Docosahexaenoic Acid Neurolipidomics (original) (raw)
. Author manuscript; available in PMC: 2011 Apr 1.
Abstract
Mediator lipidomics is a field of study concerned with the characterization, structural elucidation and bioactivity of lipid derivatives generated by enzymatic activity. Omega-3 fatty acids have beneficial effects for vision, brain function, cardiovascular function, and immune-inflammatory responses. Docosahexaenoic acid [DHA; 22:6(n-3)], the most abundant essential omega-3 fatty acid in the human body, is selectively enriched and avidly retained in the central nervous system as an acyl chain of phospholipids. Brain-ischemia reperfusion and seizures trigger rapid release of DHA and of arachidonic acid (AA) as free, unesterified fatty acids. AA in turn generates eicosanoids, and DHA forms docosanoids. The stereoselective docosanoid neuroprotectin D1 (NPD1; 10R,17S-dihydroxy-docosa-4Z,7Z,11E,15E,19Z hexaenoic acid) is formed early in brain-ischemia reperfusion. Supplementation of NPD1 (intracerebroventricularly; i.c.v.) or of DHA (i.c.v. or systemically) results in decreased infarct size, polymorphonuclear neutrophil infiltration, ischemia-induced nuclear factor kappa B (NFκB) activation, and cyclooxygenase-2 (COX-2) induction. DHA involvement in cell function includes enhancing Akt translocation and activation, and binding to a peroxisome proliferator-activated receptor-gamma (PPAR-γ) family of ligand-activated nuclear receptors. Here we present an overview of recent DHA-mediator lipidomic studies in experimental brain ischemia-reperfusion and other conditions.
1. Mediator Lipidomics
The term `lipidome' refers to the comprehensive detailed description of the composition of lipid classes and their molecular species of a given cell, part of a cell, or organ. Mediator lipidomics, on the other hand, involves the characterization, structural elucidation and discovery of lipid derivatives generated by an enzyme. A key in the development of mediator lipidomics is the advent of High Performance Lipid Chromatography - Electrospray Ionization - Mass Spectrometry/Mass Spectrometry (HPLC-ESI-MS/MS) [1]. Mediator lipidomics-based analysis on the most abundant omega-3 fatty acid family, docosahexaenoic acid (DHA), which is richly endowed and avidly retained in the central nervous system (CNS), has led to the uncovering of biologically-active DHA derivatives, termed “docosanoids”. DHA neurolipidomics goes beyond the descriptive aspects of lipidomic analysis because it has initiated the identification of DHA derivatives and the uncovering of docosanoid bioactivity using cell signaling, cell function, cell survival, and disease mechanisms as approaches. These studies have led to the identification of neuroprotectin D1 (NPD1; 10R,17S-dihydroxy-docosa-4Z,7Z,11E,15E,19Z hexaenoic acid). Here we present an overview on NPD1 bioactivity in pro-survival and inflammation resolution in the CNS, highlighting DHA-NPD1 in brain ischemia-reperfusion.
2. DHA in the Central Nervous System
Omega-3 essential fatty acid family members, DHA (22:6, n-3) [2] and linolenic acid (18:3, n-3) after dietary intake, are actively incorporated in the liver prior to distribution to various organs. Then linolenic acid is elongated and desaturated by liver hepatocytes to DHA, which in turn is activated (22:6-CoA) and acylated into phospholipids and eventually released as lipoproteins into the bloodstream [3]. DHA accretion in the CNS after liver release correlates with photoreceptor membrane biogenesis and synaptogenesis during the postnatal development [3]. DHA is involved in aging, memory formation, synaptic membrane function, photoreceptor biogenesis and function, and neuroprotection [3,4]. Epidemiologic studies indicate that diets enriched with DHA are associated with reduced risk of cognitive impairment [5]. Certain diets, such as those of vegans, vegetarians, and the elderly, contain relatively low DHA [6]. While low dietary DHA leads to progressive loss of DHA in the CNS, both the brain and retina display a striking ability to retain and actively conserve DHA even after prolonged omega-3 fatty acid dietary deficiencies [7,8]. Extended deficiencies result in decreased amounts of DHA in neuronal membranes, altering membrane fluidity and signaling properties [5]. When rats are fed low-DHA diets for one or more generations, deficits in brain functions arise [5]. The elderly are at risk for cognitive and cellular impairments associated with decreased DHA [5] as well as an enhanced susceptibility to cerebrovascular disease, including poor recovery after brain injury. Moreover in addition to decreased dietary intake and reduced liver fatty acid desaturase capacity, age-related defects in antioxidant systems contribute to increased lipid peroxidation that further reduces DHA levels [5]. Therefore, essential omega-3 fatty acid dietary deficits contribute to cognitive decline, as well as increased risk and severity of brain injury.
3. DHA is the Precursor of the Docosanoid Neuroprotectin D1
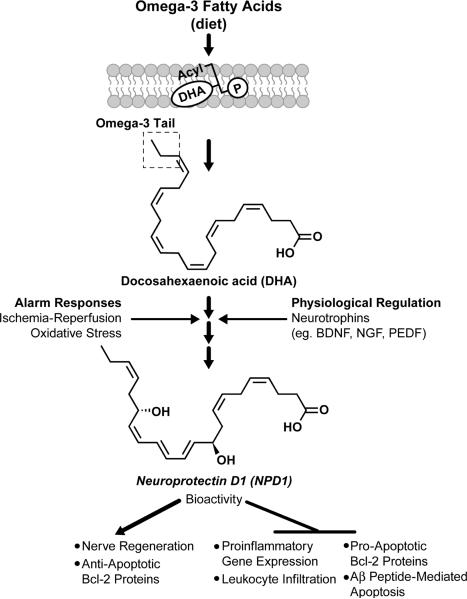
DHA is the precursor for the synthesis of NPD1. NPD1 synthesis is induced as an alarm response to oxidative stress and/or neurotrophin activation, triggering signaling for homeostatic maintenance of cellular integrity (Figure 1) [7]. After DHA is cleaved from membrane phospholipids by phospholipase A2, free (unesterified) DHA is converted into the stereospecific mediator NPD1 by lipoxygenation catalyzed by 15-lipoxygenase-1 [9] followed by epoxidation and hydrolysis reactions [10,11]. A high affinity binding site for NPD1 was described recently [10].
Figure 1.
Dietary-derived omega-3 fatty acids are channeled through the liver and blood stream to organs [2]. The central nervous system incorporates DHA into phospholipids for the biogenesis of dendritic spines, photoreceptor membranes, and other cellular membranes of the central nervous system. A membrane phospholipid containing a docosahexanoyl chain of _sn_-2 is hydrolyzed by a phospholipase A2 generating a free (unesterified) DHA (the omega-3 (n-3) tail is highlighted). Lipoxygenation is then followed by epoxidation and hydrolysis to generate neuroprotectin D1 (NPD1). NPD1 bioactivity results in downregulation of pro-inflammatory gene expression, leukocyte infiltration in brain ischemia reperfusion damage, and of pro-apoptotic Bcl-2 proteins. NPD1 induces nerve regeneration and anti-apoptotic Bcl-2 proteins.
The neurotrophins that stimulate NPD1 synthesis and secretion, promoting neuronal and/or photoreceptor cell survival, include: persephin, BDNF (brain derived neurotrophic factor), NGF (nerve growth factor), LIF (leukemia inhibitory factor), FGF2 (fibroblast growth factor 2), PEDF (pigment epithelium-derived factor), CNTF (ciliary neurotrophic factor), GDNF (glial cell derived neurotrophic factor), and NT3 (neurotrophin-3) [12].
Once formed, NPD1 markedly modulates inflammatory signaling cascades. In retinal pigment epithelial (RPE) cells, NPD1 counteracts apoptosis and markedly downregulates pro-inflammatory gene expression [11]. Specifically, NPD1 activates protective anti-apoptotic Bcl-2 proteins, including Bcl-2, Bcl-xL, and Bfl-1/A1, while inhibiting pro-apoptotic proteins BAD, BAX, Bid, and Bix [11]. Oxidative stress, as well as ischemia-reperfusion, activates pro-apoptotic Bcl-2 proteins to initiate the release of cytochrome C from mitochondria. Cytochrome C binds and activates the potent pro-apoptotic signaling molecule caspase-3. NPD1 interferes with the activation of this cascade, and prevents pro-apoptotic signaling as part of its anti-inflammatory, pro-survival repair activity [11, 13]. NPD1 has also been shown to attenuate the effects of oxidative stress induced by H2O2 plus tumor necrosis factor [11]. NPD1 is able to shunt signaling away from caspase-3 activation via activation of Bcl-2 proteins, resulting in decreased cell death [13,14].
4. Lipidomics in Experimental Stroke
Stroke is the third cause of death and the leading cause of adult disability affecting over 780,000 people in the United States each year [15]. On average, a stroke occurs every 45 seconds and someone dies as a result of stroke every 3 minutes [16]. Thrombolytic agents are the only treatment of stroke; however, these agents are indicated in only a small subset of patients and are relatively ineffective [17]. Experimental therapies that show efficacy in animal models have not been translated to humans [18]. One of the reasons that effective treatment for stroke remains elusive is the incomplete understanding of endogenous mechanisms that control damage and that set in motion repair responses.
Most strokes are ischemic due to occlusion of the middle cerebral artery and brain damage ensues mainly due to events taking place during reperfusion. Experimental models recapitulate ischemic stroke in animals by the introduction of a nylon suture through the common carotid artery that reaches and blocks the middle cerebral artery (usually during 1–2 hours). Then upon removal of the intraluminal suture, ischemia-reperfusion results in severely-damaged tissue to the infarct core, which is surrounded by the penumbra, an area with some collateral circulation. DHA mediator lipidomics applied to models of experimental brain ischemia-reperfusion has provided an insight into cell signaling that aims to counteract damage. These studies were based on the fact that brain ischemia triggers the accumulation of free (unesterified) DHA and arachidonic acid (AA) mediated by the rapid activation of phospholipase A2, which cleaves membrane phospholipid polyunsaturated acyl chains [4]. AA is then converted into eicosanoids and DHA is converted into docosanoids. The eicosanoids are composed of several mediators that regulate cell function and participate in brain injury. As an example, cyclooxygenase-2 (COX-2) catalyses the synthesis of prostaglandin E2 (PGE2). COX-2 is required for function and participates in neuroinflammation. Physiologically, COX-2 is constitutively expressed in hippocampal dendritic spines and neuronal cell bodies and is modulated by synaptic activity [19]. On the other hand, COX-2 is rapidly induced in neurons by seizures [20], cerebral ischemia [21,22] or Aβ peptide-induced neuronal damage [13]. COX-2 induction in these pathological conditions generally contributes to neuronal injury; however, downstream COX-2 signaling pathways indicate that the molecular mechanisms triggering brain injury or exerting neuroprotection are complex and not well understood. PGE2 also activates the G protein-coupled receptors (GPCRs) EP1, EP2, EP3 and EP4. EP2-receptor activation by PGE2 is neuroprotective after ischemia [21, 22], whereas EP1-receptor activation induces neurodegenerative damage [23].
Brain docosanoids were first recognized by mediator lipidomics during experimental ischemia-reperfusion [14]. These studies uncovered at least two stereospecific DHA oxygenation pathways that lead to the synthesis of novel mediators: a) NPD1, initially identified as 10,17S-docosatriene, and its precursor 17S-HDHA; and b) aspirin-triggered docosanoids or 17-R resolvins, the biosynthesis of which likely involves an acetylated COX-2. Aspirin was tested in mice undergoing brain ischemia-reperfusion since it is used prophylactically for cerebrovascular diseases and is known to be generated in anti-inflammatory lipid mediators in non-neural tissues [24–26]. Aspirin-triggered docosanoids or 17-R resolvins found in brain ischemia-reperfusion include 7,17R diHDHA, 7,8,17R triHDHA and 17R-HDHA [14]. Although the bioactivity of these DHA derivatives remains to be defined, it is of interest that when aspirin was administered 15 minutes before the onset of one hour of ischemia followed by reperfusion, a metabolic shift away from NPD1 and towards 17-R docosanoids was observed [14].
Brain ischemia-reperfusion-induced NPD1 synthesis peaks at 8 hours and then decreases, displaying increased content even after 25 hours of reperfusion. Since cerebroventricular- infused NPD1 (400 ng over 48 hours) elicits remarkable protection, it was suggested that the endogenously generated NPD1 was not sufficient to counteract the damaging actions of those conditions of ischemia-reperfusion. Thus, if the injury is mild endogenous NPD1 may counteract the damage; however, if the injury is severe the NPD1 protective bioactivity may be overwhelmed, resulting in brain damage [14].
In so far as the mechanism of action of NPD1 is concerned, it was found that NPD1 attenuates ischemia-reperfusion-triggered leukocyte infiltration, nuclear factor kappa B (NFκB) induction, COX-2 expression, and stroke volume [14]. Furthermore intravenous administration of DHA complexed with serum albumin results in increased NPD1 production in the ipsilateral hemisphere and promotes neurological recovery after ischemia reperfusion [27]. Thus NPD1 and other DHA-derived mediators are endowed with potent anti-inflammatory and pro-resolving actions in experimental models of inflammatory diseases outside of the nervous system, such as peritonitis [24,25] and acute kidney injury [26].
5. DHA induces Akt Signaling
Akt pathways are implicated in multiple cellular processes, including protein synthesis, survival, proliferation, glucose metabolism and neuronal maintenance. Akt regulates cell survival and injury-induced apoptosis. Akt achieves full activation when it is phosphorylated at both of its regulatory domains located at Ser473 and Thr308 and has reduced activity when only one of these regulatory domains is phosphorylated [28]. Akt phosphorylation results when phosphatidylinositol-4,5-bisphosphate (PIP2) is converted to phosphatidylinositol-3,4,5-triphosphate (PIP3) via phosphoinositide-3-kinase (PI3K) activity. PIP3 activates phosphoinositide-dependent protein kinase (PDK1), which phosphorylates Akt's Thr308 domain [29]. PIP3 activity and Akt phosphorylation are often disrupted after stroke, resulting in a loss of Akt activity and increased cellular injury and apoptosis [30]. The second Akt phosphorylation domain, located at Ser473, is phosphorylated as a response to cellular stress via the rictor-mTOR complex [29]. This activates Akt to full or partial function, depending on the phosphorylation state of Thr308. Once activated, Akt phosphorylates multiple substrates, including the mammalian target of rapamycin (mTOR, aka FRAP, RAFT), BAD, forkhead transcription factor (FKHR), glycogen synthase kinase 3β (GSK3β), and proline-rich Akt substrate (PRAS), and also initiates a series of cascades that drive the cell towards survival [31].
DHA induces Akt activation and translocation [32]. In the nervous system, DHA accumulates as an acyl chain in the aminophospholipids, phosphatidylethanolamine, and phosphatidylserine (PS). Neuronal membranes have been shown to possess the unique property of being able to modify PS levels with dietary DHA supplementation [33]. Supplemented DHA results in an increase of PS and has been shown to increase the rate of Akt translocation from the membrane to the cytosol after ischemia, a required step for phosphorylation and activation of Akt [32]. Translocation is induced by interaction between PS and the binding pocket of the PH domain of Akt. Serum starvation without DHA results in increased caspase 3 activity and cellular death. Supplemented DHA attenuates this effect and results in increased PS, decreased caspase-3, and decreased apoptosis [32]. Although the levels of total Akt remain constant, more pGSK-3, a downstream mediator of Akt and an indicator of Akt activity, is produced with DHA. This indicates that supplementation with DHA promotes survival, at least in part, via an increase in PS that results in greater Akt translocation and activity [32].
6. DHA is a Ligand of Nuclear Receptor Transcription Factors
Nuclear receptors are a large family of ligand-activated transcription factors that bind their agonists in the cytosol and translocate into the nucleus to induce transcription of genes that influence multiple cellular functions such as growth, metabolism, and repair. DHA has been shown to bind RXRs [34] and, along with 9-HODE and 13-HODE, also has a binding affinity with PPAR-γ nuclear receptors [35,36]. Once bound to their ligands, RXRs and PPAR-γ form activated heterodimers that induce genes for various processes, including the development/proliferation of adipose cells, improved lipid metabolism/partitioning (which results in improved insulin sensitivity), and the regulation of inflammatory mediators and immunity [37]. Specifically, RXR-PPAR-γ complexes inhibit a wide range of inflammatory mediators, including inducible nitric oxide synthase (iNOS), interleukin-12 (IL-12), interferon-γ (IFN-γ), tumor necrosis factor α (TNFα), interleukin -1β (IL-1β), matrix metalloproteinase-9 (MMP-9), and scavenger receptor A [37]. RXR-PPAR-γ activation has been shown to significantly reduce infarct volumes, improve behavioral outcomes, and reduce inflammatory COX-2 and iNOS expression in rats following middle cerebral artery occlussion [38]. PPAR-γ activation after middle cerebral artery occlussion also results in the binding/activation of 14-3-3ε PPAR response elements (PPREs) located at promoter regions of target 14-3-3ε genes. The activated PPREs increased 14-3-3ε transcription and 14-3-3ε sequestering of phosphorylated BAD, which protected the mitochondria membrane potential and decreased apoptosis after middle cerebral artery occlussion [39]. Although a direct link between DHA and the beneficial effects of RXR-PPAR-γ in the brain after middle cerebral artery occlussion has yet to be shown, direct interaction of DHA and RXR-PPAR-γ has been found in adipocytes [36] and dendritic cells [40].
7. Prospects of Docosahexaenoic Acid Neurolipidomics
Lipidomics will provide an increased understanding of the significance of DHA in aging, stroke, and during the initiation and progression of neurodegenerative diseases. This information in turn will contribute to neuroprotective, restorative, and regenerative translational approaches that take advantage of the beneficial properties of this fatty acid [13]. DHA is concentrated as acyl chains in phospholipids of the CNS and may exert beneficial actions by sustaining membrane structural properties as components of specific phospholipid molecular species. As such, DHA-rich phospholipids may modulate ion channels, receptors, or other membrane proteins. A second mechanism for DHA's beneficial actions is the formation of enzyme-catalyzed stereospecific mediators, docosanoids. NPD1 and aspirin- triggered 17R docosanoids in brain ischemia-reperfusion are critical modulators for inflammation resolution that counteract injury and aim to restore homeostasis [7,10,14].
How DHA in supplied physiologically to the CNS via the blood stream remains unclear [3]. Phosphatidylserine, which is rich in DHA, increases the translocation/activation of Akt, but DHA's action/s downstream messengers of the Akt cascade are not well defined. In addition, DHA's interaction with the nuclear receptors RXR-PPAR-γ is beginning to be defined. Also, it stills needs to be clarified exactly how neurotropins, such as BDNF, signal for NPD1 production. Does NPD1 act in an autocrine fashion? Or is NPD1 released, in turn eliciting a paracrine bioactivity? Or does NPD1 fulfill both an autocrine and a paracrine role? Furthermore, what are the capacity/limitations of aged brains to respond to DHA under ischemia-reperfusion? The understanding of DHA neurolipidomics coupled to signaling and the use of disease models of the nervous system will contribute to the development of new therapeutic modalities for stroke and other neurodegenerative diseases.
Acknowledgements
This work was supported by National Institutes of Health, National Institute of Neurological Disorders and Stroke grant NS046741. T.D.N. is a recipient of Ruth L. Kirschstein National Research Service Awards for Individual Predoctoral MD/PhD and Other Dual Doctoral Degree Fellows (F30) grant AG032841 from NIH, National Institute on Aging.
Footnotes
Conflict of Interest N.G.B. is a consultant for Resolvyx Pharmaceuticals.
References
- [1].Bazan NG, Marcheselli VL, Lu Y, Hong S, Jackson F. Lipidomic approaches to neuroprotection signaling in the retinal pigment epithelium. In: Fliesler SJ, Kisselev OG, editors. Signal Tranduction in the Retina. 2008. pp. 349–73. Chapter 15. [Google Scholar]
- [2].Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–71. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- [3].Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989;86:2903–7. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44:2221–33. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- [5].Uauy R, Dangour AD. Nutrition in brain development and aging: role of essential fatty acids. Nutr Rev. 2006;64:S24–S33. doi: 10.1301/nr.2006.may.s24-s33. [DOI] [PubMed] [Google Scholar]
- [6].Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease. Nat Clin Pract Neurol. 2009;5:140–52. doi: 10.1038/ncpneuro1044. [DOI] [PubMed] [Google Scholar]
- [7].Bazan NG. Homeostatic regulation of photoreceptor cell integrity: significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid: the Proctor Lecture. Invest Ophthalmol Vis Sci. 2007;48:4866–81. doi: 10.1167/iovs.07-0918. [DOI] [PubMed] [Google Scholar]
- [8].Neuringer M, Connor WE, Lin DS, Barstad L, Luck S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci USA. 1986;83:4021–5. doi: 10.1073/pnas.83.11.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Calandria JM, Marcheselli VL, Mukherjee PK, Uddin J, Winkler JW, Petasis NA, Bazan NG. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J Biol Chem. 2009;284:17877–82. doi: 10.1074/jbc.M109.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marcheselli VL, Mukherjee PK, Arita M, Hong S, Antony R, Sheets K, Petasis NA, Serhan CN, Bazan NG. Neuroprotectin D1/Protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and leukocytes. Prostaglandins Leukot Essent Fatty Acids. 2009 doi: 10.1016/j.plefa.2009.10.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–6. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, Bazan NG. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci U S A. 2007;104:13152–57. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–83. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–17. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- [15]. http://stroke.nih.gov/materials.
- [16].Caplan LR. Improving stroke prevention and treatment now. Cerebrum. 2006:2–11. [Google Scholar]
- [17].Adams HP, Jr, Brott TG, Furlan AJ, et al. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke. Circulation. 1996;94:1167–74. doi: 10.1161/01.cir.94.5.1167. [DOI] [PubMed] [Google Scholar]
- [18].O'Collins VE, Macleod MR, Donnan GA, et al. 1,026 Experimental Treatments in Acute Stroke. Ann Neurol. 2006;59:467–77. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- [19].Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA. 1996;93:2317–21. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Marcheselli VL, Bazan NG. Sustained induction of prostaglandin endoperoxide synthase-2 by seizures in hippocampus. Inhibition by a platelet-activating factor antagonist. J Biol Chem. 1996;271:24794–99. doi: 10.1074/jbc.271.40.24794. [DOI] [PubMed] [Google Scholar]
- [21].McCullough L, Wu L, Haughey N, et al. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24:257–68. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Liu D, Wu L, Breyer R, Mattson MP, Andreasson K. Neuroprotection by the PGE2 EP2 receptor in permanent focal cerebral ischemia. Ann Neurol. 2005;57:758–61. doi: 10.1002/ana.20461. [DOI] [PubMed] [Google Scholar]
- [23].Kawano T, Anrather J, Zhou P, et al. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–9. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- [24].Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–87. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- [26].Duffield JS, Hong S, Vaidya VS, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–11. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- [27].Belayev L, Marcheselli VL, Khoutorova L, Rodriguez de Turco EB, Busto R, Ginsberg MD, Bazan NG. Docosahexaenoic acid complexed to albumin elicits high-grade ischemic neuroprotection. Stroke. 2005;36:118–23. doi: 10.1161/01.STR.0000149620.74770.2e. [DOI] [PubMed] [Google Scholar]
- [28].Alessi DR, Andjelkovic M, Caudwell B, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–51. [PMC free article] [PubMed] [Google Scholar]
- [29].Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- [30].Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–70. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- [31].Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. J Biol Chem. 2007;282:18661–5. doi: 10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- [32].Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proc Natl Acad Sci U S A. 2005;102:10858–63. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Guo M, Stockert L, Akbar M, Kim HY. Neuronal specific increase of phosphatidylserine by docosahexaenoic acid. J Mol Neurosci. 2007;33:67–73. doi: 10.1007/s12031-007-0046-z. [DOI] [PubMed] [Google Scholar]
- [34].Lengqvist J, Mata De Urquiza A, Bergman AC, et al. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Mol Cell Proteomics. 2004;3:692–703. doi: 10.1074/mcp.M400003-MCP200. [DOI] [PubMed] [Google Scholar]
- [35].Itoh T, Fairall L, Amin K, et al. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol. 2008;15:924–31. doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Banga A, Unal R, Tripathi P, et al. Adiponectin translation is increased by the PPARgamma agonists pioglitazone and omega-3 fatty acids. Am J Physiol Endocrinol Metab. 2009;296:E480–9. doi: 10.1152/ajpendo.90892.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- [38].Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–96. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- [39].Wu JS, Cheung WM, Tsai YS, et al. Ligand-activated peroxisome proliferator-activated receptor-gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3 epsilon upregulation. Circulation. 2009;119:1124–34. doi: 10.1161/CIRCULATIONAHA.108.812537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zapata-Gonzalez F, Rueda F, Petriz J, et al. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPAR(gamma):RXR heterodimers: comparison with other polyunsaturated fatty acids. J Leukoc Biol. 2008;84:1172–82. doi: 10.1189/jlb.1007688. [DOI] [PubMed] [Google Scholar]