Cdk5 phosphorylates Cdh1 and modulates cyclin B1 stability in excitotoxicity (original) (raw)
Abstract
Anaphase-promoting complex/cyclosome (APC/C), an E3 ubiquitin ligase that destabilizes cell cycle proteins, is activated by Cdh1 in post-mitotic neurons, where it regulates axonal growth, synaptic plasticity and survival. The APC/C–Cdh1 substrate, cyclin B1, has been found to accumulate in degenerating brain areas in Alzheimer's disease and stroke. This highlights the importance of elucidating cyclin B1 regulation by APC/C–Cdh1 in neurons under stress conditions relevant to neurological disease. Here, we report that stimulation of _N_-methyl-D-aspartate receptors (NMDARs) that occurs in neurodegenerative diseases promoted the accumulation of cyclin B1 in the nuclei of cortical neurons; this led the neurons to undergo apoptotic death. Moreover, we found that the Ser-40, Thr-121 and Ser-163 triple phosphorylation of Cdh1 by the cyclin-dependent kinase-5 (Cdk5)–p25 complex was necessary and sufficient for cyclin B1 stabilization and apoptotic death after NMDAR stimulation. These results reveal Cdh1 as a novel Cdk5 substrate that mediates cyclin B1 neuronal accumulation in excitotoxicity.
Keywords: apoptosis, Cdh1, Cdk5, cyclin B1, neurons
Introduction
The anaphase-promoting complex/cyclosome (APC/C) is a multisubunit E3 ubiquitin ligase that controls cell cycle progression by assembling polyubiquitin chains on regulatory proteins, such as securin and mitotic cyclins, targeting them for degradation by the 26S proteasome (Peters, 2002). APC/C is activated in a cell cycle-dependent manner by two activators, namely Cdc20 and Cdh1 (Schwab et al, 1997; Sigrist and Lehner, 1997; Visintin et al, 1997). In early mitosis, APC/C binding to Cdc20 leads to the initiation of anaphase, whereas association with Cdh1 in late mitosis maintains APC/C activity throughout the subsequent G1 (Schwab et al, 1997; Sigrist and Lehner, 1997; Visintin et al, 1997; Yamaguchi et al, 1997, 2000; Kitamura et al, 1998; Blanco et al, 2000; Bashir et al, 2004; Wei et al, 2004; Buschhorn and Peters, 2006). As Gieffers et al (1999) found that Cdh1 and APC/C subunits, not Cdc20, are expressed in post-mitotic mammalian neurons, it has been shown that APC/C–Cdh1 is implied in fundamental nervous system processes, such as axonal growth and patterning (Konishi et al, 2004; Lasorella et al, 2006), synaptic control (Juo and Kaplan, 2004; van Roessel et al, 2004) and neuronal survival (Almeida et al, 2005).
Being terminally differentiated cells, post-mitotic neurons actively repress proteins related with the cell cycle and DNA replication, and the aberrant expression of these proteins has been associated with neuronal cell death (reviewed in Becker and Bonni, 2004). In particular, the APC/C–Cdh1 substrate, cyclin B1 (Thornton and Toczyski, 2003), has been found to accumulate in degenerating neurons of patients suffering from neurodegenerative disorders, such as Alzheimer's disease (Vincent et al, 1997; Yang et al, 2003; McShea et al, 2007; Mosch et al, 2007) and stroke (Love, 2003), as well as in experimental models of cerebral ischaemia (Erdo et al, 2004; Wen et al, 2004; Rashidian et al, 2007). Recently, we reported that APC/C–Cdh1 activity downregulates cyclin B1 protein stability in post-mitotic neurons as an essential survival mechanism (Almeida et al, 2005). In view of these findings, we sought to elucidate cyclin B1 regulation by APC/C–Cdh1 in neurons under excitotoxic conditions relevant to neurological disease.
Results and discussion
Cyclin B1 accumulation mediates neuronal apoptotic death induced by NMDAR stimulation in cortical neurons
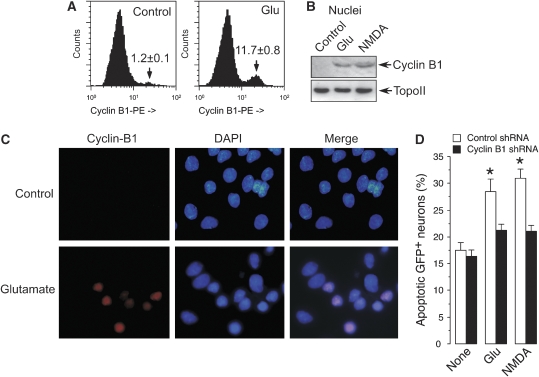
To investigate changes in cyclin B1 protein levels following an excitotoxic stimulus, post-mitotic cortical neurons were incubated with glutamate (100 μM) or NMDA (100 μM) for 5 min and the cells were examined for the presence of cyclin B1 protein 20 h after the insult. We have shown earlier that under these conditions glutamate triggers neuronal death in an _N_-methyl-D-aspartate receptor (NMDAR)-dependent manner (Almeida and Bolaños, 2001). Flow cytometric analyses using a phycoerythrin (PE)-cyclin B1-conjugated antibody (Figure 1A) revealed an ∼6.6-fold increase in cyclin B1 following glutamate treatment. Western blotting analyses confirmed that glutamate and NMDA treatments promoted cyclin B1 accumulation in the nuclei (Figure 1B). Immunocytochemical analyses confirmed cyclin B1 colocalization with condensed and fragmented nuclei in neurons treated with glutamate (Figure 1C). These results suggest that cyclin B1 accumulates in the nuclei after an excitotoxic stimulus in cortical neurons. Cyclin B1 was also found to accumulate in cortical neurons incubated in the presence of the active form of β-amyloid (βA25–35), but not in the presence of its inactive form (βA35−25) (Supplementary Figure S1A), supporting the potential relevance for cyclin B1 accumulation in neurological disease. To test whether cyclin B1 mediated neuronal death by NMDAR stimulation, we knocked down cyclin B1 using pSuper-neo.gfp small hairpin RNAs (Supplementary Figure S1B). The expression of cyclin B1 shRNA in neurons—identified as GFP+ cells—significantly prevented the increase in apoptotic death caused by glutamate or NMDA treatment, as analysed by flow cytometric analysis of annexin V+/7-amino-actinomycin D (7-AAD−) in GFP+ neurons (Figure 1D; Supplementary Figure S1C). Taken together, these results suggest that nuclear accumulation of cyclin B1 after glutamate or NMDA treatments mediates excitotoxic death.
Figure 1.
Cyclin B1 accumulation mediates neuronal apoptotic death induced by NMDAR stimulation in cortical neurons. Cortical neurons in primary culture were incubated in the presence of glutamate (100 μM) or NMDA (100 μM) for 5 min; after washing, cells were further incubated in culture medium for 20 h. (A) Analyses of cyclin B1 (phycoerythrin-conjugated) levels by flow cytometry revealed that glutamate induced an ∼6.6-fold increase in cyclin B1. (B) Western blot analysis revealed that glutamate or NMDA promoted cyclin B1 accumulation in the nuclei of neurons. (C) Immunocytochemical evidence for cyclin B1 nuclear localization in neurons by glutamate treatment ( × 100 magnification). (D) Knockdown of cyclin B1 (cyclin B1 shRNA) in neurons prevents the apoptotic death triggered by glutamate or NMDA. *P<0.05 versus none. Topoisomerase-II (TopoII, A) was used as protein loading marker.
Cdh1 modulates cyclin B1 stability after NMDAR stimulation in cortical neurons
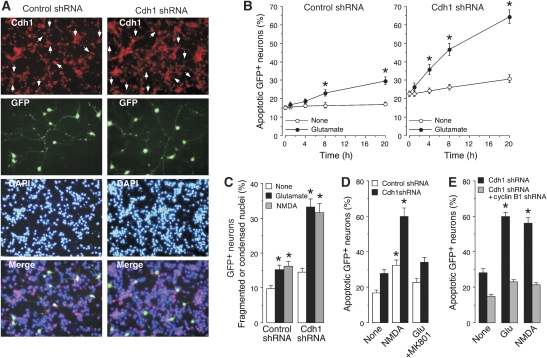
To understand the mechanism responsible for cyclin B1 accumulation in neurons treated with glutamate or NMDA, we focused on Cdh1; that is, the only APC/C activator responsible for cyclin B1 degradation in post-mitotic neurons (Almeida et al, 2005). To accomplish this, Cdh1 was knocked down using shRNA inducing cyclin B1 accumulation (Supplementary Figure S1D) and promoting a decrease in Cdh1 protein in post-mitotic neurons as assessed by immunocytochemistry (Figure 2A). We have shown earlier that Cdh1 shRNA treatment in these neurons triggers cyclin B1 accumulation in the nucleus (Almeida et al, 2005). Incubation of neurons with glutamate induced a time-dependent increase in apoptotic death (Figure 2B, left panel), an effect that was accelerated and enhanced by Cdh1 knockdown (Figure 2B, right panel). This was confirmed by fluorescence microscopic analysis of GFP+ neurons; a significant loss of neurons being observed after glutamate treatment and Cdh1 shRNA knockdown (Supplementary Figure S1E). Quantitative analyses of GFP+ neurons by flow cytometry confirmed the synergistic effect of glutamate and Cdh1 knockdown in causing a loss of GFP+ neurons (Supplementary Figure S1F, left panel); furthermore, the expression of hEmi1, an inhibitor of Cdh1-dependent APC/C activity (Hsu et al, 2002), mimicked the effect of Cdh1 knockdown (Supplementary Figure S1F, right panel). To confirm the involvement of Cdh1 in excitotoxicity, we also determined fragmented or condensed nuclei of neurons treated with glutamate or NMDA. Such treatments enhanced the proportion of fragmented or condensed nuclei, an effect that was synergistic with Cdh1 knockdown (Figure 2C) or APC/C inhibition with hEmi1 (Supplementary Figure S1G). Flow cytometric analyses confirmed the synergistic enhancement of apoptotic death caused by Cdh1 shRNA (Figure 2D) or hEmi1 (Supplementary Figure S1H) with NMDA or glutamate, the glutamate effect being prevented by the NMDAR antagonist, MK-801. Taken together, these results suggest that inhibition of APC/C–Cdh1 sensitizes neurons to apoptotic death by NMDAR stimulation. Next, we investigated whether cyclin B1 regulation by APC/C–Cdh1 was a pathway involved in excitotoxicity. As shown in Figure 2E, cyclin B1 shRNA rescued the increased apoptotic death caused by glutamate or NMDA treatments to Cdh1-silenced neurons (see also Supplementary Figure S1I). These results suggest that Cdh1 protects neurons against NMDAR-mediated excitotoxicity by preventing cyclin B1 protein accumulation.
Figure 2.
Cdh1 modulates cyclin B1 stability after NMDAR stimulation in cortical neurons. (A) Cdh1 shRNA expression induces Cdh1 depletion, as revealed by immunostaining ( × 20 magnification). (B) Cdh1 knockdown sensitizes neurons towards excitotoxic damage, as apoptotic death was accelerated and enhanced by Cdh1 shRNA expression. (C) Cdh1 knockdown synergistically enhanced the proportion of fragmented or condensed nuclei induced by glutamate after 20 h. (D) Cdh1 knockdown enhanced apoptotic death caused by glutamate or NMDA, as assessed by annexin V/7-AAD; the NMDA receptor inhibitor, MK-801, prevented glutamate-mediated apoptotic death. (E) Cyclin B1 shRNA abolished the increased apoptotic death triggered 20 h after glutamate (100 μM/5 min) or NMDA (100 μM/5 min) treatments in Cdh1-silenced neurons. *P<0.05 versus none.
NMDAR stimulation produces serine-phosphorylation and accumulation of Cdh1 in the cytosol
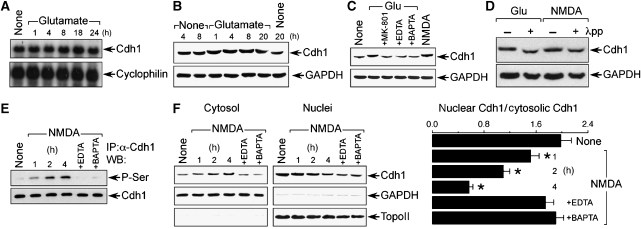
We then investigated the mechanism by which NMDAR stimulation regulated APC/C–Cdh1 activity. The mRNA abundance of Cdh1 was unaltered by glutamate treatment, at least up to 24 h post-incubation (Figure 3A). In addition, Cdh1 protein abundance remained unmodified, although its migration in a polyacrylamide gel was slowed—shifted—by glutamate treatment in a time-dependent manner (Figure 3B), suggesting that the excitotoxic stimulus triggered a post-translational modification on Cdh1 protein. The Cdh1 shift was mimicked by NMDA and abolished by the NMDA receptor antagonist, MK-801, and by the Ca2+ chelators EDTA and BAPTA (Figure 3C), indicating an NMDAR-mediated phenomenon. As the Cdh1 protein shift has been shown earlier to be a consequence of Cdh1 hyperphosphorylation (Kramer et al, 2000; Tran et al, 2008), we tested such a possibility. To do so, protein extracts obtained from glutamate- or NMDA-treated neurons were incubated with protein phosphatase. As shown in Figure 3D, phosphatase treatment converted the Cdh1 shifted band to the fast-migrating form, suggesting that glutamate and NMDA trigger Cdh1 phosphorylation. To further test this possibility, protein extracts obtained from neurons incubated with NMDA were immunoprecipitated with α-Cdh1, and western blotting was performed against a general α-phosphoserine antibody. As shown in Figure 3E, NMDA treatment produced a time- and Ca2+-dependent serine phosphorylation of Cdh1. This result, together with the observed increase in the apparent molecular mass of the shifted Cdh1 (Figure 3B) and previous observations from other laboratories (Kramer et al, 2000; Tran et al, 2008), suggests that Cdh1 would be phosphorylated, at least, on serine residue(s). Moreover, it has been reported that Cdh1 hyperphosphorylation sequesters Cdh1 in the cytosol, thus inhibiting APC/C–Cdh1 activity (Jaquenoud et al, 2002; Zhou et al, 2003). We therefore investigated this possibility. As shown in Figure 3F, NMDA promoted a time-dependent accumulation of Cdh1 in the cytosol that paralleled Cdh1 depletion in the nuclei, a phenomenon that was Ca2+ dependent. Thus, NMDAR stimulation promotes Cdh1 hyperphosphorylation, and its accumulation in the cytosol, leading to APC/C–Cdh1 inactivation.
Figure 3.
NMDAR stimulation produces serine phosphorylation and accumulation of Cdh1 in the cytosol. (A) Northern blot analysis revealed that glutamate (100 μM/5 min) did not alter Cdh1 mRNA abundance, at least up to 24 h. (B) Glutamate did not change Cdh1 protein abundance, but it caused Cdh1 mobility super-shift in the gel. (C) Cdh1 mobility super-shift at 4 h after glutamate incubation was abolished by NMDAR inhibition (using MK-801) or by removing calcium (using EDTA or BAPTA), and it was mimicked by NMDA. (D) Protein phosphatase (λpp) treatment of samples obtained from neurons at 4 h after glutamate or NMDA stimulation prevents Cdh1 mobility super-shift. (E) Protein extracts were obtained from NMDA (100 μM/5 min)-treated neurons at the indicated time points, Cdh1 was immunoprecipitated and subjected to western blotting against α-phosphoserine (P-Ser); NMDA time-dependently triggered Ser phosphorylation of Cdh1 that was prevented by removing calcium (using EDTA or BAPTA). (F) NMDA induced Cdh1 accumulation in the cytosol and depletion in the nuclei in a time-dependent manner; both effects were prevented by calcium removal (using EDTA or BAPTA); topoisomerase-II (TopoII) was undetectable in the cytosol (left panel), and GAPDH was negligibly expressed in the nuclei (middle panel); films (left and middle panels) were scanned and the nuclear/cytosolic Cdh1 band intensity ratios were calculated (right panel). *P<0.05 versus none. Cyclophilin was used as RNA loading marker, and GAPDH (B, C, D, F), Cdh1 (E) or topoisomerase-II (TopoII, F) were used as protein loading markers.
NMDAR stimulation activates Cdk5–p25, which interacts with, and phosphorylates Cdh1, leading to cyclin B1 accumulation and apoptotic death
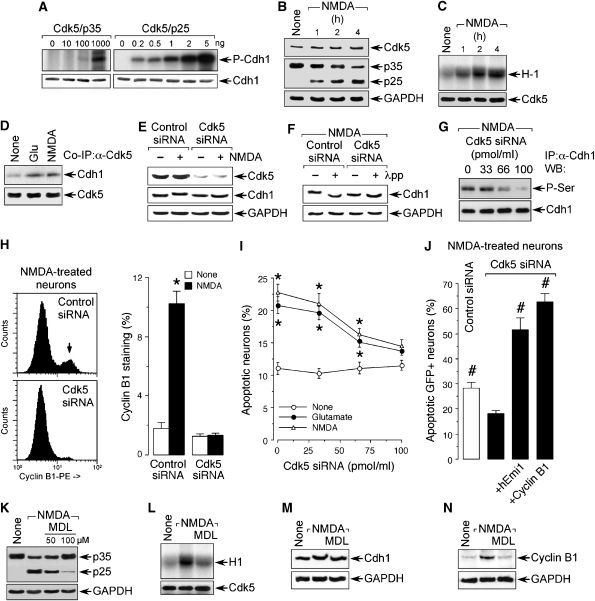
To understand the mechanism by which NMDAR stimulation triggered Cdh1 phosphorylation, we first investigated the possible involvement of Ca2+/calmodulin-dependent protein kinase-II (CaMKII). Neurons were treated with NMDA in either the absence or presence of the CaMKII inhibitor KN-62 (Tokumitsu et al, 1990). Cdh1 was then immunoprecipitated and subjected to western blotting against α-phosphoserine. As shown in Supplementary Figure S2A, the increased levels of serine-phosphorylated Cdh1 was not prevented by KN-62, ruling out CaMKII in the effect. As glycogen synthase kinase-3 (GSK-3) has been shown earlier to be associated with NMDAR-mediated excitotoxicity (Liang and Chuang, 2007), we next tested for this second possibility. As depicted in Supplementary Figure S2B, AR-A014418 (AR-A), a selective GSK-3 inhibitor (Gould et al, 2004) did not abolish NMDAR-mediated Cdh1 phosphorylation. Having ruled out CaMKII and GSK-3 as the kinases promoting Cdh1 phosphorylation, we searched for putative phosphorylation sites in the Cdh1 protein sequence at scansite (http://scansite.mit.edu). We found three high probability score potential phosphorylation consensus sites by cyclin-dependent kinases (Cdks; S/T-P-X-K/H/R) in Cdh1 conserved in mammals (Ser-40, Thr-121 and Ser-163; Supplementary Figure S2C). We inhibited Cdks with the unspecific Cdk inhibitor roscovitine, a treatment that dose-dependently prevented the gel shift and serine phosphorylation (Supplementary Figures S2D and E) of Cdh1 caused by NMDA. This result supports the notion that Cdh1 is phosphorylated by a Cdk. This observation, together with the evidence that Cdk5 is activated by calpain in a Ca2+-dependent manner after glutamate treatment to cortical neurons (Lee et al, 2000), prompted us to focus on Cdk5 as a putative kinase for Cdh1. To test this possibility, we first investigated the possible in vitro phosphorylation of Cdh1 by Cdk5. Cdk5 can be bound to either p35 or to its proteolytic product, p25, which maintains Cdk5 in a persistent activation form (Lee et al, 2000) and is known to be accumulated in the neurons of patients with Alzheimer's disease (Patrick et al, 1999). The in vitro Cdk5 kinase assay showed that both forms of Cdk5 were able to phosphorylate Cdh1, although Cdk5–p25 was about three orders of magnitude more efficient than Cdk5–p35 (Figure 4A). Next, we tested whether Cdk5 was activated in neurons treated with NMDA. To perform this, we first investigated for the possible changes in p35 and p25 protein levels under these conditions. As shown in Figure 4B, NMDAR stimulation elicited a decrease in p35 protein abundance and the accumulation of p25, suggesting the proteolytic conversion of p35 into p25 in neurons under such stress. To confirm that this was accompanied by Cdk5 activation, we determined histone H1 phosphorylation, which increased in the extracts obtained from NMDA-treated neurons (Figure 4C). These results confirm that NMDAR stimulation triggers Cdk5 activation in our experimental model. To further investigate whether Cdk5 interacted with Cdh1 in neurons, protein extracts obtained from neurons treated with NMDA or glutamate were immunoprecipitated using a α-Cdk5 antibody, followed by western blotting against Cdh1. The results (Figure 4D) show an increase in the band intensity in the co-immunoprecipitated NMDA- and glutamate-treated samples, suggesting that NMDAR stimulation promotes a Cdk5–Cdh1 interaction. To confirm that Cdk5 was the Cdk responsible for Cdh1 phosphorylation in neurons, Cdk5 was knocked down with siRNA, a treatment that was effective as from 3 days of treatment in a dose-dependent manner (Supplementary Figures S2F and G). Cdk5-knockdown neurons were then incubated with NMDA, and the Cdh1 gel shift was investigated. As shown in Figure 4E, Cdk5 silencing abolished Cdh1 gel shift by NMDA; furthermore, the Cdk5 knockdown-mediated Cdh1 gel migration was similar to that found after incubation of the protein extracts with protein phosphatase (Figure 4F). To test the possible role of Cdk5 in Cdh1 serine(s) phosphorylation, protein extracts obtained from Cdk5-silenced neurons incubated with NMDA were immunoprecipitated with α-Cdh1, and western blotting was performed against α-phosphoserine. As shown in Figure 4G, Cdk5 knockdown dose-dependently inhibited the NMDA-mediated serine(s) phosphorylation of Cdh1. Taken together, these results suggest that Cdh1 is serine-phosphorylated by Cdk5 on NMDAR stimulation.
Figure 4.
NMDAR stimulation activates Cdk5–p25, which interacts with, and phosphorylates Cdh1, leading to cyclin B1 accumulation and apoptotic death. (A) In vitro Cdk5 kinase assay showed that both Cdk5–p35 and Cdk5–p25 complexes phosphorylate Cdh1, Cdk5–p25 being about three orders of magnitude more efficient than Cdk5–p35. (B) NMDA (100 μM/5 min) time-dependently triggered p35 decrease and p25 accumulation in cortical neurons. (C) NMDAR stimulation triggers Cdk5 activation as assayed by histone 1 (H1) phosphorylation in vitro. (D) Neurons were incubated with glutamate or NMDA; after 4 h, protein extracts were immunoprecipitated with α-Cdk5 and subjected to western blotting against α-Cdk5 and α-Cdh1; glutamate and NMDA increased Cdh1 band intensity. (E) NMDA treatment produced Cdh1 mobility super-shift that was prevented by Cdk5 knockdown using Cdk5 siRNA. (F) Incubation of protein extracts with protein phosphatase (λpp) decreased the Cdh1 mobility super-shift caused by NMDA in control siRNA-treated neurons; this effect was not evident in Cdk5 siRNA-treated neurons. (G) Cdk5 siRNA dose-dependently prevented the serine phosphorylation of Cdh1 induced 4 h after NMDA treatment. (H) Cdk5 siRNA abolished the increase in cyclin B1 caused by NMDA treatment, as assessed by flow cytometry after 20 h using a cyclin B1-phycoerythrin (PE) antibody. (I) Cdk5 siRNA dose-dependently prevented glutamate- and NMDA-mediated apoptotic death, as assessed by flow cytometry using annexin V/7-ADD (20 h post-treatment). (J) Inhibition of APC/C–Cdh1 (using hEmi1 expression) or cyclin B1 overexpression counteracted the protective effect caused by Cdk5 siRNA on NMDA-mediated neuronal apoptotic death. (K) MDL, a specific inhibitor of calpain, dose-dependently prevented NMDA-mediated p35 cleavage into p25. MDL prevented (L) Cdk5 activation, (M) Cdh1 gel shift and (N) cyclin B1 accumulation caused by NMDAR stimulation. *P<0.05 versus none. #P<0.05 versus Cdk5 siRNA+NMDA (in J). GAPDH (B, E, F, K, N) was used as protein loading marker.
Next, we investigated the involvement of this Cdk5–Cdh1 interaction in cyclin B1 accumulation and apoptotic death after NMDAR stimulation. To achieve this, we analysed cyclin B1 in Cdk5-silenced neurons and observed that Cdk5 knockdown abolished the increase in cyclin B1 triggered by NMDA treatment (Figure 4H). We also found that Cdk5 siRNA dose-dependently prevented the apoptotic neuronal death triggered by NMDA or glutamate (Figure 4I). In addition, the decrease in NMDA-mediated apoptotic death promoted by Cdk5 knockdown was counteracted by both Cdh1 inhibition (hEmi1) and cyclin B1 overexpression (Figure 4J). These results confirm that NMDAR stimulation leads to cyclin B1 accumulation and excitotoxicity through Cdk5. In view that two possible forms of Cdk5 (Cdk5–p35 and Cdk5–p25) can phosphorylate Cdh1 in vitro (Figure 4A), we next aimed to elucidate which was responsible for the phosphorylation of Cdh1 and cyclin B1 accumulation on NMDAR stimulation. To perform this, neurons were treated with NMDA either in the absence or in the presence of MDL, a specific inhibitor of calpain, that is, the enzyme responsible for the conversion of p35 into p25 (Lee et al, 2000). As depicted in Figure 4K, the NMDA-mediated cleavage of p35 into p25 was inhibited by MDL in a dose-dependent manner. Furthermore, MDL prevented NMDA-mediated phosphorylation of histone H1 (Figure 4L), Cdh1 gel shift (Figure 4M) and cyclin B1 accumulation (Figure 4N). Taken together, these results confirm that NMDAR stimulation of cortical neurons triggers the calpain-mediated proteolytic conversion of p35 into p25 leading to Cdk5 activation. Cdk5–p25 complex then phosphorylates Cdh1, which remains inhibited sequestered in the cytosol (see Figure 3F) and, hence, inactivates E3 ubiquitin ligase APC/C (Jaquenoud et al, 2002; Zhou et al, 2003). As a result of this, APC/C substrate (Thornton and Toczyski, 2003), cyclin B1, stabilizes (Figures 4H and N) and trigger neuronal apoptotic death (Figure 4J), possibly by catalysing BAD Ser-128 phosphorylation, as reported by others (Konishi et al, 2002).
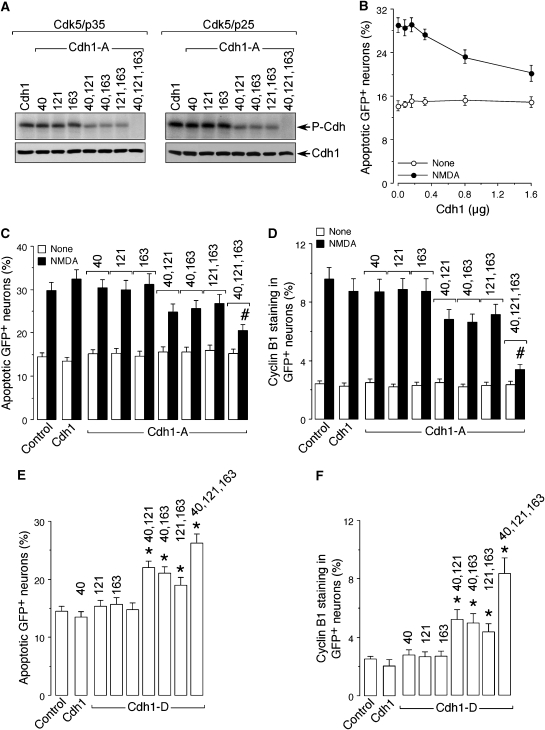
Triple phosphorylation of Cdh1 at Ser-40, Thr-121 and Ser-163 by Cdk5 is necessary and sufficient for cyclin B1 accumulation and excitotoxicity
Finally, we aimed to identify the specific residues of Cdh1 phosphorylated by Cdk5 that were responsible for cyclin B1 accumulation and apoptotic death. To achieve this, we performed site-directed mutagenesis on residues Ser-40, Thr-121 and Ser-163 of Cdh1, which ranked high probability phosphorylation scores by Cdks at http://scansite.mit.edu. These residues were mutated to Ala as single, double or triple mutations, and the resulting mutant forms of Cdh1 were expressed, affinity purified and subjected to Cdk5–p35 (1 μg) and Cdk5–p25 (1 ng) in vitro kinase assays. As shown in Figure 5A, the single mutations did not produce observable decrease in the level of Cdh1 phosphorylation, the double mutations slightly reduced Cdh1 phosphorylation, whereas the triple mutation abolished Cdh1 phosphorylation by both complexes of Cdk5. These results strongly suggest that Cdk5–p25 and Cdk5–p35 phosphorylate Cdh1 at residues Ser-40, Thr-121 and Ser-163 in vitro. To investigate whether the phosphorylation of these resides were responsible for excitotoxicity and cyclin B1 accumulation, a preliminary dose–response curve was performed using wild-type Cdh1. As shown in Figure 5B, Cdh1 expression dose-dependently prevented NMDA-mediated apoptotic death; thus, for the next experiments we used the minimum Cdh1 cDNA amount (0.32 μg/106 neurons) that did not alter NMDA-mediated apoptosis (Figures 5B and C). Expression in neurons of equal amounts of Cdh1 cDNA carrying the single mutations did not affect NMDA-mediated apoptotic death (Figure 5C) or cyclin B1 accumulation (Figure 5D); expression of Cdh1 carrying the double mutations slightly prevented NMDA-mediated apoptotic death (Figure 5C) and cyclin B1 accumulation (Figure 5D); finally, expression of the triple mutant Cdh1 almost completely abolished NMDA-mediated apoptotic death (Figure 5C) and cyclin B1 accumulation (Figure 5D). These results strongly suggest that the triple phosphorylation of Cdh1 on Ser-40, Thr-121 and Ser-163 is necessary for NMDA-mediated cyclin B1 accumulation and excitotoxicity. To further corroborate these results, we aimed to generate mutant forms of Cdh1 mimicking the phosphorylated status. To achieve this, Ser-40, Thr-121 and Ser-163 residues of Cdh1 were mutated to Asp as single, double or triple mutations, and equal amounts of wild-type or mutant forms of Cdh1 cDNA were then expressed in neurons. Expression of Cdh1 carrying the single mutations did not affect neuronal apoptotic death (Figure 5E) or cyclin B1 levels (Figure 5F); expression of the double mutations significantly increased apoptotic death (Figure 5E) and cyclin B1 levels (Figure 5F), and expression of the triple mutant further enhanced apoptotic death (Figure 5E) and cyclin B1 levels (Figure 5F) to similar values to those observed with NMDA treatment (Figures 5C and D). These results strongly suggest that the triple phosphorylation of Cdh1 on Ser-40, Thr-121 and Ser-163 is sufficient to trigger cyclin B1 accumulation and apoptotic death in cortical neurons.
Figure 5.
Triple phosphorylation of Cdh1 at Ser-40, Thr-121 and Ser-163 by Cdk5 is necessary and sufficient for cyclin B1 accumulation and excitotoxicity. Residues Ser-40, Thr-121 and Ser-163 of Cdh1 were mutated to either Ala (Cdh1-A) or Asp (Cdh1-D) by site-directed mutagenesis as single, double or triple mutations. Both the wild-type and all Ala-mutant forms of Cdh1 were expressed and affinity purified. (A) The in vitro kinase assay on the Ala mutant forms of Cdh1 by Cdk5–p35 (1 μg) and Cdk5–p25 (1 ng) shows that single mutations do not produce observable decrease in Cdh1 phosphorylation, double mutations slightly reduced Cdh1 phosphorylation, and triple mutation abolished Cdh1 phosphorylation. (B) Cdh1 expression in cortical neurons dose-dependently prevented NMDA-mediated apoptotic death, the minimum Cdh1 cDNA amount not altering NMDA-mediated apoptosis being 0.32 μg/106 neurons. This amount was used for the following experiments. (C) NMDA-mediated apoptotic death, as assessed by flow cytometry using annexin V/7-ADD (20 h post-treatment), was not prevented by the expression in neurons of equal amounts of Cdh1 cDNA carrying the single Ala mutations, it was slightly prevented by Cdh1 carrying the double Ala mutations and it was significantly prevented by Cdh1 carrying the triple Ala mutation. (D) NMDA-mediated cyclin B1 accumulation, as assessed by flow cytometry after 20 h using a cyclin B1-phycoerythrin (PE) antibody, was not prevented by the expression in neurons of equal amounts of Cdh1 cDNA carrying the single Ala mutations, it was slightly prevented by Cdh1 carrying the double Ala mutations and it was abolished by Cdh1 carrying the triple Ala mutation. (E) Neuronal apoptotic death, as assessed by flow cytometry using annexin V/7-ADD (20 h post-treatment), was not altered by the expression of equal amounts of Cdh1 cDNA carrying the Asp single mutations, it was significantly increased by Cdh1 carrying the double Asp mutations and it further increased by Cdh1 carrying the triple Asp mutation, reaching values similar to those observed with NMDA treatment (See C). (F) Cyclin B1 accumulation, as assessed by flow cytometry after 20 h using a cyclin B1-PE antibody, was not altered by the expression of equal amounts of Cdh1 cDNA carrying the Asp single mutations, it was significantly increased by Cdh1 carrying the double Asp mutations and it further increased by Cdh1 carrying the triple Asp mutation, reaching values similar to those observed with NMDA treatment (See C). *P<0.05 versus control. #P<0.05 versus control NMDA.
In conclusion, here we show that NMDAR stimulation triggers the activation of Cdk5, which promotes Cdh1 phosphorylation at residues Ser-40, Thr-121 and Ser-163 and cytosolic sequestration. As a consequence of these events, APC/C is inactivated and cyclin B1 is accumulated in the nucleus, leading to neuronal apoptotic death. Interestingly, Cdk5 phosphorylates the NMDAR NR2A subunit at Ser-1232, hence facilitating NMDAR synaptic transmission (Li et al, 2001). It is possible that NMDAR overactivation, which occurs in prolonged release of the neurotransmitter glutamate such as that occurring in neurodegenerative diseases and stroke (Bossy-Wetzel et al, 2004), could lead to a Cdk5-NMDAR activation feedback loop contributing to the propagation of the neurodegeneration response. If so, Cdk5-mediated APC/C–Cdh1 inactivation followed by cyclin B1 accumulation may be a novel pathway in neuronal death following NMDAR stimulation. This is in good agreement with the recently reported finding that the cyclin B1 counterpart kinase, Cdk1, mediates neurotoxicity by phosphorylating and activating the FOXO transcription factor (Yuan et al, 2008). As cyclin B1 accumulates in degenerating brain areas in Alzheimer's disease (Vincent et al, 1997; Konishi et al, 2002) and stroke (Wen et al, 2004)––that is, situations known to be associated with an excitotoxic-type of neuronal death (Bossy-Wetzel et al, 2004)—our results suggest that Cdk5-mediated Cdh1 inactivation would contribute to neuronal death in neurological disorders.
Materials and methods
Cell culture, transfections and treatments
Primary culture of cortical neurons were prepared from fetal Wistar rats at 16 days of gestation and cultured in DMEM (Sigma, Madrid, Spain) supplemented with 10% FCS (Roche Diagnostics, Heidelberg, Germany). At 48 h after plating, the medium was replaced with DMEM supplemented with 5% horse serum (Sigma), 20 mM D-glucose and cytosine arabinoside (10 μM) to prevent non-neuronal proliferation (Almeida et al, 2004). After 4 days in culture, neurons were transfected with plasmid constructions or siRNA using Lipofectamine 2000 (Invitrogen, Madrid, Spain), following the manufacturer's instructions (Almeida et al, 2005). After transfections, neurons were incubated with 100 μM glutamate (plus 10 μM glycine) or 100 μM NMDA (plus 10 μM glycine and 10 μM CNQX, to prevent the secondary activation of non-NMDA receptors) in buffered Hank's solution (pH 7.4), for 5 min (Almeida and Bolaños, 2001). When indicated, incubations were performed in the presence of MK-801 (Sigma), KN-62 (Calbiochem, Merck, Darmstadt, Germany), AR-A014418 (Calbiochem), roscovitine (Sigma) or MDL (MDL-28170 or calpain inhibitor-III; Calbiochem). Neurons were then washed and further incubated in culture medium for the indicated time period. Human embryonic kidney 293T (HEK293T) cells were maintained in DMEM supplemented with 10% (v/v) FCS and transfected with plasmid constructions using Lipofectamine 2000.
Plasmid constructions, site-directed mutagenesis and siRNA used
Cdh1 and cyclin B1 depletion was achieved by RNA interference using a vector-based shRNA approach. For Cdh1, we used the target sequence 5′-TGAGAAGTCTCCCAGTCAG-3′ (Almeida et al, 2005). For cyclin B1, we used the 5′-GATGGAGCTGATCCAAACC-3′ target sequence (Almeida et al, 2005). As controls, we used the firefly luciferase-targeted oligonucleotide 5′-CTGACGCGGAATACTTCGA-3′ (Almeida et al, 2005). The forward and reverse synthetic 64-nt oligonucleotides (Isogen Life Technologies, Maarsen, The Netherlands) were designed, annealed and inserted into the _Bgl_II/_Hin_dIII sites of either pSuper.GFP vectors, following the manufacturer's instructions (Oligoengine, Seatle, WA). These constructions express a 19-bp 9-nt stem-loop shRNA specifically targeted against Cdh1 (Cdh1 shRNA), cyclin B1 (cyclin B1 shRNA) or luciferase (control) mRNAs. hEmi1 was used to inhibit the APC/C complex by co-transfecting cortical primary neurons with pCS2-hEmi1 (generously donated by Dr Peter Jackson, Stanford University School of Medicine, Stanford, CA) plus pEGFP (Clontech Laboratories Inc., Palo Alto, CA), and control transfections were carried out with a truncated form of hEmi1 carrying a deletion of the zinc-binding region that causes loss of function (pCS2-Myc5-hEmi1ΔZBR, generously donated by Dr Peter Jackson) plus pEGFP. Human full-length Cdh1 cDNA was a generous gift of Dr J Pines (Gurdon Institute, University of Cambridge, UK), and was used to obtain the Ser-40, Thr-121 and Ser-163 single, double or triple Cdh1 mutants using the QuikChange XL site directed mutagenesis kit (Stratagene, La Jolla, CA, USA). Residues were mutated either to Ala (Cdh1-A; to block phosphorylation) or to Asp (Cdh1-D; to mimic a phosphorylated status). Specific depletion of Cdk5 was achieved by using small (21 bp) interfering double-stranded ribonucleotides (siRNA) specifically designed to target the coding sequence of the rat Cdk5 mRNA. The targeted sequence was 5′-AAGCCGUACCCGAUGUAUC-3′ (Zheng et al, 2007). A corresponding 21-bp luciferase siRNA was used as a control. These siRNAs were synthesized, high-pressure liquid chromatography-purified, and annealed to create the double-stranded siRNA (Dharmacon Research Inc., Lafayette, CO).
Western blot analysis
After transfections and treatments, cells were lysed in RIPA buffer (2% sodium dodecylsulphate, 2 mM EDTA, 2 mM EGTA and 50 mM Tris pH 7.5), supplemented with phosphatase inhibitors (1 mM Na3VO4 and 50 mM NaF) and protease inhibitors (100 μM phenylmethylsulphonyl fluoride, 50 μg/ml anti-papain, 50 μg/ml pepstatin, 50 μg/ml amastatin, 50 μg/ml leupeptin, 50 μg/ml bestatin and 50 μg/ml soybean trypsin inhibitor), and boiled for 5 min. Aliquots of cell extracts were subjected to SDS polyacrylamide gel (MiniProtean®; Bio-Rad) and blotted with anti-Cdh1 (AR38, a generous gift from Dr J Gannon, Clare Hall Laboratories, Cancer Research UK), anti-cyclin B1 (GNS-1; Becton Dickinson Biosciences Pharmingen, Erembodegen, Belgium), anti-topoisomeraseII (Becton Dickinson Biosciences Pharmingen), anti-phosphoserine (Zymed, Invitrogen), anti-Cdk5 (C-8, Santa Cruz Biotechnology, Heidelberg, Germany), anti-p35 (Santa Cruz Biotechnology) or anti-GAPDH (Sigma), overnight at 4°C. Signal detection was performed with an enhanced chemiluminescence kit (Pierce, Thermo Scientific, Waltham, MA, USA).
Co-immunoprecipitation assay
For co-immunoprecipitation of endogenous proteins, neurons were lysed in ice-cold buffer containing 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 10 mM EDTA, 2 mM EGTA, 1% NP-40, supplemented with the phosphatase and protease inhibitors cited above. Cell extracts were clarified by centrifugation and supernatants (500 μg protein) were incubated with anti-Cdk5 (2 μg) for 4 h at 4°C, followed by the addition of 30 μl of protein A-agarose (GE Healthcare Life Sciences, Uppsala, Sweden), for 2 h at 4°C. When Cdh1 was immunoprecipitated, supernatants (50 μg protein) were incubated with anti-Cdh1 in the presence of 10 μl of protein G-agarose (GE Healthcare Life Sciences). Inmunoprecipitates were extensively washed with lysis buffer and detected by western blot analysis (Wang et al, 2003).
Northern blot analysis
Purified (Sigma) total RNA samples were electrophoresed (15 μg of RNA per line) on a 1% (w/v) agarose-formaldehyde gel. After transfer to a GeneScreen Plus membrane (NEN Life Science, Boston, MA) and crosslinking by ultraviolet irradiation, membranes were hybridized for 18 h at 65°C in the presence of the appropriate random-primed [α-32P]dCTP-radiolabelled (Amersham Biosciences, Buckinghamshire, UK) cDNA probe and exposed to Kodak XAR-5 film. We used a cDNA probe either against human Cdh1 or against rat cyclophilin.
Flow cytometric detection of cyclin B1
After transfections and treatments, cells were fixed and permeabilized using the Fix&Perm kit from Caltag (Invitrogen) and were stained with PE mouse anti-cyclin B1 (GSN1; Santa Cruz Biotechnology), according to the protocol included in the technical data sheet.
Immunocytochemistry
Neurons grown on glass coverslips were fixed with 4% (v/v, in PBS) paraformaldehyde for 30 min and immunostained anti-Cdh1 or anti-cyclin B1 antibodies (Almeida et al, 2005). Immmunolabelling was deteted using anti-mouse-IgG-Cy3 (Sigma). Coverslips were washed and mounted in SlowFade® light antifade reagent (Molecular Probes, Oregon, USA) on glass slides.
Measurement of neuronal cell death
After treatments, neurons were stained with APC-conjugated annexin V/7-AAD (Becton Dickinson Biosciences) and were analysed on a FACScalibur flow cytometer (Becton Dickinson Biosciences), to quantitatively determine the percentage of apoptotic cells. Annexin V-APC-stained cells that were 7-AAD negative were considered apoptotic (Almeida et al, 2004, 2005). Data are expressed as a percentage of neuronal cell death. In addition, neuronal death was assayed by 4′-6-diamidino-2-phenylindole (DAPI) nuclear staining. Neurons were fixed with 4% (v/v, in PBS) paraformaldehyde for 30 min at room temperature, rinsed with PBS and incubated with DAPI (30 μM; Sigma). Results were expressed as a percentage of condensed or fragmented nuclei.
Protein phosphatase assay
Cells were lysed in buffer (50 mM Tris pH 7.5, 10 mM KCl, 10% glycerol, 137 mM NaCl, 0.1% NP-40 and protease inhibitors) either in the absence or presence of phosphatase inhibitors (1 mM Na3VO4 and 50 mM NaF) and boiled for 5 min. Aliquots of cell lysates were centrifuged at 14 000 g for 10 min. Lysates was then incubated with l-protein phosphatase buffer (50 mM Tris pH 8.0, 2 mM MgCl2) either in the absence (lysates containing phosphatase inhibitors) or presence of l-protein phosphatase (Roche Diagnostics) at 5 U/μg protein for 30 min at 30°C (Tran et al, 2008). Reactions were terminated by adding 2 × lysis buffer used for western blot analysis. Samples were boiled for 5 min and analysed by western blot.
Cdk5 kinase assay
Neurons were lysed in ice-cold buffer containing 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 10 mM EDTA, 2 mM EGTA, 1% NP-40, supplemented with the phosphatase and protease inhibitors cited above. After clearing debris by centrifugation, extracts (500 μg protein) were incubated with anti-Cdk5 (2 μg) for 4 h at 4°C, followed by the addition of 30 μl of protein A-agarose (GE Healthcare Life Sciences) for 2 h at 4°C. Immunoprecipitates were washed for four times in lysis buffer and resuspended in kinase buffer (20 mM Tris–HCl pH 7.6, 20 mM MgCl2, 2 mM MnCl2, 1 mM EDTA, 1 mM EGTA and 0.1 mM dithiothreitol) containing 20 μM ATP, 10 μCi of [γ-32P]ATP and histone H1 (50 μg/ml; Sigma). Samples were subjected to SDS–polyacrylamide gel (12%) electrophoresis and transferred proteins were visualized by autoradiography or blotted with anti-Cdk5 (Wang et al, 2003). Glutathione _S_-transferase (GST)-Cdh1, GST-Cdh1-A and GST-Cdh1-D were expressed 1 h at 37°C in 10 ml of BL21 culture after 1 mM isopropyl-β-D-thiogalactopyranoside induction. Peptides were purified on 200 μl of glutathione-agarose beads and stored at −80°C. GST-fused proteins (10 μl) and different concentrations of recombinant Cdk5–p35 or Cdk5–p25 (Cell Signaling Technology, Boston, MA) were incubated as above.
Statistical analysis
Measurements from individual cultures were always performed in triplicate. The results are expressed as mean±s.e.m. values for three different culture preparations. Statistical analysis of the results was performed by one-way analysis of variance, followed by the least significant difference multiple range test. In all cases, P<0.05 was considered significant.
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Information
Acknowledgments
CM is a recipient of a fellowship from the Junta de Castilla y León. We thank S Moreno for his helpful comments on the paper, and M Sacristan for her help with the Cdk5 kinase assay. We also thank M Resch for her excellent technical assistance. AA is funded by Fondo de Investigación Sanitaria, Instituto de Salud Carlos III (RENEVAS) and Junta de Castilla y León. JPB is funded by SAF2007-61492, CONSOLIDER, SA066A07 and Red Terapia Celular-ISCIII. This study was specifically supported by the Fondo de Investigación Sanitaria (grant FIS06/0794 to AA) Junta de Castilla y Leon (GRS244/A/08).
Competing financial interest The authors declare that they have no competing financial interest.
References
- Almeida A, Bolaños JP (2001) A transient inhibition of mitochondrial ATP synthesis by nitric oxide synthase activation triggered apoptosis in primary cortical neurons. J Neurochem 77: 676–690 [DOI] [PubMed] [Google Scholar]
- Almeida A, Bolanos JP, Moreno S (2005) Cdh1/Hct1-APC is essential for the survival of postmitotic neurons. J Neurosci 25: 8115–8121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida A, Moncada S, Bolaños JP (2004) Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol 6: 45–51 [DOI] [PubMed] [Google Scholar]
- Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M (2004) Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 428: 190–193 [DOI] [PubMed] [Google Scholar]
- Becker EBE, Bonni A (2004) Cell cycle regulation of neuronal apoptosis in development and disease. Progr Neurobiol 72: 1–25 [DOI] [PubMed] [Google Scholar]
- Blanco MA, Sanchez-Diaz A, de Prada JM, Moreno S (2000) APC(ste9/srw1) promotes degradation of mitotic cyclins in G(1) and is inhibited by cdc2 phosphorylation. EMBO J 19: 3945–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Schwarzenbacher R, Lipton SA (2004) Molecular pathways to neurodegeneration. Nat Med 10: S2–S9 [DOI] [PubMed] [Google Scholar]
- Buschhorn BA, Peters JM (2006) How APC/C orders destruction. Nat Cell Biol 8: 209–211 [DOI] [PubMed] [Google Scholar]
- Erdo F, Trapp T, Mies G, Hossmann KA (2004) Immunohistochemical analysis of protein expression after middle cerebral artery occlusion in mice. Acta Neuropathol 107: 127–136 [DOI] [PubMed] [Google Scholar]
- Gieffers C, Peters BH, Kramer ER, Dotti CG, Peters JM (1999) Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc Natl Acad Sci USA 96: 11317–11322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Einat H, Bhat R, Manji HK (2004) AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol 7: 387–390 [DOI] [PubMed] [Google Scholar]
- Hsu JY, Reimann JDR, Sørensen CS, Lukas J, Jackson PK (2002) EF2-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC–Cdh1. Nat Cell Biol 4: 358–366 [DOI] [PubMed] [Google Scholar]
- Jaquenoud M, van Drogen F, Peter M (2002) Cell cycle-dependent nuclear export of Cdh1p may contribute to the inactivation of APC/C(Cdh1). EMBO J 21: 6515–6526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juo P, Kaplan JM (2004) The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr Biol 14: 2057–2062 [DOI] [PubMed] [Google Scholar]
- Kitamura K, Maekawa H, Shimoda C (1998) Fission yeast Ste9, a homolog of Hct1/Cdh1 and Fizzy-related, is a novel negative regulator of cell cycle progression during G1-phase. Mol Biol Cell 9: 1065–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi Y, Lehtinen M, Donovan N, Bonni A (2002) Cdc2 phosphorylation of BAD links the cell cycle to the cell death machinery. Mol Cell 9: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Konishi Y, Stegmuller J, Matsuda T, Bonni S, Bonni A (2004) Cdh1-APC controls axonal growth and patterning in the mammalian brain. Science 303: 1026–1030 [DOI] [PubMed] [Google Scholar]
- Kramer ER, Scheuringer N, Podtelejnikov AV, Mann M, Peters JM (2000) Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol Biol Cell 11: 1555–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasorella A, Stegmuller J, Guardavaccaro D, Liu G, Carro MS, Rothschild G, de la Torre-Ubieta L, Pagano M, Bonni A, Iavarone A (2006) Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature 442: 471–474 [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405: 360–364 [DOI] [PubMed] [Google Scholar]
- Li BS, Sun MK, Zhang L, Takahashi S, Ma W, Vinade L, Kulkarni AB, Brady RO, Pant HC (2001) Regulation of NMDA receptors by cyclin-dependent kinase-5. Proc Natl Acad Sci USA 98: 12742–12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang MH, Chuang DM (2007) Regulation and function of glycogen synthase kinase-3 isoforms in neuronal survival. J Biol Chem 282: 3904–3917 [DOI] [PubMed] [Google Scholar]
- Love S (2003) Neuronal expression of cell cycle-related proteins after brain ischaemia in man. Neurosci Lett 353: 29–32 [DOI] [PubMed] [Google Scholar]
- McShea A, Lee HG, Petersen RB, Casadesus G, Vincent I, Linford NJ, Funk JO, Shapiro RA, Smith MA (2007) Neuronal cell cycle re-entry mediates Alzheimer disease-type changes. Biochim Biophys Acta 1772: 467–472 [DOI] [PubMed] [Google Scholar]
- Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T (2007) Aneuploidy and DNA replication in the normal human brain and Alzheimer's disease. J Neurosci 27: 6859–6867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH (1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402: 615–622 [DOI] [PubMed] [Google Scholar]
- Peters JM (2002) The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol Cell 9: 931–943 [DOI] [PubMed] [Google Scholar]
- Rashidian J, Iyirhiaro GO, Park DS (2007) Cell cycle machinery and stroke. Biochim Biophys Acta 1772: 484–493 [DOI] [PubMed] [Google Scholar]
- Schwab M, Lutum AS, Seufert W (1997) Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell 90: 683–693 [DOI] [PubMed] [Google Scholar]
- Sigrist SJ, Lehner CF (1997) Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90: 671–681 [DOI] [PubMed] [Google Scholar]
- Thornton BR, Toczyski DP (2003) Securin and B-cyclin/CDK are the only essential targets of the APC. Nat Cell Biol 5: 1090–1094 [DOI] [PubMed] [Google Scholar]
- Tokumitsu H, Chijiwa T, Hagiwara M, Mizutani A, Terasawa M, Hidaka H (1990) KN-62, 1-[N,_O_-bis(5-isoquinolinesulfonyl)-_N_-methyl-L-tyrosyl]-4-phenylpiperazine, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 265: 4315–4320 [PubMed] [Google Scholar]
- Tran K, Mahr JA, Choi J, Teodoro JG, Green MR, Spector DH (2008) Accumulation of substrates of the anaphase-promoting complex (APC) during human cytomegalovirus infection is associated with the phosphorylation of Cdh1 and the dissociation and relocalization of APC subunits. J Virol 82: 529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roessel DA, Elliott DA, Robinson IM, Prokop A, Brand AH (2004) Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell 119: 707–718 [DOI] [PubMed] [Google Scholar]
- Vincent I, Jicha G, Rosado M, Dickson DW (1997) Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain. J Neurosci 17: 3588–3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin R, Prinz S, Amon A (1997) CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278: 460–463 [DOI] [PubMed] [Google Scholar]
- Wang J, Liu S, Fu Y, Wang JH, Lu Y (2003) Cdk5 activation induces hippocampal CA1 cell death by directly phosphorylating NMDA receptors. Nat Neurosci 6: 1039–1047 [DOI] [PubMed] [Google Scholar]
- Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG (2004) Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 428: 194–198 [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Brun-Zinkernagels AM, Koulen P, Simpkins JW (2004) Transient cerebral ischemia induces aberrant neuronal cell cycle re-rentry and Alzheimer's disease-like tauopathy in female rats. J Biol Chem 279: 22684–22692 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Murakami H, Okayama H (1997) A WD repeat protein controls the cell cycle and differentiation by negatively regulating Cdc2/B-type cyclin complexes. Mol Biol Cell 8: 2475–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Okayama H, Nurse P (2000) Fission yeast Fizzy-related protein srw1p is a G(1)-specific promoter of mitotic cyclin B degradation. EMBO J 19: 3968–3977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mufson EJ, Herrup K (2003) Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer's disease. J Neurosci 23: 2557–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Becker EB, Merlo P, Yamada T, DiBacco S, Konishi Y, Schaefer EM, Bonni A (2008) Activation of FOXO1 by Cdk1 in cycling cells and postmitotic neurons. Science 319: 1665–1668 [DOI] [PubMed] [Google Scholar]
- Zheng YL, Li BS, Kanungo J, Kesavapany S, Amin N, Grant P, Pant HC (2007) Cdk5 modulation of mitogen-activated protein kinase signaling regulates neuronal survival. Mol Biol Cell 18: 404–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ching YP, Chun AC, Jin DY (2003) Nuclear localization of the cell cycle regulator CDH1 and its regulation by phosphorylation. J Biol Chem 278: 12530–12536 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Information