table 1 were identified as being circadianly expressed, a percentage comparable to previous studies that have used COSOPT (39, 43). Real-time PCR quantitatively confirmed the COSOPT results and verified that Bmal1, Per2, and Cry2 are circadian genes in skeletal muscle (Fig. 1, A–C). Furthermore, the results provided in Table 1 demonstrate that adult skeletal muscle expresses the common components of the molecular core clock (Bmal1, Per2, and Cry2) as well as several welldefined CCGs (Dbp, Tef, and Hlf).
Table 1.
Guide genes
| Gene Symbol | MMC-β |
| Bmal1 | 0.013 |
| Dbp | 0.013 |
| Rev-erbα | 0.017 |
| Nfil3 | 0.020 |
| Ccrn41 | 0.027 |
| Per2 | 0.040 |
| Rev-erbβ | 0.040 |
| Tef | 0.064 |
| Cry2 | 0.098 |
| Rora | 0.101 |
| Hlf | 0.111 |
| Bhlhb2 | 0.113 |
| Hnrpu | 0.137 |
| Weel | 0.163 |
| Per3 | 0.197 |
| Clock | 0.200 |
Fig. 1.
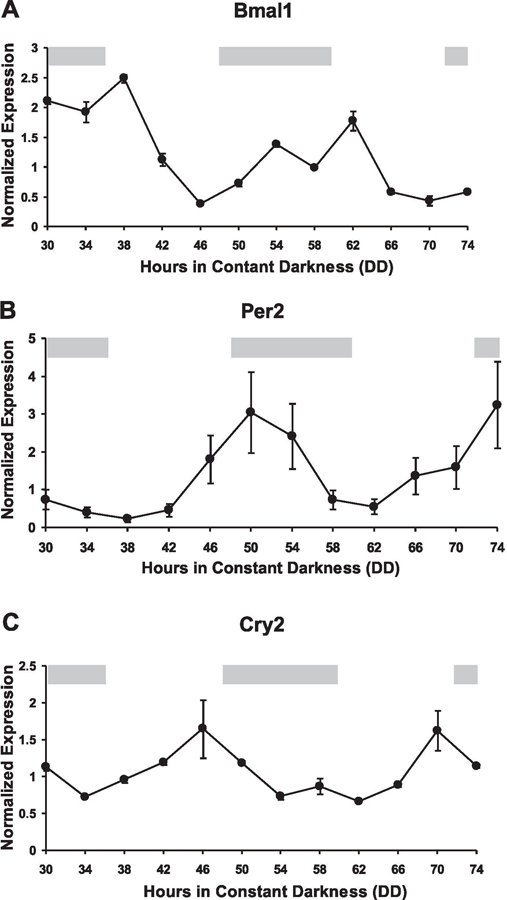
Real-time PCR verification of core clock gene expression in skeletal muscle. Real-time PCR was performed to validate the circadian expression of core clock genes identified by COSOPT from the array results. Graphical presentation of the results shows brain muscle arnt-like 1 (Bma1, A), period 2 (Per2, B), and cryptochrome (Cry2, C) transcript levels oscillate over 48 h with a circadian period indicative of a functioning circadian clock in adult skeletal muscle. Independent duplicate samples were run at each of 12 time points and normalized to GAPDH expression as described in METHODS. The light gray bars across the top of each graph represent the presumptive night with the intervening white spaces representing presumptive day.
A total of 215 genes were identified as having a circadian pattern of expression in adult skeletal muscle at a MMC-β ≤0.15 (Supplemental Table S1)1. A comparison of the circadian transcriptome among skeletal muscle, SCN, liver, and heart revealed only three genes (Per2, Dbp, and Dec1) in common among the four tissues (Supplemental Table S2). Per2 is a critical component of the core clock, while Dbp and Dec1 (Bhlhb2) are verified CCGs regulated by the CLOCK: BMAL1 heterodimer (41, 50, 54, 72). The small overlap in circadian genes between different tissues is consistent with previous profiling studies and highlights the tissue-specific characteristics of circadian programs of gene expression (43, 60).
Comparisons of circadian genes between skeletal muscle and liver or skeletal muscle and SCN identified 31 and 20 transcripts, respectively, in common, with many of the shared transcripts being elements of the core clock or known CCGs (Supplemental Table S2). It was somewhat surprising that there was less overlap between two striated muscle tissues, skeletal muscle and heart, than what was found between skeletal muscle and liver (60). The remarkable tissue specificity of circadian genes is thought to reflect the unique physiological requirements of a particular tissue as well as the specific environmental stimuli received by a tissue.
The tissue specificity of the skeletal muscle circadian transcriptome was highlighted by the presence of such muscle-specific genes as Myod1, Ucp3, Atrogin1 (Fbxo32), Pdk4, and Myh1 (myosin IIx). To identify additional skeletal muscle-enriched genes, the 215 circadian probe sets were analyzed, using GNF SymAtlas database (http://symatlas.gnf.org/SymAtlas/) with criteria set to select those genes expressed in skeletal muscle at levels fivefold above the average expression in all 61 tissues profiled. The results of this analysis revealed 14 genes (7%) were above the cut-off with more than half (8 out of 14) being expressed 10-fold above the mean and highly muscle specific (62) (Supplemental Table S3). Interestingly, three of the genes, Neurl, Asb2, and Atrogin1 (Fbxo32), have all been shown to be part of specific E3 ubiquitin ligase complexes (15, 17, 30, 32). Neurl and Atrogin1 (Fbxo32) are known to be involved in Notch signaling and associated with the maintenance of muscle mass, respectively, whereas the function of Asb2 is less well studied. In addition to Asb2, a second uncharacterized ankyrin repeat and SOCS box-containing (Asb) family member, Asb11, showed the highest degree of muscle-specific expression (56.2-fold above the mean) of all circadian genes.
The Ky (kyphoscoliosis peptidase) gene was another highly enriched gene in skeletal muscle that exhibits a circadian pattern of expression (MMC-β 0.074; ~35-fold enriched; see Supplemental Table S3). The ky/ky mouse suffers from a degenerative myopathy that is distinguished by a severe thoraco-lumbar kyphoscoliosis. Surprisingly, these mice are unable to mount a hypertrophic response to functional overload induced by synergist ablation (6). The exact function of the KY protein is not known but evidence from a recent study suggests it is a cytoskeleton protein with protease activity that interacts with a number of different sarcomeric proteins such as filamin C, myosin binding protein C, and titin (5). Based on these findings the authors proposed that Ky may have a role in some forms of limb-girdle muscular dystrophies that have not yet been mapped to a specific chromosomal loci (5).
Temporal clustering, based on time of peak expression, of the 215 circadian genes was performed to determine if the genes were equally distributed over 24 h. Since the analysis was performed over two circadian cycles, the replicate values for each probe set were averaged (n = 4) for the equivalent circadian time points (i.e., CT18 = DD30 and DD54, CT22 = DD34 and DD58, etc.). As shown in Fig. 2, genes were equally distributed between subjective day (n = 102) and subjective night (n = 113). More than a third of the genes, however, showed peak expression at the middle of the subjective night (CT18), a time period when nocturnal animals such as mice are most physically active and feeding. Though the mechanism(s) for this peak is unknown, one possible explanation is the increased contractile activity and/or associated changes in cellular metabolism that occur at night serve to transcriptionally target these genes in skeletal muscle.
Fig. 2.
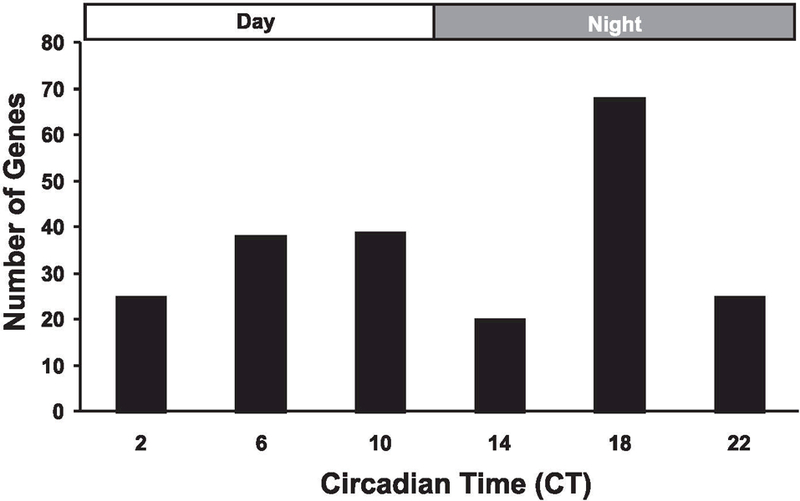
Temporal distribution of circadian genes by peak expression. Cycling genes were clustered according to their respective circadian time of peak expression. Genes were equally distributed between subjective day [circadian time 2 (CT2), CT6, and CT10: n = 103] and subjective night (CT14, CT18, and CT22: n = 112). Almost one-third (n = 68) of the cycling transcripts had peak expression at CT18, a time period of high physical activity and feeding.
The circadian data set was annotated and each gene was assigned a GO term representing a biological process (Supplemental Table S1). As shown in Fig. 3, circadian genes in skeletal muscle encompass a broad range of biological processes such as cell adhesion, transport, metabolism, cell cycle, signaling, protein metabolism, cytoskeleton organization, and transcription. Over half of the genes were classified under one of four GO designations; the largest group represented was transcription (18%) followed by signaling (15%), protein metabolism (12%), and substrate metabolism (9%). As with many expression profiling studies, a significant number of the transcripts were labeled as “uncharacterized” because no information was available regarding a demonstrated or predicted function. These uncharacterized transcripts were not significantly different in terms of mean expression level (P = 0.385) or MMC-β value (p = 0.266) than the circadian data set as a whole; thus, their characterization will be important for fully understanding the function of circadian rhythms in adult skeletal muscle.
Fig. 3.
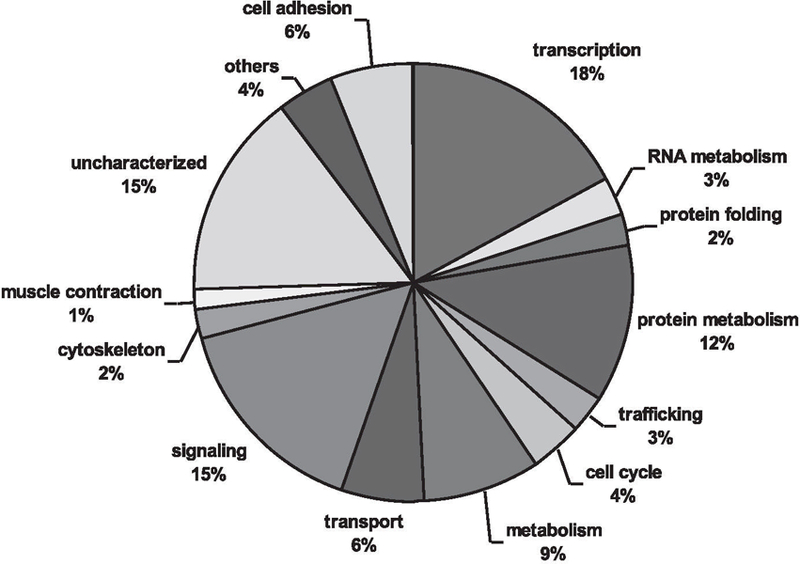
Gene ontology of skeletal muscle circadian transcriptome. A pie chart showing the distribution of circadian genes by biological process. Gene annotation was performed using GNF SymAtlas and literature searches. The “others” designation represents biological processes that occurred infrequently in the circadian transcriptome and included (frequency in parentheses) blood coagulation (2), chitin metabolism (1), DNA repair (1), meiosis (2), methyl-transferase (1), and defense response (2). The list of genes within each biological cluster is shown in Supplemental Table S1.
To determine how the peak expression of each biological process might vary over the course of a circadian cycle, GO terms were distributed according to their respective gene’s peak expression. For most transcripts of a particular GO, peak expression was evenly distributed between subjective day and night (Table 2). For example, genes with a GO protein metabolism designation, 13 displayed peak expression during the day, while 12 showed peak expression during presumptive night. Further inspection of the protein metabolism group revealed~50% of the genes were associated with the ubiquitin cycle, and, of these, almost two-thirds had peak expression during the presumptive night including the muscle-specific ubiquitin ligases Atrogin1 and MuRF1. Genes assigned to GO designations cell cycle, protein folding, and cytoskeleton organization also had their peak expression grouped almost exclusively during subjective night (Table 2).
Table 2.
Temporal distribution of gene ontology
| Gene Ontology | CT2 | CT6 | CT10 | CT14 | CT18 | CT22 | Total |
| Transcription | 2 | 6 | 12 | 3 | 10 | 4 | 37 |
| Signaling | 3 | 9 | 3 | 0 | 13 | 5 | 33 |
| Metabolism-protein | 5 | 2 | 6 | 2 | 5 | 5 | 25 |
| Metabolism | 4 | 3 | 2 | 1 | 7 | 2 | 19 |
| Transporter | 1 | 3 | 2 | 2 | 3 | 2 | 13 |
| Cell adhesion | 4 | 3 | 1 | 2 | 2 | 1 | 13 |
| Cell cycle | 0 | 1 | 0 | 1 | 4 | 2 | 8 |
| RNA metabolism | 0 | 2 | 1 | 2 | 1 | 0 | 6 |
| Trafficking | 0 | 3 | 1 | 0 | 2 | 0 | 6 |
| Protein folding | 0 | 0 | 0 | 1 | 4 | 0 | 5 |
| Cytoskeleton organization | 0 | 0 | 1 | 2 | 2 | 0 | 5 |
| Muscle contraction | 2 | 0 | 0 | 1 | 0 | 0 | 3 |
| Others | 2 | 0 | 3 | 0 | 3 | 1 | 9 |
| Uncharacterized | 2 | 6 | 7 | 3 | 12 | 3 | 33 |
| Total | 25 | 38 | 39 | 20 | 68 | 25 | 215 |
Circadian oscillation of Myod1 transcript.
The largest group of circadian genes were those categorized as “regulation of transcription, DNA-dependent,” consisting of a number of different transcription factor families such as PAS domain (Per2), PAS-bHLH (Arnt and Bmal1), bHLH (Myod1, Dec1, Hey1 and Mxi1), bZIP (Dbp, Tef, Hlf and Nfil3), Zn-finger (Rora, Rev-erbα., Rev-erbβ, Nr1b2 and Bcl6), SOCS-box (Asb4 and 11), and coactivators (Ctpb2 and Nrip1) (Supplemental Table S5). Within this group, the Myod1 transcript was identified as having a robust circadian pattern of expression. The identification of Myod1 as a circadian gene was an exciting finding because it expands the potential function of Myod1 beyond its known role in myogenesis to include an active role in the daily maintenance of adult skeletal muscle. As with many of the circadian genes involved with transcription, Myod1 peak expression occurred during subjective night at CT18 (Supplemental Table S4). The circadian expression of Myod1 transcript may be conserved between mouse and human as suggested by a recent study that found daily variation in Myod1 expression in human skeletal muscle (65). The circadian function of Myod1 remains to be determined, but the possible conservation of Myod1 circadian expression between species indicates an important role in skeletal muscle.
Circadian regulation of protein metabolism.
Circadian genes involved in protein synthesis, modification, or degradation were grouped together under the GO term “protein metabolism” and, as suggested by their overrepresentation (12%) in the circadian data set, appear to have an important role in circadian rhythms of skeletal muscle. Two related circadian genes, Ddit4 and Ddit4l (more commonly known as Redd1 and 2 or Rpt801 and 801l, respectively), are stress-induced proteins that modulate protein synthesis by inhibiting mammalian target of rapamycin (mTOR) activity during hypoxia and energy deprivation (8, 11, 57). Ddit4l was of particular interest because it is highly muscle specific (Supplemental Table S3), is significantly downregulated during the initial phase of muscle hypertrophy (J. J. McCarthy and K. A. Esser, unpublished observations) and was initially identified in a screen for genes upregulated during early stages of skeletal muscle atrophy (45). This evidence suggests Ddit4l is a muscle-specific regulator of mTOR activity that serves as a nodal point to integrate the circadian regulation of protein synthesis with daily stresses encountered by the muscle such as fluctuations in cellular energy charge and nutrient availability.
About half of the genes with the GO designation protein metabolism are involved in some way with protein degradation via the ubiquitin cycle (Neurl, Atrogin, MuRF1, Trim23, Fbxl7, Psma1, Hnrpu, Ubc, Usp2, Asb2, and Wsb1). Identification of the two ubiquitin ligases Atrogin1 (Fbxo32) and MuRF1 (Trim63) as circadian genes was of great interest given their central role in models of skeletal muscle atrophy (7, 22). Asb2 and Wsb1 are both SOCS-box containing proteins known to be part of E3 ubiquitin ligase complexes (17, 21, 30). The function of Asb2 is unknown, but it is qualitatively very similar to Atrogin1 in that it is a circadianly expressed, muscle-specific ubiquitin ligase. Wsb1, in complex with Elongin BC-Cul5-Rbx1, regulates thyroid hormone activity in response to Hedgehog signaling by D2 ubiquitination (12). The peak expression of these four ubiquitin ligases occurs within a 4-h window with Atrogin1, MuRF1, and Wsb1, peaking at CT22 and Asb2 expression peaking 4 h later at CT2. The coordinated circadian expression of these ubiquitin ligases is unique to the skeletal muscle transcriptome and suggests that there is an important need to insure the proper temporal degradation of proteins in skeletal muscle (43).
Circadian control of lipid metabolism.
One of the largest GO groups of circadianly regulated genes in skeletal muscle consisted of genes associated with substrate metabolism. Previous expression profiling studies have linked circadian rhythms with metabolism; however, unlike what has been shown for the SCN and liver, the majority of the metabolic transcripts cycling in skeletal muscle are involved in lipid metabolism and homeostasis (see Table 3) (2, 43). The cycling of genes important for lipid metabolism in skeletal muscle is not completely surprising given skeletal muscle is one of the most metabolically demanding tissues in the body that relies heavily on fatty acids as a fuel source.
Table 3.
Circadian genes involved with lipid metabolism
| Gene Symbol | Description | Reference No. |
| Rora | transcription factor: control of lipid homeostasis in skeletal muscle | 31 |
| _Rev-erb_β | transcription factor: lipid and energy homeostasis in skeletal muscle | 47 |
| _PGC-1_β | transcription factor: regulates expression MCAD, key enzyme in FA oxidation | 20 |
| Myod1 | transcription factor: regulates expression of UCP3 | 58 |
| Ucp3 | transporter: facilitates FA oxidation | 37 |
| Pank1 | enzyme: catalyze rate-limiting step in CoA synthesis | 49 |
| Dbt | enzyme: catalyze conversion of a-keto acids into acyl-CoA | 10 |
| Dgat2 | enzyme: catalyze final step of triglyceride synthesis | 9 |
| Acat2 | enzyme: involved in cholesterol absorption and synthesis | 4 |
| Idh1 | enzyme: provide NADPH for cholesterol and FA synthesis | 55 |
| Ces3 | enzyme: hydrolysis of triglycerides | 59 |
| Pnpla3 | enzyme: triacylglycerol lipase; | 19 |
| S3–12 | coat protein: involved in packaging of triglycerides | 69 |
| Fabp9 | predicted FA binding protein | |
A number of the circadian genes involved in lipid homeostasis are transcription factors that have also been shown to regulate the fidelity of the molecular core clock. Studies have demonstrated the nuclear receptors Rora and Rev-Erbα/β function as a transcriptional activator and repressor, respectively, of Bmal1, an essential member of the core clock (16, 52). Other studies have shown that, independent of their circadian function, Rora and Rev-Erbβ are master regulators of lipid homeostasis in skeletal muscle (31, 47). It may be, as was recently suggested, that these nuclear receptors are part of a gene regulatory network that coordinates lipid metabolism and circadian rhythms within skeletal muscle (48).
The _PGC-1α._-related gene, PGC-1β, showed a circadian oscillation in expression. PGC-1β is involved in the regulation of lipid metabolism by functioning as a ligand for ERR (estrogen-related receptor) in the activation of medium chain acyl CoA dehydrogenase (MCAD) gene transcription (20, 56). MCAD has a pivotal role in lipid metabolism as it serves as the initial step in mitochondrial fatty acid β-oxidation (23). In transgenic mice that overexpress PGC-1β, skeletal muscle had elevated levels of MCAD and the mice were resistant to diet-induced obesity (20).
Evidence from mice with a targeted deletion of PGC-1 β clearly demonstrates the importance of PGC-1β in skeletal muscle (33). Stereological analysis of soleus muscle from 12-wk-old wild-type and PGC-1β null mice showed mitochondrial volume was down 18% in the knockout, which resulted in a reduction in state 3 and 4 mitochondrial respiration and decreased Cox4 and Cox5b gene expression. These results are very similar, but more mild, to the mitochondrial phenotype seen in the _Bmal1_-/- and Clock mutant mice (J. L. Andrews and K. A. Esser, unpublished observations). In these clock-compromised mice, mitochondrial volume was reduced by 40%, and, in the Clock mutant, decreased expression of mRNA for complexes IV, VI, and VII of the electron transport chain were found (Cox4i1, Cox6a2, Cox6b2, Cox7a2l, Cox7b, and Cox7c; see Supplemental Table S6). The more severe mitochondrial phenotype in the Clock mutant compared with the PGC-1β knockout may be the result of a loss of PGC-1β cycling (see Fig. 4) combined with a significant decrease in PGC-1α. expression; PGC-1α. expression was reduced by 56% in the Clock mutant (Supplemental Table S6).
Fig. 4.
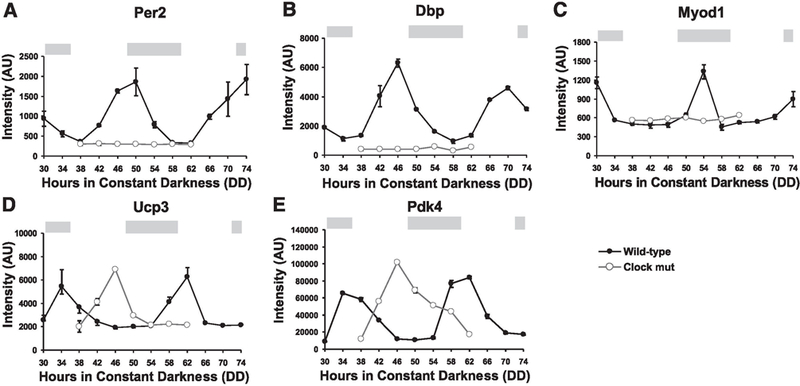
Altered expression of circadian genes in Clock mutant. Approximately 30% of the circadian genes in the Clock mutant showed a loss in cycling or a phase shift in peak expression. Graphs comparing wild-type vs. Clock mutant expression shows a loss of cycling (A–C) or a shift in the time of peak expression (D, E). The absence of circadian oscillation in the Clock mutant is shown for Per2 (A), Dbp (B), and Myod1 (C), three known clock-controlled genes. A phase shift in peak expression in the Clock mutant is shown for Ucp3 (D) and Pdk4 (E), both muscle-enriched circadian genes. The light gray bars across the top of each graph represent the presumptive night with the intervening white spaces representing presumptive day.
The expression of the muscle-specific transporter Ucp3 was also found to have a circadian oscillation. Studies to date suggest that Ucp3 acts to facilitate fatty acid oxidation in skeletal muscle cells (37). Myod1 may be involved in the regulation of Ucp3 circadian expression given that Ucp3 peak expression occurs 8 h after peak Myod1 expression (Supplemental Table S4). This notion is further supported by the finding that Myod1 is capable of activating Ucp3 transcription in a peroxisome proliferator-activated receptor-dependent manner (58). Finally, in the Clock mutant, Ucp3 expression is dramatically phase shifted, suggesting circadian expression of Myod1 is required for proper phasing of Ucp3 peak expression (Fig. 4D). If Myod1 is involved in the circadian regulation of Ucp3 expression, this indicates that it is the phasic/peak expression of Myod1 that is critical as there was no difference in the average expression of Myod1 in muscle of wild-type and Clock mutant mice.
The remaining circadian genes involved in lipid metabolism are enzymes that catalyze the synthesis or hydrolysis of fatty acids (Table 3). Pantothenate kinase (Pank), Dbt, Dgat2, Acat2, S3–12, and Idh1 have all been shown to promote the synthesis of fatty acids and/or cholesterol (4, 10, 49, 55, 69). The intracellular concentration of CoA, an essential cofactor in long-chain fatty acid metabolism, is primarily regulated by Pank1, which catalyzes what is considered to be the first rate-limiting step in CoA biosynthesis (49). Dbt is a subunit of the branched-chain α-keto dehydrogenase complex that catalyzes the conversion of α-keto acids to acyl-CoA, which, in turn, Dgat2 uses with diacylglycerol to synthesize triglycerides (9, 10). In contrast, Ces3 and Pnpla3 (adiponutrin) catalyze the hydrolysis of intracellular stores of triglycerides (19, 59). Curiously, Dgat2, Ces3, and Pnpla3 all have peak expression at CT18 (midphase of subjective night), suggesting there is an important need to balance the production and utilization of triglycerides during this period of high levels of physical activity.
Analysis of gene expression in Clock mutant.
To determine the effect of a compromised core clock on gene expression in skeletal muscle, DNA microarray analysis of skeletal muscle from the Clock mutant mouse was performed. The Clock mutation is a dominant negative (antimorphic) mutation that deletes exon 19 and causes an internal deletion of 51aa in the COOH-terminal activation domain of the CLOCK protein. (14, 27). To obtain a more complete picture of how skeletal muscle gene expression was affected in the Clock mutant, we examined expression profiles for both cycling and noncycling genes. Cycling genes with altered expression in the Clock mutant represent potential CCGs, whereas a change in the expression of a noncycling gene suggests the gene may be regulated by a CCG, whose expression itself has been altered in the Clock mutant.
A survey of circadian gene expression in the Clock mutant revealed ~30% of the genes were either no longer cycling or their phase was shifted, with the remainder of genes showing no clear change in their pattern of expression. For example, Per2 and Dbp are established target genes of Clock whose expression became noncyclical in the Clock mutant (Fig. 4, A and B) (50, 63). In contrast, some genes in the Clock mutant still showed a daily oscillation but were phase shifted as illustrated by Ucp3 and Pdk4 (Fig. 4, C and D). The molecular mechanism underlying a phase shift in gene expression is not known but may be the result of altered feeding or activity behaviors in the Clock mutant mouse or may results from a change in expression of a clock-controlled transcription factor. These are complex issues of transcriptional regulation and remain to be experimentally tested.
A comparison of mean expression of noncircadian genes between wild-type and Clock mutant found 35% (3,146 genes out of 9,002 genes expressed) of the genes had a significant change in mean expression (P ≤ 0.001); 79% (2,473 genes) of the genes were significantly downregulation in mean expression in the Clock mutant compared with wild-type (Supplemental Table S6). In contrast, a total of 673 transcripts (21%) were significantly upregulated in the Clock mutant (Supplemental Table S7). Many of the downregulated transcripts in the Clock mutant were muscle-specific genes important for me-chanical function (Table 4). As shown in Table 4, many structural muscle genes such as actin, dystrophin, titin, myosin heavy chain IIx, and myosin heavy chain IIb had decreased expression in the Clock mutant. In addition, components of the thin filament regulatory complex, troponin I and troponin C, were also downregulated. These losses in expression were accompanied by a similar reduction in expression of important metabolic genes like muscle creatine kinase, phosphofructokinase, and several members of the mitochondrial ATP synthase F0/F1 complex (Table 4). The misregulation of these highly expressed muscle genes is consistent with the loss of specific tension observed in the EDL muscle of the Clock mutant (J. L. Andrews and K. A. Esser, unpublished observations).
Table 4.
Genes down regulated in Clock mutant important for muscle function and phenotype
| Gene Symbol | Description | % Change |
| Structural | ||
| Acta | actin, α1, skeletal muscle | −27 |
| Dag1 | dystroglycan1 | −34 |
| Dmd | dystrophin | −52 |
| Sgce | sarcoglycan, ε | −31 |
| Sgcg | sarcoglycan, γ | −34 |
| Tmod1 | tropomodulin 1 | −62 |
| Tnnc2 | troponin C2, fast | −28 |
| Tnni2 | troponin 1, skeletal, fast 2 | −30 |
| Tpm1 | tropomyosin 1, α | −50 |
| Ttn | titin | −40 |
| Myh1 | myosin, heavy polypeptide 1, skeletal muscle, adult (2X) | −49 |
| Myh4 | myosin, heavy polypeptide 4, skeletal muscle, adult (2B) | −19 |
| Mylpf | myosin light chain, phosphorylatable, fast skeletal muscle (MLC-2) | −43 |
| Myom1 | myomesin 1 | −85 |
| Myom2 | myomesin 2 | −19 |
| Metabolism | ||
| Ckm | creatine kinase, muscle | −40 |
| Pfkm | phosphofructokinase, muscle | −29 |
| Ppargc1a | peroxisome proliferative activated receptor, γ, coactivator 1α (PGC-1α) | −56 |
| Atp5a1 | ATP synthase, mitochondrial F1 complex, α subunit, isoform 1 | −25 |
| Atp5b | ATP synthase, mitochondrial F1 complex, β subunit | −26 |
| Atp5c1 | ATP synthase, mitochondrial F1 complex, γ polypeptide 1 | −51 |
| Atp5e | ATP synthase, mitochondrial F1 complex, ε subunit | −40 |
| Atp5g1 | ATP synthase, mitochondrial F0 complex, subunit c, isoform 1 | −25 |
| Atp5j2 | ATP synthase, mitochondrial F0 complex, subunit f, isoform 2 | −46 |
| Atp5k | ATP synthase, mitochondrial F1F0 complex, subunit e | −38 |
| Atp5o | ATP synthase, mitochondrial F1 complex, O subunit | −44 |
| Calcium handling | ||
| Atp2a1 | ATPase, Ca2+ transporting, cardiac muscle, fast twitch 1 | −26 |
| Calm1 | calmodulin 1 | −17 |
| Calm2 | calmodulin 2 | −47 |
| Calm3 | calmodulin 3 | −33 |
| Pvalb | parvalbumin | −26 |
The GOToolBox resource was used to determine if any biological processes were over- or underrepresented in the data set comprising transcripts with altered expression in the Clock mutant (38). By using the genomic frequency of each GO term as a reference, the program performs an analysis to identify functional clusters of GO terms significantly (P < 0.01) enriched or depleted within a data set. The most notable enriched biological process was muscle contraction, which was consistent with the large number of contractile genes underexpressed in Clock mutant skeletal muscle. Most of the other enriched biological processes identified were concerned with protein metabolism (protein biosynthesis, protein folding, and ribosome biogenesis) and substrate utilization (carbohydrate metabolism and β-oxidation). The overrepresentation of these processes suggests deficiencies in synthesis and energy metabolism, resulting from a loss in gene expression, may be the primary cause for the decreased contractile gene expression observed in the Clock mutant. Surprisingly, for genes upregulated in the Clock mutant, no GO terms were significantly enriched or depleted.
Supplementary Material
S3
ACKNOWLEDGMENTS
Present addresses: J. L. Andrews, Eli Lilly, Indianapolis, IN 46285; J. B. Hogenesch, Dept. of Pharmacology, Institute Translational Medicine and Therapeutics, Univ. of Pennsylvania School of Medicine, Philadelphia, PA 19104; K. A. Esser, Dept. of Physiology, Univ. of Kentucky, Lexington, KY 40536.
GRANTS
This work was supported by the Novartis Research Foundation (J. B. Hogenesch and J. R. Walker), a predoctoral National Research Service Award from the National Institute of Neurological Disorders and Stroke (to B. H. Miller), National Institutes of Health (NIH) Grants AR-050717 (to K. A. Esser) and U01 MH-61915 (to J. S. Takahashi), and Silvio O. Conte Center NIH Grant P50 MH-074924 (to J. S. Takahashi). J. S. Takahashi is an Investigator and E. L. McDearmon is a Research Associate in the Howard Hughes Medical Institute.
Footnotes
1
The online version of this article contains supplemental material.
REFERENCES
- 1.Akashi M, Takumi T. The orphan nuclear receptor RORalpha regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol 12: 441–448, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol 12: 540–550, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson RA, Joyce C, Davis M, Reagan JW, Clark M, Shelness GS, Rudel LL. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J Biol Chem 273: 26747–26754, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Beatham J, Romero R, Townsend SK, Hacker T, van der Ven PF, Blanco G. Filamin C interacts with the muscular dystrophy KY protein and is abnormally distributed in mouse KY deficient muscle fibres. Hum Mol Genet 13: 2863–2874, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Blanco G, Coulton GR, Biggin A, Grainge C, Moss J, Barrett M, Berquin A, Marechal G, Skynner M, van Mier P, Nikitopoulou A, Kraus M, Ponting CP, Mason RM, Brown SD. The kyphoscoliosis (ky) mouse is deficient in hypertrophic responses and is caused by a mutation in a novel muscle-specific protein. Hum Mol Genet 10: 9–16, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG Jr. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev 18: 2893–2904, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, Farese RV Jr. Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J Biol Chem 276: 38870–38876, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Chuang DT, Hu CC, Ku LS, Niu WL, Myers DE, Cox RP. Catalytic and structural properties of the dihydrolipoyl transacylase component of bovine branched-chain alpha-keto acid dehydrogenase. J Biol Chem 259: 9277–9284, 1984. [PubMed] [Google Scholar]
- 11.Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem 280: 9769–9772, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Dentice M, Bandyopadhyay A, Gereben B, Callebaut I, Christoffolete MA, Kim BW, Nissim S, Mornon JP, Zavacki AM, Zeold A, Capelo LP, Curcio-Morelli C, Ribeiro R, Harney JW, Tabin CJ, Bianco AC. The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol 7: 698–705, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Florea L, Hartzell G, Zhang Z, Rubin GM, Miller W. A computer program for aligning a cDNA sequence with a genomic DNA sequence. Genome Res 8: 967–974, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280: 1564–1569, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98: 14440–14445, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillaumond F, Dardente H, Giguere V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms 20: 391–403, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Heuze ML, Guibal FC, Banks CA, Conaway JW, Conaway RC, Cayre YE, Benecke A, Lutz PG. ASB2 is an Elongin BC-interacting protein that can assemble with Cullin 5 and Rbx1 to reconstitute an E3 ubiquitin ligase complex. J Biol Chem 280: 5468–5474, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Hubbell E, Liu WM, Mei R. Robust estimators for expression analysis. Bioinformatics 18: 1585–1592, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem 279: 48968–48975, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Kamei Y, Ohizumi H, Fujitani Y, Nemoto T, Tanaka T, Takahashi N, Kawada T, Miyoshi M, Ezaki O, Kakizuka A. PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc Natl Acad Sci USA 100: 12378–12383, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamura T, Burian D, Yan Q, Schmidt SL, Lane WS, Querido E, Branton PE, Shilatifard A, Conaway RC, Conaway JW. Muf1, a novel Elongin BC-interacting leucine-rich repeat protein that can assemble with Cul5 and Rbx1 to reconstitute a ubiquitin ligase. J Biol Chem 276: 29748–29753, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Kedar V, McDonough H, Arya R, Li HH, Rockman HA, Patterson C. Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl Acad Sci USA 101: 18135–18140, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly DP, Kim JJ, Billadello JJ, Hainline BE, Chu TW, Strauss AW. Nucleotide sequence of medium-chain acyl-CoA dehydrogenase mRNA and its expression in enzyme-deficient human tissue. Proc Natl Acad Sci USA 84: 4068–4072, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kendziorski CM, Zhang Y, Lan H, Attie AD. The efficiency of pooling mRNA in microarray experiments. Biostatistics 4: 465–477, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Kent WJ. BLAT–the BLAST-like alignment tool. Genome Res 12: 656–664, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerlavage A, Bonazzi V, di Tommaso M, Lawrence C, Li P, Mayberry F, Mural R, Nodell M, Yandell M, Zhang J, Thomas P. The Celera discovery system. Nucleic Acids Res 30: 129–136, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian clock gene. Cell 89: 641–653, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kita Y, Shiozawa M, Jin W, Majewski RR, Besharse JC, Greene AS, Jacob HJ. Implications of circadian gene expression in kidney, liver and the effects of fasting on pharmacogenomic studies. Pharmacogenetics 12: 55–65, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet 15, _Spec No_2: R271–R277, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Kohroki J, Nishiyama T, Nakamura T, Masuho Y. ASB proteins interact with Cullin5 and Rbx2 to form E3 ubiquitin ligase complexes. FEBS Lett 579: 6796–6802, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Lau P, Nixon SJ, Parton RG, Muscat GE. RORalpha regulates the expression of genes involved in lipid homeostasis in skeletal muscle cells: caveolin-3 and CPT-1 are direct targets of ROR. J Biol Chem 279: 36828–36840, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Le Borgne R, Remaud S, Hamel S, Schweisguth F. Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS Biol 3: e96, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, Zhang D, Litwin SE, Zaha VG, Fountain KT, Boudina S, Jimenez-Linan M, Blount M, Lopez M, Meirhaeghe A, Bohlooly YM, Storlien L, Stromstedt M, Snaith M, Oresic M, Abel ED, Cannon B, Vidal-Puig A. Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol 4: e369, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol 47: 593–628, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Lockhart DJ, Dong H, Byrne MC, Follettie MT, Gallo MV, Chee MS, Mittmann M, Wang C, Kobayashi M, Horton H, Brown EL. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat Biotechnol 14: 1675–1680, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet 5: 407–441, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacLellan JD, Gerrits MF, Gowing A, Smith PJ, Wheeler MB, Harper ME. Physiological increases in uncoupling protein 3 augment fatty acid oxidation and decrease reactive oxygen species production without uncoupling respiration in muscle cells. Diabetes 54: 2343–2350, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Martin D, Brun C, Remy E, Mouren P, Thieffry D, Jacq B. GOToolBox: functional analysis of gene datasets based on Gene Ontology. Genome Biol 5: R101, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martino T, Arab S, Straume M, Belsham DD, Tata N, Cai F, Liu P, Trivieri M, Ralph M, Sole MJ. Day/night rhythms in gene expression of the normal murine heart. J Mol Med 82: 256–264, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, Andrews JL, Antoch MP, Walker JR, Esser KA, Hogenesch JB, Takahashi JS. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci USA 104: 3342–3347, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noshiro M, Furukawa M, Honma S, Kawamoto T, Hamada T, Honma K, Kato Y. Tissue-specific disruption of rhythmic expression of Dec1 and Dec2 in clock mutant mice. J Biol Rhythms 20: 404–418, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, Kondo S, Nikaido I, Osato N, Saito R, Suzuki H, Yamanaka I, Kiyosawa H, Yagi K, Tomaru Y, Hasegawa Y, Nogami A, Schonbach C, Gojobori T, Baldarelli R, Hill DP, Bult C, Hume DA, Quackenbush J, Schriml LM, Kanapin A, Matsuda H, Batalov S, Beisel KW, Blake JA, Bradt D, Brusic V, Chothia C, Corbani LE, Cousins S, Dalla E, Dragani TA, Fletcher CF, Forrest A, Frazer KS, Gaasterland T, Gariboldi M, Gissi C, Godzik A, Gough J, Grimmond S, Gustincich S, Hirokawa N, Jackson IJ, Jarvis ED, Kanai A, Kawaji H, Kawasawa Y, Kedzierski RM, King BL, Konagaya A, Kurochkin IV, Lee Y, Lenhard B, Lyons PA, Maglott DR, Maltais L, Marchionni L, McKenzie L, Miki H, Nagashima T, Numata K, Okido T, Pavan WJ, Pertea G, Pesole G, Petrovsky N, Pillai R, Pontius JU, Qi D, Ramachandran S, Ravasi T, Reed JC, Reed DJ, Reid J, Ring BZ, Ringwald M, Sandelin A, Schneider C, Semple CA, Setou M, Shimada K, Sultana R, Takenaka Y, Taylor MS, Teasdale RD, Tomita M, Verardo R, Wagner L, Wahlestedt C, Wang Y, Watanabe Y, Wells C, Wilming LG, Wynshaw-Boris A, Yanagisawa M, Yang I, Yang L, Yuan Z, Zavolan M, Zhu Y, Zimmer A, Carninci P, Hayatsu N, Hirozane-Kishikawa T, Konno H, Nakamura M, Sakazume N, Sato K, Shiraki T, Waki K, Kawai J, Aizawa K, Arakawa T, Fukuda S, Hara A, Hashizume W, Imotani K, Ishii Y, Itoh M, Kagawa I, Miyazaki A, Sakai K, Sasaki D, Shibata K, Shinagawa A, Yasunishi A, Yoshino M, Waterston R, Lander ES, Rogers J, Birney E, Hayashizaki Y. Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature 420: 563–573, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Peng X, Wood CL, Blalock EM, Chen KC, Landfield PW, Stromberg AJ. Statistical implications of pooling RNA samples for microarray experiments. BMC Bioinformatics 4: 26, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pisani DF, Leclerc L, Jarretou G, Marini JF, Dechesne CA. SMHS1 is involved in oxidative/glycolytic-energy metabolism balance of muscle fibers. Biochem Biophys Res Commun 326: 788–793, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Pruitt KD, Maglott DR. RefSeq and LocusLink: NCBI gene-centered resources. Nucleic Acids Res 29: 137–140, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramakrishnan SN, Lau P, Burke LJ, Muscat GE. Rev-erbbeta regulates the expression of genes involved in lipid absorption in skeletal muscle cells: evidence for cross-talk between orphan nuclear receptors and myokines. J Biol Chem 280: 8651–8659, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Ramakrishnan SN, Muscat GE. The orphan Rev-erb nuclear receptors: a link between metabolism, circadian rhythm and inflammation? Nucl Recept Signal 4: e009, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramaswamy G, Karim MA, Murti KG, Jackowski S. PPARalpha controls the intracellular coenzyme A concentration via regulation of PANK1alpha gene expression. J Lipid Res 45: 17–31, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet 38: 369–374, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Sangoram AM, Saez L, Antoch MP, Gekakis N, Staknis D, Whiteley A, Fruechte EM, Vitaterna MH, Shimomura K, King DP, Young MW, Weitz CJ, Takahashi JS. Mammalian circadian autoregulatory loop: a timeless ortholog and mPer1 interact and negatively regulate CLOCKBMAL1-induced transcription. Neuron 21: 1101–1113, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, McNamara P, Naik KA, FitzGerald GA, Kay SA, Hogenesch JB. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 43: 527–537, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Schibler U The daily rhythms of genes, cells and organs. Biological clocks and circadian timing in cells. EMBO Rep 6, Spec No: S9–13, 2005. [DOI] [PMC free article] [PubMed]
- 54.Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF Jr, Reppert SM. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 19: 1261–1269, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Shechter I, Dai P, Huo L, Guan G. IDH1 gene transcription is sterol regulated and activated by SREBP-1a and SREBP-2 in human hepatoma HepG2 cells: evidence that IDH1 may regulate lipogenesis in hepatic cells. J Lipid Res 44: 2169–2180, 2003. [DOI] [PubMed] [Google Scholar]
- 56.Sladek R, Bader JA, Giguere V. The orphan nuclear receptor estrogenrelated receptor alpha is a transcriptional regulator of the human mediumchain acyl coenzyme A dehydrogenase gene. Mol Cell Biol 17: 5400–5409, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sofer A, Lei K, Johannessen CM, Ellisen LW. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol 25: 5834–5845, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Solanes G, Pedraza N, Iglesias R, Giralt M, Villarroya F. Functional relationship between MyoD and peroxisome proliferator-activated receptor-dependent regulatory pathways in the control of the human uncoupling protein-3 gene transcription. Mol Endocrinol 17: 1944–1958, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Soni KG, Lehner R, Metalnikov P, O’Donnell P, Semache M, Gao W, Ashman K, Pshezhetsky AV, Mitchell GA. Carboxylesterase 3 (EC 3.1.1.1) is a major adipocyte lipase. J Biol Chem 279: 40683–40689, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature 417: 78–83, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Straume M DNA microarray time series analysis: automated statistical assessment of circadian rhythms in gene expression patterning. Methods Enzymol 383: 149–166, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogen-esch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA 101: 6062–6067, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci USA 99: 7728–7733, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, Hashimoto S. A transcription factor response element for gene expression during circadian night. Nature 418: 534–539, 2002. [DOI] [PubMed] [Google Scholar]
- 65.Vissing K, Andersen JL, Schjerling P. Are exercise-induced genes induced by exercise? FASEB J 19: 94–96, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, Dove WF, Pinto LH, Turek FW, Takahashi JS. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 264: 719–725, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker JR, Hogenesch JB. RNA profiling in circadian biology. Methods Enzymol 393: 366–376, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Woelfle MA, Ouyang Y, Phanvijhitsiri K, Johnson CH. The adaptive value of circadian clocks: an experimental assessment in cyanobacteria. Curr Biol 14: 1481–1486, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Wolins NE, Quaynor BK, Skinner JR, Schoenfish MJ, Tzekov A, Bickel PE. S3–12, Adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem 280: 19146–19155, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. Nuclear receptor expression links the circadian clock to metabolism. Cell 126: 801–810, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Zambon AC, McDearmon EL, Salomonis N, Vranizan KM, Johansen KL, Adey D, Takahashi JS, Schambelan M, Conklin BR. Timeand exercise-dependent gene regulation in human skeletal muscle. Genome Biol 4: R61, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng B, Larkin DW, Albrecht U, Sun ZS, Sage M, Eichele G, Lee CC, Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400: 169–173, 1999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S3