Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice (original) (raw)
. Author manuscript; available in PMC: 2011 Mar 25.
SUMMARY
Itch is the least well understood of all the somatic senses, and the neural circuits that underlie this sensation are poorly defined. Here we show that the atonal-related transcription factor Bhlhb5 is transiently expressed in the dorsal horn of the developing spinal cord and appears to play a role in the formation and regulation of pruritic (itch) circuits. Mice lacking Bhlhb5 develop self-inflicted skin lesions and show significantly enhanced scratching responses to pruritic agents. Through genetic fate-mapping and conditional ablation we provide evidence that the pruritic phenotype in Bhlhb5 mutants may be due to selective loss of a subset of inhibitory interneurons in the dorsal horn. Our findings suggest that Bhlhb5 is required for the survival of a specific population of inhibitory interneurons that regulate pruritis and provide evidence that the loss of inhibitory synaptic input results in abnormal itch.
INTRODUCTION
Both itch and pain serve crucial roles: they alert the organism to potential harm and trigger behavioral responses that prevent injury. However, our perceptual experience of these two sensations is qualitatively distinct, and our responses to them are very different. Itch is a skin-specific sensation that provokes the desire to scratch, thereby removing potentially harmful agents, such as a parasite, from the skin’s surface. In contrast, pain can occur in any part of the body and, when it originates from the skin, triggers withdrawal, thereby removing us from harm’s way (Yosipovitch et al., 2007). Both pain and itch can, however, become chronic pathological conditions (Ikoma et al., 2006). Unfortunately, effective treatment for chronic pain or itch is lacking, and progress in the development of new therapies is hampered by insufficient knowledge of the neural circuits that underlie these distinct sensations.
Our understanding of itch is limited, and the existence of itch-specific circuits is controversial (reviewed in (McMahon and Koltzenburg, 1992; Schmelz, 2008). It has been hypothesized, for example, that itch might be distinguished from pain based simply on the pattern of firing or rate of discharge of C-fiber primary sensory neurons because many C-fibers respond both to algesic (pain-inducing) and pruritic (itch-inducing) compounds (Simone et al., 2004). However, recent work has supported an alternative theory—a so-called labeled-line for itch, in which a specific subset of C-fibers selectively conveys the sensation of itch from the skin to the spinal cord, where it is relayed in a unique projection pathway to the brain. A subpopulation of primary sensory neurons that respond selectively to the pruritic agent histamine are present in humans (Schmelz et al., 1997), as are second-order spinal projection neurons that respond to histamine in the cat (Andrew and Craig, 2001). Additional evidence that itch and pain are mediated by distinct neurons has emerged recently with reports that gastrin releasing peptide receptor (GRPR)-expressing neurons in the spinal cord are specifically responsive to pruritic stimuli (Sun and Chen, 2007), and more compellingly that selective ablation of these neurons eliminates itch but not pain (Sun et al., 2009).
Despite these advances, pruritic circuitry remains poorly understood. In particular, although itch information is integrated and modulated within the dorsal horn of the spinal cord, the neurons involved in regulating itch await discovery. For instance, it is a common experience that inducing pain (e.g. by scratching) relieves itch (Ikoma et al., 2006). Conversely, relieving pain (e.g. with mu-opioids) induces itch (Umeuchi et al., 2003). The mutually antagonistic relationship between itch and pain suggests the involvement of inhibitory circuits within the dorsal spinal cord. However, such circuits have not been identified or characterized. A second type of modulation that occurs in the dorsal spinal cord is central sensitization, a type of sensory ‘learning’ involving long-lasting changes in circuit properties that increase pain sensitivity (Woolf, 1983). This type of sensory plasticity occurs through multiple mechanisms, including an increase in the excitability of spinal cord neurons that mediate pain as well as a reduction in inhibitory synaptic input onto these cells (disinhibition) (reviewed in (Latremoliere and Woolf, 2009). While central sensitization in response to itch (Ikoma et al., 2004), like that observed in response to pain, is thought to occur primarily in the dorsal spinal cord, the mechanisms involved are not known. Thus, there is a fundamental gap in knowledge with regard to the most basic spinal cord circuits that mediate pruritis.
Here we describe a mouse model involving mutation of the transcription factor Bhlhb5 that shows dramatically heightened responses to pruritic stimuli, enabling us to investigate the neural circuitry that underlies itch. Bhlhb5 is a neural-specific basic helix-loop-helix (bHLH) transcription factor related to the Drosophila proneural factor atonal (Ross et al., 2003). Compared to other well-studied members of this family, such as the NeuroD, Neurogenin and Olig transcription factors, little is known about the function of Bhlhb5 in the nervous system. Previous work has identified an important role for Bhlhb5 in the retina, where it is required for the survival of some amacrine and cone bipolar cells (Feng et al., 2006). In addition, Bhlhb5 regulates the acquisition of area-specific fates in the cortex (Joshi et al., 2008). However, the function of Bhlhb5 in somatosensory systems has not been investigated.
Within the spinal cord, Bhlhb5 is transiently expressed in subsets of post-mitotic neurons, suggesting a possible role in the assembly of spinal circuits. We investigated this idea by generating a series of mutant mice in which the function of the Bhlhb5 gene is disrupted. We show that animals lacking Bhlhb5 develop self-inflicted skin lesions and provide evidence that these lesions are due to heightened itch. To understand the molecular basis for this behavioral phenotype, we use a genetic fate mapping strategy in which Bhlhb5-expressing cells are permanently marked in vivo and uncover a crucial role for Bhlhb5 in the survival of a specific population of neurons in the superficial laminae of the dorsal horn. Furthermore, through conditional ablation, we provide evidence suggesting that loss of Bhlhb5 within inhibitory neurons in the dorsal horn results in the development of pathological skin lesions. Together, these data suggest a model in which disinhibition in the dorsal spinal cord results in abnormal itch.
RESULTS
Bhlhb5 is expressed within the dorsal horn of the spinal cord and mice lacking this factor develop skin lesions
To gain insight into the mechanisms that regulate neuronal circuit assembly in the spinal cord we examined Bhlhb5 expression in the developing spinal cord by immunohistochemistry using several newly-generated Bhlhb5-directed antibodies (Figures S1A, S1B and S1C). Bhlhb5 was observed in a sub-population of late-born neurons that migrate to the superficial layers of the dorsal horn (Figure 1A), as well as transiently expressed earlier in the embryo in V1, V2 and dI6 interneurons (Figures S1D and S1E), consistent with previous reports (Liu et al., 2007). Co-localization studies using glutamatergic and GABAergic neuronal markers (Lmx1b and Pax2), respectively (Cheng et al., 2005) revealed that approximately one-third of Bhlhb5-expressing neurons in the dorsal horn are excitatory and two-thirds inhibitory (Figures 1B and 1C). Expression of Bhlhb5 in the dorsal horn was prolonged, beginning shortly after the neurons exit mitosis (~E13.5) and persisting for up to two weeks postnatally. This extended expression of Bhlhb5 suggests a possible role for Bhlhb5 in the later aspects of neuronal differentiation, such as circuit assembly.
Figure 1. Bhlhb5 is transiently expressed in excitatory and inhibitory neurons in the dorsal spinal cord.
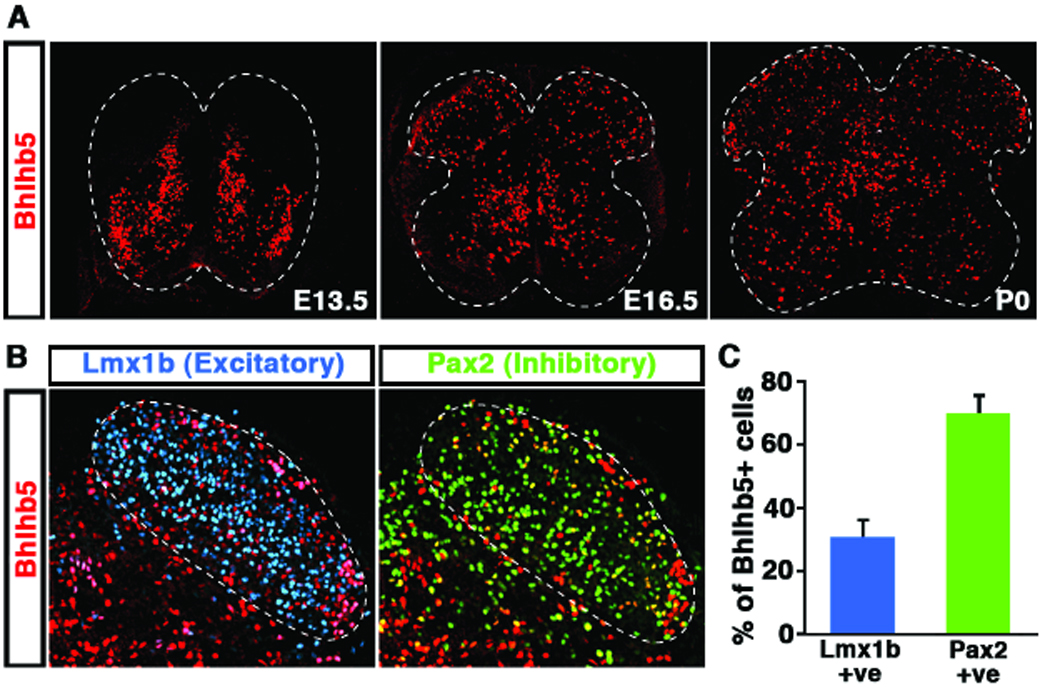
A) Expression of Bhlhb5 protein in the mouse spinal cord embryonic day (E) 13.5, E16.5 and postnatal day 0 (P0) using anti-Bhlhb5 antibodies. The generation of Bhlhb5 antibodies used in this study are described in Figures S1A, S1B and S1C. B) Co-staining of Bhlhb5 protein (red) with antibodies to the excitatory marker Lmx1b (blue) and the inhibitory marker Pax2 (green) in the dorsal horn of the spinal cord at P0. Dotted line denotes the dorsal horn region that was quantified in (C) C) Quantification of the proportion of Bhlhb5-expressing neurons (Bhlhb5+) that co-localize with either Lmx1b or Pax2 indicates that Bhlhb5 is expressed in both excitatory and inhibitory neurons of the dorsal spinal cord. (n = 3 littermate pairs, counting 10 matched lumbar sections/pair).
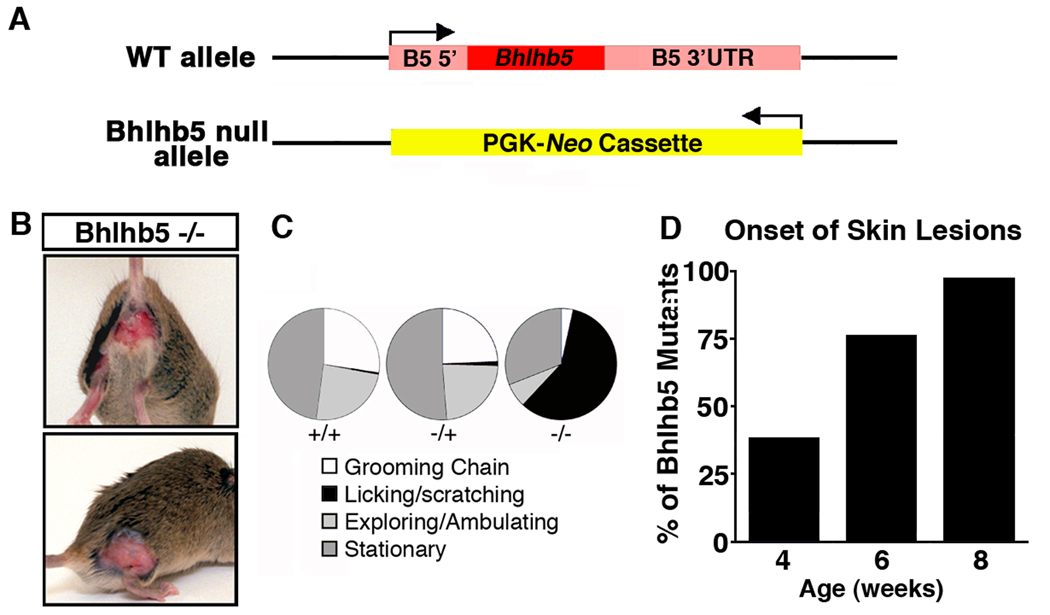
To investigate the function of Bhlhb5 in the developing spinal cord we generated a Bhlhb5 knockout mouse (Figure 2A and Figure S2). Constitutive loss of Bhlhb5 gave rise to mice that appeared to have severe somatosensory defects, as evidenced by self-inflicted skin lesions (Figure 2B). We observed these mice carefully and found that the skin lesions were due to excessive licking and scratching (Figure 2C). By four weeks of age, one third of Bhlhb5 mutants had developed skin lesions, and at eight weeks of age, skin lesions were observed in almost every Bhlhb5 knockout mouse (Figure 2D) irrespective of genetic background (129/Sv, C57Bl/6 and CD1). The skin lesions were frequently found on the perineum and the haunches (Figure S3A), but occasionally observed in many other regions, such as orafacial area. In contrast, skin lesions were never observed in wild type or heterozygous mice.
Figure 2. Loss of Bhlhb5 gives rise to mice that develop self-inflicted skin lesions due to excessive licking and scratching.
A) Schematic illustrating the Bhlhb5 knockout allele in which the Bhlhb5 gene is replaced by a neomycin expression cassette. Arrow indicates transcriptional start site. Also see Figure S2. B) Photos illustrating the skin lesions on Bhlhb5 knockout mice. C) Behavioral analysis of wild type (+/+), heterozygous (+/−) or Bhlhb5 null (−/−) with pre-existing skin lesions showing proportion of time spent at each activity over a 20-minute period indicates that Bhlhb5 −/− mice spend the majority of their time licking and scratching at the site of lesion, whereas wild type and heterozygous mice divide their time between normal head-to-toe grooming (grooming chain), exploring/ambulating or resting (stationary). Note that, prior to the onset of skin lesions, Bhlhb5 −/− mice do not display abnormal scratching or licking behavior. However, once a very small skin irritation develops, Bhlhb5 −/− mice lick and scratch persistently such that a skin lesion develops very soon thereafter. D) Time course of skin lesion onset reveals that the majority of Bhlhb5 mutants develop skin lesions between 4 and 8 weeks. Also see Figures S3A and S3D.
We analyzed the epidermis of Bhlhb5 knockout mice prior to the development of skin lesions but found that there was no evidence of abnormal innervation, as revealed by PGP9.5 staining (Figure S3B and S3C). Furthermore, electron micrographs of dorsal roots showed no evidence of neuropathy in Bhlhb5 mutants (Figure S3D).
Bhlhb5 −/− mice show heightened responses to itch-inducing agents
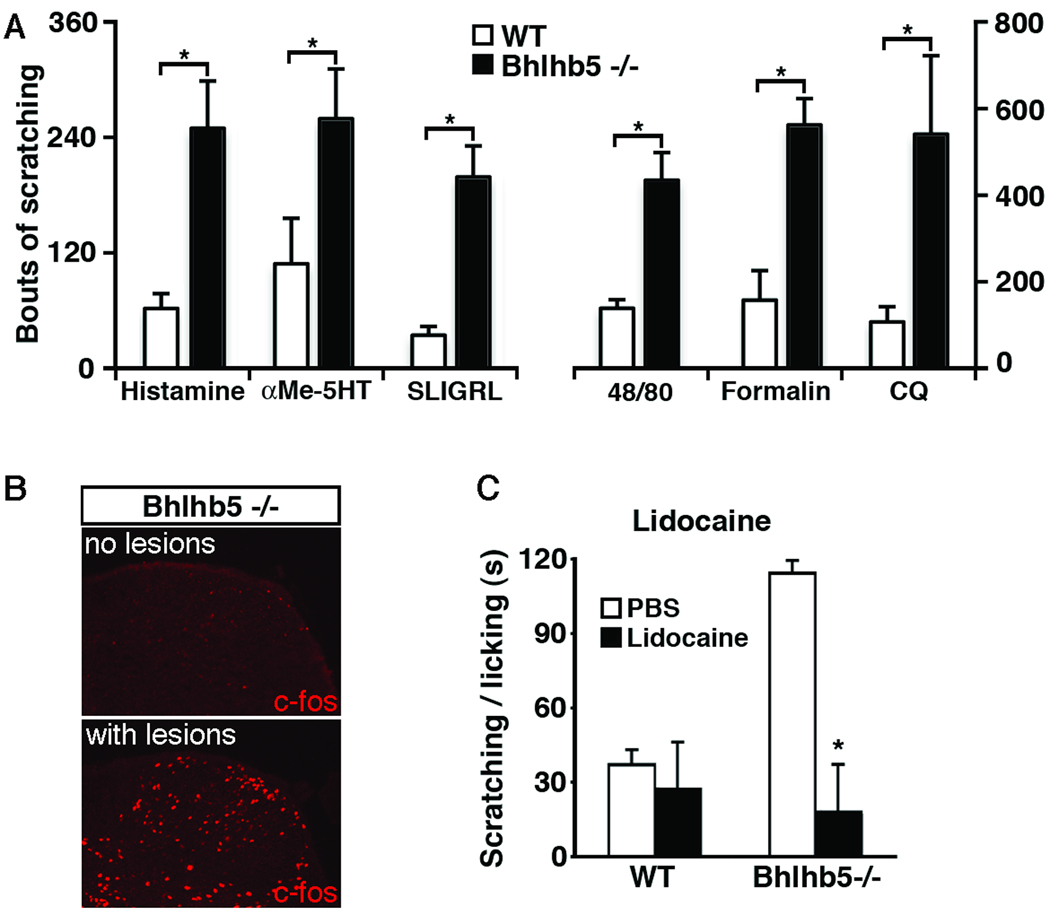
Based on the skin lesions and the scratching behavior observed in Bhlhb5 −/− mice, we hypothesized that these mice might be responding to an itch-like sensation. To test itch responsiveness, we examined the scratching behavior of mice that had received intradermal injections of pruritic agents into the nape of the neck. Because the presence of skin lesions was found to be a potentially confounding factor in the behavioral assays, (Figures S3F, S3G and S3H), these experiments were performed using young (four week-old) Bhlhb5 mutant mice, prior to the onset of skin lesions. Although the signaling events that initiate itch in the skin are not well understood, they occur through at least two independent pathways (Davidson et al., 2007). One mechanism is mediated by histamine receptors, which can be activated by histamine itself or by compound 48/80, a chemical that mediates histamine release from mast cells (Kuraishi et al., 1995). The second mechanism is mediated by the Protease-activated receptor2 (PAR2), which can be activated by peptide agonists such as SLIGRL-NH2 (Reddy et al., 2008). Bhlhb5 mutants showed elevated itch responses upon activation of either histamine-dependent or PAR2- dependent mechanisms (Figure 3A). Thus, multiple pruritogens, acting through at least two distinct mechanisms, give rise to significantly elevated itch responses in Bhlhb5 mutant mice. In addition, we tested α-methyl-5-hydroxytryptamine (αMe5HT), a serotonin analog that causes inflammation and pruritis when administered peripherally (Yamaguchi et al., 1999), chloroquine, an antimalarial drug that causes itch (as a side effect) through direct activation of sensory neurons (Sowunmi et al., 1989; Liu et al, 2009), and formalin, a pain-inducing chemical that has been found to elicit scratching when injected intradermally (Imamachi et al., 2009). Regardless of the pruritic agent used, Bhlhb5 −/− mice showed significantly more scratching behavior than wild type mice (Figure 3A).
Figure 3. Bhlhb5 −/− mice have elevated itch responses.
A) Bhlhb5 −/− mice show significantly enhanced scratching responses following the intradermal injection of pruritic agents into the nape of the neck. Agents tested were histamine, a serotonin analogue (αMe-5HT), a PAR2 agonist (SLIGRL-NH2), Compound 48/80 (48/80), formalin, and chloroquine (CQ). Experiments were performed with 4 – 5 littermate pairs that were 3 – 4 weeks old, prior to the development of skin lesions in Bhlhb5 −/− mice. Data are presented as mean ± SEM and * indicates significant difference relative to controls (p < 0.05, t-test) B) Abnormal expression of c-Fos in the dorsal horn corresponds to the site of skin lesions in Bhlhb5 −/− mice. Immunostaining with anti-c-Fos antibodies reveals elevated c-Fos expression in a segment of the dorsal horn that corresponds to the sites of a skin lesion (S1), but not in matched segment from a Bhlhb5 −/− mouse that lacks lesions. C) Local injection of lidocaine causes the abnormal scratching and licking behavior to attenuate in Bhlhb5 −/− mice. Bhlhb5 −/− mice with lesions of the perineum were used. All mice received an injection of either PBS (control) or lidocaine (to block sensory input) into the perineum. Injection of lidocaine in wild type mice had no effect on licking and scratching behavior.
The excessive scratching response observed in the Bhlhb5 −/− mice was consistent with the possibility that the skin lesions that occur in these mice might be due to a heightened sensation of itch. In support of this hypothesis, we found that the presence of skin lesions is associated with elevated neuronal activity in the dorsal horn, a region of the nervous system in which pruritic information is thought to be integrated. Specifically, the immediate-early gene c-fos was up-regulated in the dorsal spinal cord of Bhlhb5 mutants with skin lesions relative to Bhlhb5 mutant mice that lacked lesions (Figure 3B). Moreover, c-fos expression was observed in segments of the dorsal horn of spinal cord that correspond to the site of lesions, and not in other levels of the spinal cord (data not shown). Next, we reasoned that if Bhlhb5 −/− mutant mice with skin lesions were compelled to scratch by a sensation of itch then they would cease scratching if itch sensation was blocked. To test this idea, we used lidocaine to block sensory input from primary sensory neurons (Holstege, 2008). Strikingly, we found that loss of sensory input through the use of lidocaine completely attenuated the licking/scratching behavior directed toward the lesioned area (Figure 3C). Based on this finding, together with the observation that Bhlhb5 mutants respond excessively to itch-inducing stimuli, we theorize that the skin lesions are caused by a site-specific itch that leads to the abnormal scratching and/or licking behaviors. Although we cannot know with certainty how these mice are feeling, their scratching behavior is suggestive of itch. For simplicity, we refer hereafter to the sensation that is driving the scratching behavior as itch.
Bhlhb5 −/− mice have similar acute nociceptive responses but show evidence of enhanced central sensitization
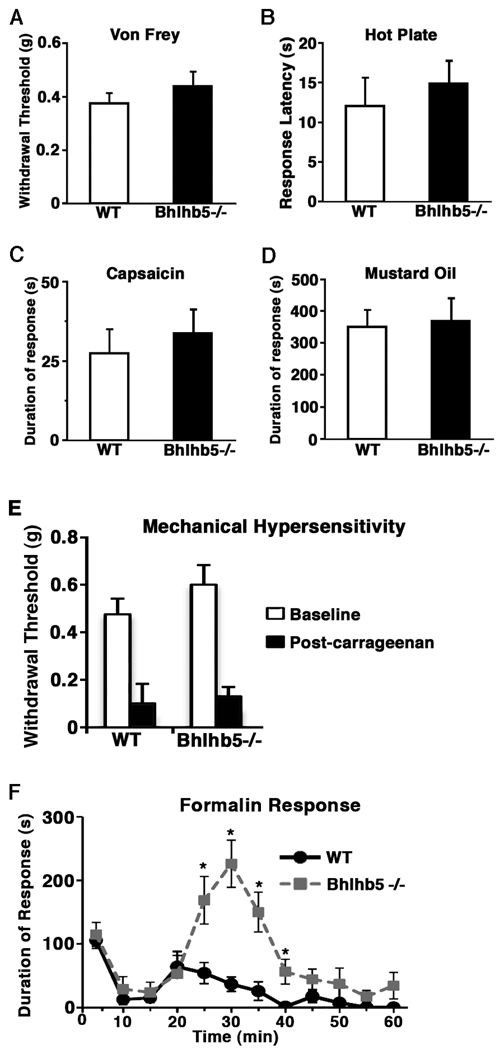
Next we investigated whether the Bhlhb5 −/− mice also respond abnormally to other somatosensory modalities such as touch and pain. Upon stimulation with von Frey filaments to measure mechanical sensitivity, wild type and Bhlhb5 −/− mice showed similar paw-withdrawal thresholds (Figure 4A). When placed on a hot plate, wild type and Bhlhb5−/− mice responded with a similar latency, indicating that thermal sensitivity is unchanged in Bhlhb5 mutants (Figure 4B). Similarly, wild type and Bhlhb5 mutant animals showed no significant differences in the duration of their paw-licking behavior upon the intraplantar injection of capsaicin or mustard oil, a TRPV1 and TRPA1 agonist respectively, indicating that loss of Bhlhb5 does not affect nociceptive responses to chemical algesics (Figures 4C and 4D). Finally, in assays for inflammation-induced mechanical hypersensitivity, no significant differences were observed between genotypes, both wild type and Bhlhb5 mutant littermates showed similar levels of heightened sensitivity following intraplantar injections of carrageenan, suggesting that mechanical allodynia is also unaffected by the loss of Bhlhb5 (Figure 4E). Together, these results suggest that the responses to various types of noxious sensory stimuli—mechanical, thermal and chemical— is not significantly different between wild type and Bhlhb5 mutants, at least in young (4-week old) mice.
Figure 4. Bhlhb5 −/− mice show evidence of enhanced central sensitization.
A) Mechanical sensitivity as assessed by measuring the withdrawal threshold upon application of von Frey fibers to the plantar surface of the hindpaw. B) Thermal sensitivity as assessed by measuring the response latency when placed on a 55°C hot plate. C–D) Acute nociception as assessed by measuring the duration of licking following the injection of capsaicin (C) or mustard oil (D) into the hindpaw. E) Mechanical hypersensitivity as assessed by measuring the withdrawal threshold prior to and two hours following the injection of carrageenan into the hindpaw. F) Upon injection of 5% formalin into the hindpaw, wild type (WT) and Bhlhb5 −/− mice show a similar response in the early phase (0 – 20 min); however, during the late phase (20 – 40 min), Bhlhb5 −/− mice show significantly more time licking their hindpaw, suggestive of enhanced central sensitization in these mice. Data are presented as mean ± SEM and * indicates a significant difference relative to controls at the same time point (p < 0.05, Mann-Whitney U-test). These experiments were performed with 6 – 8 littermate pairs that were 4 weeks old, prior to the development of skin lesions in Bhlhb5 −/− mice. (When older mice were used in these studies, Bhlhb5 −/− mice were found to have reduced responses in all behavioral assays, possibly due to the presence of skin lesions on these mice, which may have been a confounding factor (Figures S3E, S3F, and S3G).
While Bhlhb5 mutant mice showed no significant changes in their responses to most nociceptive sensory assays, the formalin test was a marked exception. In this paradigm, a biphasic behavioral response (primarily licking of the hindpaw) is seen in response to the intraplantar injection of 5% formalin. The immediate, early-phase response is due acute activation of TRPA1 expressing primary nociceptors (McNamara et al., 2007), whereas the late-phase is believed to be due to activity-dependent central sensitization (Tjolsen et al., 1992) as well as ongoing afferent input (Puig and Sorkin, 1996). Using this test we found that, although the response observed in the early-phase was similar between genotypes, the response observed during the late phase was significantly different. Specifically, the licking behavior during the second phase of the formalin test was almost 4-fold greater in Bhlhb5 mutants than in wild type littermates (Figure 4F).
Formalin is a reactive chemical that activates TRPA1, (McNamara et al., 2007) causing widespread tissue damage and inducing pain in animals and man (Dubuisson and Dennis, 1977). Many inflammatory mediators are released in response to formalin at doses used in the standard test, including ATP and H+ from leaking membranes, histamine from degranulated mast cells, and peptide agonists from activated proteases (Tjolsen et al., 1992). Thus, we hypothesized that, in addition to activating nociceptors, formalin treatment may also cause the release of agents that activate pruritoceptors. Consistent with this, formalin elicits primarily a scratching response rather than a wiping response when injected intradermally into the cheek (Figure S3E). This behavioral assay is thought to distinguish between pain and itch, since the noxious irritant capsaicin elicits wiping by the forepaw whereas the pruritogen histamine evokes scratching by the hindpaw (Shamida and Lamotte, 2008). Thus, the finding that formalin induces scratching in this assay suggests that formalin may elicit itch in addition to pain. If so, the heightened response to formalin observed in Bhlhb5 −/− mice might reflect, at least in part, an abnormal itch sensation. Alternatively, it is possible that the Bhlhb5 −/− phenotype may include a pain component, but if so, it appears to be restricted to formalin, and not heat, capsaicin, mustard oil or carrageenan.
Conditional ablation points to a possible role for the dorsal horn of the spinal cord in the abnormal itch of Bhlhb5 −/− mice
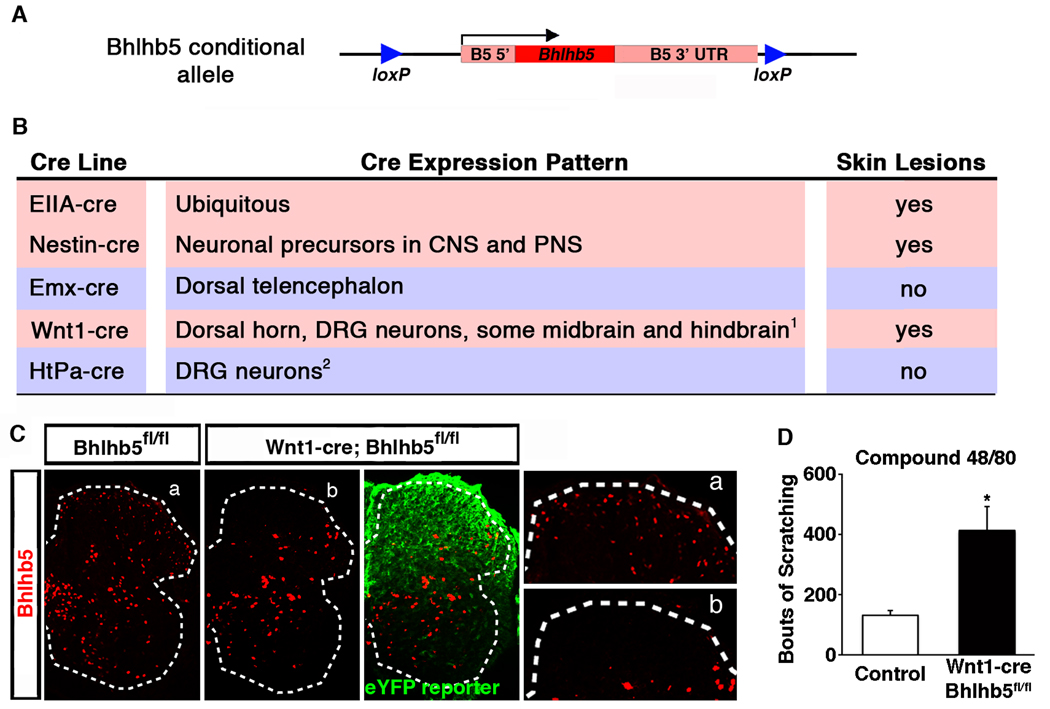
Since itch circuits are poorly characterized but are thought to involve numerous regions of the nervous system, we used conditional ablation to help identify the neurons whose dysfunction causes excessive itch in Bhlhb5 mutants. Specifically, we generated a Bhlhb5 allele that was flanked by loxP sites (Figures 5A and S4) and crossed it to a variety of cre-expressing mouse lines in which cre-mediated recombination occurs within discrete regions of the nervous system. The selective disruption of Bhlhb5 in neuronal precursors by crossing the conditional Bhlhb5 knockout to a Nestin-cre line (Tronche et al., 1999) gave rise to mice with skin lesions, suggesting that the scratching behavior in the Bhlhb5 −/− mice is neural in origin (Figure 5B) and excluding the possibility that the self-injurious grooming behavior in the mutant mice was due to topical dermatitis. One of the regions of the nervous system in which Bhlhb5 is highly expressed is in the dorsal telencephalon (Joshi et al., 2008). However, loss of Bhlhb5 in the dorsal telencephalon using the Emx-cre line (Gorski et al., 2003) was not sufficient for the development of skin lesions (Figure 5B), suggesting that higher cortical function was not involved in this phenotype. In contrast, the loss of Bhlhb5 resulting from Wnt1-driven cre expression (Rico et al., 2002) was found to be sufficient for the development of skin lesions (Figure 5B) and the incidence of these lesions was identical in frequency, location and severity to those observed in the Bhlhb5 knockout mouse (data not shown). Analysis of Bhlhb5 protein expression following Wnt1-cre-mediated recombination confirmed that Bhlhb5 was selectively lost from the dorsal but not the ventral horn of the spinal cord, as expected based on the distribution of Wnt1-cre-mediated reporter expression (Figure 5C). In addition, Wnt1-cre;Bhlhb5fl/fl mice showed significantly elevated scratching responses in response to the pruritic agent compound 48/80 (Figure 5D). Together these data indicate that loss of Bhlhb5 within the Wnt1-cre expression domain is responsible for the development of abnormal itch.
Figure 5. Conditional ablation points to a possible role of the dorsal horn in the abnormal itch in Bhlhb5 mutant mice.
A) Schematic illustrating the conditional Bhlhb5 knockout allele in which the Bhlhb5 gene is floxed by lox P sites (►), enabling cre-mediated recombination. Arrow indicates transcriptional start site. Also see Figure S4. B) Table listing the cre-expressing mouse lines used to cause selective ablation of Bhlhb5, together with the expression pattern of cre in each line and whether the subsequent animals develop skin lesions following cre-mediated recombination of Bhlhb5. 1Wnt1-cre also causes recombination in the cerebellum, and other neural crest-derived cells in addition to dorsal root ganglia (DRG) neurons. 2HtPa-cre causes recombination other neural crest-derived cells in addition to DRG neurons. Also see Figure S5. The expression pattern of each cre line was confirmed using cre-responsive reporter mice (data not shown). C) Wnt1-cre-mediated loss of Bhlhb5. Lumbar sections from P0 animals stained with antibodies for Bhlhb5 (red) and eYFP (green). Sections from control (left) and mutant mouse (right) are homozygous floxed at the Bhlhb5 locus (Bhlhb5fl/fl). In addition, the mutant mouse harbors the Wnt1-cre allele and the Rosa26 cre-responsive eYFP reporter (eYFP reporter). Insets are enlarged on right. eYFP staining reveals cells in which cre-mediated recombination has occurred. Note that Bhlhb5 staining is absent from the dorsal horn of mutants, but not the ventral horn. D) Conditional loss of Bhlhb5 in the Wnt1-cre expression domain results in significantly elevated scratching responses following the intradermal injection of compound 48/80 intradermally into the nape of the neck relative to homozygous floxed control mice. Data are presented as mean ± SEM and * indicates significant difference relative to controls (p < 0.05, t-test). Though conditional loss of Bhlhb5 pointed to a possible role of the dorsal horn in the abnormal itch, do gross defects in the dorsal spinal cord were observed in Bhlhb5 mutant mice (Figure S6).
Although Wnt1-cre causes recombination in several of regions in the nervous system (see Figure 5B), there were two regions in particular known to mediate pruritis: the dorsal root ganglia (DRG), which contains primary pruritoceptive neurons that initiate itch, and the dorsal horn of the spinal cord, which contains interneurons and projection neurons that integrate pruritic information and relay it to the brain (Sun et al., 2009). To help distinguish which of these two regions might be responsible for the self-injurious behavior in Bhlhb5 mutant mice, we used the HtPa-cre line, which results in the loss of Bhlhb5 in all neural crest derived cells (including all DRG neurons), but not in neurons of the spinal cord (Figure S5; Pietri et al., 2003). HtPa-cre-mediated ablation of Bhlhb5 was not sufficient, however, for the development of skin lesions (Figure 5B). Together, these findings suggest that the loss of Bhlhb5 within the dorsal horn is necessary for heightened itch in Bhlhb5 mutant mice.
A sub-population of Bhlhb5-expressing neurons is absent from the superficial dorsal horn of Bhlhb5 mutant mice
While the conditional ablation studies raised the possibility that defects within the dorsal horn of the spinal cord lead to abnormal itch in Bhlhb5 mutant mice, the cellular basis for this phenotype was unknown. Mice lacking Bhlhb5 were found to have no obvious or major disruption of dorsal horn anatomy based on expression of a variety of spinal cord neuronal markers, including PKCγ, calbindin, calretinin, Substance P, IB4 and CGRP (Figure S6). Recent reports have suggested that DRG neurons that express gastrin-related peptide (GRP) are specific for itch (Sun and Chen, 2007). However, when we looked specifically at this population, we found that the number of GRP-expressing neurons in the DRG was unchanged in Bhlhb5 mutants (data not shown), and the innervation by GRP-expressing neurons in the dorsal horn appeared unaffected by the loss of Bhlhb5 (Figure S6G and S6H).
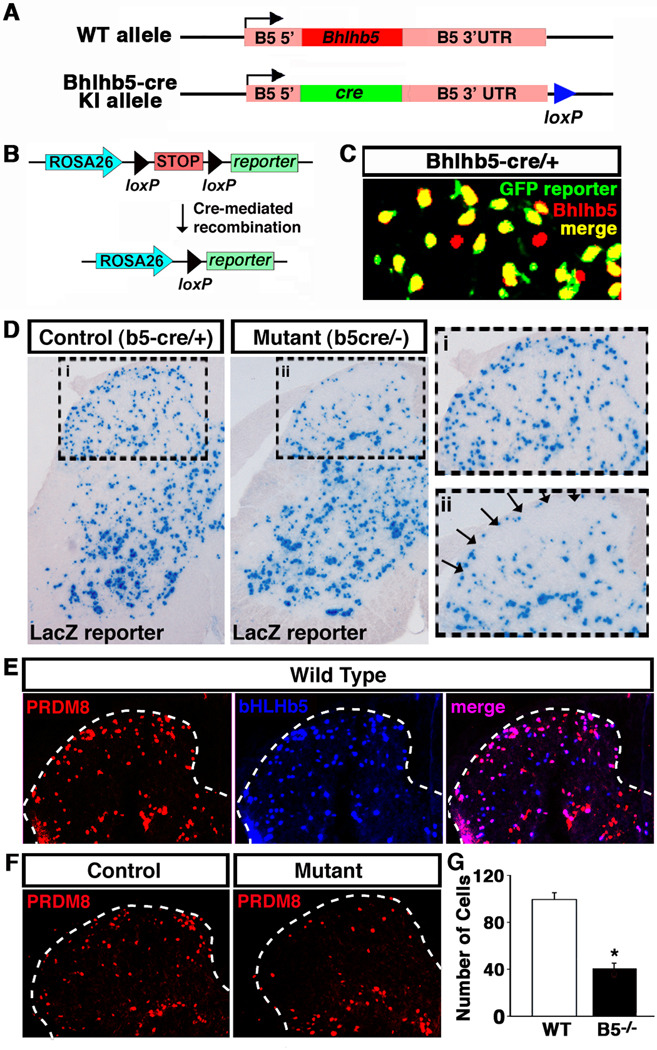
The absence of an easily discernable defect in the dorsal spinal cord of Bhlhb5 mutants was not altogether unexpected given that Bhlhb5 is expressed in just a small subset of those neurons. To determine whether there is a subtle defect in itch circuitry within the dorsal spinal cord, we needed an approach to selectively define the cells that express Bhlhb5 and observe the subsequent the fate of these cells in mice lacking Bhlhb5. Toward this end, we generated a Bhlhb5-cre knockin animal (Bhlhb5-cre), in which the coding region of the Bhlhb5 gene was replaced with cre (Figures 6A and S7). Upon crossing this Bhlhb5-cre line with a cre-responsive reporter line, cells in which the Bhlhb5 gene has been activated become permanently marked with the reporter (Figure 6B), allowing the fate of Bhlhb5-expressing cells to be resolved at a cellular level and followed throughout the life of the animal.
Figure 6. A sub-population of Bhlhb5-expressing neurons is absent from the superficial dorsal horn of Bhlhb5 mutant mice.
A) Schematic illustrating the Bhlhb5-cre knockin allele in which the coding region of the Bhlhb5 gene was replaced with the coding region of the cre recombinase gene. Also see Figure S7. B) Diagram illustrating mechanism whereby the Bhlhb5-cre allele causes irreversible recombination of the Rosa26 reporter, resulting in the removal of a stop signal and expression of the reporter (βgalactosidase or eYFP). C) Specificity of the Bhlhb5-cre allele. Representative region of dorsal spinal cord from P0 mice that are heterozygous for the Bhlhb5-cre allele and harbor the Rosa-eYFP reporter were stained with antibodies to Bhlhb5 (red) and eYFP (green). Note that at this time point, almost all labeled cells are double-labeled (yellow), indicating cre-mediated recombination has occurred in Bhlhb5-expressing cells. D) Bhlhb5-cre knockin reveals fate of Bhlhb5-cre marked cells in the spinal cord of control (Bhlhb5-cre/+) and mutant (Bhlhb5-cre/−) mice. Cre-responsive Rosa26-LacZ reporter is used to mark cells in which Bhlhb5-cre was expressed. Sections are from representative lumbar spinal cord hemisections from P28 mice. Dorsal horn is enlarged in inset for control (i) and mutant (ii) mice. Arrows indicate region of cell loss in mutant mice. Quantification reveals an ~50% loss of lacZ-expressing cells in the superficial dorsal horn of the spinal cord (data not shown, n = 4 pairs of mice, counting 20 matched lumber section/pair). See also Figure S8A. Note that no sensory phenotypes are observed in control mice that are heterozygous for Bhlhb5, and the dorsal horn appears grossly normal based on the analysis of a number of other neural markers (Figure S6). E) Co-immunostaining with antibodies against Prdm8 (red) and Bhlhb5 (blue) reveal that these two factors show a very high degree of colocalization (merge; purple) in the dorsal horn of mice at P0, suggesting that Prdm8 is a marker for Bhlhb5-expressing cells. F) Representative lumbar sections from P0 mice stained with antibodies to Prdm8 reveal that there is a loss of Prdm8-expressing neurons in Bhlhb5 mutants relative to controls. G) Quantification of Prdm8-positive (+ve) cells in the superficial dorsal horn of control (Con) and Bhlhb5 mutant (Mut) mice. (n = 3 pairs of mice, counting 10 matched lumber sections/pair).
For these experiments, we used three different cre-responsive indicator alleles (Rosa26-lacZ, Rosa26-eYFP or Z/EG reporters) (Soriano, 1999) to visualize genetically marked neurons. For simplicity, we refer to these neurons as B5-cre-lacZ, B5-cre-eYPF, or B5-cre-Z/EG marked neurons, respectively. Analysis of reporter expression in newborn mice that are heterozygous for the Bhlhb5-cre allele revealed that B5-cre-eYFP and Bhlhb5 were almost completely co-localized, indicating that reporter expression faithfully recapitulated the endogenous expression pattern of Bhlhb5 (Figure 6C).
Upon crossing the Bhlhb5-cre line with the Rosa26-lacZ reporter, we found that lacZ marked a small subset of neurons throughout all laminae of the spinal cord in adult mice (Figure 6D). In mice lacking Bhlhb5, most regions of the spinal cord showed a similar pattern of B5-cre-lacZ cells. However, specifically in the superficial laminae, we found significantly fewer B5-cre-lacZ neurons in the Bhlhb5 mutants (Figure 6D). Quantification revealed that approximately 50% of neurons that would normally have expressed Bhlhb5 were missing in the superficial dorsal horn of adult mice lacking Bhlhb5. Furthermore, this cell loss was observed throughout the rostro-caudal axis of the spinal cord (data not shown). To verify this finding and to rule out potential effects specific to the Rosa locus, we used the Z/EG line as a reporter and we again observed significant loss of cell bodies and neuropil in Bhlhb5 mutants relative to control animals (Figure S8A). Co-immunostaining with CGRP (a marker of lamina I and outer lamina II) and IB4 (a marker of inner lamina II) revealed a decrease in the number of B5-cre-Z/EG marked cells within the superficial layers in mutant mice. Given that the decrease in genetically labeled cells is observed using cre reporters that are expressed from different promoters and distinct loci, it is unlikely that these effects are due to mis-regulation of the reporter alleles.
While a loss of Bhlhb5 neurons seemed the most likely explanation for these findings, it remained possible that the reduction in the number of Bhlhb5-cre-labeled neurons in the Bhlhb5 mutant reflected merely mis-regulation of the Bhlhb5-cre allele. To address this possibility, we searched for another marker of Bhlhb5-expressing cells with which to confirm our findings. Our studies in other regions of the nervous system (to be described elsewhere) revealed that Bhlhb5 was largely co-expressed with Prdm8, a putative zinc-finger containing transcription factor. To assess whether Prdm8 could be used as a marker for Bhlhb5-expressing cells in the spinal cord, we performed double-immunolabeling experiments and found that Bhlhb5 and Prdm8 showed approximately 85% co-localization (Figure 6E). Next, we examined the number of Prdm8-expressing cells in the dorsal spinal cord of Bhlhb5 mutant mice and found that they were reduced by approximately 50% (Figures 6F and 6G), corroborating that a sub-population of Bhlhb5 neurons is absent from the superficial dorsal spinal cord in Bhlhb5 mutant mice.
Cell loss in Bhlhb5 mutant mice reflects increased programmed cell death
There are several possible explanations for the decrease in Bhlhb5cre-marked neurons in the superficial lamina of the dorsal horn in Bhlhb5cre/− mice relative to Bhlhb5cre/+ mice. The neurons might never have been born, they might have migrated to the wrong lamina, or they might have died during development.
Given that Bhlhb5 is expressed exclusively in post-mitotic neurons (data not shown and (Liu et al., 2007)), it seemed unlikely that a loss of this factor would affect mitosis. However, to rule out this possibility, we compared control mice (Bhlhb5-cre/+; Rosa26-eYFP) to Bhlhb5 mutant mice (Bhlhb5-cre/−; Rosa26-eYFP), analyzing the number of neurons that were co-labeled with BrdU, to label dividing neurons, and eYFP, to label neurons that were genetically marked by the Bhlhb5-cre allele. Neurons were labeled with BrdU at either E12.5 or E13.5, the time during which superficial dorsal horn neurons are born, and analyzed at E17.5, after they have migrated into superficial laminae (Mizuguchi et al., 2006; Wildner et al., 2006). These experiments revealed no difference in the number of genetically marked neurons that were BrdU-positive between control and Bhlhb5 mutant mice, indicating that loss of Bhlhb5 has no effect on the number of dorsal horn neurons that are born (data not shown).
We next investigated the possibility that the decrease in the number of neurons in the superficial dorsal spinal cord of Bhlhb5 mutant mice was due to a defect in migration. Towards this end, we quantified the number of cells that express B5-cre-eYFP in control and Bhlhb5 mutant animals at P0. This analysis revealed that there was an ~50% decrease in the number of B5-cre-eYFP marked neurons in the superficial dorsal horn of Bhlhb5 mutants but that no difference in the number of B5-cre-eYFP marked neurons in any other region of the spinal cord in control and mutant mice (Figures 7A, 7B, 7C, and 7D). The observed decrease in the number of B5-cre-eYFP marked neurons in the superficial dorsal horn of the Bhlhb5 mutant mice, without a corresponding increase in other regions of the spinal cord, suggests that neurons that are absent from the superficial dorsal horn have not migrated to another region of the spinal cord.
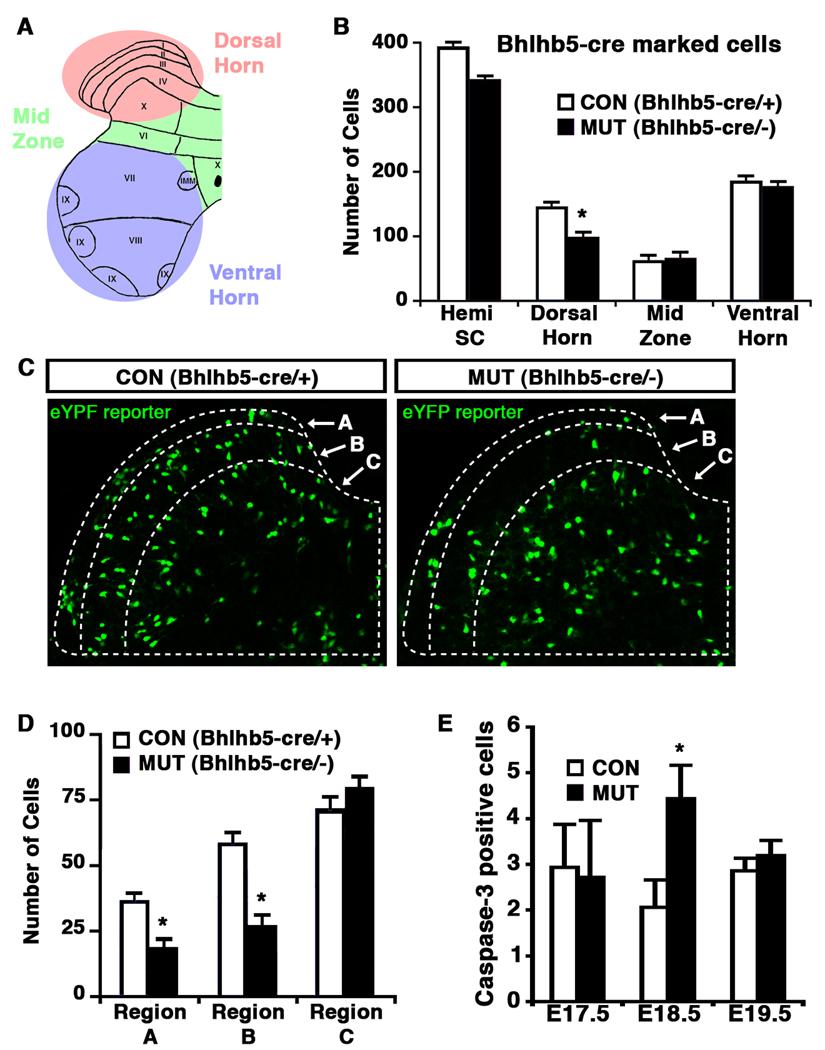
Figure 7. Cell loss in Bhlhb5 mutant mice reflects increased programmed cell death.
A) Schematic illustrating regions within a hemi-spinal cord (dorsal horn, mid zone and ventral horn) that were used for quantification. B–D) Quantification of Bhlhb5-cre marked cells in the lumbar spinal cord of control (Bhlhb5-cre/+) and mutant (Bhlhb5-cre/−) mice, visualized with the cre-responsive Rosa26-eYFP reporter at P0. B) The number of Bhlhb5-cre marked cells observed in various regions of a spinal cord hemisection was quantified. Significantly fewer Bhlhb5-cre marked cells were found in the dorsal horn of Bhlhb5 mutants relative to controls. No significant changes were observed in other regions of the spinal cord. C) Representative images from the dorsal horn of control and mutant mice showing Bhlhlb5-cre marked cells. The dorsal horn was further subdivided into regions based on anti-calretinin antibody staining (which marks region B; data not shown). Note that Rexed’s laminae are not fully formed at this stage and most lamina-specific markers are not expressed. Nevertheless, region A corresponds roughly to future lamina I and region B to future lamina II. D) Quantification of Bhlhb5-cre marked neurons in regions A, B and C, as defined above. There is a significant reduction in the number of Bhlhb5-cre marked neurons within regions A and B of the dorsal horn in mutants relative to controls. No significant change was observed within region C. E) Analysis of apoptotic cells in the dorsal horn of control and mutant animals at E17.5, E18.5 and E19.5, as indicated. Apoptotic neurons in the dorsal horn were identified using an antibody against cleaved caspase-3. Significantly more cleaved caspase-3 positive cells were observed in the mutant dorsal horn at E18.5 relative to littermate controls. For B, D and E, 3 – 5 pairs of littermates were analyzed and data represent mean +/− SEM (*, p < 0.05, Mann-Whitney U-test). Further analysis reveals that the absence of Bhlhb5-expressing neurons in the dorsal horn is due to the loss of both inhibitory and excitatory neurons (Figure S8B).
Finally, we investigated the possibility that Bhlhb5-cre marked neurons might be lost in Bhlhb5 mutant mice through programmed cell death. To address this, we counted the number of neurons that express activated caspase-3, a marker of apoptosis (Cryns and Yuan, 1998) and found that there was a small but significant increase in the number of apoptotic cells in the superficial dorsal horn of Bhlhb5 mutant mice relative to controls at E18.5, with no significant difference at E17.5 or E19.5 (Figure 7E). The timing of this cell death was in agreement with the observation that Bhlhb5 mutants display a clear loss of Bhlhb5-cre marked cells at P0 (Figure 7C), but not at (or prior to) E17.5 (data not shown). These findings indicate that the loss of Bhlhb5-cre-labeled cells in Bhlhb5 mutants is likely due to apoptosis during development, and suggest that Bhlhb5 is required for the survival of neurons in spinal cord pruritic circuits.
Inhibitory neurons underlie abnormal itch in Bhlhb5 −/− mice
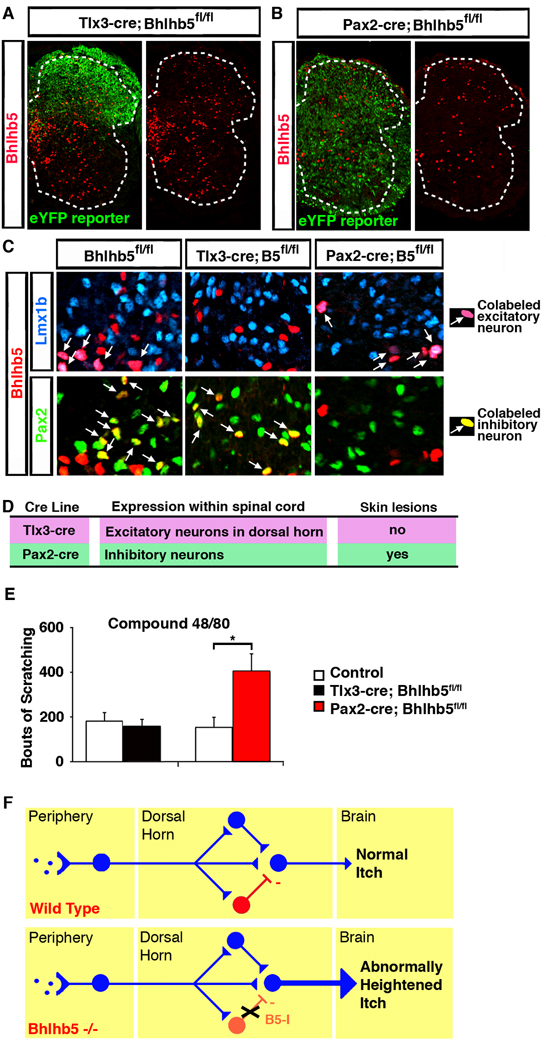
To investigate whether excitatory or inhibitory neurons are lost upon ablation of Bhlhb5, we double-labeled the B5-cre-eYFP neurons with antibodies for excitatory (Lmx1b) or inhibitory (Pax2) neurons (Cheng et al., 2005) and found a partial loss of both glutamatergic and GABAergic neurons (Figure S8B). To ascertain which subset is involved in the pruritic phenotype in Bhlhb5 mice, we used two additional cre lines, Tlx3-cre and Pax2-cre (Ohyama and Groves, 2004; Xu et al., 2008). Tlx3-cre causes recombination within regions of the nervous system that includes excitatory neurons within the dorsal horn (Figures 8A and 8C), whereas Pax2-cre causes recombination within regions of the nervous system that include most inhibitory neurons throughout the spinal cord (Figures 8B and 8C). To confirm that these alleles were behaving as expected, double-labeling with markers for glutamatergic (Lmx1b-expressing) and GABAergic (Pax2-expressing) neurons was performed. These experiments revealed that, upon Tlx3-cre-mediated recombination, Bhlhb5 was no longer expressed in excitatory neurons in the dorsal horn, though it remained expressed in inhibitory neurons in this region (Figure 8C). Conversely, upon Pax2-cre-mediated recombination, Bhlhb5 was no longer expressed in inhibitory neurons, though it remained expressed in excitatory neurons of the spinal cord (Figure 8C). Mice lacking Bhlhb5 selectively in glutamatergic neurons within the dorsal horn neither developed skin lesions nor showed heightened itch responses (Figures 8D and 8E), suggesting that Bhlhb5 function within excitatory neurons is not required for the pruritic phenotype. However, use of the Pax2-cre line to ablate Bhlhb5 gave rise to mice that both developed skin lesions and showed heightened responses to pruritic agents (Figure 8D and 8E). These findings suggest that that loss of Bhlhb5 in inhibitory neurons is sufficient to produce persistent abnormal itch (Figure 8F).
Figure 8. Abnormal itch in Bhlhb5 mutant mice may be due to loss of Bhlhb5 from inhibitory neurons of the dorsal horn.
A–B) Loss of Bhlhb5 upon either Tlx3-cre (A) or Pax2-cre (B) mediated excision in conditional Bhlhb5 mutant mice (Bhlhb5fl/fl). Lumbar hemisection from P0 mouse stained with antibodies that recognize Bhlhb5 (red) or eYFP (green). C) Specificity of the Tlx3-cre and Pax2-cre alleles. Representative region of dorsal spinal cord from P0 mice of the indicated genotypes that are stained with antibodies that recognize Bhlhb5 (red), Lmx1b (blue), or Pax2 (green). In control animals, Bhlhb5 colocalizes with both Lmx1b and Pax2, as indicated by the arrows. Upon Tlx3-cre-mediated excision within excitatory neurons, Bhlhb5 remains co-localized with the excitatory marker, Pax2 but not the inhibitory marker, Lmx1b. Conversely, upon Pax2-cre-mediated excision within inhibitory neurons, Bhlhb5 remains co-localized with the excitatory marker, Lmx1b but not the inhibitory marker, Pax2. D) Table describing the Tlx3-cre and Pax2-cre mouse lines, together with the expression pattern of cre within the spinal cord for each line, and whether the subsequent animals develop skin lesions following cre-mediated recombination of Bhlhb5. (Note that cre expression in these animals is not exclusive to the spinal cord; each line also causes some recombination in other regions of the nervous system including parts of the hindbrain.) E) Conditional loss of Bhlhb5 in the Pax2-cre domain, but not the Tlx3-cre domain, results in significantly elevated scratching responses following the intradermal injection of compound 48/80 into the nape of the neck relative to littermate controls lacking cre alleles. Data are presented as mean ± SEM and * indicates significant difference relative to controls (p < 0.05, t-test). F) Model: Disinhibition gives rise to abnormally heightened itch in Bhlhb5 −/− mice. In wild type mice, pruritic agents at the skin’s surface activate a subset of itch-mediating DRG neurons that project from the periphery to the dorsal horn of the spinal cord. This pruritic information is modulated by excitatory (blue) and inhibitory (red) neurons in the dorsal horn before being relayed to the brain and experienced as the sensation of normal itch. In Bhlhb5 mutant mice, there is a loss of inhibitory neurons in the dorsal horn. Thus, reduced inhibitory synaptic input in the dorsal horn (disinhibition) results in a greater itch signal that is conveyed to the brain, resulting in abnormally heightened itch and that may ultimately give rise to pathological skin lesions.
To further test the idea that abnormal circuitry within the dorsal horn causes the pruritic phenotype, we analyzed c-fos expression (as a marker of neuronal) activation following the injection of either the pruritic agent, compound 48/80, or formalin. Using this approach, we found significantly more fos-expressing cells in the dorsal horn of Bhlhb5 mutant mice, relative to controls (Figures S8C, S8D S8E and S8F), consistent with the idea that neurons of the dorsal horn process information aberrantly in Bhlhb5 mutant mice.
DISCUSSION
We have generated a mouse model that develops self-inflicted skin lesions and we provide evidence that this is due to abnormal itch responsiveness. Using a genetic fate mapping strategy, we provide insight into the cellular basis for this phenotype, showing that Bhlhb5 is required for the survival of a select group of neurons within the dorsal horn. Furthermore, through conditional ablation, we provide evidence that the loss of Bhlhb5 within inhibitory interneurons produces the abnormal scratching behavior that gives rise to pathological lesions.
The expression of Bhlhb5 expression within the dorsal horn is restricted to approximately 5% of neurons yet its loss in the dorsal horn is very likely responsible for the abnormal itch response we detect in Bhlhb5 mutant mice. We have identified a specific population of inhibitory neurons in the most superficial laminae of the spinal cord that require Bhlhb5 for survival and appear to be critical for regulating the normal manifestation of itch. In addition, we provide evidence that the loss of inhibitory neurons in the dorsal horn can give rise to persistent itch through a mechanism of disinhibition—decreased inhibitory synaptic input in lamina I and II of the dorsal spinal cord (Figure 9). Thus, our data suggest that itch behavior is the result both of a peripheral activation of primary afferent pruritoceptors and of central modulatory circuits. When pruritic circuits in the dorsal horn are disinhibited, as is seen in Bhlhb5 mutant mice, the activation of pruritoceptors causes persistent itch resulting in pathological lesions.
Bhlhb5 −/− mice show a dramatically elevated response in the late-phase of the formalin test, a finding widely thought to be due, at least in part, to enhanced central sensitization, (Tjolsen et al., 1992). This type of plasticity, which occurs for both itch and pain, heightens aversive sensation through long-term changes in circuit properties (Ikoma et al., 2004; Latremoliere and Woolf, 2009; Woolf, 1983). We speculate that the abnormal itch observed in Bhlhb5 mutant mice may be due to an enhanced central sensitization in response to activation of itch circuits that arises from a loss of inhibitory input in lamina I and II of the dorsal spinal cord. Thus, it is possible that normal everyday scratching might, in mutant mice with this form of aberrant central sensitization, lead to the development of a pathological itch-scratch-itch cycle. If so, this may explain why Bhlhb5 −/− mice develop lesions in discrete areas, despite the fact that these mice have a loss of Bhlhb5-expressing neurons throughout the entire rostro-caudal axis of the dorsal horn.
The finding that local anesthetic applied to the site of lesion attenuates the scratching behavior in Bhlhb5 mutant mice (Fig. 4D) strongly suggests that activity in primary sensory neurons is involved in the itch response. However, the itch phenotype in Bhlhb5 mutant mice is clearly not caused by this peripheral activity alone, since HtPa-cre mediated loss of Bhlhb5 in DRG neurons does not result in skin lesions (Fig. 5b). Rather, our studies suggest that the heightened itch in Bhlhb5 mutant mice is due to the loss of Bhlhb5 in inhibitory neurons of the dorsal spinal cord (Fig. 8). Thus, we propose that peripheral neurons initiate an itch signal that is abnormally amplified in the dorsal horn of the spinal cord, resulting in scratching responses that culminate in the development of pathological skin lesions (Fig. 9). In addition, it is possible that changes in the spinal cord of Bhlhb5 mutant mice may have an indirect effect on the activity of primary sensory neurons. This idea has precedent in models of neuropathic pain where it has recently been observed that primary nociceptors become hyperactive as a consequence a spinal cord injury (Carlton et al., 2009).
The behavioral assays for this study were performed primarily with 4 week-old mice, an age where the circuits of the spinal cord are thought to be mature (Fitzgerald, 2005). At this age, wild type and Bhlhb5 mutant mice show no significant differences in their responses to a number of pain assays (Fig. 3), but Bhlhb5 mutant mice show heightened responses to pruritogens and formalin (Fig. 4), consistent with the idea that there is a selective disruption of sensory circuits in the dorsal horn of Bhlhb5 mutant mice. In contrast, a few weeks later—subsequent to the development of skin lesions— Bhlhb5 mutant mice show somewhat higher pain thresholds than wild type controls (Fig. S3E, S3F and S3G). One possibility is that the increased pain threshold in older mice is a secondary consequence of the skin lesions. Though the scratching behavior is likely due to itch, the ensuing tissue damage might also cause pain, and either of these sensations could be a confounding factor in behavioral assays for nociception. It remains possible, however, that Bhlhb5 mutant mice develop reduced sensitivity to pain with a latent onset. Future studies will be required to clarify this issue.
Several recent studies have revealed that one of the central mechanisms underlying the development of pain hypersensitivity is reduced inhibitory synaptic input in the dorsal horn of the spinal cord. For instance, pharmacological blockade of either of GABA or glycine receptors enhances the electrophysiological responses to the activation of primary nociceptors (Baba et al., 2003; Torsney and MacDermott, 2006). Moreover, a reduction of inhibitory synaptic transmission has been shown to play a role in pain sensitization through a variety of mechanisms, including a loss of GABAergic interneurons in the dorsal horn (Moore et al., 2002; Scholz et al., 2005) and the inhibition of glycine receptor function (Zeilhofer, 2005). However, while multiple studies have shown that pain is aggravated by reduced synaptic inhibition in the dorsal spinal cord, whether analogous mechanisms regulate pruritis was unknown. Our study clarifies this issue by uncovering a role for disinhibition in the regulation of itch.
The only itch-specific neurons in the spinal cord identified at a molecular level to date are those that express the gastrin-releasing peptide receptor (GRPR) (Sun et al., 2009). Beyond these, other neurons within itch circuits of the dorsal horn remain completely unknown, underscoring a fundamental gap in current knowledge with regard to pruritic circuitry. Our study begins to bridge this gap by providing evidence that a distinct population of inhibitory interneurons within the dorsal horn functions to inhibit itch and that loss of these neurons results in persistent itch that gives rise to pathological lesions. Thus, Bhlhb5 inhibitory neurons may represent the first inhibitory component of puritic circuits to be identified genetically. In addition, the Bhlhb5-cre mouse provides a genetic marker with which to identify this population of cells. Using this molecular handle, it should be possible to define the molecular, electrophysiological and morphological characteristics of these neurons and characterize exactly, how, where and when they regulate itch. We speculate that chronic severe itch in some patients may result from a similar kind of disinihibiton to that described here, rather than from the increased activation of pruritoceptors as is commonly assumed. If so, the inhibitory neurons identified in this study may ultimately provide a cellular target for the development of therapies for chronic itch.
EXPERIMENTAL PROCEDURES
See Supplemental Experimental Procedures for details on the generation of Bhlhb5-directed antibodies, animal husbandry, colony management, immunohistochemistry, Xgal staining and the generation of Bhlhb5 null, Bhlhb5 conditional knockout, and Bhlhb5-cre knockin mice.
Behavioral Assays
Where appropriate, mice were habituated for 20 min/day over several days. For observational studies, habituated mice were placed individually a clear cage and videotaped for 20 min and scored for their behavior. To assess mechanical sensitivity, calibrated von Frey fibers were applied to the plantar surface of the hindpaws of mice. The smallest monofilament that evoked paw-withdrawal responses on five out of ten trials was taken as the mechanical threshold. To determine thermal pain threshold, mice were placed on a 55 °C hot plate and the response latency to paw licking or jumping was recorded. For tests of acute nociception, capsaicin (2.5 µg) or mustard oil (0.75%) were injected into the plantar subcutaneously into the plantar surface of the hindpaw in a 20 µl volume. For the formalin tests, the mice received 25 µl intraplantar injection of 5% formalin, and the licking behavior of the injected paw was recorded in five minute intervals for 1 hr for the subsequent hour. To test for mechanical hypersensitivity the paw withdrawal threshold was determined 2 hrs after the intraplantar injection of 2% carrageenan into the hindpaw in a 20 µl volume. To test for pruritic responses, each of the pruritic compounds were injected intradermally into the nape of the neck in a 50 µl volume and the bouts of scratching that occurred over the subsequent hour were quantified. The pruritic compounds used were histamine (1 µmol), α-methyl-5-hydroxytryptamine (30 µg), SLIGRL-NH2 (100 nmol), compound 48/80 (100 µg) formalin (5%), and chloroquine (200 µg). To investigate the involvement of sensory feedback, Bhlhb5 −/− mice that had pre-existing skin lesions in the perineum (or wild type littermates) mice were injected with 200 µl of 0.3% lidocaine (or PBS, as a control) subcutaneously into the perineum. Ten minutes later, the scratching and licking behavior directed toward the perineum was assessed every 10 s for a total of 10 min.
Quantification of neurons
For neuronal quantification experiments, 3 – 5 pairs of P0 littermates were analyzed. Ten matched lumbar sections per animal were used for quantification, spanning 2000 microns. Cell counts in various regions of the spinal cord were conducted using the cell count function of Metamorph (Molecular Devices). Metamorph cell scoring parameters were validated by manual counts and were kept constant across all conditions. To quantify apoptotic neurons, mice were analyzed in a similar fashion and cleaved caspase-3 positive cells in the dorsal horn were counted manually. All counts were conducted blind to genotype.
Supplementary Material
01
ACKNOWLEDGEMENTS
We thank E. C. Griffith for critical readings of the manuscript; D. Harmin for help with statistical analysis. M. Gee and P. Zhang for assistance with mouse colony management; the MRDDRC Gene Manipulation Core (M. Thompson, Y. Zhou, and H. Ye); the MRDDRC Imaging Core (L. Bu); and the MRDDRC Histology Core (M. Liana). This work was supported by a Jane Coffin Childs Fellowship and a Dystonia Medical Research Foundation Fellowship to S.E.R., NIH grant R01-NS-048276 to M.E.G., and the Developmental Disabilities Mental Retardation Research Center grant NIH-P30-HD-18655.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. 2001;4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Du J, Tan HY, Nesic O, Hargett GL, Bopp AC, Yamani A, Lin Q, Willis WD, Hulsebosch CE. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain. 2009;147:265–276. doi: 10.1016/j.pain.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Samad OA, Xu Y, Mizuguchi R, Luo P, Shirasawa S, Goulding M, Ma Q. Lbx1 and Tlx3 are opposing switches in determining GABAergic versus glutamatergic transmitter phenotypes. Nat Neurosci. 2005;8:1510–1515. doi: 10.1038/nn1569. [DOI] [PubMed] [Google Scholar]
- Cryns V, Yuan, J. Proteases to die for. Genes Dev. 1998;12:1551–1570. doi: 10.1101/gad.12.11.1551. [DOI] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Feng L, Xie X, Joshi, PS, Yang Z, Shibasaki K, Chow RL, Gan L. Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development. 2006;133:4815–4825. doi: 10.1242/dev.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121:341–354. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Fartasch M, Heyer G, Miyachi Y, Handwerker H, Schmelz M. Painful stimuli evoke itch in patients with chronic pruritus: central sensitization for itch. Neurology. 2004;62:212–217. doi: 10.1212/wnl.62.2.212. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi PS, Molineaux BJ, Feng L, Xie X, Macklis JD, Gan L. Bhlhb5 regulates the post-mitotic acquisition of area identities in layers II – V of the developing neocortex. Neuron. 2008;60:258–272. doi: 10.1016/j.neuron.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Liu Z, Chen T, Li H, Qiang B, Yuan J, Peng X, Qiu M. Selective expression of Bhlhb5 in subsets of early-born interneurons and late-born association neurons in the spinal cord. Dev Dyn. 2007;236:829–835. doi: 10.1002/dvdy.21061. [DOI] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Koltzenburg M. Itching for an explanation. Trends Neurosci. 1992;15:497–501. doi: 10.1016/0166-2236(92)90102-e. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi R, Kriks S, Cordes R, Gossler A, Ma Q, Goulding M. Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nat Neurosci. 2006;9:770–778. doi: 10.1038/nn1706. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- Pietri T, Eder O, Blanche M, Thiery JP, Dufour S. The human tissue plasminogen activator-Cre mouse: a new tool for targeting specifically neural crest cells and their derivatives in vivo. Dev Biol. 2003;259:176–187. doi: 10.1016/s0012-1606(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico B, Xu B, Reichardt LF. TrkB receptor signaling is required for establishment of GABAergic synapses in the cerebellum. Nat Neurosci. 2002;5:225–233. doi: 10.1038/nn808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE, Greenberg ME, Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- Schmelz M. Itch and pain. Neurosci Biobehav Rev. 2008 doi: 10.1016/j.neubiorev.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamida SG, Lamotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol. 2004;91:213–222. doi: 10.1152/jn.00527.2003. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sowunmi A, Walker O, Salako LA. Pruritus and antimalarial drugs in Africans. Lancet. 1989;2:213. doi: 10.1016/s0140-6736(89)90391-7. [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Umeuchi H, Togashi Y, Honda T, Nakao K, Okano K, Tanaka T, Nagase H. Involvement of central mu-opioid system in the scratching behavior in mice, and the suppression of it by the activation of kappa-opioid system. Eur J Pharmacol. 2003;477:29–35. doi: 10.1016/j.ejphar.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Wildner H, Muller T, Cho SH, Brohl D, Cepko CL, Guillemot F, Birchmeier C. dILA neurons in the dorsal spinal cord are the product of terminal and non-terminal asymmetric progenitor cell divisions, and require Mash1 for their development. Development. 2006;133:2105–2113. doi: 10.1242/dev.02345. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Xu Y, Lopes C, Qian Y, Liu Y, Cheng L, Goulding M, Turner EE, Lima D, Ma Q. Tlx1 and Tlx3 coordinate specification of dorsal horn pain-modulatory peptidergic neurons. J Neurosci. 2008;28:4037–4046. doi: 10.1523/JNEUROSCI.4126-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci Res. 1999;35:77–83. doi: 10.1016/s0168-0102(99)00070-x. [DOI] [PubMed] [Google Scholar]
- Yosipovitch G, Carstens E, McGlone F. Chronic itch and chronic pain: Analogous mechanisms. Pain. 2007;131:4–7. doi: 10.1016/j.pain.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU. The glycinergic control of spinal pain processing. Cell Mol Life Sci. 2005;62:2027–2035. doi: 10.1007/s00018-005-5107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01