Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans (original) (raw)
Abstract
The low barometric pressure at high altitude causes lower arterial oxygen content among Tibetan highlanders, who maintain normal levels of oxygen use as indicated by basal and maximal oxygen consumption levels that are consistent with sea level predictions. This study tested the hypothesis that Tibetans resident at 4,200 m offset physiological hypoxia and achieve normal oxygen delivery by means of higher blood flow enabled by higher levels of bioactive forms of NO, the main endothelial factor regulating blood flow and vascular resistance. The natural experimental study design compared Tibetans at 4,200 m and U.S. residents at 206 m. Eighty-eight Tibetan and 50 U.S. resident volunteers (18–56 years of age, healthy, nonsmoking, nonhypertensive, not pregnant, with normal pulmonary function) participated. Forearm blood flow, an indicator of systemic blood flow, was measured noninvasively by using plethysmography at rest, after breathing supplemental oxygen, and after exercise. The Tibetans had more than double the forearm blood flow of low-altitude residents, resulting in greater than sea level oxygen delivery to tissues. In comparison to sea level controls, Tibetans had >10-fold-higher circulating concentrations of bioactive NO products, including plasma and red blood cell nitrate and nitroso proteins and plasma nitrite, but lower concentrations of iron nitrosyl complexes (HbFeIINO) in red blood cells. This suggests that NO production is increased and that metabolic pathways controlling formation of NO products are regulated differently among Tibetans. These findings shift attention from the traditional focus on pulmonary and hematological systems to vascular factors contributing to adaptation to high-altitude hypoxia.
Keywords: circulation, endothelium
The low barometric pressure at high altitude causes lower arterial oxygen content among Tibetan highlanders, who maintain normal levels of oxygen use as indicated by basal and maximal oxygen consumption levels that are consistent with sea level predictions (1–3). Hypothetically, the unavoidably low supply of oxygen in the air and the blood could be offset by increasing blood flow to improve oxygen delivery. Blood flow is determined by numbers, length, and diameter of blood vessels that in turn are largely determined directly or indirectly by levels of NO, a potent vasodilator synthesized in the endothelial cells lining the vessels (4–7). Tibetans have high levels of NO synthesis in the lungs (8), and pulmonary blood flow correlated with NO in a sample studied at 4,200 m (8, 9). This suggests the hypothesis that Tibetan highlanders offset hypoxia with higher systemic blood flow and higher levels of circulating, biologically active metabolites of NO. After synthesis by the endothelium, NO rapidly undergoes reaction in the blood to form products that have circulatory and metabolic effects, including nitrite, nitrate, nitrosothiol proteins (proteins containing NO-cysteine covalent bonds), and α-nitrosyl hemoglobin (HbFeIINO), in which NO occupies the heme binding site for oxygen in hemoglobin (5, 10–13). A sample of 88 Tibetans at 4,200 m had forearm blood flow more than double that of a sample of 50 sea level residents at 206 m and circulating concentrations of biologically active forms of NO >10-fold higher. These results highlight blood flow and its regulation as central components of Tibetans' adaptation to high-altitude hypoxia.
Results
Arterial Oxygen Content, Delivery, and Forearm Blood Flow.
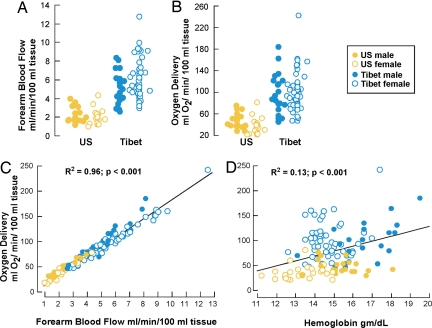
Eighty-eight Tibetan native residents at 4,200 m and 50 U.S. sea level residents at 206 m (all healthy, normotensive, nonsmoking, nonpregnant volunteers, 18–55 years of age) participated in this natural experiment (Table 1). Tibetans were shorter and lighter and had lower arterial oxygen saturation and content. Tibetan men and women had higher forearm blood flow as compared with the sea level group (Fig. 1A and Table 2). Sea level blood flow rates were in the previously reported range (10, 14, 15). Forearm blood flow did not correlate with age, body mass index, arterial oxygen content, or blood pressure in either sample (all P > 0.05). Importantly, Tibetans had greater forearm blood flow and yet maintained lower vascular resistance as compared with those at sea level (Table 2). As a consequence of the greater tissue blood flow and higher hemoglobin concentration, Tibetans delivered more than two times more oxygen to the capillary beds of the forearm despite lower arterial oxygen content as compared with sea level (Fig. 1 B–D).
Table 1.
Tibetan (4,200 m) and U.S. sea level (206 m) sample characteristics (mean ± SEM)
| Subjects | n | Age, years | Height, m | Weight, kg | Hemoglobin, g/dl | O2 saturation, % of hemoglobin | Arterial O2 content,* ml of O2/g of hemoglobin | Pulse, beats per minute | Systolic blood pressure, mm Hg | Diastolic blood pressure, mm Hg | Mean arterial pressure, † mm Hg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tibetan males | 25 | 32 ± 2 | 1.62 ± 0.01 | 47 ± 1 | 16.5 ± 0.3 | 83.7 ± 0.7 | 18.2 ± 0.2 | 74 ± 2 | 113 ± 2 | 77 ± 2 | 89 ± 1 |
| Tibetan females | 63 | 30 ± 1 | 1.54 ± 0.05 | 44.5 ± 0.5 | 14.6 ± 0.1 | 85.2 ± 0.5 | 17.4 ± 0.2 | 79 ± 1 | 113 ± 1 | 76 ± 1 | 89 ± 1 |
| U.S. males | 23 | 35 ± 2 | 1.78 ± 0.01 | 86 ± 2 | 15.5 ± 0.2 | 96.9 ± 0.2 | 20.8 ± 0.3 | 70 ± 2 | 127 ± 2 | 76 ± 1 | 93 ± 1 |
| U.S. females | 27 | 38 ± 2 | 1.64 ± 0.01 | 68 ± 3 | 13.3 ± 0.2 | 98.1 ± 0.2 | 19.3 ± 0.4 | 72 ± 1 | 114 ± 2 | 69 ± 1 | 84 ± 1 |
| P value‡ | 0.2 | <0.001 | <0.001 | 0.01 | <0.001 | <0.001 | 0.13 | <0.001 | 0.7 | 0.06 | |
| P value§ | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.9 | <0.001 | 0.004 |
Fig. 1.
Hemodynamics and oxygen delivery among Tibetan and sea level populations. (A and B) Tibetan forearm blood flow (A) and oxygen delivery (B) were greater than sea level controls. (C) The greater forearm blood flow of Tibetans compared with the sea level population accounts for the greater oxygen delivery (_R_2 = 0.96 and P < 0.001). (D) Higher hemoglobin concentrations of Tibetans as compared with sea level population contributes modestly to greater oxygen delivery (_R_2 = 0.13 and P < 0.001).
Table 2.
Tibetan and U.S. sea level forearm hemodynamics and oxygen delivery (mean ± SEM)
| Subjects | Blood flow, ml/min/100 ml of tissue | Vascular resistance,* mm Hg/ml/min/100 ml of tissue | Oxygen delivery, † ml of O2/min/100 ml of tissue | Blood flow during 50% fractional insipired oxygen, ml/min/100 ml of tissue | Blood flow during exercise, ‡ ml/min/100 ml of tissue |
|---|---|---|---|---|---|
| Tibetan males | 4.9 ± 0.3 | 21 ± 2 | 96 ± 8 | 4.9 ± 0.6§ | 11.0 ± 0.7 |
| Tibetan females | 5.7 ± 0.2 | 17.2 ± 0.9 | 98 ± 5 | 4.5 ± 0.2¶ | 9.4 ± 0.3 |
| U.S. males | 2.3 ± 0.1 | 44 ± 3 | 47 ± 3 | Not done | 3.1 ± 0.8 |
| U.S. females | 2.0 ± 0.1 | 47 ± 3 | 36 ± 3 | Not done | 3.1 ± 0.5 |
| P value‖ | <0.001 | <0.001 | <0.001 | <0.001 | |
| P value** | <0.001 | <0.001 | <0.001 | <0.001 |
Effects of Oxygen Supplementation and Exercise on Forearm Blood Flow.
Experiments designed to investigate blood flow regulation tested for the presence of hypoxic vasodilation and exercise-induced vasodilation. First, the presence of a hypoxia-induced vasodilation was determined by oxygen supplementation. Experimental relief from hypoxia by inhalation of 50% oxygen caused Tibetans to achieve oxygen saturations ≥98% and caused a small reduction of forearm blood flow and systolic blood pressure among Tibetan women, but not men (Table 2). Diastolic blood pressure was not affected by oxygen breathing, but Tibetans experienced a 16% decline in pulse with oxygen breathing (pulse while breathing supplemental oxygen: Tibetan men, 62 ± 2; Tibetan women, 66 ± 1 beats per minute). These findings suggest modest systemic hypoxic vasodilation and tachycardia; however, even after relief of hypoxia by supplemental oxygen, forearm blood flow of the Tibetans remained double that of sea level controls (Table 2). Experimentally increasing oxygen demand with 5 min of forearm exercise increased forearm blood flow of participants at both altitudes; however, Tibetans had much greater flow increase than sea level individuals (Table 2). Taken together, the response to relief of hypoxia and the response to the increased demands of exercise demonstrate that blood flow of Tibetans is actively regulated in the expected ways around a much higher baseline.
Circulating NO Reaction Products: Plasma Nitrite and Nitrate.
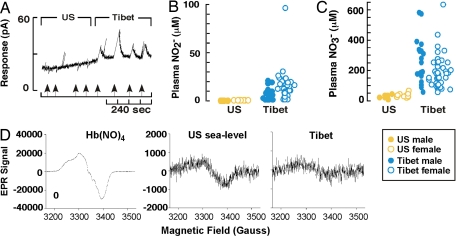
Greater tissue blood flow of Tibetans could be related to greater numbers or length of vessels and/or vasodilation. NO is the main vasoregulator of blood flow to capillaries where oxygen is released to tissues, and plasma nitrite and nitrate concentrations are stable and reliable measures of systemic NO production (5, 6, 16–18). Hence, circulating reaction products of NO were evaluated in Tibetans. Nitrite and nitrate were measured in deproteinated and delipidated plasma from Tibetan and sea level samples initially by an amperometric NO sensor method after release of NO from nitrite or from total nitrite and nitrate. Tibetan men and women had average plasma nitrite of 4.8 ± 1.4 μM and 11 ± 2 μM, respectively, whereas sea level men and women had undetectable levels (Fig. 2 A–C). Measurements based on the Griess reaction confirmed the high levels of nitrite in Tibetan plasma. To detect nitrite in sea level samples and to definitively confirm the unexpectedly high levels of nitrite in the Tibetan sample, a more sensitive high-performance liquid chromatography assay was performed and confirmed >10-fold-higher nitrite among Tibetans and low levels for sea level samples as previously reported [Tibet, 12 ± 2 μM (n = 7); sea level, 0.55 ± 0.03 μM (n = 10)] (13). As for nitrate, Tibetan men and women had 10-fold-higher plasma levels of 234 ± 31 μM and 158 ± 13 μM, as compared with 23 ± 4 μM and 30 ± 4 μM for their sea level counterparts (P < 0.001) (Fig. 2C). High-performance liquid chromatography analyses confirmed higher plasma nitrate among Tibetans [Tibet, 237 ± 98 μM (n = 7); sea level, 26 ± 7 (n = 10); P = 0.02]. Tibetan women had higher nitrite and lower nitrate than Tibetan men whereas there were no gender differences in nitrite or nitrate in the sea level samples. The nitrate levels of the sea level samples were similar to previous reports using the same techniques (13, 19). Tibetan plasma nitroso protein levels were found to be only modestly higher than sea level [Tibet, 47 ± 9 nM (n = 7); sea level, 14.5 ± 0.5 nM (n = 8); P = 0.02]. This suggests that the metabolic pathways governing NO product formation are different in the Tibetan and the sea level samples.
Fig. 2.
NO products in the circulation of Tibetan and sea level populations. (A) Amperometric detection (pA) of plasma NO release from nitrite. Arrows identify sample injections. (B and C) Tibetan nitrite (B) and nitrate (C) levels in plasma samples were higher than sea level controls (all P < 0.001). (D) EPR determination of HbFeIINO in red blood cell samples. EPR spectra of the tetranitrosyl HbFeIINO standard is shown in Left. EPR spectra of blood obtained from a Tibetan participant (Right) revealed lower levels of HbFeIINO complex than blood obtained from a sea level volunteer (Center). Data shown are representative of the mean values of the population samples (P < 0.03).
The plasma nitrite and nitrate concentrations of Tibetans are unprecedented for healthy people. Average concentrations of nitrite and nitrate exceeded those typically seen in septic shock patients (20), although Tibetans had no sign of systemic inflammation (C-reactive protein; data not shown) (9) Consistent with and confirmatory of the orders of magnitude greater nitrate concentrations in vivo, Tibetans had higher urinary nitrate than sea level controls (urine nitrate/creatinine: Tibet, 105 ± 49 mmol/mol; sea level, 7.8 ± 0.6 mmol/mol; P < 0.001). The high urine nitrate was consistent with intact renal clearance of nitrate and excluded potential causes of increased nitrate on the basis of renal function. Overall, these findings indicate a much greater total body nitrite and nitrate in Tibetans and led us to evaluate whether other NO reaction products were also increased.
Red Blood Cell Nitrite, Nitrate, Nitroso Proteins, and Nitrosyl Products.
NO and/or plasma nitrite and nitrate may enter the red blood cell, where reactions lead to formation of nitroso proteins and nitrosyl products (5, 11). Thus, in a subgroup of samples, cellular nitroso proteins (RXNO, mainly SNO-hemoglobins) and nitrosyl products (HbFeIINO), and levels of nitrite and nitrate, were measured. Tibetans had abundant red blood cell nitroso protein levels whereas the sea level sample had very low levels [Tibet, 2,409 ± 705 nM (n = 7); sea level, <10 nM (n = 3); P < 0.001]. The Tibetan sample had intracellular nitrosoprotein levels that were much higher than the plasma levels whereas the sea level sample had intracellular levels roughly the same as the plasma levels. Thus, it appears that the pathways governing the formation and diffusion of NO products differ between the two samples. Tibetan red blood cell nitrite was similar to sea level (Tibet, 0.66 ± 0.08 μM; sea level, 0.50 ± 0.07 μM; P = 0.6), whereas red blood cell nitrate levels were higher (Tibet, 43 ± 14 μM; sea level, 4.9 ± 0.4 μM; P = 0.03). Despite the greater nitrate and nitroso levels, Tibetans had lower levels of iron nitrosyl complexes in red blood cells than sea level cells as evaluated by electron paramagnetic resonance (EPR) spectroscopy (% of total Hb that is the nitrosyl product, HbFeIINO: Tibet, 0.03 ± 0.01; sea level, 0.18 ± 0.05; P = 0.03) (Fig. 2D). Together, the predominantly cell-associated nitroso proteins but lower levels of iron nitrosyl levels indicate that nitroso protein products preferentially form within Tibetan red blood cells. Overall, the metabolic pathways converting NO to other products are regulated differently among Tibetans than among sea level natives.
Exogenous Sources of NO Reaction Products.
High-nitrate meals or drinks can increase circulating NO metabolites because of enterosalivary circulation of nitrate and reduction by oral commensal bacteria (21, 22). To exclude a dietary source of circulating NO products, nitrite and nitrate levels were measured in household water supply and meals from 10 participants. Water, tea, and barley beer had undetectable or low levels (<15 μM nitrite and <70 μM nitrate). Likewise, foods contained low levels of nitrite or nitrate (<0.4 mg/kg nitrite and <125 mg/kg nitrate). Overall, the average daily consumption was not at a level expected to significantly increase circulating nitrate or nitrite (17, 22). These results suggest that endogenous NO production is up-regulated.
Factors Regulating NO Synthesis: l-Arginine and Arginase.
Because most plasma nitrite, and much of plasma nitrate, is derived from endothelial NO synthesis (eNOS) (5, 6, 16–18), the high nitrite and nitrate concentrations detected likely indicate higher endothelial NO formation in Tibetans. Circulating red blood cells synthesize NO (23); however, lower levels of intracellular nitrite and nitrate, as compared with plasma levels, suggested that formation was not primarily within red blood cells. Although information about eNOS expression and endothelial enzymatic activity is difficult to obtain in field studies, measure of factors that regulate NO synthesis can provide a useful hint. Availability of l-arginine, the substrate for eNOS, is a major determinant of the amount of NO synthesized by eNOS in vivo (24). The intracellular l-arginine availability for NO synthesis is determined by both uptake from blood and intracellular metabolism, in particular via arginase enzymes (24–26). Here, l-arginine availability was estimated by measures of circulating l-arginine and arginase activity. Plasma l-arginine levels in the Tibetan circulation were similar to reported sea level values (54 ± 5 μM arginine). However, Tibetans' plasma arginase activity was less than half of sea level controls [Tibetans, 18 ± 2 milliunits/ml (n = 84); sea level, 50 ± 11 milliunits/ml (n = 20); P < 0.001], and arginase activity was inversely related to plasma nitrate levels (r = −0.24 and P = 0.01). These findings support the concept that Tibetans may have more intracellular l-arginine available to cause higher endogenous NO synthesis.
Discussion
The primary finding of this study is that Tibetans at 4,200 m have substantially higher blood flow that is achieved without the potential cost of hypertension or elevated vascular resistance. The greater blood flow enables greater oxygen delivery and offsets the low arterial oxygen content unavoidably associated with high-altitude residence. The second main finding is that NO and its products, which control blood flow and cellular respiration, are present in the circulation of Tibetans at remarkably high levels as compared with the sea level population. These findings reorient attention from the traditional focus on pulmonary and hematological factors to vascular factors for adaptation to chronic hypoxia.
The l-arginine/NO synthase pathway is the most likely explanation for high concentrations of circulating NO products in Tibetans. Although a precise quantitative assessment of NO formation in Tibetan highlanders compared with the sea level control group is difficult to make without further knowledge about possible differences in NO synthase enzyme kinetics, NO metabolism in blood and tissues, and NO product elimination characteristics, a rough estimate can be made based on plasma and urine levels considering that nitrate is the final end product of NO metabolism in vivo (17–19). Based on the plasma nitrate levels determined in this study and its distribution volume in 30% of body weight (18, 19), the average total body pool of nitrate is estimated to correspond to 3.4 and 2.1 mmol in Tibetan men and women and just 0.53 and 0.47 mmol in sea level men and women, respectively. The total body pool of nitrate estimated for sea level participants in this study is similar to previous reports (18, 19). Based on urine nitrate levels and assuming similar renal function and insignificant contributions from dietary sources, Tibetans appear to have >4-fold-higher NO production than sea level controls. The differences in NO production among the Tibetans and sea level controls estimated by nitrate excretion are consistent with the relative increase of total body pool of nitrate estimated by plasma nitrate levels, i.e., 6.5-fold and 4.4-fold higher in Tibetan men and women than in sea level men and women, respectively.
Although the nature and significance of the bioactive pools of NO products in blood remain controversial (10, 12), the global increase of NO products in Tibetans provides strong support for the concept that NO is critical for oxygen transport and delivery in this chronically hypoxic population. The very high levels of NO observed in Tibetans may have effects in addition to those demonstrated for vascular tone, blood flow, and oxygen delivery. At sea level ambient oxygen availability, NO has well established effects to optimize intracellular oxygen utilization; high levels of NO decrease oxygen consumption and enhance coupled respiration and ATP content while increasing mitochondrial numbers through biogenesis (6, 27, 28). In fact, healthy sea level subjects ingesting high levels of nitrate daily for 3 days achieve a significant reduction of oxygen consumption at submaximal exercise through improved efficiency of energy production during cellular respiration (29). These studies at sea level suggest that the high levels of NO products in Tibetans may affect metabolic as well as circulatory parameters. However, studies of muscle ultrastructure found that Sherpas (a Tibetan high-altitude subpopulation) and Tibetans born and raised at 1,300 m have fewer mitochondria per volume of muscle tissue and a greater muscle capillary density than lowland natives of different ethnic groups (30, 31). Although the NO levels of these samples are unknown, the findings suggest that the populations have intrinsic differences in muscle ultrastructure and/or that hypoxia influences NO effects.
Acclimatization of sea level populations to hypoxia during exercise or acute exposure to high altitude or illness provides insight into some of the mechanisms that preserve oxygen delivery to tissues. Although not to the magnitude achieved by Tibetans in this study, well trained athletes at sea level acquire enhancement of endogenous NO production and forearm blood flow in response to the repetitive intermittent increases in oxygen demand during endurance training. The acclimatization to exercise is associated with improvements in oxygen delivery through blood flow and increased tissue capillary density but is likewise accompanied by improvements in cellular respiration and oxygen consumption during exercise (14, 15, 32, 33). Previous studies have identified that a transient and acute fractional increase in blood flow occurs upon initial ascent of healthy sea level men and women to altitude, which is related to cardiovascular effects (34, 35). However, increased tissue blood flow is not sustained and returns to near normal sea level values within 7–10 days. Similarly, patients with hypoxia related to lung diseases also acclimatize. Although the number of capillaries per muscle fiber in patients with tissue hypoxia due to chronic obstructive pulmonary disease is generally less than in healthy controls (36), ≈30% of patients are able to maintain a high capillary-to-muscle fiber ratio and tissue blood flow and thus avoid the rapid muscle fatigue during exercise seen in patients with low capillary density (37). Strikingly, capillary length and surface area within the diaphragm are markedly increased in emphysema patients as compared with controls, which apportions greater tissue blood flow and oxygen delivery to this continuously active essential muscle (38). These examples of vascular acclimatization of sea level populations to hypoxia suggest that the microvasculature responses may occur relatively rapidly. The current study evaluated Tibetans only at high altitude and cannot determine whether the high NO and tissue blood flow are reversible at prolonged exposure to low altitude, although experiments suggest these features are not immediately relieved by oxygen supplementation. Nevertheless, these adaptive responses enable Tibetans chronically exposed to high-altitude hypoxia to circumvent the limitations of reduced oxygen availability to achieve work capacities similar to sea level dwellers.
The very high systemic blood flow, rate of oxygen delivery, and levels of NO further underscore the contrasting patterns of functional adaptation found among Tibetans as compared with Andean highlanders (8, 39). For example, at the same altitude, Tibetans have lower hemoglobin concentration and percent of oxygen saturation of hemoglobin and do not exhibit the hypoxic pulmonary vasoconstriction characteristic of their Andean counterparts (9). Higher levels of NO synthesis and NO-regulated vasodilation and blood flow in the pulmonary and systemic vasculature may be important mechanisms underlying the distinctive, effective Tibetan response to high-altitude hypoxia. In conclusion, the newly discovered importance of high blood flow and circulating levels of NO products among Tibetans increases understanding of the mechanisms of human adaptation to extreme ambient hypoxia and predicts that treatments based on similar strategies may be effective for patients with diseases associated with tissue hypoxia at any altitude.
Materials and Methods
High-Altitude Population.
Data collection took place from June to August 2002 in Panam Xiang, a rural agropastoral district of Xigatse Prefecture, Tibet Autonomous Region, at 4,200 m as previously reported (9). A field laboratory was established where 88 normotensive, nonsmoking (by self-report and verification by exhaled carbon monoxide level), healthy, nonpregnant, high-altitude native Tibetan volunteers 18–55 years of age with normal pulmonary function provided measures of height, weight, blood pressure, forearm blood flow, exhaled breath, venous blood, and percent oxygen saturation of hemoglobin. A second data collection period during June 2005 in the same location involved five men and five women with the same characteristics, 19–32 years of age, who provided measures of exhaled breath, blood pressure, urine, reports of 24-h dietary intake, and aliquots of the foods. Six people participated in both studies. Hemoglobin concentration was determined in duplicate by using the cyanmethemoglobin technique (hemoglobinometer from Hemocue, Angelholm, Sweden) immediately after drawing a venous blood sample. Percent oxygen saturation of hemoglobin was determined by pulse oximetry (Criticare Model 503; Criticare Systems, Waukeshau, WI) as the average of six readings taken 10 seconds apart as in our previous studies (40). The research described herein adheres to the principles of the Declaration of Helsinki and Title 45 of the U.S. Code of Federal Regulations, Part 46, Protection of Human Subjects. The Institutional Review Board of the Cleveland Clinic and Case Western Reserve University approved the protocol, and informed consent was obtained from all participants.
Sea Level Population.
Two low-altitude reference samples composed of healthy (by self-report), nonsmoking volunteers with normal lung function, 18–55 years of age, were measured at the Cleveland Clinic (Cleveland, OH) at 206 m. A sample of 20 volunteers provided data on age, height, weight, exhaled NO, and venous blood in 2003 as reported previously (9). In 2005, a sample of 50 healthy volunteers screened with the same criteria provided data on height, weight, blood pressure, forearm blood flow, exhaled breath, venous blood, percent oxygen saturation of hemoglobin, and urine.
Ambient Conditions.
The ambient conditions at the time of morning calibrations in Tibet during 2002 have been reported (9). The ambient conditions at the time of morning calibrations in Tibet during 2005 were 18.5°C average temperature, 31% relative humidity, 2.4 ppb NO in ambient air, and 469 Torr barometric pressure. The average temperature at the time of forearm blood flow measurements was 19 ± 0.3°C. In Cleveland, ambient conditions were 23°C, 29% relative humidity, in a climate-controlled room. Measurements were made when ambient NO was <10 ppb. The barometric pressure in Cleveland was 754 Torr.
Blood Collection and Food Sampling.
Blood was collected into heparinized tubes for plasma separation. Tibetan study participants provided aliquots of food and drinks from their own meals. Food was mainly grain. The staple food was toasted barley flour. High-nitrate-containing foods (such as leafy green vegetables) were rare. Fluids were mainly butter tea and barley beer.
NO-Related Products.
Three distinct methods were used to measure nitrite and nitrate in plasma samples: (i) an amperometric NO sensor in combination with acidified iodide for the detection of NO derived from nitrite, or from total nitrite and nitrate after cadmium/copper-mediated reduction of nitrate to nitrite (ISO-NOP, Nitralyzer II; World Precision Instruments, Sarasota, FL) (26); (ii) nitrite and nitrate (after reduction) using the classical Griess reaction (41); and (iii) nitrite and nitrate quantification using a dedicated HPLC system (ENO-20; Eicom) (13). All three methods were in good agreement with high concordance for plasma nitrite and nitrate values (all comparisons _R_2 > 0.6 and P < 0.005). Nitrite and nitrate concentrations were determined in all samples after removal of fatty/particulate materials and proteins by ZnSO4/NaOH using the amperometric technique, and by Griess reaction and HPLC. For the determination of total nitroso products (RXNO), plasma was preincubated with NEM/EDTA for thiol blockade to prevent artificial nitrosation before nitrite removal by sulfanilamide and subsequent analysis by gas phase chemiluminescence (42, 43). During the field study, samples of venous blood were sometimes centrifuged for separation of plasma after sitting at ambient temperature for up to 6 h. Nitrite is converted to nitrate in blood. The finding of high levels of nitrite in the Tibetan samples despite the delays in separating plasma from red blood cells suggests that the original levels of nitrite may have been even higher than those observed during analysis. Comparing the levels of nitrite and nitrate among samples processed within minutes to those processed hours after collection revealed no significant differences in the levels of nitrite or nitrate. Therefore, the estimates of nitrite are perhaps biased toward low values. In a subgroup of samples for which erythrocytes were also available, erythrocyte nitrite, nitrate, and RXNO were evaluated. Erythrocytes were lysed 1:4 in hypotonic water containing 10 mM NEM/2.5 mM EDTA. For total nitrite and nitrate analysis, the lysate was further diluted 1:2 with methanol and centrifuged for removal of hemoglobin; for nitroso analysis, samples were injected into an iodine/iodide-containing reaction solution after preincubation with acidified sulfanilamide for 15 min and analyzed by gas phase chemiluminescence as described elsewhere (43). Because sample volume was limiting, no evaluation of the sensitivity of the RXNO pool to degradation by HgCl2 (for determination of the nitrosothiol portion of the signal) was performed.
The extent of artificial nitrosation that may have occurred because of inevitable delays between blood sampling, centrifugation, and the freeze/thaw process under field conditions was investigated in a separate set of experiments carried out in human blood from U.S. sea level controls (resident in Boston or Cleveland). Blood was spiked with 30, 100, or 300 μM nitrite or nitrate, with different delays imposed between sampling and processing (i.e., centrifugation and freezing) to match those experienced under field conditions. No nitroso products were detected upon spiking with nitrate. Nitrite-mediated nitrosation was highest immediately after addition of nitrite to blood and gradually declined with increased delay between sampling and processing, but levels did not exceed 0.5% in plasma and 1.9% in red blood cells under any condition. Thus, although the higher concentration of nitroso species in Tibetan plasma may be a result of their high constitutive nitrite level, the levels of nitroso compounds in red blood cells far exceed those achievable by constitutive nitrite.
Iron Nitrosyls.
The presence of HbFeIINO in heparinized red blood cells was evaluated by EPR spectra recorded on a Bruker ESP 300 EPR spectrometer equipped with an ER 035 NMR G meter and a Hewlett-Packard 5352B microwave frequency counter. All spectra were obtained at 150 K by using a microwave power of 2 mW, microwave frequency of 9.45 GHz, modulation amplitude of 5.0 G, and modulation frequency of 100 kHz. Ten to 20 scans per sample were accumulated to improve signal-to-noise ratio, and spin quantitations were calculated by double integration as compared with a HbFeIINO standard analyzed under the same measurement conditions. To evaluate the stability of the HbFeIINO complex in aerobic conditions, another sample was prepared following the same procedure but transferred to an Eppendorf tube exposed to room air for 30 min before freezing. Spin quantitation indicated that 70% of the original spin concentration was recovered. Full reduction by dithionite was used to evaluate potential higher oxidation states of HbFeNO.
Blood Flow.
Forearm blood flow (milliliters of blood per 100 ml of tissue per minute) was measured noninvasively by strain gauge venous occlusion plethysmography (44). The mercury strain gauge plethysmography technique (model EC5R Strain Gauge and Photoplethysmograph; D.E. Hokanson) consists of a thin mercury-filled rubber tube encircling the widest part of the forearm that stretches as the volume of the forearm changes with each heartbeat. The stretch increases the electrical resistance in the tube that is conveyed in analog form for calculations (noninvasive vascular program). A wrist cuff inflated to suprasystolic blood pressure served to exclude circulation from the hand, and a rapid, automatic blood pressure cuff was inflated to 50 mm Hg every 7 seconds. This allowed arterial blood to enter the forearm circulation for 7 seconds while preventing venous outflow, resulting in a distension of the forearm proportional to the inflow. Increase in forearm blood flow after 5 min of exercise with a hard rubber ring offering 11.3 kg of resistance quantified blood flow responses to increased oxygen demands. Exercise consisted of 5 min of repetitive handgrips of 10 seconds of contraction of the dynamometer followed by 5 seconds of relaxation. The average of a series of five determinations taken immediately at the end of the 5 min of exercise quantifies the blood flow response to exercise. Forearm blood flow was also measured after 10 min of breathing 50% fractional inspired oxygen in Tibetan subjects.
Arginase Activity.
Arginase activity in plasma was determined as the conversion of [14C-guanidino]l-arginine (PerkinElmer, Boston, MA) to [14C]urea, which was further converted to 14CO2 by urease and trapped as Na2 14CO3 for scintillation counting as described previously (26).
Acknowledgments
We acknowledge the valuable assistance of D. Laskowski, J. Sharp, M. Baaklini, S. A. A. Comhair, K. P. Strohl, I. Kushner, and D. Rzewnicki. The research was supported by National Science Foundation Grants NSF0452326 and NSF0215747 and National Institutes of Health Grants HL60917, HL69029, M01 RR018390, and M01 RR00080.
Footnotes
The authors declare no conflict of interest.%
This article is a PNAS Direct Submission.
References
- 1.Beall CM. In: Mountain Biodiversity: A Global Assessment. Komer C, Spehn EM, editors. New York: Parthenon; 2002. pp. 199–210. [Google Scholar]
- 2.Beall CM, Henry J, Worthman C, Goldstein MC. Am J Hum Biol. 1996;8:361–370. doi: 10.1002/(SICI)1520-6300(1996)8:3<361::AID-AJHB7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Vogel JA, Patton JF, Mello RP, Daniels WL. J Appl Physiol. 1986;60:494–500. doi: 10.1152/jappl.1986.60.2.494. [DOI] [PubMed] [Google Scholar]
- 4.Gigante B, Morlino G, Gentile MT, Persico MG, De Falco S. FASEB J. 2006;20:970–972. doi: 10.1096/fj.05-4481fje. [DOI] [PubMed] [Google Scholar]
- 5.Ignarro LJ. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 6.Moncada S, Higgs EA. Handb Exp Pharmacol. 2006;176:213–254. doi: 10.1007/3-540-32967-6_7. [DOI] [PubMed] [Google Scholar]
- 7.Williams JL, Cartland D, Hussain A, Egginton S. J Physiol. 2006;570:445–454. doi: 10.1113/jphysiol.2005.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beall CM, Laskowski D, Strohl KP, Soria R, Villena M, Vargas E, Alarcon AM, Gonzales C, Erzurum SC. Nature. 2001;414:411–412. doi: 10.1038/35106641. [DOI] [PubMed] [Google Scholar]
- 9.Hoit BD, Dalton ND, Erzurum SC, Laskowski D, Strohl KP, Beall CM. J Appl Physiol. 2005;99:1796–1801. doi: 10.1152/japplphysiol.00205.2005. [DOI] [PubMed] [Google Scholar]
- 10.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, et al. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 11.Kelm M. Biochim Biophys Acta. 1999;1411:273–289. doi: 10.1016/s0005-2728(99)00020-1. [DOI] [PubMed] [Google Scholar]
- 12.McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, et al. Nat Med. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 13.Rassaf T, Bryan NS, Kelm M, Feelisch M. Free Radical Biol Med. 2002;33:1590–1596. doi: 10.1016/s0891-5849(02)01183-8. [DOI] [PubMed] [Google Scholar]
- 14.Alomari MA, Welsch MA, Prisby RD, Lee CM, Wood RH. Int J Sports Med. 2001;22:361–365. doi: 10.1055/s-2001-15654. [DOI] [PubMed] [Google Scholar]
- 15.Green DJ, Cable NT, Fox C, Rankin JM, Taylor RR. J Appl Physiol. 1994;77:1829–1833. doi: 10.1152/jappl.1994.77.4.1829. [DOI] [PubMed] [Google Scholar]
- 16.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Godecke A, Schrader J, Schulz R, et al. Free Radical Biol Med. 2003;35:790–796. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 17.Rhodes P, Leone AM, Francis PL, Struthers AD, Moncada S, Rhodes PM. Biochem Biophys Res Commun. 1995;209:590–596. doi: 10.1006/bbrc.1995.1541. [DOI] [PubMed] [Google Scholar]
- 18.Wagner DA, Schultz DS, Deen WM, Young VR, Tannenbaum SR. Cancer Res. 1983;43:1921–1925. [PubMed] [Google Scholar]
- 19.Jungersten L, Ambring A, Wall B, Wennmalm A. J Appl Physiol. 1997;82:760–764. doi: 10.1152/jappl.1997.82.3.760. [DOI] [PubMed] [Google Scholar]
- 20.Dhillon SS, Mahadevan K, Bandi V, Zheng Z, Smith CW, Rumbaut RE. Chest. 2005;128:1706–1712. doi: 10.1378/chest.128.3.1706. [DOI] [PubMed] [Google Scholar]
- 21.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. New Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 22.Pannala AS, Mani AR, Spencer JP, Skinner V, Bruckdorfer KR, Moore KP, Rice-Evans CA. Free Radical Biol Med. 2003;34:576–584. doi: 10.1016/s0891-5849(02)01353-9. [DOI] [PubMed] [Google Scholar]
- 23.Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, Kumara I, Gharini P, Kabanova S, Ozuyaman B, et al. Blood. 2006;107:2943–2951. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 24.Ignarro LJ, Buga GM, Wei LH, Bauer PM, Wu G, del Soldato P. Proc Natl Acad Sci USA. 2001;98:4202–4208. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Meininger CJ, Hawker JR, Jr, Haynes TE, Kepka-Lenhart D, Mistry SK, Morris SM, Jr, Wu G. Am J Physiol. 2001;280:E75–E82. doi: 10.1152/ajpendo.2001.280.1.E75. [DOI] [PubMed] [Google Scholar]
- 26.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, et al. FASEB J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 27.Clementi E, Nisoli E. Comp Biochem Physiol. 2005;142:102–110. doi: 10.1016/j.cbpb.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 28.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, et al. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 29.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Acta Physiol (Oxford, UK) 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 30.Kayser B, Hoppeler H, Desplanches D, Marconi C, Broers B, Cerretelli P. J Appl Physiol. 1996;81:419–425. doi: 10.1152/jappl.1996.81.1.419. [DOI] [PubMed] [Google Scholar]
- 31.Kayser B, Hoppeler H, Claassen H, Cerretelli P. J Appl Physiol. 1991;70:1938–1942. doi: 10.1152/jappl.1991.70.5.1938. [DOI] [PubMed] [Google Scholar]
- 32.Franzoni F, Galetta F, Morizzo C, Lubrano V, Palombo C, Santoro G, Ferrannini E, Quinones-Galvan A. Clin Sci (London) 2004;106:329–335. doi: 10.1042/CS20030229. [DOI] [PubMed] [Google Scholar]
- 33.Klausen K, Andersen LB, Pelle I. Acta Physiol Scand. 1981;113:9–16. doi: 10.1111/j.1748-1716.1981.tb06854.x. [DOI] [PubMed] [Google Scholar]
- 34.Weil JV, Byrne-Quinn E, Battock DJ, Grover RF, Chidsey CA. Clin Sci. 1971;40:235–246. doi: 10.1042/cs0400235. [DOI] [PubMed] [Google Scholar]
- 35.Zamudio S, Douglas M, Mazzeo RS, Wolfel EE, Young DA, Rock PB, Braun B, Muza SR, Butterfield GE, Moore LG. Am J Physiol. 2001;281:H2636–H2644. doi: 10.1152/ajpheart.2001.281.6.H2636. [DOI] [PubMed] [Google Scholar]
- 36.Whittom F, Jobin J, Simard PM, Leblanc P, Simard C, Bernard S, Belleau R, Maltais F. Med Sci Sports Exerc. 1998;30:1467–1474. doi: 10.1097/00005768-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Saey D, Michaud A, Couillard A, Cote CH, Mador MJ, LeBlanc P, Jobin J, Maltais F. Am J Resp Crit Care Med. 2005;171:1109–1115. doi: 10.1164/rccm.200408-1005OC. [DOI] [PubMed] [Google Scholar]
- 38.Poole DC, Mathieu-Costello O. J Appl Physiol. 1997;82:599–606. doi: 10.1152/jappl.1997.82.2.599. [DOI] [PubMed] [Google Scholar]
- 39.Beall CM. Proc Natl Acad Sci USA. 2007;104(Suppl 1):8655–8660. doi: 10.1073/pnas.0701985104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beall CM, Strohl KP, Blangero J, Williams-Blangero S, Decker MJ, Brittenham GM, Goldstein MC. Hum Biol. 1997;69:597–604. [PubMed] [Google Scholar]
- 41.Schmidt H, Kelm M. In: Methods in Nitric Oxide Research. Feelisch M, Stamler J, editors. Chichester, UK: Wiley; 1996. pp. 491–497. [Google Scholar]
- 42.Feelisch M, Rassaf T, Mnaimneh S, Singh N, Bryan NS, Jourd'Heuil D, Kelm M. FASEB J. 2002;16:1775–1785. doi: 10.1096/fj.02-0363com. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Bryan NS, MacArthur PH, Rodriguez J, Gladwin MT, Feelisch M. J Biol Chem. 2006;281:26994–27002. doi: 10.1074/jbc.M603953200. [DOI] [PubMed] [Google Scholar]
- 44.Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Circulation. 1993;87:1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]