Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) (original) (raw)
. Author manuscript; available in PMC: 2008 Nov 26.
Abstract
Although activated spinal cord glia contribute importantly to neuropathic pain, how nerve injury activates glia remains controversial. It has recently been proposed, on the basis of genetic approaches, that toll-like receptor 4 (TLR4) may be a key receptor for initiating microglial activation following L5 spinal nerve injury. The present studies extend this idea pharmacologically by showing that TLR4 is key for maintaining neuropathic pain following sciatic nerve chronic constriction injury (CCI). Established neuropathic pain was reversed by intrathecally delivered TLR4 receptor antagonists derived from lipopolysaccharide. Additionally, (+)-naltrexone, (+)-naloxone, and (-))-naloxone, which we show here to be TLR4 antagonists in vitro on both stably transfected HEK293-TLR4 and microglial cell lines, suppressed neuropathic pain with complete reversal upon chronic infusion. Immunohistochemical analyses of spinal cords following chronic infusion revealed suppression of CCI-induced microglial activation by (+)-naloxone and (-))-naloxone, paralleling reversal of neuropathic pain. Together, these CCI data support the conclusion that neuron-to-glia signaling through TLR4 is important not only for initiating neuropathic pain, as suggested previously, but also for maintaining established neuropathic pain. Furthermore, these studies suggest that the novel TLR4 antagonists (+)-naloxone and (-))-naloxone can each fully reverse established neuropathic pain upon multi-day administration. This finding with (+)-naloxone is of potential clinical relevance. This is because (+)-naloxone is an antagonist that is inactive at the (-))-opioid selective receptors on neurons that produce analgesia. Thus, these data suggest that (+)-opioid antagonists such as (+)-naloxone may be useful clinically to suppress glial activation, yet (-))-opioid agonists suppress pain.
Keywords: (+)-naloxone, (+)-naltrexone, microglia, stereoselectivity
Introduction
Investigations over the past 15 years support the idea that glia (microglia and astrocytes) are importantly involved in neuropathic pain (Watkins et al., 2007). That glial activation is causal to neuropathic pain is supported by attenuation of such pain by a variety of glially active drugs with diverse mechanisms of action (Hashizume et al., 2000; Milligan et al., 2003; Sweitzer et al., 2006; Ledeboer et al., 2007; Mika et al., 2007). Furthermore, blockade of products of activated glia (e.g. proinflammatory cytokines) likewise reverses neuropathic pain (Sweitzer et al., 2001; Milligan et al., 2003).
Although it is clear that peripheral nerve injury activates spinal cord glia, the signals that initiate this activation are controversial. Several neuron-to-glia activation signals have been proposed, including fractalkine acting via microglial CX3CR1 (Milligan et al., 2004; Verge et al., 2004) and ATP acting via the microglial P2X4 receptor (Tsuda et al., 2005; Trang et al., 2006) or P2X7 receptor (McGaraughty et al., 2007).
An additional mechanism by which glia may be activated is via stimulation of a member of the toll-like receptor (TLR) family, TLR4. In the central nervous system, TLR4 is expressed on microglia and perhaps astrocytes and endothelial cells, but not on neurons (Miyake, 2007). Although TLR4 is known as the receptor that detects endotoxin [lipopolysaccharide (LPS)], it also detects host cell stress and damage that causes the release of host DNA, RNA, heat shock proteins, cell membrane components and the like (Miyake, 2007). Regarding neuropathic pain, it has been proposed that sensory neuron damage leads to the release of such substances and that these stimulate microglial TLR4 in spinal cord, initiating microglial activation (Tanga et al., 2005). This was tested using TLR4 knockout and TLR4 point mutation mice after L5 spinal nerve transection and by administering TLR4 antisense oligodeoxynucleotide to reduce spinal TLR4 expression. These manipulations, all occurring prior to nerve injury, decreased neuropathic pain, suppressed spinal microglial activation markers, and decreased neuropathy-induced increases in spinal proinflammatory cytokines (Tanga et al., 2005).
The purpose of the present studies was three-fold: first, to test the generality of TLR4 involvement in neuropathic pain by exploring whether TLR4 is involved in pain arising from a second classic neuropathy model, namely, sciatic chronic constriction injury (CCI); second, to define whether TLR4 blockade could reverse well-established neuropathic pain, not simply prevent its initial development (Tanga et al., 2005), as reversal is the more clinically relevant endpoint; and third, to explore whether pharmacological approaches, rather than the genetic manipulations previously used (Tanga et al., 2005), may be effective for targeting TLR4 so to resolve neuropathic pain. Here we tested intrathecal administration of two variants of LPS that bind to but fail to activate TLR4, and (-))- and (+)-isomers of the opioid antagonists naloxone and naltrexone, which we show to non-stereoselectively block TLR4 signaling in vitro. If these opioid antagonists were found to be effective in neuropathic pain, it could provide a novel, blood-brain barrier-permeable, small molecule approach for neuropathic pain control.
Materials and methods
Subjects
Pathogen-free adult male Sprague-Dawley rats (n = 6 rats/group for each experiment; 300-375 g; Harlan Labs, Madison, WI, USA) were used in all experiments. Rats were housed in temperature-controlled (23 ± 3°C) and light-controlled (12-h light/12-h dark cycle; lights on at 07:00 h) rooms with standard rodent chow and water available ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Colorado at Boulder.
Drugs
(+)-Naloxone and (+)-naltrexone were obtained from the National Institute on Drug Abuse (Research Triangle Park, NC and Bethesda, MD, USA). (-))-Naloxone was purchased from Sigma (St Louis, MO, USA). Sterile endotoxin-free isotonic saline (Abbott Laboratories, North Chicago, IL, USA) was the vehicle for all opioids. LPS from an msbB Escherichia coli mutant (a TLR4 antagonist due to its lack of the myristoyl fatty acid moiety of lipid A) and LPS-RS (a TLR4 antagonist naturally produced by Rhodobacter sphaeroides) were purchased from Invivogen (San Diego, CA, USA). Where applicable, drugs were prepared and are reported as free base concentrations. Vehicles were administered in equal volumes to the drugs under test.
Pain threshold
All testing was conducted blind with respect to group assignment. Rats received at least three 60-min habituations to the test environment prior to behavioral testing. The von Frey test (Chaplan et al., 1994) was performed within the sciatic innervation region of the hindpaws as previously described in detail (Chacur et al., 2001; Milligan et al., 2001). Assessments were made prior to (baseline) and at specific times after experimental manipulations, as detailed in each experiment. A logarithmic series of 10 calibrated Semmes-Weinstein monofilaments (von Frey hairs; Stoelting, Wood Dale, IL, USA) was applied randomly to the left and right hindpaws to define the threshold stimulus intensity required to elicit a paw withdrawal response. Log stiffness of the hairs was determined by log10 (milligrams × 10) and ranged from 3.61 (4.07 g) to 5.18 (15.136 g). The behavioral responses were used to calculate absolute threshold (the 50% paw withdrawal threshold) by fitting a Gaussian integral psychometric function using a maximum-likelihood fitting method (Harvey, 1986; Treutwein & Strasburger, 1999), as described in detail previously (Milligan et al., 2000, 2001). This fitting method allows parametric analyses that otherwise would not be appropriate (Milligan et al., 2000, 2001).
CCI
Neuropathic pain was induced using the CCI model of partial sciatic nerve injury (Bennett & Xie, 1988). CCI was performed at the mid-thigh level of the left hindleg as previously described (Milligan et al., 2004). In brief, four sterile chromic gut sutures (cuticular 4-0 chromic gut, FS-2; Ethicon, Somerville, NJ, USA) were loosely tied around the gently isolated sciatic nerve, during the same operation as for intrathecal catheter placements (above). Drug testing was delayed until 10-14 days after surgery to ensure that neuropathic pain was well established prior to the initiation of drug delivery.
Acute and chronic catheter implantation, and intrathecal and subcutaneous drug administration
The method of acute intrathecal drug administration and the construction and implantation of the indwelling intrathecal catheters was based on that described previously (Milligan et al., 1999). Briefly, intrathecal operations were conducted under isoflurane anesthesia (Phoenix Pharmaceuticals, St Joseph, MO, USA) by threading sterile polyethylene-10 tubing (PE-10 Intramedic Tubing; Becton Dickinson Primary Care Diagnostics, Sparks, MD, USA) guided by an 18-gauge needle between the L5 and L6 vertebrae. The catheter was inserted such that the proximal catheter tip lay over the lumbosacral enlargement. For acute intrathecal drug delivery, the catheters were pre-loaded with drugs at the distal end in a total volume of no greater than 25 μL and delivered over 20-30 s once the catheter was in position. Intrathecal doses of mutant LPS and LPS-RS were 20 μg, and those of (+)-naloxone and (+)-naltrexone were 60 μg. For indwelling catheters, the catheters were preloaded with the respective drug treatment so as not to delay the delivery of drug following completion of surgery. The needle was removed and the catheter was sutured to the superficial musculature of the lower back. The catheters were 17 cm in length, and were attached to a pre-loaded osmotic minipump (Alzet, 2001, Cupertino, CA, USA). Chronic intrathecal (+)-naloxone, 25 and 60 μg/h, was delivered for 4 days. For intrathecal (-))-naloxone, 60 μg/h was delivered for 4 days. For acute subcutaneous administration of (+)-naloxone, rats were given 100 mg/kg in a 2 mL/kg dose volume.
Immunohistochemistry
Following the final behavioral testing, rats were transcardially perfused first with isotonic saline and then with fresh 4% paraformaldehyde/0.1 m phosphate buffer (pH 7.4). After dissection and immersion post-fixation in 4% paraformaldehyde/0.1 m phosphate buffer (pH 7.4) for an additional 24 h, the lumbar spinal cords were then dehydrated in 30% sucrose with 0.1% azide at 4°C until slicing. For immunohistochemistry, sections were treated to suppress endogenous peroxidase and to prevent staining of endogenous biotin. The sections were incubated in either primary mouse monoclonal anti-rat glial fibrillary acidic protein (GFAP) (1 : 500; to visualize astrocyte activation; ICN Biomedicals, Costa Mesa, CA, USA) or primary mouse monoclonal anti-rat CD11b/c (OX-42; 1 : 500; to visualize microglial activation as upregulation of complement receptor type 3; BD Pharmingen, San Jose, CA, USA) for 24 h at 4°C. The slides were then incubated in secondary biotinylated goat anti-mouse IgG antibody (1 : 500; Jackson ImmunoResearch, West Grove, PA, USA) overnight at 4°C. Finally, sections were reacted using the avidin-biotin complex procedure (Vector Elite kit; 1 : 100 in phosphate-buffered saline-Triton; 2 h; Vector Laboratories, Burlingame, CA, USA) and 3′,3′-diaminobenzidine (Sigma-Aldrich). Glucose oxidase (Sigma-Aldrich; type V-s; 0.02%) and β-d-glucose (0.1%) were used to generate hydrogen peroxide. Nickel(I) ammonium sulfate was added to the 3′,3′-diaminobenzidine solution (0.025% w/v) to intensify the reaction product. One series of sections was processed as described above, except that the primary antibody was omitted from the incubation buffer (omission controls). The slides were dried overnight, cleared, and coverslipped. Slides were viewed with an Olympus Vannox II bright-field microscope. Images were collected with a Cohu CCD camera coupled to a computer equipped with NIH Image software (version 1.60). Brightness and contrast were kept constant with the images not altered. The intensity of the light source was calibrated each day.
TLR4 cell line culture and reporter protein assay
A human embryonic kidney-293 (HEK-293) cell line stably transfected to express human TLR4 at high levels was purchased from Invivogen (293-htlr4a-md2cd14; here referred to as HEK-TLR4). These cells are stably transfected by Invivogen with multiple genes involved in TLR4 recognition, which include TLR4 and the co-receptors MD2 and CD14. In addition, these cells stably express an optimized alkaline phosphatase reporter gene under the control of a promoter inducible by several transcription factors such as nuclear factor kappaB and activator protein 1. Secreted alkaline phosphatase (SEAP) protein is produced as a consequence of TLR4 activation.
HEK-TLR4 cells were grown at 37°C (5% CO2; VWR incubator model 2300) in 10-cm dishes (Greiner Bio-One, CellStar 632171; Monroe, NC, USA) in normal supplement selection media [Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA), HEK-TLR4 selection (Invivogen), penicillin 10 000 U/mL (Invitrogen), streptomycin 10 mg/mL (Invitrogen), normocine (Invivogen), and 200 nm l-glutamine (Invitrogen)]. The cells were then plated for 48 h in 96-well plates (Microtest 96-well plate, flat bottom, Becton Dickinson; 5 × 103 cells/well) with the same medium. After 48 h, supernatants were removed and replaced with 180 μL of sterile artificial cerebrospinal fluid (124 mm NaCl, 5 mm KCl, 0.1 mm CaCl2.2H2O, 3.2 mm MgCl2.6H2O, 25 mm NaHCO3, 10 mm glucose, pH 7.4) to model in vivo conditions. The drugs under test were then added in 20 μL and incubated for 24 h. Supernatants (15 μL) were then collected from each well for immediate assay.
SEAP in the supernatants was assayed using the Phospha-Light System (Applied Biosystems) according to the manufacturer’s instructions. This is a chemiluminescence assay that incorporates Tropix CSPD chemiluminescent substrate. The 15-μL test samples are diluted in 45 μL of 1× dilution buffer, transferred to 96-well plates (Thermo, Walthma, MA, USA), heated at 65°C in a water bath (Model 210; Fisher Scientific, Pittsburgh, PA, USA) for 30 min, and then cooled on ice to room temperature. Assay buffer (50 μL/well) is added and, 5 min later, reaction buffer (50 μL/well) is added and allowed to incubate for 20 min at room temperature. The light output is then measured in a microplate luminometer (#IL213.1191; Dynex Technologies, Chantilly, VA, USA).
HAPI cell culture and mRNA quantification
A rat microglial cell line (HAPI) (Cheepsunthorn et al., 2001) was grown at 37°C (5% CO2) in 10-cm dishes in normal supplement selection media (Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, penicillin 10 000 U/mL, streptomycin 10 mg/mL, normocine, and 200 nm l-glutamine). The cells were then plated for 24 h in 96-well plates at 8 × 106 cells/well with the same medium. After 24 h, supernatants were removed and replaced with 180 μL of artificial cerebrospinal fluid to model in vivo conditions. The drugs under test were then added in 20 μL and incubated for 4 h. At this time, supernatants were removed, 100 μL of Trizol reagent (Invitrogen) was added to each well, and plates were frozen at -80°C until later analysis. Samples were then centrifuged (12 000 g) at 4°C. After chloroform and isopropyl alcohol isolation steps, samples were vortexed, incubated, and centrifuged (12 000 g) at 4°C. Nucleic acid precipitates were washed twice in 75% ethanol and centrifuged (7500 g) at 4°C. UV spectrophotometry was used to assess purity and concentration. Samples were treated with DNase (DNA-free kit; Ambion), and this was followed by requantitation before cDNA synthesis. Amplification of cDNA was performed using the QuantiTect SYBR Green polymerase chain reaction (PCR) Kit (Qiagen) in iCycler iQ 96-well PCR plates (Bio-Rad) on a MyiQ Single Color Real-Time PCR Detection System (Bio-Rad). The reaction mixture (26 μL) was composed of 1× QuantiTect SYBR Green PCR Master Mix (containing the fluorescent dye SYBR Green I, 2.5 mm MgCl2, dNTP mix, and HotStart Taq DNA polymerase), 10 nm fluorescein, 500 nm each of forward and reverse primers, 25 ng of cDNA, and nuclease-free H2O. The reaction conditions were an initial 15 min at 95°C, followed by 40 cycles of 15 s at 94°C, 30 s at 55-60°C, and 30 s at 72°C. Melt curve analyses were conducted to assess uniformity of product formation, primer-dimer formation, and amplification of non-specific products. Linearity and efficiency of PCR amplification were assessed using standard curves generated by increasing amounts of cDNA. SYBR Green 1 fluorescence (PCR product formation) was monitored in real time using the MyiQ Single Color Real-Time PCR Detection System (Bio-Rad). The threshold for detection of PCR product was set in the log-linear phase of amplification, and the threshold cycle (CT, the number of cycles to reach threshold of detection) was determined for each reaction. The levels of the target mRNAs were quantified, using blinded procedures, relative to the level of the housekeeping gene encoding glyceraldehyde-3-phosphate-dehydrogenase using the comparative CT (ΔCT) method. Expression of the housekeeping gene was not significantly altered by experimental treatment.
Statistics
Data from the von Frey test were analysed as the interpolated 50% thresholds (absolute threshold) in log base 10 of stimulus intensity (monofilament stiffness in milligrams × 10). Pre-drug baseline measures were analysed by one-way anova. Post-drug timecourse measures were analysed by repeated measures two-way anovas followed by Bonferroni post hoc tests, where appropriate. For immunohistochemistry densitometry, analysis of glial activation was done using the percentage of field black procedure as previously described in detail (Milligan et al., 2001). Cell culture data were analysed by anova. Statistical comparisons are indicated on the figures for clarity, and significance was set at P < 0.05.
Results
Experiment 1. Reversal of CCI-induced neuropathic pain by acute intrathecal delivery of the TLR4 antagonists, mutant LPS and LPS-RS
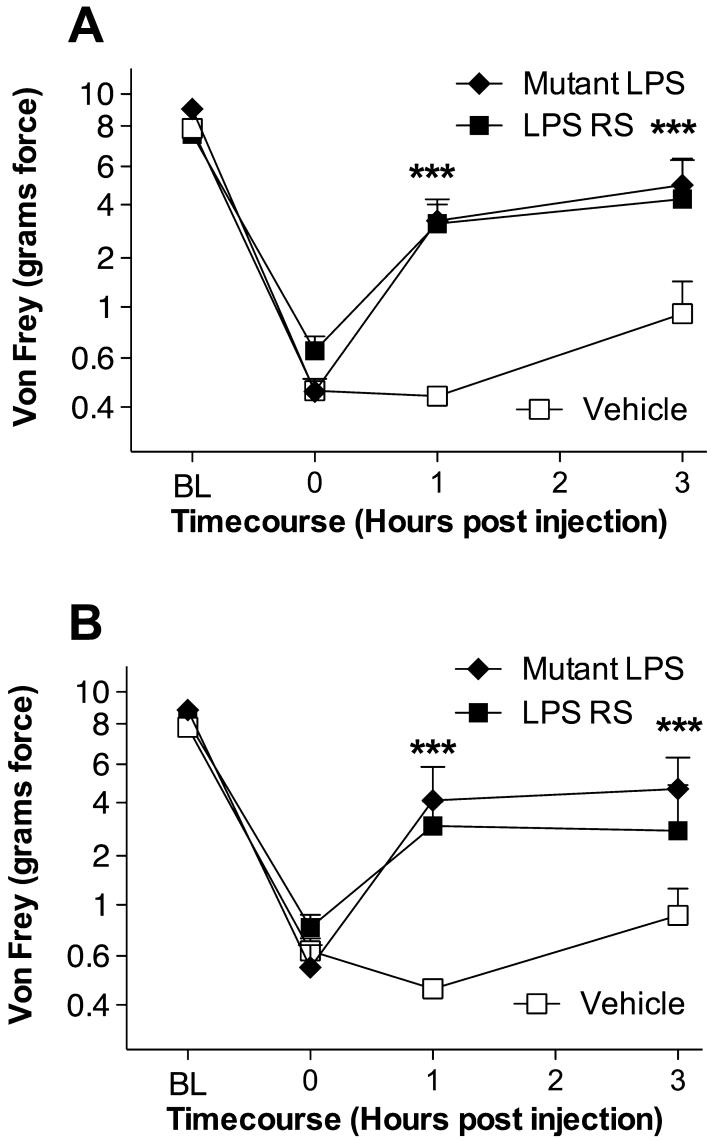
As a first test of whether TLR4 significantly contributes to neuropathic pain (mechanical allodynia) induced by CCI, the effect of a single dose of an intrathecally administered TLR4 receptor antagonist (mutant LPS or LPS-RS) vs. an equal volume of vehicle was examined. No differences were observed between groups in the response thresholds recorded for the hindleg ipsilateral (Fig. 1A) or contralateral (Fig. 1B) to sciatic nerve injury either pre-surgery [baseline (BL)] or pre-drug recorded 14 days after CCI (time 0). Upon completion of the pre-drug assessment, rats were intrathecally given either 20 μg of a TLR4 antagonist (either non-signaling mutant LPS or LPS-RS; dose based on pilot studies) or vehicle, and response thresholds were determined 1 and 3 h later. As seen in Fig. 1, both TLR4 antagonists reliably reversed both ipsilateral and contralateral mechanical allodynia over the timecourse tested.
Fig. 1.
Reversal of chronic constriction injury (CCI)-induced neuropathic pain by acute intrathecal delivery of the toll-like receptor (TLR)4 antagonists, mutant lipopolysaccharide (LPS), and LPS-RS. After baseline (BL) testing, rats received CCI of one sciatic nerve at the mid-thigh level. After pre-drug testing (0 h) 14 days later to confirm the development of bilateral CCI-induced mechanical allodynia, rats were intrathecally given either 20 μg of mutant LPS (filled diamonds), 20 μg of LPS-RS (filled squares), or an equal volume of vehicle (open squares). Behavioral responses recorded 1 and 3 h later revealed reliable attenuation of both ipsilateral (A) and contralateral (B) mechanical allodynia by this TLR4 antagonist. ***P < 0.001 as compared to vehicle (saline) controls.
Experiment 2. Non-classic blockade of LPS-induced TLR4 signaling in HEK-293 cells in vitro by naloxone and naltrexone
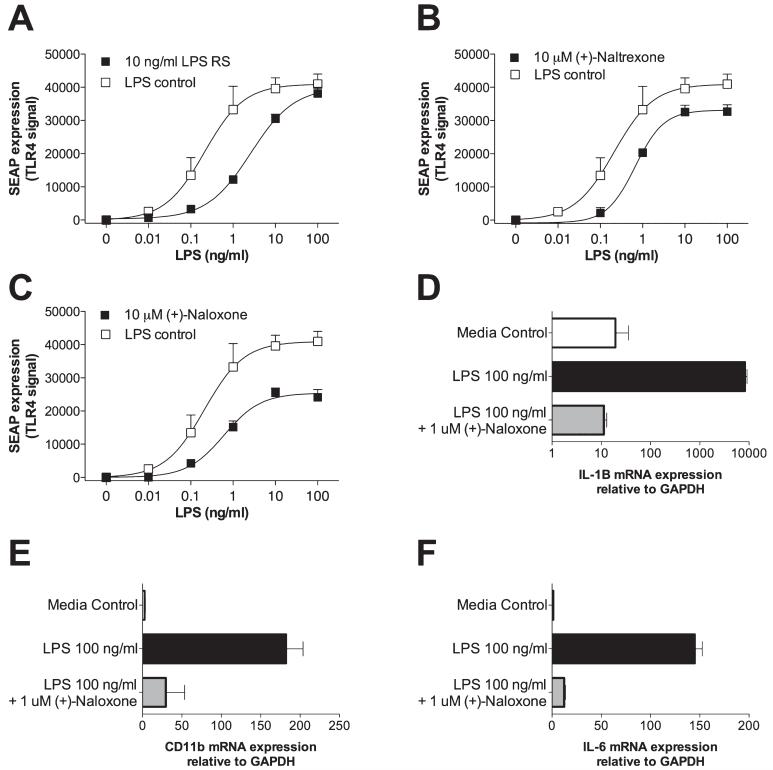
Although mutant LPS and LPS-RS are commercially available TLR4 antagonists appropriate for research purposes, they have no potential to be clinically relevant, given that they would be neither orally active nor blood-brain barrier permeable. Given this limitation of currently available TLR4 antagonists, we explored whether naloxone and/or naltrexone could exert non-classic (non-stereoselective) effects on TLR4 signaling. This choice was based, in part, on prior reports that both (-))-naloxone and (+)-naloxone equally inhibited LPS-induced microglial production of superoxide, nitric oxide, and tumor necrosis factor (Liu et al., 2000, 2006). To test this, we challenged HEK-TLR4 cells with LPS (0, 0.01, 0.1, 1, 10 or 100 ng/mL) and either vehicle (LPS control), 10 ng/mL LPS-RS (LPS antagonist used as a positive control), 10 μm (+)-naltrexone, 10 μm (-))-naltrexone, 10 μm (+)-naloxone or 10 μm (-))-naloxone, with doses based on pilot studies. All drugs produced a significant attenuation of LPS-induced SEAP expression (Fig. 2A-C). LPS-RS, as anticipated, produced a competitive inhibition of TLR4 activity, based on the shape of the inhibition curve obtained (Fig. 2A). Both naloxone and naltrexone produced reliable non-classic, non-competitive inhibition of SEAP expression, with comparable antagonism being produced by both the (+)- and (-))-isomers of each [Fig. 2B and C; only the non-classic effect of the (+)-isomers are illustrated for simplicity]. Thus, naloxone and naltrexone non-stereoselectively block TLR4 signaling.
Fig. 2.
Non-classic blockade of lipopolysaccharide (LPS)-induced toll-like receptor (TLR) signaling and microglial activation. HEK-TLR4 cells were incubated with LPS (log doses from 0 to 100 ng/mL) along with either vehicle (LPS control), 10 ng/mL LPS-RS (A), 10 μm (+)-naltrexone (B), or 10 μm (+)-naloxone (C). All drugs inhibited TLR4 signaling, as reflected by secreted alkaline phosphatase (SEAP) levels, with competitive antagonism observed using LPS-RS and non-competitive antagonism by (+)-naltrexone and (+)-naloxone on the basis of the shapes of the resultant curves. Comparable antagonisms were documented for (-))-naltrexone and (-))-naloxone, but are not included here for clarity. In a separate study, the HAPI microglial cell line was used to examine the effects of (+)-naloxone on microglial activation by LPS. Whereas 100 ng/mL LPS produced elevations in mRNA for CD11b (microglial activation marker) (E), interleukin (IL)-1β (D), and IL-6 (F), 1 μm (+)-naloxone abolished each of these LPS-induced effects. GAPDH, glyceraldehyde-3-phosphate-dehydrogenase.
Experiment 3. Non-classic blockade of LPS-induced proinflammatory cytokine gene expression in microglial cells in vitro by (+)-naloxone
To confirm and extend the prior reports by Liu et al. (2000, 2006), we tested whether LPS-induced microglial activation [as measured by increased gene expression of CD11b (microglial activation marker), interleukin (IL)-1β, and IL-6] could be non-classically inhibited by (+)-naloxone. Microglia were chosen for study in vitro because they are the predominant central nervous system cell type expressing TLR4 (Miyake, 2007). (+)-Naloxone, rather than (+)-naltrexone, was chosen for study on the basis of the indication that (+)-naloxone may be the stronger inhibitor of TLR4 signaling of the two (Experiment 2, above). Microglial cells were challenged with either no drug (media control), 100 ng/mL LPS, or 100 ng/mL LPS plus 1 μm (+)-naloxone. Whereas LPS reliably increased mRNA expression of IL-1, CD11b and IL-6 (center bars, Fig. 2D-F), (+)-naloxone non-classically abolished the elevated expression of all three indicators of microglial activation (lower bars of Fig. 2D-F).
Experiment 4. Reversal of CCI-induced neuropathic pain by acute delivery of neuronally inactive (+)-naloxone and (+)-naltrexone, novel TLR4 antagonists
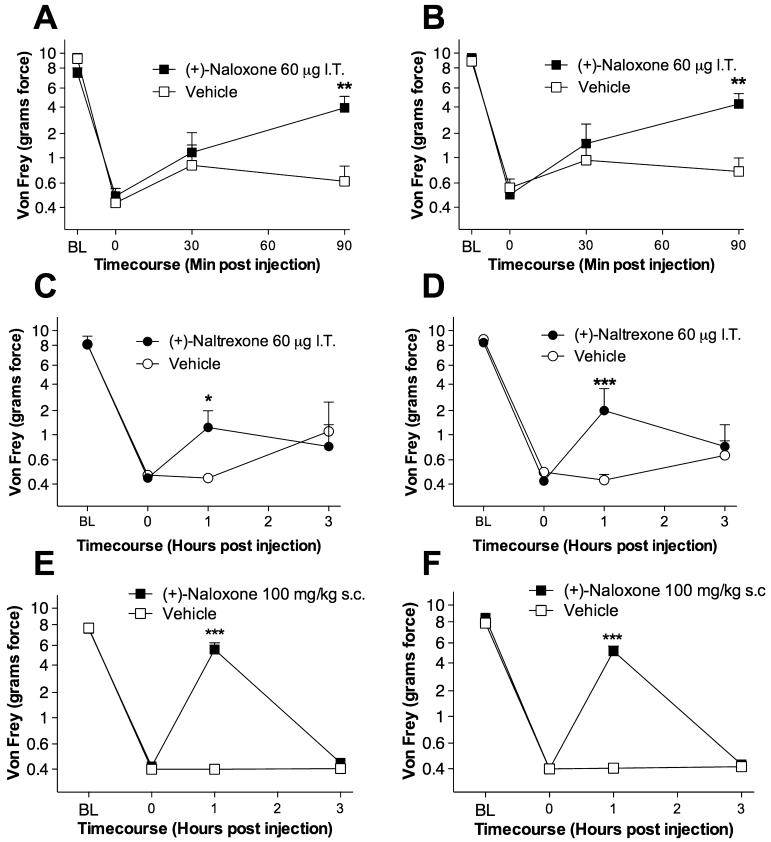
On the basis of the positive findings of Experiments 2 and 3, above, which support non-classic blockade of TLR4 signaling by naloxone and naltrexone, the procedures of Experiment 1 were repeated, with the exception that intrathecally delivered (+)-naloxone and (+)-naltrexone (each 60 μg in separate groups) were administered instead of mutant LPS or LPS-RS. In addition, (+)-naloxone was also administered systemically (100 mg/kg subcutaneously). For the intrathecal studies, a briefer post-injection timecourse was assessed for (+)-naloxone than for (+)-naltrexone, given the shorter drug half-life expected. As seen in Fig. 3, all groups exhibited comparable response thresholds pre-surgery (BL) and 14 days post-surgery prior to drug administration (time 0). Intrathecal (+)-naloxone significantly attenuated both ispilateral and contralateral neuropathic pain (mechanical allodynia) 90 min after drug administration (Fig. 3A and B), and (+)-naltrexone reliably attenuated both ipsilateral and contralateral neuropathic pain 1 h after drug administration (Fig. 3C and D). Systemic (+)-naloxone also succeeded in significantly attenuating neuropathic pain 1 h after drug administration (Fig. 3E and F).
Fig. 3.
Reversal of chronic constriction injury (CCI)-induced neuropathic pain by acute delivery of neuronally inactive (+)-naloxone and (+)-naltrexone, novel toll-like receptor (TLR)4 antagonists. After baseline (BL) testing, rats received CCI of one sciatic nerve at the mid-thigh level. After pre-drug testing (0 h) 14 days later to confirm the development of bilateral CCI-induced mechanical allodynia, rats were intrathecally (IT) given 60 μg of either (+)-naloxone (filled squares, A and B), (+)-naltrexone (filled circles, C and D), 100 mg/kg subcutaneous (+)-naloxone (filled squares, E and F) or vehicle (open symbols, A-F). Behavioral responses recorded after drug administration revealed reliable attenuation of both ipsilateral (A, C and E) and contralateral (B, D and F) mechanical allodynia by these novel TLR4 antagonists. *P < 0.05, **P < 0.01, ***P < 0.001 as compared to vehicle (saline) controls.
Experiment 5. Persistent reversal of CCI-induced neuropathic pain by sustained intrathecal delivery of neuronally inactive (+)-naloxone and (+)-naltrexone
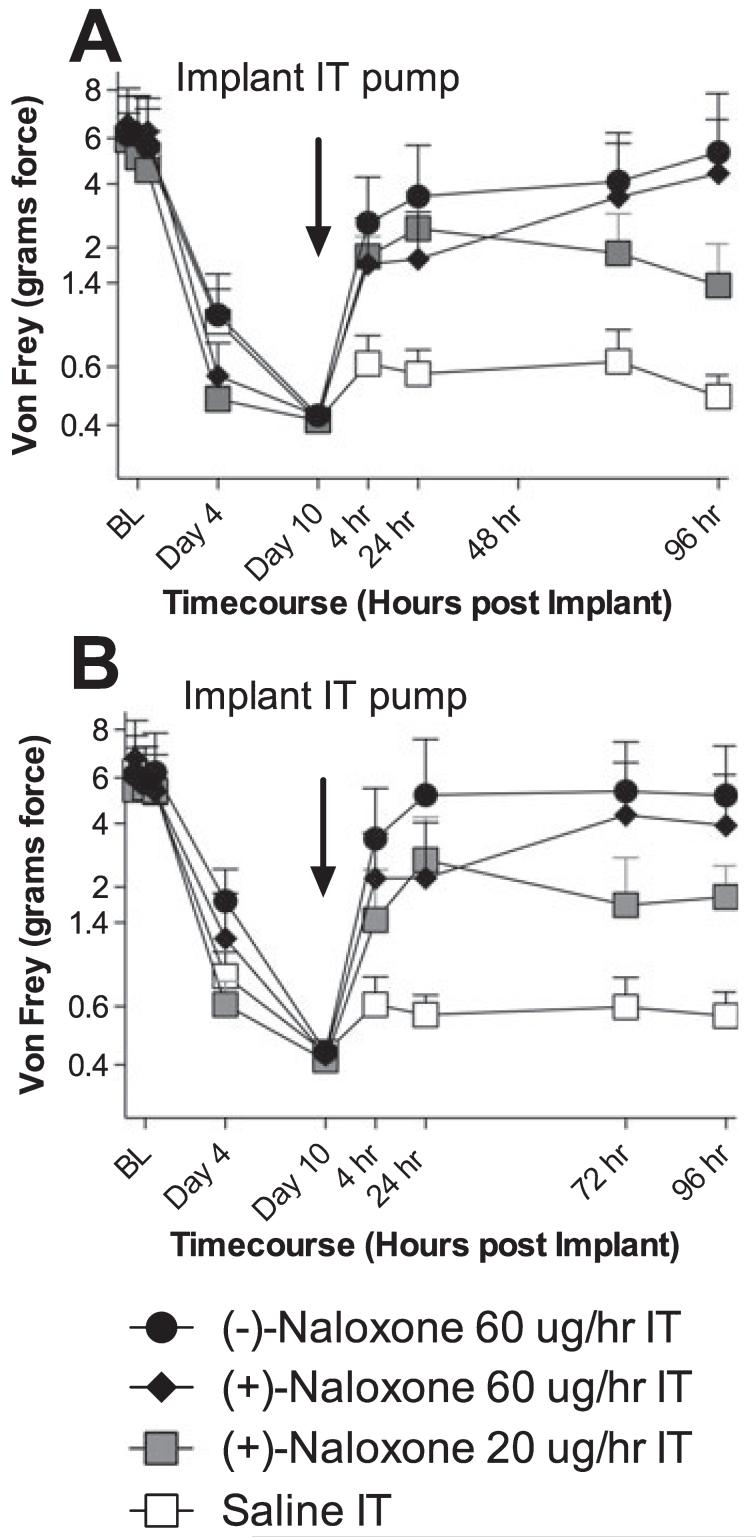
Given the initial suggestion of potentially greater efficacy of (+)-naloxone than of (+)-naltrexone in reversing CCI-induced neuropathic pain in Experiment 2, (+)-naloxone was chosen for study in this last behavioral experiment. Here, the effects of sustained osmotic minipump intrathecal infusion of (+)-naloxone (20 or 60 μg/h), (-))-naloxone (60 μg/h) or an equal volume of vehicle were compared on CCI-induced mechanical allodynia, where intrathecal infusions began 10 days after sciatic nerve injury and continued for 4 days. As seen in Fig. 4, no differences in pre-surgery response thresholds (BL) or pre-drug (day 10 after CCI) response thresholds were observed across groups. As compared to sustained intrathecal vehicle, (+)-naloxone (20 and 60 μg/h) and (-))-naloxone (60 μg/h) each reliably reversed neuropathic pain, with complete reversal of response thresholds to pre-surgery BL levels in both ipsilateral and contralateral hindpaws by 60 μg/h (+)-naloxone and (+)-naltrexone after 4 days of drug delivery.
Fig. 4.
Persistent reversal of chronic constriction injury (CCI)-induced neuro-pathic pain by sustained intrathecal (IT) delivery of neuronally inactive (+)-naloxone and (+)-naltrexone. After baseline (BL) testing, rats received CCI of one sciatic nerve at the mid-thigh level. After behavioral assessments on days 4 and 10 to confirm the development of bilateral CCI-induced mechanical allodynia, rats were implanted with osmotic minipumps to produce sustained IT administration of either (-))-naloxone (filled circles, 60 μg/h), (+)-naloxone (filled diamonds, 60 μg/h), (+)-naloxone (filled squares, 20 μg/h), or vehicle (open squares). Behavioral responses recorded over time after drug administration revealed reliable attenuation of both ipsilateral (A) and contralateral (B) mechanical allodynia by these novel toll-like receptor 4 antagonists with complete reversal of allodynia by 60 μg/h (-))-naloxone and 60 μg/h (+)-naloxone by 96 h. Treatment effects compared to vehicle: P < 0.0001.
Experiment 6. Sustained intrathecal delivery of neuronally inactive (+)-opioid antagonists suppresses the expression of a microglial activation marker
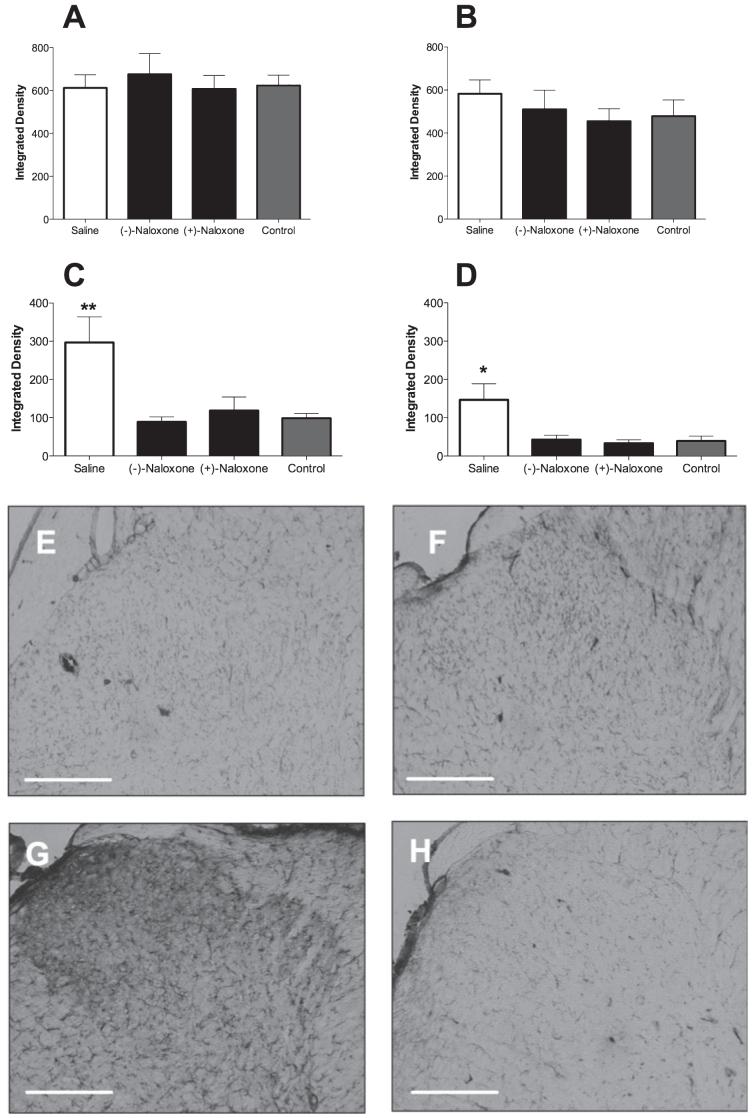
Given the complete reversal of mechanical allodynia produced by 60 μg/h (+)-naloxone and (-))-naloxone as compared to vehicle controls in Experiment 5, L5 spinal cords of these three groups of rats and a naïve control group were collected upon completion of the behavioral assessments (above) and processed for astrocyte (GFAP) and microglia (CD11b/c) activation markers. Analysis by densitometry revealed no reliable suppression of astrocytic GFAP by either (+)-naloxone or (-))-naloxone in either dorsal horn (Fig. 5A and B). In contrast, (+)-naloxone and (-))-naloxone each suppressed the expression of CD11b/c by microglia in both the ipsilateral (Fig. 5C, E and F) and contralateral (Fig. 5D) dorsal horn, as compared to saline-treated neuropathic pain rats and naïve controls (Fig. 5C, D, G and H).
Fig. 5.
Sustained intrathecal delivery of neuronally inactive (+)-opioid antagonists suppresses the expression of a microglial activation marker. The L5 spinal cords of chronic constriction injury (CCI) rats receiving sustained intrathecal administration of 60 μg/h(-))-naloxone, 60 μg/h (+)-naloxone or vehicle for 4 days in Experiment 3 (behavior shown in Fig. 3) were analysed for expression of astrocyte [glial fibrillary acidic protein (GFAP); A and B] and microglia (CD11b/c; C-H) activation markers. As quantified by densitometry, neither (+)-naloxone nor (-))-naloxone affected GFAP expression in ipsilateral (A) or contralateral (B) dorsal horns, as compared to vehicle controls. In contrast, both isomers of naloxone produced reliable suppression of CD11b/c bilaterally, as compared to neuropathic vehicle-treated animals (C and D). Representative sections (10×) of ipsilateral dorsal horns of neuropathic (CCI) rats treated intrathecally with 60 μg/h (+)-naloxone (E), 60 μg/h(-))-naloxone (F) and vehicle (G) are shown. A comparable section from a naïve control is illustrated in H. *P < 0.05, **P < 0.01 as compared to naive controls. Scale bar: 500 μm.
Discussion
The present series of studies explored pharmacological approaches for reversing neuropathic pain via blockade of TLR4. The one prior study that implicated TLR4 in L5 spinal nerve transection-induced neuropathic pain used genetic approaches to disrupt TLR4 signaling for the initiation of signaling to microglia (Tanga et al., 2005). Here we demonstrate, using the CCI model, that acute and/or multi-day sustained administration of TLR4 antagonists reverse neuropathic pain as well. This effect was observed using structurally diverse antagonists: non-signaling mutant LPS and LPS-RS, known to be receptor antagonists of TLR4 (Invivogen), (+)-naltrexone, (+)-naloxone, and (-))-naloxone. Use of in vitro HEK-293 cells stably transfected to express TLR4 linked to the expression of a reporter protein (SEAP) provided a means to show that naloxone and naltrexone each non-stereoselectively and non-competitively block TLR4 signaling. Supporting this evidence that (+)- and (-))-isomers of these compounds are TLR4 antagonists is the finding that (+)-naloxone also abolished LPS-induced mRNA for CD11b, IL-1 and IL-6 in microglial cells in vitro. Whether the blockade of TLR4 function by (+)- and (-))-opioid antagonist isomers reflects an action at the level of the TLR4 receptor per se vs. disruption of downstream signaling is currently unknown and under investigation. What is clear from the present studies is that sustained intrathecal administration of (+)-naloxone and (-))-naloxone exerted identical effects in vivo, namely: (i) a progressive reversal of neuropathic pain over time, resulting in full reversal of pain responsivity to pre-CCI levels; and (ii) a robust suppression of a microglial (but not astrocyte) activation marker in spinal cord dorsal horn, as compared to vehicle controls. This progressive reversal of neuropathic pain by (+)-naloxone and (-))-naloxone makes their actions notably different from those of other microglial inhibitors such as minocycline, which can prevent but not reverse neuropathic pain (Raghavendra et al., 2003; Ledeboer et al., 2005).
The only commercially available TLR4 antagonists are the mutant LPS and LPS-RS used here and an inhibitor of a TLR4 downstream signaling molecule, toll-interleukin 1 receptor domain-containing adaptor protein (TIRAP), which is essential for the LPS-induced IkB-α phosphorylation and nuclear factor kappaB activation that leads to the production of proinflammatory cytokines. As noted above, mutant LPS and LPS-RS were shown in the present experiments to reverse neuropathic pain. In addition, we have recently reported that intrathecal administration of both mutant LPS and the TIRAP inhibitor enhance acute morphine analgesia (Hutchinson et al., 2007). Together, these findings provide evidence that activation of TLR4 both opposes opioid analgesia and enhances neuropathic pain (Hutchinson et al., 2007). As neither the mutant LPS, LPS-RS nor the TIRAP inhibitor would be orally active or cross the blood-brain barrier, their potential clinical utility is limited.
The fact that (+)- and (-))-opioid antagonists are orally active, readily cross the blood-brain barrier, suppress spinal glial activation and reverse neuropathic pain is exciting in its implications. Whereas classic opioid receptors expressed by neurons are highly stereoselective for binding (-))-opioid isomers, TLR4 (expressed predominantly on microglia) is not. This fact is key, in that it predicts that neuronally inactive (+)-opioid antagonists such as (+)-naloxone and (+)-naltrex-one could be developed into clinically viable drugs for potentiating opioid analgesia and suppressing neuropathic pain.
Although mutant LPS and LPS-RS are selective TLR4 receptor antagonists, it is not yet clear whether (+)- and (-))-opioid antagonists are equally restricted in their actions. There are approximately 11 members in the TLR family, all of which are pattern recognition receptors. This means that various TLR family members are differentially ‘tuned’ for detecting, and becoming activated in response to, evolutionarily conserved patterns expressed by bacteria, viruses, mycoplasma, and other pathogens (Miyake, 2007). In addition, TLR4 and TLR2 have recently been discovered to also become activated in response to ‘endogenous danger signals’. These are substances released by stressed, damaged and dead cells that are not normally found in the extracellular fluid (Miyake, 2007). The sensing of endogenous danger signals by TLR4 and TLR2 led to the discovery, using genetic approaches, that both TLR4 and TLR2 are involved in neuropathic pain (Tanga et al., 2005; Kim et al., 2007). Whereas we have focused our initial studies on TLR4 (Hutchinson et al., 2007), ongoing studies are exploring whether the function of TLR2 or other members of the TLR family may be sensitive to (+)- and/or (-))-opioids as well. These studies, in addition to ongoing exploration of whether (+)- and (-))-opioid isomers act at the TLR receptor itself vs. at downstream sites in the intracellular signaling cascade, will help define the specificity vs. generality of the effects observed.
Proinflammatory cytokines and their receptors, such as IL-1 and IL-1 receptor, have established causal roles in mediating allodynia in neuropathic pain rat models, with IL-1 receptor antagonist reversing exaggerated pain states (Milligan et al., 2001, 2003; Sweitzer et al., 2001). Given that TLR2, TLR4 and IL-1 share the same downstream signaling cascade (Yamamoto et al., 2002), and their receptor signaling is increased during allodynic states, as is that of IL-1, inhibition of TLR signaling may be expected to have the same beneficial action as cytokine receptor antagonism. As we have demonstrated here, TLR4 antagonism, at least, does reverse allodynia, consolidating the importance of the Toll/IL-1 signaling cascade in exaggerated pain states.
Neuronally active (-))-naloxone did not lead to an exacerbation of neuropathic pain in the present studies, suggesting that endogenously released opioids do not play an important role in modulating neuropathic pain under these conditions. Although neuropathy-induced alterations in endogenous opioid peptide biosynthesis and opioid receptor density in nociceptive pathways have been reported (Przewlocki & Przewlocka, 2005), and endogenous opioidergic systems suppress pain enhancement in a spinal cord injury model (Hao et al., 1998) and perhaps following peripheral neuropathy under some conditions (Nichols et al., 1996), the present data did not indicate enhanced allodynia in response to intrathecally administered (-))-naloxone. This failure of neuronally active (-))-opioid antagonists to enhance neuropathic pain is in agreement with the findings of others (Siegan & Sagen, 1998; Nozaki & Kamei, 2006; Ambriz-Tututi & Granados-Soto, 2007).
It is also notable that prior studies have not reported suppression of neuropathic pain by naloxone or naltrexone. Although the present study is the first test of the effect of intrathecal or systemic (+)-naloxone and (+)-naltrexone in neuropathic pain, intrathecal (-))-naloxone and (-))-naltrexone have previously been tested in neuropathic pain control groups included in the study of various analgesic drugs. Prior studies have almost exclusively used far lower doses of intrathecal (-))-naloxone and (-))-naltrexone than the 60-μg doses tested here, with no effects on neuropathic pain being noted. In contrast, Ambriz-Tututi & Granados-Soto (2007) tested an acute injection of intrathecal (-))-naltrexone at a dose of 50 μg in neuropathic rats. Although the effects of (-))-naltrexone were never directly compared to vehicle in a single experiment, examination of their data (expressed only as area under the curve across the entire 4-h behavioral timecourse; Figs 2B and 5B) suggests strongly that (-))-naltrexone did suppress mechanical allodynia as compared to vehicle. The fact that these results indicate a reduction in neuropathic pain by (-))-naltrexone using a 4-h area under the curve analysis is notable, in comparison to the present results, given: (i) the small but reliable effect observed here using 60-μg intrathecal (+)-naltrexone; and (ii) the transient nature of the attenuation of neuropathic pain in response to acute (+)-naltrexone (only at the 1-h time-point). It was for such reasons that naloxone, not naltrexone, was chosen for study with chronic intrathecal infusion (Experiment 5), as naloxone appeared to be more effective. Indeed, (+)-naloxone and (-))-naloxone were observed to completely reverse CCI-induced mechanical allodynia. Notably, this reversal occurred progressively across days. This may be a key reason why no prior study has reported reversal of neuropathic pain with opioid antagonists, as none to our knowledge has employed constant infusion across such a prolonged timecourse.
The present studies on TLR4 involvement in neuropathic pain extend our work documenting that TLR4 is also importantly involved in glial dysregulation of opioid actions. Those studies demonstrate that, similarly to neuropathic pain (Tanga et al., 2005), morphine upregulates TLR4 expression in microglia in vitro and in vivo (Hutchinson et al., 2007). Also similar to results reported here for neuropathic pain, intrathecal administration of either the mutant LPS TLR4 receptor antagonist or an inhibitor of downstream signaling through TIRAP potentiated intrathecal morphine analgesia (Hutchinson et al., 2007). Strikingly, a broad range of clinically relevant opioids (morphine, methadone, meperidine, fentanyl, oxycodone) were all observed to activate TLR4 (Hutchinson et al., 2007). Taken together with the fact that neuropathic pain arising from at least L5 spinal nerve transection (Tanga et al., 2005) and CCI (present study) is created, at least in part, via activation of TLR4 contributing to microglial activation, these data suggest that TLR4 may prove to be a target worth exploring for improving clinical pain control.
Acknowledgements
This work was supported by an International Association for the Study of Pain International Collaborative grant, an American Australian Association Merck Company Foundation Fellowship, a National Health and Medical Research Council CJ Martin Fellowship (ID 465423) and NIH Grants DA015642, DA017670 and DE017782. A portion of this work was supported by the NIH Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
Abbreviations
BL
baseline
CCI
chronic constriction injury
GFAP
glial fibrillary acidic protein
HEK
human embryonic kidney
IL
interleukin
LPS
lipopolysaccharide
PCR
polymerase chain reaction
SEAP
secreted alkaline phosphatase
TIRAP
toll-interleukin 1 receptor domain-containing adaptor protein
TLR
toll-like receptor
References
- Ambriz-Tututi M, Granados-Soto V. Oral and spinal melatonin reduces tactile allodynia in rats via activation of MT2 and opioid receptors. Pain. 2007;132:273–280. doi: 10.1016/j.pain.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. doi: 10.1016/S0304-3959(01)00354-2. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35:53–62. doi: 10.1002/glia.1070. [DOI] [PubMed] [Google Scholar]
- Hao JX, Yu W, Xu XJ. Evidence that spinal endogenous opioidergic systems control the expression of chronic pain-related behaviors in spinally injured rats. Exp. Brain Res. 1998;118:259–268. doi: 10.1007/s002210050280. [DOI] [PubMed] [Google Scholar]
- Harvey LOJ. Efficient estimation of sensory thresholds. Behav. Res. Methods Istrum. Comput. 1986;18:623–632. [Google Scholar]
- Hashizume H, Rutkowski MD, Weinstein JN, DeLeo JA. Central administration of methotrexate reduces mechanical allodynia in an animal model of radiculopathy/sciatica. Pain. 2000;87:159–169. doi: 10.1016/S0304-3959(00)00281-5. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence and reward. ScientificWorldJ. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J. Biol. Chem. 2007;282:14975–14983. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW. Ibudilast (AV-411). A new class of therapeutic candidate for neuropathic pain and opioid withdrawal syndromes. Expert Opin. Investig. Drugs. 2007;16:935–950. doi: 10.1517/13543784.16.7.935. [DOI] [PubMed] [Google Scholar]
- Liu B, Du L, Kong LY, Hudson PM, Wilson BC, Chang RC, Abel HH, Hong JS. Reduction by naloxone of lipopolysaccharide-induced neurotoxicity in mouse cortical neuron-glia co-cultures. Neuroscience. 2000;97:749–756. doi: 10.1016/s0306-4522(00)00057-9. [DOI] [PubMed] [Google Scholar]
- Liu W, Wang CH, Cui Y, Mo LQ, Zhi JL, Sun SN, Wang YL, Yu HM, Zhao CM, Feng JQ, Chen PX. Inhibition of neuronal nitric oxide synthase antagonizes morphine antinociceptive tolerance by decreasing activation of p38 MAPK in the spinal microglia. Neurosci. Lett. 2006;410:174–177. doi: 10.1016/j.neulet.2006.08.091. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Namovic MT, Donnelly-Roberts DL, Harris RR, Zhang XF, Shieh CC, Wismer CT, Zhu CZ, Gauvin DM, Fabiyi AC, Honore P, Gregg RJ, Kort ME, Nelson DW, Carroll WA, Marsh K, Faltynek CR, Jarvis MF. P2X7-related modulation of pathological nociception in rats. Neuroscience. 2007;146:1817–1828. doi: 10.1016/j.neuroscience.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Mika J, Osikowicz M, Makuch W, Przewlocka B. Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur. J. Pharmacol. 2007;560:142–149. doi: 10.1016/j.ejphar.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Hinde JL, Mehmert KK, Maier SF, Watkins LR. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J. Neurosci. Methods. 1999;90:81–86. doi: 10.1016/s0165-0270(99)00075-8. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O’Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J. Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O’Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR. Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J. Neurosci. 2003;23:1026–1040. doi: 10.1523/JNEUROSCI.23-03-01026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O’Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur. J. Neurosci. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- Miyake K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin. Immunol. 2007;19:3–10. doi: 10.1016/j.smim.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Nichols ML, Bian D, Ossipov MH, Malan TP, Jr, Porreca F. Antiallodynic effects of a CCKB antagonist in rats with nerve ligation injury: role of endogenous enkephalins. Neurosci. Lett. 1996;215:161–164. doi: 10.1016/0304-3940(96)12964-5. [DOI] [PubMed] [Google Scholar]
- Nozaki C, Kamei J. Possible involvement of opioidergic systems in the antinociceptive effect of the selective serotonin reuptake inhibitors in sciatic nerve-injured mice. Eur. J. Pharmacol. 2006;552:99–104. doi: 10.1016/j.ejphar.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Przewlocka B. Opioids in neuropathic pain. Curr. Pharm. Des. 2005;11:3013–3025. doi: 10.2174/1381612054865055. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- Siegan JB, Sagen J. Adrenal medullary transplants attenuate sensorimotor dysfunction in rats with peripheral neuropathy. Pharmacol. Biochem. Behav. 1998;59:97–104. doi: 10.1016/s0091-3057(97)00326-2. [DOI] [PubMed] [Google Scholar]
- Sweitzer S, Martin D, DeLeo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103:529–539. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, Pahl JL, DeLeo JA. Propentofylline attenuates vincristine-induced peripheral neuropathy in the rat. Neurosci. Lett. 2006;400:258–261. doi: 10.1016/j.neulet.2006.02.058. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl Acad. Sci. USA. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang T, Beggs S, Salter MW. Purinoceptors in microglia and neuropathic pain. Pflugers Arch. 2006;452:645–652. doi: 10.1007/s00424-006-0074-5. [DOI] [PubMed] [Google Scholar]
- Treutwein B, Strasburger H. Fitting the psychometric function. Percept. Psychophys. 1999;61:87–106. doi: 10.3758/bf03211951. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in ‘small’ glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur. J. Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Glia as the ‘bad guys’: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav. Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]