Vive les differences! Individual variation in neural mechanisms of executive control (original) (raw)
. Author manuscript; available in PMC: 2011 Apr 8.
Published in final edited form as: Curr Opin Neurobiol. 2010 Apr 8;20(2):242–250. doi: 10.1016/j.conb.2010.03.002
Summary
Investigations of individual differences have become increasingly important in the cognitive neuroscience of executive control. For instance, individual variation in lateral prefrontal cortex function (and that of associated regions) has recently been used to identify contributions of executive control processes to a number of domains, including working memory capacity, anxiety, reward/motivation, and emotion regulation. However, the origins of such individual differences remain poorly understood. Recent progress toward identifying the genetic and environmental sources of variation in neural traits, in combination with progress identifying the causal relationships between neural and cognitive processes, will be essential for developing a mechanistic understanding of executive control.
It is a ubiquitous fact that individuals differ from each other both psychologically and biologically. Investigating and exploiting individual differences has been a standard research tradition within psychology [1], but has only recently become more strongly emphasized in cognitive neuroscience. The trend is especially prominent in studies of executive control (Figure 1). Here we review a variety of well-established and novel individual difference approaches, the unique methodological considerations that accompany such approaches, and the utility of such approaches both for understanding the neural mechanisms of executive control and the underlying sources of individual variation.
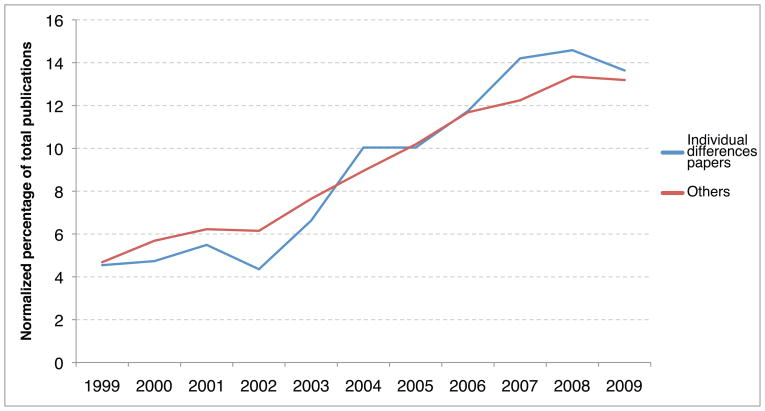
Figure 1. A trend toward increasing use of individual differences measures in executive control cognitive neuroscience research over the past 10 years.
The graph illustrates a shift from proportionally fewer individual difference studies early in the decade to a proportionally larger number of individual difference studies over the last several years. The blue line illustrates individual difference studies of the neural basis of executive control, while the red line illustrates such research not including individual difference terms (see below). The publication data were normalized by dividing the number of publications per year by the total number of publications (between 1999 and 2009) in each category.
Source: Scopus. Search used: (“individual differences” OR IQ OR PERSONALITY OR “individual variability”) AND (“cognitive control” OR “executive control” OR “working memory” OR “response inhibition” OR attention) AND (fMRI OR MRI OR ERP OR EEG OR PET OR MEG OR TMS)
The recent surge in the use of individual difference approaches in cognitive neuroscience likely stems from the increased experimental and inferential power afforded by such approaches. In particular, individual difference analyses provide a convenient means for testing brain-behavior relationships that is complementary to experimental manipulation. As a simple example, activity in a brain region hypothesized to implement interference control might be expected to show within-subject sensitivity to high vs. low interference, yet it may also show between-subject correlation with task performance (Figure 2). Since within-subject and between-subject variance components are statistically independent, individual differences analyses can provide convergent evidence for one’s theoretical hypothesis.
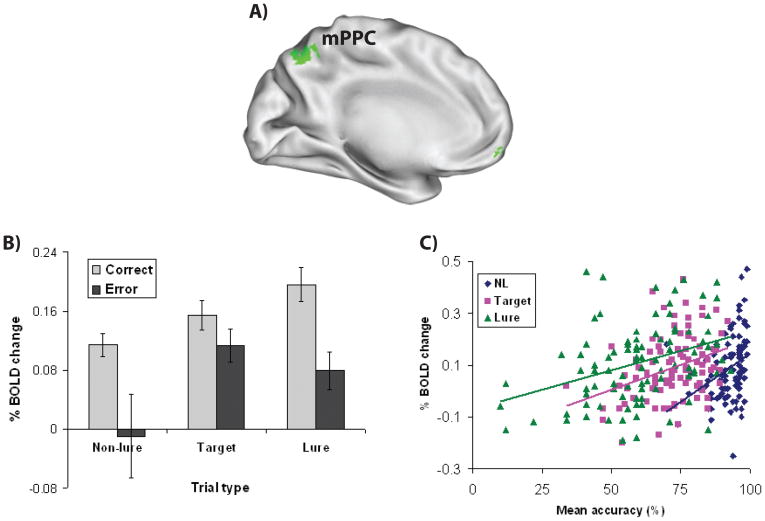
Figure 2. Complementary within-subject and between-subject effects may be present within a single region.
In a large fMRI study of working memory (N = 94; unpublished data), A) medial posterior parietal cortex (mPPC) showed a convergence of within-subject and between-subject effects, B) including both trial-by-trial differences in response accuracy (greater activation for correct responses than in correct responses on high interference [lure] as well as low interference [non-lure, target] trials) and C) individual differences in mean response accuracy (the correlation is plotted separately for each trial type, and only for correct responses).
Another important reason to conduct individual differences research is to better understand the existence of highly variable cognitive traits. Such prominent individual differences likely play a critical role in certain cognitive domains, such as executive control. Executive control is increasingly thought of as a construct tightly linked with classic individual difference dimensions of general cognitive ability that have been studied in psychological research for decades–for example, general “fluid” intelligence (gF) and working memory capacity (WMC) [2–4]. Similarly, constructs within the domains of personality and emotion processing (e.g., reward sensitivity, trait anxiety, and emotion regulation) have been recently linked to differences in executive control [5–7]. Thus, as described below, investigators interested in the theoretical mechanisms of cognitive and affective individual differences have been conducting cognitive neuroscience studies of executive control in order to provide support for such theories [8–12].
In the remainder of this review, we discuss some of the applications of the individual differences approaches in selective domains related to executive control, as well as recent efforts to understand the origins and mechanisms that produce individual differences in these domains. Before turning to this discussion, however, we first describe a number of general methodological issues and developments that are central to consider when conducting and interpreting cognitive neuroscience studies based on individual differences (see [13] for a more extensive review).
Methodological considerations
A first issue to consider in conducting individual difference analyses is the size and nature of the sample. Although the typical sample size in cognitive neuroscience studies of executive control is in the range of 15–25 subjects, larger samples may be required in order to consistently detect individual differences effects [14]. The problem is two-fold: 1) studies with small sample sizes will tend to identify only a small fraction of population-level effects in any given sample; and 2), because of increased sampling error, effect sizes derived from small samples will tend to be inflated—often grossly so [15]. The combination of these two problems is apt to lead to an illusory perception that activations are spatially selective and extremely strong [14]; in fact the underlying distribution of effects may be spatially diffuse and much weaker.
A second issue to consider is whether to use an unselected sample (i.e., continuous range), or to pre-select participants into extreme-groups based on a relevant individual difference variable. The latter approach is statistically valid, and generally increases power to detect linear relationships—though at the cost of an increased risk of mischaracterizing the magnitude and functional shape of identified effects [16]. Note, however, that in contrast to the extreme-groups design, it is never advisable to dichotomize an unselected sample into high-low groups post-hoc (e.g., based on median or tertile split), since post-hoc splits are statistically inferior to continuous analyses in virtually all respects [17].
A third issue to consider is to what extent the individual difference measure being used reflects state versus trait influences. Neuroimaging studies most commonly relate brain activation to behavioral or physiological measures that are assessed concurrently (e.g., in-scanner task performance); however, it is important to appreciate that differences in such measures may be heavily context-dependent. For example, people may perform better or worse on an executive control task not only because of stable differences in inherent ability, but also because of transient differences in mood, fatigue level, motivation, cognitive effort, etc. Conversely, studies that employ standard, well-established measures with demonstrable stability (e.g., IQ or Extraversion) are in a better position to make inferences about the trait-like nature of any differences in brain activation. As discussed further below, an exciting variant of the latter approach is to use genotypic differences (which are necessarily stable) to predict intermediate phenotypic variation assessed with neural measurements.
A fourth issue to consider concerns the reliability of the neural measures used in individual difference analyses. The strength of observed individual difference correlations is critically constrained by the reliability of both the independent and dependent variables (e.g., brain activity). Unfortunately, reliability estimates are not typically computed in individual differences-based cognitive neuroscience studies. When such estimates have been conducted, reliability coefficients have only rarely approached levels considered adequate in the psychometric literature [for reviews, see 18,19]. More generally, because neural measurement reliability is likely to be highly context-dependent (i.e., might vary significantly across brain regions, samples, tasks, etc), it must be computed for each new sample and brain region of interest. Integrating basic reliability estimation into existing software packages (e.g., calculating split-half coefficients across even and odd runs) would allow researchers to explicitly report reliability estimates in a manner consistent with behavioral studies, effectively providing a quality check on the plausibility of reported individual difference results.
A final methodological consideration is the primary statistical approach for detecting individual difference effects. Although by far the most common approach is simple univariate correlation, there has been an increasing shift in the functional neuroimaging community towards more powerful and sophisticated methods, such as those that rely upon multivariate techniques. These include approaches that focus on individual differences in anatomical connectivity, using diffusion MRI [20], functional connectivity [21], and effective connectivity, utilizing techniques such as partial least squares regression (PLS) [22], structural equation modeling (SEM) [23], and dynamic causal modeling (DCM) [24], as well as more general ways of characterizing the complexity of brain networks through graph-theoretic methods [25,26]. Another powerful approach is statistical mediation, which provides a test of whether brain activity in a region (or set of regions) mediates the relationship between two observable variables (e.g., a stable trait index and behavioral performance) [11], or between the effect of another brain region on behavior [27]. Recently, tools have become available to test for such effects across the whole brain [28], potentially leading to a more widespread use of such approaches in the future.
Individual difference approaches in cognitive neuroscience studies of executive control
The use of individual difference approaches has been employed to clarify the role of executive control mechanisms in a number of relevant cognitive and affective domains. Rather than attempt an exhaustive review, we use this space to highlight a few notable investigations that elegantly illustrate this general principle.
Working memory capacity
One area of increased study relates to notions of working memory capacity – the number of items that can be successfully stored and utilized over short durations. A critical question has been the extent to which individual differences in capacity reflect the function of a core storage system (i.e., buffer size or efficacy), or rather an attentional control mechanism that may function to govern access to this system (i.e., filtering out irrelevant information and preventing interference). Both fMRI and ERP studies have pointed to the lateral inferior parietal sulcus (IPS) as a core storage system, in that individual differences in capacity predicts the working memory load level that produces asymptotic activity in this region [29,30]. More recent work has also demonstrated that some of these effects can be explained in terms of attentional filtering effects mediated by lateral prefrontal cortex (PFC) and basal ganglia [9,10]. In one elegant account combining computational modeling and brain imaging approaches [31], the IPS serves to maintain the separability and integrity of WM representations, while the lateral PFC provides a non-specific excitatory drive input that can dynamically boost IPS capacity, and that may be the fundamental source of WM individual difference effects. Additional evidence suggests an important contribution of dopaminergic modulation in the basal ganglia and PFC to these individual difference effects, as increased dopamine synthesis in the caudate predicts higher WM capacity [32] and, in older adults, increased delay-related PFC activation and WM performance [33].
Trait anxiety
Individual difference approaches have also been used to draw links between executive control functions and stable traits that have been traditionally linked to non-cognitive dimensions such as affect and personality. For example, in the domain of anxiety, one prominent theoretical account suggests that high-anxious individuals may utilize top-down control mechanisms in an inefficient manner, thus showing increased sensitivity to distractor interference [6]. Recent neuroimaging studies have confirmed and extended this idea. In one study, trait anxiety was associated with a reduction in DLPFC activation in response to conflict triggered by distractor interference, selectively under conditions in which attention was not perceptually constrained, and thus available to be captured by distractors [34]. A second study focused on temporal dynamics to demonstrate that the inefficiency of cognitive control in anxiety might be reflected as reduced sustained but increased transient activation to events (particularly distractors) in the PFC and related components of the brain cognitive control network [8].
Reward/Motivation
The domain of reward and motivation provides another opportunity to investigate the interplay between affect-related individual differences and executive control. Personality traits reflecting reward sensitivity and motivation have been found to significantly modulate components of brain reward circuitry during periods of reward anticipation and delivery [35–37]. More critically, in studies in which reward motivation is manipulated during tasks with high cognitive demands, individual differences in reward sensitivity predict the magnitude of motivation-related activation increases primarily in components of the brain cognitive control network, such as lateral and dorsomedial PFC regions, rather than in reward circuitry [38,39]. This pattern was shown most directly in a recent study in which a statistical mediation approach was employed to demonstrate that the temporal dynamics of activation in right dorsolateral PFC could directly predict the relationship between individual differences in reward sensitivity and the magnitude of working memory performance enhancement observed under reward motivation conditions [40]. These results suggest that affect-related personality traits might govern the efficacy by which reward signals trigger the updating and representation of cognitive goals.
Emotion regulation
Individual differences approaches have also been utilized to understand the neural mechanisms of emotion regulation. One attractive theoretical model is that cognitive control mechanisms in the lateral PFC contribute to emotion regulation by providing a top-down attentional bias over on-going emotional responses and evaluation (putatively implemented in subcortical regions such as the amygdala and ventral striatum) based on the current behavioral goal [5]. Recent studies have supported this model using statistical mediation techniques, showing that PFC-amygdala relationships are mediated differently in depressed vs. non-depressed individuals [41], mediate individual differences in autonomic arousal associated with regulation efforts [42], and also can predict individual differences in regulation success [28]. Interestingly, in the latter study it was found that the PFC-amygdala interaction predicted reduced regulation success, while a second PFC-nucleus accumbens pathway predicted increased success, thus potentially reflecting up-regulation of positive emotions [43].
The origins of individual differences variation: Genetics, environment, and neural mechanisms
A major goal of individual differences research is to identify the sources of variation underlying the observed variation of interest. Behavioral genetics studies have demonstrated that approximately half of the variance in executive control ability can be accounted for by heritable influences [44–46, but see 47]. However, attempts to relate specific genetic polymorphisms directly to cognitive-behavioral differences in executive control function have met with little success, as most identified candidates explain at best a small fraction of variance in executive function [48]. Cognitive neuroscience may provide a solution to this problem by identifying intermediate phenotypes: neurobiological mechanisms that serve as bridging constructs from which to relate genetic and behavioral variation more sensitively than direct gene-behavior correlations. For instance, a strong relationship has been identified between variants of specific genes and individual differences in the activation dynamics of PFC and associated neural circuits during WM and executive control tasks [49].
One such gene, catechol O-methyltransferase (COMT), codes for an enzyme that degrades dopamine in PFC and has a prominent single nucleotide polymorphism (val158met) (Figure 3A). The low-enzyme-activity allele (met) is associated with enhanced WM capacity and attentional focus [50,51] and, more recently, it has been associated with more efficiency (lower amplitude) in sustained WM-related activity in PFC [52], likely due to higher tonic dopamine presence in PFC. In contrast, while the high-enzyme-activity allele (val) is associated with less attentional focus, it allows for greater cognitive flexibility [53] possibly due to greater sensitivity to transient dopamine bursts, which are thought to initiate WM updates [54,55]. Importantly, these findings point to a mechanistic understanding of the contribution of specific COMT variants to individual differences in neurobiology and behavior.
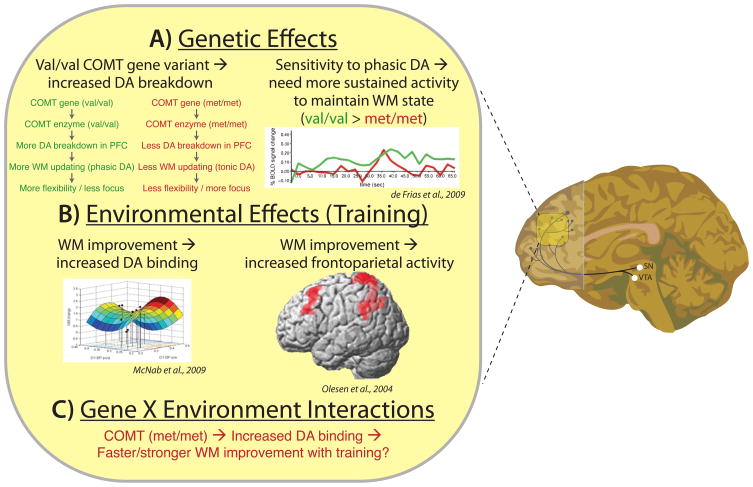
Figure 3. Explaining individual variation in PFC function: An example of how neural mechanisms can bridge the gap between genetic/environmental factors and individual differences.
A) PFC activity covaries with the COMT val158met variant (on right) [52]. One explanation for this correlation involves the differential breakdown of dopamine in PFC across val (more breakdown) and met (less breakdown) genotype variants (on left). B) PFC dopamine receptor binding (left) and PFC activity during a WM task (right) are modulated by practice performing particular WM tasks [66,67], suggesting that frequently performed day-to-day tasks (e.g., frequently dialing phone numbers from memory) may affect PFC function, which in turn may increase WM capabilities. C) Further research is necessary to establish clear gene X environment interactions between COMT variants, PFC function, and individual differences in behavior. We suggest that the met variant, which involves slower breakdown of DA, may promote an increase in DA receptor binding (and a possible increase in DA receptors) to allow for faster/stronger increases in WM capacity with practice.
Having a mechanistic account of a gene-behavior relationship provides several advantages beyond simply identifying a complex set of biomarkers. For instance, it has been shown that the met COMT variant is associated both with greater WM capacity [50] as well as trait anxiety [56]. This initially appears to be a somewhat random association, yet a mechanistic understanding of how these COMT variants influence neural processing demonstrates that dopamine (the molecule affected by the COMT enzyme) influences both WM (in PFC) and affect (in striatum) [57]. We expect that mechanistic understanding of other gene-behavior relationships can provide similar insights, as well as novel predictions that can improve understanding even further.
Another major advantage of taking a mechanistic perspective is the ability to identify computational trade-offs in genetic variants. Identifying these trade-offs can lend insight into why major variants exist in the population in the first place. For instance, the COMT val158met genotypes trade off between efficient WM updating (val) and robust WM maintenance (met) (see Figure 3A). This prominent bimodal distribution in the population may exist because evolution favored optimization of one or the other variant depending on particular environmental contexts [58].
To illustrate, consider several ways in which this trade-off may express itself in actual behavior. The Dual Mechanisms of Control theory [59,60] suggests that val individuals might tend towards a reactive cognitive control strategy characterized by increased flexibility yet reduced preparation, while met individuals might use a more proactive control strategy characterized by reduced flexibility yet increased preparation. The reactive strategy is likely much faster in unpredictable contexts (thus creating evolutionary pressure toward the val allele in chaotic environments), while the proactive strategy is likely faster and more effective in predictable contexts (creating an opposing evolutionary pressure toward the met allele when the environment is stable). The tremendous variability of human experience likely drove the population toward two extremes in this computational trade-off. Similarly, the COMT val158met genotype may influence the trade-off between exploration (greater for val) and exploitation (greater for met) [61], which are also linked to differential evolutionary advantages/risks depending on the environmental context. For instance, a bias towards exploration can lead to greater advantages when the available resources are becoming rapidly depleted, whereas a bias towards exploitation is optimal when the environment is stable and has already been adequately sampled [62,63].
Nevertheless, genetic factors are unlikely to fully account for most individual differences. Environmental influences, such as practice effects, likely account for a large component of variance as well. Supporting this conclusion, it has been shown that WM capacity increases with practice performing particular executive control tasks [64,65]. This practice effect has been shown to increase activity [66] and dopamine receptor density [67] in a fronto-parietal network linked to executive control functions (Figure 3B). These examples demonstrate an important role for life experiences on individual differences, and illustrate the importance of cognitive neuroscience in identifying the exact mechanisms by which different environments can result in observed individual differences.
In addition to bridging the gap between genetic and environmental sources of variation, cognitive neuroscience can also identify links between these factors as they interact to produce individual differences in behavior. Evidence for this kind of gene X environment interaction is emerging from studies of learning and associated neural changes. Recent studies have demonstrated that there is a relationship between learning and brain plasticity in white matter [68], gray matter [69], and functional connectivity [70], as well as between brain plasticity and genetics [71]. However, it remains unclear if these gene-influenced differences in plasticity actually affect experience-dependent learning. Research into the genetic determinants of brain plasticity and the effects of brain plasticity on learning will be essential for addressing this issue, and for understanding the neural bases of individual differences in behavior more generally.
Conclusion
The increased utilization of individual differences approaches in cognitive neuroscience research has advanced our understanding of how neural mechanisms of executive control contribute to a variety of domains, including working memory capacity, personality, motivation, and emotion regulation. Although individual difference approaches involve special methodological considerations and challenges, they represent a complementary approach to standard experimental manipulations that provides increased inferential and explanatory power when testing hypotheses relating the efficacy of putative control mechanisms to successful behavioral performance. More importantly, cognitive neuroscience-based individual differences approaches may provide a bridging level of description and analysis that facilitates understanding of the causal mechanisms linking physiological effects of both genotype expression and experience-dependent changes (i.e., environmental factors) to cognitive and behavioral variation in executive control.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
● Of special interest
●● Of outstanding interest
- 1.Underwood BJ. NORTHWESTERN UNIV EVANSTON ILL DEPT OF PSYCHOLOGY. Individual differences as a crucible in theory construction. 1974. [Google Scholar]
- 2.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin and Review. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- 3.Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- 4.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex ‘frontal lobe’ tasks: A latent variable analysis. Cognitive psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 5.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- 7.Gray JR, Burgess GC, Schaefer A, Yarkoni T, Larsen RJ, Braver TS. Affective personality differences in neural processing efficiency confirmed using fMRI. Cogn Affect Behav Neurosci. 2005;5:182–190. doi: 10.3758/cabn.5.2.182. [DOI] [PubMed] [Google Scholar]
- 8.Fales CL, Barch DM, Burgess GC, Schaefer A, Mennin DS, Gray JA, Braver TS. Anxiety and cognitive efficiency: Differential modulation of transient and sustained neural activity during a working memory task. Cognitive, Affective, and Behavioral Neuroscience. 2008;8:239–253. doi: 10.3758/cabn.8.3.239. [DOI] [PubMed] [Google Scholar]
- 9.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- 10.Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- 11.Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- 12.DeYoung CG, Shamosh NA, Green AE, Braver TS, Gray JR. Intellect as distinct from Openness: differences revealed by fMRI of working memory. J Pers Soc Psychol. 2009;97:883–892. doi: 10.1037/a0016615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ●● 13.Yarkoni T, Braver TS. Cognitive neuroscience approaches to individual differences in working memory and executive control: Conceptual and methodological issues. In: Gruszka M, Szymura, editors. Handbook of Individual Differences in Cogntition. in press. Reviews a number of important methodological and conceptual considerations when conducting individual differences analyses of fMRI data, focusing particularly on applications to executive control. Issues covered include statistical power, reliability, and the relation between between-subject and within-subject analyses, among others. [Google Scholar]
- 14.Yarkoni T. Big correlations in little studies: Inflated fMRI correlations reflect low statistical power. Perspectives on Psychological Science. 2009;4:294–298. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- 15.Ioannidis JP. Why most discovered true associations are inflated. Epidemiology. 2008;19:640–648. doi: 10.1097/EDE.0b013e31818131e7. [DOI] [PubMed] [Google Scholar]
- 16.Preacher KJ, Rucker DD, MacCallum RC, Nicewander WA. Use of the extreme groups approach: A critical reexamination and new recommendations. Psychological Methods. 2005;10:178–192. doi: 10.1037/1082-989X.10.2.178. [DOI] [PubMed] [Google Scholar]
- 17.MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological Methods. 2002;7:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- 18.Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- 19.Yarkoni T, Braver TS. Cognitive neuroscience approaches to individual differences in working memory and executive control: Conceptual and methodological issues. In: Gruszka A, Matthews G, Szymura B, editors. Handbook of Individual Differences in Cognition: Attention, Memory and Executive Control. in press. [Google Scholar]
- 20.Cohen MX, Schoene-Bake JC, Elger CE, Weber B. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci. 2009;12:32–34. doi: 10.1038/nn.2228. [DOI] [PubMed] [Google Scholar]
- 21.Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Protzner AB, McIntosh AR. The interplay of stimulus modality and response latency in neural network organization for simple working memory tasks. J Neurosci. 2007;27:3187–3197. doi: 10.1523/JNEUROSCI.4963-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schlosser RG, Wagner G, Sauer H. Assessing the working memory network: studies with functional magnetic resonance imaging and structural equation modeling. Neuroscience. 2006;139:91–103. doi: 10.1016/j.neuroscience.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 24.Rowe JB, Eckstein D, Braver T, Owen AM. How does reward expectation influence cognition in the human brain? J Cogn Neurosci. 2008;20:1980–1992. doi: 10.1162/jocn.2008.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. Brain Anatomical Network and Intelligence. PLoS Computational Biology. 2009:5. doi: 10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Heuvel MP, Mandl RCW, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;13 doi: 10.1002/hbm.20737. Advance online publication. Retrieved May 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim SL, Padmala S, Pessoa L. Segregating the significant from the mundane on a moment-to-moment basis via direct and indirect amygdala contributions. Proc Natl Acad Sci U S A. 2009;106:16841–16846. doi: 10.1073/pnas.0904551106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ● 28.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. A suite of newly developed tools for mediation/moderation analysis of fMRI data used to identify dissociable prefrontal-subcortical pathways that mediated the effects of cognitive reappraisal on emotional experience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- 30.Todd JJ, Marois R. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cogn Affect Behav Neurosci. 2005;5:144–155. doi: 10.3758/cabn.5.2.144. [DOI] [PubMed] [Google Scholar]
- ● 31.Edin F, Klingberg T, Johansson P, McNab F, Tegner J, Compte A. Mechanism for top-down control of working memory capacity. Proc Natl Acad Sci U S A. 2009;106:6802–6807. doi: 10.1073/pnas.0901894106. Computational analyses along with supporting neuroimaging data demonstrating that the core mechanism of working memory capacity might be lateral inhibitory interactions within parietal regions, but that individual differences in capacity might reflect variation in a dynamic top-down excitatory drive signal to this system from the lateral prefrontal cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cools R, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci. 2008;28:1208–1212. doi: 10.1523/JNEUROSCI.4475-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landau SM, Lal R, O’Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb Cortex. 2009;19:445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ● 34.Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2009;12:92–98. doi: 10.1038/nn.2242. Surprising evidence that trait anxiety is associated with a reduced response in prefrontal cortex to response conflict even in the absence of threat, but only under conditions of low perceptual load, suggesting deficient allocation of attentional control when these are not fully engaged by the task at hand. [DOI] [PubMed] [Google Scholar]
- 35.Hahn T, Dresler T, Ehlis AC, Plichta MM, Heinzel S, Polak T, Lesch KP, Breuer F, Jakob PM, Fallgatter AJ. Neural response to reward anticipation is modulated by Gray’s impulsivity. Neuroimage. 2009;46:1148–1153. doi: 10.1016/j.neuroimage.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 36.Linke J, Kirsch P, King AV, Gass A, Hennerici MG, Bongers A, Wessa M. Motivational orientation modulates the neural response to reward. Neuroimage. 2010;49:2618–2625. doi: 10.1016/j.neuroimage.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Simon JJ, Walther S, Fiebach CJ, Friederich HC, Stippich C, Weisbrod M, Kaiser S. Neural reward processing is modulated by approach- and avoidance-related personality traits. Neuroimage. 2010;49:1868–1874. doi: 10.1016/j.neuroimage.2009.09.016. [DOI] [PubMed] [Google Scholar]
- ● 38.Engelmann JB, Damaraju E, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Front Hum Neurosci. 2009;3:4. doi: 10.3389/neuro.09.004.2009. Evidence that individual differences in personality traits indexing reward sensitivity modulate both sustained and transient neural activation dynamics during visuospatial attention, under task conditions in which reward incentives are used to manipulate motivational state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locke HS, Braver TS. Motivational influences on cognitive control: Behavior, brain activation, and individual differences. Cognitive, Affective, and Behavioral Neuroscience. 2008;8:99–112. doi: 10.3758/cabn.8.1.99. [DOI] [PubMed] [Google Scholar]
- 40.Jimura K, Locke HS, Braver TS. Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. in submission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urry HL, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. Neuroimage. 2009;47:852–863. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proc Natl Acad Sci U S A. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ando J, Ono Y, Wright MJ. Genetic structure of spatial and verbal working memory. Behav Genet. 2001;31:615–624. doi: 10.1023/a:1013353613591. [DOI] [PubMed] [Google Scholar]
- 45.Luciano M, Wright M, Smith GA, Geffen GM, Geffen LB, Martin NG. Genetic covariance among measures of information processing speed, working memory, and IQ. Behav Genet. 2001;31:581–592. doi: 10.1023/a:1013397428612. [DOI] [PubMed] [Google Scholar]
- ●● 46.Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. in press, advance online publication. An accessible and comprehensive overview of intelligence, with a notable integration of recent neurobiological studies with the older and more extensive tradition of psychometric research in this domain. [Google Scholar]
- ●● 47.Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of experimental psychology General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. Intriguing evidence that a latent executive function variable comprising measures of inhibition, working memory updating and task-set shifting had 99% of variance explained by a genetic contribution. Note that WM capacity was not included in their statistical model, leaving open the possibility that it can be strongly influenced by practice (as suggested by others) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Reviews Neuroscience. 2006;7:818. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- 49.Greene CM, Braet W, Johnson KA, Bellgrove MA. Imaging the genetics of executive function. Biological Psychology. 2008;79:30–42. doi: 10.1016/j.biopsycho.2007.11.009. [DOI] [PubMed] [Google Scholar]
- ●● 50.Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. Review and theoretical synthesis of literature relating the COMT genetic polymorphism to individual variation between tonic vs. phasic modes of dopamine transmission, and consequent effects on working memory maintenance vs. updating functions. [DOI] [PubMed] [Google Scholar]
- 51.Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- ●● 52.de Frias C, Marklund P, Eriksson E, Larsson A, Oman L, Annerbrink K, Bäckman L, Nilsson L, Nyberg L. Influence of COMT Gene Polymorphism on fMRI-assessed Sustained and Transient Activity during a Working Memory Task. Journal of cognitive neuroscience. 2009 doi: 10.1162/jocn.2009.21318. Reports that the COMT val158met gene variants affect lateral PFC dynamics during WM, with more sustained activity for the val variant, potentially involving greater DA breakdown and, according to the authors, greater PFC activity necessary for WM maintenance (due to increased difficulty). Greater transient activity for the met variant is reported in hippocampus, consistent with greater difficulty updating WM for met individuals. [DOI] [PubMed] [Google Scholar]
- 53.Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, Goldman RS, Hoptman MJ, Sheitman B, Lindenmayer JP. Neurocognitive correlates of the COMT Val158Met polymorphism in chronic schizophrenia. Biological Psychiatry. 2002;52:701–707. doi: 10.1016/s0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- 54.Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Current Opinion in Neurobiology. 2002;12:223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- 55.Hazy TE, Frank MJ, O’Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:1601. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmack K, Schlagenhauf F, Sterzer P, Wrase J, Beck A, Dembler T, Kalus P, Puls I, Sander T, Heinz A. Catechol-O-methyltransferase val158met genotype influences neural processing of reward anticipation. Neuroimage. 2008;42:1631–1638. doi: 10.1016/j.neuroimage.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 57.Cools R. Role of dopamine in the motivational and cognitive control of behavior. The Neuroscientist. 2008;14:381. doi: 10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- 58.Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Molecular Psychiatry. 2009 doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- 59.Braver TS, Gray JR, Burgess GC. Explaining the many varieties of working memory variation: Dual mechanisms of cognitive control. Variation in working memory. 2007:76–106. [Google Scholar]
- ●● 60.Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. Outlines a theory of variability in cognitive control in which both situational fluctuations and individual differences might be explained by flexible shifts between proactive and reactive modes of control, characterized by anticipatory/sustained vs. transient/stimulus-driven engagement of lateral PFC and associated regions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ● 61.Frank MJ, Doll BB, Oas-Terpstra J, Moreno F. Prefrontal and striatal dopaminergic genes predict individual differences in exploration and exploitation. Nature Neuroscience. 2009;12:1062–1068. doi: 10.1038/nn.2342. An elegant combination of molecular genetic and computational neuroscience approaches, reveals that polymorphisms in distinct genes controlling prefrontal and striatal dopamine function differentially modulate the basic trade-off between exploitation and exploration during a novel reinforcement learning paradigm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. Journal of Comparative Neurology. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- 63.Cohen JD, McClure SM, Yu AJ. Should I stay or should I go? How the human brain manages the trade-off between exploitation and exploration. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:933. doi: 10.1098/rstb.2007.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. Journal of clinical and experimental neuropsychology. 2002;24:781–791. doi: 10.1076/jcen.24.6.781.8395. [DOI] [PubMed] [Google Scholar]
- 65.Westerberg H, Klingberg T. Changes in cortical activity after training of working memory--a single-subject analysis. Physiol Behav. 2007;92:186–192. doi: 10.1016/j.physbeh.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 66.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature Neuroscience. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- ● 67.Mcnab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in Cortical Dopamine D1 Receptor Binding Associated with Cognitive Training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. Increased D1 binding potential in PFC and parietal cortices accompanying increased WM capacity from training. [DOI] [PubMed] [Google Scholar]
- 68.Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nature Neuroscience. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ceccarelli A, Rocca MA, Pagani E, Falini A, Comi G, Filippi M. Cognitive learning is associated with gray matter changes in healthy human individuals: A tensor-based morphometry study. Neuroimage. 2009:1–18. doi: 10.1016/j.neuroimage.2009.07.009. [DOI] [PubMed] [Google Scholar]
- ● 70.Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci USA. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. Demonstrates that resting state functional connectivity MRI (fs-fcMRI) measures can be influenced by practice. Unlike gross anatomical measures, fs-fcMRI likely reflects functional connection weights across many synapses, providing important complementary information about changes in stable functional characteristics of the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, Rothwell JC. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. The Journal of Physiology. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]