Interleukin-2 Receptor Signaling in Regulatory T Cell Development and Homeostasis (original) (raw)
. Author manuscript; available in PMC: 2008 Nov 30.
Abstract
Interleukin-2 (IL2) was initially identified from supernatants of activated lymphocytes over 30 years ago [1]. In the ensuing 15 years, the cDNAs for both IL2 and the three chains of the interleukin-2 receptor (IL2R) were cloned [2–7]. Subsequently, many of the downstream biochemical pathways activated by the IL2 receptor complex were identified [8] and the structure of IL2 bound to this tripartite receptor complex was solved [9]. Thus, we now have a very good understanding of how each chain contributes to high affinity IL2 binding and signal transduction [10,11]. In contrast, over the past 30 years the role that IL2 plays in regulating lymphocyte function has involved many surprising twists and turns. For example, IL2 has been shown, paradoxically, to regulate both lymphocyte proliferation and lymphocyte death. In this review, we briefly outline the original findings suggesting a role for IL2 as a T cell growth factor, as well as subsequent studies pointing to its function as an initiator of Activation-induced Cell Death, but then focus on the newly appreciated role for IL2 and IL2R signaling in the development and homeostasis of regulatory T cells.
Interleukin-2 plays an important role in multiple aspects of T cell biology. Binding of IL2 to its receptor was initially demonstrated to be critical for inducing the proliferation of T cells in vitro [12]. Subsequent studies demonstrated that IL2R signaling leads to the activation of many genes associated with cell proliferation such as c-myc and fos [13]. These findings led to the idea that signaling via the IL2R complex would be critical for mounting effective immune responses in vivo and established IL2 as a T cell growth factor. However, the concept of IL2 as a critical factor required for T cell proliferation was essentially overturned in the early 1990s when mice lacking the IL2 gene were first developed. The initial characterization of these mice demonstrated that in vitro proliferation of ConA-stimulated _IL2_−/− T cells was impaired, but much more modestly than one would have predicted, suggesting the presence of alternative T cell growth factors [14]. It also was readily apparent that in vivo, T cell proliferation was clearly not impaired; _IL2_−/− mice exhibited massive lympho-proliferation resulting in splenomegaly and lymphoadenopathy. In addition, _IL2_−/− mice rapidly developed signs of autoimmunity including, most prominently, a disease resembling ulcerative colitis [15]. These autoimmune manifestations ultimately resulted in death of 100% of _IL2_−/− mice between 12–25 weeks of age. The reasons for this surprising onset of autoimmunity were at first unclear, but one potential explanation was provided by Michael Leonardo who demonstrated that IL2 plays an important role in sensitizing T cells for Activation-induced cell death [16]. When mice lacking the IL2Rα or IL2Rβ chain were generated, they too exhibited multi-organ autoimmune disease resulting in early death [17,18]. Moreover, examination of _IL2Rα_−/− mice demonstrated a significant defect in activation-induced cell death [17] suggesting that the primary mechanism that accounted for deregulated T cell homeostasis, and the onset of autoimmunity, was a failure of IL2-induced T cell death.
The data suggesting that IL2 prevents autoimmunity via an effect on activation-induced cell death was compelling. However, new discoveries regarding a novel subset of CD4+ T cells began to shed doubt on whether the failure of IL2-induced T cell death was sufficient to account for the defects seen in IL2 deficient mice. Specifically, in the mid 1990s Sakaguchi and colleagues identified a population of CD4+ T cells that expressed high levels of CD25 and potently suppressed the proliferation of activated T cells in vitro. Moreover, they found that injection of CD4+CD25− T cells into T-cell depleted host mice rapidly induced autoimmune disease; importantly, disease could be prevented or even reversed by the transfer of CD4+CD25+ T cells [19]. These studies established CD4+CD25+ T cells as the elusive suppressor T cells, more commonly referred to now as regulatory T cells (or Tregs), that were initially proposed over 35 years ago [20,21]. The discovery of this novel T cell subset characterized by high level IL2R expression, in conjunction with the observation that _IL2_−/− and _IL2Rβ_−/− mice lacked CD4+CD25+ T cells, suggested that IL2-dependent signals were required for either Treg development, homeostasis, or function. Subsequent studies aimed at identifying genes involved in autoimmune disease have found distinct alleles of IL2 or IL2Rα associated with autoimmunity and defects in Tregs. For example, the insulin-dependent diabetes susceptibility locus 3 (idd3) in the NOD mouse has been shown to map to the IL2 gene; importantly, Tregs from susceptible mice were found to have decreased regulatory T cell function [22]. Likewise, the idd4 locus in NOD mice has been suggested to map to alterations in the downstream IL2 effector STAT5 [23]. Finally, studies of human diabetes, multiple sclerosis and Graves’ Disease have been mapped to distinct alleles for IL2Rα [24–26]. These observations led to an explosive interest in regulatory T cells resulting in significant insights to all aspects of Treg biology. In the ensuing few pages we will specifically focus on the role IL2 plays in the differentiation and homeostasis of so-called natural Tregs that develop in the thymus.
Role of IL2 in the Development of CD4+CD25+Foxp3+Tregs
Seminal studies from three separate labs eventually established that the critical defect in IL2- or IL2R-deficient mice resulted from the absence of functional Tregs [27–30]. Compellingly, Malek and colleagues demonstrated that transfer of limited numbers of CD4+CD25+ T cells into _IL2Rβ_−/− mice prevented autoimmune disease. These findings clearly demonstrated that the defect in _IL2Rβ_−/− mice was not cell autonomous, but rather due to the absence of a functional Treg population [28]. These studies pointed to a critical role for the IL2R in Treg biology. However, whether the effect of the IL2R was on Treg development, homeostasis, or function remained unclear and has been quite controversial.
Malek and colleagues initially proposed that the IL2R was uniquely required for Treg development. Specifically, they developed a transgenic mouse model in which transcription of the _IL2Rβ_-chain was driven by the _lck_-proximal promoter, resulting in a thymic-restricted expression pattern. Crossing this transgene onto the _IL2Rβ_−/− background restored a population of CD4+CD25+ T cells in the thymus and periphery that suppressed lymphoproliferation and the lethal autoimmune disease normally observed in _IL2Rβ_−/− mice [31]. These findings suggested that IL2Rβ-dependent signals were required for Treg development in the thymus, but were dispensable for their homeostasis and function in the periphery. Subsequent studies in which neutralizing antibodies to IL2 were injected into neonatal mice demonstrated a reduction in numbers of CD4+CD25+ Tregs in the thymus [32], further supporting a role for IL2 in Treg development. In contrast, work by LaFaille and colleagues demonstrated that both splenocytes and purified CD4+CD8− thymocytes from _IL2_−/− mice could suppress the induction of disease in a mouse model of EAE [33]. This effect was not observed when CD4+ T cells were obtained from _IL2Rα_−/− mice. These studies indicated that there is a population of CD4+ T cells in both the thymus and spleen of _IL2_−/− mice that can exert regulatory activity when IL2 is restored. The initial explanation for these findings was that IL2 was required for Treg function. However, an alternative possibility was that IL2 was required for Treg homeostasis and that potentially reduced numbers of Tregs found in _IL2_−/− mice were rapidly expanded following transfer into IL2-competent host mice. Thus, early studies on the role of IL2 and the IL2R in Treg development led to apparently contradictory findings.
A major problem with the initial studies on the role of IL2 in Treg development was the use of CD25 as a surrogate marker for this cell population. Although relatively selective, CD25 is also upregulated on activated effector T cells [34]. Moreover, it was not clear whether all Tregs would be found within the CD25+ subset of CD4+ T cells. To more accurately dissect the role of IL2 in Treg biology, a unique marker was needed that could be utilized to track developing regulatory T cells in the thymus and mature Tregs in the periphery. Recent pioneering work by several groups identified a member of the forkhead-box family of transcription factors called Foxp3 which fulfills such a role. Specifically, the naturally occurring scurfy mouse mutant, which develops lethal multi-organ autoimmune disease, was found to have mutations in the foxp3 gene that were predicted to disrupt Foxp3 function; restoration of wild type Foxp3 in these mice prevented disease [35]. Likewise, the genetic defect in human patients suffering from the autoimmune disease IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) were found to stem from mutations in the human foxp3 gene [36,37]. Subsequent studies by the Ramsdell, Rudensky and Sakaguchi labs independently demonstrated that Foxp3 was both necessary and sufficient for Treg development [38–40]. Importantly, these studies also established Foxp3 as a unique marker for Tregs in the mouse. More recently, the Rudensky and Flavell labs generated mouse models in which cells that express Foxp3 can be directly identified using the fluorescent proteins GFP or DsRed respectively [41,42]. Using these mouse models and recent advances in intracellular flow cytometry for Foxp3, the controversial role of IL2 and IL2R signaling in Treg development and homeostasis is beginning to be clarified.
Using a Foxp3-GFP fusion knock-in mouse model Fontenot et al observed that CD4+Foxp3+ cells were clearly present in _IL2_−/− mice, although they failed to express CD25. Identical results were obtained in _IL2Rα_−/− mice. In both _IL2_−/− and _IL2Rα_−/− mice, numbers of thymic Tregs in young animals were slightly reduced (~2-fold) while peripheral Tregs were present in relatively normal numbers [43]. Similar conclusions were simultaneously reached by D’Cruz and Klein using a transgenic TCR system and intracellular staining for Foxp3 protein. In these studies, they found that thymic selection into the Treg lineage was not impaired in _IL2_−/− mice, but the peripheral maintenance of these cells was dramatically affected [44]. Although the studies by Fontenot et al and D’Cruz and Klein appeared to contradict the original findings of Malek and colleagues regarding the role of IL2Rβ in Treg development, more recent work examining Tregs in _IL2Rβ_−/− mice appears to have resolved this discrepancy. Specifically, using intracellular staining for Foxp3 we too have found that young _IL2_−/− and _IL2Rα_−/− mice have relatively normal numbers of thymic and peripheral Tregs. In contrast, mice in which IL2Rβ signaling is disrupted have a more dramatic defect in the population of regulatory T cells in the thymus and secondary lymphoid organs [45]. Similar results using _IL2Rβ_−/− mice have recently been obtained by Ziegler and colleagues [46]. To examine this in more detail, we generated _IL2_−/− _x IL15_−/− mice and observed that they had a comparable decrease in regulatory T cell numbers to that seen in _IL2Rβ_−/− mice [45]. These results demonstrate that IL2 and IL15 act as redundant factors, which are both capable of regulating Treg development via interactions involving the IL2Rβ chain. Importantly, these results provide an explanation for the earlier apparently discrepant findings of Malek and Lafaille. Namely, IL2 by itself is not required for Treg development due to the redundant role of IL15. However, the IL2Rβ chain is required for efficient Treg development as it is a critical component of both the IL2 and IL15 receptors. Finally, it is important to point out that other γc-dependent cytokines, such as IL7, may allow for inefficient Treg development. This is based on the observation that while _IL2Rβ_−/− mice show a clear reduction in Tregs in both the thymus and peripheral lymphoid organs, a residual population of these cells can still be seen. In contrast, _γc_−/− mice are devoid of Tregs [43,45]. Taken together, these studies illustrate a predominant role for the IL2Rβ chain in driving the development of Tregs.
Role of IL2 in the Homeostasis of CD4+Foxp3+ Tregs
The initial findings that thymic restricted expression of the IL2Rβ chain was sufficient to restore peripheral Treg numbers suggested that IL2Rβ-dependent signals might not be required for homeostasis of peripheral Tregs [31]. A potential caveat to these studies was the observation that the IL2Rβ transgene was weakly expressed in peripheral T cells. IL2Rβ expression levels were sufficient for the induction of very transient STAT5 activation but, importantly, did not induce clear biological responses [47]. Perhaps more tellingly, when regulatory T cells from these mice were transferred into _IL2Rβ_−/− mice (which lack Tregs but do produce IL2) they did not survive nor did they prevent autoimmunity [28]. Subsequent studies found that mature Tregs in mice expressing the thymic-restricted IL2Rβ chain proliferated poorly when compared to WT Tregs. Mixed bone marrow chimera experiments, in which the ability of cells expressing the thymic-restricted IL2Rβ chain versus WT cells were compared, were also carried out. In those studies, cells expressing the thymic restricted IL2Rβ chain contributed well to the thymic Treg compartment but were not effective at repopulating peripheral lymphoid organs when compared to WT Tregs [48]. Taken together, these studies suggested that IL2Rβ-dependent signals are required for homeostasis of Tregs as well.
A remaining question is whether IL2, as opposed to the IL2R (which can bind both IL2 and IL15), is also required for Treg homeostasis. As mentioned above, relatively normal numbers of Tregs are found in both _IL2_−/− and _IL2Rα_−/− mice indicating that IL2 may not be critical for Treg homeostasis [43–45]. In addition, purified Tregs injected into _rag2_−/− mice underwent homeostatic proliferation that was not affected by IL2 neutralization, indicating that under lymphopenic conditions IL2 is not critical for Treg homeostasis [49]. However, it is important to note that Tregs do disappear in older _IL2_−/− and _IL2Rα_−/− mice (>8 weeks of age). Furthermore, gene microarray studies comparing Tregs from WT and _IL2_−/− mice suggested that IL2 plays a predominant role in regulating metabolic activity and survival of Tregs [43]. Supporting these findings, Setoguchi et al carried out studies in which they injected Balb/c mice with IL2 neutralizing antibodies. In these studies they found a modest reduction of CD4+CD25+ T cells in the thymus, but a profound reduction in CD4+CD25+ T cells in secondary lymphoid organs such as the spleen and lymph nodes. Furthermore, this reduction of CD4+CD25+ T cells resulted in the spontaneous induction of autoimmune gastritis in BALB/c mice; similarly, IL2 neutralization in NOD mice led to an exacerbation of the diabetes phenotype that naturally develops in these animals [49]. We have obtained similar results when injecting neutralizing IL2 antibodies into C57Bl/6 mice; peripheral CD4+Foxp3+ Treg numbers were clearly reduced. Importantly, however, we found that this treatment only affected the CD4+CD25+ subset of Tregs, suggesting that other cytokines may play a role in maintaining homeostasis of CD4+CD25−Foxp3+ Tregs (MAB and MAF, unpublished observations). Taken together we believe the available data suggest a model (Fig. 1) in which IL2 plays a critical role in governing normal homeostasis of Tregs. This most likely reflects the downregulation of the IL7Rα [50,51] and IL15Rα chain (KBV and MAF, submitted) on mature Tregs, which renders them more dependent on IL2 for survival and expansion. Importantly, we have found that Tregs in _IL2_−/−mice have upregulated expression of both the IL15Rα and IL7Rα chains (KBV and MAF, submitted); this most likely accounts for the ability of Tregs to survive (at least initially) in _IL2_−/− mice. Likewise, under lymphopenic conditions, IL15 and IL7 appear to be sufficient to maintain Treg homeostasis [49]. However, in wild type mice, in which Tregs express very low levels of the IL7Rα and IL15Rα chains and thus compete quite poorly with naïve and effector T cell subsets for endogenous IL7 and IL15, homeostasis of Tregs is predominately governed by IL2.
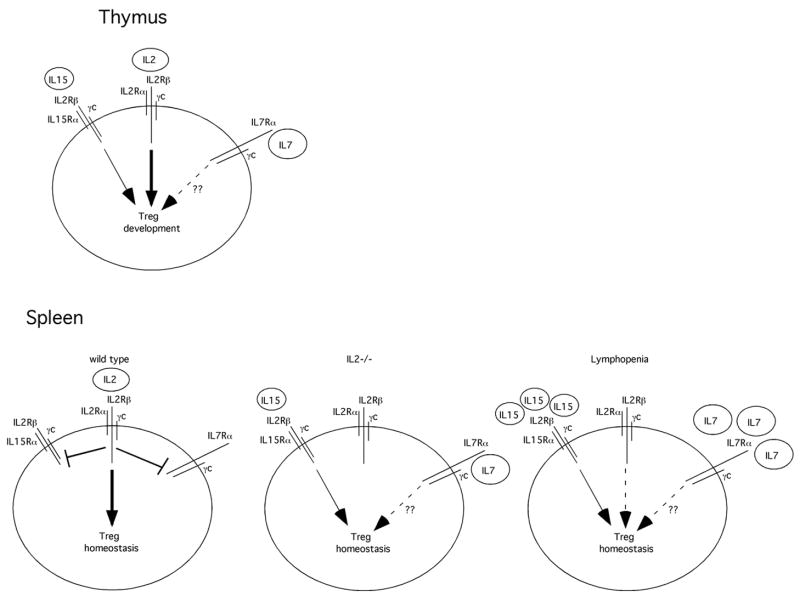
Figure 1. Model of Cytokine-dependent Treg Development and Homeostasis.
In the thymus, IL2 plays the predominant role in driving Treg development. In the absence of IL2, IL15 is sufficient for regulatory T cell development. Other γc-dependent cytokines such as IL7 may play a minor role in this process and most likely account for the few Tregs found in _IL2Rβ_−/− mice as compared to _γc_−/− mice in which Tregs are absent. In the spleen of wild type mice, IL2-dependent signals lead to the downregulation of both the IL7Rα and IL15Rα chains. This renders mature Tregs primarily dependent on IL2 for their survival and expansion. In contrast, in _IL2_−/− mice, IL15Rα and IL7Rα remain expressed at high levels and allow for survival of mature Tregs. Likewise, under lymphopenic conditions, such as occurs upon introduction of Tregs into _rag2_−/− mice, Tregs do survive and expand. This most likely occurs due the absence of other T cell subsets thereby leading to increased amounts of available IL15 and IL7 that can stimulate Treg survival despite lower expression of IL15Rα and IL7Rα receptors by those cells. In addition, the absence of IL2 signals may lead to re-expression of the IL15Rα and IL7Rα rendering these cells more sensitive to IL15 and IL7.
IL2R Signaling and Tregs
The IL2 receptor-signaling complex is composed of three distinct subunits, the α-chain (CD25), β-chain (CD122), and γ-chain (CD132) each of which have unique roles in facilitating IL2-dependent signal transduction. The IL2Rα chain has a very short cytoplasmic domain and does not transmit intracellular signals [52]. Instead, IL2Rα acts to confer high affinity binding of IL2 to the IL2R complex (Kd for IL2Rβ/γ c = 10−9 M versus 10−11 M for the IL2Rα/β/γc tripartite receptor)[10,34]. In contrast, the IL2Rβ and γc chains play the predominate role in transducing intracellular signals. Neither of these chains has intrinsic enzymatic function; rather, they associate with the intracellular tyrosine kinases Jak1 and Jak3, respectively [53–56]. Ligand binding results in the activation of Jak1 and Jak3 which then phosphorylate multiple tyrosine residues found in the cytoplasmic tail of the IL2Rβ chain [57]. Therefore, the primary component of the IL2R complex that functions to convert extracellular ligand binding into intracellular signals is the IL2Rβ chain.
Taniguchi and colleagues first identified three distinct regions of the IL2Rβ-chain that were critical for IL2-dependent signal transduction using expression of mutant IL2 receptor constructs in the mouse pro-B cell line BAF-BO3 [58]. These regions include a serine rich region, an acidic region, and a carboxy-terminal region, referred to as the S, A, and H regions, respectively (Fig. 2). Numerous studies have shown that the primary role of the S-region in IL2R signaling is binding of JAK-1 to conserved motifs referred to as box1 and box 2 [59,60]. The association of Jak-1 with the IL2Rβ chain, along with the association of JAK-3 with the γc chain, leads to phosphorylation of tyrosine residues in the A and H-regions following receptor activation. Thus, the primary role of the A- and H-regions in IL2R signaling is to serve as docking sites for SH2-domain-containing adaptor or effector molecules. Three of these intracellular tyrosine residues, which are conserved across multiple species, appear to play particularly important roles. The first of these is the most membrane-proximal tyrosine residue (Tyr-338 in the human receptor; Tyr-341 in the murine receptor). Several groups demonstrated that Tyr-338 is critical for the recruitment of the adaptor protein Shc to the receptor complex [61–63]. This leads to recruitment of the adaptor protein Grb2 and activation of the Ras/Raf/Mek/Erk signaling cascade. In addition, the adaptor protein Gab2 is also recruited via SHC, and results in the recruitment and activation of PI3K [64]. These findings indicate that the A region is utilized to induce MAPK and PI3K signaling pathways. In contrast, the H-region, which contains two tyrosine resides, Tyr-392 and Tyr-510, appears to be responsible for activation of STAT5. Expression of IL2R mutants lacking either the A or H regions demonstrated that either receptor was capable of preventing autoimmunity when introduced into _IL2Rβ_−/− T cells in vivo [65,66]. This led to the suggestion that the Ras/PI3K and STAT5 pathways were redundant with regard to Treg development and homeostasis. However, subsequent work cast some doubt on this interpretation. Specifically, the role of the tyrosine residues in both the A- and the H-regions were further dissected using IL2R constructs containing various tyrosine to phenylalanine mutations. Using this strategy, Gaffen et al demonstrated that the transcription factor STAT5 could be activated by three tyrosine residues in the IL2Rβ-chain (Tyr-338 in the A-region, Tyr-392, and Tyr-510 in the H-region)[67]. Consistent with the initial studies examining IL2R signal transduction, STAT5 appears to bind most avidly to phosphorylated Tyr-510 and to a lesser extent to Tyr-392. Supporting these findings, IL2Rβ mutants containing just Tyr-392 or Tyr-510 were capable of activating STAT5. However, although direct binding of STAT5 to Tyr-338 based phospho-peptides has not yet been demonstrated, an IL2Rβ-mutant containing just Tyr-338 was capable of activating STAT5. Thus, both the A- and H-regions are capable of activating STAT5 suggesting that perhaps STAT5 plays a key role in Treg development.
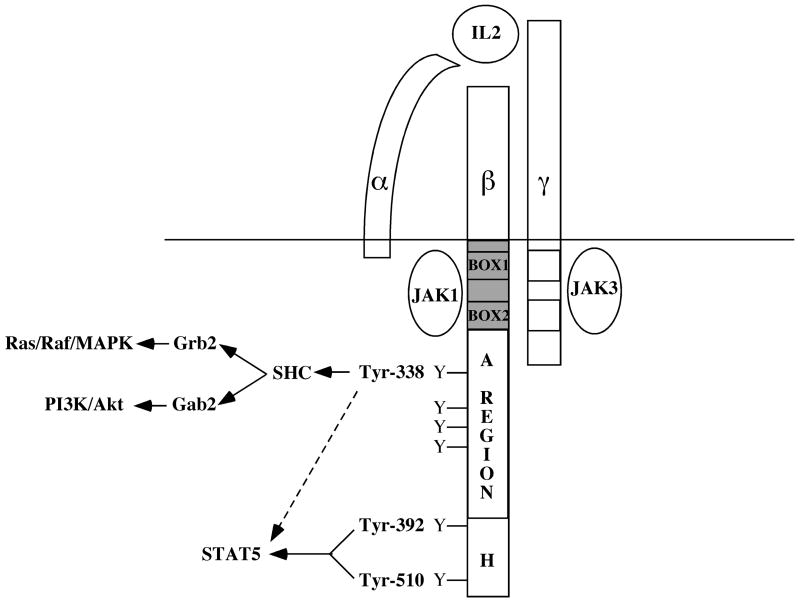
Figure 2. Signaling Components of the IL2 Receptor Complex.
The IL2R is composed of three distinct subunits - IL2Rα, IL2Rβ, and IL2Rγ. The IL2Rβ-chain consists of three previously identified signaling domains, the A-, H-, and S-regions. The tyrosine-containing A and H regions are labeled, while the JAK1 binding domain or S-region is shaded in gray. Phosphorylation of Tyr-338 in the A region recruits the adaptor protein SHC; SHC then binds Grb2 and Gab2, leading to activation of the Ras-Raf-MAPK and PI3K/Akt signaling pathways, respectively. Phosphorylation of Tyr-392 and Tyr-510 in the H region recruits STAT5 and leads to its activation; in the absence of these two tyrosine residues Tyr-338 can also induce STAT5 activation.
Given that IL2 induces multiple signal transduction pathways, it is important to know which of these pathways are actually active in Tregs. A key study by Bensinger et al demonstrated that activated CD4+CD25+ effector T cells induced the PI3K, Ras and STAT5 signaling pathways in response to IL2 stimulation. In contrast, freshly purified CD4+CD25+ Tregs exclusively activated STAT5 when stimulated with exogenous IL2 [68]. Supporting a role for STAT5 in Treg development and homeostasis, Burchill et al reported that transgenic mice expressing a constitutively active form of STAT5 in lymphocytes exhibit a significant increase in Tregs in both the thymus and secondary lymphoid organs [69]. In contrast, Treg numbers are reduced in mice expressing a STAT5 hypomorphic allele [69–71]. These studies pointed to a key role for STAT5 in promoting Treg homeostasis but did not address whether STAT5 regulated Treg development.
The generation of mice in which both STAT5a and STAT5b were completely deleted was required to examine the role of STAT5 in Treg development. Mice in which the STAT5a and STAT5b genes were flanked by loxP sites were ultimately generated by Hennighausen and colleagues [72]. Deletion of STAT5 in all tissues led to multiple defects, including impaired erythropoesis, and perinatal lethality [72]. To examine the role of STAT5 in T cells, we crossed STAT5a/b floxed mice to CD4-Cre transgenic animals thereby allowing for selective ablation of STAT5 in T cells. We found that T cells lacking STAT5 were incapable of differentiating into Tregs, strongly supporting a role for STAT5 in Treg development [45]. Similar findings were reported by O’Shea and colleagues [73]. Thus, STAT5 is necessary for Treg development.
To determine whether IL2Rβ-dependent STAT5 signaling was sufficient to drive Treg development and survival, Yang and colleagues generated a series of IL2Rβ mutants which selectively activated just the STAT5 or Ras/PI3K pathways [45]. Hematopoietic stem cells from _IL2Rβ_−/− mice were transduced with retroviruses expressing these mutants and then used to generate bone marrow chimeras to examine the role of these two pathways in Treg development. These studies demonstrated that activation of STAT5 alone was sufficient to restore Treg development while activation of the Ras/PI3K pathway was not ([45] and JY and MAF, unpublished results). Supporting these studies, we found that expression of a constitutively active STAT5 transgene restored Treg development in both _IL2Rβ_−/− and γ_c_−/− mice [45]. Taken together, these findings illustrate that IL2Rβ-dependent activation of STAT5 is both necessary and sufficient for the development and homeostasis of Tregs.
Although mouse models in which levels of STAT5 activation are manipulated indicate a critical role for STAT5 in Treg development, the exact means by which STAT5 directs this process has yet to be fully elucidated. One possible mechanism by which STAT5 signaling could influence Treg biology is through the induction or maintenance of the Treg lineage specification factor Foxp3. Recently Zorn et al identified a consensus STAT5 binding motif in the first intron of the human foxp3 gene locus that is conserved throughout evolution. Introduction of luciferase constructs containing this motif into fibroblasts, in the presence of a constitutively active form of STAT5 or STAT3, resulted in the induction of luciferase activity [74]. These findings demonstrated that this motif was potentially functional, but did not demonstrate whether STAT5 or STAT3 actually bound these sites in vivo. We independently identified the same consensus STAT5 binding motif in the first intron of the foxp3 gene, as well as several closely spaced evolutionarily conserved STAT5 binding motifs in the 5′ region of the murine foxp3 promoter (Fig. 3). Using chromatin immunoprecipitation assays, we detected binding of STAT5 to the promoter site, but not the intronic sites in sorted CD4+CD25+ T cells [45]. A recent report by O’Shea and colleagues confirmed our chromatin immunoprecipitation studies with regard to STAT5 binding to the foxp3 promoter site. However, they also reported significant binding of STAT5 to the conserved intronic STAT5 binding sites, as well as a site located several kb 5′ of the foxp3 transcription start site. These latter studies also demonstrated that STAT3 was completely dispensable for foxp3 transcription and Treg development [73]. Taken together, these studies clearly demonstrate that STAT5 can bind to the foxp3 gene. However, additional experiments are required to determine the biological significance of these STAT5 binding sites.
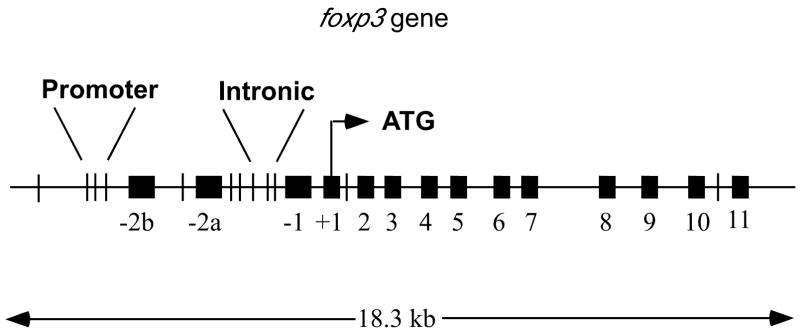
Figure 3. STAT Binding Motifs in the Murine foxp3 gene.
The murine foxp3 gene locus contains many motifs to which activated STAT molecules can bind. Black boxes represent exons while vertical lines represent consensus STAT binding motifs. The three closely linked sites (separated by 7 base pairs) in the promoter and the three sites labeled intronic are conserved across species.
In summary, studies over the last few years have helped illuminate the key role that IL2 and IL2Rβ-dependent signals play in Treg development and homeostasis. However, many questions still remain. For example, although STAT5 has been shown to bind to distinct sites in the foxp3 gene, the functional relevance of these binding sites remains to be determined. Likewise, transcription factors that potentially cooperate with STAT5 to promote foxp3 transcription remain to be identified. Finally, how TCR-dependent and IL2Rβ-dependent signals interact to promote Treg development remains to be established. The answer to these questions will provide a more complete picture of how IL2Rβ-dependent signals entrain regulatory T cell development and homeostasis.
Acknowledgments
This work was supported by grants from the NIH (AI061165) and by a Pew Scholar Award, a Cancer Research Investigator Award and a Leukemia and Lymphoma Society Scholar Award to M.A.F. M.A.B. was supported by an NIH training grant (2T32-AI07313).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi T, Matsui H, Fujita T, Takaoka C, Kashima N, Yoshimoto R, Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983;302:305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- 3.Leonard WJ, Depper JM, Crabtree GR, Rudikoff S, Pumphrey J, Robb RJ, Kronke M, Svetlik PB, Peffer NJ, Waldmann TA, et al. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984;311:626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- 4.Nikaido T, Shimizu A, Ishida N, Sabe H, Teshigawara K, Maeda M, Uchiyama T, Yodoi J, Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984;311:631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- 5.Cosman D, Cerretti DP, Larsen A, Park L, March C, Dower S, Gillis S, Urdal D. Cloning, sequence and expression of human interleukin-2 receptor. Nature. 1984;312:768–771. doi: 10.1038/312768a0. [DOI] [PubMed] [Google Scholar]
- 6.Hatakeyama M, Tsudo M, Minamoto S, Kono T, Doi T, Miyata T, Miyasaka M, Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA’s. Science. 1989;244:551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- 7.Takeshita T, Asao H, Ohtani K, Ishii N, Kumaki S, Tanaka N, Munakata H, Mankamura M, Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992;257:379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- 8.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Rickert M, Garcia KC. Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science. 2005;310:1159–1163. doi: 10.1126/science.1117893. [DOI] [PubMed] [Google Scholar]
- 10.Smith KA. The interleukin 2 receptor. Annu Rev Cell Biol. 1989;5:397–425. doi: 10.1146/annurev.cb.05.110189.002145. [DOI] [PubMed] [Google Scholar]
- 11.Lin JX, Leonard WJ. Signaling from the IL-2 receptor to the nucleus. Cytokine Growth Factor Rev. 1997;8:313–332. doi: 10.1016/s1359-6101(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 12.Hatakeyama M, Tsudo M, Minamoto S, Kono T, Doi T, Miyata T, Miyasaka M, Taniguchi T. Interleukin-2 receptor β chain gene: generation of three receptor forms by cloned human α and β chain cDNA. Science. 1989;244:551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- 13.Miyazaki T, Liu Z-J, Kawahara A, Minami Y, Yamada K, Tsujimoto Y, Barsoumian EL, Perlmutter RM, Taniguchi T. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc and lck cooperate in hematopoietic cell proliferation. Cell. 1995 doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 14.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 15.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 16.Lenardo MJ. Interleukin-2 programs mose αβ T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 17.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor αchain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 20.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–755. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 21.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology. 1970;18:723–737. [PMC free article] [PubMed] [Google Scholar]
- 22.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VE, Gonzalez-Munoz A, Clark J, Veijola R, Cubbon R, Chen SL, Rosa R, Cumiskey AM, Serreze DV, Gregory S, Rogers J, Lyons PA, Healy B, Smink LJ, Todd JA, Peterson LB, Wicker LS, Santamaria P. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nature genetics. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davoodi-Semiromi A, McDuffie M, Litherland S, Clare-Salzler M. Truncated pStat5B is associated with the Idd4 locus in NOD mice. Biochemical and biophysical research communications. 2007;356:655–661. doi: 10.1016/j.bbrc.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 24.Vella A, Cooper JD, Lowe CE, Walker N, Nutland S, Widmer B, Jones R, Ring SM, McArdle W, Pembrey ME, Strachan DP, Dunger DB, Twells RC, Clayton DG, Todd JA. Localization of a type 1 diabetes locus in the IL2RA/CD25 region by use of tag single-nucleotide polymorphisms. American journal of human genetics. 2005;76:773–779. doi: 10.1086/429843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Risk Alleles for Multiple Sclerosis Identified by a Genomewide Study. N Engl J Med. 2007 doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 26.Brand OJ, Lowe CE, Heward JM, Franklyn JA, Cooper JD, Todd JA, Gough SC. Association of the interleukin-2 receptor alpha (IL-2Ralpha)/CD25 gene region with Graves’ disease using a multilocus test and tag SNPs. Clinical endocrinology. 2007;66:508–512. doi: 10.1111/j.1365-2265.2007.02762.x. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Zhou YW, Kato M, Mak TW, Nakashima I. Normal regulatory alpha/beta T cells effectively eliminate abnormally activated T cells lacking the interleukin 2 receptor beta in vivo. The Journal of experimental medicine. 1999;190:1561–1572. doi: 10.1084/jem.190.11.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 29.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 30.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nature reviews. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 31.Malek TR, Porter BO, Codias EK, Scibelli P, Yu A. Normal lymphoid homeostasis and lack of lethal autoimmunity in mice containing mature T cells with severely impaired IL-2 receptors. J Immunol. 2000;164:2905–2914. doi: 10.4049/jimmunol.164.6.2905. [DOI] [PubMed] [Google Scholar]
- 32.Bayer AL, Yu A, Adeegbe D, Malek TR. Essential role for interleukin-2 for CD4(+)CD25(+) T regulatory cell development during the neonatal period. The Journal of experimental medicine. 2005;201:769–777. doi: 10.1084/jem.20041179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. The Journal of experimental medicine. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greene WC, Leonard WJ. The human interleukin-2 receptor. Annu Rev Immunol. 1986;4:69–95. doi: 10.1146/annurev.iy.04.040186.000441. [DOI] [PubMed] [Google Scholar]
- 35.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nature genetics. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 36.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nature genetics. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 37.Bennett CL, Brunkow ME, Ramsdell F, O’Briant KC, Zhu Q, Fuleihan RL, Shigeoka AO, Ochs HD, Chance PF. A rare polyadenylation signal mutation of the FOXP3 gene (AAUAAA-->AAUGAA) leads to the IPEX syndrome. Immunogenetics. 2001;53:435–439. doi: 10.1007/s002510100358. [DOI] [PubMed] [Google Scholar]
- 38.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4(+)CD25(+) T regulatory cells. Nature immunology. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 39.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4(+)CD25(+) regulatory T cells. Nature immunology. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 40.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 41.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 42.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature immunology. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 44.D’Cruz LM, Klein L. Development and function of agonist-induced CD25(+)Foxp3(+) regulatory T cells in the absence of interleukin 2 signaling. Nature immunology. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 45.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 46.Soper DM, Kasprowicz DJ, Ziegler SF. IL-2Rbeta links IL-2R signaling with Foxp3 expression. European journal of immunology. 2007;37:1817–1826. doi: 10.1002/eji.200737101. [DOI] [PubMed] [Google Scholar]
- 47.Yu A, Malek TR. Selective availability of IL-2 is a major determinant controlling the production of CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2006;177:5115–5121. doi: 10.4049/jimmunol.177.8.5115. [DOI] [PubMed] [Google Scholar]
- 48.Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2007;178:4062–4071. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- 49.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. The Journal of experimental medicine. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. The Journal of experimental medicine. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. The Journal of experimental medicine. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hatakeyama M, Minamoto S, Taniguchi T. Intracytoplasmic phosphorylation sites of Tac antigen (p55) are not essential for the conformation, function, and regulation of the human interleukin 2 receptor. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:9650–9654. doi: 10.1073/pnas.83.24.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russell SM, Johnston JA, Noguchi M, Kawamura M, Bacon CM, Friedmann M, Berg M, McVicar DW, Witthuhn BA, Silvennoinen O, Goldman AS, Schmalstieg FC, Ihle JN, O’Shea JJ, Leonard WJ. Interaction of IL-2Rβ and gammac chains with Jak1 and Jak3: Implications for XSCID and XCID. Science. 1994;266:1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 54.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu Z-J, Oishi I, Silvennoinen O, Witthuhn BA, Ihle JN, Taniguchi T. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 55.Witthuhn BA, Silvennoinen O, Miura O, Lai KS, Cwik C, Liu ET, Ihle JN. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- 56.Johnston JA, Kawamura M, Kirken RA, Chen Y-Q, Blake TB, Shibuya K, Ortaldo JR, McVicar DW, O’Shea JJ. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 57.Sharon M, Gnarra JR, Leonard WJ. The β-chain of the IL-2 receptor (p70) is tyrosine-phosphorylated on YT and HUT-102B2 cells. J Immunol. 1989;143:2530–2533. [PubMed] [Google Scholar]
- 58.Hatakeyama M, Mori H, Doi T, Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989;59:837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- 59.Liu KD, Lai SY, Goldsmith MA, Greene WC. Identification of a variable region within the cytoplasmic tail of the IL-2 receptor beta chain that is required for growth signal transduction. The Journal of biological chemistry. 1995;270:22176–22181. doi: 10.1074/jbc.270.38.22176. [DOI] [PubMed] [Google Scholar]
- 60.Zhu MH, Berry JA, Russell SM, Leonard WJ. Delineation of the regions of interleukin-2 (IL-2) receptor beta chain important for association of Jak1 and Jak3. Jak1-independent functional recruitment of Jak3 to Il-2Rbeta. The Journal of biological chemistry. 1998;273:10719–10725. doi: 10.1074/jbc.273.17.10719. [DOI] [PubMed] [Google Scholar]
- 61.Evans GA, Howard OM, Erwin R, Farrar WL. Interleukin-2 induces tyrosine phosphorylation of the vav proto-oncogene product in human T cells: lack of requirement for the tyrosine kinase lck. Biochemistry Journal. 1993;294:339–342. doi: 10.1042/bj2940339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friedmann MC, Migone TS, Russell SM, Leonard WJ. Different interleukin 2 receptor beta-chain tyrosines couple to at least two signaling pathways and synergistically mediate interleukin 2-induced proliferation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2077–2082. doi: 10.1073/pnas.93.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ravichandran KS, Igras V, Shoelson SE, Fesik SW, Burakoff SJ. Evidence for a role for the phosphotyrosine-binding domain of Shc in interleukin 2 signaling. Proceedings of the National Academy of Sciences (USA) 1996;93:5275–5280. doi: 10.1073/pnas.93.11.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gadina M, Sudarshan C, Visconti R, Zhou YJ, Gu H, Neel BG, O’Shea JJ. The docking molecule gab2 is induced by lymphocyte activation and is involved in signaling by interleukin-2 and interleukin-15 but not other common gamma chain-using cytokines. The Journal of biological chemistry. 2000;275:26959–26966. doi: 10.1074/jbc.M004021200. [DOI] [PubMed] [Google Scholar]
- 65.Van Parijs L, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. Uncoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 66.Fujii H, Ogasawara K, Otsuka H, Suzuki M, Yamamura K, Yokochi T, Miyazaki T, Suzuki H, Mak TW, Taki S, Taniguchi T. Functional dissection of the cytoplasmic subregions of the IL-2 receptor betac chain in primary lymphocyte populations. Embo J. 1998;17:6551–6557. doi: 10.1093/emboj/17.22.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaffen SL, Lai SY, Ha M, Liu X, Hennighausen L, Greene WC, Goldsmith MA. Distinct tyrosine residues within the interleukin-2 receptor beta chain drive signal transduction specificity, redundancy, and diversity. The Journal of biological chemistry. 1996;271:21381–21390. doi: 10.1074/jbc.271.35.21381. [DOI] [PubMed] [Google Scholar]
- 68.Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, Thompson CB, Burchill MA, Farrar MA, Turka LA. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burchill MA, Goetz CA, Prlic M, O’Neil JJ, Harmon IR, Bensinger SJ, Turka LA, Brennan P, Jameson SC, Farrar MA. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J Immunol. 2003;171:5853–5864. doi: 10.4049/jimmunol.171.11.5853. [DOI] [PubMed] [Google Scholar]
- 70.Antov A, Yang L, Vig M, Baltimore D, Van Parijs L. Essential role for STAT5 signaling in CD25+CD4+ regulatory T cell homeostasis and maintenance of self-tolerance. J Immunol. 2003;171:3435–3441. doi: 10.4049/jimmunol.171.7.3435. [DOI] [PubMed] [Google Scholar]
- 71.Snow JW, Abraham N, Ma MC, Herndier BG, Pastuszak AW, Goldsmith MA. Loss of tolerance and autoimmunity affecting multiple organs in STAT5A/5B-deficient mice. J Immunol. 2003;171:5042–5050. doi: 10.4049/jimmunol.171.10.5042. [DOI] [PubMed] [Google Scholar]
- 72.Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L. Inactivation of Stat5 in mouse mammary epithelieum during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol. 2004;24:8037–8047. doi: 10.1128/MCB.24.18.8037-8047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O’Shea JJ. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, Frank DA, Ritz J. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]