Self-renewal and expansion of single transplanted muscle stem cells (original) (raw)
. Author manuscript; available in PMC: 2010 Aug 10.
Published in final edited form as: Nature. 2008 Sep 17;456(7221):502–506. doi: 10.1038/nature07384
Abstract
Adult muscle satellite cells have a principal role in postnatal skeletal muscle growth and regeneration1. Satellite cells reside as quiescent cells underneath the basal lamina that surrounds muscle fibres2 and respond to damage by giving rise to transient amplifying cells (progenitors) and myoblasts that fuse with myofibres. Recent experiments showed that, in contrast to cultured myoblasts, satellite cells freshly isolated3–5 or satellite cells derived from the transplantation of one intact myofibre6 contribute robustly to muscle repair. However, because satellite cells are known to be heterogeneous4,6,7, clonal analysis is required to demonstrate stem cell function. Here we show that when a single luciferase-expressing muscle stem cell is transplanted into the muscle of mice it is capable of extensive proliferation, contributes to muscle fibres, and Pax7+luciferase+ mononucleated cells can be readily re-isolated, providing evidence of muscle stem cell self-renewal. In addition, we show using in vivo bioluminescence imaging that the dynamics of muscle stem cell behaviour during muscle repair can be followed in a manner not possible using traditional retrospective histological analyses. By imaging luciferase activity, real-time quantitative and kinetic analyses show that donor-derived muscle stem cells proliferate and engraft rapidly after injection until homeostasis is reached. On injury, donor-derived mononucleated cells generate massive waves of cell proliferation. Together, these results show that the progeny of a single luciferase-expressing muscle stem cell can both self-renew and differentiate after transplantation in mice, providing new evidence at the clonal level that self-renewal is an autonomous property of a single adult muscle stem cell.
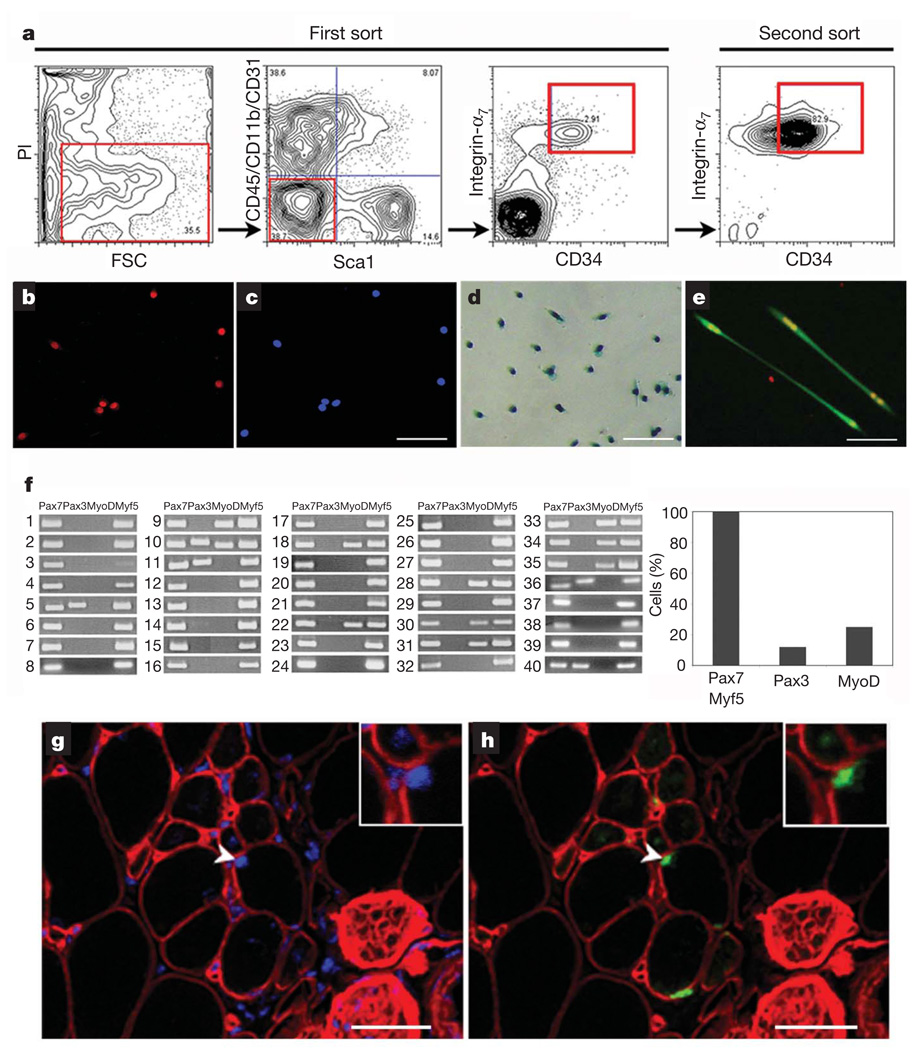
We reasoned that prospective isolation of muscle stem cells (MuSCs) in conjunction with a dynamic analysis of their fate in vivo would greatly enhance our understanding of their potential to regenerate damaged muscle. Accordingly, we tested various fluorescence-activated cell sorting (FACS) fractionation procedures3–5,7 and determined that, after depletion of CD45 (also known as Ptprc), CD11b (Itgam), Sca1 (Ly6a) and CD31 (Pecam1), a combination of endogenous markers—CD34 and integrin-α7—enriched for a muscle cell population of morphologically round cells that uniformly expressed the satellite-cell-specific transcription factor Pax7 (Fig. 1a–c). When isolated from Myf5-nLacZHet mice (in which the nLacZ reporter gene has been introduced into the locus of the myogenic transcription factor Myf5 gene, expression of which is characteristic of activated satellite cells) and plated in vitro, these cells were activated (β-galactosidase+ (β-gal+); ref. 8 and Fig. 1d) and differentiated to form multinucleated myotubes (Fig. 1e). To determine the heterogeneity of this cell population, we performed single-cell reverse transcription followed by polymerase chain reaction for four transcripts for each of 40 FACS-isolated MuSCs (Fig. 1f and Supplementary Methods). Remarkably, all cells (100%) within this population expressed Pax7 andMyf5, characteristic of satellite cells9,10. Of these, 25% also expressed MyoD (also known as Myod1), a marker of committed progenitors11, and 12% expressed Pax3, a marker of progenitors intermediate between satellite cells and myoblasts in hindlimb muscles12,13. These data show that the CD34+integrin-α7+ population is not fully homogeneous, because a subset of cells expressed commitment markers. To test the function of this muscle cell population in vivo, cells were transplanted into tibialis anterior muscles of non-obese diabetic/severely combined immuno-deficient (NOD/SCID) mice depleted of endogenous satellite cells by 18 Gy irradiation14,15. Four weeks after transplantation, muscles were damaged with notexin (NTX)16,17, and Myf5–β-gal+ donor-derived cells were subsequently detected in the satellite cell position underneath the basal lamina of myofibres (Fig. 1g, h). This classical histological analysis demonstrated that this population of MuSCs homed to the satellite cell niche and responded appropriately to muscle damage by upregulating expression of the Myf5 transcription factor.
Figure 1. Characterization of the integrin-α7+CD34+ fraction as muscle stem cells.
a, Flow cytometry analysis of freshly isolated muscle cells. Living cells were gated for forward scatter (FSC) and propidium iodide (PI) negativity (left panel). Cells negative for blood markers CD45 and CD11b, for endothelial marker CD31 and for mesenchymal marker Sca1 were gated (middle-left panel), and within this population the integrin-α7+CD34+ fraction was sorted (right panels). b, c, Forty-eight hours after isolation, muscle integrin-α7+CD34+ cells were stained for Pax7 (left: Pax7, red; right: nuclei, blue). Scale bar, 80 µm. d, After 5 days of culture in growth medium, integrin-α7+CD34+ cells isolated from Myf5-nLacZHet mice showed β-gal activity. Scale bar, 80 µm. e, After 3 days of culture in differentiation medium, integrin-α7+CD34+ cells (from _GFP_-transgenic mice) differentiated to form myotubes (green) that expressed myogenin (red). Scale bar, 80 µm. f, Single MuSCs (1–40) were individually sorted and reverse transcription followed by polymerase chain reaction performed as described in Supplementary Methods. The results show that this population consistently and homogeneously expresses Pax7 and Myf5, the expected transcriptional profile for satellite cells8–10. In contrast, both MyoD and Pax3 expression are heterogeneous, indicating the presence of committed progenitors, in accordance with previously published reports11–13. g, h, Freshly isolated integrin-α7+CD34+ cells from Myf5-nLacZHet mice were transplanted into recipient mice. One month after transplant, recipient muscles were damaged by NTX injection and 5 days later immunofluorescence of transverse tissue sections revealed Myf5–β-gal+ cells engrafted in the satellite cell position (arrowhead and inset). β-gal, green; laminin, red; nuclei, blue. Scale bars, 20 µm.
We reasoned that a dynamic assay could complement histological analyses by providing insights into the kinetics and extent of proliferation of transplanted MuSCs. We therefore developed a non-invasive bioluminescence imaging assay to monitor MuSCs by mating Myf5-nLacZHet mice with firefly luciferase (FLuc) transgenic mice18. In these studies, cell number was assessed as the bioluminescence signal derived from constitutive luciferase activity, and the activity of the Myf5 promoter was assayed histologically as β-gal activity. The linearity, sensitivity and reproducibility of the bioluminescence assay for quantifying cell numbers was validated in vitro (Supplementary Fig. 1) and in vivo (Fig. 2a). The minimum number of cells detectable above control uninjected legs was 10,000 (Fig. 2a).
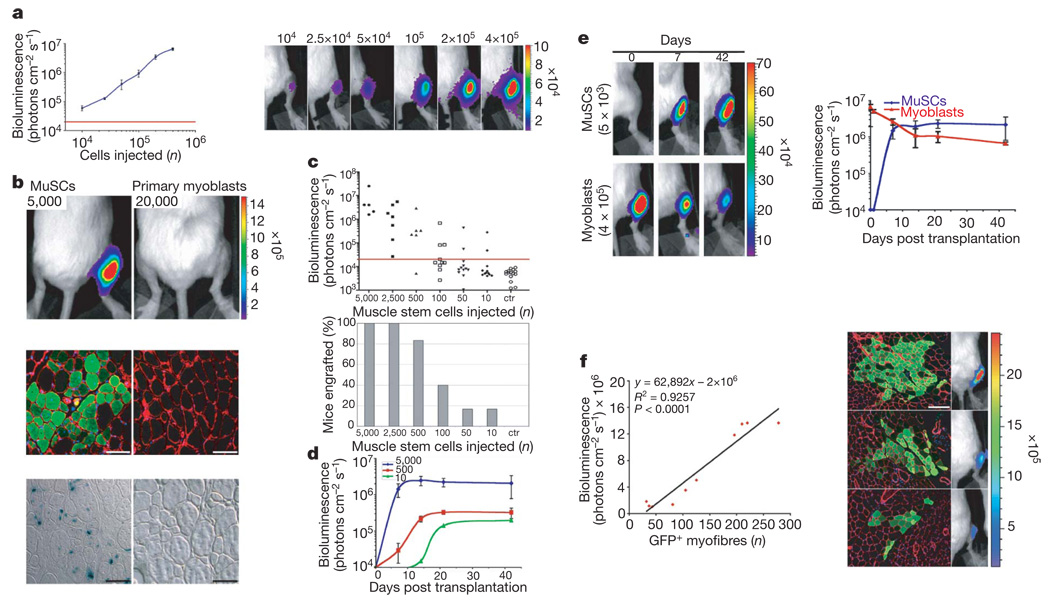
Figure 2. MuSC engraftment monitored by in vivo non-invasive bioluminescence imaging.
a, Increasing numbers of myoblasts isolated from Myf5-nLacZ/FLuc mice were injected into the tibialis anterior muscles of NOD/SCID recipients and imaged 2 h after injection. Shown is a graph of bioluminescence data represented as average ± s.e.m. (n = 4; P ≤ 0.05; left) and a bioluminescent image of representative injected mice, with the number of cells injected indicated at the top (bioluminescence values are indicated as photons cm−2 s−1 × 104; right). b, Top, freshly isolated MuSCs or cultured myoblasts from double-transgenic mice were injected into recipients and imaged four weeks after transplantation (n = 4; P < 0.005; bioluminescence values are indicated as photons cm−2 s−1 × 105). Middle panels, immunofluorescence of luciferase expression in transverse muscle sections reveal the contribution of MuSCs, not of myoblasts, to muscle fibres. Laminin, red; luciferase, green; nuclei, blue. Scale bars, 100 µm. Bottom, 5 days before tissue harvesting, muscles were damaged with NTX. β-gal staining revealed Myf5–β-gal+ cells in muscles transplanted with MuSCs, not with myoblasts. Scale bars, 100 µm. c, Different numbers of MuSCs were injected into muscles of recipients (n = 50), and engraftment was measured by imaging four weeks after transplantation (uninjected legs are shown as negative control, ctr). A scattered graph of bioluminescent values of individual mice (top) and a histogram graph showing the percentages of mice with successful engraftment for each number of cells injected are shown (bottom). d, Engraftment of MuSCs (5,000, 500, 10) was monitored by imaging over a period of six weeks after transplantation (average ± s.e.m.; n = 3; P < 0.05). e, Freshly isolated MuSCs or cultured myoblasts were transplanted into recipients and engraftment was monitored by imaging over a period of six weeks after transplantation. Bioluminescent images of representative injected mice acquired at the indicated days are shown (bioluminescence values are indicated as photons cm−2 s−1 × 104; left). Graph of bioluminescence measurements (average ± s.e.m., n = 5; P < 0.05, right). f, MuSCs from GFP/FLuc transgenic mice were transplanted into recipients. Four weeks later, mice were analysed by bioluminescence imaging and for histology. Regression analysis shows a significant (n = 10, P < 0.0001) correlation between the number of GFP+ myofibres and luciferase activity in individual mice (left). Representative images of immunofluorescence (GFP, green; laminin, red; nuclei, blue; scale bar, 120 µm) and bioluminescence imaging (bioluminescence values are indicated as photons cm−2 s−1 × 105; right).
To validate bioluminescence imaging as an assay for MuSC function in vivo, we compared freshly isolated integrin-α7+CD34+ MuSCs with cultured primary myoblasts, because previous studies by others indicated that these two cell types differ markedly3. We injected either 5,000 freshly isolated MuSCs or a fourfold excess of 20,000 cultured myoblasts19–22, both isolated from the Myf5-nLacZ/FLuc transgenic mice, into irradiated legs of NOD/SCID recipients. Four weeks after transplantation, myoblasts were barely detectable (0.2 ± 0.01 × 105 photons cm−2 s−1; Fig. 2b, top panels), indicating that their numbers had declined, whereas freshly isolated MuSCs yielded robust luciferase activity (29.0 ± 7.0 × 105 photons cm−2 s−1), a signal corresponding to ~3 × 105 cells (Fig. 2b, top panels), which is approximately a 60-fold expansion (~6 doublings). Histological analysis revealed luciferase+ myofibres in muscles of mice injected with freshly isolated MuSCs, but not myoblasts (Fig. 2b, middle panels). Histochemistry of NTX-damaged muscles revealed the presence of Myf5–β-gal+ cells, indicative of activated satellite cells, after injection of uncultured MuSCs, but not myoblasts (Fig. 2b, bottom panels). Together, these results confirm that freshly isolated MuSCs, but not myoblasts, successfully engraft, proliferate and give rise to committed progenitors that contribute to muscle fibres.
To determine the proportion of cells with engraftment potential in this muscle cell population, we transplanted different numbers of freshly isolated MuSCs into irradiated tibialis anterior muscles. Bioluminescence was assayed four weeks after transplantation, and successful engraftment was defined as persistence of a signal >20,000 photons cm−2 s−1, significantly above the background signal detected in control uninjected legs (Fig. 2c). More than 80% of mice showed engraftment when high numbers of MuSCs (500–5,000) were transplanted; however, even when as few as 10 cells were transplanted, 16% (2 out of 12 mice) showed engraftment (Fig. 2c). This percentage is probably the result of several hurdles such as the heterogeneity of the cell population (Fig. 1f), the survival rate of the cells after the isolation and injection procedures, and the threshold of detection by bioluminescence imaging. Notably, the signal plateaued in all cases (Fig. 2d), as reported for haematopoiesis23, presumably reflecting a proliferation of cells until the need is met, after which a combination of cell death and quiescence leads to tissue homeostasis. The plateau occurred later and at a lower level when fewer cells were injected, suggesting that over time endogenous radiation-resistant satellite cells15 increasingly compete with transplanted MuSCs for engraftment. In contrast with the marked increase in cell numbers that accompanies engraftment of freshly isolated MuSCs, cultured myoblasts decline in numbers within the first few days and then persist without detectable proliferation (Fig. 2e), consistent with previously published reports24. Furthermore, when MuSC-transplanted muscles with different engraftment levels were analysed, a linear correlation between the number of donor-derived myofibres and the bioluminescence signal was observed (Fig. 2f), further validating this dynamic assay as an ideal tool to quantify donor-derived cells in vivo.
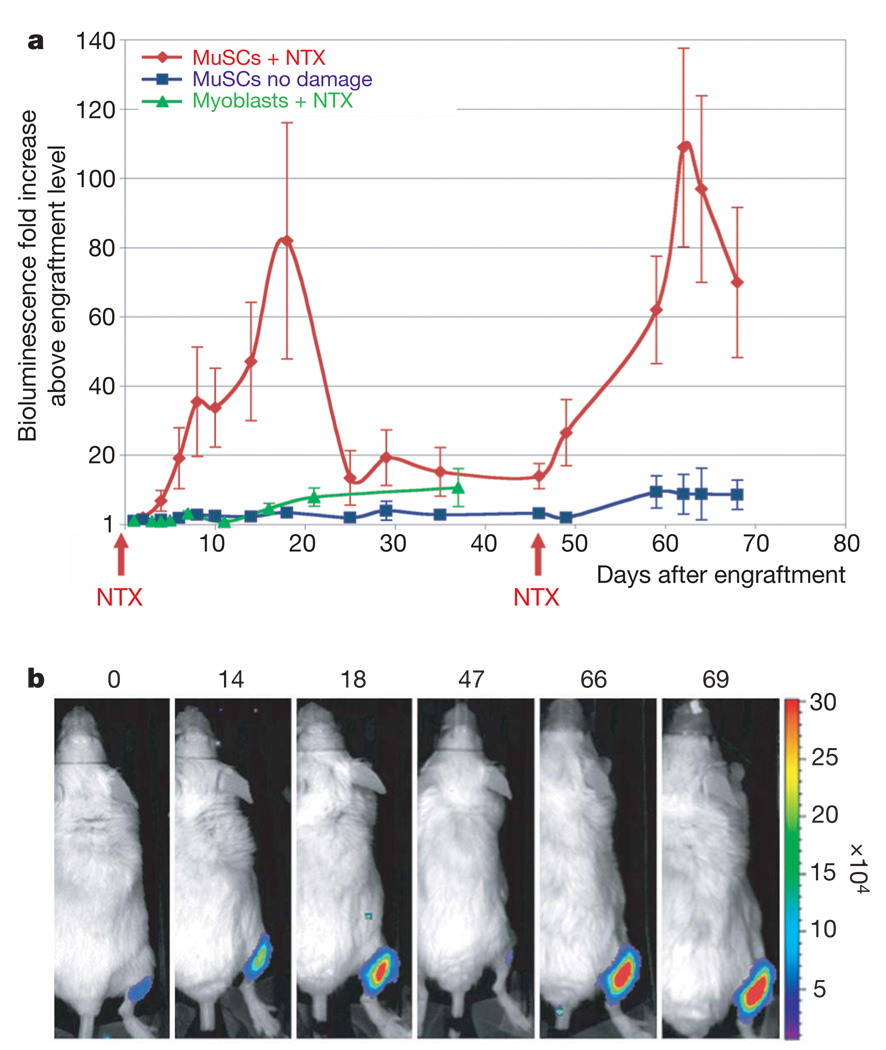
A functional property of adult stem cells is the ability to repeatedly respond to tissue injury by giving rise to substantial numbers of proliferative progenitors. Accordingly, irradiated NOD/SCID mice were injected with 10 or 500 MuSCs (Fig. 3 and Supplementary Fig. 2). After engraftment, to evaluate the response of different numbers of MuSCs to injury, bioluminescence values for each mouse were normalized to the bioluminescence value obtained at the plateau of engraftment for that mouse. Mice were divided into two groups; one of these received NTX damage whereas the other did not. After NTX injury, transplanted cells underwent a second wave of ~80-fold expansion and, after a second injury with NTX, a third wave of ~100-fold expansion was observed, assessed as luciferase activity relative to the activity at the engraftment plateau. A peak was observed ~15 days post injury in each case (Fig. 3a, b and Supplementary Fig. 2), consistent with the timing of regeneration of 18-Gy-irradiated NOD/SCID muscles evident by morphology, by the presence of Myf5–β-gal+ donor-derived cells and by the presence of regenerating myofibres expressing embryonic myosin heavy chain (Supplementary Fig. 3). This bioluminescence assay revealed a drop in cell number at the end of each regenerative wave of cell expansion, suggesting that cell death may counterbalance stem and progenitor cell proliferation to achieve homeostasis post injury. In contrast, luciferase activity in undamaged control mice remained relatively constant after engraftment (Fig. 3a and Supplementary Fig. 2). These results demonstrate that transplanted MuSCs can respond to serial injury with successive waves of progenitor expansion. The magnitude of the response to two sequential notexin injections suggests that stem cell function persisted over time.
Figure 3. MuSC proliferation in response to muscle tissue damage.
a, A low number of MuSCs (10–500) was transplanted into recipients (n = 10). After the engraftment plateau was reached (as in Fig. 2d), mice were divided into two groups, and the tibialis anterior muscles of one group were damaged by NTX injection, resulting in a substantial increase in cell numbers. At 47 days after the first damage, a second NTX injection led to a second increase in cell number, whereas no significant change was detected in the undamaged group. As a comparison, high numbers of primary myoblasts (4 × 105) were transplanted into recipients and four weeks later muscles were damaged by NTX injection. The average bioluminescence fold increase ± s.e.m. is shown (n = 5; P < 0.05). b, Bioluminescent images of a representative animal transplanted with MuSCs and damaged with NTX acquired on the days indicated (top). Bioluminescence values are indicated as photons cm−2 s−1 × 104.
To determine the basis for the accumulation of the bioluminescence signal in the course of regeneration, we analysed cell proliferation and apoptosis. Transverse sections of transplanted tibialis anterior muscles at days 7, 13 and 19 days post injury, revealed that donor-derived cells were proliferating as shown by the expression of the proliferative marker Ki67 in GFP+ donor-derived cells (Supplementary Fig. 4). Thus, the ongoing proliferation over the three-week time period post injury supports the progressive accumulation of new donor-derived cells over time detected by bioluminescence. In contrast with proliferation, apoptosis slowly increased, and was highest at 19 days, suggesting that cell death could account for the decline in bioluminescence signal (Supplementary Fig. 5).
A potential caveat in these experiments is that the increase in luciferase signal might be due not only to changes in cell number but also to changes in the expression of the luciferase gene or increased access of luciferin to luciferase+ cells in a damaged tissue. To address these possibilities, muscles previously transplanted with myoblasts, as in Fig. 2e, were damaged by NTX injection. Instead of the bioluminescence signal increasing, it remained constant (Fig. 3a), indicating that luciferase activity is not affected by tissue damage and confirming that myoblasts are unable to achieve detectable proliferation in response to injury (Fig. 3a). Finally, we compared in vitro undifferentiated myoblasts and tibialis anterior myofibres isolated from FLuc transgenic mice and found that, per microgram of DNA, enzyme activity did not differ (Supplementary Fig. 6 and Supplementary Methods). These results demonstrate that luciferase activity constitutes a readout of muscle cell numbers.
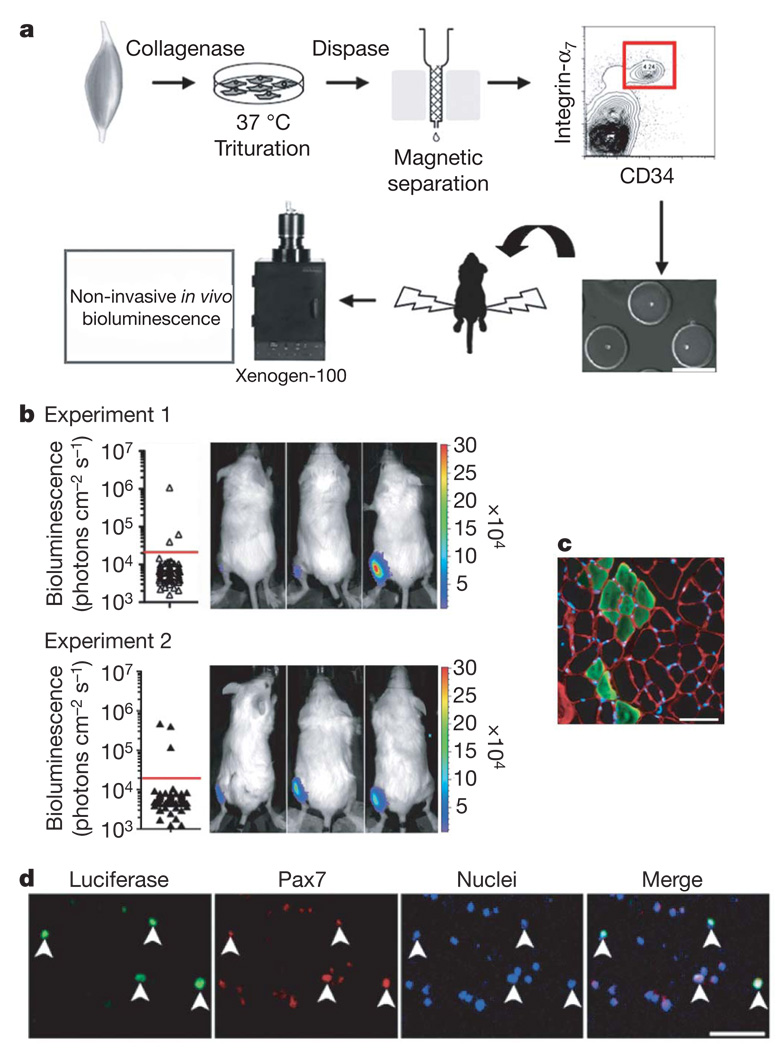
To establish definitively that the MuSCs are capable of self-renewal in vivo, transplantation and analysis of the progeny of a single cell are required, the gold standard established for haematopoietic stem cells23,25. Because more than one cell (10–500 cells per muscle) was transplanted in the experiments described above, we could not rule out the possibility that different MuSCs gave rise to the three successive waves of progenitor proliferation (during engraftment and after two NTX injections) without ever giving rise to another muscle stem cell. To test this possibility, we performed single MuSC transplantation experiments (Fig. 4a and Supplementary Methods). In each of 2 independent experiments, 3 out of a total of 72 single cells (4%) exhibited engraftment above background levels four weeks after transplantation (Fig. 4b). These results revealed that the progeny of a single adult MuSC are capable of remarkable proliferation during engraftment, as in the six mice a signal approximately equivalent to 21,000–84,000 cells (~14–17 doublings) was detected. Notably, the bioluminescence assay enabled these single-cell studies, as detection of a 4% frequency using classical histological analyses would have been extremely labour-intensive. Histological analysis revealed that in those mice with a detectable bioluminescence signal, the progeny of a single cell differentiated and fused with mature myofibres (Fig. 4c). To determine whether self-renewal had occurred in vivo, we dissected the muscles and identified at least 50 donor-derived luciferase+ cells per mouse expressing the satellite cell transcription factor Pax7 (81 ± 7% of total re-isolated luciferase+ cells) two months after transplantation of a single cell (Fig. 4d). These results demonstrate that a single integrin-α7+CD34+ MuSC can self-renew, giving rise to a population of mononucleated Pax7+ cells that stably reside in recipient muscles.
Figure 4. Transplantation of a single MuSC demonstrates self-renewal capacity.
a, Scheme of single MuSC transplantation. Cells were isolated from Myf5-nLacZ/FLuc transgenic mice by FACS as in Fig. 1a, segregated as single cells in hydrogel microwells (< 10 µl per microwell; scale bars, 150 µm), individually picked by micromanipulation and transplanted into tibialis anterior muscles of recipient mice. b, Two independent experiments are shown (_n_= 6/144), and in each case 3 out of 72 single cell transplants resulted in engraftment above background detected by imaging mice four weeks after transplantation (left). Bioluminescent images of the 6 positive recipients after single MuSC transplantation (bioluminescence values are indicated as photons cm−2 s−1 × 104; right).
c, Immunofluorescence of luciferase expression in transverse muscle sections from the mice in b revealed the contribution of single MuSC progeny to muscle fibres. Laminin, red; luciferase, green; nuclei, blue. Scale bars, 50 µm. d, Muscle cells were re-isolated from mice transplanted with single cells, and donor-derived luciferase+Pax7+ cells were detected. Scale bars, 100 µm.
Here we provide evidence that adult MuSCs have self-renewal capacity in vivo by demonstrating that after transplantation a single cell is capable of both producing copies of itself and generating more specialized progenitors. Moreover, the bioluminescence imaging used in this report revealed the time course and unexpected magnitude of the proliferative response of which adult MuSCs and their progeny are capable in vivo, in contrast to myoblasts (Supplementary Fig. 7). This assay should have broad application for quantitative comparisons among cell types after delivery to solid tissues in diverse disease and injury models.
METHODS SUMMARY
Muscle stem cell isolation
Tibialis anterior muscles of mice were subjected to enzymatic dissociation (first collagenase 0.2% and then dispase (0.04 units ml−1), Sigma) for 90 min, after which non-muscle tissue was gently removed under a dissection microscope. The cell suspension was filtered through a 70 µm nylon filter (Falcon) and incubated with the following biotinylated antibodies: CD45, CD11b, CD31 and Sca1 (BD Bioscience). Streptavidin beads (Miltenyi Biotech) were then added to the cells together with the following antibodies: integrin-α7–phycoerythrin (PE; a gift from F. Rossi) and CD34–Alexa647 (eBioscience), after which magnetic depletion of biotin-positive cells was performed. The (CD45−CD11b−CD31−Sca1−)CD34+integrin-α7+ population was then fractionated twice by flow cytometry (DIVA-Van, Becton-Dickinson). Primary myoblasts were isolated as described previously20.
Bioluminescence imaging
For imaging, a Xenogen-100 device was used, as described previously18. In brief, the system comprises a light-tight imaging chamber, a charge-coupled device camera with a cryogenic refrigeration unit and the appropriate computer system (Living-Image Software; Xenogen). After intraperitoneal injection of luciferin in 100 µl of PBS (0.1 mmol per kg body weight, Xenogen), we acquired images continuously for 30 min and stored them for subsequent analysis. We analysed images at 15 min after luciferin injection.
Immunofluorescence and histology
Muscle tissues were prepared for histology as described previously17. For immunofluorescence, rabbit anti-β-galactosidase (Molecular Probes), rabbit anti-luciferase (Abcam), rabbit anti-GFP (Molecular Probes), rat anti-laminin (Upstate Technologies), mouse anti-Pax7 (Developmental Mouse Hybridoma Bank), mouse anti-myogenin (Pharmingen), rat anti-Ki67 (DAKO), mouse anti-embryonic-myosin-heavy-chain (F1.652)26 and TUNEL (Apop TAG Red kit, Chemicon) were used.
Statistical analysis
Data are presented as mean ± s.e.m. Comparisons between groups used the Student’s _t_-test assuming two-tailed distributions, with an alpha level of 0.01–0.05.
Supplementary Material
Supplementary Fig.
Acknowledgements
We thank O. Alkan for developing the single cell RT–PCR protocol, K. Koleckar, M. Pajcini and T. Doyle for technical support, T. Brazelton, F. Rossi and S. Corbel for comments on the manuscript, J. Ramunas for statistical analysis, and M. Lutolf and P. Gilbert for fabricating the hydrogel microwells. We also thank F. Rossi for providing the integrin-α7–PE-conjugated antibody, and C. Contag for providing the FLuc mice. This work was supported by NIH grants AG009521, AG024987 and by the Baxter Foundation.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions A.S., H.M.B. and R.D. designed the research, and A.S. and R.D. performed the experiments with support from P.K. S.V. performed single-cell RT–PCR. A.S. analysed the data. A.S., R.D. and H.M.B. discussed the results and wrote the paper.
References
- 1.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 2.Mauro A. Satellite cells of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montarras D, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 4.Kuang S, et al. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerletti M, et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins CA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Sherwood RI, et al. Isolation of adult mouse myogenic precursors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Tajbakhsh S, et al. Gene targeting the myf-5 locus with nlacZ reveals expression of this myogenic factor in mature skeletal muscle fibers as well as early embryonic muscle. Dev. Dyn. 1996;206:291–300. doi: 10.1002/(SICI)1097-0177(199607)206:3<291::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Seale P, et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 10.Zammit PS, et al. Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp. Cell Res. 2002;281:39–49. doi: 10.1006/excr.2002.5653. [DOI] [PubMed] [Google Scholar]
- 11.Zammit PS, et al. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell. 2002;3:397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 13.Boutet SC, et al. Regulation of Pax3 by proteasomal degradation of monoubiquitinated protein in skeletal muscle progenitors. Cell. 2007;130:349–362. doi: 10.1016/j.cell.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 14.Wakeford S, Watt DJ, Partridge TA. X-irradiation improves mdx mouse muscle as a model of myofiber loss in DMD. Muscle Nerve. 1991;14:42–50. doi: 10.1002/mus.880140108. [DOI] [PubMed] [Google Scholar]
- 15.Heslop L, Morgan JE, Partridge TA. Evidence for a myogenic stem cell that is exhausted in dystrophic muscle. J. Cell Sci. 2000;113:2299–2308. doi: 10.1242/jcs.113.12.2299. [DOI] [PubMed] [Google Scholar]
- 16.Harris JB, Johnson MA. Further observations on the pathological responses of rat skeletal muscle to toxins isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin. Exp. Pharmacol. Physiol. 1978;5:587–600. doi: 10.1111/j.1440-1681.1978.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 17.Sacco A, et al. IGF-I increases bone marrow contribution to adult skeletal muscle and enhances the fusion of myelomonocytic precursors. J. Cell Biol. 2005;171:483–492. doi: 10.1083/jcb.200506123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wehrman TS, et al. Luminescent imaging of β-galactosidase activity in living subjects using sequential reporter-enzyme luminescence. Nature Methods. 2006;3:295–301. doi: 10.1038/nmeth868. [DOI] [PubMed] [Google Scholar]
- 19.Blau HM, Hughes SM. Retroviral lineage markers for assessing myoblast fate in vivo. Adv. Exp. Med. Biol. 1990;280:201–203. doi: 10.1007/978-1-4684-5865-7_22. [DOI] [PubMed] [Google Scholar]
- 20.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rando TA, Pavlath GK, Blau HM. The fate of myoblasts following transplantation into mature muscle. Exp. Cell Res. 1995;220:383–389. doi: 10.1006/excr.1995.1329. [DOI] [PubMed] [Google Scholar]
- 22.Gussoni E, Blau HM, Kunkel LM. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nature Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- 23.Cao YA, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc. Natl Acad. Sci. USA. 2004;101:221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barberi T, et al. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nature Med. 2007;13:642–648. doi: 10.1038/nm1533. [DOI] [PubMed] [Google Scholar]
- 25.Osawa M, et al. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 26.Silberstein L, et al. Developmental progression of myosin gene expression in cultured muscle cells. Cell. 1986;46:1075–1081. doi: 10.1016/0092-8674(86)90707-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig.