Multi-Institutional Phase II Study of Selumetinib in Patients With Metastatic Biliary Cancers (original) (raw)
Abstract
Purpose
Biliary cancers (BCs) carry a poor prognosis, but targeting the RAS/RAF/mitogen-activated protein kinase kinase (MEK)/extracellular signal-related kinase (ERK) pathway is of significance. Selumetinib is an inhibitor of MEK1/2, so this trial was designed to determine the safety and efficacy of selumetinib in BC.
Patients and Methods
This was a multi-institutional phase II study of selumetinib at 100 mg given orally twice per day to patients with advanced BC. The primary end point was response rate. All patients were required to provide tissue before enrolling. The levels of phosphorylated ERK (pERK) and AKT (pAKT) were assessed by immunohistochemistry. Tumors were genotyped for the presence of BRAF- and/or _RAS-_activating mutations.
Results
Twenty-eight eligible patients with a median age of 55.6 years were enrolled. Thirty-nine percent of patients had received one prior systemic therapy. Three patients (12%) had a confirmed objective response. Another 17 patients (68%) experienced stable disease (SD), 14 of whom (56%) experienced prolonged SD (> 16 weeks). Patients gained an average nonfluid weight of 8.6 pounds. Median progression-free survival was 3.7 months (95% CI, 3.5 to 4.9) and median overall survival was 9.8 months (95% CI, 5.97 to not available). Toxicities were mild, with rash (90%) and xerostomia (54%) being most frequent. Only one patient experienced grade 4 toxicity (fatigue). All patients had tissue available for analysis. No BRAF V600E mutations were found. Two patients with short-lived SD had KRAS mutations. Absence of pERK staining was associated with lack of response.
Conclusion
Selumetinib displays interesting activity and acceptable tolerability in patients with metastatic BC. Our results warrant further evaluation of selumetinib in patients with metastatic BC.
INTRODUCTION
Biliary cancers (BCs) are the second most common primary liver cancers.1 The tumor arises from the ductular epithelium of the biliary tree within the liver (intrahepatic), the extrahepatic ducts (extrahepatic), or the gallbladder.2 Intrahepatic cancer is steadily increasing in the Western world.1,2 Most patients present with advanced disease and die within a few months of diagnosis because of severe cachexia.2 Survival rate at 5 years is less than 5% and has remained unchanged for 30 years. Historically, there has been no satisfactory treatment available for patients with metastatic BC; patients faced a low response rate and poor survival.2–4 A recent phase II/III randomized study (Advanced Biliary Cancer [ABC] 01/02) suggested a superior outcome when cisplatin was added to gemcitabine versus gemcitabine alone.5 A subgroup analysis suggested that the observed advantage might be derived from the large proportion of patients (25%) with locally advanced disease.5
The RAS/RAF/MEK/ERK signaling pathway plays a central role in the regulation of cellular processes, including proliferation, apoptosis, and metabolism.6,7 This pathway is one of the most important and best understood mitogen-activated protein (MAP) kinase signal transduction pathways and is activated by a diverse group of extracellular signals, including growth factor receptors (eg, epidermal growth factor receptor, platelet-derived growth factor receptor) and cytokines.8 Activated RAS triggers the phosphorylation and activation of the RAF kinase, which then phosphorylates MEK1 and MEK2 on two serine residues.9 Activated MEK phosphorylates its only known substrates: ERK1 and ERK2. Phosphorylated ERK (pERK) dimerizes and translocates to the nucleus,10 where it is involved in several important cellular functions.
RAS and BRAF mutations are rarely found together in tumors, and this absence of overlap implies an important role for the RAS/RAF/MEK cascade in tumor formation.11,12 Although RAS mutations do not clearly determine whether a cell line will be sensitive to MEK inhibition, BRAF mutations are frequently associated with the more sensitive phenotype13 and may constitute a key survival mechanism for those cells.14 Selumetinib (AZD6244, ARRY-142886; AstraZeneca, Manchester, United Kingdom) is a second-generation, potent, selective, orally available, and uncompetitive small molecule inhibitor of the MAP kinase, MEK1/2.15 Selumetinib's activity was examined in a panel of human cancer cell lines that showed broad activity, particularly in lines containing the BRAF V600E–activating mutation.16
There is evidence that the frequencies of mutations of RAS and RAF in BC are distinctly different. In one study evaluating 69 patients with BC, 31 (45%) and 15 (22%) had KRAS mutations or V600E BRAF missense mutation, respectively; no patients had both.17 Mutations of the RAS genes have been observed in 10% to 57% of gallbladder carcinomas.18 In one study from Greece, mutations were observed in seven (335) of 21 gallbladder carcinomas.19 In another study, the V600E somatic mutation of BRAF was absent in all 62 archival biliary tract cancers analyzed.20
Considering these findings, we hypothesized that selumetinib would be active in patients with advanced BC. We also hypothesized that beneficial clinical effects of selumetinib would correlate with the presence of activating mutations in BRAF, activation of ERK, and/or lack of activation of the AKT pathway. We conducted and report here a phase II study of single-agent selumetinib in BC to evaluate its efficacy and tolerability at a dose of 100 mg given orally twice daily.
PATIENTS AND METHODS
Eligible patients were required to have histologically confirmed advanced biliary tract carcinoma. All patients were required to have either fresh or paraffin-embedded tissue from tumor blocks before enrolling onto the study. Patients had to have measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST),21 ≤ 1 prior systemic anticancer therapy; patients with prior cryotherapy, radiofrequency ablation, ethanol injection, transarterial chemoembolization, or photodynamic therapy were included provided that greater than 6 weeks had elapsed and indicator lesion(s) were outside the area of prior treatment. Additional criteria included life expectancy ≥ 12 weeks, Eastern Cooperative Oncology Group performance status of less than 2, and the ability to take and absorb oral medications. Patients had to have normal organ function, including total bilirubin ≤ 2 times the upper limit of normal and AST/ALT ≤ 3 times the upper limit of normal. Selected exclusion criteria included prior treatment with MEK inhibitors or sorafenib; major surgery within three weeks; brain metastases; history of malignancy other than BC within the previous three years except for adequately treated basal cell carcinoma, squamous cell cancer, or carcinoma of the cervix; uncontrolled intercurrent illness; pregnancy; HIV infection; QT interval greater than 500 milliseconds; and concomitant medication that can prolong the QT interval.
Study Design
This was a National Cancer Institute (NCI)/Cancer Therapy Evaluation Program (CTEP) –sponsored phase II, open label, multicenter trial led by The Ohio State University with the participation of Vanderbilt University, Emory University, and University of North Carolina. Selumetinib was provided by NCI/CTEP. The primary objective of this study was to determine the overall response rate (complete response and partial response) as defined by RECIST.21 Tissue samples were required from all patients before enrollment. Secondary objectives included evaluation of toxicity, overall survival (OS), progression-free survival (PFS), assessment of BRAF and KRAS mutations and measurement of pERK and pAKT as indicators for the activation of relevant pathways.
Selumetinib Administration and Dose Modifications
The starting dose and schedule of selumetinib was 100 mg given orally twice daily in a mix and drink formulation in 28-day cycles without interruption. Treatment was administered on an outpatient basis. The two parts of the formulation were selumetinib, supplied as a powder in glass bottles/vials, and an aqueous solution of the Captisol vehicle (Cydex Pharmaceuticals, Lenexa, KS), which was mixed and reconstituted as a suspension immediately before use. There were two levels of dose reductions planned (50 mg twice per day and 50 mg once per day) with patients taken off the study for additional dose reductions.
Assessment of Response and Toxicity
Radiologic assessment was done by computed tomography or magnetic resonance imaging (as long as the same consistent measure was used serially) every 8 weeks, and responses were measured according to RECIST.21 Toxicities were defined by the NCI–Common Terminology Criteria of Adverse Events version 3.0.
Correlative Studies
Tissue samples.
Formalin-fixed/paraffin-embedded (FFPE) or fresh frozen tissue samples were obtained from all patients before treatment. Portions of fresh frozen tissue were later fixed in formalin and paraffin embedded. Slides of 4 or 10 μm were prepared from FFPE blocks and used for immunohistochemistry or DNA extraction, respectively. From each paraffin block, one or more sections was stained with hematoxylin and eosin, and tumor-containing regions were identified by a pathologist.
Immunohistochemistry
FFPE tissue blocks were stained for pAKT and pERK as previously described.22 Briefly, FFPE tissue sections were dewaxed, soaked in alcohol, and incubated in hydrogen peroxide after microwaving in antigen-unmasking solution (Vector Lab, Burlingame, CA). Samples were then incubated with anti-pAKT (Ser473 specific) or anti-pERK (Thr 202/Tyr 204) antibodies from Cell Signaling Technology (Beverly, MA). Staining was performed with the Vectastain Universal Quick Kit (Vector Lab) using the manufacturer's protocol. Negative controls were produced with omission of primary antibodies, and positive controls included tissue samples previously examined and identified as positive for pAKT or pERK. Staining was scored (scale 0 to 3) by four independent investigators in a blinded review.
DNA Extraction and Genotyping
FFPE tissue was scraped from slides and placed into 200 μL 0.5% Tween 20. Samples were heated to 90°C for 10 minutes and then digested with proteinase K (0.2 mg/mL) at 55°C for 3 hours. After digestion, 400 μL of 5% Chelex 100 (Bio-Rad, Hercules, CA) in Tris-EDTA was added, and samples were heated to 99°C for 10 minutes followed by centrifugation at 4°C and 10,500 rotations per minute for 15 minutes. Supernatants were extracted with chloroform before ethanol precipitation of DNA. Extracted genomic DNA was quantified and evaluated for KRAS and BRAF mutations. DNA Extraction and allele-specific polymerase chain reaction mutational assays (using KRAS and BRAF kits [DxS, Manchester, United Kingdom]) were conducted at AstraZeneca in the United Kingdom to identify KRAS (G12C, A, D, V, S, R, G13D) and BRAF (V600E) mutational status.
Statistical Methods
Simon's two-stage minimax design was used; the true overall response rate was set at 10% and 30%, respectively, under null and alternative hypothesis. With type I and type II error rates both at 10%, a total of 25 evaluable patients were needed. If two or more responses were seen in the 16 patients from the first stage, the study would proceed to the second stage. If more than four responses were observed in the whole cohort, the agent would be considered promising. If efficacy goals were met in the first two stages, an additional 10 evaluable patients were to be treated to allow the evaluation of correlative end points with adequate statistical power. The Kaplan-Meier method was used to analyze data on secondary end points including PFS and OS. For each patient, the staining scores for pERK or pAKT from four raters were averaged and then dichotomized. For different subgroups created based on baseline pERK or pAKT status, median PFS and OS, together with their 95% CIs, were reported. Group difference was assessed with log-rank test. All data analyses were conducted using SAS (SAS/STAT User's Guide, Version 9, 1990; SAS Institute, Cary, NC). P values less than .05 were considered statistically significant.
RESULTS
Patient Characteristics
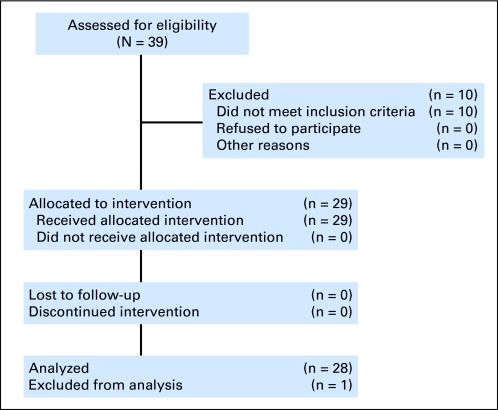
Twenty-nine patients were enrolled between December 2007 and January 2009. Patient characteristics are listed in Table 1. Of the 29 patients enrolled, 25 were evaluable for response. One patient was found to be ineligible, because of an incorrect diagnosis on pathologic review, and was removed from study 2 weeks after therapy. All remaining 28 patients were evaluable for toxicity and survival analysis (Fig 1). Representing the majority of patients enrolled onto the study, 17 (61%) had intrahepatic cholangiocarcinoma. Eleven (39%) had exposure to prior chemotherapy. All patients had metastatic disease, and the average number of target lesions was 4. As of August 2010, there were no patients treated on the study, and seven patients were alive.
Table 1.
Patient Demographics and Characteristics
| Characteristic | No. of Patients (n = 28*) |
|---|---|
| Sex | |
| Male | 19 |
| Female | 9 |
| Age, years | |
| Median | 55.6 |
| Range | 26-79 |
| Race/ethnicity | |
| White | 27 |
| African American | 1 |
| ECOG performance status | |
| 0 | 11 |
| 1 | 17 |
| Prior treatment | |
| X-ray treatment | 4 |
| Surgery | 8 |
| Chemotherapy | 11 |
| Metastatic | 7 |
| Adjuvant | 4 |
| Gemcitabine-based | 8 |
| Other† | 3 |
| Biologic therapy‡ | 2 |
| Biliary drainage/stenting | 4 |
| Disease site | |
| Intrahepatic | 17 |
| Gallbladder | 7 |
| Extrahepatic | 4 |
| Metastasis site | |
| Liver only | 7 |
| Liver and other§ | 16 |
| Other¶ | 5 |
| Number of target lesions | |
| Average | 4 |
| Range | 1-13 |
Fig 1.
CONSORT diagram.
Treatment Toxicity
The most common toxicities (Table 2) included rash (90%), xerostomia (54%), and nausea (51%). Most toxicities were grade 1 or 2. Only one patient suffered grade 4 toxicity (fatigue). No ocular toxicities were reported. All toxicities were manageable and reversible. Only 14% (4 of 28) of patients required dose reductions because of grade 3 fatigue, diarrhea, rash, or cellulitis.
Table 2.
Common Toxicities (n = 28)
| Toxicity | All Toxicities (%) | Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | Grade 4 (%) |
|---|---|---|---|---|---|
| Rash | 90 | 68 | 18 | 4 | 0 |
| Xerostomia | 54 | 25 | 29 | 0 | 0 |
| Nausea | 51 | 39 | 4 | 8 | 0 |
| Vomiting | 50 | 25 | 21 | 4 | 0 |
| Fatigue | 49 | 25 | 16 | 4 | 4 |
| Diarrhea | 45 | 4 | 25 | 16 | 0 |
| Bloating | 43 | 18 | 21 | 4 | 0 |
| Dry skin | 40 | 32 | 8 | 0 | 0 |
| Dysgeusia | 29 | 21 | 8 | 0 | 0 |
| Mucositis | 24 | 16 | 8 | 0 | 0 |
| Flatulence | 20 | 16 | 4 | 0 | 0 |
| Thrombocytopenia | 12 | 8 | 4 | 0 | 0 |
| Pruritus | 16 | 12 | 4 | 0 | 0 |
| Alopecia | 12 | 12 | 0 | 0 | 0 |
| Hypokalemia | 12 | 8 | 4 | 0 | 0 |
Treatment Efficacy
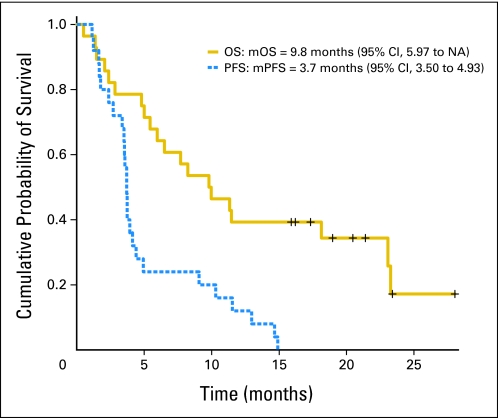
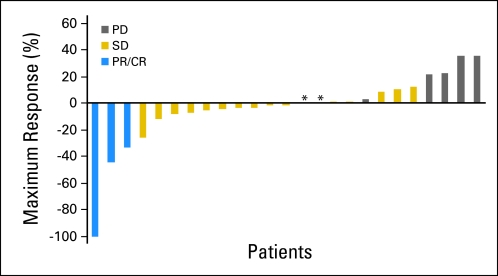
A median of 4 cycles were administered per patient (range, 1 to 16). Three patients had an objective response, including three with confirmed partial response (one of those had an unconfirmed complete response; Table 3 ). Seventeen patients (68%) experienced stable disease (SD), including 11 (44%) with SD duration ≥ 16 weeks and three (12%) with duration of more than 1 year. Study radiographs and responses were independently reviewed and found acceptable by CTEP. Median PFS (mPFS) was 3.7 months (95% CI, 3.5 to 4.9), and median OS (mOS) was 9.8 months (95% CI, 5.97 to not available; Fig 2). Figure 3 shows that the majority of patients (52%) experienced target lesion decrease. There was no correlation between rash or CA 19-9 levels with efficacy parameters. Exposure to prior therapy did not seem to influence the rate or duration of response. OS was lower for patients with prior exposure to therapy versus for patients with no prior exposure (5.43 v 18.1 months). Patients receiving selumetinib on this study experienced an average of 5% confirmed nonfluid weight gain (results are being published separately).
Table 3.
Efficacy Results
| Result | No. of Patients | % |
|---|---|---|
| Response* | ||
| Overall response rate | 3 | 12 |
| Complete response | 0 | 0 |
| Partial response† | 3 | 12 |
| Stable disease | 17 | 68 |
| Stable disease > 16 weeks | 14 | 56 |
| Progressive disease | 5 | 20 |
| Nonevaluable | 3 | 12 |
| mPFS, months | 3.7 | |
| 95% CI | 3.5 to 4.9 | |
| pAKT < 1 | 3.6 | |
| pAKT ≥ 1 | 3.7‡ | |
| pERK < 1 | 3.5 | |
| pERK ≥ 1 | 3.7§ | |
| mOS, months | 9.8 | |
| 95% CI | 5.97 to NA | |
| pAKT < 1 | 6.5 | |
| pAKT ≥ 1 | 9.9¶ | |
| pERK < 1 | 5.4 | |
| pERK ≥ 1 | 11.4∥ | |
| Weight gain, pounds# | ||
| Mean | 8.6 | |
| Range | 1.0-33.0 |
Fig 2.
Kaplan-Meier plots for overall survival (OS) and progression-free survival (PFS). mOS, median OS; NA, not available; mPFS, median PFS.
Fig 3.
Waterfall plot showing maximum percentage decrease in target lesions. (*) Patients with 0% change as maximum response. PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response.
Biologic Markers
Tissue specimens were available for all patients (n = 28). One sample for a patient with a best response of SD was found to contain insufficient tumor for correlative studies.
Immunohistochemistry Analysis of pERK and pAKT
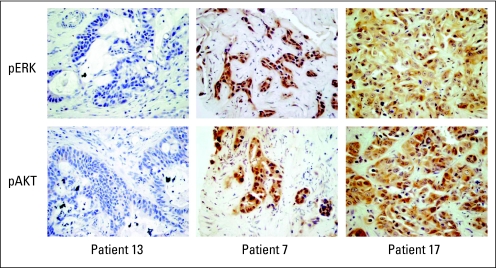
Staining for pAKT and pERK were evaluated in tumor samples from 27 individual patients. Independent scoring from four investigators ranged from 0 to 3 for pERK and pAKT (0 indicated no staining for the specific marker), and mean scores were calculated. The intraclass correlation coefficient between raters was 0.963 for pAKT and 0.977 for pERK, which suggests good agreement. There was no correlation between absent or low versus high pAKT or pERK status (average score, < 1 v ≥ 1) and mPFS. There was a numeric advantage with mOS with pERK ≥ 1 or pAKT ≥ 1 compared with pERK less than 1 or pAKT less than 1, although the difference did not reach statistical significance (Table 3). Because tumors with an average score between 0 and 1 had some scores of 0 and others higher, we also analyzed the data when grouping the samples as no detectable immunostaining (scored 0 by all reviewers) versus having detectable immunostaining. When analyzed in this manner, positive immunostaining for pERK was associated with improved OS (P = .0083, Appendix Tables A1 and A2, online only). It is notable that none of the 11 patients with pERK scores less than 1 were responders, and four of five patients with progressive disease were included in this group (Table 4). Representative immunohistochemistry results are shown in Appendix Figure A1 (online only).
Table 4.
Results for Immunohistochemistry, Response, PFS, and OS
| Patient ID | Best Response | PFS (months) | OS (months) | Mean pAKT Score* | Mean pERK Score* |
|---|---|---|---|---|---|
| 1 | SD | 14.7 | 31.0† | 0.5 | 0.75 |
| 2 | PD | 1.6 | 5.4 | 1.0 | 0.5 |
| 3 | SD | 4.9 | 7.6 | 2.25 | 0.0 |
| 4 | NE | 1.4 | 2.1 | 0.25 | 0.25 |
| 5 | PD | 1.2 | 2.1 | 1.5 | 0.0 |
| 6 | SD | 9.1 | 23.1 | 2.75 | 3.0 |
| 7 | PR | 4.4 | 8.2 | 1.0 | 1.0 |
| 8 | NE | NE | 10.0 | 2.0 | 1.0 |
| 9 | PD | 1.7 | 2.8 | 1.5 | 0.0 |
| 10 | SD | 3.6 | 23.3 | 0.0 | 0.875 |
| 11 | SD | 3.5 | 23.4† | 0.0 | 0.625 |
| 12 | PD | 1.1 | 1.5 | 0.0 | 0.0 |
| 13 | SD | 3.7 | 6.5 | 0.0 | 0.0 |
| 14 | PR | 10.3 | 24.4† | 1.5 | 2.0 |
| 15 | SD | 11.5 | 23.5† | 2.25 | 2.75 |
| 16 | SD | 14.9 | 18.0 | 3.0 | 2.0 |
| 17 | NE | NE | 0.5 | 2.75 | 3.0 |
| 18 | SD | 13.0 | 22.0† | NA | NA |
| 19 | SD | 3.4 | 11.5 | 1.0 | 1.0 |
| 20 | PR | 3.5 | 6.0 | 2.0 | 2.875 |
| 21 | PD | 2.0 | 19.4 | 1.125 | 2.625 |
| 22 | SD | 3.7 | 9.8 | 3.0 | 2.5 |
| 23 | SD | 2.7 | 19.2† | 0.75 | 1.0 |
| 24 | SD | 4.1 | 5.0 | 0.5 | 0.25 |
| 25 | SD | 4.1 | 11.3 | 0.5 | 2.0 |
| 26 | SD | 3.7 | 18.9† | 1.25 | 2.875 |
| 27 | SD | 3.9 | 4.8 | 0.0 | 1.5 |
| 28 | SD | 2.4 | 2.4 | 0.875 | 2.25 |
Mutational Analysis of BRAF and KRAS
One or more slides (10 μm) from each block was used for DNA extraction and genotyping. Slides from 28 individuals were genotyped for BRAF and KRAS mutations. BRAF evaluation was successful on all 28 samples, and 25 of 28 samples were sufficient for KRAS. All samples had wild-type BRAF sequencing. Of the 25 samples successfully evaluated for KRAS G12/13X mutations, two were positive, which indicates the presence of G12S and G12D mutations.
DISCUSSION
BC remains a challenging cancer. The rationale for this study was based on the demonstration of a potential role for BRAF and KRAS signaling pathways in the carcinogenesis of BC. Results from this study reveal interesting activity for selumetinib as a single agent in this patient population with metastatic BC that included patients (39%) with prior exposure to systemic therapy. There were three objective responses and another 14 patients with meaningful SD. Additionally, both mPFS and mOS compare favorably with published historical controls.2–5 Consistent with the PFS and response results was the target lesion decrease in 52% of patients as shown in Figure 3.
Selumetinib was fairly well tolerated with predominantly GI and cutaneous toxicities. Fourteen percent of treated patients required dose reduction for grade 3 diarrhea, fatigue, rash, or cellulitis. We did not observe any ocular toxicity with this MEK inhibitor.
In our population, no patients had a BRAF V600E mutation, and a there was a low occurrence (approximately 8%) of KRAS G12/13X mutations. Although other published studies suggest these mutations can be found in up to 60% of patients with BC,17–20 our study is the only prospective analysis of BRAF/KRAS mutational status in this patient population in the United States. Several published studies suggest that the presence of an activating mutation of BRAF in cancer cell lines predicts sensitivity to MEK inhibition in vitro. In our study and despite the presence of several patients who experienced clinical benefit, there were no BRAF V600E mutations. Of note, our study included a larger proportion of patients with intrahepatic disease compared with the general population of BC patients. This could limit the generalizability of our findings, including the interpretation of the molecular correlates, especially the mutational analyses.
Another finding in our study was the lack of a clear correlation between pERK or pAKT immunostaining and mPFS. Interestingly, patients with pERK score lower than 1 included 80% of patients whose disease progressed and none of the patients who experienced objective response. In addition, OS was lower in patients in whom pERK immunostaining was absent. Patients with pERK ≥ 1 or pAKT ≥ 1 had an improved but not statistically significant survival advantage. These findings raise the possibility that pERK and/or pAKT may have prognostic but not predictive relevance and suggest that tumor sample AKT activity may not predict resistance. We recognize that immunohistochemistry is qualitative and that staining can be heterogeneous. However, it is important to note that the agreement in scores between the independent observers was high and tissue samples were available from 95% of study patients, including every patient who experienced objective response or whose disease progressed. Given the exploratory nature of the correlative analyses and the limitation of such analyses in the setting of a small sample size, additional studies using robust and more objective measures are needed to confirm this potentially important finding. Finally, sequential biopsies in patients on therapy were not available to confirm pharmacodynamic inhibition of ERK activation.
Given the absence of clear predictors from the correlative studies presented, several other mechanisms may explain the interesting activity noted with selumetinib in BC. Alternative genetic or epigenetic alterations for this malignancy, such as activation as a result of BRAF gene mutations at other nucleotide positions or newly described MEK mutations,23,24 are certainly possible. A recent study identified transcriptional pathway signatures that could predict for MEK addiction and response to selumetinib with no absolute correlation with mutational or phosphoprotein markers of BRAF/MEK, RAS, or PI3K.25 Finally, immune modulation can potentially explain the observed activity of selumetinib, including the documented nonfluid weight gain. Selumetinib has been previously shown to inhibit secretion of interleukin (IL) -626 as well as of other cytokines including IL-1 and tumor necrosis factor. IL-1, IL-6, and tumor necrosis factor have all been implicated in the origins of cachexia in cancer.27–29 Cholangiocarcinoma cells constitutively secrete IL-6, a vital cytokine for cholangiocarcinogenesis, with a major role on survival signaling pathways and growth of these cells,30,31 and inhibition of IL-6 has been shown to attenuate growth of cholangiocarcinoma cell lines.32
A review of published results on selumetinib in various malignancies revealed few reported responses,15,33–38 specifically in melanoma (< 5%),36 lung cancer (5%),38 and thyroid cancer (< 3%).35 In our study, 12% of patients experienced objective responses, and 56% experienced prolonged SD; the majority of those with prolonged SD had target lesion decrease. Our study fell short of achieving the number of responses required to consider moving forward with single-agent selumetinib in patients with BC. One may argue that our choice of primary end point was not optimal, and our target response rate may have been ambitious. Nonetheless, with the presence of preliminary evidence of activity for selumetinib, development of this agent is warranted in combination with other promising agents, such as inhibitors of epidermal growth factor receptor39 or AKT.40,41
In conclusion, MEK inhibition with selumetinib was well tolerated and shows evidence of promising activity in BC. Correlative studies confirming the potential negative predictive value of absence of ERK phosphorylation and identifying new positive predictors of clinical response are needed to better understand the mechanisms of activity and to better select patients for treatment. The results of the present study suggest that selumetinib monotherapy has clinical activity in BC and may represent a particularly promising compound for inclusion in combinatorial strategies.
Appendix
Table A1.
Survival Results for pAKT With IHC Reference Point at Zero
| IHC Reference Point | OS | PFS | ||||
|---|---|---|---|---|---|---|
| Median (months) | 95% CI | P | Median (months) | 95% CI | P | |
| 0 | 6.5 | 4.8 to NA | .86 | 3.57 | 3.53 to NA | .24 |
| > 0 | 9.88 | 5.97 to NA | 3.7 | 3.34 to NA |
Table A2.
Survival Results for pERK With IHC Reference Point at Zero
| IHC Reference Point | OS | PFS | ||||
|---|---|---|---|---|---|---|
| Median (months) | 95% CI | P | Median (months) | 95% CI | P | |
| 0 | 2.83 | 2.07 to NA | .0083 | 1.67 | 1.23 to NA | .14 |
| > 0 | 11.4 | 8.23 to NA | 3.7 | 3.53 to 10.3 |
Fig A1.
Representative immunohistochemistry results from patients 13, 7, and 17 to demonstrate the range of staining. pERK, phosphorylated ERK; pAKT, phosphorylated AKT.
Footnotes
See accompanying editorial on page 2298 and article on page 2350
Supported by Grant No. NO1-CM62207 from the National Cancer Institute, National Institutes of Health, Bethesda, MD.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00553332.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Gilian Ellison, AstraZeneca (C) Consultant or Advisory Role: None Stock Ownership: None Honoraria: Matthew D. Ringel, AstraZeneca Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Tanios Bekaii-Saab, Mitch A. Phelps, Xiaobai Li, L. Austin Doyle, Michael Grever, Matthew D. Ringel, Miguel A. Villalona-Calero
Provision of study materials or patients: Tanios Bekaii-Saab, Laura Goff, John Sae Wook Kauh, Bert H. O'Neil, Mark Bloomston
Collection and assembly of data: Tanios Bekaii-Saab, Motoyasu Saji, Laura Goff, John Sae Wook Kauh, Bert H. O'Neil, Stephanie Balsom, Catherine Balint, Ryan Liersemann
Data analysis and interpretation: Tanios Bekaii-Saab, Mitch A. Phelps, Xiaobai Li, Motoyasu Saji, Vasily V. Vasko, Mark Bloomston, William Marsh, Gilian Ellison, Matthew D. Ringel
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. doi: 10.1016/S0140-6736(05)67530-7. [DOI] [PubMed] [Google Scholar]
- 3.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415–423. doi: 10.1634/theoncologist.2007-0252. [DOI] [PubMed] [Google Scholar]
- 4.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: A pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valle J, Wasan H, Palmer DH, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 6.O'Neill E, Kolch W. Conferring specificity on the ubiquitous Raf/MEK signalling pathway. Br J Cancer. 2004;90:283–288. doi: 10.1038/sj.bjc.6601488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nature Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 8.Janssen KP, Abal M, Abala M, et al. Mouse models of K-ras-initiated carcinogenesis. Biochim Biophys Acta. 2005;1756:145–154. doi: 10.1016/j.bbcan.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Ahn NG, Nahreini TS, Tolwinski NS, et al. Pharmacologic inhibitors of MKK1 and MKK2. Methods Enzymol. 2001;332:417–431. doi: 10.1016/s0076-6879(01)32219-x. [DOI] [PubMed] [Google Scholar]
- 10.Khokhlatchev AV, Canagarajah B, Wilsbacher J, et al. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- 11.Espinosa AV, Porchia L, Ringel MD. Targeting BRAF in thyroid cancer. Br J Cancer. 2007;96:16–20. doi: 10.1038/sj.bjc.6603520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 13.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 14.Wickenden JA, Jin H, Johnson M, et al. Colorectal cancer cells with the BRAF(V600E) mutation are addicted to the ERK1/2 pathway for growth factor-independent survival and repression of BIM. Oncogene. 2008;27:7150–7161. doi: 10.1038/onc.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adjei AA, Cohen RB, Franklin W, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–2146. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706–712. doi: 10.1136/gut.52.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves ME, DeMatteo RP. Genes and viruses in hepatobiliary neoplasia. Semin Surg Oncol. 2000;19:84–93. doi: 10.1002/1098-2388(200009)19:2<84::aid-ssu2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Saetta AA, Papanastasiou P, Michalopoulos NV, et al. Mutational analysis of BRAF in gallbladder carcinomas in association with K-ras and p53 mutations and microsatellite instability. Virchows Arch. 2004;445:179–182. doi: 10.1007/s00428-004-1046-9. [DOI] [PubMed] [Google Scholar]
- 20.Goldenberg D, Rosenbaum E, Argani P, et al. The V599E BRAF mutation is uncommon in biliary tract cancers. Mod Pathol. 2004;17:1386–1391. doi: 10.1038/modpathol.3800204. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Hou P, Ji M, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–3116. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 23.Marks JL, Gong Y, Chitale D, et al. Novel MEK1 mutation identified by mutational analysis of epidermal growth factor receptor signaling pathway genes in lung adenocarcinoma. Cancer Res. 2008;68:5524–5528. doi: 10.1158/0008-5472.CAN-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emery CM, Vijayendran KG, Zipser MC, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009;106:20411–20416. doi: 10.1073/pnas.0905833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dry JR, Pavey S, Pratilas CA, et al. Transcriptional pathway signatures predict MEK addiction and response to selumetinib (AZD6244) Cancer Res. 2010;70:2264–2273. doi: 10.1158/0008-5472.CAN-09-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tai YT, Fulciniti M, Hideshima T, et al. Targeting MEK induces myeloma-cell cytotoxicity and inhibits osteoclastogenesis. Blood. 2007;110:1656–1663. doi: 10.1182/blood-2007-03-081240. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Zhang D, Zhou Y, Wu L, et al. Association of IL-6 gene polymorphisms with cachexia susceptibility and survival time of patients with pancreatic cancer. Ann Clin Lab Sci. 2008;38:113–119. [PubMed] [Google Scholar]
- 28.Zhang D, Zheng H, Zhou Y, et al. Association of IL-1beta gene polymorphism with cachexia from locally advanced gastric cancer. BMC Cancer. 2007;7:45. doi: 10.1186/1471-2407-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Argilés JM, Busquets S, Toledo M, et al. The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care. 2009;3:263–268. doi: 10.1097/SPC.0b013e3283311d09. [DOI] [PubMed] [Google Scholar]
- 30.Wehbe H, Henson R, Meng F, et al. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res. 2006;66:10517–10524. doi: 10.1158/0008-5472.CAN-06-2130. [DOI] [PubMed] [Google Scholar]
- 31.Meng F, Yamagiwa Y, Ueno Y, et al. Over-expression of interleukin-6 enhances cell survival and transformed cell growth in human malignant cholangiocytes. J Hepatol. 2006;44:1055–1065. doi: 10.1016/j.jhep.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J, Tadlock L, Gores GJ, et al. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology. 1999;30:1128–1133. doi: 10.1002/hep.510300522. [DOI] [PubMed] [Google Scholar]
- 33.Banerji U, Camidge DR, Verheul HM, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16:1613–1623. doi: 10.1158/1078-0432.CCR-09-2483. [DOI] [PubMed] [Google Scholar]
- 34.Bennouna J, Lang I, Valladares-Ayerbes M, et al. A phase II, open-label, randomised study to assess the efficacy and safety of the MEK1/2 inhibitor AZD6244 (ARRY-142886) versus capecitabine monotherapy in patients with colorectal cancer who have failed one or two prior chemotherapeutic regimens. Invest New Drugs. doi: 10.1007/s10637-010-9392-8. [epub ahead of print on Feb 2, 2010] [DOI] [PubMed] [Google Scholar]
- 35.Lucas AS, Cohen EE, Cohen RB, et al. Phase II study and tissue correlative studies of AZD6244 (ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma (IRPTC) and papillary thyroid carcinoma (PTC) with follicular elements. J Clin Oncol. 2010;28(suppl):430s. abstr 5536. [Google Scholar]
- 36.Dummer R, Robert C, Chapman PB, et al. AZD6244 (ARRY-142886) vs temozolomide (TMZ) in patients (pts) with advanced melanoma: An open-label, randomized, multicenter, phase II study. J Clin Oncol. 2008;26(suppl):491s. abstr 9033. [Google Scholar]
- 37.O'Neil BH, Williams-Goff LW, Kauh J, et al. A phase II study of AZD6244 in advanced or metastatic hepatocellular carcinoma. J Clin Oncol. 2009;27(suppl) abstr e15574. [Google Scholar]
- 38.Tzekova V, Cebotaru C, Ciuleanu TE, et al. Efficacy and safety of AZD6244 (ARRY-142886) as second/third-line treatment of patients (pts) with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2008;26(suppl):431s. abstr 8029. [Google Scholar]
- 39.Philip PA, Mahoney MR, Allmer C, et al. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol. 2006;24:3069–3074. doi: 10.1200/JCO.2005.05.3579. [DOI] [PubMed] [Google Scholar]
- 40.Leelawat K, Leelawat S, Narong S, et al. Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4 induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2007;13:1561–1568. doi: 10.3748/wjg.v13.i10.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jimeno A, Rubio-Viqueira B, Amador ML, et al. Dual mitogen-activated protein kinase and epidermal growth factor receptor inhibition in biliary and pancreatic cancer. Mol Cancer Ther. 2007;6:1079–1088. doi: 10.1158/1535-7163.MCT-06-0448. [DOI] [PubMed] [Google Scholar]