Mitotic Functions of Kinesin-5 (original) (raw)
. Author manuscript; available in PMC: 2011 May 1.
Published in final edited form as: Semin Cell Dev Biol. 2010 Jan 28;21(3):255–259. doi: 10.1016/j.semcdb.2010.01.019
Abstract
In all eukaryotic cells, molecular motor proteins play essential roles in spindle assembly and function. The homotetrameric kinesin-5 motors in particular generate outward forces that establish and maintain spindle bipolarity and contribute to microtubule flux. Cell cycle dependent phosphorylation of kinesin-5 motors regulates their localization to the mitotic spindle. Analysis of live cells further shows that kinesin-5 motors are highly dynamic in the spindle. Understanding the interactions of kinesin-5 motors with microtubules and other spindle proteins is likely to broaden the documented roles of kinesin-5 motors during cell division.
Keywords: Eg5, mitosis, spindle, kinesin, dynein
Introduction
One of the first mitotic motors to be identified was the kinesin, BimC (Enos and Morris, 1990). This kinesin was identified in a temperature-sensitive fungal library screen in search of strains that were defective in cellular division at the restrictive temperature (Morris, 1976). A similar screen carried out in fission yeast identified a related kinesin, Cut7 (Hagan and Yanagida, 1990). Mutations in either of these motor proteins blocked spindle pole body separation and thus prevented the successful completion of mitosis (Enos and Morris, 1990; Hagan and Yanagida, 1990). Subsequent work has identified BimC/Cut7 orthologs in Xenopus (Eg5), S. cerevisiae (Cin8p and Kip1p), Drosophila (KLP61F), human (hsEg5), C. elegans (BMK-1), and Arabidopsis (AtKRP125a,b,c and AtF16L2 ); with the exception of C. elegans, the gene product plays a critical role in mitosis (Bannigan et al., 2007; Bishop et al., 2005; Blangy et al., 1995; Heck et al., 1993; Hoyt et al., 1992; Le Guellec et al., 1991; Reddy and Day, 2001; Roof et al., 1992; Sawin et al., 1992). This group of related kinesins, subsequently classified as the kinesin-5 family (Lawrence et al., 2004), localizes to spindle microtubules and structures present at spindle poles.
Structurally the kinesin-5 polypeptide consists of an N-terminal head domain, which contains the motor; an internal stalk domain, capable of forming coiled coils; and a C-terminal tail domain (Le Guellec et al., 1991). Four of these ~125kDa monomers associate to form a homotetrameric complex with motor domains positioned at each end of the tetramer’s long axis (Blangy et al., 1995; Cole et al., 1994; Kashina et al., 1996). Such an arrangement allows kinesin-5 motors to crosslink and slide apart antiparallel microtubules, a behavior that has been directly observed in vitro (Kapitein et al., 2005). Eg5 is a relatively slow motor, moving at ~2-3um/min, and has been shown to be moderately processive (Cole et al., 1994; Kwok et al., 2006; Sawin et al., 1992). This is in contrast to other mitotic motors, notably dynein, which is capable of rapid minus end-directed microtubule-based transport in vitro (~75um/min) (Paschal et al., 1987), although dynein-dependent transport within the spindle is considerably slower (~6um/min) (Heald et al., 1996; Rusan et al., 2002). The biophysical properties of Eg5 in vitro (Kapitein et al., 2008; Kapitein et al., 2005; Valentine and Gilbert, 2007) will not be reviewed here.
Contribution of Kinesin-5 motors to the establishment and maintenance of bipolar spindles
In every model system analyzed, with the exception of C. elegans (Bishop et al., 2005), spindle assembly requires kinesin-5 activity (Bannigan et al., 2007; Blangy et al., 1995; Heck et al., 1993; Hoyt et al., 1992; Le Guellec et al., 1991; Reddy and Day, 2001; Roof et al., 1992; Sawin et al., 1992). In fungi, kinesin-5 motor activity is additionally required for maintenance of a bipolar spindle prior to anaphase and elongation of the spindle during anaphase B (Hoyt, 1994). In S. cerevisiae, for example, previously separated spindle pole bodies collapse in response to kinesin-5 inhibition (Saunders and Hoyt, 1992).
Similar to fungi, kinesin-5 orthologs in Xenopus and Drosophila contribute to both spindle assembly and maintenance. Addition of monastrol to Xenopus bipolar spindles induces rapid collapse of the bipolar array into a monopole. In this system, poles move together at a rate of ~1um/min although spindles that are ‘trapped’ between the coverslip and slide shortened more slowly (Kapoor et al., 2000). In Drosophila embryos, the kinesin-5, KLP61F, is required to maintain metaphase spindle length and to drive anaphase B spindle elongation (Sharp et al., 2000) (Sharp et al., 1999). In this system, bipolar spindles in cells injected with anti-KLP61F antibodies collapsed into monopoles at a rate of 5.7um/min (Sharp et al., 1999). However, in Drosophila embryos, centrosome movement to opposite sides of the nuclear envelope (prior to NEB) proceeds even in the presence of anti-KLP61F antibodies (Sharp et al., 1999). In these prophase cells, initial centrosome separation requires cytoplasmic dynein (Robinson et al., 1999). Mechanistically, cortical dynein pulling on astral microtubules is thought to generate outward forces that drive centrosome separation (Sharp et al., 1999); similar processes may operate in mammalian cells as well (Vaisberg et al., 1993). In the C. elegans embryo, inhibition of BMK-1, the sole kinesin-5 in this organism, does not block mitosis. In these cells, BMK-1 functions to modulate strong cortical pulling forces, acting as a brake, thus restricting centrosome separation (Saunders et al., 2007) (Grill et al., 2003).
In cultured mammalian cells, kinesin-5 activity is required for the establishment of a bipolar spindle, but the contribution of Eg5 to maintenance of bipolar spindle morphology is somewhat less clear. In BS-C1 and HeLa cells containing bipolar spindles, addition of monastrol (Mayer et al., 1999) or anti-Eg5 antibodies, respectively, did not result in spindle collapse (Blangy et al., 1995; Kapoor et al., 2000). In LLC-Pk1 epithelial cells expressing GFP-tubulin (Rusan et al., 2001), however, addition of monastrol to metaphase spindles results in spindle shortening though not complete collapse to a monopolar phenotype (Ferenz et al., 2009). Although it is possible that different cell lines respond differentially to inhibition of Eg5, an alternative possibility is that the modest spindle shortening (~30%) apparent in LLC-Pk1 cells was overlooked in the earlier studies. Taken together, these results show that Eg5 is required to establish and maintain spindle bipolarity, but in mammalian cells additional plus-end directed motor proteins likely aid in such maintenance. These results paired with the localization of Eg5 to antiparallel microtubules also imply that kinesin-5 contributes to bipolar spindle assembly and maintenance by generating an outward force.
Contribution of kinesin-5 motors to the balance of forces in the mitotic spindle
The pioneering studies of kinesin-5 motors in fungi further demonstrated that these motors can be counteracted by opposing forces; in other words, kinesin-5 motors were capable of engaging in antagonistic relationships. The first example of such antagonistic activity was the observation that mutations in the S. cerevisiae minus-end directed kinesin Kar3 could partially suppress the collapsed spindle phenotype resulting from loss of Cin8p and Kip1p (Saunders and Hoyt, 1992). Similar situations have been demonstrated in additional fungal and animal systems (Mountain et al., 1999; O’Connell et al., 1993; Pidoux et al., 1996; Sharp et al., 1999). Importantly, motor proteins capable of antagonizing kinesin-5 proteins are not limited to the Kar3 type. For example, the loss or inhibition of Eg5 can be rescued through loss or inhibition of dynein (Ferenz et al., 2009; Gaglio et al., 1996; Mitchison et al., 2005; Tanenbaum et al., 2008).
Although the existence of a balance of forces in the metaphase spindle is now well established, the site, or sites, where forces are generated has not been examined, in part due to the difficulties in determining where motors are active within the live cell. To address this issue, we recently used a spindle assembly assay (Tulu et al., 2006) to test the hypothesis that antagonistic motors generate force at overlapping antiparallel microtubules. In these experiments, the initial distance between centrosomes, and hence the degree of overlap between antiparallel microtubules, is highly variable and can therefore be used to test the effects of motor protein inhibition on the outcome of spindle assembly. We predicted, and confirmed, that Eg5 inhibited cells formed bipolar not monopolar arrays when spindle assembly initiated with well-separated (>5.5 um) centrosomes. The results of our live cell studies, paired with in silico modeling, support our hypothesis that Eg5-based antagonistic motor activity requires overlapping antiparallel microtubules in the mammalian mitotic spindle (Ferenz et al., 2009). Additional experiments in other model systems are needed to ascertain if this model is generally applicable.
Contribution of Kinesin-5 motors to spindle flux
Following spindle formation, microtubule marking experiments have revealed the presence of a unique form of microtubule motion, called spindle flux, that results from the coordinated addition and loss of tubulin subunits from opposite ends of spindle microtubules (Mitchison, 1989). With the exception of yeast (Maddox and Salmon, 2000), flux has been observed in all eukaryotic systems examined to date (at rates between 0.5 and 3.0 um/min) during both metaphase and anaphase (Desai et al., 1998; Dhonukshe et al., 2006; LaFountain et al., 2004; Maddox et al., 2002; Mitchison, 1989; Mitchison and Salmon, 1992; Sawin and Mitchison, 1991; Zhai et al., 1995). Motion that is consistent with flux is also apparent in mammalian prophase cells (Ferenz and Wadsworth, 2007). Though inhibition of flux in human tissue culture cells does not prevent mitotic progression, it does drastically increase the number of lagging anaphase chromosomes (Ganem et al., 2005). This is consistent with the known relationship between poleward flux and anaphase chromosome-to-pole motion (Rogers et al., 2005; Zhai et al., 1995) and more recent data indicating that flux is responsible for the temporal synchrony of chromosome segregation (Matos et al., 2009). In human cells, flux may additionally make a contribution to centrosome separation (Toso et al., 2009).
Given the ability of kinesin-5 motors to crosslink and slide antiparallel microtubules, the presence of these motors at the spindle midzone ideally positions them to contribute to spindle flux. In favor of this possibility, inhibition of Eg5 eliminates flux in metaphase spindles assembled in Xenopus egg extracts (Miyamoto et al., 2004; Shirasu-Hiza et al., 2004). In the same system, displacement of the flux depolymerase from spindle poles results in spindle elongation at rates consistent with Eg5-mediated microtubule-microtubule sliding (Gaetz and Kapoor, 2004). In contrast to these results, however, inhibition of Eg5 with monastrol in metaphase Ptk or LLC-Pk1 cells results in only a modest reduction in flux (Cameron et al., 2006; Ferenz and Wadsworth, 2007). Furthermore, flux remains operational in mammalian monopolar spindles that lack antiparallel microtubules (Cameron et al., 2006; Ferenz and Wadsworth, 2007) implying that the mechanistic contribution kinesin-5 makes to flux may vary by model system. Because centrosomes can be the site of force generation in mammalian cells (Waters et al., 1996), flux has been modeled as a feeder/chipper in which Eg5 (located at spindle poles) feeds microtubules to a depolymerase, also localized at spindle poles (Cassimeris, 2004). The differential activity of Eg5 regarding flux in Xenopus and mammalian systems may relate to the high proportion of antiparallel microtubules found in Xenopus spindles.
Unexpectedly, inhibition of Eg5 did not reduce the rate of flux in prophase cells. Instead, the frequency of poleward (P) vs away-from-the-pole (AP) motion was altered following inhibition of Eg5 (from ~54% P motion in control to ~12% in monastrol treated prophase cells) (Ferenz and Wadsworth, 2007). These results support the possibility that Eg5 additionally functions to cross-link and tether spindle microtubules (Kapitein et al., 2005). If Eg5 is the dominant cross-linker in prophase spindles, then treatment with monastrol could result in microtubules becoming untethered, leading to the increase in AP motions of microtubules driven by the action of antagonistic motors (Ferenz and Wadsworth, 2007). In contrast, other proteins may function redundantly with Eg5 to tether microtubules in metaphase cells, so loss of Eg5 function would not result in an increase in the frequency of AP motion (Ferenz and Wadsworth, 2007).
Mitotic localization and regulation of kinesin-5 motors
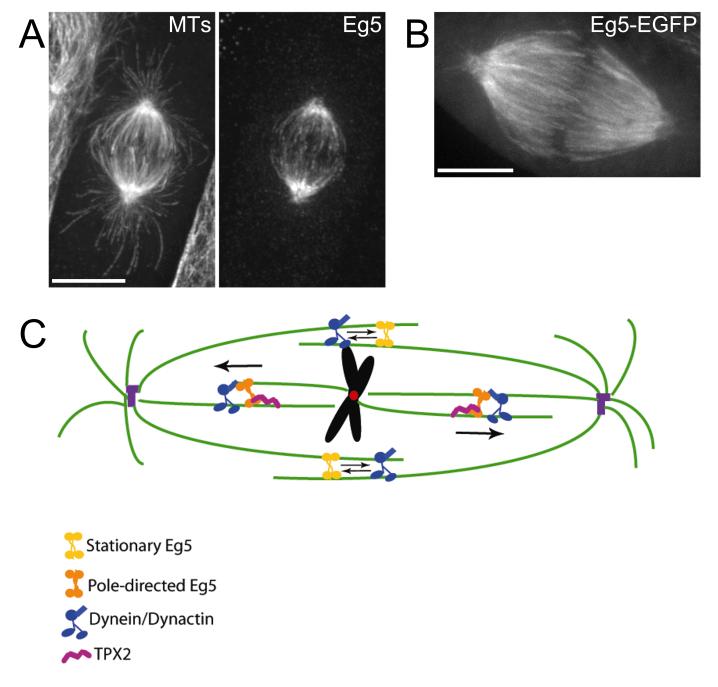
Kinesin-5 proteins localize to spindle microtubules, with an enrichment at centrosomes or spindle pole bodies, but are not detectable on astral microtubules (Figure 1). Although the spindle localization is consistent with a mitotic function, the concentration of the protein at spindle poles rather than at regions of microtubule overlap is somewhat unexpected. However, this observation is consistent with the possibility that kinein-5 functions on both parallel, and antiparallel microtubules (Kapitein et al., 2005; van den Wildenberg et al., 2008). This possibility is supported by observations showing that inhibition of kinesin-5 leads to disruption of centrosomes and spindle poles (Groen et al., 2008).
Figure1.
Localization and activity of Eg5 in mammalian cells. (A) Indirect immunofluorescence of an LLC-Pk1 cell stained with antibodies to tubulin (MTs) and Eg5. Notice that Eg5 is enriched near spindle poles and excluded from astral microtubules. (B) Image of a living LLC-Pk1 cell expressing Eg5-EGFP. Bars = 10 um. (C) Model showing Eg5 activity in the mitotic spindle. Static Eg5 at the spindle equator (yellow) crosslinks antiparallel microtubules and generates a force that opposes dynein/dynactin (blue). In the half spindle, interactions with TPX2 (purple) may target Eg5 (yellow) to parallel microtubules and interactions with dynein may promote poleward motion.
During interphase, Eg5 is diffusely cytoplasmic. What is the cue that transitions Eg5 to its mitotic location? Kinesin-5 family members were originally referred as to BimC motors because sequence comparisons showed similarity between BimC and its orthologs in the motor region and in a conserved region in the C-terminal tail, referred to as the ‘BimC box’, which contains a consensus site for phosphorylation by Cdk1 (Blangy et al., 1995). Mutation of a conserved threonine to alanine within the BimC box prevents motor localization to the spindle, demonstrating that the association of Eg5 with spindle microtubules is regulated by cell-cycle dependent phosphorylation (Blangy et al., 1995; Cahu et al., 2008; Sawin and Mitchison, 1995). In addition to Cdk1, recent work shows that the NIMA-family kinase, Nek6, phosphorylates a small fraction of Eg5 and rescue experiments show that this phosphorylation is important for the mitotic function of Eg5 (Rapley et al 2008). Finally, in C. elegans, the Eg5 ortholog BMK-1 (which lacks the conserved threonine in the BimC box) interacts with and is phosphorylated by the aurora B kinase, AIR-2, in the C-terminal tail domain and this interaction is important for the localization of BMK-1 to spindle microtubules (Bishop et al., 2005).
Dynamics of kinesin-5 motors
Although genetic and biochemical approaches have provided important information about the mitotic functions of molecular motors, an ultimate goal is to visualize the dynamic behavior and interactions of spindle components in live cells. Several recent studies have provided such information for kinesin-5 motors in live cells. In yeast, which have an intranuclear spindle composed of relatively few microtubules, Cin8p contributes to chromosome congression by stimulating catastrophe transitions at microtubule plus ends (Gardner et al., 2008). In Xenopus, photoactivation studies show that Eg5 is static in the spindle midzone, consistent with a role for the motor in sliding antiparallel microtubules (Uteng et al., 2008). However, in the half-spindle, Eg5 was observed to move poleward in a dynein-dependent manner (Uteng et al., 2008). In Drosophila embryos, fluorescence recovery after photobleaching and fluorescence speckle microscopy have shown that KLP61F-GFP turns-over very rapidly, both near the equator and poles, and can undergo short poleward runs in the half-spindle (Cheerambathur et al., 2008). The dynamic behavior of KLP61F is similar to the rapid turnover of microtubules in these cells and support a model in which kinesin-5 motors can both crosslink and slide antiparallel microtubules (Cheerambathur et al., 2008).
The dynamic behavior of kinesin-5 motors is likely regulated by interactions with microtubules and with other spindle components. Eg5 has been shown to bind to the p150 subunit of dynactin, to the spindle assembly factor TPX2 and is a component of the HURP complex (Blangy et al., 1997; Eckerdt et al., 2008; Koffa et al., 2006). How might these interactions contribute to Eg5 function? One possibility is that binding of Eg5 to two antiparallel microtubules in the spindle midzone may restrict interactions with other spindle components. Conversely, an interaction of Eg5 with the p150 subunit of dynactin in the half spindle could promote poleward transport of Eg5 that was not actively generating outward forces (Uteng et al., 2008). Similarly, an interaction between Eg5 and TPX2 (which is enriched toward spindle poles) may promote the interaction of Eg5 with parallel microtubules (Figure 2). The phosphorylation state of Eg5, and its binding partners, are likely to contribute to the regulation of these interactions. Additional experiments in live cells throughout mitosis are necessary to understand the regulation of Eg5 in mitotic cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bannigan A, Scheible W, Lukowitz W, Fagerstrom C, Wadsworth P, Somerville C, Baskin TI. A conserved role for kinesin-5 in plant mitosis. J. Cell Sci. 2007;120:1–6. doi: 10.1242/jcs.009506. [DOI] [PubMed] [Google Scholar]
- Bishop JD, Han Z, Schumacher JM. The Caenorhabditis elegans Aurora B kinase AIR-2 phosphorylates and is required for the localization of a BimC kinesin to meiotic and mitotic spindles. Mol. Bio. Cell. 2005;16:742–756. doi: 10.1091/mbc.E04-08-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangy A, Arnaud L, Nigg EA. Phosphorylation by p34cdc2 protein kinase regulates binding of the kinesin related motor HsEg5 to the dynactin subunit p150glued. J. Biol. Chem. 1997;272:19418–19424. doi: 10.1074/jbc.272.31.19418. [DOI] [PubMed] [Google Scholar]
- Blangy A, Lane HA, d’Herin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Cahu J, Olichon A, Hentrich C, Schek H, Drinjakovic J, Zhang C, Doherty-Kirby A, Lajoie G, Surrey T. Phosphorylation by Cdk1 increases the binding of Eg5 to microtubules in vitro and in Xenopus egg extract spindles. PLos ONE. 2008;3:e3936. doi: 10.1371/journal.pone.0003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LA, Yang G, Cimini D, Canman JC, Kisurina-Evgenieva O, Khodjakov A, Danuser G, Salmon ED. Kinesin 5-independent poleward flux of kinetochore microtubules in PtK1 cells. J. Cell Biol. 2006;173:173–179. doi: 10.1083/jcb.200601075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L. Cell division: Eg’ing on microtubule flux. Curr. Biol. 2004;14:R1000–R1002. doi: 10.1016/j.cub.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Cheerambathur DK, Brust-Mascher I, Civelekoglu-Scholey G, Scholey JM. Dynamic partitioning of mitotic kinesin-5 cross-linkers between microtubule-bound and freely diffusing states. J. Cell Biol. 2008;182:429–436. doi: 10.1083/jcb.200804100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DG, Saxton WM, Sheehan KB, Scholey JM. A “Slow” homotetrameric kinesin-related motor protein purified from Drosophila embryos. J. Biol. Chem. 1994;269:22913–22916. [PMC free article] [PubMed] [Google Scholar]
- Desai A, Maddox PS, Mitchison TJ, Salmon ED. Anaphase chromosome movement and poleward spindle flux occur at similar rates in Xenopus extract spindles. J. Cell Biol. 1998;141:703–713. doi: 10.1083/jcb.141.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Vischer N, Gadella TWJ., Jr. Contribution of microtubule growth polarity and flux to spindle assembly and functioning in plant cells. J. Cell Sci. 2006;119:3193–3205. doi: 10.1242/jcs.03048. [DOI] [PubMed] [Google Scholar]
- Eckerdt F, Eyers PA, Lewellyn AL, Prigent C, Maller JL. Spindle pole regulation by a discrete Eg5-interacting domain in TPX2. Curr. Biol. 2008;18:519–525. doi: 10.1016/j.cub.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enos AP, Morris NR. Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell. 1990;60:1019–1027. doi: 10.1016/0092-8674(90)90350-n. [DOI] [PubMed] [Google Scholar]
- Ferenz N, Paul R, Fagerstrom C, Mogilner A, Wadsworth P. Dynein antagonizes Eg5 by crosslinking and sliding antiparallel microtubules. Curr. Biol. 2009 doi: 10.1016/j.cub.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenz NP, Wadsworth P. Prophase microtubule arrays undergo flux-like behavior in mammalian cells. Molec. Biol. Cell. 2007;18:3993–4002. doi: 10.1091/mbc.E07-05-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz J, Kapoor TM. Dynein/dynactin regulate metaphase spindle length by targeting depolymerizing activities to spindle poles. J. Cell Biol. 2004;166:465–471. doi: 10.1083/jcb.200404015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaglio T, Saredi A, Bingham JB, Hasbani MJ, Gill SR, Schroer TA, Compton DA. Opposing motor activities are required for the organization of the mammalian mitotic spindle pole. J. Cell Biol. 1996;135:399–414. doi: 10.1083/jcb.135.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem NJ, Upton K, Compton DA. Efficient mitosis in human cells lacking polewards microtubule flux. Curr. Biol. 2005;15:1827–1832. doi: 10.1016/j.cub.2005.08.065. [DOI] [PubMed] [Google Scholar]
- Gardner MK, Bouck DC, Paliulis LV, Meehl JB, O’Toole ET, Haase J, Soubry A, Joglekar AP, Winey M, Salmon ED, Bloom K, Odde DJ. Chromosome congression by kinesin-5 motor-mediated disassembly of longer kinetochore microtubules. Cell. 2008;135:894–906. doi: 10.1016/j.cell.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill SW, Howard J, Schaffer E, Stelzer EHK, Hyman A. The distribution of active force generators controls mitotic spindle position. Science. 2003;301:518–521. doi: 10.1126/science.1086560. [DOI] [PubMed] [Google Scholar]
- Groen AC, Needleman D, Brangwynne C, Gradinaru C, Fowler B, Mazitschek R, Mitchison TJ. A novel small-molecule inhibitor reveals a possible role of kinesin-5 in anastral spindle-pole assembly. J. Cell Sci. 2008;121:2293–2300. doi: 10.1242/jcs.024018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I, Yanagida M. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature. 1990;347:563–566. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Heck MMS, Pereira A, Pesavento P, Yannoni Y, Spradling AC, Goldstein LSB. The kinesin-like protein KLP61F is essential for mitosis in Drosophila. J. Cell Biol. 1993;123:665–679. doi: 10.1083/jcb.123.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt MA. Cellular roles of kinesin and related proteins. Curr. Op. Cell Biol. 1994;6:63–68. doi: 10.1016/0955-0674(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Hoyt MA, He L, Loo KK, Saunders WS. Two Saccharomyces cerevisiae kinesin-rrelated gene products required for mitotic spindle assembly. J. Cell Biol. 1992;118:109–120. doi: 10.1083/jcb.118.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Kwok BH, Weinger JS, Schnmidt CF, Kapoor TM, Peterman EJG. Microtubule cross-linking triggers the directional motility of kinesin-5. J. Cell Biol. 2008 doi: 10.1083/jcb.200801145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Peterman EJG, Kwok BH, Kim JH, Kapoor TM, Schmidt CF. The bipolar mitotic kinesin Eg5 moves on both microtubles that it crosslinks. Nature. 2005;435:114–118. doi: 10.1038/nature03503. [DOI] [PubMed] [Google Scholar]
- Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 2000:975–988. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashina AS, Baskin RJ, Cle DG, Wedaman KP, Saxton WM, Scholey JM. A bipolar kinesin. Nature. 1996;379:270–272. doi: 10.1038/379270a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffa MD, Casanova CM, Santarella R, Kocher T, Wilm M, Mattaj IW. HURP is part of a ran-dependent complex involved in spindle formation. Curr. Biol. 2006;16:743–754. doi: 10.1016/j.cub.2006.03.056. [DOI] [PubMed] [Google Scholar]
- Kwok BH, Kapitein LC, Kim JH, Peterman EJG, Schmidt CF, Kapoor TM. Allosteric inhibition of kinesin-5 modulates its processive directional motility. Nat. Chem. Biol. 2006;2:480–485. doi: 10.1038/nchembio812. [DOI] [PubMed] [Google Scholar]
- LaFountain JR, Jr., Cohan CS, Siegel AJ, LaFountain DJ. Direct visualization of microtubule flux during metaphase and anaphase in crane-fly spermatocytes. Mol. Biol. Cell. 2004;15:5724–5732. doi: 10.1091/mbc.E04-08-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence CJ, Dawe RK, Christie KR, Cleveland DW, Dawson SC, Endow SA, Goldstein LSB, Goodson HV, Hirokawa N, Howard J, Malmberg RL, McIntosh JR, Miki H, Mitchison TJ, Okada Y, Reddy ASN, Saxton WM, Schliwa M, Scholey JM, Vale RD, Walczak CE, Wordeman L. A standardized kinesin nomenclature. J. Cell Biol. 2004;167:19–22. doi: 10.1083/jcb.200408113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guellec R, Paris RB, Couturier A, Roghi C, Phillipe M. Mol. Cell Biol. 1991;11:3395–3398. doi: 10.1128/mcb.11.6.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox P, Desai A, Oegema K, Mitchison TJ, Salmon ED. Poleward microtubule flux is a major component of spindle dynamics and anaphase a in mitotic Drosophila embryos. Curr. Biol. 2002;12:1670–1674. doi: 10.1016/s0960-9822(02)01183-1. [DOI] [PubMed] [Google Scholar]
- Maddox P, Salmon ED. The polarity and dynamics of microtubule assembly in the budding yeast Saccharomyces cerevisiae. Nat. Cell Biol. 2000;2:36–41. doi: 10.1038/71357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos I, Pereira AJ, Lince-Faria M, Cameron LA, Salmon ED, Maiato H. Synchronizing chromosome segregation by flux-dependent force equalization at kinetochores. J. Cell Biol. 2009;186:11–26. doi: 10.1083/jcb.200904153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- Mitchison T, Maddox P, Gaetz J, Groen A, Shirasu M, Desai A, Salmon ED, Kapoor TM. Roles of polymerization dynamics, opposed motors, and a tensile element in governing the length of Xenopus extract meiotic spindles. Mol. Biol. Cell. 2005;16:3064–3076. doi: 10.1091/mbc.E05-02-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J. Cell Biol. 1989;109:637–652. doi: 10.1083/jcb.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Salmon ED. Poleward kinetochore fiber movement occurs during both metaphase and anaphase-A in newt lung cell mitosis. J. Cell Biol. 1992;119:569–582. doi: 10.1083/jcb.119.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto DT, Perlman Z, Burbank KS, Groen AC, Mitchison TJ. The kinesin Eg5 drives poleward microtubule flux in Xenopus laevis egg extract spindles. J. Cell Biol. 2004;167:813–818. doi: 10.1083/jcb.200407126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NR. Mitotic mutants of Aspergillis nidulans. Genet. Res. 1976;26:237–254. doi: 10.1017/s0016672300016049. [DOI] [PubMed] [Google Scholar]
- Mountain V, Simerly C, Howard L, Ando A, Schatten G, Compton DA. The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. J. Cell Biol. 1999;147:351–365. doi: 10.1083/jcb.147.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell MJ, Meluh PB, Rose MD, Morris NR. Supprression of the bimC mitotic spindle defect by deletion of klpA, a kar43 related kinesin like protein in Aspergillus nidulans. J. Cell Biol. 1993;120:153–162. doi: 10.1083/jcb.120.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal BM, Shpetner HS, Vallee RB. Map1c is a microtubule activated ATPase which translocates microtubules in vitro and has dynein like properties. J. Cell Biol. 1987;105:1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux AL, LeDizet M, Cande WZ. Fission yeast pkl1 is a kinesin-related protein involved in mitotic spindle function. Mol. Bio. Cell. 1996;7:1639–1655. doi: 10.1091/mbc.7.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AS, Day IS. Kinesin in the Arabidopsis genome: A comparative analysis among eukaryotes. BMC Genomics. 2001;2:2–14. doi: 10.1186/1471-2164-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JT, Wojcik EJ, Sanders MA, McGrail M, Hays TS. Cytoplasmic dynein is required for the nuclear attachment and migration of centrosomes during mitosis in Drosophila. J. Cell Biol. 1999;146:597–608. doi: 10.1083/jcb.146.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GC, Rogers SL, Sharp DJ. Spindle microtubules in flux. J. Cell Sci. 2005;118:1105–1116. doi: 10.1242/jcs.02284. [DOI] [PubMed] [Google Scholar]
- Roof DM, Meluh PB, Rose MD. Kinesin-related proteins required for the assembly of the mitotic spindle. J. Cell Biol. 1992;118:95–108. doi: 10.1083/jcb.118.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusan N, Tulu US, Fagerstrom C, Wadsworth P. Microtubule rearrangment in prophase/prometaphase cells requires cytoplasmic dynein. J. Cell Biol. 2002;158:997–1003. doi: 10.1083/jcb.200204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusan NM, Fagerstrom C, Yvon AC, Wadsworth P. Cell cycle dependent changes in microtubule dynamics in living cells expressing GFP-alpha tubulin. Mol. Biol. Cell. 2001;12:971–980. doi: 10.1091/mbc.12.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AM, Powers J, Strome S, Saxton WM. Kinesin-5 acts as a brake in anaphase spindle elongation. Curr. Biol. 2007;17:R453–R454. doi: 10.1016/j.cub.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders WS, Hoyt MA. Kinesin-related proteins required for the structural integrity of the mitotic spindle. Cell. 1992;70:451–458. doi: 10.1016/0092-8674(92)90169-d. [DOI] [PubMed] [Google Scholar]
- Sawin KE, LeGuellec K, Philippe M, Mitchison TJ. Mitotic spindle organization by a plus-end directed microtubule motor. Nature. 1992;359:540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- Sawin KE, Mitchison TJ. Poleward microtubule flux in mitotic spindles assembled in vitro. J. Cell Biol. 1991;112:941–954. doi: 10.1083/jcb.112.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE, Mitchison TJ. Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle. Proc. Natl. Acad. Sci. USA. 1995;92:4289–4293. doi: 10.1073/pnas.92.10.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Rogers GC, Scholey JM. Microtubule motors in mitosis. Nature. 2000;407:41–47. doi: 10.1038/35024000. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Yu KR, Sisson JC, Sullivan W, Scholey JM. Antagonistic microtubule-sliding motors position mitotic centrosomes in Drosophila early embryos. Nat Cell Bio. 1999;1:51–54. doi: 10.1038/9025. [DOI] [PubMed] [Google Scholar]
- Shirasu-Hiza M, Perlman ZE, Wittmann T, Karsenti E, Mitchison TJ. Eg5 causes elongation of meiotic spindles when flux-associated microtubule depolymerization is blocked. Curr. Biol. 2004;14:1941–1945. doi: 10.1016/j.cub.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Tanenbaum ME, Macurek L, Galjart N, Medema RH. Dynein, Lis1 and CLIP170 counteract Eg5-dependent centrosome separation during bipolar spindle assembly. EMBO. 2008;27:3235–3245. doi: 10.1038/emboj.2008.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toso A, Winter JR, Garrod AJ, Amaro AC, Meraldi P, McAinsh AD. Kinetochore-generated pushing forces separate centrosomes during bipolar spindle assembly. J. Cell Biol. 2009;184:365–372. doi: 10.1083/jcb.200809055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulu US, Fagerstrom C, Ferenz NP, Wadsworth P. Molecular requirements for kinetochore-associated microtubule formation in mammalian cells. Curr. Biol. 2006;16:536–541. doi: 10.1016/j.cub.2006.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uteng M, Hentrich C, Bieling P, Surrey T. Poleward transport of Eg5 by dynein-dynactin in Xenopus egg extract spindles. J. Cell Biol. 2008;182:715–726. doi: 10.1083/jcb.200801125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisberg EA, Koonce MP, McIntosh JR. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J. Cell Biol. 1993;123:849–858. doi: 10.1083/jcb.123.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine MT, Gilbert SP. To step or not to step? How biochemistry and mechanics influence processivity in kinesin and Eg5. Curr. Op. Cell Bio. 2007;19:75–81. doi: 10.1016/j.ceb.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wildenberg SMJL, Tao L, Kapitein LC, Schmidt CF, Scholey JM, Peterman EJG. The homotetrameric kinesin-5 KLP61F preferentially crosslinks microtubules into antiparallel orientations. Curr. Biol. 2008;18:1860–1864. doi: 10.1016/j.cub.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JC, Mitchison TJ, Rieder CL, Salmon ED. The kinetochore microtubule minus-end disassembly associated with poleward flux produces a force that can do work. Mol. Biol. Cell. 1996;7:1547–1558. doi: 10.1091/mbc.7.10.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y, Kronebusch PJ, Borisy GG. Kinetochore microtubule dynamics and the metaphase-anaphase transition. J. Cell Biol. 1995;131:721–734. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]