The role of a murine transplantation model of atherosclerosis regression in drug discovery (original) (raw)
. Author manuscript; available in PMC: 2015 Nov 28.
Published in final edited form as: Curr Opin Investig Drugs. 2009 Mar;10(3):232–238.
Abstract
Atherosclerosis is the leading cause of death worldwide. To date, the use of statins to lower LDL levels has been the major intervention used to delay or halt disease progression. These drugs have an incomplete impact on plaque burden and risk, however, as evidenced by the substantial rates of myocardial infarctions that occur in large-scale clinical trials of statins. Thus, it is hoped that by understanding the factors that lead to plaque regression, better approaches to treating atherosclerosis may be developed. A transplantation-based mouse model of atherosclerosis regression has been developed by allowing plaques to form in a model of human atherosclerosis, the apoE-deficient mouse, and then placing these plaques into recipient mice with a normolipidemic plasma environment. Under these conditions, the depletion of foam cells occurs. Interestingly, the disappearance of foam cells was primarily due to migration in a CCR7-dependent manner to regional and systemic lymph nodes after 3 days in the normolipidemic (regression) environment. Further studies using this transplant model demonstrated that liver X receptor and HDL are other factors likely to be involved in plaque regression. In conclusion, through the use of this transplant model, the process of uncovering the pathways regulating atherosclerosis regression has begun, which will ultimately lead to the identification of new therapeutic targets.
Keywords: Atherosclerosis, chemokine [C-C motif] receptor 7, migration, HDL, liver X receptor, regression
Introduction
The idea that human atheromata can regress has met considerable resistance over the decades [1]. The reason for this may have been that advanced atherosclerotic plaques in humans and in animal models contain calcification and fibrosis, characteristics that were regarded as irreversible. Regardless, it is indisputable that the ability to induce atherosclerosis regression is a desirable clinical goal for several reasons. First, atherosclerosis is the leading underlying cause of death worldwide [1–3]. Second, currently available interventions to lower plasma concentrations of LDL incompletely reduce the risk for clinical cardiovascular endpoints (by approximately 30 to 45%), and therefore new therapeutics that further reduce this risk are needed [2,3]. In this review, the history of atherosclerosis regression research is summarized, with a focus on a recently developed mouse model. The potential of this model as a discovery tool is also described.
The pathogenesis of atherosclerosis
Initiation of atherogenesis
Atherosclerotic plaques begin as fatty streaks underlying the endothelium of large arteries. Currently, it is thought that a key initiating event in the formation of fatty streaks is the retention of atherogenic apolipoprotein B (apoB)-containing lipoproteins (LDL and remnants of VLDL and chylomicrons) by the extracellular matrix in the artery wall [4]. While in the plasma these lipoproteins are protected from oxidation, they are thought to become susceptible to enzymatic and non-enzymatic modifications when retained in the artery wall. A number of potential oxidant-generating systems, such as myeloperoxidase, nitric oxide synthase and 15-lipoxygenase (15-LO), have been investigated that could directly or indirectly oxidize apoB, and the lipids of LDL and remnant lipoproteins (reviewed in reference [5]).
Fatty streak formation occurs in the arterial intima. The streaks are areas rich in macrophages that have taken up enough retained and newly entered apoB-containing lipoproteins to become overloaded with cholesterol; these cells have a foamy appearance and hence, are known as 'foam cells'. In addition to cholesterol, macrophages also become enriched in modified and oxidized lipids, causing cellular activation. Activated macrophages and foam cells secrete cytokines and chemokines (eg, IL-1, IL-6, TNFα and monocyte chemotactic protein-1) that increase the expression of adhesion molecules on endothelial cells, to which monocytes stick (reviewed in reference [6]). These adhesion molecules also promote monocyte entry into the intimal space of the artery, where these cells eventually become activated macrophages. This activity creates a perpetuating cycle in which activated macrophages and foam cells help to recruit more circulating monocytes, which ultimately become activated macrophages and foam cells that recruit still more cells. One of the first endothelial adhesion molecules to be implicated in monocyte recruitment was vascular cell adhesion molecule-1, based on the increased expression levels of this molecule on endothelial cells over plaque-prone areas, its preferential recruitment of monocytes, and its pattern of regulation by proinflammatory stimuli [7,8]. In addition, E-selectin and P-selectin also appear to contribute to monocyte entry [7].
Ultimately, the chemokines and cytokines produced by activated macrophages and foam cells also cause adverse effects on endothelial and smooth muscle cells, such as decreased nitric oxide production and increased proliferation, respectively, as well as the recruitment of other types of leukocytes in addition to monocytes; these leukocytes include T-cells and mast cells that add to the inflammatory environment of a progressing plaque [9].
Plaque progression
The transition from the relatively simple fatty streak to a more complex plaque is characterized by the migration of proliferating smooth muscle cells from the medial layer of the artery wall, past the internal elastic lamina, and into the intimal, or subendothelial, space. One benefit of the smooth muscle cell migration is that these cells can secrete extracellular matrix, particularly collagen, which can form a fibrous cap under the endothelium, keeping plaques stable. Conversely, one disadvantage of the migration is that the intimal smooth muscle cells may also proliferate and take up modified lipoproteins, contributing to foam cell formation [10,11].
Another aspect of plaque progression is influenced by interactions between macrophages and T-cells that result in a broad range of cellular and humoral responses, and the acquisition of many features of a chronic inflammatory state [9]. Significant cross-talk seems to occur among the cellular elements of developing plaques. T-cells in plaques are thought to be activated, expressing both Th1 and Th2 cytokines [12]. Similarly, macrophages, endothelial cells and smooth muscle cells appear to be activated and contribute to the inflammatory state, based on their expression of MHC class II molecules and numerous inflammatory products, such as TNFα, IL-6 and monocyte chemotactic protein-1 [10].
The net rate of accumulation of cholesterol in the plaque reflects the difference between the rate of cholesterol deposition, and its rate of extraction from the plaque by HDLs. High concentrations of cholesterol may be associated with the apoptosis and necrosis of plaque macrophages and foam cells. Cholesterol then accumulates in the extracellular domain and forms the lipid (or necrotic) core of advanced plaques. The core also contains tissue factor, which, until plaque rupture, is segregated from the circulating clotting elements. Because plaque macrophages secrete matrix metalloproteases that can digest the fibrous cap, vulnerability to rupture is thought to result when the cap is eroded to below a critical thickness. A ruptured plaque triggers a thrombotic event, frequently occlusive enough to cause a myocardial infarction (reviewed in reference [13]).
Regression of atherosclerosis: A brief review of progress in preclinical models
Early studies in animal models
The first interventional study demonstrating substantial shrinkage of atherosclerotic plaques was conducted in cholesterol-fed rabbits over 50 years ago [14]. To mobilize tissue stores of cholesterol, animals received intravenous bolus injections of phosphatidylcholine, an acceptor of cholesterol. After less than 1.5 weeks of treatment, the arterial plaques were fewer and smaller. Interestingly, approximately 75% of the arterial cholesterol stores had been removed. Over the next two decades, similar arterial benefits from the injection of phospholipids were reported by a number of research groups using a variety of atherosclerotic animal models, including primates [15]. Such exciting observations were questioned by other studies in other animal models and humans that cast doubt on the possibility of atherosclerotic regression [16].
A turning point in this research was a short-term study conducted in squirrel monkeys by Maruffo and Portman [17], and more extensive research by Armstrong et al [18,19]. The latter research group demonstrated that advanced arterial plaques in cholesterol-fed rhesus monkeys underwent shrinkage and remodeling during long-term follow up (up to 40 months) after a switch to low-fat or linoleate-rich diets [18,19]. In 1976, success in atherosclerosis regression was again achieved in rabbits, following reversion to a normal chow diet, in combination with the administration of hypolipidemic agents [20]. A series of studies also achieved shrinkage of atheromata in rabbits via injections of HDL or artificial HDL-like complexes (eg, see references [21,22]). The success of these studies gradually reduced the skepticism that had greeted earlier studies.
Recent studies in mice
Model development and characterization
While informative, the above-mentioned animal models are not the best candidates for determining the molecular factors responsible for the pathogenesis of atherosclerosis or the regression process because of the expense and inconvenience of maintaining colonies, and the inability to conduct genetic manipulations. The mouse, which does not have these restrictions, has therefore become the most widely-used model for atherosclerosis research. Because mice have a naturally strong resistance to atherosclerosis, given their low LDL and high HDL levels, changes using gene inactivation through homologous recombination ('knock out') were needed.
The two basic mouse models of atherosclerosis are the apoE-null (apoE−/−) mouse [23,24] and the LDL-receptor-null (LDLR−/−) mouse [25]. In these models, the normally low plasma apoB-lipoprotein levels are increased to atherogenic levels through the elimination of either a ligand (apoE) or a receptor (LDLR) for lipoprotein clearance. A cholesterol-enriched diet (Western diet) can further increase the plasma apoB-lipoprotein levels in these mice, resulting in accelerated plaque formation in the major arteries. These models have served as the basis for numerous studies, mainly focusing on the identification of factors that play a role in atheroma formation and progression.
Plaque regression studies in mouse models have lagged behind studies investigating atherosclerotic progression. Some studies of atherosclerotic progression were conducted using somatic adenoviral gene transfer (eg, see references [26,27]); however, this approach has been limited, mainly because of either the eventual loss of transgene expression or relatively low expression, even using second-generation viral vectors, partly due to immune responses to the vectors [28,29].
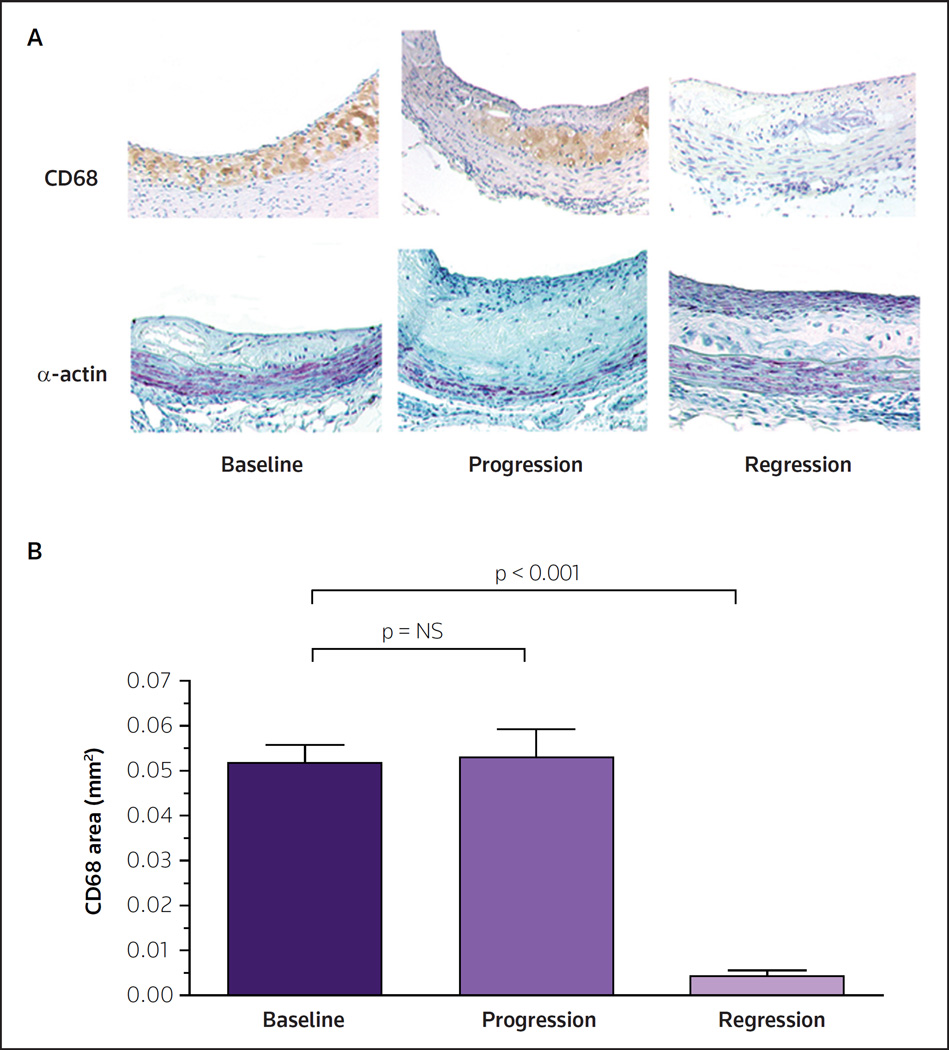
A mouse model to study regression that would avoid the problems of transient or low levels of the factor under study (eg, LDL, HDL and apoE) was therefore needed. This was accomplished by the transplantation of either a thoracic aortic segment [30] or an aortic arch segment [31] from apoE−/− mice (an extremely pro-atherogenic milieu consisting of high plasma apoB-lipoprotein levels and low HDL-cholesterol levels) into wild-type (WT) recipient mice. The donor segment was transplanted to the abdominal aorta, and blood flow was routed through the graft. Using this method, dyslipidemia was corrected indefinitely and the new plasma lipoprotein environment of the plaques was sustained in the graft long term. After the desired time period, the graft was removed, and changes in the plaques were compared with non-transplanted baseline animals using standard techniques (morphometrics and immunohistochemistry). As illustrated in Figure 1, after transplantation into the WT recipient, regression of the plaque was rapidly observed (as judged by changes in the plaque content of CD68+ cells; CD68 is a marker of macrophages and foam cells). In contrast, when the recipient was an apoE−/− mouse, further disease progression was observed [30–33].
Figure 1. Regression of advanced atherosclerotic plaques in the mouse transplantation model.
Mice null for apolipoprotein E were fed a Western diet for 40 weeks to promote advanced atherosclerosis. Aortic arches from these mice were either harvested and analyzed by (A) histochemical and (B) histomorphometric methods, or were transplanted into apolipoprotein E-null ('progression') or wild-type ('regression') recipient mice. After 9 weeks, the same analyses were conducted on the aortic arches from transplantation recipients. The histochemical results for the foam cell marker CD68 (brown) and the vascular smooth cell marker α-actin (purple) are illustrated. The images demonstrate the immunostaining of representative aortic plaques in cross section. Note the low levels of foam cells and the appearance of a fibrous cap in the regression group. In contrast to the results in the regression mice, the progression group demonstrated a persistence of foam cells and no development of a fibrous cap. As shown in panel B, the visual histochemical staining suggesting a large depletion of foam cells was confirmed by quantitative analysis of the plaque area stained for CD68.
NS not significant
(Adapted with permission from Lippincott Williams & Wilkins and Trogan E, Fayad ZA, Itskovich VV, Aguinaldo JGS, Mani V, Fallon JT, Chereshnev I, Fisher EA: Serial studies of mouse atherosclerosis by in vivo magnetic resonance imaging detect lesion regression after correction of dyslipidemia. Arterioscler Thromb Vasc Biol (2004) 24(9):1714–1719. © 2004 Lippincott Williams & Wilkins)
Transplanting either early (fatty streaks) or more advanced plaques into WT recipients substantially reduced CD68+ cell content (by as much as 90%), while also increasing the number of smooth muscle cells, particularly in the fibrous cap region below the endothelium [30–34]. The loss of CD68+ cells was surprisingly rapid, with large decreases (up to 50%) observed as early as 3 days after transplantation [33,35]. With advanced plaques, all pathological features regressed after 9 weeks, including CD68+ cells, smooth muscle cells and necrotic cores, as assessed by immunohistochemical assays [30–32]. This evidence of regression, even in advanced plaques, suggests that the negative results of numerous previous regression attempts reflects a failure to sufficiently improve the lipoprotein environment of the plaque. It should be noted that these past attempts to model plaque regression have included dietary interventions; however, apoE−/− and LDLR−/− mice remain hyperlipidemic even when fed low-fat and low-cholesterol-containing diets [23–25].
Molecular changes in regressing plaques
This mouse model of atherosclerotic regression allows for the characterization of the cellular and molecular features of regressing plaques. The relevance to human plaques is predicated by the overlap between the human and mouse genomes (> 95% similar), implying that critical factors identified in a mouse model of atherosclerosis will also play important roles in the human disease. To begin to characterize the molecular changes in the mouse transplant model, CD68+ cell trafficking studies were conducted and demonstrated that the rapid loss of these cells from regressing plaques was caused by the migration of CD68+ cells into regional and systemic lymph nodes [33,35]. Particularly striking was the observation that the low cholesterol plasma milieu in WT mice provoked CD68+ cells (traditionally thought to be macrophages and foam cells) to display markers of dendritic cells, which are monocyte-derived cells that can migrate from tissues to lymph nodes [33,35]. This was a surprising result because it had been previously thought that macrophages and dendritic cells represented terminally differentiated states [36].
Using laser capture microdissection (LCM) techiques to specifically remove CD68+ cells from plaques [37], it was observed that, in the regression environment, mRNA for chemokine [C-C motif] receptor 7 (CCR7), which is required for dendritic cell migration, was induced [33,38]. Significantly, the injection of WT recipient animals with antibodies against the two CCR7 ligands CCL19 and CCL21 inhibited the majority of CD68+ cells from migrating from the aortic transplant plaques, thereby establishing a functional role for CCR7 in plaque regression [33]. As expected, the mRNA concentrations of several proteins implicated in atherothrombosis, such as vascular cell adhesion protein 1, monocyte chemotactic protein-1 and tissue factor, were decreased in CD68+ cells during regression [33]. Contrary to expectation, however, the level of mRNA for the nuclear receptor liver X receptor α (LXRα; which is induced in vitro by oxidized sterols [39]) significantly increased in vivo, as did the anti-atherogenic target of LXRα, the ATP-binding cassette transporter A1 (ABCA1) [33]. These increases were surprising because in studies in vitro, LXRα and ABCA1 expression decrease when macrophages are in a low cholesterol environment.
Systemic administration of an LXR agonist causes plaque regression in LDLR−/− mice [40] and, based on the LCM data presented above, it was questioned whether the upregulation of LXR and CCR7 under regression conditions (WT recipient) was a coincidence or indicated a functional relationship. Recent data in mice suggest that a functional relationship exists and that regression caused by an LXR agonist is largely CCR7-dependent [JE Feig, EA Fisher, unpublished data]. Studies to further define the relationships between LXR, CCR7 and plaque regression are ongoing.
Effects of HDL on regression
WT recipients have low levels of apoB-lipoproteins and high HDL levels compared with apoE−/− donors. Both lipoprotein factors could promote plaque regression, and the mouse transplant model has been used to distinguish between the contributions of these molecules in regression. Diseased aortic segments from apoE−/− donors were transplanted into human apoAI transgene expressing apoE−/− mice that have WT plasma levels of HDL-cholesterol (65 mg/dl), but high levels of non-HDL cholesterol (> 500 mg/dL) [34]. Over 5 months, the transplanted plaques demonstrated foam-cell depletion and the formation of a fibrous cap [34], both changes that would be expected to stabilize a vulnerable plaque in the clinical setting; however, the degree of regression observed in this model was lower than that observed in studies using WT recipients [33]. The results to date suggest that raising plasma functional HDL levels through enhanced apoAI production favorably remodels atheromata, but that a stronger effect is achieved when high levels of functional HDL are combined with large reductions in the concentration of circulating apoB-lipoproteins, which is in agreement with the results of clinical studies of statins in patients with coronary atherosclerosis [41,42].
Microarray analysis of regressing plaques
It is likely that many factors and pathways are involved in plaque regression. To begin exploring these factors and pathways, microarray experiments have been conducted in which CD68+ cells from donor (baseline), apoE−/− recipient and WT recipient mice were collected. In this study, dramatically different molecular signatures characterizing progression versus regression were observed in the different cell populations [JE Feig, O Puig, V Reiser, EA Fisher, unpublished data]. For example, genes related to a migratory phenotype, such as components of the contractile apparatus, were significantly upregulated, with genes of the cellular adhesion apparatus being downregulated. Both of these findings are consistent with previous data noted above that regression is characterized by cells migrating from plaques.
The transplant model of regression as a drug discovery tool
The transplant regression model can be used as a drug discovery tool in numerous ways. Perhaps the most obvious is the 'mining' of microarray data. The mouse and human genomes are highly similar, making it likely, though not certain, that key factors in atherosclerosis will be common to both species. Thus, further examination of the microarray data will undoubtedly uncover changes in atherosclerosis relevant to humans in a variety of pathways, including those related to lipid metabolism, inflammation and cellular stress. Compounds that regulate these pathways, or factors within them, may ultimately result in new therapeutic agents.
Another approach related to microarray data, but more targeted, is to test the roles of specific candidate factors based on the processes that are involved in plaque regression that are identified through the analysis of the plaques in this mouse model. For example, the finding that cell migration is involved in plaque regression suggested that factors regulating migration in monocyte-derived cells may be important to regression. This was the method used to investigate CCR7, which is now established as a possible therapeutic target. Changes in factors of the inflammatory pathways may similarly point to new, promising therapeutic targets.
A lower 'throughput' approach that may be valuable in particular circumstances is to use the model not to discover or validate factors that are potential therapeutic candidates, but to test agents to determine additive effects to lowering non-HDL-cholesterol or raising HDL-cholesterol levels. For example, it is increasingly recognized that the functionality of HDL determines whether the molecule is atheroprotective, and that this may be independent of HDL levels [43]. Transplantation into recipient mice with low levels of HDL-cholesterol (such as the apoE−/− or the apoAI−/− mice, depending on the desired level of non-HDL-cholesterol) that have been treated with placebo or an HDL-elevating compound could be conducted. The effects on plaque regression can then be compared with the degree of elevation in HDL.
It should be noted that in the future the testing of anti-atherosclerotic agents may be accomplished by non-invasive, serial imaging. Currently, however, there is poor resolution of plaque composition by imaging, and maximum information on the effects of a candidate agent would still require examination of tissue [32]. For agents expected to be used in combination with a statin, the transplant model would have use in simulating aggressive statin treament (using WT recipients), because the apoE−/− and LDLR−/− mice, lacking an important ligand for the LDLR (apoE) or the receptor itself, do not have a robust plasma cholesterol-lowering response to statin treatment.
Conclusion
The regression of atherosclerosis is a tantalizing clinical goal that based on information from preclinical studies should be achievable. As important as the statins have been in reducing cardiovascular risk, the significant residual risk of heart disease that remains with the use of current treatments suggests that new drugs that target additional pathways related to plaque regression are needed. Most likely these drugs would be used in combination with existing therapeutics to achieve the greatest impact on risk reduction. Through the use of the mouse transplant model, pathways that have the potential to become targets in the treatment of atherosclerosis have been identified. In addition to the proposed uses for this model, there is the potential that novel uses can be developed.
Acknowledgements
Research on atherosclerosis regression in the authors' laboratory has been supported by grants from the NIH (EAF: HL61814, HL084312, DK087945; JEF: AG029748). The authors also would like to thank their colleague, Dr Kevin Jon Williams, for many stimulating discussions.
References
•• of outstanding interest
• of special interest
- 1.Blankenhorn DH, Hodis HN. George Lyman Duff Memorial Lecture. Arterial imaging and atherosclerosis reversal. Arterioscler Thromb. 1994;14(2):177–192. doi: 10.1161/01.atv.14.2.177. [DOI] [PubMed] [Google Scholar]
- 2.Williams KJ, Feig JE, Fisher EA. Cellular and molecular mechanisms for rapid regression of atherosclerosis: From bench top to potentially achievable clinical goal. Curr Opin Lipidol. 2007;18(4):443–450. doi: 10.1097/MOL.0b013e32823bcb15. [DOI] [PubMed] [Google Scholar]
- 3.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: Insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5(2):91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- 4.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15(5):551–561. doi: 10.1161/01.atv.15.5.551. •• A provocative synthesis of the order of events in the initiation and subsequent progression of an atherosclerotic plaque.
- 5.Glass CK, Witztum JL. Atherosclerosis. The road ahead. Cell. 2001;104(4):503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 6.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation. 2007;116(16):1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 7.Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102(1):145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251(4995):788–791. doi: 10.1126/science.1990440. •• A classic paper on the role of endothelial adhesion molecules in atherogenesis.
- 9.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: Lessons from mouse models. Nat Rev Immunol. 2008;8(10):802–815. doi: 10.1038/nri2415. • A timely review on the different types of leukocytes involved in atherosclerosis.
- 10.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 11.Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci USA. 2003;100(23):13531–13536. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 13.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 14.Friedman M, Byers SO, Rosenman RH. Resolution of aortic atherosclerotic infiltration in the rabbit by phosphatide infusion. Proc Soc Exp Biol Med. 1957;95(3):586–588. doi: 10.3181/00379727-95-23300. •• An early demonstration that atherosclerosis could be regressed by increasing what was later recognized to be cholesterol efflux from plaques.
- 15.Williams KJ, Werth VP, Wolff JA. Intravenously administered lecithin liposomes: A synthetic antiatherogenic lipid particle. Perspect Biol Med. 1984;27(3):417–431. doi: 10.1353/pbm.1984.0031. [DOI] [PubMed] [Google Scholar]
- 16.Stein Y, Stein O. Does therapeutic intervention achieve slowing of progression or bona fide regression of atherosclerotic lesions? Arterioscler Thromb Vasc Biol. 2001;21(2):183–188. doi: 10.1161/01.atv.21.2.183. [DOI] [PubMed] [Google Scholar]
- 17.Maruffo CA, Portman OW. Nutritional control of coronary artery atherosclerosis in the squirrel monkey. J Atheroscler Res. 1968;8(2):237–247. doi: 10.1016/s0368-1319(68)80060-2. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong ML. Evidence of regression of atherosclerosis in primates and man. Postgrad Med J. 1976;52(609):456–461. doi: 10.1136/pgmj.52.609.456. •• This and related articles from Dr Armstrong (see reference [19]) are historically important in that they demonstrate that plaque regression is possible in humans and non-human primates.
- 19.Armstrong ML, Warner ED, Connor WE. Regression of coronary atheromatosis in rhesus monkeys. Circ Res. 1970;27(1):59–67. doi: 10.1161/01.res.27.1.59. [DOI] [PubMed] [Google Scholar]
- 20.Wissler RW, Vesselinovitch D. Studies of regression of advanced atherosclerosis in experimental animals and man. Ann NY Acad Sci. 1976;275:363–378. doi: 10.1111/j.1749-6632.1976.tb43368.x. [DOI] [PubMed] [Google Scholar]
- 21.Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85(4):1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazaki A, Sakuma S, Morikawa W, Takiue T, Miake F, Terano T, Sakai M, Hakamata H, Sakamoto Y, Natio M, et al. Intravenous injection of rabbit apolipoprotein A-I inhibits the progression of atherosclerosis in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol. 1995;15(11):1882–1888. doi: 10.1161/01.atv.15.11.1882. [DOI] [PubMed] [Google Scholar]
- 23.Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. •• This reference, along with reference [24], reports the first robust mouse models of atherosclerosis.
- 24.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 25.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92(2):883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desurmont C, Caillaud JM, Emmanuel F, Benoit P, Fruchart JC, Castro G, Branellec D, Heard JM, Duverger N. Complete atherosclerosis regression after human apoE gene transfer in apoE-deficient/nude mice. Arterioscler Thromb Vasc Biol. 2000;20(2):435–442. doi: 10.1161/01.atv.20.2.435. [DOI] [PubMed] [Google Scholar]
- 27.Harris JD, Schepelmann S, Athanasopoulos T, Graham IR, Stannard AK, Mohri Z, Hill V, Hassall DG, Owen JS, Dickson G. Inhibition of atherosclerosis in apolipoprotein-E-deficient mice following muscle transduction with adeno-associated virus vectors encoding human apolipoprotein-E. Gene Ther. 2002;9(1):21–29. doi: 10.1038/sj.gt.3301615. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Jooss KU, Su Q, Ertl HC, Wilson JM. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3(2):137–144. [PubMed] [Google Scholar]
- 29.DeMatteo RP, Chu G, Ahn M, Chang E, Burke C, Raper SE, Barker CF, Markmann JF. Immunologic barriers to hepatic adenoviral gene therapy for transplantation. Transplantation. 1997;63(2):315–319. doi: 10.1097/00007890-199701270-00024. [DOI] [PubMed] [Google Scholar]
- 30.Reis ED, Li J, Fayad ZA, Rong JX, Hansoty D, Aguinaldo JG, Fallon JT, Fisher EA. Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein E-deficient mouse in a novel transplantation model. J Vasc Surg. 2001;34(3):541–547. doi: 10.1067/mva.2001.115963. • The first transplant mouse model of atherosclerosis was described in this paper.
- 31.Chereshnev I, Trogan E, Omerhodzic S, Itskovich V, Aguinaldo JG, Fayad ZA, Fisher EA, Reis ED. Mouse model of heterotopic aortic arch transplantation. J Surg Res. 2003;111(2):171–176. doi: 10.1016/s0022-4804(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 32.Trogan E, Fayad ZA, Itskovich VV, Aguinaldo JG, Mani V, Fallon JT, Chereshnev I, Fisher EA. Serial studies of mouse atherosclerosis by in vivo magnetic resonance imaging detect lesion regression after correction of dyslipidemia. Arterioscler Thromb Vasc Biol. 2004;24(9):1714–1719. doi: 10.1161/01.ATV.0000139313.69015.1c. • This paper described an example of non-invasive imaging of mouse atherosclerosis by MRI.
- 33.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in apoE-deficient mice. Proc Natl Acad Sci USA. 2006;103(10):3781–3786. doi: 10.1073/pnas.0511043103. •• This paper, aong with reference [35], establishes the migration of monocyte-derived cells from plaques in a CCR7-dependent process as one potential mechanism of plaque regression.
- 34.Rong JX, Li J, Reis ED, Choudhury RP, Dansky HM, Elmalem VI, Fallon JT, Breslow JL, Fisher EA. Elevating high-density lipoprotein cholesterol in apolipoprotein E-deficient mice remodels advanced atherosclerotic lesions by decreasing macrophage and increasing smooth muscle cell content. Circulation. 2001;104(20):2447–2452. doi: 10.1161/hc4501.098952. •• A direct demonstration that the selective normalization of HDL levels reverses atherosclerotic disease.
- 35.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci USA. 2004;101(32):11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 37.Trogan E, Fisher EA. Laser capture microdissection for analysis of macrophage gene expression from atherosclerotic lesions. Methods Mol Biol. 2005;293:221–231. doi: 10.1385/1-59259-853-6:221. • This paper describes an early application of LCM in atherosclerosis research.
- 38.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99(1):23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 39.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: Opening the X-files. Science. 2001;294(5548):1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 40.Levin N, Bischoff ED, Daige CL, Thomas D, Vu CT, Heyman RA, Tangirala RK, Schulman IG. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler Thromb Vasc Biol. 2005;25(1):135–142. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- 41.Nicholls SJ, Tuzcu EM, Sipahi I, Grasso AW, Schoenhagen P, Hu T, Wolski K, Crowe T, Desai MY, Hazen SL, Kapadia SR, et al. Statins, high-density lipoprotein cholesterol, and regression of coronary atherosclerosis. J Am Med Assoc. 2007;297(5):499–508. doi: 10.1001/jama.297.5.499. [DOI] [PubMed] [Google Scholar]
- 42.Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: The ASTEROID trial. J Am Med Assoc. 2006;295(13):1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 43.Ansell BJ, Fonarow GC, Fogelman AM. The paradox of dysfunctional high-density lipoprotein. Curr Opin Lipidol. 2007;18(4):427–434. doi: 10.1097/MOL.0b013e3282364a17. •• An excellent review of recent clinical trials, and the distinction between HDL levels and HDL function.