Th2 cytokine-Induced Alterations in Intestinal Smooth Muscle Function Depend on Alternatively Activated Macrophages (original) (raw)
. Author manuscript; available in PMC: 2010 Oct 14.
Published in final edited form as: Gastroenterology. 2008 Apr 4;135(1):217–225.e1. doi: 10.1053/j.gastro.2008.03.077
Abstract
Background & Aims
Enteric nematode infection induces a strong Th2 cytokine response and is characterized by increased infiltration of various immune cells including macrophages. The role of these immune cells in host defense against enteric nematode infection, however, remains poorly defined. The present study investigated the role of macrophages and the arginase pathway in nematode-induced changes in intestinal smooth muscle function and worm expulsion.
Methods
Mice were infected with Nippostrongylus brasiliensis, and were injected intravenously with clodronate-containing liposome to deplete macrophages or given S-(2-boronoethyl)-I-cysteine in the drinking water to inhibit arginase activity. Segments of intestinal smooth muscle were suspended in organ baths to determine responses to acetylcholine, 5-HT, or nerve stimulation. The phenotype of macrophages was monitored by measuring mRNA expression of the specific molecular markers via real-time PCR or viewed by immunofluoresence staining.
Results
Nippostrongylus brasiliensis infection increased the infiltration of macrophages and induced the up-regulation of specific markers for alternatively activated macrophages by a mechanism dependent on IL-4 or IL-13 activation of Stat6. Elimination of alternatively activated macrophages by treatment of mice with clodronate-liposomes blocked smooth muscle hyper-contractility and increased smooth muscle thickness, and impaired worm expulsion. In addition, specific inhibition of arginase activity interfered with smooth muscle contractility, but only partially affected the protective immunity of the host.
Conclusions
These data show that the phenotype of macrophages is determined by the local immune environment and that alternatively activated macrophages play a major role in the effects of Th2 cytokines, IL-4 and IL-13, on intestinal smooth muscle function.
The gut is one of the richest sources of macrophages in the body where they play a fundamental role in host defense through the recognition, phagocytosis, and killing of microorganisms1. Macrophages undergo distinct pathways of activation and display different phenotypes depending on the cytokine microenvironment2. The best characterized macrophages are the classically activated macrophages (CAMϕ) that are induced by the Th1 cytokine, IFN-γ plus microbial lipopolysaccharide (LPS). CAMϕ are one of the major effector cells in Th1 immune responses and play an essential role in protection against intracellular pathogens through the production of nitric oxide (NO) by inducible nitric oxide synthase (NOS-2), the marker for CAMϕ3. Less well defined are the alternatively activated macrophages (AAMϕ) induced by Th2 cytokines, IL-4 and IL-13, acting through the type 2 IL-4 receptor. AAMϕ are characterized by highly up-regulated arginase I, mannose receptor (CD206), as well as the secretion of chitinase and “found in inflammatory zone” (FIZZ) family members as YM1, FIZZ1, and FIZZ24, 5. AAMϕ are implicated in allergic, cellular and humoral responses to parasitic and extracellular pathogens2. In fact, AAMϕ are recruited to the site of tissue invasion of the parasitic worm, Heligmosomoides polygyrus, in the submucosa of the duodenum, where they regulate larval metabolism and exit from the tissue6.
Enteric nematode infection is characterized by an intestinal smooth muscle hyper-contractility that is dependent on IL-4/IL-13 and receptor-mediated activation of Stat6 signaling pathway7, 8. Infection also causes intestinal smooth muscle hypertrophy in which insulin-like growth factor-1 (IGF-1) is thought to play an important role9. We hypothesize that intestinal macrophages play a critical role in nematode infection-induced intestinal smooth muscle hyper-contractility that contribute to worm expulsion. To investigate this possibility, we evaluated: (i) the role of IL-4/IL-13 and Stat6 signaling in Nippostrongylus brasiliensis (N. brasiliensis) infection-induced macrophage recruitment and activation; (ii) effect of macrophage depletion on infection-induced intestinal smooth muscle hyper-contractility and smooth muscle morphology, and worm expulsion; and (iii) the involvement of arginase I pathway of macrophage activation on nematode-induced alterations in gut function. The results of these investigations demonstrated that infection induced a recruitment of AAMϕ that is dependent IL-4/IL-13 activating Stat6 signaling pathway. Alternatively activated macrophages are involved in the infection-induced smooth muscle functional and morphological changes, and contribute to worm expulsion from the intestinal lumen. Furthermore, inducible arginase I is required for the AAMϕ to act as a regulator of intestinal smooth muscle function during worm infection.
Materials and Methods
Mice
BALB/c female wild type (WT) mice were purchased from the Small Animal Division of the National Cancer Institute. Severe compromised immunodeficiency (SCID) mice were purchased from The Jackson Laboratory (Bar Harbor, ME 04609). Mice deficient in Stat6 (Stat6−/−), IL-4 (IL-4−/−), and IL-13 (IL-13−/−) were obtained from the breeding colonies at the University of Cincinnati, as a generous gift from Dr. Fred Finkelman, or National Institutes of Health, respectively. These studies were conducted in accordance with principles set forth in the Guide for Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, National Research Council, Health and Human Services Publication (National Institutes of Health 85-23, revised 1996), and the Beltsville Animal Care and Use Committee, 2003.
Administration of IL-13
Mice (n= 5/group) were injected iv with saline or 10µg of IL-13 daily for 7 days. The amount of cytokine administered was based on the observation that daily injection of immunocompetent BALB/c mice with this dose of IL-13 enhances worm expulsion10.
Nippostrongylus brasiliensis infection and worm expulsion
Infective, third stage larvae of N. brasiliensis (L3) (specimens on file at the U.S. National Parasite Collection, U.S. National Helminthological Collection, Collection 81930, Beltsville, MD) were propagated and stored at room temperature in fecal/charcoal/peat moss culture plates until used8. Groups of mice were inoculated subcutaneously with 500 L3 and studied 9 days later. The timing of the studies following infection with N. brasiliensis correlates with the time of the maximal effects on gut function and coincides with worm expulsion8. Appropriate age-matched controls were performed for each infection. Worm egg production were determined as described previously10, but total adult worms were detected qualitatively by scanning the intestinal surface with a dissecting scope to preserve the tissue for appropriate physiological analysis. In general, mice completely expel worms by day 9 after inoculation, and therefore, the presence of adult worms in the intestine indicates a delay in expulsion.
Liposome-mediated macrophage depletion and inhibition of arginase activity in vivo
Methods used to deplete macrophage and inhibit arginase activity in vivo were described previously6. Briefly, clodronate- (Cl2MDP) or control PBS-containing liposomes were administrated (0.2 ml, i.v. at days 0, 1, 3, 5, 7, and 9 after inoculation) to mice to deplete macrophages. Liposomes were generated as previously described using phosphatidylcholine (LIPOID E PC; Lipoid GmbH, Ludwigshafen, Germany) and cholesterol (Sigma, St. Louis, MO)11, 12. Cl2MDP was a kind gift of Roche Diagnostics GmbH (Mannheim, Germany). For arginase inhibition, mice were given 0.2% S-(2-boronoethyl)-I-cysteine (BEC) via drinking water at day 2–9 post infection.
In vitro contractility
In vitro smooth muscle contractility was measured as described previously8. Briefly, one centimeter segments of jejunum were flushed of their intestinal contents, suspended longitudinally in individual 8 ml-organ baths, and maintained in oxygenated Kreb’s solution at 37°C. One end of the tissue was attached to an isometric tension transducer (Model FT03; Grass Medical Instruments, Quincy, MA) and the other to the bottom of the bath. Tissues were stretched to a load of 19.6 mN (2g). Tension was recorded using a Grass model 79 polygraph (Grass Medical Instruments, Quincy, MA) and expressed as force per cross sectional area13. After equilibration, intestinal smooth muscle responses to 5-HT (100µM), or acetylcholine (10nM-0.1mM) were examined. The amplitude of spontaneous contractions was also measured over a 2-min period immediately before addition of agonists.
RNA extraction, cDNA synthesis and real-time quantitative polymerase chain reaction (PCR)
Total RNA was prepared from full thickness sections of jejunum unless indicated. In some cases, the mucosa layer was dissected away from the muscle layer and both layers were processed separately for RNA isolation. Detailed methods for RNA isolation, PCR, and primer sequences, are presented in the supplemental material.
Immunofluorescent staining
Frozen blocks of mid-jejunum were prepared using the Swiss-roll technique and stored at −80°C. Tissue sections (4 µm) were cut from frozen blocks using an HM505E cryostat (Richard-Allan Scientific, Kalamazoo, MI). Details of the methods for immunofluorescence staining are provided in the supplemental material. The images were taken by establishing settings for the samples from the individual vehicle groups and using the same conditions to evaluate the samples from the infected or treated groups. Fluorescent channels were photographed separately and then merged together to locate the AAMϕ. Comparisons were made only among slides prepared on the same day. Smooth muscle thickness was determined in Giemsa-stained sections for each treatment group.
Solutions and drugs
Krebs buffer contained (in mM) 4.74 KCl, 2.54 CaCl2, 118.5 NaCl, 1.19 NaH2PO4, 1.19 MgSO4, 25.0 NaHCO3, and 11.0 glucose. All drugs were obtained from Sigma (St Louis, MO) unless indicated otherwise. On the day of the experiment, 5-HT was dissolved in water and appropriate dilutions were made.
Data analysis
Agonist responses were fitted to sigmoid curves (Graphpad, San Diego, CA). Statistical analysis was performed using one-way ANOVA followed by Neuman-Keuls test to compare the responses, mRNA expression, and smooth muscle thickness among the different treatment groups. Appropriate vehicle-, time-, and age-matched controls were performed for each group.
Results
Nippostrongylus brasiliensis infection induced an accumulation of AAMϕ
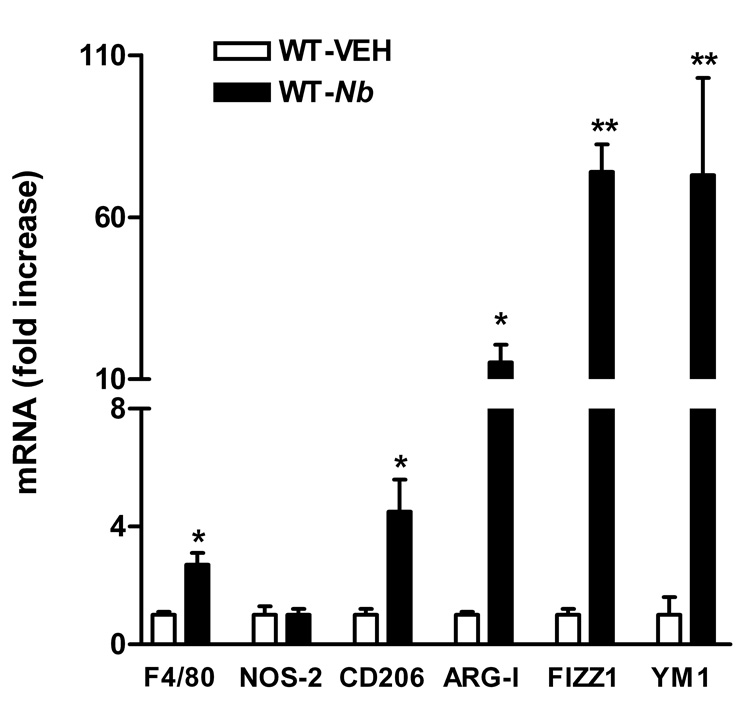
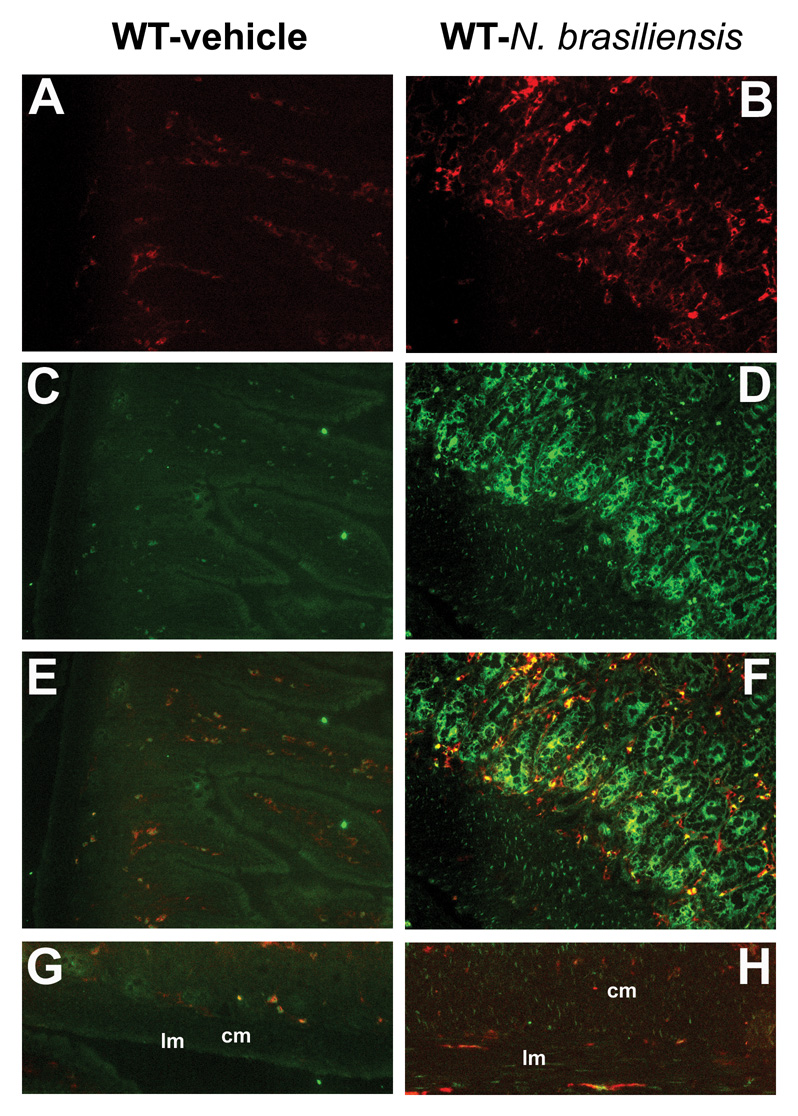
Infection with N. brasiliensis induced a polarized Th2 cytokine response with elevated production of IL-4 and IL-13 at 9 days post infection, but no change in the expression of IFN-γ14. The mRNA expression of F4/80, a general macrophage molecular marker, was up-regulated after infection (Figure 1), indicating the recruitment of macrophages to the small intestine. Additional studies were performed to examine the macrophage phenotype by measuring the expression of macrophage molecular markers. In the whole thickness sections of jejunum, infection up-regulated the expression of AAMϕ molecular markers (Figure 1), including CD206, arginase I, FIZZ1, and YM1. The expression of NOS-2, the CAMϕ marker, remained unchanged after N. brasiliensis infection (Figure 1). The infection-induced up-regulation of AAMϕ molecular markers was also observed in samples from smooth muscle dissected from mucosa/submusoca, including CD206 (4.2±1.6 fold), arginase I (16.6±4.2 fold), and FIZZ1 (17.1±12.1 fold). These data indicate that the accumulated macrophages in intestine after N. brasiliensis infection display AAMϕ, rather than CAMϕ phenotype. To visualize macrophage infiltration, sections of intestine were stained with anti-F4/80-Alexa and anti-CD206-FITC. In control mice, there were a few F4/80+/CD206+ cells located mainly in the lamina propria of the intestine (Figure 2E). In infected mice, the increase in the number of F4/80+/CD206+ macrophages was observed in both mucosal (Figure 2F) and the smooth muscle layers (Figure 2H).
Figure 1.
N. brasiliensis infection induced changes in the mRNA expressions of macrophage molecular markers. Mice were inoculated subcutaneously with 500 N. brasiliensis (Nb) infective third stage larvae or treated with vehicle (VEH), and studied 9 days later. Intestinal strips were taken from the mice for total RNA extraction. Real-time quantitative PCR was performed to measure the mRNA expression. The fold increases were relative to the individual vehicle groups (VEH) after normalization to 18s rRNA. * p<0.05, **p<0.01 compared with the respective WT-VEH (n≥5 for each group).
Figure 2.
Increased infiltration and alternative activation of macrophages in the whole section (A–F) or smooth muscle layer (G, H) of small intestine from mice infected with N. brasiliensis. Mice were inoculated subcutaneously with 500 N. brasiliensis infective third stage larvae (B, D, F, H) or treated with vehicle (A, C, E, G), and studied 9 days later. Frozen tissue blocks of mid-jejunum were prepared and the sections were cut for immunofluoresence staining for anti-F4/80-Alexa647 (A, B) or anti-CD206-FITC (C, D). Fluorescent channels were photographed separately and then merged together to locate the alternatively activated macrophages in the whole section of the small intestine (E, F). For smooth muscle layer, only the merged picture is shown (G, H). All the pictures are the representatives from each group of at least 5 mice. Original magnification, x200; lm: longitudinal smooth muscle layer; cm: circular smooth muscle layer.
Immune-mediated macrophage infiltration and activation
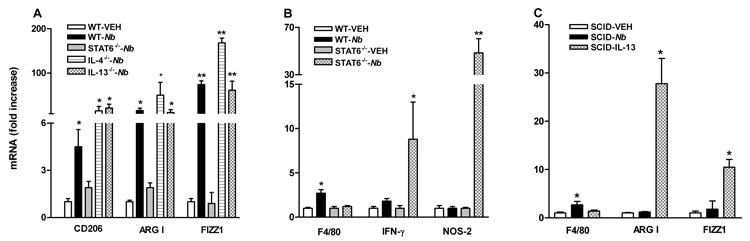
To determine the role of Th2 cytokines IL-4/IL-13 and the Stat6 signaling pathway in the development of AAMϕ, IL-4−/−, IL-13−/−, or Stat6−/− mice were infected with N. brasiliensis. The mRNA expression of arginase I, CD206, FIZZ1, or NOS-2 was similar in all strains in uninfected mice (data not shown). Infection-induced up-regulation of CD206, arginase I, or FIZZ1 in WT mice was not seen in Stat6−/−, but remained elevated in IL-4−/− or IL-13−/− mice (Figure 3A), indicating that the infection-induced accumulation and development of AAMϕ can be mediated by either IL-4- or IL-13 activation of Stat6 signaling. It should be noted that N. brasilinesis are cleared effectively in IL-4−/− mice10, but not in IL-13−/− mice15.
Figure 3.
Dependence of infection-induced macrophage recruitment and activation on IL-4/IL-13 activating Stat6 and innate verse adaptive immune response. WT, SCID, IL-4−/−, IL-13−/−, or Stat6−/− mice were inoculated subcutaneously with 500 N. brasiliensis (Nb) infective third stage larvae or treated with vehicle (VEH), and studied 9 days later. One group of SCID mice was given (i.v.) exogenous IL-13 for 7 days. Intestinal strips were taken for total RNA extraction. Real-time quantitative PCR was performed to measure the mRNA expression. The fold increases were relative to the individual vehicle groups (VEH) after normalization to 18s rRNA. * p<0.05, **p<0.01 compared with the respective WT-VEH (n≥5 for each group).
The expression of IFN-γ was not affected by N. brasiliensis infection in WT, but was up-regulated significantly in infected Stat6−/− mice (Figure 3B). Correspondingly, NOS-2 expression was significantly up-regulated (Figure 3B), indicating the intestinal macrophages in infected Stat6−/− mice display the CAMϕ phenotype. This result confirmed the classical activation of macrophage in a Th1 microenvironment. F4/80 expression in Stat6−/−-infected mice was detectable, but not significantly different from that in uninfected Stat6−/−mice (Figure 3B), indicating that the accumulation of AAMϕ is Stat6-dependent. In addition, these data suggest that the increased production of NOS-2 in infected Stat6−/− mice is derived from resident macrophages.
To determine the role of innate verses adaptive immune response in the infection-induced accumulation and/or activation of macrophages, SCID mice were infected with N. brasiliensis. Infection up-regulated mRNA expression of F4/80, but not arginase I and FIZZ1, in SCID mice (Figure 3C), indicating that the recruitment of macrophages during infection was independent of T and B cells, and was mediated by innate immune response. On the other hand, AAMϕ require T and B cell-dependent adaptive immune response to produce adequate amounts of Th2 cytokines; therefore, we determined the effect of exogenous administration of IL-13 to SCID mice. IL-13 up-regulated the expression of AAMϕ markers as expected (Figure 3C). N. brasiliensis infection also slightly up-regulated the expression of IL-4 (2.8±1.5 fold) and IL-13 (16±12 fold) in SCID mice, but the levels were significantly lower than those in WT infected mice14.
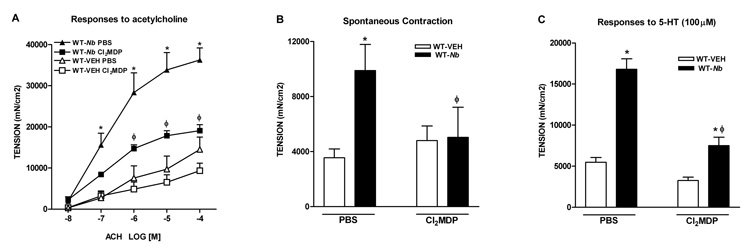
Depletion of macrophages abolished nematode infection-induced intestinal smooth muscle function and morphology
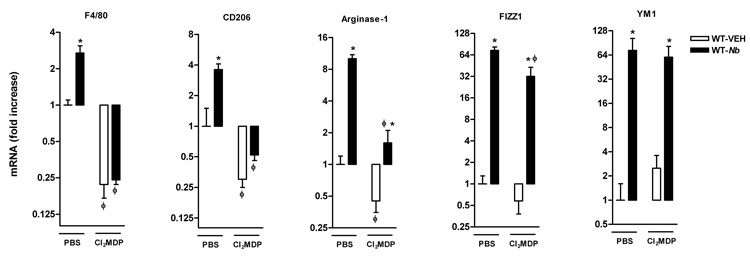
We showed previously that nematode infection induced an intestinal smooth muscle hyper-contractility that was dependent on Th2 cytokines, IL-4 and IL-13 acting on Stat68. To determine if macrophages contribute to the infection-induced changes in intestinal smooth muscle function, mice were treated with Cl2MDP-liposome to deplete macrophages. Compared to mice treated with PBS, Cl2MDP treatment significantly decreased F4/80 expression both in uninfected and _N. brasiliensis_-infected mice, indicating Cl2MDP effectively depleted not only infection-elicited, but also the resident macrophages in the small intestine (Figure 4). Similarly, Cl2MDP treatment decreased the constitutive expression of CD206 and arginase I in uninfected mice, and abolished the infection-induced up-regulation of these markers (Figure 4). It is noteworthy that FIZZ1 and YM1 are two commonly cited AAMϕ molecular markers; however, our data showed that N. brasiliensis infection-induced up-regulation of FIZZ1 was slightly, but significantly, attenuated and that the up-regulation of YM1 was unaffected by Cl2MDP treatment suggesting that cells, other than AAMϕ, can express these genes in the intestine (Figure 4).
Figure 4.
Clodronate-liposome treatment depleted both resident and recruited macrophages in the intestine, indicated by the decreased mRNA expressions of macrophage markers in uninfected mice, or abolishing of the upregulation of the markers in _N. brasiliensis_–infected mice. Mice were infected with N. brasiliensis (Nb) or treated with vehicle (VEH). Clodronate- (Cl2MDP) or control PBS- (PBS) containing liposomes were administrated (i.v., 0.2 ml at days 0, 1, 3, 5, 7, and 9 after inoculation) to deplete macrophages. Intestinal strips were taken for total RNA extraction. Real-time quantitative PCR was performed to measure the mRNA expression. The fold increases were relative to the individual vehicle groups (VEH) after normalization to 18s rRNA. *p<0.05 vs the respective WT-VEH; ϕp<0.05 vs the respective PBS (n≥5 for each group).
Consistent with previous studies, N. brasiliensis infection induced a smooth muscle hyper-contractility8, 14, 16. Cl2MDP treatment did not significantly affect the intestinal smooth muscle function in uninfected mice, but attenuated or abolished the smooth muscle hyper-contractility to acetylcholine (Figure 5A) and to 5-HT (Figure 5C) as well as the increased amplitude of spontaneous contractions (Figure 5B) in _N. brasiliensis_-infected mice. In addition, the attenuated smooth muscle responses to 5-HT was associated with a lower 5-HT2A mRNA expression (2.9 ± 0.4 in WT-_Nb_-Cl2MDP verses 7.3 ± 0.3 in WT-_Nb_-PBS, p<0.05, n≥4). We showed previously that 5-HT2A is the major receptor responsible for the infection-induced smooth muscle hypercontractility to 5-HT16.
Figure 5.
Depletion of macrophages by clodronate-liposome treatment attenuated nematode infection-induced intestinal smooth muscle hypercontractility. Mice were infected with N. brasiliensis (Nb) or treated with vehicle (VEH). Clodronate- (Cl2MDP) or control PBS- (PBS) containing liposomes were administrated (i.v., 0.2 ml at days 0, 1, 3, 5, 7, and 9 after inoculation) to deplete macrophages. Intestinal strips were taken from the mice and suspended longitudinally in organ baths for in vitro contractility studies in response to (A) acetylcholine (ACH), (C) serotonin (5-HT), or (B) for spontaneous contraction.
*p<0.05 vs the respective WT-VEH; ϕp<0.05 vs the respective PBS (n≥5 for each group).
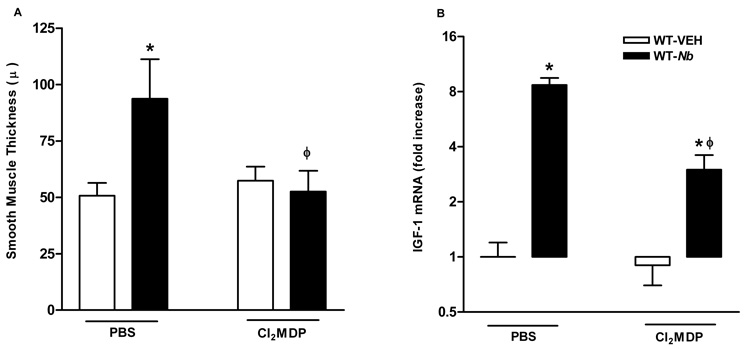
N. brasiliensis infection also resulted in increase in intestinal smooth muscle thickness that may be due to hypertrophy as well as hyperplasia. Cl2MDP treatment did not affect the smooth muscle thickness in uninfected control mice, but abolished nematode infection-induced changes smooth muscle morphology (Figure 6A). This effect was associated with a significant reduction of the mRNA expression of IGF-1 (Figure 6B), a growth factor known to be involved in smooth muscle cell proliferation9.
Figure 6.
Depletion of macrophages abolished nematode infection-induced increase in intestinal smooth muscle thickness (A) and was associated with a reduced mRNA expression of IGF-1 (B). Mice were infected with N. brasiliensis (Nb) or treated with vehicle (VEH). Clodronate-(Cl2MDP) or control PBS- (PBS) containing liposomes were administrated (i.v., 0.2 ml at days 0, 1, 3, 5, 7, and 9 after inoculation) to deplete macrophages. Changes in smooth muscle thickness were assessed in Giemsa-stained sections (A); or whole tissue was processed for the measurement of mRNA expression of IGF-1 by real-time quantitative PCR (B). *p<0.05 vs the respective WT-VEH; ϕp<0.05 vs the respective PBS (n≥5 for each group).
Arginase is required for the infection-induced alternations in intestinal smooth muscle
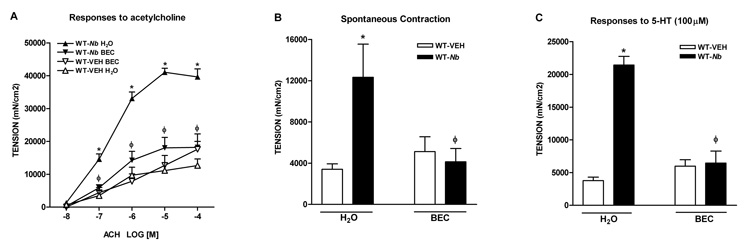
Alternatively activated macrophages are associated with the up-regulation of arginase I, an inducible enzyme that catalyzes L-arginine to produce proline. Up-regulation of arginase I in AAMϕ is implicated in Th2-mediated pathologies including allergy and nematode infection. N. brasiliensis infection induced a significant increase in the expression of arginase I that was dependent on Stat6 (Figure 3A). To investigate if arginase I is one of the effector molecules that mediate host’s protective immunity against N. brasiliensis, mice were administrated BEC to block arginase activity. BEC is a general inhibitor for both arginase I and II; however, N. brasiliensis infection significantly increased the expression of arginase I (Figure 1) but inhibited the expression of arginase II (1.0 ± 0.1 in WT-VEH verses 0.3 ± 0.0 in WT-Nb, p<0.05, n>5), suggesting the inhibitory effect of BEC was primarily on arginase I. BEC treatment alone did not affect intestinal smooth muscle function in control mice (Figure 7). In contrast, this treatment abolished or attenuated the infection-induced smooth muscle hyper-contractile responses to acetylcholine (Figure 7A) and 5-HT (Figure 7C), and the increased amplitude of spontaneous contractions (Figure 7B).
Figure 7.
Inhibition of arginase abolished nematode infection-induced intestinal smooth muscle hypercontractility. Mice were infected with N. brasiliensis (Nb) or treated with vehicle (VEH), and were given 0.2% S-(2-boronoethyl)-I-cysteine (BEC) via drinking water at day 2–9 post infection for arginase inhibition in vivo. Intestinal strips were taken from the mice and suspended longitudinally in organ baths for in vitro contractility studies in response to (A) acetylcholine (ACH, 10nM-0.1mM), (C) serotonin (5-HT, 100µM), or (B) for spontaneous contraction. *p<0.05 vs the respective WT-VEH H2O; ϕp<0.05 vs the respective WT-Nb H2O (n≥5 for each group).
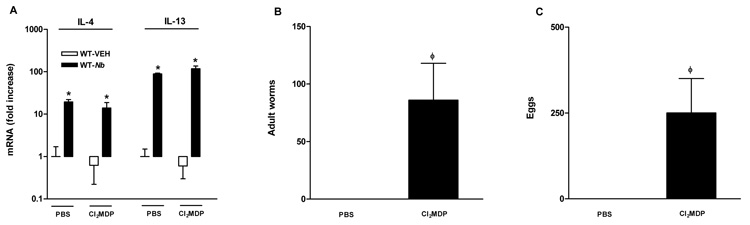
Macrophages or arginase were not required for N. brasiliensis infection-induced up-regulation of IL-4/IL-13
Macrophages are known to be one of the major sources of some cytokines that direct the adaptive immune response to pathogens. To determine whether the effects of macrophage depletion or arginase inhibition on intestinal smooth muscle function are mediated by alterations in cytokine production, we determined the expression of key Th1 and Th2 cytokines. Expression of IL-4, IL-13, and IFN-γ in the small intestine was not affected by Cl2MDP or BEC treatment from either uninfected or _N. brasiliensis_-infected mice (Figure 8A and data not shown). Macrophage markers were also monitored to examine if inhibition of arginase altered the development of AAMϕ. BEC treatment did not affect significantly the mRNA expression of CD206, arginase I, FIZZ1, or NOS-2 in either uninfected or _N. brasiliensis_-infected mice (data not shown), indicating that inhibition of arginase did not change the phenotype of macrophage activation.
Figure 8.
Depletion of macrophages resulted in impaired expulsion of N. brasiliensis, but did not affect N. brasiliensis infection-induced upregulation of IL-4 or IL-13. Mice were infected with N. brasiliensis (Nb) or treated with vehicle (VEH). Clodronate- (Cl2MDP) or control PBS-(PBS) containing liposomes were administrated (i.v., 0.2 ml at days 0, 1, 3, 5, 7, and 9 after inoculation) to deplete macrophages. Intestinal strips were taken for the measurement of the mRNA expression of IL-4 and IL-13 by real-time quantitative PCR (A). Separate groups of mice were infected with N. brasiliensis and treated with Cl2MDP. At the day 9 post infection, the intestine was collected for worm counting (B) and feces was collected for egg counting (C). *p<0.05 vs the respective WT-VEH; ϕp<0.05 vs the respective PBS (n≥5 for each group).
Macrophages, arginase, and worm expulsion
Adult worm numbers in the small intestine and egg production from feces were monitored to determine if the macrophage depletion or BEC treatment impaired worm expulsion. At day 9 post infection, there were no worms in the small intestine of mice treated with PBS while there were worms in the small intestine as well as eggs in the feces in mice treated with Cl2MDP (Figure 8B, C). In 3 out of 5 mice treated with BEC, there were numerous worms visible throughout the small intestine; however, there were no eggs in the feces of these mice. These observations indicate that treatment with either Cl2MDP or BEC impaired the protective responses against N. brasiliensis infection in the intestine.
Discussion
Enteric nematode infection is associated with CD4+ T cell-dependent infiltration of a number of immune cells including eosinophils, mast cells, and macrophages at the area of infection. In the present study, we demonstrate that this immune-mediated recruitment and development of AAMϕ in the small intestine is dependent largely on IL-4/IL-13 and activation of Stat6. More importantly, these AAMϕ link Th2 cytokine production to infection-induced alterations in gut function. A distinguishing feature of AAMϕ is the metabolism of L-arginine to ornithine via arginase I. This study is the first to show that AAMϕ and the arginase pathway play a significant role in gastrointestinal smooth muscle responses to nematode infection.
Intestinal macrophages are continuously replenished by circulating monocytes1 and play key roles in mucosal homeostasis as well as host defense. Monocytes entering the healthy intestinal mucosa acquire a specific “tolerogenic” phenotype characterized by a down regulation of receptors for LPS, and the toll like receptors as well as specific chemokine receptors17, 18. In response to enteric pathogens, cytokine-induced up-regulation of MCP-1 plays a key role in recruitment of additional circulating monocytes to the intestine and differentiation of these infiltrating macrophages. Their location in the lamina propria is strategic in the mucosal response to pathogens that cross the epithelial barrier. There is also a population of resident macrophages in the smooth muscle layer that were shown to impact smooth muscle function in response to endotoxin/LPS19 or oxidative stress20. It is well established that macrophages undergo alternative activation in the context of strong Th2 cytokine environments, including helminth infection, asthma, or allergy2. Consistent with previous studies, we showed here that N. brasiliensis infection induced an increased infiltration and accumulation of AAMϕ, characterized by up-regulation of F4/80, CD206, arginase I, FIZZ1, and YM1. The requirement of Th2 cytokines IL-4- or IL-13-mediated activation of Stat6 for infection-induced accumulation and activation of AAMϕ was established by showing that N. brasiliensis failed to up-regulate AAMϕ markers in Stat6−/− mice. The significantly elevated AAMϕ marker mRNA expression in IL-4−/− or IL-13−/− mice indicates that either cytokine alone is capable of eliciting the full development of AAMϕ.
Intestinal macrophages are involved in both innate and adaptive immune response. A recent study implicated AAMϕ in the innate immune response to N. brasiliensis infection in the lung showing that early up-regulation of marker expression was dependent on Th2 cytokines released from resident granulocytes rather than T cells21. We used SCID mice to investigate the role of the innate versus adaptive immune response to infection-induced recruitment and development of AAMϕ in the small intestine. _N. brasiliensis_-infected SCID mice showed a small, but significant, increase in IL-4 and IL-13 expression as well as an increased infiltration of macrophages. These macrophages, however, did not express markers of the AAMϕ phenotype suggesting that the levels of IL-4 and IL-13 in SCID mice are insufficient to induce development of AAMϕ. It is of interest, that treatment of SCID mice with exogenous IL-13 was able to induce the AAMϕ phenotype and increase smooth muscle hyper-contractility. Unlike N. brasiliensis infection, IL-13 did not appear to increase macrophage infiltration. Thus, IL-13 has the ability to work independently of the adaptive immune system to induce an increase in smooth muscle function that is associated with an elevated expression of AAMϕ markers in resident macrophages.
The results of the current study are consistent with other reports showing that AAMϕ influence the immune outcome during infections. Alternatively activated macrophages are linked to the suppression of T cell responsiveness in chronic infections22 and are important for down regulation of Th2 mediated immune pathology. In the present study, macrophage depletion did not change either Th1 (IFN-γ) or Th2 (IL-4, IL-13) cytokine expression, suggesting that macrophages are not involved in the initiation of the cytokine response elicited by N. brasiliensis infection, but are part of the downstream events in response to increased Th2 cytokine production.
Host resistance to enteric parasites is associated with significant Th2- and Stat6-dependent changes in intestinal physiology8. We and others showed previously that infection with several different enteric nematodes induced a stereotypic elevation in intestinal smooth muscle responses to acetylcholine, serotonin, agonists of protease-activated receptors, and to nerve stimulation7, 8, 14, 16, 23. The contribution of macrophages to Th1-mediated changes in smooth muscle contractility is well established20, 24. To determine the functional role of AAMϕ on N. brasiliensis infection, we used Cl2MDP-liposome to deplete macrophages. Cl2MDP treatment effectively depleted not only infection-elicited, but also the resident macrophages evidenced by the decreased expression of macrophage markers in both infected and uninfected mice. Although macrophage-depleted uninfected mice displayed similar intestinal smooth muscle function to that untreated controls, the intestinal smooth muscle hyper-contractility observed in _N. brasiliensis_-infected mice treated with PBS-liposomes was absent in Cl2MDP-treated infected mice. These data indicate that the resident macrophages do not play a major role in the constitutive regulation of intestinal smooth muscle contractility, but are required absolutely for the hyper-contractility in nematode infection. Additionally, these macrophage-depleted mice had an impaired ability to expel worms, confirming the contribution of AAMϕ in host defense against nematode infection6 albeit by more than one mechanism. Infection also induces a STAT6-dependent increase in the smooth muscle responses to 5-HT, an effect associated with an up-regulation of the 5-HT2A receptor16. The present study shows that this elevated 5-HT2A expression is dependent, in part, on the presence of AAMϕ emphasizing the importance of the interaction between macrophages and smooth muscle in the Th2-mediated hyper-contractility to 5-HT.
Alternatively activated macrophages express/secrete a number of proteins that could be responsible for the protective effects of macrophages against nematode infection. Of these molecules, arginase I is the enzyme distinguishes AAMϕ from CAMϕ, leading to hydrolysis of L-arginine to ornithine25, a precursor for polyamine biosynthesis via ornithine decarboxylase, or proline via ornithine aminotransferase. Both of these pathways are implicated in cell proliferation and collagen production. Indeed, increased arginase activity is linked to airway hyper responsiveness or decreased airway smooth muscle relaxation in asthma26, 27. Moreover, elevated arginase I expression increases airway smooth muscle proliferation by mechanisms involving the production of polyamines28. Although up-regulation of arginase I is a common feature in nematode infection29, its function in host defense against nematode infection remains unclear. We showed here that administration of BEC in _N. brasiliensis_-infected mice abolished the intestinal smooth muscle hyper-contractility and impaired worm expulsion. Although BEC also inhibits arginase II, the significantly down-regulated arginase II versus up-regulated arginase I expression in _N. brasiliensis_-infected mice suggests that the effects of BEC were mainly through the inhibition of arginase I. The precise mechanism by which arginase regulates intestinal smooth muscle function was not investigated in the current study. Both arginase I and NOS use the same substrate L-arginine suggesting that arginase inhibition could affect the NO production. It is, however, unlikely that NO production is altered in BEC-treated mice as BEC had no effect on smooth muscle contractility in control mice. The results of the present study indicate that influx of AAMϕ with increased arginase activity play a major role in infection-induced hyper-contractility and increased smooth muscle thickness.
In conclusion, the results of the present study link the up-regulation of Th2 cytokine and activation of Stat6 with the accumulation of AAMϕ that control smooth muscle contractility and morphology via arginase I metabolism of arginine. We recently observed that mice deficient in IL-13Rα1 express arginase I after infection with N. brasiliensis but do not expel worms from the intestine30. This is consistent with the observation that IL-4−/− or IL-13−/− mice express CD206, FIZZ1, and arginase I after N. brasiliensis infection, but only IL-13−/− mice fail to expel worms. Thus, AAMϕ, like eosinophils, mast cells, and goblet cells that develop in response to nematode infection in the intestine, respond to orchestrated cues in the local environment to affect functional activity of surrounding cells such as smooth muscle and also contribute to protective immunity against infection.
Supplementary Material
01
Acknowledgement
The authors wish to thank Dr. Debra Donaldson, Respiratory Disease, Wyeth Research, Cambridge, MA for the generous gift of IL-13, and Dr. Fred D Finkelman, University of Cincinnati, for providing IL-13 deficient mice.
_Grant Support_-- This work was supported by NIH grants RO1-AI/DK49316 (T.S-D), USDA CRIS project #1235-52000-053 (JFU), and NIH grants R01-AI031678 (WCG). The opinions and assertions in this article are those of the authors and do not necessarily represent those of the U. S. Department of Agriculture.
Abbreviations
IL
interleukin
IL-4−/−
IL-4-deficient
IL-13−/−
IL-13-deficient
N. brasiliensis
Nippostrongylus brasiliensis
PCR
polymerase chain reaction
Stat6−/−
signal transducer and activator of transcription 6-deficient
Th2
type 2 T helper cells
WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
Reference List
- 1.Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–159. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 2.Gordon S. ALTERNATIVE ACTIVATION OF MACROPHAGES. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 3.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 4.Raes G, Noel W, Beschin A, Brys L, De BP, Hassanzadeh GH. FIZZ1 and Ym as tools to discriminate between differentially activated macrophages. Dev Immunol. 2002;9:151–159. doi: 10.1080/1044667031000137629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raes G, De BP, Noel W, Beschin A, Brombacher F, Hassanzadeh GG. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol. 2002;71:597–602. [PubMed] [Google Scholar]
- 6.Anthony RM, Urban JF, Jr, Alem F, Hamed HA, Rozo CT, Boucher JL, Van RN, Gause WC. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akiho H, Blennerhassett P, Deng Y, Collins SM. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G226–G232. doi: 10.1152/ajpgi.2002.282.2.G226. [DOI] [PubMed] [Google Scholar]
- 8.Zhao A, McDermott J, Urban JF, Jr, Gause W, Madden KB, Yeung KA, Morris SC, Finkelman FD, Shea-Donohue T. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. 2003;171:948–954. doi: 10.4049/jimmunol.171.2.948. [DOI] [PubMed] [Google Scholar]
- 9.Kuemmerle JF, Zhou H, Bowers JG. IGF-I stimulates human intestinal smooth muscle cell growth by regulation of G1 phase cell cycle proteins. Am J Physiol Gastrointest Liver Physiol. 2004;286:G412–G419. doi: 10.1152/ajpgi.00403.2003. [DOI] [PubMed] [Google Scholar]
- 10.Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 11.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 12.Van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93–99. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- 13.Zhao A, Bossone C, Pineiro-Carrero V, Shea-Donohue T. Colitis-induced alterations in adrenergic control of circular smooth muscle in vitro in rats. J Pharmacol Exp Ther. 2001;299:768–774. [PubMed] [Google Scholar]
- 14.Zhao A, Morimoto M, Dawson H, Elfrey JE, Madden KB, Gause WC, Min B, Finkelman FD, Urban JF, Jr, Shea-Donohue T. Immune Regulation of Protease-Activated Receptor-1 Expression in Murine Small Intestine during Nippostrongylus brasiliensis Infection. J Immunol. 2005;175:2563–2569. doi: 10.4049/jimmunol.175.4.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8:339–342. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhao A, Urban JF, Jr, Morimoto M, Elfrey JE, Madden KB, Finkelman FD, Shea-Donohue T. Contribution of 5-HT2A receptor in nematode infection-induced murine intestinal smooth muscle hypercontractility. Gastroenterology. 2006;131:568–578. doi: 10.1053/j.gastro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Hausmann M, Kiessling S, Mestermann S, Webb G, Spottl T, Andus T, Scholmerich J, Herfarth H, Ray K, Falk W, Rogler G. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 18.Spoettl T, Hausmann M, Herlyn M, Gunckel M, Dirmeier A, Falk W, Herfarth H, Schoelmerich J, Rogler G. Monocyte chemoattractant protein-1 (MCP-1) inhibits the intestinal-like differentiation of monocytes. Clinical & Experimental Immunology. 2006;145:190–199. doi: 10.1111/j.1365-2249.2006.03113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hori M, Kita M, Torihashi S, Miyamoto S, Won KJ, Sato K, Ozaki H, Karaki H. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am J Physiol Gastrointest Liver Physiol. 2001;280:G930–G938. doi: 10.1152/ajpgi.2001.280.5.G930. [DOI] [PubMed] [Google Scholar]
- 20.Wehner S, Behrendt FF, Lyutenski BN, Lysson M, Bauer AJ, Hirner A, Kalff JC. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut. 2007;56:176–185. doi: 10.1136/gut.2005.089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reece JJ, Siracusa MC, Scott AL. Innate immune responses to lung-stage helminth infection induce alternatively activated alveolar macrophages. Infect Immun. 2006;74:4970–4981. doi: 10.1128/IAI.00687-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor MD, Harris A, Nair MG, Maizels RM, Allen JE. F4/80+ Alternatively Activated Macrophages Control CD4+ T Cell Hyporesponsiveness at Sites Peripheral to Filarial Infection. J Immunol. 2006;176:6918–6927. doi: 10.4049/jimmunol.176.11.6918. [DOI] [PubMed] [Google Scholar]
- 23.Vallance BA, Blennerhassett PA, Collins SM. Increased intestinal muscle contractility and worm expulsion in nematode-infected mice. Am J Physiol. 1997;272:G321–G327. doi: 10.1152/ajpgi.1997.272.2.G321. [DOI] [PubMed] [Google Scholar]
- 24.Torihashi S, Ozaki H, Hori M, Kita M, Ohota S, Karaki H. Resident macrophages activated by lipopolysaccharide suppress muscle tension and initiate inflammatory response in the gastrointestinal muscle layer. Histochemistry and Cell Biology. 2000;113:73–80. doi: 10.1007/s004180050009. [DOI] [PubMed] [Google Scholar]
- 25.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 26.Meurs H, McKay S, Maarsingh H, Hamer MA, Macic L, Molendijk N, Zaagsma J. Increased arginase activity underlies allergen-induced deficiency of cNOS-derived nitric oxide and airway hyperresponsiveness. Br J Pharmacol. 2002;136:391–398. doi: 10.1038/sj.bjp.0704725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King NE, Rothenberg ME, Zimmermann N. Arginine in asthma and lung inflammation. J Nutr. 2004;134:2830S–2836S. doi: 10.1093/jn/134.10.2830S. [DOI] [PubMed] [Google Scholar]
- 28.Wei LH, Wu G, Morris SM, Jr, Ignarro LJ. Elevated arginase I expression in rat aortic smooth muscle cells increases cell proliferation. Proc Natl Acad Sci U S A. 2001;98:9260–9264. doi: 10.1073/pnas.161294898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair MG, Guild KJ, Artis D. Novel effector molecules in type 2 inflammation: lessons drawn from helminth infection and allergy. J Immunol. 2006;177:1393–1399. doi: 10.4049/jimmunol.177.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, Stevens S, Valenzuela DM, Murphy AJ, Yancopoulos GD, Urban JF, Donnelly RP, Wynn TA. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor [alpha]1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01