Measurement of Human Immunodeficiency Virus Type 1 Preintegration Transcription by Using Rev-Dependent Rev-CEM Cells Reveals a Sizable Transcribing DNA Population Comparable to That from Proviral Templates (original) (raw)
Abstract
Preintegration transcription is an early process in human immunodeficiency virus type 1 infection and has been suggested to occur at a low level. The templates have also been suggested to represent a small population of nonintegrated viral DNA, particularly the two-long-terminal-repeat (2-LTR) circles. However, these determinations were made by either using PCR amplification of viral transcripts in bulk cell populations or utilizing the LTR-driving reporter cells that measure the synthesis of Tat. The intrinsic leakiness of LTR often makes the measurement of low-level viral transcription inaccurate. Since preintegration transcription also generates Rev, to eliminate the nonspecificity associated with the use of LTR alone we have developed a novel Rev-dependent indicator cell, Rev-CEM, to measure preintegration transcription based on the amount of Rev generated. In this report, using Rev-CEM cells, we demonstrate that preintegration transcription occurs on a much larger scale than expected. The transcribing population derived from nonintegrated viral DNA was comparable (at approximately 70%) to that derived from provirus in a productive viral replication cycle. Nevertheless, each nonintegrated viral DNA template exhibited a significant reduction in the level of transcriptional activity in the absence of integration. We also performed flow cytometry sorting of infected cells to identify viral templates. Surprisingly, our results suggest that the majority of 2-LTR circles are not active in directing transcription. It is likely that the nonintegrated templates are from the predominant DNA species, such as the full-length, linear DNA. Our results also suggest that a nonintegrating lentiviral vector can be as effective as an integrating vector in directing gene expression in nondividing cells, with the proper choice of an internal promoter.
Replication of the human immunodeficiency virus (HIV) requires stable integration of the viral genome into the human chromatin, and yet the natural process of HIV infection generates large quantities of nonintegrated viral DNA that accumulate in lymphoid tissues, the brain, and peripheral CD4 lymphocytes (7, 9, 42, 51, 55). Under cell culture conditions, nonintegrated HIV DNA also accumulates in T cells and macrophages (28, 44, 47). These DNA molecules exhibit low levels of transcriptional activity (13, 53, 58, 63) but are fully competent at transcribing all classes of viral transcripts, including both the early, multiply spliced and the late, singly spliced and nonspliced transcripts (28, 62). The nonintegrated viral DNA can also direct limited syntheses of viral early products such as Nef, Tat, and Rev (28, 62, 63). The transcription and translation of these regulatory genes prior to integration have been suggested to be a normal, early process in the HIV life cycle (62, 63) and may facilitate viral infection (20, 28, 63). For example, Nef generated from nonintegrated viral DNA can downregulate CD4 (20) and lower the threshold of T-cell activation (14, 21, 50, 63). In infected macrophages, nonintegrated viral DNA can also stimulate the secretion of proinflammatory cytokines and chemokines such as IP-10, which may promote viral infection and pathogenesis (28). Additionally, it has recently been shown that transcription from nonintegrated HIV type 1 (HIV-1) DNA can functionally complement proviruses to prevent potential losses of HIV genetic diversity (18, 59).
The demonstration of nonintegrated HIV transcription has stimulated a recent surge in testing nonintegrating lentiviral vectors for the safer delivery of therapeutic genes for gene therapy (30, 33, 45, 48, 56, 57, 64) and vaccination (39). The nonintegrating vectors have demonstrated remarkably high efficiency in mediating stable expression from internally promoted transgenes in nondividing cells (45, 48, 64). Nevertheless, the molecular mechanism regulating preintegration transcription is not well characterized, and the DNA templates are currently unknown.
Nonintegrated HIV DNA exists in three forms: the one-long-terminal-repeat (1-LTR) circle, the 2-LTR circle, and linear DNA. Additionally, viral particles produced in laboratories by transfection have also contained some plasmid DNA. The circular forms were originally thought to be precursors for retroviral integration (43, 52). However, direct evidence from a cell-free in vitro integration system (5) and other studies (12, 34) conclusively demonstrated that linear DNA is the precursor for retroviral integration. The 2-LTR circles have also been suggested to be the nonintegrated templates for transcription. For example, the detection of preintegration transcription coincided with a marked increase in the numbers of 2-LTR circles when the viral integrase was disrupted (13, 58, 62) or inhibited (22). Furthermore, a study described in a recent report detected a novel viral transcript across the LTR-LTR junction in infected cells (6), suggesting that transcription from 2-LTR circles may actually occur. The circular DNA forms represent a minor population; in particular, the 2-LTR circles constitute approximately 0.03 to 5% of total viral DNA during the initial stage of viral replication (8, 62). Thus, it is possible that the observed nonintegrated transcription might represent a very small number of circular viral DNA molecules; thus, the majority of nonintegrated viral DNA might be transcriptionally inactive in the absence of integration.
Previously, we have also measured preintegration transcription in bulk cell populations by the use of reverse transcriptase-PCR and Western blot analysis. We quantified one of the early proteins, Nef, and demonstrated that nonintegrated HIV-1 DNA expresses approximately 6 to 10% of the amount of Nef expressed by the wild-type (Wt) viral DNA (28, 62, 63). These previous methods, although semiquantitative, were not capable of determining the percentages of viral templates. Alternatively, earlier studies used HeLa-CD4-LTR-beta-galactosidase (HeLa-CD4-LTR-β-Gal) indicator cells to measure the transcribing population and showed that D116N, a nonintegrating virus, generated only approximately 10% blue cells in comparison with the results seen with an infection with a similar level of the Wt virus (13, 58). We repeated these experiments using another LTR-based indicator cell, CEM-green fluorescent protein (CEM-GFP) (19), and obtained a similar result (unpublished data). These data suggest that 90% of the active DNA templates in HIV-1 infection would become silent if not integrated. However, these indicator cells utilize an integrated HIV-1 LTR promoter to drive the expression of reporter genes, and the intrinsic leakiness of the HIV LTR promoter often makes the measurement inaccurate. First, the LTR possesses basal levels of transcription in the absence of Tat. The false-positive signals, although typically at low levels, are difficult to distinguish from the low-level signals generated from nonintegrated viral DNA. Second, the LTR promoter not only responds to Tat but also reacts to numerous cellular transcription factors (16, 25). External environmental stimuli, such as cytokines, mitogens (2), or even the HIV envelope protein itself (37), can trigger Tat-independent reporter expression. Thus, specific and accurate measurement of nonintegrated transcription is difficult to achieve using these LTR-based cell lines.
To avoid the nonspecificity of the use of HIV Tat alone as an indicator for measuring preintegration transcription, we have established a novel system utilizing another HIV regulatory protein, Rev. The Rev protein is known to regulate viral gene expression by specific interaction with the HIV Rev-responsive element (RRE) on the 3′ half of the unspliced and the partially spliced viral transcripts and mediates their nuclear export and translation (11, 36). Results determined in our previous studies have suggested the presence of low levels of Rev generated from nonintegrated HIV DNA (28, 62). Therefore, we anticipated that preintegration transcription could also be measured based on the amount of Rev generated. In this report, we describe the quantification of HIV preintegration transcription using Rev-dependent Rev-CEM cells.
MATERIALS AND METHODS
Plasmids and DNA cloning.
Plasmids pNL4-3 and pNL4-3/D116N were kindly provided by Malcolm Martin. The env mutant, pNL4-3(KFS), was kindly provided by Eric Freed (15). The env and integrase double mutant, pNL4-3(KFS)/D116N, was constructed by introducing a point mutation (Asp116 to Asn) into the integrase catalytic domain of pNL4-3(KFS) as previously described (13). pHCMV-G, which expresses the vesicular stomatitis virus glycoprotein, has been previously described (65). pNL-ΔΨ-Env was constructed by inserting the env gene of HIV-1NL4-3 into the lentiviral pNL-RRE-SA vector (66). The packaging signal was further deleted by cutting with KasI plus BssHII and religating. p89.6-LTR-D116N was constructed by replacing the LTR portion of pNL4-3(KFS)/D116N with that of the HIV clone p89.6 by the use of the BssHII and XhoI sites. The following plasmids were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: pCMV-rev from Marie-Louise Hammarskjöld and David Rekosh (32) and p89.6 from Ronald G. Collman (10).
Viruses and cells.
HIV-1NL4-3 (1) and HIV-1NL4-3/D116N (13) were generated by transfection of plasmid pNL4-3 or pNL4-3/D116N into HEK293T cells by the use of Lipofectamine 2000 (Invitrogen, Carlsbad, CA) as previously described (66). The vesicular stomatitis virus-glycoprotein (VSV-G) pseudotyped viruses were produced by cotransfection of HEK293T cells (3 × 106) with 10 μg of pHCMV-G and 10 μg of plasmid pNL4-3(KFS) or pNL4-3(KFS)/D116N. Envelope-typed HIV-1 was produced by cotransfection of HEK293T cells with 10 μg of pNL-ΔΨ-Env and 10 μg of pNL4-3(KFS) or pNL4-3(KFS)/D116N. Viral supernatant was harvested at 48 h postcotransfection, centrifuged for 15 min at 500 × g to remove cellular debris, filtered through a 0.45-μm-pore-size filter, and then stored at −80οC. Levels of p24 in viral supernatant were measured using a Perkin Elmer Alliance p24 antigen enzyme-linked immunosorbent assay kit (Perkin Elmer, Waltham, MA). Plates were kinetically read using an ELx808 automatic microplate reader (Bio-Tek Instruments) at 630 nm.
The Rev-CEM Rev-dependent cell line has been described previously (60, 61). Rev-CEM was derived from CEM-SS cells, which lack APOBEC3G/F. The following cell lines and reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: CEM-SS from Peter L. Nara (38); CEM-GFP from Jacques Corbeil (19); and 118-D-24 integrase inhibitor (54). All cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA), penicillin (50 U/ml), and streptomycin (50 mg/ml). For infection, unless otherwise specified, 2 × 105 cells were incubated with 100 to 500 ng (p24) of virus for 2 h at 37°C. Cells were washed one to two times and then resuspended in fresh culture medium.
DNA purification.
Genomic DNA was extracted using an SV total RNA isolation kit (Promega, Madison, WI) as recommended by the manufacturer. The kit (with minor modifications) was also used for extracting DNA from virion particles. Briefly, 500 μl of viral supernatant was mixed with 500 μl of 8 M guanidium thiocyanate. The solution was gently mixed, and 2 ml of dilution buffer was added, followed by the addition of 857 μl of 95% ethanol. Samples were loaded onto spin columns and spun at maximum speed for 1 min. Supernatant was discarded, and the columns were washed with 600 μl of 70% ethanol followed by washing with 400 μl of 70% ethanol. Finally, DNA was eluted with 50 μl of nuclease-free water. A 10-μl volume of the sample was used for real-time PCR analysis.
PCR and real-time PCR.
The sequences of the PCR primers used in this work are available upon request. For PCR measurement of 2-LTR circles, primers LTR-1180-1161 and LTR-8809 were used. These primers were specific to pNL4-3(KFS)/D116N and did not amplify DNA from uninfected Rev-CEM cells. PCR was performed using a 50-μl volume containing 1× PCR buffer (Ambion, Austin, TX), 125 μM (each) deoxynucleoside triphosphates (dNTPs), 1.5 mM Mg2+, 50 pmol of primers, and 1 to 2 U of SuperTaq Plus polymerase (Ambion, Austin, TX). The reaction was carried out with a 3-min hot start at 94°C followed by 40 cycles of 94°C for 10 s, 68°C for 20 s, and 68°C for 2 min, with a final extension at 68°C for 10 min. Real-time PCR quantification of HIV-1NL4-3 virion DNA was performed using a Bio-Rad IQ5 real-time PCR detection system as previously described (28) and primers 5′ LTR-U5 and 3′ Gag and the TaqMan probe 6-carboxyfluorescein-U5/Gag (FAM-U5/Gag). DNA standards were prepared using pLTR-2C (28). A standard curve was generated by measuring 1 to 106 copies of pLTR-2C diluted in 100 ng of total cellular DNA purified from uninfected CEM-SS cells.
The Rev-CEM cell carries an integrated lentiviral vector, pNL-GFP-RRE-SA, that is derived from pNL4-3. To avoid any background amplification from Rev-CEM cells, the LTR region of pNL4-3(KFS)/D116N was replaced with the LTR of p89.6 to generate a new HIV clone, p89.6-LTR-D116N, so that the LTR region of p89.6-LTR-D116N could be selectively amplified. Following infection of Rev-CEM with HIV-189.6-LTR-D116N, 2-LTR circles were measured by real-time PCR using primers U9362-9393 and U9362-54 and the probe FAM-U9362-10-BHQ-1. These primers amplify only DNA from cells infected with HIV-189.6-LTR-D116N and not DNA from uninfected Rev-CEM cells. The 2-LTR-circle PCR standard was generated by chemical synthesis of the LTR-LTR junction (BlueHeron Biotechnology, Bothell, WA) and cloning into a pUC vector. Real-time PCR was performed using a 30-μl reaction mixture containing 1× Gene Expression Master Mix (Applied Biosystems, Foster City, CA), 300 nM (each) primers, and 300 nM probe, at 50°C for 2 min, 95°C for 10 min, and then 40 cycles of 15 s at 95°C and 60 s at 60°C. Total cellular DNA was normalized based on the amplification of the β-actin pseudogene by the use of β-actin primers (Ambion, Austin, TX) and a Quantifast SYBR green PCR kit (Qiagen, Valencia, CA).
Alu/real-time PCR.
To construct an Alu/real-time PCR standard, a 1.3-kb fragment containing the PKG promoter and the neomycin resistance gene from pMSCVneo (Clontech, Mountain View, CA) was inserted into a lentiviral vector, pNL-RRE-SA (61). The resulting plasmid, pNLneo, was used to generate lentivirus and infect HeLa cells at a low (0.05 to 0.1) multiplicity of infection. Transduced cells were cultured in 100 mg/ml of G418 for more than a month to ensure that levels of nonintegrated DNA were diminished and that only integrated DNA remained. DNA from the G418-resistant HeLa cells (HeLa-NLneo) was extracted, and copies of viral DNA were measured using real-time PCR. The integrated DNA copy number for HeLa-NLneo cells was estimated to be around 1.4 copies per cell. Alu/real-time PCR standards were prepared by diluting HeLa-NLneo DNA from uninfected HeLa cells. A total cellular DNA volume equivalent to the DNA amount from 15,000 cells was used in reactions to keep the number of Alu sites constant.
For measuring integration, total genomic DNA was purified and used for Alu-HIV PCR amplification or Alu/real-time PCR amplification as previously described (40). For Alu-HIV PCR amplification, the Alu primer and HIV primer (1180-1161) were used. PCR was performed in a 50-μl volume containing 1× PCR buffer, 125 μM (each) dNTPs, 1.5 mM Mg2+, 100 pmol of primers, and 2 U of SuperTaq Plus polymerase (Ambion, Austin, TX). The reaction was carried out with a 3-min hot start at 94°C followed by 25 cycles of 94°C for 10 s, 65°C for 20 s, and 68°C for 3 min. Levels of background one-way amplification from nonintegrated DNA were determined using the HIV primer (1180-1161) alone. Following the first round of PCR amplification, an aliquot equivalent to one-fifth of the Alu-PCR product was serially diluted for the nested PCR, which was performed using primers HIV/tat/rev5 and LTR gag2 in a 50-μl volume containing 1× PCR buffer, 125 μM dNTPs, 1.5 mM Mg2+, 50 pmol of primers, and 2 U of SuperTaq Plus polymerase (Ambion, Austin, TX) for 30 cycles of 20 s at 94°C and 50 s at 68°C. Controls for background amplification of nonintegrated viral DNA were achieved by PCR in the absence of the Alu primer (HIV only). A cellular β-actin pseudogene was amplified to ensure that equal amounts of cellular DNA were used.
For Alu/real-time PCR amplification, the Alu primer and the gag primer were used. PCR was performed in a 50-μl volume containing 1× PCR buffer, 125 μM dNTPs, 1.5 mM Mg2+, 100 pmol of primers, and 2 U of SuperTaq Plus polymerase (Ambion, Austin, TX). The reaction was carried out with a 3-min hot start at 94°C followed by 25 cycles of 94°C for 10 s, 65°C for 20 s, and 68°C for 3 min. Levels of background one-way amplification from nonintegrated DNA were determined using the gag primer alone. Following the first round of PCR amplification, an aliquot equivalent to one-fifth of the Alu-PCR products was used for real-time PCR analysis of viral DNA as described above, using primers 5′ LTR-U5 and 3′ Gag and the TaqMan probe FAM-U5/Gag. Integrated viral DNA copy numbers were calculated based on the integrated viral DNA standard prepared from HeLa-NLneo.
Flow cytometry and cell sorting.
Cells were mixed with an equal volume of 1% paraformaldehyde and incubated for 15 min at room temperature. Following incubation, cells were transferred to a new tube for flow cytometric analysis. For cell-sorting experiments, live HIV-infected cells were sorted using GFP levels and the exclusion sort mode. Flow cytometry and cell sorting were performed using FACSCalibur (BD Biosciences, San Jose, CA) and FACSAria (BD Biosciences, San Jose, CA) systems equipped with 405, 488, and 638 laser lines and DIVA version 4.12 acquisition software. Gates for single cells were defined based on forward scatter height and forward scatter width as well as on side scatter height and side scatter width. Expression of GFP was measured against forward scatter (FSC), and the sorting gate was defined using GFP-positive cells. From a single sample of infected cells, GFP-positive and GFP-negative cells were sorted into two separate tubes. Postsort analysis was performed using CellQuest (BD Biosciences, San Jose, CA) and FlowJo (Tree Star, San Carlos, CA) software.
RESULTS
Application of the Rev-dependent human T-cell line, Rev-CEM, to measurement of low-level transcription from nonintegrated HIV-1 DNA.
We previously described a Rev-dependent cell line that was constructed by transduction of a Rev-dependent lentiviral vector into a human T cell, CEM-SS (60, 61). The Rev-CEM cell line carries a GFP reporter (pNL-GFP-RRE-SA) that was placed under the control of Rev by introducing multiple splicing sites and the RRE (Fig. 1A). This arrangement regulates GFP as a late gene, in similarity to a viral structural gene that is dependent on Rev for translation. Similar RRE-containing constructs carrying the CAT gene have been shown to be dependent on the presence of Rev, and the minimal RRE is an 88-bp fragment encompassing a Rev binding site (23, 24). The RRE region in our lentiviral vector is a full-length 857-bp fragment. The improved specificity of Rev-regulated reporter expression was manifested early during our cell cloning process. Initially, 129 clones were randomly selected following transduction with the lentiviral particle vNL-GFP-RRE-SA, and 23 of the selected cells were found to carry the pNL-GFP-RRE-SA lentiviral vector. None of these 23 clones exhibited GFP expression in the absence of HIV-1, and all of them became positive for GFP upon HIV-1 infection. One clone, G11.1.16, was selected and further recloned three times and then renamed Rev-CEM. Detailed characterization of Rev-CEM has been described elsewhere (60), and the cell data have recently been deposited into the NIH AIDS Research and Reference Reagent program.
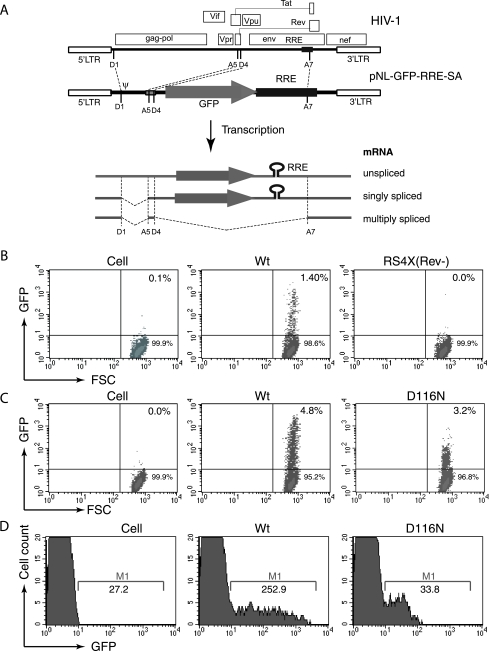
FIG. 1.
Application of the Rev-CEM Rev-dependent cell in measurement of preintegration transcription. (A) Rev-CEM carries a Rev-dependent lentiviral vector, pNL-GFP-RRE-SA. Landmarks from left to right are as follows: D1, HIV splicing donor 1; ψ, the HIV RNA genome packaging signal sequence; A5 and D4, HIV splicing acceptor 5 and donor 4; A7, HIV splicing acceptor 7. Viral transcripts are represented below the construct. Only the unspliced and singly spliced transcripts expressed GFP. (B) Rev-CEM cells were infected with equal p24 levels of HIV-1NL4-3 (Wt) or a Rev mutant, RS4X(Rev-). The percentages of GFP-positive cells were measured by flow cytometry at 48 h. (C) Rev-CEM cells were infected with the Wt virus (1.4 × 106 cpm per million cells [reverse transcriptase unit]) or an integrase mutant, D116N (1.1 × 106 cpm per million cells). The percentages of GFP-positive cells were measured at 48 h with flow cytometry. (D) Histoplot results for the infection described for panel C, showing the average GFP intensities of the GFP-positive cells. M-1, GFP-positive cell region.
Rev-dependent GFP expression in Rev-CEM is tightly regulated, unlike that of Tat-dependent cells such as CEM-GFP (19) and HeLa-CD4-LTR-β-Gal (29), which exhibit background reporter expression in the absence of HIV-1. Additionally, Rev-CEM does not respond to nonviral stimulations, i.e., stimulation by mitogens or cytokines such as phytohemagglutinin, phorbol myristate acetate, interleukin-7, granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor alpha, whereas the Tat-dependent cells responded to stimulation by factors such as phorbol myristate acetate and tumor necrosis factor alpha (60). This high stringency of Rev-CEM cells permitted us to measure low-level viral transcription, such as the transcription from nonintegrating virus, without interference from background LTR expression. As shown in Fig. 1C, we infected Rev-CEM with an integration-negative virus, D116N, and detected GFP expression using flow cytometry. As a control, infection with a Rev-negative virus, RS4x(Rev-) (46), did not generate GFP signal (Fig. 1B), demonstrating the specificity and requirement of Rev for GFP expression. Nevertheless, GFP expression resulting from D116N infection was uniformly at a lower level in comparison with that resulting from the Wt infection, which generated a range of GFP-positive cells at various intensities (across 2 orders of magnitude) (Fig. 1D). This was likely a result of variations in the integration sites (26). These data suggested that nonintegrated viral DNA templates possess relatively homogeneous, low-level transcriptional activity.
Characterization of preintegration transcription using Rev-CEM cells.
To further confirm that the low-level GFP signals generated from D116N infection of Rev-CEM were indeed dependent on viral processes and on newly synthesized, nonintegrated viral DNA, we performed a series of control experiments. Because virion particles prepared by transfection contain some plasmid DNA on the surface that could be introduced into Rev-CEM cells and act as templates, we treated virion particles with Benzonase. This treatment has been suggested to reduce the presence of plasmid DNA to a level undetectable by PCR (49). As shown in Fig. 2A, even after aggressive Benzonase treatment that diminished virion DNA levels by 6 orders of magnitude, we observed a minimal decrease in GFP expression (Fig. 1B), excluding a role of contaminating plasmid DNA in mediating GFP expression in Rev-CEM cells. As is consistent with this result, the reverse transcriptase inhibitor zidovudine (AZT) was able to inhibit GFP expression after both D116N and Wt infection (Fig. 2C and D), suggesting that the transcription was dependent on the presence of newly synthesized viral DNA. In addition, the AZT inhibition also excluded the possibility that contaminating Rev, although it is not a virion protein, may result in GFP expression.
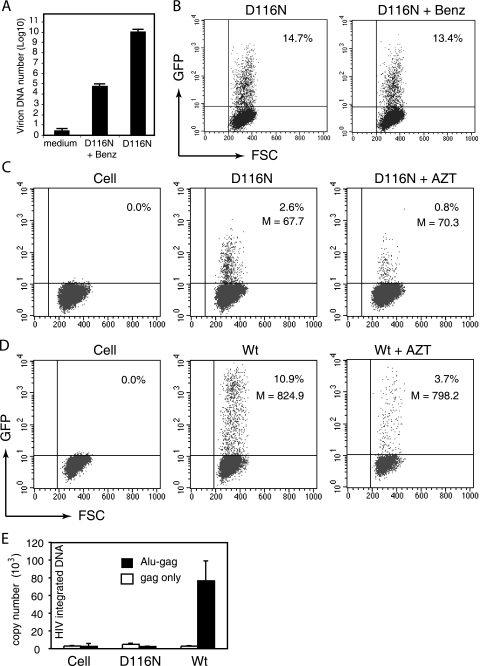
FIG. 2.
Characterization of preintegration transcription using Rev-CEM. (A and B) Preintegration transcription does not result from plasmid DNA contaminating virion particles. (A) HIV-1NL4-3/D116N (D116N) particles were treated with Benzonase (Benz; 125 U/ml) or subjected to mock treatment for 15 min at 37°C. Virion DNA was then purified and quantified by real-time PCR as described in Materials and Methods. (B) The same viruses, with or without Benzonase treatment, were also used to infect Rev-CEM cells, and the percentages of GFP-positive cells were measured by flow cytometry at 48 h. (C and D) Preintegration transcription is dependent on the presence of newly synthesized viral DNA. (C) Rev-CEM cells were left untreated or treated with the reverse transcriptase inhibitor AZT (50 μM) for 12 h and then infected with D116N. The percentages and average GFP intensities (M) of GFP-positive cells were measured at 48 h with flow cytometry. (D) As controls, cells were also infected with the Wt virus to demonstrate inhibition by AZT. (E) Preintegration transcription from nonintegrated viral DNA templates. CEM-SS cells were infected with equal p24 levels of the VSV-G pseudotyped Wt or D116N virus. Cells were lysed 48 h after infection, and total cellular DNA was extracted and quantified with Alu/real-time PCR for integrated viral DNA as described in Materials and Methods. Controls for background amplification of nonintegrated viral DNA were prepared by PCR in the presence (Alu-gag) or absence (gag only) of the Alu forward primer.
Since some integrase mutants can integrate at a low level of efficiency in the absence of a functional integrase (17), we also performed Alu/real-time PCR to detect viral integration. Even with a viral dosage that can generate approximately 10% GFP-positive Rev-CEM cells (data not shown), we were not able to detect integration in D116N infection, whereas with the same dosage we detected abundant integration in the Wt infection (Fig. 2E). With these results, we felt confident that the low-level GFP expression observed in D116N infection was bona fide viral transcriptional activity from newly synthesized, nonintegrated viral DNA. In addition, pseudotyping with the VSV-G envelope has been known to greatly enhance HIV-1 infectivity, likely as a result of higher viral titers generated by transfection or enhanced viral entry through endocytosis (3, 35). We observed a similar enhancement of the effect of VSV-G pseudotyping on GFP expression in the results from both Wt and D116N infections (Fig. 3).
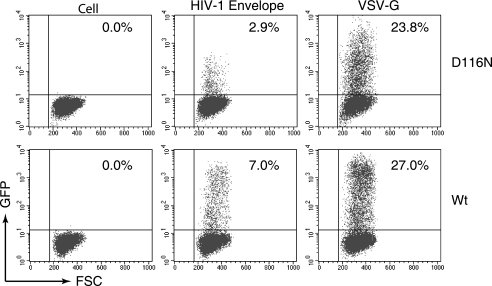
FIG. 3.
Effects of VSV-G pseudotyping on preintegration transcription. Single-cycle replicating HIV-1 particles were produced by cotransfection of an envelope-negative construct, pNL4-3(KFS) or pNL4-3(KFS)(D116N), with an HIV-1 envelope construct, pNL-ΔΨ-Env, or with a VSV-G construct, pHCMV-G. Equal p24 levels of the viral particles carrying either the HIV-1 envelope or VSV-G were used to infect Rev-CEM cells. The percentages of GFP-positive cells were measured by flow cytometry at 48 h.
Measurement of preintegration transcription using Rev-CEM cells revealed a sizable transcribing DNA population comparable to that seen with proviral templates.
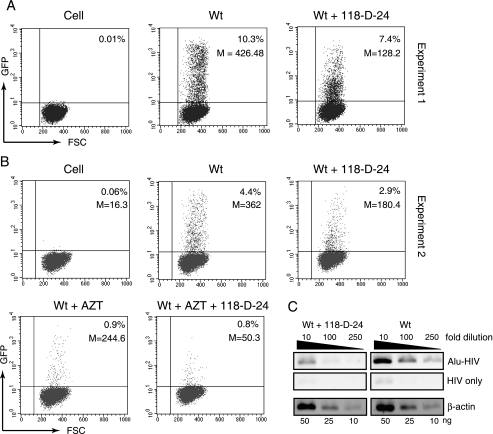
To use Rev-CEM cells to quantify preintegration transcription, we infected Rev-CEM cells with the Wt virus or D116N while using equal amounts of p24. To limit viral replication to a single round, we also pseudotyped both viruses with VSV-G. Following infection, cells were analyzed by flow cytometry at 48 h for GFP expression. As shown in Fig. 4, in each of three independent infection experiments, infection of Rev-CEM cells with the Wt virus generated 20.5%, 12.1%, and 11.3% GFP-positive cells, respectively. Surprisingly, infection with D116N generated much higher percentages of GFP-positive cells than we expected, with levels of 11.8%, 8.4%, and 8.3%, respectively, seen in the three independent experiments, and the fractions of GFP-positive cells generated by D116N infection were 58%, 69%, and 73% of those produced by the Wt viral infection. These levels were much higher than the 11.5% that was previously measured using the HeLa-CD4-LTR-β-Gal indicator cells (13). These results suggested that the numbers of nonintegrated, transcribing DNA molecules in D116N infection were not small in comparison with the numbers seen using the proviral templates in a Wt infection. Nevertheless, the average GFP intensity for D116N-infected cells was much lower than for the Wt-infected cells (52.29 ± 4.18 versus 468.99 ± 61.71 [averages of results of three infections]). Similar results were also observed when the Wt infection was inhibited with an integrase inhibitor, 118-D-24 (54). In two independent experiments, the inhibitor reduced the numbers of GFP-positive cells to only 71.8% and 65.9% of the numbers generated by the Wt infection, whereas it decreased the GFP intensities from 426.5 to 128.2 and from 362 to 180.4, respectively (Fig. 5A and B).
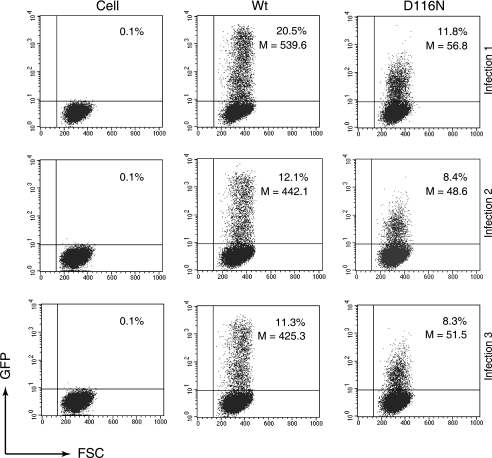
FIG. 4.
The population of transcribing templates in D116N infection in comparison with Wt infection populations. Rev-CEM cells were infected with equal p24 levels of the VSV-G-pseudotyped Wt or D116N viruses. The percentages and average GFP intensities of GFP-positive cells were measured by flow cytometry at 48 h. Uninfected Rev-CEM cells were used as a control. Data represent results from three independent infections. D116N-infected cells had a much lower GFP intensity than Wt-infected cells (52.29 ± 4.18 in D116N versus 468.99 ± 61 in Wt cells [averages of the results of three infections]), whereas the percentage of the GFP-positive population remained comparable (9.5% in D116N versus 14.6% in Wt cells [averages of the results of three infections]). Similar results were obtained with viral particles carrying the envelope of HIV-1NL4-3 (data not shown).
FIG. 5.
Effects of the integrase inhibitor 118-D-24 on HIV-1 transcription in Rev-CEM cells. (A) The integrase inhibitor 118-D-24 (50 μM) was used to treat Rev-CEM cells for 4 h prior to HIV-1 infection. Cells treated with 118-D-24 or left untreated were then infected with equal p24 levels of the Wt (VSV-G pseudotyped) virus for 2 h; following infection and washing, cells were cultured in the continuous presence or absence of 118-D-24, respectively. The percentages and average GFP intensities (M) were measured by flow cytometry at 48 h. Uninfected, drug-treated Rev-CEM cells were used as a control. (B) Repeat of the same infection with a lower viral dosage. For additional controls, cells were also treated with AZT (50 μM) or 118-D-24 plus AZT and then identically infected. (C) Alu-HIV PCR measurement of 118-D-24 inhibition of HIV integration. CEM-SS cells were infected exactly as described for panel B in the presence or absence of 118-D-24. Total cellular DNA was purified at 48 h postinfection and then amplified with Alu-HIV PCR as described in Materials and Methods. The cellular β-actin pseudogene was also amplified to ensure that equal amounts of DNA were used.
The pattern of inhibition by the integrase inhibitor was also markedly different from that by the reverse transcriptase inhibitor AZT, as measured by results obtained using Rev-CEM cells. In contrast to 118-D-24, AZT significantly diminished the percentage of GFP-positive cells from 4.4% to 0.9% in Wt infection (Fig. 5B), but it only slightly decreased the GFP intensity from 362 to 244.6. It is reasonable that in the experiments using AZT inhibition, levels of viral DNA synthesis were diminished, but the small amount of residual DNA template generated in a cell would be fully functional for integration and transcription, whereas in 118-D-24 inhibition, although the synthesis of viral DNA was not affected, most of the DNA templates did not integrate and their transcriptional activity was reduced (Fig. 5). Based on these results, we conclude that in the absence of integration, the population of transcribing templates largely remains (at approximately 70%) but that each template exhibits a significant reduction in activity.
2-LTR circles are not the templates for preintegration transcription.
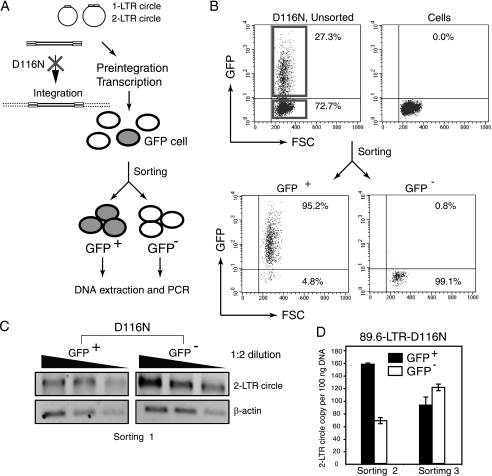
With the success of the use of Rev-CEM to measure preintegration transcription, it became possible to determine the active viral DNA templates. Because D116N infection generates a sizable GFP population carrying active templates, these GFP cells can be isolated and characterized. Previous studies have suggested that the 2-LTR circles might be the templates used for preintegration transcription (6, 13, 58). There was a significant increase in the numbers of 2-LTR circles in cells when integration was prevented (13, 58, 62) or inhibited (22). In particular, it has also been shown that an RNA transcript crossing the LTR-LTR junction exists in infected cells, suggesting that the 2-LTR circular DNA might be transcriptionally active (6). Based on these previous data, we expected that if the 2-LTR circles were the templates, they would be predominantly maintained within the GFP-positive population in D116N infection. Thus, we performed flow cytometer sorting of GFP-positive cells following infection of Rev-CEM cells with D116N. As shown in Fig. 6, we separated cells into GPF-positive and GFP-negative populations (purity above 95%) (Fig. 6A and 6B). Total cellular DNA was then extracted from each population and subjected to PCR amplification for 2-LTR circles (Fig. 6C). To our surprise, the 2-LTR circles were not exclusively concentrated within the GFP-positive population; rather, they appeared to be distributed roughly at the same level between GFP-positive and GFP-negative cells (Fig. 6C). We repeated this experiment using another clone of D116N (89.6-LTR-D116N) that we constructed (see Materials and Methods) to facilitate greater accuracy of quantification of the 2-LTR circles with real-time PCR (Fig. 6D). Again, the two independent sorting experiments confirmed that the 2-LTR circles were not predominantly distributed in the GFP-positive cells (Fig. 6D); the average data from the two experiments showed that 56.5% of 2-LTR circles were in the GFP-positive cells whereas 43.5% of them were in the GFP-negative cells. In experiments conducted under one of our sets of infection conditions (Fig. 6C), the percentage of GFP-positive cells was 7%, and 93% of infected cells were negative for GFP. Based on the distribution of 2-LTR circles between GFP-positive and GFP-negative cells, we estimated that the majority of 2-LTR circles were not active, since they were in the GFP-negative cells. We also calculated the copy number of 2-LTR circles contained in approximately 100 GFP-positive cells. The copy number of 2-LTR circles was less than 1 per 100 cells. We concluded that the majority of the GFP-positive cells (99%) did not contain 2-LTR circles.
FIG. 6.
Flow cytometry sorting and PCR quantification of 2-LTR circles. (A) Schematic representation of flow cytometry cell sorting of D116N-infected Rev-CEM cells and subsequent quantification of 2-LTR circles by PCR. (B) An example of one sorting experiment. Cells were infected with D116N, washed, cultured for 48 h, and then analyzed by flow cytometry. Cells within the red frames were sorted into GFP-positive and GFP-negative populations. Sorted cells were reanalyzed by flow cytometer to check cell purity. (C) PCR amplification of 2-LTR circles following sorting and DNA extraction. In this sorting experiment, Rev-CEM cells were infected with D116N. The GFP-positive cells represented 7% of the cell population. (D) To facilitate real-time PCR quantification of 2-LTR circles, the LTR region in mutant D116N (HIV-1NL4-3/D116N) was replaced with the LTR of HIV-189.6 (see Materials and Methods). Rev-CEM cells were infected with the resulting 89.6-LTR-D116N virus, washed, cultured for 48 h, and then sorted. The levels of GFP-positive cells in sortings 2 and 3 were 27.3% and 23.0%, respectively.
DISCUSSION
For this report, we measured HIV-1 preintegration transcription utilizing a novel Rev-dependent indicator cell line, Rev-CEM. The use of Rev eliminated the nonspecificity intrinsically associated with the HIV LTR/Tat system and permitted sensitive and accurate analyses of preintegration transcription. Using Rev-CEM, we confirmed that preintegration transcription occurs. We also demonstrated that, in contrast to previous suggestions, the nonintegrated viral DNA templates represent a sizable population (approximately 70%) comparable to the populations seen with the normal transcribing templates in a productive viral replication cycle. Nevertheless, each viral DNA template exhibited a significant reduction in activity in the absence of integration. These results suggest that a clear distinction exists between nonintegrated viral DNA and integrated templates in directing viral gene transcription. Indeed, it has previously been shown that there is a marked difference between nonintegrated DNA and provirus in the requirement for the activation of transcription. The Tat-associated histone acetyltransferase activity is preferentially important for the transactivation of integrated but not nonintegrated HIV-1 LTR, supporting the idea of Tat-dependent transactivation for provirus and Tat-independent transactivation for nonintegrated DNA (4, 41). Presumably, the inability of viral DNA to integrate may result in a universally lower capacity for transcription from each episomal LTR.
The 2-LTR circles have been suggested to be the templates for preintegration transcription (6). Our results are in conflict with this suggestion. The discrepancy might result from different methods used for detecting gene expression. We observed no Rev expression from the 2-LTR circles but do not exclude the possibility that other viral genes could be transcribed from them. Nevertheless, although the LTR-LTR transcripts were detected (6), the biological purpose of the LTR-LTR transcription is currently unknown. These viral transcripts might represent short, abortive transcription events occurring around the viral promoter region (27). It is known that short viral transcripts do exist in infected cells (31). In our Rev-CEM system, the detection of GFP expression strictly requires the transcription and translation of functional Rev protein from D116N. Our sorting experiments are also consistent with our quantification results showing that in D116N infection results, there was a sizable GFP-positive population comparable to that seen in a Wt infection (Fig. 4); thus, it is possible that the transcribing templates in both cases were from a predominant DNA population which consists largely of the full-length, linear DNA, in either integrated or nonintegrated form. This raises the possibility that the same viral DNA subpopulation reaches the nucleus, where the viral DNA can both transcribe prior to integration and subsequently integrate. Nevertheless, direct evidence from alternative approaches is required to further test this hypothesis.
Given the significant percentage of active templates demonstrated in this study, the importance and overall contribution of nonintegrated viral DNA in viral pathogenesis should not be underestimated. Accumulation of nonintegrated viral DNA is a prominent feature of HIV infection. During the asymptomatic phase of HIV infection, in the peripheral CD4 T cells of patients, the virus was predominantly harbored as full-length, nonintegrated DNA (7, 9). In the brains of patients with AIDS and dementia, levels of nonintegrated viral DNA are more than 10 times higher than levels of integrated proviral DNA (42). It is possible that the nonintegrated viral DNA may produce low levels of viral proteins such as Tat and Nef in various tissues such as the brain and may contribute to viral pathogenesis (42, 55). Additionally, as suggested recently, transcription from nonintegrated viral DNA is not merely an irrelevant phenomenon distant from the productive HIV replication cycle. These two virological processes are intimately intertwined to maintain critical functions such as the preservation of HIV genetic diversity (18, 59).
The demonstration of a large population of transcribing templates in nonintegrating HIV infection highlights the great potential of nonintegrating lentiviral vectors for efficient expression of therapeutic genes in terminally differentiated, noncycling cells. Our results indicate that a nonintegrating lentiviral vector, with so many active templates, can be as efficient as an integrating vector when a proper internal promoter is used to compensate for the low LTR activity. This has been recently demonstrated in studies showing that nonintegrating lentiviral vectors showed surprisingly high efficiency (equivalent to that of Wt vectors) in mediating expression from internally promoted transgenes in ocular and neuronal cells (45, 48, 64).
Acknowledgments
We thank D. Rekosh for suggestions and comments, U. O'Doherty for Alu/real-time PCR protocols, J. Guernsey for editorial assistance, and the NIH AIDS Research and Reference Reagent Program for reagents.
S. Iyer was supported by a GMU Provost Fellowship. This work was supported by Public Health Service grant AI069981 from NIAID to Y.W. and by the NICHD Intramural Program (L.B.M. and A.B.).
Footnotes
▿
Published ahead of print on 24 June 2009.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguilar-Cordova, E., J. Chinen, L. Donehower, D. E. Lewis, and J. W. Belmont. 1994. A sensitive reporter cell line for HIV-1 tat activity, HIV-1 inhibitors, and T cell activation effects. AIDS Res. Hum. Retrovir. 10295-301. [DOI] [PubMed] [Google Scholar]
- 3.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 715871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benkirane, M., R. F. Chun, H. Xiao, V. V. Ogryzko, B. H. Howard, Y. Nakatani, and K. T. Jeang. 1998. Activation of integrated provirus requires histone acetyltransferase. p300 and P/CAF are coactivators for HIV-1 Tat. J. Biol. Chem. 27324898-24905. [DOI] [PubMed] [Google Scholar]
- 5.Brown, P. O., B. Bowerman, H. E. Varmus, and J. M. Bishop. 1987. Correct integration of retroviral DNA in vitro. Cell 49347-356. [DOI] [PubMed] [Google Scholar]
- 6.Brussel, A., and P. Sonigo. 2004. Evidence for gene expression by unintegrated human immunodeficiency virus type 1 DNA species. J. Virol. 7811263-11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukrinsky, M. I., T. L. Stanwick, M. P. Dempsey, and M. Stevenson. 1991. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science 254423-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler, S. L., M. S. Hansen, and F. D. Bushman. 2001. A quantitative assay for HIV DNA integration in vivo. Nat. Med. 7631-634. [DOI] [PubMed] [Google Scholar]
- 9.Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387183-188. [DOI] [PubMed] [Google Scholar]
- 10.Collman, R., J. W. Balliet, S. A. Gregory, H. Friedman, D. L. Kolson, N. Nathanson, and A. Srinivasan. 1992. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J. Virol. 667517-7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Agostino, D. M., B. K. Felber, J. E. Harrison, and G. N. Pavlakis. 1992. The Rev protein of human immunodeficiency virus type 1 promotes polysomal association and translation of gag/pol and vpu/env mRNAs. Mol. Cell. Biol. 121375-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellis, J., and A. Bernstein. 1989. Retrovirus vectors containing an internal attachment site: evidence that circles are not intermediates to murine retrovirus integration. J. Virol. 632844-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelman, A., G. Englund, J. M. Orenstein, M. A. Martin, and R. Craigie. 1995. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J. Virol. 692729-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenard, D., W. Yonemoto, C. de Noronha, M. Cavrois, S. A. Williams, and W. C. Greene. 2005. Nef is physically recruited into the immunological synapse and potentiates T cell activation early after TCR engagement. J. Immunol. 1756050-6057. [DOI] [PubMed] [Google Scholar]
- 15.Freed, E. O., E. L. Delwart, G. L. Buchschacher, Jr., and A. T. Panganiban. 1992. A mutation in the human immunodeficiency virus type 1 transmembrane glycoprotein gp41 dominantly interferes with fusion and infectivity. Proc. Natl. Acad. Sci. USA 8970-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia, J. A., F. K. Wu, R. Mitsuyasu, and R. B. Gaynor. 1987. Interactions of cellular proteins involved in the transcriptional regulation of the human immunodeficiency virus. EMBO J. 63761-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaur, M., and A. D. Leavitt. 1998. Mutations in the human immunodeficiency virus type 1 integrase D,D(35)E motif do not eliminate provirus formation. J. Virol. 724678-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelderblom, H. C., D. N. Vatakis, S. A. Burke, S. D. Lawrie, G. C. Bristol, and D. N. Levy. 2008. Viral complementation allows HIV-1 replication without integration. Retrovirology 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gervaix, A., D. West, L. M. Leoni, D. D. Richman, F. Wong-Staal, and J. Corbeil. 1997. A new reporter cell line to monitor HIV infection and drug susceptibility in vitro. Proc. Natl. Acad. Sci. USA 944653-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillim-Ross, L., A. Cara, and M. E. Klotman. 2005. Nef expressed from human immunodeficiency virus type 1 extrachromosomal DNA downregulates CD4 on primary CD4+ T lymphocytes: implications for integrase inhibitors. J. Gen. Virol. 86765-771. [DOI] [PubMed] [Google Scholar]
- 21.Glushakova, S., J. Munch, S. Carl, T. C. Greenough, J. L. Sullivan, L. Margolis, and F. Kirchhoff. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4+ T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 7510113-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hazuda, D. J., P. Felock, M. Witmer, A. Wolfe, K. Stillmock, J. A. Grobler, A. Espeseth, L. Gabryelski, W. Schleif, C. Blau, and M. D. Miller. 2000. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science 287646-650. [DOI] [PubMed] [Google Scholar]
- 23.Hope, T. J., D. McDonald, X. J. Huang, J. Low, and T. G. Parslow. 1990. Mutational analysis of the human immunodeficiency virus type 1 Rev transactivator: essential residues near the amino terminus. J. Virol. 645360-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, X. J., T. J. Hope, B. L. Bond, D. McDonald, K. Grahl, and T. G. Parslow. 1991. Minimal Rev-response element for type 1 human immunodeficiency virus. J. Virol. 652131-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones, K. A., and B. M. Peterlin. 1994. Control of RNA initiation and elongation at the HIV-1 promoter. Annu. Rev. Biochem. 63717-743. [DOI] [PubMed] [Google Scholar]
- 26.Jordan, A., P. Defechereux, and E. Verdin. 2001. The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J. 201726-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kao, S. Y., A. F. Calman, P. A. Luciw, and B. M. Peterlin. 1987. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature 330489-493. [DOI] [PubMed] [Google Scholar]
- 28.Kelly, J., M. H. Beddall, D. Yu, S. R. Iyer, J. W. Marsh, and Y. Wu. 2008. Human macrophages support persistent transcription from unintegrated HIV-1 DNA. Virology 372300-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J. Virol. 662232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King, W., M. D. Patel, L. I. Lobel, S. P. Goff, and M. C. Nguyen-Huu. 1985. Insertion mutagenesis of embryonal carcinoma cells by retroviruses. Science 228554-558. [DOI] [PubMed] [Google Scholar]
- 31.Landry, S., M. Halin, S. Lefort, B. Audet, C. Vaquero, J. M. Mesnard, and B. Barbeau. 2007. Detection, characterization and regulation of antisense transcripts in HIV-1. Retrovirology 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis, N., J. Williams, D. Rekosh, and M. L. Hammarskjold. 1990. Identification of a _cis_-acting element in human immunodeficiency virus type 2 (HIV-2) that is responsive to the HIV-1 rev and human T-cell leukemia virus types I and II rex proteins. J. Virol. 641690-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, Z., J. Dullmann, B. Schiedlmeier, M. Schmidt, C. von Kalle, J. Meyer, M. Forster, C. Stocking, A. Wahlers, O. Frank, W. Ostertag, K. Kuhlcke, H. G. Eckert, B. Fehse, and C. Baum. 2002. Murine leukemia induced by retroviral gene marking. Science 296497. [DOI] [PubMed] [Google Scholar]
- 34.Lobel, L. I., J. E. Murphy, and S. P. Goff. 1989. The palindromic LTR-LTR junction of Moloney murine leukemia virus is not an efficient substrate for proviral integration. J. Virol. 632629-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo, T., J. L. Douglas, R. L. Livingston, and J. V. Garcia. 1998. Infectivity enhancement by HIV-1 Nef is dependent on the pathway of virus entry: implications for HIV-based gene transfer systems. Virology 241224-233. [DOI] [PubMed] [Google Scholar]
- 36.Malim, M. H., J. Hauber, S. Y. Le, J. V. Maizel, and B. R. Cullen. 1989. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature 338254-257. [DOI] [PubMed] [Google Scholar]
- 37.Merzouki, A., P. Patel, S. Cassol, M. Ennaji, P. Tailor, F. R. Turcotte, M. O'Shaughnessy, and M. Arella. 1995. HIV-1 gp120/160 expressing cells upregulate HIV-1 LTR directed gene expression in a cell line transfected with HIV-1 LTR-reporter gene constructs. Cell. Mol. Biol. (Noisy-le-Grand) 41445-452. [PubMed] [Google Scholar]
- 38.Nara, P. L., W. C. Hatch, N. M. Dunlop, W. G. Robey, L. O. Arthur, M. A. Gonda, and P. J. Fischinger. 1987. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res. Hum. Retrovir. 3283-302. [DOI] [PubMed] [Google Scholar]
- 39.Negri, D. R., Z. Michelini, S. Baroncelli, M. Spada, S. Vendetti, V. Buffa, R. Bona, P. Leone, M. E. Klotman, and A. Cara. 2007. Successful immunization with a single injection of non-integrating lentiviral vector. Mol. Ther. 151716-1723. [DOI] [PubMed] [Google Scholar]
- 40.O'Doherty, U., W. J. Swiggard, D. Jeyakumar, D. McGain, and M. H. Malim. 2002. A sensitive, quantitative assay for human immunodeficiency virus type 1 integration. J. Virol. 7610942-10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada, M., and K. T. Jeang. 2002. Differential requirements for activation of integrated and transiently transfected human T-cell leukemia virus type 1 long terminal repeat. J. Virol. 7612564-12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pang, S., Y. Koyanagi, S. Miles, C. Wiley, H. V. Vinters, and I. S. Chen. 1990. High levels of unintegrated HIV-1 DNA in brain tissue of AIDS dementia patients. Nature 34385-89. [DOI] [PubMed] [Google Scholar]
- 43.Panganiban, A. T., and H. M. Temin. 1984. Circles with two tandem LTRs are precursors to integrated retrovirus DNA. Cell 36673-679. [DOI] [PubMed] [Google Scholar]
- 44.Pauza, C. D., J. E. Galindo, and D. D. Richman. 1990. Reinfection results in accumulation of unintegrated viral DNA in cytopathic and persistent human immunodeficiency virus type 1 infection of CEM cells. J. Exp. Med. 1721035-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Philippe, S., C. Sarkis, M. Barkats, H. Mammeri, C. Ladroue, C. Petit, J. Mallet, and C. Serguera. 2006. Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proc. Natl. Acad. Sci. USA 10317684-17689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riggs, N. L., and J. C. Guatelli. 1996. Production and characterization of high-titer stocks of rev-defective HIV-1. Virology 217602-606. [DOI] [PubMed] [Google Scholar]
- 47.Robinson, H. L., and D. M. Zinkus. 1990. Accumulation of human immunodeficiency virus type 1 DNA in T cells: results of multiple infection events. J. Virol. 644836-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saenz, D. T., N. Loewen, M. Peretz, T. Whitwam, R. Barraza, K. G. Howell, J. M. Holmes, M. Good, and E. M. Poeschla. 2004. Unintegrated lentivirus DNA persistence and accessibility to expression in nondividing cells: analysis with class I integrase mutants. J. Virol. 782906-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sastry, L., Y. Xu, R. Cooper, K. Pollok, and K. Cornetta. 2004. Evaluation of plasmid DNA removal from lentiviral vectors by benzonase treatment. Hum. Gene Ther. 15221-226. [DOI] [PubMed] [Google Scholar]
- 50.Schrager, J. A., and J. W. Marsh. 1999. HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc. Natl. Acad. Sci. USA 968167-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaw, G. M., B. H. Hahn, S. K. Arya, J. E. Groopman, R. C. Gallo, and F. Wong-Staal. 1984. Molecular characterization of human T-cell leukemia (lymphotropic) virus type III in the acquired immune deficiency syndrome. Science 2261165-1171. [DOI] [PubMed] [Google Scholar]
- 52.Shoemaker, C., S. Goff, E. Gilboa, M. Paskind, S. W. Mitra, and D. Baltimore. 1980. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc. Natl. Acad. Sci. USA 773932-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stevenson, M., T. L. Stanwick, M. P. Dempsey, and C. A. Lamonica. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 91551-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Svarovskaia, E. S., R. Barr, X. Zhang, G. C. Pais, C. Marchand, Y. Pommier, T. R. Burke, Jr., and V. K. Pathak. 2004. Azido-containing diketo acid derivatives inhibit human immunodeficiency virus type 1 integrase in vivo and influence the frequency of deletions at two-long-terminal-repeat-circle junctions. J. Virol. 783210-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teo, I., C. Veryard, H. Barnes, S. F. An, M. Jones, P. L. Lantos, P. Luthert, and S. Shaunak. 1997. Circular forms of unintegrated human immunodeficiency virus type 1 DNA and high levels of viral protein expression: association with dementia and multinucleated giant cells in the brains of patients with AIDS. J. Virol. 712928-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vargas, J., Jr., G. L. Gusella, V. Najfeld, M. E. Klotman, and A. Cara. 2004. Novel integrase-defective lentiviral episomal vectors for gene transfer. Hum. Gene Ther. 15361-372. [DOI] [PubMed] [Google Scholar]
- 57.Vargas, J., Jr., M. E. Klotman, and A. Cara. 2008. Conditionally replicating lentiviral-hybrid episomal vectors for suicide gene therapy. Antivir. Res. 80288-294. [DOI] [PubMed] [Google Scholar]
- 58.Wiskerchen, M., and M. A. Muesing. 1995. Human immunodeficiency virus type 1 integrase: effects of mutations on viral ability to integrate, direct viral gene expression from unintegrated viral DNA templates, and sustain viral propagation in primary cells. J. Virol. 69376-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu, Y. 2008. The second chance story of HIV-1 DNA: unintegrated? Not a problem! Retrovirology 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu, Y., M. H. Beddall, and J. W. Marsh. 2007. Rev-dependent indicator T cell line. Curr. HIV Res. 5395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, Y., M. H. Beddall, and J. W. Marsh. 2007. Rev-dependent lentiviral expression vector. Retrovirology 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu, Y., and J. W. Marsh. 2003. Early transcription from nonintegrated DNA in human immunodeficiency virus infection. J. Virol. 7710376-10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu, Y., and J. W. Marsh. 2001. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science 2931503-1506. [DOI] [PubMed] [Google Scholar]
- 64.Yáñez-Muñoz, R. J., K. S. Balaggan, A. MacNeil, S. J. Howe, M. Schmidt, A. J. Smith, P. Buch, R. E. MacLaren, P. N. Anderson, S. E. Barker, Y. Duran, C. Bartholomae, C. von Kalle, J. R. Heckenlively, C. Kinnon, R. R. Ali, and A. J. Thrasher. 2006. Effective gene therapy with nonintegrating lentiviral vectors. Nat. Med. 12348-353. [DOI] [PubMed] [Google Scholar]
- 65.Yee, J. K., A. Miyanohara, P. LaPorte, K. Bouic, J. C. Burns, and T. Friedmann. 1994. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc. Natl. Acad. Sci. USA 919564-9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young, J., Z. Tang, Q. Yu, D. Yu, and Y. Wu. 2008. Selective killing of HIV-1-positive macrophages and T cells by the Rev-dependent lentivirus carrying anthrolysin O from Bacillus anthracis. Retrovirology 536. [DOI] [PMC free article] [PubMed] [Google Scholar]