Cadherin Adhesion, Tissue Tension and Non-Canonical Wnt Signaling Regulate Fibronectin Matrix Organization (original) (raw)
. Author manuscript; available in PMC: 2010 Mar 1.
Summary
In this study we demonstrate that PCP signaling regulates morphogenesis in Xenopus embryos in part, through the assembly of the fibronectin (FN) matrix. We outline a regulatory pathway that includes cadherin adhesion and signaling through Rac and Pak culminating in actin reorganization, myosin contractility and tissue tension, which in turn directs the correct spatiotemporal localization of FN into a fibrillar matrix. Increased mechanical tension promotes FN fibril assembly in the blastocoel roof (BCR) while reduced BCR tension inhibits matrix assembly. These data support a model for matrix assembly in tissues where cell-cell adhesions play an analogous role to the focal adhesions of cultured cells by transferring to integrins the tension required to direct FN fibril formation at cell surfaces.
Introduction
The extracellular matrix (ECM) protein FN is central to a variety of biological processes required for embryogenesis including cell adhesion, directional cell migration, gene expression and the control of cell division and survival (Dzamba et al., 2002; Hynes, 1990; Mosher, 1989). Mice are unable to complete embryogenesis without FN (George et al., 1993) highlighting the importance of this ECM protein in development. In the Xenopus embryo FN is required for the morphogenetic movements of gastrulation; cells undergoing motility are in contact with FN matrix (Davidson et al., 2004) and inhibition of FN assembly disrupts the directed cellular rearrangements needed to drive epiboly and axial extension (Davidson et al., 2006; Marsden and DeSimone, 2001; Marsden and DeSimone, 2003).
The assembly of FN is regulated both spatially and temporally in the embryo. Fibrillogenesis does not begin until the onset of gastrulation and occurs only on the free surfaces of blastocoel roof (BCR) cells that line the blastocoel cavity (Lee et al., 1984). Soluble FN is present in the blastocoel fluid and all cells at this stage express integrin α5β1, the primary integrin responsible for FN assembly (Gawantka et al., 1992; Joos et al., 1995; Lee et al., 1984). Thus, the presence of both FN and its receptor are alone insufficient to initiate fibrillogenesis.
Although the regulation of FN assembly in vivo is not well understood, the process has been studied extensively in cultured cells. Current models of FN assembly (Mao and Schwarzbauer, 2005a; Pankov et al., 2000) suggest that fibrillogenesis is a multistep process. First, dimeric FN binds to cell surfaces through integrins. Integrin α5β1 is the primary receptor responsible for FN assembly but other integrins can support assembly when activated (Sechler et al., 2000; Wennerberg et al., 1996; Wu et al., 1995; Wu et al., 1996). Cytoskeletal tension triggers a conformational change in integrin-bound FN dimers that exposes FN-FN interaction sites that promote assembly (Baneyx et al., 2002; Gao et al., 2003; Ingham et al., 1997; Zhong et al., 1998).
The biomechanical context in which assembly occurs in 3-dimensional microenvironments and embryos is different from that of cells cultured on planar substrates (Cukierman et al., 2001; Mao and Schwarzbauer, 2005b). Cells of the BCR are anchored to one another in a multilayered sheet and, therefore, the mechanism by which tension is generated and transmitted to FN dimers in order to promote fibrillogenesis in the embryo is likely to be different from that employed by cultured cells.
Many cellular processes including convergence extension, oriented cell division and mobilization of Dishevelled (Dsh) are dependent on both FN matrix (Davidson et al., 2006; Marsden and DeSimone, 2001; Marsden and DeSimone, 2003; Munoz et al., 2006) and non-canonical Wnt/planar cell polarity (PCP) signaling (Munoz et al., 2006; Tada et al., 2002; Wallingford et al., 2000). Disruption of the PCP pathway leads to disorganization of the ECM (Goto et al., 2005). In this study we establish whether Wnt/PCP signaling regulates FN assembly and investigate mechanisms by which cell-cell adhesion and tissue tension contribute to this process.
Results
Non-canonical Wnt signaling regulates convergent extension and FN fibril assembly
Both Wnt 11 signaling through the non-canonical Wnt pathway and FN matrix have been reported to regulate convergent extension movements. (Davidson et al., 2006; Marsden and DeSimone, 2003; Tada and Smith, 2000). We therefore investigated whether Wnt 11 regulates FN deposition and fibril assembly. An extensive fibrillar FN matrix forms on the surface of untreated control dorsal marginal zones (DMZ) taken from intact embryos (Figure 1A). Control animal cap explants obtained from stage 8 embryos extend following treatment with activin (Figure 1B) as predicted from earlier studies (Symes and Smith, 1987). Animal caps expressing dominant negative Wnt11 (dnWnt11) did not extend (Figure 1D) and did not assemble fibrils (Figure S1). Correspondingly, FN was localized to cell peripheries in DMZs expressing dnWnt11 but fibrils did not form across DMZ cell surfaces (Figure 1C). Wnt11 is reported to activate both the canonical and non-canonical Wnt pathways (Tada and Smith, 2000; Tao et al., 2005) and Dsh is a common downstream effector of both pathways (Wallingford and Habas, 2005). The DIX domain of Dsh is required for canonical Wnt signaling through β-catenin whereas non-canonical Wnt signaling acts through the DEP domain (Axelrod et al., 1998). We coinjected transcripts encoding dnWnt11 along with Dsh constructs lacking either the DIX domain (DshΔDIX) or the DEP domain (DshΔDEP). DshΔDIX, but not DshΔDEP, was able to rescue both FN assembly and animal cap extension in the presence of dnWnt11 (Figure 1E-H; S1). Thus, non-canonical Wnt PCP-signaling is required for FN fibrillogenesis in DMZs and tissue extension in animal caps but canonical Wnt signaling is not.
Figure 1. Non-canonical Wnt signaling regulates FN assembly and cadherin adhesion.
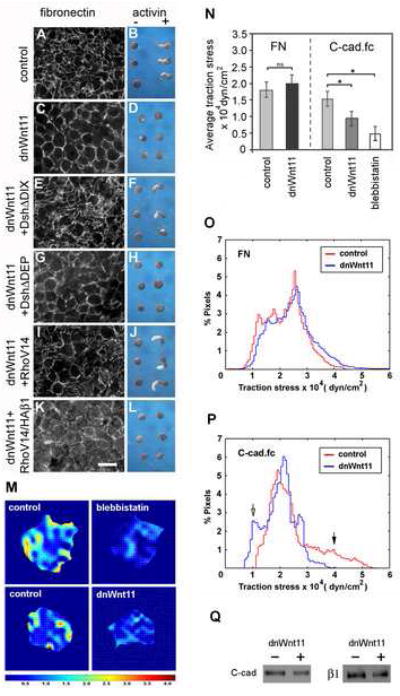
(A,C,E,G,I, K) FN fibril assembly in dorsal marginal zones from stage 11 embryos was analyzed by immunofluoresence microscopy using anti-FN pAb 32. (A) FN forms a network of fibrils in control dorsal marginal zones by this stage. (C) dnWnt11 inhibits fibril assembly but FN is deposited at cell boundaries. (E) FN fibril assembly is rescued by Dsh lacking the DIX domain. (G) Dsh lacking the DEP domain is unable to rescue fibril assembly. (I) Constitutively active RhoV14 also rescues FN assembly. (K) dn integrin HAβ1 blocks RhoV14 rescue of fibril assembly. Scale bar in K=50 μm. (B,D,F,H,J,L) Animal cap extension assays plus (+) or minus (-) activin. (B) Control activin-treated animal caps extend. (D) Caps expressing dnWnt11 do not extend. (F) Dsh construct lacking the DIX domain rescues cap extension. (H) Dsh lacking the DEP domain does not rescue cap extension. (J) Constitutively active RhoV14 rescues extension. (L) RhoV14 is unable to rescue extension when co-expressed with HAβ1. (M) Representative traction stress maps for BCR cells plated on substrates coated with C-Cad.fc. Colors express the magnitude of traction stress (× 10 4 dyn/cm2) mapped to cell regions.
(N) Average traction stress per cell (+/- S.D.) for control, blebbistatin treated and dnWnt11 expressing cells. (*) p < 0.001. (ns) not significant.
(O-P) Distribution of traction stress for control and dnWnt11 expressing cells on (O) FN and (P) C-cad.fc substrates. Vertical axis values represent percentage of total pixels with a specific stress value. For panels (N-P) averages were calculated from 10-15 individual cell measurements. Frequencies of low (open arrow) and high (closed arrow) stress attributed to dnWnt11 and control cells, respectively, are indicated.
(Q) Western blot to compare cell surface levels of C-cad and β1 integrins in intact biotinylated BCRs, (+) or (-) dnWnt 11.
Because the small GTPases Rho and Rac are downstream of Dsh in the PCP pathway (Habas et al., 2003; Habas et al., 2001), we tested whether constitutively active forms of these proteins could rescue the effects of dnWnt. Co-expression of RhoV14 (or RacV12, data not shown) with dnWnt 11 rescued both FN assembly (Figure 1I) and activin induced animal cap extension (Figure 1J). Integrin function is required for GTPase rescue of the dnWnt11 phenotype because inhibition of FN fibrillogenesis with the HAβ1 dominant negative integrin construct Marsden and DeSimone, 2003) prevented animal cap extension even in the presence of RhoV14 (Figure 1 K,L). More FN punctae were observed at cell surfaces in these samples (Fig. 1K), which may reflect a change in integrin association with the actin cytoskeleton caused by the dominant negative HAβ1 construct (LaFlamme et al., 1994).
Cadherin-dependent traction stress is reduced in cells expressing dnWnt11
Because the dnWnt11 experiments indicated that PCP signaling could regulate FN fibril assembly, an integrin and tension-dependent process, we next investigated whether cell-matrix and/or cell-cell adhesion were altered in cells expressing dnWnt11. For these studies we focused attention on cells of the BCR, which are the first to assemble FN fibrils in vivo. In order to more directly compare the functional consequences of any adhesive differences we utilized traction force microscopy (Butler et al., 2002), which provides an instantaneous readout of the mechanical interaction between a cell and its substrate. This enabled us to establish the distribution of traction stresses generated by individual cells that have engaged either their cadherins or integrins (Figure 1M-P). BCRs were dissociated and the cells plated on elastic substrates (containing fluorescent beads as fiduciary markers) with known elastic modulus, coated either with the extracellular domain of C-cadherin fused to the Fc domain of IgG (C-cad.fc) or with FN. Cells were allowed to spread and then treated with trypsin to free them from the substrate. Bead positions before and after cell detachment were recorded, analyzed and average traction stresses computed (Figure 1N).
BCR cells applied an average traction stress of between ~1.8 and 2.0 × 104 dyn/cm2 on FN substrates; differences between control and dnWnt11 cells were not significant (Figure 1N). In addition, no significant differences in the distribution of forces generated by these cells were observed (Figure 1O). In contrast, the average traction stress generated by control cells on C-cad.fc was approximately 1.5 × 104 dyn/cm2 while stress applied by dnWnt11 cells on C-cad.fc was reduced by ~40% relative to controls (Figure 1M,N). BCR cells treated with blebbistatin were used as a baseline control for reduced cell contractility in the traction force assay (Figure 1M,N). These cells remained adherent to the C-cad substrate but average traction stress was reduced by more than 60%.
Although the changes in average traction stress of control and dnWnt11 cells on C-cad.fc were significant (Figure 1N), more dramatic differences in the distribution of forces in these cells became apparent when the data were instead plotted as a histogram representing percent pixels at defined traction stress values (Fig. 1P). Some regions of control cells generated forces in excess of 4.0 × 104 dyn/cm2 (Figure 1P, closed arrow). In contrast, there were regions of dnWnt11 cells that had much reduced traction stress relative to controls (Figure 1P, open arrow).
Cell-surface biotinylation of dissected BCRs demonstrated that the levels of both C-cadherin (C-cad) and integrin β1 in cells expressing dnWnt11 were reduced to 53% that of controls (Figure 1Q; N=3; SEM +/- 18% for C-cadherin and SEM +/- 12% for integrin β1). However, it is unclear whether this was in part due to differences in labeling efficiency given the altered morphology of the BCR under these conditions.
Cadherins regulate FN assembly
Because interfering with Wnt/PCP signaling reduced cadherin adhesion and affected FN assembly, we next asked whether FN fibril assembly in the BCR might also be linked to normal changes in cell-cell adhesion. At the onset of gastrulation (stage 10), FN appears in a punctate pattern on cell surfaces with a few short fibrils occasionally observed between cells (Figure 2A,D). FN is assembled into fibrillar structures over time and by stage 11.5 (Figure 2E,H) forms an extensive meshwork across the BCR. The assembly of FN coincides with apparent changes in cell-cell contacts. At the onset of gastrulation, BCR cells are rounded and C-cadherin staining is seen at cell borders and in radially-directed cell protrusions (Figure 2A,B arrows) typical of nascent cell-cell junctions (Adams et al., 1996; Vasioukhin et al., 2000); F-actin staining is also detected at these locations (Figure 2C). By stage 11.5, BCR cells are polygonal, C-cadherin localizes to the periphery of the cells and circumferential F- actin staining is much more pronounced (Figure 2 F,G). This suggests that adherens junctions mature during the course of gastrulation, comparable to the maturation of cell-cell contacts seen in other systems (Adams et al., 1998; Hirano et al., 1987).
Figure 2. Cadherin adhesion regulates FN fibril assembly.
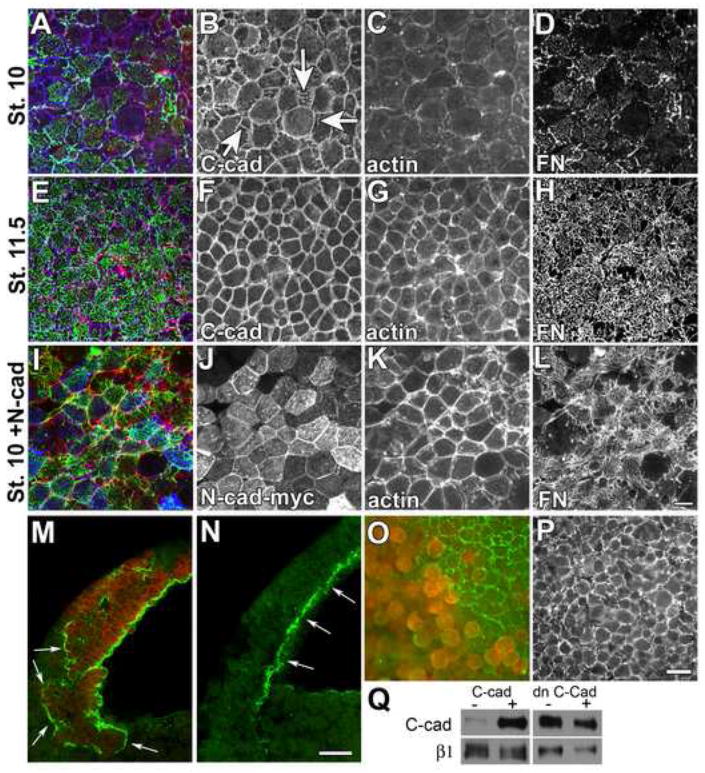
Confocal projections (15 μM) of BCRs from embryos fixed at (A-D) early (st. 10) or (E-H) late (st. 11.5) gastrula stage. BCRs were stained with antibodies against FN (green), C-cad (blue), and phalloidin to visualize filamentous actin (red). (A,E,I) Composite of all 3 fluorochromes. At stage 10, C-cad is noted at cell edges (B) and in linear arrays perpendicular to the cell edge (B, arrows), little cortical actin is present (C) and FN is arranged in punctae at cell surfaces (D). Cells in the BCR are rounded at stage 10. By stage 11, cells are polygonal in shape (compare B and F), actin staining is predominantly cortical (G) and FN is arranged in a fibrillar network (H). (I-L) Embryos expressing N-cad at stage 10 are similar to control embryos at stage 11.5; cells are polygonal (J), actin is organized at the cell cortex (K), and FN fibrils are assembled at the cell surface (L). Scale bar=25 μm in (L).
(M,N). Saggital confocal sections of stage 10 embryos injected in one blastomere at the two cell stage with transcripts encoding a myc-epitope tagged N-cad (M), compared to an uninjected sibling control (N). Embryos were immunostained with antisera to FN (green) and mAb 9E10 to detect N-Cad-myc (red). (M, arrows) FN assembles in ectopic locations at the borders between non-expressing cells and cells expressing N-Cad. (N, arrows) In normal embryos FN is restricted to the surface facing the blastocoel cavity. Scale bar =50 μm in (N).
(O, P). BCRs dissected from stage 11 embryos injected in one blastomere at the two cell stage with mRNA encoding (O) a dominant negative cadherin construct consisting of the IL2 receptor extracellular domain fused to the C-cad transmembrane and cytoplasmic domains or, (P) uninjected sibling control embryos. Isolated BCRs were immunostained with pAb32 to FN (green) and a mAb to the IL2 receptor (red). (O) FN matrix is absent from cells (red) expressing the dominant negative cadherin while, (O, P) FN fibril assembly is underway in control cells at this stage. Scale bar =50μm in (P).
(Q) Western blot of cell surface levels of C-cad and β1 integrins in intact BCRs over expressing C-cad or dn C-cad.
Increased expression of cadherins has been demonstrated to increase cell-cell adhesive strength (Duguay et al., 2003; Steinberg and Takeichi, 1994), surface tension (Foty and Steinberg, 2005) and the assembly of cortical actin in Xenopus embryos (Tao et al., 2007). We therefore investigated whether increased cell-cell adhesion could drive FN fibril assembly at earlier stages. Transcripts encoding myc epitope-tagged N-cadherin (N-cad) were injected into one cell of a 2-cell embryo at the animal pole (Figure 2I-L). N-cad-myc expression (Figure 2I,J) resulted in the precocious assembly of FN fibrils at stage 10 (Figure 2I,L) with many more fibrils noted between cells and across cell surfaces (compare 2D,L). Expression of N-cad also led to a dramatic change in BCR cell shape at this stage from round to hexagonal (compare 2B and 2J) and this was associated with increased F-actin at cell peripheries (Figure 2K). The shape and organization of the actin cytoskeleton in cells expressing N-cad more closely resembled BCR cells at stage 11.5 than sibling stage 10 BCR cells (compare Figures 2C,G and K). Cells expressing N-cad were, however, similar in size to stage 10 sibling controls, indicating that the changes in FN assembly observed were not due simply to an increase in the rate of development. These matrix-promoting effects were not limited to N-cad because expression of C-cadherin, E-cadherin and cadherin-11 each resulted in similar phenotypes (not shown). Thus, FN assembly is likely due to a general cadherin-dependent increase in cell adhesion rather than a specific effect of N-cad. Increased cadherin adhesion was also observed to redirect the normal location of FN fibrils in the embryo. Confocal sections of intact embryos revealed that FN was assembled around patches of cells expressing N-cad (Figure 2M, arrows) and not restricted to surfaces of cells facing the blastocoel as in control embryos (Figure 2N, arrows).
To investigate whether endogenous cadherins were involved in promoting FN fibril assembly, a dominant negative C-cadherin (dn C-cad) construct was expressed in embryos. This construct contains the extracellular and transmembrane domains of the interleukin-2 (IL2) receptor fused to the cytoplasmic tail of C-cad. A similar construct in which the E-cadherin tail is fused to the IL2 receptor competes with wild type cadherins for binding of cytoplasmic factors such as catenins but is unable to function in homophilic adhesion (Gottardi et al., 2001). As expected, cells in regions of the BCR that expressed the dn C-cad were rounded and had FN present at cell surfaces but it did not assemble into fibrils (Figure 2O). Adjacent regions of the BCR lacking expression of the construct initiated fibril assembly (Figure 2O) as seen in uninjected stage-matched (stage 11) controls (Figure 2P). These experiments demonstrate that cadherin adhesion is required for FN assembly.
We next confirmed that levels of C-cad at BCR cell surfaces were increased following overexpression of the wild-type C-cad construct (Figure 2Q). C-cad was used for these experiments because N-cad is not normally expressed at these stages. A representative example is shown. As expected, C-cad levels at the cell surface typically increased approximately 8-fold in embryos injected with 250 pg of C-cad transcript whereas β1 integrin levels were largely unchanged. Surface levels of both C-cad and β1 integrin were reduced slightly in embryos expressing the dn C-cad construct and this may have contributed to the lack of FN fibrils under these particular conditions. However, no significant increase in cell surface levels of C-cad was noted during the stages in which FN fibrils are normally being deposited in untreated control embryos (Figure S2). Thus, cadherin-dependent cell-cell adhesion at these stages is likely regulated through alterations in C-cad adhesive activity (Brieher and Gumbiner, 1994; Gumbiner, 2005) rather than changes in levels of expression at the cell surface. An alternative mechanism for regulation of cell-cell adhesion is Wnt/PCP dependent changes in cadherin trafficking and turnover (Ulrlich et al, 2005).
Cell-cell contact and adhesion increase traction stress on FN
The hypothesis to emerge from these experiments was that increased cell-cell adhesion and cohesivity contribute to fibril assembly by altering tissue tension in the BCR. Because FN fibril assembly is ultimately an integrin-dependent process, we asked whether a change in traction stress on FN could be detected in BCR cells overexpressing C-cad. Single BCR cells overexpressing C-cad (Figure 3 C,D) typically appeared less “round” than control cells (Figure 3 A,B) when plated on FN-coated elastic substrates, however, they were indistinguishable from one another in terms of average traction stress (Figure 3A,C,I). Interestingly, when control BCR cells were placed on the substrate at densities that permitted cell-cell contact, traction stress on the FN substrate was increased relative to single cells (Figure 3 E,F,I). “Clustered” cells overexpressing C-cad also applied more traction stress on FN than single cells (~1.7 × 104 dyn/cm2 vs. ~2.6 × 104 dyn/cm2) but the expression of C-cad did not significantly increase average traction stress over that calculated for clustered control cells. (Figure 3 G, H, I). Cells in C-cad clusters were, however, more spread than controls (1058 mm2 +/- 100 mm2 vs. 870 mm2 +/- 113 mm2, n=40, p <0.004) and took on a polygonal shape reminiscent of fibril-assembling cells in the intact BCR (e.g., Figure 2F,J). Because clustered cells overexpressing C-cad occupied a greater area we can conclude that the total force applied to the substrate by these cells on a per cell basis (i.e., total elastic energy transferred to the substrate) was greater than that of controls. Histogram comparing C-cad overexpressing cells alone or in clusters further illustrates differences in the distribution of traction stress on the FN substrate (Figure 3J). Thus, these data support the hypothesis that increased cell-cell adhesion is linked to a change in tissue tension that further contributes to changes in integrin-FN traction forces.
Figure 3. Traction force mapping of single vs. clustered cells on FN.
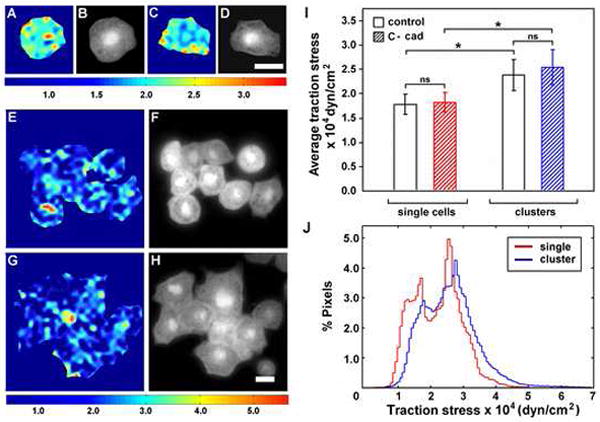
(A-H) BCR cells on FN. (A-B, E-F) control cells and (C-D, G-H) cells overexpressing C-Cad. (A,C,E,G) Traction stress maps and (B,D,F,H) phase contrast images of same field. Colors express the magnitude of traction stress (× 10 4 dyn/cm2) mapped to cell regions. Note that the range of stresses represented by the color scale in (A-D) is different from scale in (E-H). Scale bars = 25 μm.
(I) Cell-cell contact increases the average traction stress on FN exerted by both control cells and cells over expressing C-cad. Average traction stress per cell (+/-S.D.). (*) denotes statistical significance (p < 0.001). (ns) not significant.
(J) Distribution of traction stress comparing single and clustered cells over expressing C-cad on FN substrates. Vertical axis represents percentage of total pixels with a certain stress value. Data derived from a total of 30 individual cell measurements (groups of 4-9 cells/cluster) per condition. Single cell data in (I) derived from 9 cells (3 separate experiments).
Rac activity is required for the FN Fibril-Promoting Activity of N-cadherin
Activated forms of both Rho and Rac were able to rescue convergent extension and the inhibition of FN assembly observed following expression of dnWnt11 (Figure 1I,J and data not shown). Additionally, because small GTPases are known to play key roles in regulating both adhesion and the cytoskeleton (Burridge and Wennerberg, 2004; Hall, 2005) we investigated whether promotion of FN fibril formation by ectopic N-cad required GTPase activity. Transcripts encoding dominant negative Rac (N17), Rho (N19), or Cdc42 (N17; data not shown) were co-injected with transcripts encoding myc epitope-tagged N-cad into one blastomere of 2-cell stage embryos. Embryos were cultured to stage 10, fixed and stained for FN. Only RacN17 prevented the precocious assembly of FN fibrils by N-cad (compare Figure 4A to D) even though both dominant negative GTPases were expressed at equivalent levels (Figure 4G). Stage-appropriate localization of pericellular FN was observed in cells co-expressing RacN17 and N-cad.
Figure 4. Rac activity is required for cadherin to promote FN assembly.
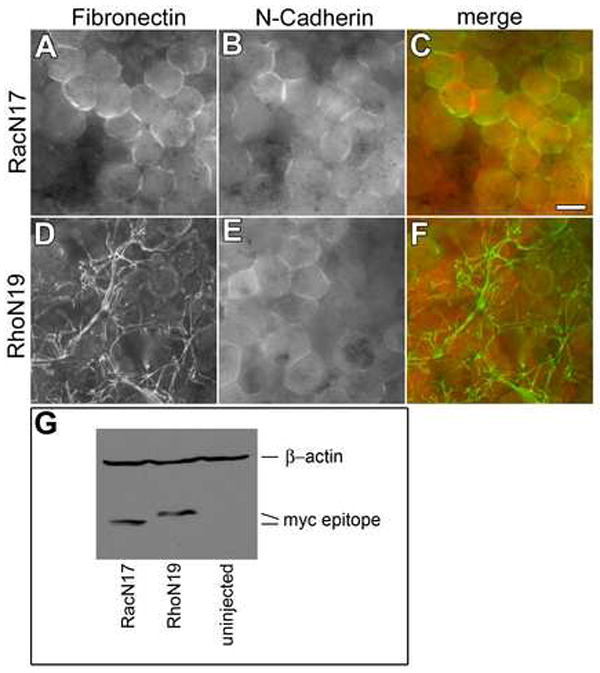
Embryos injected with mRNA encoding N-cadherin and either (A-C) dominant negative RacN17 or (D-F) RhoN19 were fixed at stage 10. BCRs immunostained with (A,D,C,F, green) anti-FN pAb 32 or, (B,E,C,F, red) mAb 9E10 to detect areas of myc epitope-tagged N-cad. Co-expression of dominant negative Rac but not dominant negative Rho inhibited the fibril promoting effects of N-cad expression (compare A-C to D-F). Bar=25μm. (G) Western blot analysis of expression levels of myc epitope-tagged RacN17 Rac and RhoN19. β-actin used as loading control.
Pak activity is required for FN assembly
Because Rac inhibition specifically abrogated the effects of N-cad over-expression on FN assembly, we asked whether Rac activity was normally required for FN fibrillogenesis. Cells expressing myc-epitope tagged RacN17 were unable to assemble FN fibrils (Figure 5A,B,D). Rac inhibition led to dramatic changes in the cytoskeleton, with F-actin observed throughout the cytoplasm rather than localized to the cell periphery (e.g., compare 5C to 5G). Thus, Rac is required for FN and actin assembly under normal conditions.
Figure 5. Rac and Pak activity are required for FN fibril assembly.
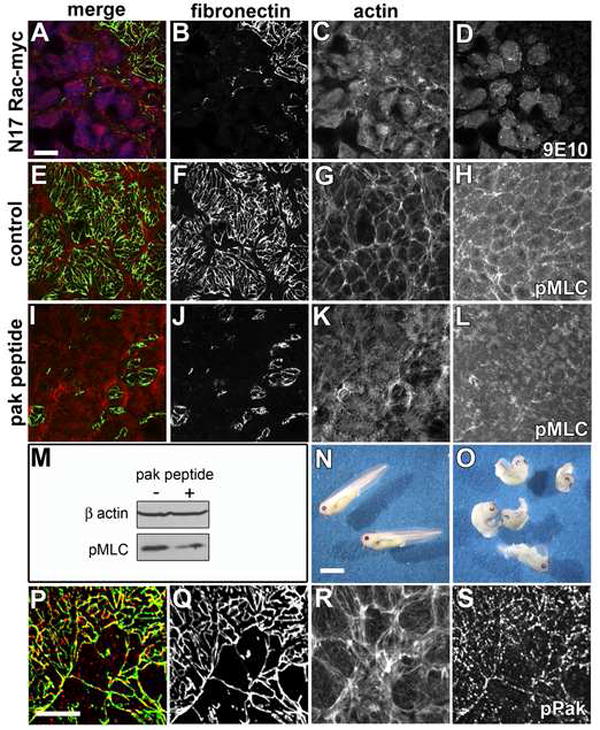
(A-D) Confocal images of BCRs from stage 11.5 embryos expressing dominant negative Rac N17 and immunolabeled with (A green, B) pAb32 to FN, (A blue, D) mAb 9E10 to detect cells expressing myc epitope tagged RacN17 and, (A red, C) phalloidin. Cells expressing RacN17 are unable to assemble FN fibrils. Actin filaments are detected throughout the cytoplasm and are no longer enriched at the periphery of cells. (E-H) Control or (I-L) inhibitory Pak peptides were delivered at blastula stage 8 by intrablastocoelic injection and the embryos cultured to late gastrula stage. (E-G, I-K) Confocal images of stage 11.5 BCRs immunostained as in (A-C). Inhibition of both FN fibril assembly and dramatic reorganization of the actin cytoskeleton was observed in embryos injected with the Pak inhibitory peptide (compare F,G to J,K). (H) Phospho-MLC (pMLC) was detected at cell peripheries in stage-matched sibling embryos injected with control peptide. (L) pMLC was greatly reduced in Pak peptide injected BCR cells. (M) Western blot of embryo lysates from control (-) and Pak peptide (+) injected embryos. β-actin included as loading control. (N) Embryos injected with control peptide develop normally while (O) embryos injected with the PAK inhibitory peptide were shortened along the anterior posterior axis. (P-S) Confocal images of BCRs from stage 11.5 embryos immunostained for (P-green, Q) FN, and (P-red, S) phospho-Pak and, (R) F-actin with phalloidin. (P) Phosphorylated Pak detected as punctae that extensively colocalize with FN fibrils. Scale bar in (A) and (P) =25μm. Scale bar in (N) =1 mm.
Pak kinases are downstream effectors of Rac that regulate the organization of the cytoskeleton (Bokoch, 2003). In endothelial cells, Pak is critical for myosin phosphorylation and contractility (Stockton et al., 2007; Stockton et al., 2004), functions that are required for assembly of both actin stress fibers and FN fibrils. To examine the potential involvement of Pak kinases in FN assembly, we blocked Pak function with an inhibitory peptide that prevents its association with the Nck SH3 domain (Kiosses et al., 2002). Embryos were injected at stage 8 with either the Pak inhibitory peptide or a control peptide in which two prolines essential for SH3 binding were mutated. The control peptide had no effect on FN matrix (Figure 5E,F) or development (Figure 5N). By contrast, the inhibitory peptide inhibited FN assembly (Figure 5I,J) and resulted in a shortened anterior-posterior axis, similar to embryos deficient in convergent extension (Figure 5O). The active peptide also resulted in actin filaments that stretched across the cells instead of the normal circumferential arrangement seen in controls (Figure 5 compare panels G and K). Phosphorylated myosin light chain (pMLC) was localized to cell contacts of control BCR cells (Figure 5H) but was reduced by the inhibitory Pak peptide (Figure 5L). The reduction in pMLC was confirmed by western blotting of embryo lysates (Figure 5M); the Pak peptide reduced MLC phosphorylation by approximately 50%. To confirm the effects of the Pak peptide, embryos were also injected with a plasmid encoding the Pak autoinhibitory domain (Stockton et al., 2004) in separate experiments. Blocking Pak kinase activity via this method gave similar results (data not shown). Confocal imaging of BCR cells labeled with an antibody that is specific for a phosphoepitope involved in Pak activation (Ser141) revealed extensive co-localization with FN fibrils (Figure 5P-S).
Mechanical tension on the BCR regulates FN assembly
Our data suggest that mechanical tension is involved in the regulation of FN fibril assembly. This was addressed directly using approaches designed to physically increase or decrease tension across the BCR and, in response, to establish if the timing and/or location of fibril assembly was altered. First, a short open-ended micropipette was used to make a hole in the BCR at stage 8 in order to reduce intra-blastocoelic hydrostatic pressure by equilibrating with the external media. The micropipette was left in place during gastrulation with the open end submerged in Danilchik’s medium containing 100 μg/ml FN, a concentration sufficient to permit assembly on BCR cells in vitro (Winklbauer, 1998) (Figure 6A). This ensured that embryos retained concentrations of FN in the blastocoel sufficient to support fibril assembly even when partially “deflated”. When sibling embryos reached stage 11.5, both punctured and control embryos were fixed and stained for FN. In punctured embryos, FN assembled along the edges of BCR cells (Figure 6C) but failed to form the extensive fibrillar network seen in control BCRs (Figure 6B,D). Labeling with the anti-α5 integrin pAb 881 also revealed that the BCR cells in punctured embryos were more round than BCR cells in control embryos (Figure 6E, F). The shape of the BCR cells from punctured embryos at stage 11.5 closely resembled those from stage 10 embryos (e.g. Figure 3A-D).
Figure 6. Tissue tension regulates FN assembly.
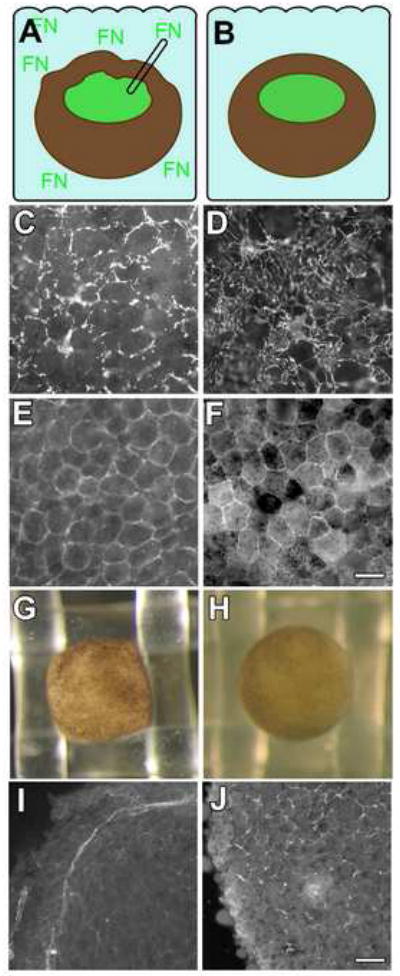
(A) Embryos were punctured with a fine glass pipette open at both ends prior to the onset of gastrulation (stage 10), and left in place until stage 11.5 with exogenous FN present in the culture medium. (B) Sibling control embryos were cultured under the same conditions without puncturing. BCRs were fixed and immunostained (C,D) using mAb 4H2, and (E,F) pAb 881 to detect FN and α5 integrin, respectively. (C) FN is detected at cell edges in punctured embryos but (D) arranged in fibrils in controls. (E) α5 integrins localized to cell membranes in punctured embryos as in controls (F), but control cells are arranged in a more polygonal pattern. Scale bar in (F) =50 μm.
(G-J) Increased tension on the BCR promotes assembly. Individual mid-blastula (st. 8) embryos were (G) forced into a nitex grid or (H) placed on top of the grid and cultured until stage 10 and fixed. (I) FN fibrils are precociously assembled on the BCR in a pattern that corresponds to the perimeter of the nitex grid where the embryo is compressed. (J) pre-fibrillar FN is localized to cell edges in controls, typical of normal embryos at this stage. Scale bar in (J) =75 μm.
Correspondingly, increased mechanical tension in the BCR was able to promote early FN fibril assembly. Embryos at stage 8 were inserted into the openings of nylon mesh (mesh opening = 1×1 mm; embryo diameter = 1.5 mm), causing the BCR to bulge and the animal pole to become “cube-shaped” (Figure 6G). Control sibling embryos were maintained on top of the mesh without squeezing (Figure 6H). When controls reached stage 10, the embryos were fixed in place, the BCRs removed, and fibril assembly assayed by immunofluoresence. Precocious fibrils were observed along edges of the BCR constrained by the mesh (Figure 6I) whereas FN remained pericellular and non-fibrillar in the BCRs of control embryos (Figure 6J). Taken together, these data show that decreased mechanical tension inhibited FN fibrillogenesis, while increased mechanical force accelerated FN matrix assembly.
Discussion
The assembly of FN into fibrils is dependent on the generation of cell tension (Halliday and Tomasek, 1995; Pankov et al., 2000; Zhang et al., 1997; Zhong et al., 1998). The integrin-dependent process by which cellular tension is transmitted to FN dimers to initiate fibril assembly has been well documented in cultured cells and is generated through cell attachment and cytoskeletal anchoring via focal adhesions (Mao and Schwarzbauer, 2005a). This mode of matrix assembly is more likely representative of mesenchymal cells and fibroblasts in mature connective tissues but does not easily explain how FN can be assembled at the free surfaces of multicelled tissues. In these situations tissue tension is also generated through increased cell-cell contact and cohesivity. We propose an alternative model of FN matrix assembly in tissues that involves the active translocation of integrin/FN-dimer complexes from sites of cell-cell contact toward the centers of the free surfaces of adjacent cells (Davidson et al. 2008) in order to initiate FN-FN interactions and fibril assembly (Figure 7).
Figure 7. Model for regulation of FN assembly in tissues.
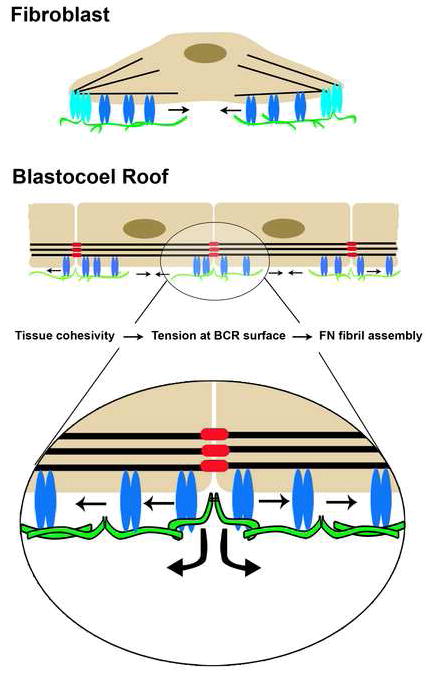
The current view of FN matrix assembly is based on observations of cells (e.g., fibroblasts) in culture. The cell is anchored to the actin cytoskeleton via integrins in focal adhesions (light blue). An increase in local mechanical tension is transmitted to α5β1 integrins (blue) with bound dimeric FNs (green) and as integrin/FN complexes move centripetally (arrows) from focal adhesions, cryptic sites in FN are exposed that promote FN-FN assembly. On the BCR and in epithelia, the analogous role of the focal adhesion in culture is supplanted by associations of the actin cytoskeleton with cadherins (red) at adherens junctions. Local mechanical tension is transmitted to α5β1 with bound FN dimers. Cryptic sites in FN are exposed and promote FN-FN assembly as integrin/FN complexes move centripetally (arrows) from sites of cell-cell contact and across the free surfaces of adjacent cells in a Rac/Pak dependent process. It is possible that a single FN dimer could be bound to integrins on adjacent cells moving in opposite directions or to two integrins within a single cell, the latter of which is more similar to movement out of the focal contact in cultured cells.
Both PCP signaling (Wallingford et al., 2000) and FN (Davidson et al., 2006; Marsden and DeSimone, 2003) are required for gastrulation movements in Xenopus. In the current study we show that normal assembly of FN matrix is inhibited following expression of dnWnt11. We propose that PCP signaling acts upstream to regulate FN fibrillogenesis by increasing cadherin adhesive activity and tension in BCR cells. Thus, one function of the PCP pathway in these embryos is to regulate FN matrix assembly in the marginal zone of the BCR. We were unable to complement morphologic descriptions of fibrillogenesis in the embryo with biochemical assays such as deoxycholate (DOC) solubility of FN, which is used routinely in mammalian cell culture systems to assess FN fibril formation (McKeown-Longo and Mosher, 1983). In both cultured mammalian cells (Wierzbicka-Patynowiski and Schwarzbauer, 2003) and in the embryo, FN is first observed as diffuse punctae across cell surfaces. With time, both in cultured cells and on the BCR, fine fibrils are found initially at cell-cell junctions. These newly assembled FN fibrils are soluble in 2% DOC but with time become detergent insoluble. The fibrils we have identified morphologically at gastrulation are DOC soluble but by neurula stages they display DOC insolubility (Figure S3) suggesting that this progression is, in fact, similar to the progression of FN assembly and DOC solubility reported for cultured cells. Moreover, we recently reported that FN fibrils are required for radial intercalation and epiboly in the blastocoel roof and that non-fibrillar FN promotes high speed migration of mesendodermal cells (Rozario et al. in press). Therefore, while early embryonic fibrillar and non-fibrillar FNs are indistinguishable in terms of DOC solubility, differences in biological functions supported by these two physical states of FN are evident in vivo.
The first assembly of FN during Xenopus embryogenesis occurs on the BCR at the onset of gastrulation (Lee et al., 1984). C-cadherin adhesion is greater in BCR cells than in other regions of the embryo (Reintsch and Hausen, 2001) consistent with the idea that cadherin adhesive activity may regulate spatially tissue cohesivity (Foty and Steinberg, 2005) and thus, tissue tension and FN assembly (Figure 7). The formation of mature adherens junctions is characterized by the remodeling of filopodial contacts between cells into continuous linear junctions and the formation of a cortical actin belt (Adams et al., 1998; Bershadsky, 2004; Tao et al., 2007; Vasioukhin et al., 2000). We observed similar changes in the BCR suggesting that nascent adherens junctions are maturing during the course of gastrulation.
Artificially increasing levels of cadherin expression on the BCR leads to precocious FN and cortical actin assembly, however levels of C-cadherin expressed at the cell surface do not normally change during the course of gastrulation (Brieher and Gumbiner, 1994). Therefore, junctional maturation at the onset of gastrulation is unlikely to be regulated by a simple alteration in cadherin expression levels. Cadherin activity is subject to regulation by inside-out signaling, but the molecular mechanisms of this are not well understood (Gumbiner, 2005). Our results, and those of others (Torres et al., 1996; Ulrich et al., 2005) demonstrate that non-canonical Wnt signaling is one mechanism by which cadherin adhesion is regulated in embryos. The cells of the BCR change shape from round to polygonal as gastrulation proceeds indicating that the cells are under increased tension (Lecuit and Lenne, 2007). Increased expression of cadherins accelerates this change in shape. Conversely, inhibition of cadherin leads to rounded cells suggesting that cadherins are responsible for generating and maintaining tension within the BCR.
We used traction force microscopy to investigate adhesion-dependent cell and tissue tension in both individual and clustered BCR cells. The most interesting and important conclusion to be drawn from these data is that cell-cell contact and adhesion results in a significant increase in traction stress on a FN substrate. Interestingly, cadherin overexpression in clustered cells did not result in an increase in the average traction stress exerted on the FN substrate compared to control clusters. These cells did, however, have increased surface area in contact with the FN substrate and were more likely to form clusters than control cells. We were unable to measure traction stress on FN substrates in clusters of cells expressing dnWnt11 or dn-cadherin because reduced cell-cell adhesion under these conditions precluded cluster formation. Because cell clustering itself leads to greater traction stress these findings suggest that Wnt and cadherin effects on assembly may result from multiple modes of influence on tissue tension.
The traction force experiments together with the whole embryo analyses, suggest that the fibril-promoting affects of cadherin overexpression are likely to come from organization and recruitment of the cortical actin cytoskeleton (Tao et al., 2007), which in turn sets the stage for the increase in tissue tension required for integrin-dependent fibril assembly. Cadherins are known to regulate F-actin assembly both in cultured cells (Kovacs et al., 2002; Kobielak et al., 2004) and in Xenopus embryos (Tao et al., 2007). Cadherin ligation has also been shown to induce myosin phosphorylation in cultured cells (Shewan et al., 2005). It is this actin and myosin-dependent tension that promotes FN fibril assembly. In the current study, mechanical deformations of the embryo regulated FN fibril assembly further demonstrating the likely importance of tissue tension to this process.
The small GTPase Rac is a critical component of the pathway through which cadherins contribute to tissue tension. Both cadherin ligation (Arthur et al., 2002; Lampugnani et al., 2002; Noren et al., 2001) and Wnt/PCP signaling (Habas et al., 2003) can promote the activation of the small GTPases Rac and Rho. While Rho has been shown to promote FN fibril assembly in cultured cells (Zhang et al., 1997; Zhong et al., 1998) by promoting contractility through the phosphorylation of MLC (Yoneda et al., 2007), in our system Rac is the critical GTPase for FN assembly. Tension is generated via regulation of the actin cytoskeleton and MLC phosphorylation by Rac and its downstream effector Pak. Inhibiton of either Rac or Pak abrogated cortical actin assembly in BCR cells. Activated Pak colocalized with FN fibrils. When Pak was inhibited the phosphorylation of MLC at cell-cell junctions was reduced, consistent with previous observations in endothelial cells (Stockton et al., 2007; Stockton et al., 2004). Taken together, these data indicate that Pak is the key downstream effector of Rac in this system regulating cell tension and FN assembly.
In summary, we provide evidence that tissue tension regulated by adherens-like contacts between blastomeres is key to the assembly of FN fibrils and that developmental control of cadherin adhesive activity is a critical step in this process. Wnt/PCP signaling is one way in which C-cadherin adhesive activity is regulated to enhance cell-cell junction formation. Subsequent activation of the small GTPase Rac activates Pak and likely other effectors as well. These Rac pathways lead to actin polymerization and myosin phosphorylation that together increase mechanical tension. Tension is transmitted through integrin α5β1, resulting in increased FN matrix assembly. This fibrillar FN provides an important co-stimulus with the PCP pathway and cadherin-dependent polarized cell behaviors to drive cell movements at gastrulation.
Experimental procedures
Embryos and animal caps
X. laevis embryos were obtained using standard methods, grown in 0.1X Modified Barth’s Saline (MBS; Sive et al., 2000) and staged according to Nieuwkoop and Faber (1994). Animal caps extension assays were undertaken as described in Symes and Smith (1987).
cDNA constructs
Constructs used in this study included: integrin HAβ1 (Marsden and DeSimone, 2003); dnWnt11 (Jim Smith, University of Cambridge); Dishevelled (Randy Moon, University of Washington); N-cadherin-pCS2+MT (Chris Kintner, Salk Institute); X-cad-11 pCS2+ (Doris Wedlich, Karlsruhe University); C-cad pCS2+MT, E-cad pSP64T, IL2R-C-Cadherin pCS2+, and the C-cad.fc expression construct (Barry Gumbiner, University of Virginia); human Rac, Rho and Cdc42 constructs were subcloned into pCS2+MT; Pak autoinhibitory domain in pDSRed1-N1 (Stockton et al., 2004).
Fibril Assembly Assay
Transcripts (25-250 pg depending on construct) were injected into the animal pole of one blastomere at the 2-4 cell stage. To target DMZs, both dorsal blastomeres were injected at the 4 cell stage. Embryos were cultured in 0.1X MBS then fixed in 4% paraformaldehyde in PBS or 2% trichloroacetic acid in 0.1X MBS. At least 10 BCRs were examined by immunofluorescence per experimental condition. Experiments were repeated 3 or more times. Primary antibodies for immunofluorescence: pAb 32 (Lee et al., 1984) and mAb 4H2 anti-FN, 9E10 anti-myc, pAb anti-C-cad (Barry Gumbiner, University of Virginia), pAb 881 anti-integrin α5 (Joos et al., 1995), anti-IL2 receptor and anti-phospho (Ser141) Pak 1/2/3 (Biosource International), anti-phospho (Ser19) myosin light chain 2 (Cell Signaling Technology) and an anti-hemaglutinin site A mAb (Judith White, University of Virginia). Secondary Abs were obtained from Molecular Probes. F-actin was labeled with Alexa 488 or Alexa 546 phalloidin. Digital images were captured using Openlab software and a Hammatsu Orca on a Zeiss Axiophot or with a Nikon C1 confocal microscope. FN was considered fibrillar when extended across cell surfaces and nonfibrillar when pericellular and/or present as punctae (Davidson et al., 2008). In some instances, embryos were fixed, bisected and cleared using benzylbenzoate/benzyl alcohol.
Inhibitory peptides
The Pak inhibitory and control peptides are described in (Kiosses et al., 2002). 30 nl of control or inhibitory peptide (200 μg/ml) was injected into the blastocoel at stage 8 and embryos processed for immunofluorescence at stage 11.5.
Traction force microscopy (TFM)
The constrained subcase of the Fourier Transform Traction Cytometry (FTTC) was used to calculate traction fields for different experimental conditions. The theoretical justification and experimental and computational methods of this approach have been described in detail (Butler et al., 2002). For the current studies, polydimethylsiloxane substrates (Sylgard, Dow-Corning,Midland, MI) were prepared by spreading a 100 μm thick layer of premixed base-cure Sylgard solution on coverglasses and cured at 85° C overnight. A second, 3-7 μm thin layer containing yellow-green fluorescent beads of 1 μm diameter (Molecular Probes/Invitrogen, Carlsbad, CA) was spread on the top of the already cured layer, and cured as previously. The elastic properties of these substrates were assessed by standard stress-strain experiments: a drop of cured PDMS was compressed between two glass coverslips, the applied force and measured geometric deformations yielding Young’s modulus. The elastic modulus of such substrates was tuned by varying the ratio of cure and base components of the Sylgard PDMS, an optimal composition was reached at 1:60 -1:70 ratio where noticeable bead displacement was recorded while a migratory cell (mesendoderm) still maintained its normal behavior.
The elastic substrates were coated with the protein of choice, either 5 μg/ml C-cad.fc (Niessen and Gumbiner, 2002) or 20 μg/ml bovine plasma FN in PBS. BCR cells from embryos injected with 10,000 MW Texas Red dextran (Molecular Probes/Invitrogen, Carlsbad, CA) were seeded on to the substrate and allowed to attach for 30-40 minutes. The substrates were transferred to the stage of an AxioObserver microscope (Carl Zeiss Microimaging, Thornwood, NY) fitted with an OptiGrid Structured Light System (Qioptic Imaging Solutions, Fairport, NY). Fluorescent images of the cell and underlying beads were taken, then the cell was released by treating it with 0.05% trypsin EDTA and the image of the beads recorded again. The displacements of beads were determined by matching the same regions from the two images with beads using normalized 2D cross correlation and Fast Fourier Transform (original image: 1024 × 1024 pixel, correlation windows: 64 × 64 pixels, displacement map resolution 16 pixels). The traction field was evaluated with the elasticity theory by carrying out a Fourier transform (FT) on the displacement field, calculating the FT of the traction field (a simple multiplication with the FT of the Boussinesq solution), then obtaining the traction field by inverse FT. The traction field was adjusted by applying a constraint on the traction field to be zero on the outside of the cell boundary, recalculating the tractions with an iterative procedure until convergence was reached. The scripts used to analyze the images and perform the numerical calculations were custom written using Matlab Software (Mathworks, Inc., Natick, MA).
Cell Surface Labeling
BCRs from 25 control or experimental embryos were biotinylated using EZ link sulfo-NHS biotin (Pierce) in 1x MBS containing 0.1X Ca++ and Mg++. Reaction was halted by washing in 100 mM glycine in 1x MBS, and BCRs lysed (1% NP40, 50 mM tris pH 7.4, 100 mM NaCl containing protease inhibitors; Sigma P2714). Cleared supernatants (20 min. at 14,000 rpm) incubated with Neutravidin beads (Pierce) overnight at 4° C, proteins eluted in SDS sample buffer containing 0.4 M urea, separated by SDS PAGE (7%) and western blotted. Membranes were sequentially probed with mAbs 8C8 (β1 integrin) and 6B6 (C-cad). Each experiment was repeated 2-3 times and relative expression levels normalized to total biotinylated protein (Fuji Imagequant).
Western Blots
To detect pMLC embryos were lysed in 25 mM tris, 100 mM NaCl, 1 mM EDTA, 1mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 0.1% SDS, 0.5% deoxycholate, 1% NP40); to detect myc-tagged N17Rac and N19Rho BCRs were lysed in 100 mM NaCl, 50 mM tris pH 7.4, 1% NP40 and protease inhibitors (Sigma P2714). Lysates representing 2 embryo equivalents (pMLC) or 2 BCR equivalents (Rac and Rho) were resolved on 12% SDS-PAGE gels, transferred to nitrocellulose and probed with mAbs against pMLC2 (Ser19) or myc (9E10), and anti-β–actin mAb used as loading control.
Supplementary Material
Acknowledgments
The authors are grateful to the many colleagues who provided key reagents. This work was supported by USPHS grant HD26402, and by a research grant from the Office of the Vice President for Research and Graduate Studies at the University of Virginia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142:1105–1119. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CL, Nelson WJ, Smith SJ. Quantitative analysis of cadherin-catenin-actin reorganization during development of cell-cell adhesion. J Cell Biol. 1996;135:1899–1911. doi: 10.1083/jcb.135.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur WT, Noren NK, Burridge K. Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol Res. 2002;35:239–246. doi: 10.4067/s0716-97602002000200016. [DOI] [PubMed] [Google Scholar]
- Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci U S A. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A. Magic Touch: how does cell-cell adhesion trigger actin assembly? Trends in Cell Biology. 2004;14:589–593. doi: 10.1016/j.tcb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-Activated Kinases. Annual Review of Biochemistry. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Brieher WM, Gumbiner BM. Regulation of C-cadherin function during activin induced morphogenesis of Xenopus animal caps. J Cell Biol. 1994;126:519–527. doi: 10.1083/jcb.126.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Butler JP, Tolic-Norrelykke IM, Fabry B, Fredberg JJ. Traction fields, moments, and strain energy that cells exert on their surroundings. Am J Cell Physiol. 2002;282:595–605. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking Cell-Matrix Adhesions to the Third Dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Dzamba BJ, Keller R, Desimone DW. Live imaging of cell protrusive activity, and extracellular matrix assembly and remodeling during morphogenesis in the frog, Xenopus laevis. Dev Dyn. 2008;237:2684–2692. doi: 10.1002/dvdy.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Keller R, DeSimone DW. Assembly and remodeling of the fibrillar fibronectin extracellular matrix during gastrulation and neurulation in Xenopus laevis. Dev Dyn. 2004;231:888–895. doi: 10.1002/dvdy.20217. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Marsden M, Keller R, DeSimone DW. Integrin α5β1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr Biol. 2006;16:833–844. doi: 10.1016/j.cub.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Duguay D, Foty RA, Steinberg MS. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev Biol. 2003;253:309–323. doi: 10.1016/s0012-1606(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Dzamba BJ, Bolton MA, DeSimone DW. The integrin family of cell adhesion molecules. In: Beckerle M, editor. Cell Adhesion. Oxford, UK: Oxford University Press; 2002. pp. 100–154. [Google Scholar]
- Gao M, Craig D, Lequin O, Campbell ID, Vogel V, Schulten K. Structure and functional significance of mechanically unfolded fibronectin type III1 intermediates. Proc Natl Acad Sci U S A. 2003;100:14784–14789. doi: 10.1073/pnas.2334390100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawantka V, Ellinger-Ziegelbauer H, Hausen P. Beta 1-integrin is a maternal protein that is inserted into all newly formed plasma membranes during early Xenopus embryogenesis. Development. 1992;115:595–605. doi: 10.1242/dev.115.2.595. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Goto T, Davidson L, Asashima M, Keller R. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr Biol. 2005;15:787–793. doi: 10.1016/j.cub.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Wong E, Gumbiner BM. E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J Cell Biol. 2001;153:1049–1060. doi: 10.1083/jcb.153.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Halliday NL, Tomasek JJ. Mechanical properties of the extracellular matrix influence fibronectin assembly in vitro. Exp Cell Res. 1995;217:109–117. doi: 10.1006/excr.1995.1069. [DOI] [PubMed] [Google Scholar]
- Hirano S, Nose A, Hatta K, Kawakami A, Takeichi M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J Cell Biol. 1987;105:2501–2510. doi: 10.1083/jcb.105.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Fibronectins. New York: Springer-Verlag; 1990. [Google Scholar]
- Ingham KC, Brew SA, Huff S, Litvinovich SV. Cryptic self-association sites in type III modules of fibronectin. J Biol Chem. 1997;272:1718–1724. doi: 10.1074/jbc.272.3.1718. [DOI] [PubMed] [Google Scholar]
- Joos TO, Whittaker CA, Meng F, DeSimone DW, Gnau V, Hausen P. Integrin alpha 5 during early development of Xenopus laevis. Mech Dev. 1995;50:187–199. doi: 10.1016/0925-4773(94)00335-k. [DOI] [PubMed] [Google Scholar]
- Kiosses WB, Hood J, Yang S, Gerritsen ME, Cheresh DA, Alderson N, Schwartz MA. A dominant-negative p65 PAK peptide inhibits angiogenesis. Circ Res. 2002;90:697–702. doi: 10.1161/01.res.0000014227.76102.5d. [DOI] [PubMed] [Google Scholar]
- Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol. 2002;12:379–382. doi: 10.1016/s0960-9822(02)00661-9. [DOI] [PubMed] [Google Scholar]
- LaFlamme SE, Thomas LA, Yamada SS, Yamada KM. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J Cell Biol. 1994;126:1287–98. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell. 2002;13:1175–1189. doi: 10.1091/mbc.01-07-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit T, Lenne PF. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev Mol Cell Biol. 2007;8:633–644. doi: 10.1038/nrm2222. [DOI] [PubMed] [Google Scholar]
- Lee G, Hynes R, Kirschner M. Temporal and spatial regulation of fibronectin in early Xenopus development. Cell. 1984;36:729–740. doi: 10.1016/0092-8674(84)90353-2. [DOI] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005a;24:389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE. Stimulatory effects of a three-dimensional microenvironment on cell-mediated fibronectin fibrillogenesis. J Cell Sci. 2005b;118:4427–4436. doi: 10.1242/jcs.02566. [DOI] [PubMed] [Google Scholar]
- Marsden M, DeSimone DW. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development. 2001;128:3635–3647. doi: 10.1242/dev.128.18.3635. [DOI] [PubMed] [Google Scholar]
- Marsden M, DeSimone DW. Integrin-ECM interactions regulate cadherin-dependent cell adhesion and are required for convergent extension in Xenopus. Curr Biol. 2003;13:1182–1191. doi: 10.1016/s0960-9822(03)00433-0. [DOI] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol. 1983;93:466–472. doi: 10.1083/jcb.97.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DF, editor. Fibronectin. New York: Academic Press; 1989. [Google Scholar]
- Munoz R, Moreno M, Oliva C, Orbenes C, Larrain J. Syndecan-4 regulates non-canonical Wnt signalling and is essential for convergent and extension movements in Xenopus embryos. Nat Cell Biol. 2006;8:492–500. doi: 10.1038/ncb1399. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Gumbiner BM. Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J Cell Biol. 2002;156:389–399. doi: 10.1083/jcb.200108040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop P, Faber J. Normal Table of Xenopus laevis (Daudin) New York: Garland Publishing; 1994. [Google Scholar]
- Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276:33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of alpha(5)beta(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JW, DeSimone DW. Xenopus embryonic cell adhesion to fibronectin: position-specific activation of RGD/synergy site-dependent migratory behavior at gastrulation. J Cell Biol. 1996;134:227–240. doi: 10.1083/jcb.134.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reintsch WE, Hausen P. Dorsoventral differences in cell-cell interactions modulate the motile behaviour of cells from the Xenopus gastrula. Dev Biol. 2001;240:387–403. doi: 10.1006/dbio.2001.0478. [DOI] [PubMed] [Google Scholar]
- Rozario T, Dzamba B, Weber GF, Davidson LA, DeSimone DW. The physical state of fibronectin matrix differentially regulates morphogenetic movements in vivo. Dev Biol. 2009 doi: 10.1016/j.ydbio.2008.12.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechler JL, Cumiskey AM, Gazzola DM, Schwarzbauer JE. A novel RGD-independent fibronectin assembly pathway initiated by alpha4beta1 integrin binding to the alternatively spliced V region. J Cell Sci. 2000;113(Pt 8):1491–1498. doi: 10.1242/jcs.113.8.1491. [DOI] [PubMed] [Google Scholar]
- Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Steinberg MS, Takeichi M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci U S A. 1994;91:206–209. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton R, Reutershan J, Scott D, Sanders J, Ley K, Schwartz MA. Induction of vascular permeability: beta PIX and GIT1 scaffold the activation of extracellular signal-regulated kinase by PAK. Mol Biol Cell. 2007;18:2346–2355. doi: 10.1091/mbc.E06-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton RA, Schaefer E, Schwartz MA. p21-activated kinase regulates endothelial permeability through modulation of contractility. J Biol Chem. 2004;279:46621–46630. doi: 10.1074/jbc.M408877200. [DOI] [PubMed] [Google Scholar]
- Symes K, Smith JC. Gastrulation movements provide an early marker of mesoderm induction in Xenopus. Development. 1987;101:339–349. [Google Scholar]
- Tada M, Concha ML, Heisenberg CP. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin Cell Dev Biol. 2002;13:251–260. doi: 10.1016/s1084-9521(02)00052-6. [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC. Xwnt11 is target of Xenopus Brachyury: regulation of gastrulaton movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Tao Q, Nandadasa S, McCrea PD, Heasman J, Wylie C. G-protein-coupled signals control cortical actin assembly by controlling cadherin expression in the early Xenopus embryo. Development. 2007;134:2651–2661. doi: 10.1242/dev.002824. [DOI] [PubMed] [Google Scholar]
- Tao QYC, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie C, Lin X, Heasman J. Maternal Wnt11 Activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Torres MA, Yang-Snyder JA, Purcell SM, DeMarais AA, McGrew LL, Moon RT. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol. 1996;133:1123–1137. doi: 10.1083/jcb.133.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fassler R. Beta 1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol. 1996;132:227–238. doi: 10.1083/jcb.132.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Schwarzbauer JE. The ins and outs of fibronectin matrix assembly. J Cell Sci. 2003;116:3269–3276. doi: 10.1242/jcs.00670. [DOI] [PubMed] [Google Scholar]
- Winklbauer R. Conditions for fibronectin fibril formation in the early Xenopus embryo. Dev Dyn. 1998;212:335–345. doi: 10.1002/(SICI)1097-0177(199807)212:3<335::AID-AJA1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wu C, Fields AJ, Kapteijn BA, McDonald JA. The role of alpha 4 beta 1 integrin in cell motility and fibronectin matrix assembly. J Cell Sci. 1995;108(Pt 2):821–829. doi: 10.1242/jcs.108.2.821. [DOI] [PubMed] [Google Scholar]
- Wu C, Hughes PE, Ginsberg MH, McDonald JA. Identification of a new biological function for the integrin alpha v beta 3: initiation of fibronectin matrix assembly. Cell Adhes Commun. 1996;4:149–158. doi: 10.3109/15419069609014219. [DOI] [PubMed] [Google Scholar]
- Yoneda A, Ushakov D, Multhaupt HA, Couchman JR. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol Biol Cell. 2007;18:66–75. doi: 10.1091/mbc.E06-08-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Magnusson MK, Mosher DF. Lysophosphatidic acid and microtubule destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Mol Biol Cell. 1997;8:1415–1425. doi: 10.1091/mbc.8.8.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.