Increased Very Low Density Lipoprotein Secretion, Hepatic Steatosis, and Insulin Resistance (original) (raw)
. Author manuscript; available in PMC: 2012 Sep 1.
Published in final edited form as: Trends Endocrinol Metab. 2011 May 26;22(9):353–363. doi: 10.1016/j.tem.2011.04.007
Abstract
Insulin resistance (IR) not only affects regulation of carbohydrate metabolism, but all aspects of lipid and lipoprotein metabolism. IR is associated with increased secretion of very low density lipoproteins (VLDL) and increased plasma triglycerides, as well as hepatic steatosis, despite the increased VLDL secretion. Here, we link IR with increased VLDL secretion and hepatic steatosis at both the physiologic and molecular levels. Increased VLDL secretion, together with the downstream effects on high density lipoprotein cholesterol and low density lipoprotein size is pro-atherogenic. Hepatic steatosis is a risk for steatohepatitis and cirrhosis. Understanding the complex inter-relationship between IR and these abnormalities of liver lipid homeostasis may provide insights relevant to new therapies for these increasing clinical problems.
Insulin resistance and dysregulated metabolism: not just a problem of carbohydrate metabolism
IR, a major abnormality underlying type 2 diabetes mellitus, is defined as the pathophysiological condition of reduced insulin responsiveness in liver, muscle, and adipose tissue (1;2). Although IR is typically considered in the context of carbohydrate metabolism, it is also a major driving force for the metabolic syndrome and the concomitant high risk for cardiovascular diseases (CVD) (2;3). Increased CVD in the metabolic syndrome is linked significantly to the lipid and lipoprotein abnormalities that are characteristic of individuals with IR (4). The dyslipidemia in IR is characterized by a triad of lipid abnormalities: 1) increased plasma concentrations of very low density lipoprotein (VLDL)-triglyceride (TG) and apolipoprotein B100 (apoB100; the full-length product of translation of the apob gene in the liver); 2) low levels of high density lipoprotein (HDL) cholesterol and apolipoprotein A1; and 3) relatively normal levels of low density lipoproteins (LDL) cholesterol with increased numbers of predominantly small, dense LDL particles. The latter two abnormalities are associated with the increased plasma VLDL levels via the actions of cholesterol ester transfer protein (CETP), which mediates the exchange of core lipids (TG and cholesterol ester (CE)) between VLDL and HDL or VLDL and LDL (5). These pathways will not be addressed in this review.
Here we focus on the current understanding of why there is increased hepatic assembly and secretion of VLDL particles in IR. Additionally, we review current understanding of the hepatic steatosis that also occurs in IR, despite the increased secretion of VLDL particles. Integral to these questions, we believe, is apoB100, which is required for the assembly and secretion of hepatic-derived VLDL, intermediate density lipoproteins (IDL), and LDL particles in humans. ApoB48 is a truncated translation product of the apob gene that is synthesized only in the small intestine in humans and is required for the assembly and secretion of chylomicrons, the very large TG-rich lipoproteins that transport dietary fat and cholesterol (apoB48 is also synthesized and secreted from rodent livers). Although we will touch on abnormalities in apoB48 secretion in IR, this review will focus mainly on apoB100. When we are referring to pathways and processes that affect both apoB100 and apoB48, we will simply use the term apoB.
The complexity of apoB100 and apoB48, both in terms of their structures (6) and itineraries in the secretory pathways of the hepatocyte and small intestine, respectively, (7–9) enables these proteins to efficiently and effectively carry out their major role: to transport lipids, particularly TG, to peripheral tissues. Unfortunately, as described in detail below, these same complexities lay the foundation of both the increased secretion of VLDL (and chylomicrons) and the predisposition to hepatic steatosis in people with IR.
Sources of hepatic TG in normal and IR states
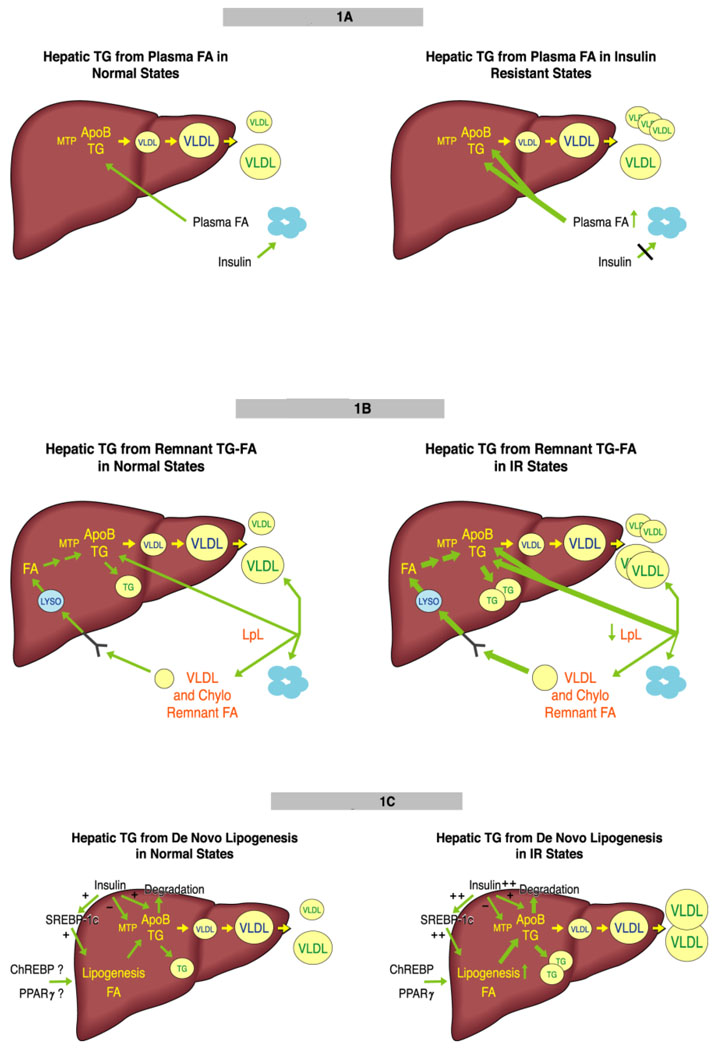
If the role of VLDL is to transport excess energy, mainly in the form of TG, out of the liver, it is not surprising that VLDL assembly and secretion is a substrate-dependent process that is highly regulated by the availability of TG (7;8;10). Hepatic TG derives from: 1) uptake of circulating albumin-bound fatty acids (FA); 2) uptake of circulating remnants of VLDL and chylomicron (the product oflipoprotein lipase (LpL)-mediated hydrolysis of the core TG in chylomicrons), with their remaining TG-FA; and 3) de novo synthesis of FA in the liver (lipogenesis). [FIGURE 1]
Fig 1.
Hepatic TG derives from three sources. (a) The major source is from lipolysis of adipose tissue TG. FA is released, bound to albumin, and circulates to all organs, with the large majority taken up by the liver through several pathways. The major regulation of adipose tissue lipolysis is via insulin signaling (left). In IR, there is increased release of FA throughout the day, with increased delivery to the liver. Studies in individuals with hepatic steatosis indicate that about 80% of hepatic and VLDL TG come from plasma FA. Studies in mice suggest that plasma FA can stimulate apoB secretion, even when the delivery of FA is not great enough to stimulate TG secretion. The result of IR-mediated perturbations is secretion of more VLDL that may also carry more TG (right). (b) VLDL and chylomicrons (not shown) deliver TGFA to adipose tissue via the action of LpL. Approximately 80% of TG in these lipoproteins is removed during this process. The resulting remnant lipoproteins can deliver their remaining TGFA to the liver using several pathways, mainly the LDL receptor. The TGFA enters the lysosome and FA are released (left). In IR, LpL activity may be modestly reduced, leading to TGFA-enriched remnants that deliver more FA to the liver. The result of this IR-mediated perturbation is secretion of more VLDL that can also be larger. (right). (c) When the liver has more energy than it requires, glucose is converted to FA by DNL. Insulin plays the major regulatory role in this process via stimulation of SREBP-1c expression and maturation. Insulin may inhibit MTP expression, thus down-regulating VLDL assembly, as well as targeting apoB for degradation. ChREBP and PPARγ may also stimulate DNL (left). In IR, insulin levels are increased and, although the liver does not respond to insulin in terms of suppression of glucose production and release, DNL is increased. It is not clear if insulin’s negative actions on MTP and apoB are maintained in IR. ChREPB and PPARγ probably add to the stimulation of DNL. The result of increased DNL is the secretion of the same number of larger VLDL (right).
Impaired insulin signaling and increased FA flux into the liver
It is well established from both in vitro and in vivo studies over several decades that moderate increases in FA delivery to hepatocytes or the liver, respectively, stimulate assembly and secretion of VLDL (Figure 1A) (9–14). It is generally accepted that FA levels in blood and FA flux to the liver are increased in insulin resistant humans with and without type 2 diabetes (14;15), although this finding is not universal (16). The higher amounts of FA are thought to result from the failure of insulin to suppress lipolysis of adipose tissue TG (17); insulin-mediated suppression may vary, however, in different tissue depots, with visceral adipose tissue being more resistant to insulin than lower limb adipose tissue (18). Visceral adipose tissue has been suggested to play a key role in the abnormalities of hepatic lipid metabolism observed in IR. However, the large majority of FA flux to the liver derives from upper body non-visceral fat, although it reaches the liver mostly via the portal vein (19).
Recent studies have demonstrated important nuances in the role of FA in stimulating VLDL secretion. Intravenous infusions of oleic acid (OA) bound to albumin stimulate apoB secretion in mice without stimulating TG secretion, whereas delivery of a similar absolute amount of OA derived from endocytosis of remnant-like particles during infusion of Intralipid (an emulsion of TG and phospholipids [PL]) did not affect apoB secretion (20). Several fold more OA had to be delivered via Intralipid to stimulate apoB secretion, and at those levels of OA, TG secretion was also increased (20). The mechanism whereby small quantities of OA bound to albumin stimulate apoB secretion without increasing TG secretion is unclear, but may explain why, in people with IR, increased apoB100 secretion by the liver may occur with little or no increase in TG secretion (21). Additionally, the type of FA may be crucial as palmitic acid (PA) and decosahexanoic acid (DHA) may have different effects from OA (discussed later in this review). An area that needs further exploration are the pathways whereby FA are taken up by the liver; both diffusion and receptor mediated mechanisms are likely to be important (22–24). The status of these pathways in IR has not been studied in depth, although recent reports suggest upregulation of some of the involved receptors in humans with hepatic steatosis (25;26).
Impaired metabolism of VLDL and chylomicron remnants in IR
Increased fasting and increased postprandial TG levels are characteristic abnormalities in IR (27). It was assumed for many years that increased postprandial TG was due to the combination of defective lipolysis of chylomicrons and VLDL, and increased VLDL secretion (27;28). Recent studies have, however, demonstrated that increased assembly and secretion of apoB48-containing chylomicrons in fructose fed hamsters (29) and humans during periods of hyperinsulinemia (30) is an additional factor. Increased FA flux to the small intestine, as well as IR, appears to play key roles in the intestine, just as they do in the liver (30;31). Thus, the small intestine appears to respond to IR in a manner similar to the liver (32). In addition to increased secretion of VLDL and chylomicrons, moderate reductions in LpL activity and increased secretion of apolipoprotein CIII (apo CIII), an inhibitor of LpL, into plasma in IR both contribute to less efficient lipolysis of VLDL and chylomicron TG (33), resulting in TG-enriched VLDL and chylomicron remnants that are removed by the liver (Figure 1B). Based on in vivo work with Intralipid (20) and early studies with cultured hepatocytes (34), this would be expected to result in increased assembly and secretion of VLDL apoB100 and TG.
Recent work on the “spillover” of dietary-derived chylomicron TG-FA has added further complexity to the role of postprandial lipids in the regulation of hepatic lipid metabolism (35;36). If modest, but significant quantities of FA generated by the lipolysis of chylomicron TG escapes “local” re-esterification in adipose tissue and are taken up, instead by the liver, they would act similarly to any other albumin-bound FA, and could stimulate apoB100 secretion irrespective of the stimulation of TG secretion.
Increased de novo FA synthesis in insulin resistance
The third source of hepatic TG is de novo lipogenesis (DNL) (Figure 1C). Although most of the detailed studies that we will refer to regarding the regulation of hepatic DNL have been conducted in rodents, lipogenesis does contribute significantly to VLDL secretion in humans, where it is increased in individuals with obesity and IR (37;38). Regulation of hepatic DNL by the transcription factor, sterol response element binding protein-1c (SREBP-1c), has been defined in great detail (39). SREBP-1c regulates nearly all genes involved in FA and TG synthesis, including acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), long-chain elongase (ELOVL6), stearoyl-CoA desaturase 1 (SCD1), and mitochondrial glycerol 3-phosphate acyltransferase (GPAT) (40). A major regulator of SREBP-1c is the liver x receptor (LXR) (41), which stimulates SREBP-1c transcription by binding to a LXR-response element in the promoter. LXR also directly stimulates transcription of the genes for both FAS gene (42) and the carbohydrate responsive element binding protein (ChREBP) (43), which regulates hepatic lipogenesis in a way that is complementary to SREBP-1c (44) (see below).
Insulin, at least in part through stimulation of LXR plays a central role in the regulation of hepatic SREBP-1c gene expression (40;41;43;45). Insulin also increases the cleavage of SREBP-1c to its mature, nuclear form via an LXR-independent mechanism (46). The role of insulin is best exemplified by the fasting–re-feeding paradigm in which mice that have been fasted 24 hrs are re-fed with high carbohydrate chow and show a dramatic stimulation of the key genes and proteins in the lipogenic pathway; insulin is critical to this response (47;48). Of great interest and importance to the pathophysiology of increased VLDL secretion and hepatic steatosis is the selective IR that occurs in the liver regarding insulin’s ability to regulate hepatic glucose and lipid metabolism (49). The basis for the continued ability of insulin to stimulate DNL at the same time that the hormone is unable to suppress hepatic gluconeogenesis has been a focus of research for the past decade. Although a detailed description of the studies done is beyond the scope of this review, it is clearly important to summarize this body of work and the models that have been proposed.
Differential insulin signaling through IRS-1 and IRS-2 has been proposed as a basis of selective insulin hepatic insulin resistance, where IRS-1 signaling through SREBP-1c remains active while IRS-2 mediated suppression of gluconeogenesis via deactivation of FoxO1 is lost (50). However, recent studies indicate that both IRS-1 and IRS-2 play important roles in regulating both glucose and lipid metabolism in the liver (51;52). Another proposal for selective insulin resistance is that while AKT plays a central role in transmitting insulin signaling to the carbohydrate pathway, other kinases are important for the regulation of DNL, particularly the atypical PKClambda(zeta) (53–55) and PKCbeta (53). Other studies, however, have provided strong evidence that AKT2 is critical for insulin stimulation of DNL and the development of hepatic steatosis (56;57). If the latter studies are correct, then how does selective IR occur if AKT2 is central to both insulin-mediated suppression of gluconeogenesis and insulin-stimulated DNL?
Several groups have studied the potential role of FoxO1 in DNL and hepatic steatosis, but at this point in time, genetic manipulations with gain and loss of FoxO1 function have produced an unclear picture (58–60). Thus, while FoxO1 may play a role in VLDL secretion via regulation of microsomal triglyceride transfer protein (see below), its role as a direct regulator of DNL remains to be determined. A potentially important alternative insulin signaling pathway for DNL is the mammalian target of rapamycin complex 1 (mTORC1) pathway. mTORC1 is a protein complex centrally involved in protein synthesis and cell growth that is sensitive to nutrient flux. This could be the pathway for the selective insulin-stimulated induction of SREBP-1c expression in an otherwise insulin resistant liver (61;62).
Another area of recent interest involves the role of micro-RNAs in lipid metabolism (63). In particular, both in vitro (64) and in vivo (65) studies have implicated miR-122 as an inhibitor of genes in the DNL pathway. In contrast, miR-33, which is transcribed from an SREBP2 intron, inhibits transcription of several genes involved in FA oxidation as well as the gene for cholesterol transporter, ABCA1 (66). Most recently, Trib1, a gene identified by GWAS as potentially involved in lipid metabolism, has been shown to regulate DNL, VLDL secretion, and plasma lipid levels in mice (67). How IR might affect micro-RNAs or Trib1 is unknown. Lastly, several groups have provided evidence linking endoplasmic reticulum (ER) stress to increased lipogenesis; this will be discussed later in this review.
In addition to SREBP-1c, two other transcription factors play roles in hepatic fat metabolism, particularly in states of IR. ChREBP, noted earlier, is regulated mainly by glucose, but is thought to play a complementary role in the regulation of hepatic DNL (44). The fact that LXR, which is a key regulator of SREBP-1c, also regulates ChREBP (43) provides further evidence for a network of regulation in which both glucose and insulin are important. In the insulin resistant ob/ob mouse model, ChREBP is required for complete activation of SREBP-1c, Acc, Fas, Elovl6, and Scd1 activation (68). In this model, ChREBP knockdown not only reduced the rate of DNL but also increased FA oxidation. ChREBP-deficient mice have reduced rates of both glucose and FA synthesis (69). Overall, several studies suggest that ChREBP can be responsible for as much as 50% of DNL under certain conditions. Most relevant to this review, in IR, especially when glucose flux through the liver is increased, the role of ChREBP in stimulating DNL may be exaggerated.
Hepatic peroxisome proliferators activated receptor gamma (PPARγ) might be another important regulator of DNL and TG synthesis (70–72). I Increased hepatic DNL in the apoB/BATless mouse, a model of IR, increased VLDL secretion, and hepatic steatosis, was associated with increased hepatic expression of PPARγ2, but not SREBP-1c or ChREBP (73). Furthermore, after knockdown of _Ppar_γ expression with anti-sense oligonucleotide, Fas and Acc mRNA levels as well as biochemically measured DNL and hepatic TG content were reduced, indicating a role for hepatic expression of PPARγ2 in the regulation of hepatic DNL and TG synthesis. Of note, neither Fas nor Acc are known to have PPARγ response elements in their promoters, suggesting an indirect effect of PPARγ2 on DNL. Relevant to states of IR, Pparγ2 gene expression can be stimulated in primary mouse hepatocytes by insulin and OA (74).
Disposition of hepatic TG and IR
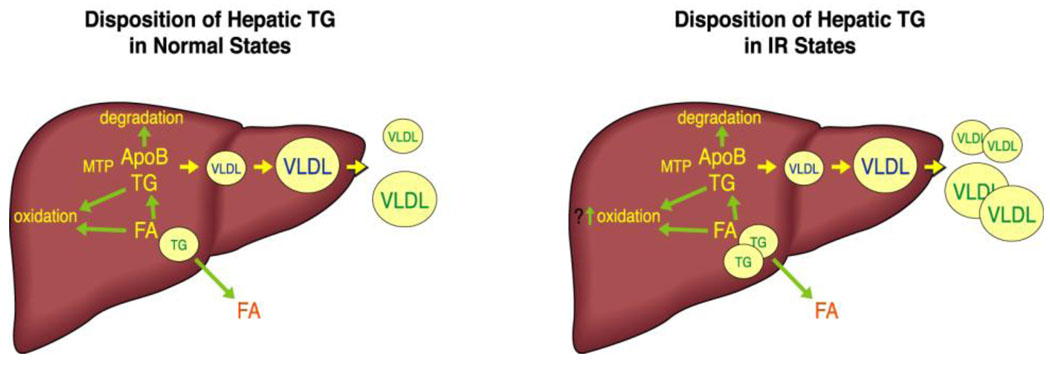
We have reviewed the three major pathways for delivery to and synthesis within the liver of FA and TG, as well as the dysregulation of each of those pathways in states of IR. Once TG begins to accumulate in the liver, there are also three pathways that handle its disposition: oxidation, storage, and secretion. It is clear that dysregulation of any of these pathways can result in abnormalites in both VLDL secretion and hepatic lipid content. We will examine each of these pathways, with focus on their roles in IR-associated increases in VLDL secretion and in hepatic steatosis. (FIGURE 2)
Fig 2.
TG that is synthesized in the liver can be disposed of by three processes. FA can be released from TG and oxidized: FA are a major source of energy for the liver. FA can be released from TG and then re-esterified into TG and assembled, with apoB, into VLDL for secretion. Recent studies in mice suggest that hepatocytes can also secrete FA into the blood; there is no evidence for this in humans. Finally, TG can be stored in lipid droplets (left). In IR, increased TG storage in lipid droplets results from increased availability of FA from plasma, remnants, and DNL. There is no evidence that steatosis results from decreased FA oxidation. In fact, the modest evidence that exists in humans suggests that FA oxidation is increased in people with IR. Secretion of TGFA is also increased in IR (right).
Hepatic FA oxidation and IR
Genetic or pharmacologic manipulation of hepatic FA oxidation results in altered hepatic TG content in numerous studies. However, there is minimal evidence that reduced FA oxidation is important for the increased rates of VLDL secretion and hepatic steatosis in diet-induced obesity in mice or in humans with IR (16). In fact, several studies point to increased (75) or normal (76) levels of hepatic FA oxidation in obesity and IR states. This, of course, does not rule out approaches to increase FA oxidation as potential therapies for the dyslipidemia/steatosis of IR.
Lipid droplets, FA oxidation, and VLDL secretion
The past decade has witnessed an explosion of new information regarding lipid droplets, first in adipocytes and more recently in hepatocytes. Rather than simply being passive depots for storage of excess energy, lipid droplets are complex and active components of the metabolic network that regulates energy metabolism in cells (77;78). The physiology of lipid droplets is mostly dependent on a family of proteins with similar structures; these include perilipin, adipocyte differentiation related protein (ADRP), tail interacting protein of 47Kda (TIP-47), S3–12, and oxidative tissue-enriched PAT protein (OXPAT). Recently, these have been renamed as members of the Perilipin Family (PLIN 1–5) (79). Other proteins of interest include members of the Cide family (80) and the FIT proteins (81). Genetic manipulations of several droplet coat proteins have been shown to affect hepatic lipid metabolism. For example, loss of PLIN 2 (ADRP) is associated with lower hepatic TG and increased VLDL secretion (82) whereas overexpression of hepatic ADRP has the opposite effect (83). Genetic manipulation of Cidec, also known as fat specific protein (FSP27) was associated with increased steatosis (forced Cidec expression) or decreased steatosis (Cidec knockdown) in hepatocytes. These observations are concordant with the increased amounts of FSP27 in fatty livers of ob/ob mice as well as the reduced FSP27 levels in livers of PPARγ-deficient mice.
In addition to lipid droplet-associated proteins, intracellular lipases play roles in regulating hepatic TG content. ATGL-deficient mice (84) have hepatic steatosis, in addition to increased adipose tissue and significant cardiac fat accumulation. Increased expression of ATGL in cultured hepatocytes results in increased FA oxidation, while knockdown of expression is associated with decreased FA oxidation (85). In these experiments, however, VLDL secretion was unaffected. Overexpression of ATGL in the liver was associated with reduced hepatic fat and increased FA oxidation and secretion (86). The role of HSL in the liver, where it is expressed at low levels, is less clear. In the whole body HSL-deficient mouse, although hepatic TG content was reduced, plasma FA levels were also decreased, making it difficult to identify the basis for less hepatic fat (13). Overexpresssion of HSL in the liver, however, results in increased FA oxidation and secretion along with lower hepatic TG content, suggesting a possible role for this enzyme (86). Even less information is available regarding how much droplet proteins and lipases are expressed in livers that are IR and/or steatotic. It is obvious, however, that this will be an emerging area of important new insights regarding the relationship between IR, VLDL secretion, and hepatic steatosis.
Recent studies of adiponutrin/patatin-like phospholipase domain-containing protein 3 (PNPLA3) are an example of the increasing importance of lipid droplet biology. A common SNP in PLNPA3 was identified in the Dallas Heart Study as being associated with significantly greater prevalence of non-alcoholic fatty liver disease (NAFLD) (87). This finding has been confirmed in several populations (88), but although gene expression is regulated by SREBP-1c and possibly ChREBP (89), there is no relationship between the presence of the SNP and either IR or the metabolic syndrome (87;90). The fact that PNPLA3 appears to be a TG-hydrolase and the presence of the SNP abolishes that activity in cultured cells (91), suggests that PNPLA3 plays a role as a facilitator of hepatic steatosis when other factors such as IR or alcohol drive TG synthesis (92). The recent finding that PNPLA3-deficient mice do not develop a fatty liver supports this view (93).
Assembly and secretion of VLDL and IR
Regulation of the assembly and secretion of VLDL
The assembly and secretion of VLDL, a large macromolecular aggregate of thousands of lipid molecules and several proteins, is extremely complex (7–9;94). Much of this complexity derives from the structure of apoB, but the physiologic basis for complex regulation is that VLDL is a vehicle for transporting energy from the liver to the periphery and, therefore, should be regulated by the metabolic status of the liver. There is general, but not complete, agreement that assembly of VLDL occurs in two-steps. The first step involves targeting of nascent, lipid-poor apoB100 for complete translocation into the lumen of the endoplasmic reticulum (ER) and away from co- or early post-translational degradation by the proteasome (95;96); this first step is dependent on some minimal lipidation and microsomal triglyceride transfer protein (MTP) (8). Exogenous FA-albumin uptake by the liver may play a stimulatory role at this point that is independent of their role as substrate for core lipid synthesis (20) (Figure 1A). The second step involves the addition of core lipids, mainly TG. Existing experimental data support models with either a continuous addition of core lipids while apoB100 is associated with the ER membrane, or a single fusion of a nascent apoB100 particle with a lipid droplet in the lumen of the ER (9). During the second step, MTP appears necessary for the transfer of TG from the ER membrane into the lumen (97) where lipid droplets can form without apoB (98;99). In contrast, MTP may not be necessary for the “fusion” of luminal lipid droplets into nascent VLDL (100;101). How this fusion occurs is unclear, although an intra-ER luminal lipase, triglyceride-hydrolase 1 (TGH1) has been proposed as the catalyst (102). The site of later addition of lipid is also debated, with some data (including our own) (103) indicating the ER as the site, while other data support post-ER compartments, including the Golgi, as the site where mature VLDL are formed (104). Although second-step lipid loading can provide some protection for apoB100 from degradation, this process mainly determines the size of the VLDL that is secreted. There are a number of other pathways that can degrade apoB post-translationally in the ER and post-ER (8), including autophagy (105–107). Of note, several lines of evidence suggest that TG derived from DNL increase the size but not the number of VLDL secreted by the liver (108–110); this contrasts with the potential for FA taken up from the circulation to directly stimulate apoB secretion (20).
Of interest is the finding in cultured rat hepatocytes that overexpression of apoCIII, which can occur in states of IR, may facilitate secretion of apoB and TG (111). However, reduced apoCIII production during treatment with pioglitazone did not lower in vivo measures of apoCIII secretion in humans although lower apoCIII secretion was associated with better lipolysis of VLDL (112).
Regulation of VLDL secretion by insulin in normal and IR states
The role of insulin in the regulation of hepatic VLDL secretion is central both to the normal regulation of nutrient flux into and out of the liver and to the abnormalities present in IR. Thus insulin is a key regulator of:1) FA flux to the liver; 2) hepatic DNL; 3) FA oxidation in the liver; 4) MTP synthesis; and 5) apoB degradation. We have addressed the first three already and will focus now on insulin’s effects on MTP and on apoB degradation, particularly in IR states.
Regulation of MTP by insulin
MTP is an 88 kDa ER resident chaperone protein that, when heterodimerized with protein disulfide isomerase (PDI), catalyzes the transfer of neutral lipids (TG, Pl, CE) to nascent apoB, a rate-limiting step in hepatic VLDL production (113). MTP gene expression is negatively regulated by insulin in a dose-, and time-dependent manner in HepG2 cells (114). Suppression of MTP by insulin occurs, at least partly, via FoxO1; insulin normally leads to phosphorylation of FoxO1 and its exclusion from the nucleus, resulting in the inhibition of hepatic MTP expression. However, in IR, FoxO1 phosphorylation is reduced and this transcription factor localizes preferentially in the nucleus, enhancing MTP expression (115). The loss of insulin-mediated inhibition of MTP gene expression could play a role in VLDL overproduction in states of IR. Indeed, expression of a constitutively active FoxO1 increased VLDL secretion in mice (116). Insulin may also inhibit MTP via a MAPK pathway (117). Foxa2 has also been suggested, along with PGC-1β, as a regulator of MTP gene expression (118); there is controversy regarding insulin-mediated signaling to Foxa2, however. Other suggested regulators of MTP, which could be altered by IR, including hepatocyte nuclear factor 4 (HNF4) and peroxisome proliferator-activated receptor alpha (PPARα) (119; 120). The complex nature of the relationship between insulin signaling, MTP expression and VLDL secretion is exemplified in our studies of the liver specific insulin receptor knockout mouse (LIRKO). In this model, FoxO1 should reside completely in the nucleus; however, MTP gene expression was only minimally increased and VLDL TG secretion was reduced by 50% (121). Furthermore, there were no changes in Mtp mRNA or protein in the apoB/BATless mouse, a model of IR and increased VLDL secretion (73). In contrast, M gene expression and protein are increased in ob/ob mice (122).
Regulation of apoB secretion by insulin
In addition to the role of insulin in the regulation of FA oxidation, DNL and MTP, insulin can directly affect the secretion of apoB100 by targeting it for degradation; apoB48 appears to be much less sensitive to insulin, at least in cultured liver cells. During the past 25 years, studies in cells, small animals, and humans have demonstrated a key role for hepatic insulin signaling in the short-term regulation of VLDL assembly and secretion (9; 123). In cultured hepatocytes, acute exposure of cells to insulin inhibits the secretion of both triglyceride (TG) and apoB despite concomitant stimulation of TG synthesis (123;124). Acute inhibition of VLDL secretion from hepatocytes by insulin results from direct targeting of apoB100 for degradation, at least in part, via PI3-kinase (PI3-K) mediated mechanisms (125–127), although recent data suggests this is not via AKT1 (128). The recent identification of the autophagy pathway as a site of post-ER degradation of aberrantly folded or aggregated apoB100 (94;105; 129) suggest that this may be a mechanism for insulin-mediated degradation as well. In addition to cellular studies, in vivo acute glucose-stimulated hyperinsulinemia suppressed secretion of VLDL in rats (130), while short-term hyperinsulinemia with euglycemia inhibited the secretion of VLDL in normal humans (131;132). Hyperinsulinemia also inhibits intestinal secretion of apoB48 (133), although under the conditions of this experiment, suppression of circulating FA also had a significant effect.
If insulin targets apoB for degradation, why is there increased VLDL and chylomicron assembly and secretion in states of IR (134)? The answer can probably be found in a combination of loss of responsiveness to insulin over time and the countervailing stimulation of apoB secretion by hepatic TG. Thus, chronic exposure of cultured hepatocytes to insulin causes a loss of its inhibitory effects on apoB secretion (135;136). Similarly, VLDL secretion was not inhibited by short-term hyperinsulinemia in obese individuals (132) or people with type 2 diabetes mellitus (137), two states characterized by IR. These results suggest the development of resistance to insulin signaling in the pathway for apoB degradation when hyperinsulinemia is chronic. Mice with liver-specific loss of PTEN have both excessive insulin signaling and severe hepatic steatosis: TG. secretion is increased in these mice (138). Increased VLDL secretion has also been observed in several other in vivo mouse models where chronic hyperinsulinemia exists together with increased hepatic TG (73;122). It is difficult, therefore, to separate IR in the pathway to apoB degradation from a stronger stimulatory force to assemble and secrete VLDL arising from steatosis (139). Increased FA flux to the liver in some of these models or disease states may also counteract the inhibitory effects of insulin on apoB secretion. Relevant to this point, insulin-mediated inhibition of VLDL secretion is attenuated by pre-treatment with an LXR agonist, which increases hepatic TG content (140). Studies of LIRKO mice (121), showed that chronic, complete loss of insulin signaling was associated with increased apoB secretion, supporting to some extent a chronic role for insulin in the degradation of apoB, at least in livers with normal TG content. Overall, the results of these many studies indicate that acute hyperinsulinemia, such as in the immediate postprandial state, can inhibit VLDL secretion, whereas in states of IR and chronic systemic hyperinsulinemia, a combination of loss of responsiveness to insulin-targeting of apoB for degradation and/or the stimulation of apoB secretion by increased hepatic TG and/or increased FA flux to the liver, results in increased VLDL secretion and dyslipidemia.
Hepatic steatosis in IR
The remaining question is why does TG accumulate in insulin resistant livers even though VLDL assembly and secretion are increased (134;139)? VLDL apoB and TG secretion rates are increased 3–5 times in humans with IR compared to normal individuals (141;142); shouldn’t this range of secretion be adequate to prevent accumulation of hepatic TG, or is there a limitation to the secretion of VLDL in states of IR? One limitation would be that increased hepatic TG, rather than stimulating increased assembly of VLDL particles, would stimulate the assembly of larger particles, but not more particle; this would relieve some of the stress but there is probably a limit to the size of VLDL that can be produced. As noted earlier, TG derived from DNL appears to increase particle size without increasing the number of particles secreted (108–110), and DNL is increased in states of IR (37), providing more TG for VLDL secretion (38).
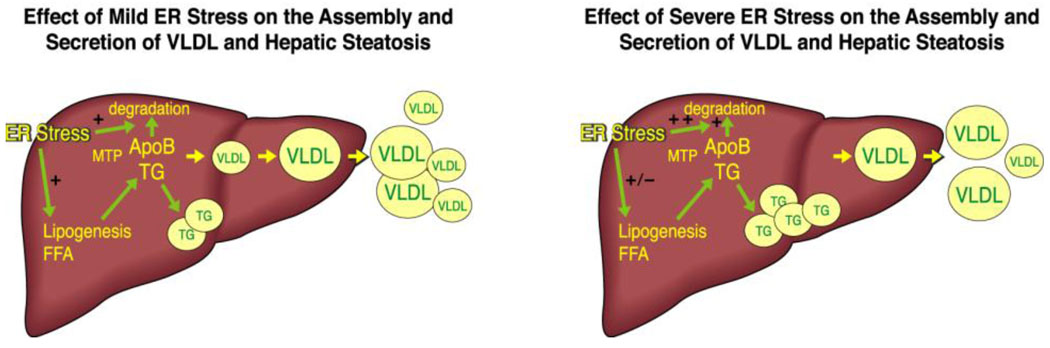
An additional basis for the counter-intuitive association of hepatic steatosis and increased VLDL secretionis ER stress. Detailed descriptions of ER stress and the associated uncoupled protein response (UPR) are beyond the scope of this review and are readily available (143;144). Several recent reports have examined the increasing evidence for a critical role of ER stress and the UPR in metabolic diseases, including IR and hepatic steatosis (145–148). Relevant to this review, FA-induced ER stress can inhibit apoB assembly and secretion directly (149). In both in vitro and in vivo studies, induction of ER stress by OA-targeted apoB100 for cotranslational and posttranslational degradation and decreased its secretion, (Figure 3). Importantly, the association between OA-induced ER stress and apoB secretion was parabolic in nature: modest ER stress was actually associated with increased apoB secretion (because OA-mediated stimulation of apoB was greater than ER stress-mediated inhibition) whereas more significant ER stress led to a loss of OA stimulation and actual inhibition of secretion. In a glucosamine-induced model of ER stress apoB is targeted for both ER-associated and post-ER degradation (150) as well as reduced synthesis (151). Adding further complexity is the finding that increased apoB entry into the ER lumen, as would occur if the first step in VLDL secretion was increased, may trigger ER stress (152). In any event, these findings are compatible with the concomitant occurrence of hepatic steatosis and increased VLDL secretion: ie, people with fatty livers typically secrete more VLDL than normal individuals but, because of ER-stress and the UPR (149), they do not secrete enough to meet the demand for TG transport. Additionally, there may be a point, particularly if non-alcoholic steatohepatitis replaces hepatic steatosis, that apoB secretion will fall even further, exacerbating the process (153). On the other hand, if autophagy is a pathway for the degradation of apoB (105–107), there may be concomitant protection from hepatic steatosis (154)
Fig 3.
ER stress and the associated UPR were first described as responses to inadequate energy in the cell leading to misfolding of proteins in the ER. Recent studies have demonstrated the ER stress can result from “over-nutrition” in the cell, particularly in terms of the delivery of FA. ER stress itself may stimulate DNL, leading to more FA in the hepatocytes. When ER stress is mild or moderate, the increased availability of TG and FA will stimulate both hepatic storage of TG and VLDL secretion (left). If ER stress is more severe, the UPR may actually inhibit VLDL secretion via either increasing co-translational degradation of apoB by the proteasome or stimulating post-ER degradation of apoB and VLDL, possibly via autophagy. In this scenario, which is often coincident with IR, VLDL secretion may be increased, but not enough to prevent significant steatosis, even though DNL may no longer be stimulated (right).
If the complexity reviewed above was not adequate, it now appears that ER stress and the UPR can also significantly affect hepatic lipid synthesis and steatosis (145;147;148;155;156). The rationale for a link between ER dysfunction, the UPR, and increased DNL can seem at first, to be counterproductive, because a major lipid synthesized is TG, which would need to be secreted via a dysfunctional ER. However, the synthesis of phosphatidylcholine, a major ER membrane PL, at a time when more ER is required, does make sense (157). Most importantly, studies to date indicate that the degree of ER stress and the extent to which the UPR compensates for ER stress determine the outcome. Severe ER stress resulting from genetic deletion of components of the UPR, together with a stressor such as tunicamycin, suppresses both DNL and fatty acid oxidation, with steatosis resulting from the decreased oxidation. On the other hand, during more “physiologic” ER stress, the UPR may stimulate DNL (158;159). To add further complexity to these new findings, XBP1, a key player in the UPR, appears to also play a role in the regulation of DNL via mechanisms that are independent of ER stress (155;160). Clearly, more work is needed to better define the role of ER stress in livers of IR rodents and humans.
An area we have not discussed, but one that almost certainly plays an important role in hepatic steatosis, is the state of increased systemic inflammation that accompanies IR (145;161). Altered Kupfer cells (162), stellate cells (163) and circulating mononuclear cells that infiltrate the liver (164) have all been implicated as facilitators of TG accumulation in the liver. This rapidly evolving field adds an additional dimension to the associations that have been discussed here.
Concluding Remarks
In this overview we attempted to provide a foundation for increased understanding of the commonly observed combination of increased VLDL secretion and hepatic steatosis in states of IR. The regulation of assembly and secretion of VLDL comprises a complexity appropriate for the creation of a large, multimolecular transport vehicle whose “raison d’etre” is to help maintain nutrient homeostasis in the liver. It is not surprising, therefore, that IR, which results from, and causes, disturbances in nutrient metabolism, has complex effects on VLDL secretion. Unfortunately, the complexity inherent in these systems has made it difficult to translate targets identified in cells and animal models to treatments of humans. Future studies will hopefully begin to consolidate the present large body of knowledge to reveal central targets amenable to effective therapies. Until that time, and preferential to the use of pharmacologic approaches, reduced energy intake coupled with increased energy expenditure are our “best bets” to improve IR and its consequences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119:S10–S16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reaven GM. Why Syndrome X? from Harold Himsworth to the insulin resistance syndrome. Cell Metab. 2005;1:9–14. doi: 10.1016/j.cmet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with Type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 4.Chahil TJ, Ginsberg GN. Diabetic dyslipidemia. Endocrinol Metab Clin N Am. 2006;35:491–510. doi: 10.1016/j.ecl.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Barter PJ, Brewer HB, Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterio. Thromb. & Vasc. Biol. 2003;23:160–167. doi: 10.1161/01.atv.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 6.Segrest JP, Jones MK, Mishra VK, Anantharamaiah GM, Garber DW. ApoB-100 has a pentapartite structure composed of three amphipathic alpha-helical domains alternating with two amphipathic beta-strand domains. Detection by the computer program LOCATE. Arterioscler Thromb Vasc Biol. 1994;14:1674–1685. doi: 10.1161/01.atv.14.10.1674. [DOI] [PubMed] [Google Scholar]
- 7.Fisher EA, Ginsberg HN. Complexity in the secretory pathway: The assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem. 2002;277:17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 8.Fisher EA, Pan M, Chen X, Wu X, Wang H, Jamil H, Sparks JD, Williams KJ. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J Biol Chem. 2001;276:27855–27863. doi: 10.1074/jbc.M008885200. [DOI] [PubMed] [Google Scholar]
- 9.Ginsberg HN, Fisher EA. The ever-expanding role of degradation in the regulation of apolipoprotein B metabolism. J Lipid Res. 2009 Suppl:S162–S166. doi: 10.1194/jlr.R800090-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olofsson S-O, Stillemark-Billton P, Asp L. Intracellular assembly of VLDL. Two major steps in separate cell compartments. Trends Cardiovasc. Med. 2000;10:338–345. doi: 10.1016/s1050-1738(01)00071-8. [DOI] [PubMed] [Google Scholar]
- 11.Barter PJ, Nestel PJ. Precursors of plasma triglyceride fatty acids in obesity. Metabolism. 1973;22:779–783. doi: 10.1016/0026-0495(73)90048-6. [DOI] [PubMed] [Google Scholar]
- 12.Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158–166. doi: 10.1172/JCI117633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haemmerle G, Zimmermann R, Strauss JG, Kratky D, Riederer M, Knipping G, Zechner R. Hormone-sensitive lipase deficiency in mice changes the plasma lipid profile by affecting the tissue-specific expression pattern of lipoprotein lipase in adipose tissue and muscle. J Biol Chem. 2002;277:12946–12952. doi: 10.1074/jbc.M108640200. [DOI] [PubMed] [Google Scholar]
- 14.Fabbrini E, Mohammed BS, Magkos F, Korenblat KM, Patterson BW, Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basu A, Basu R, Shah P, Vella A, Rizza RA, Jensen MD. Systemic and regional free fatty acid metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab. 2001;280:E10000–E10006. doi: 10.1152/ajpendo.2001.280.6.E1000. [DOI] [PubMed] [Google Scholar]
- 16.Bickerton AS, Roberts R, Fielding BA, Tornqvist H, Blaak EE, Wagenmakers AJ, Gilbert M, Humphreys SM, Karpe F, Frayn KN. Adipose tissue fatty acid metabolism in insulin-resistant men. Diabetologia. 2008;51:1936. doi: 10.1007/s00125-008-1040-x. [DOI] [PubMed] [Google Scholar]
- 17.Jensen MD, Nielsen S. Insulin dose response analysis of free fatty acid kinetics. Metabolism. 2007;56:68–76. doi: 10.1016/j.metabol.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Meek SE, Nair KS, Jensen MD. Insulin regulation of regional free fatty acid metabolism. Diabetes. 1999;48:10–14. doi: 10.2337/diabetes.48.1.10. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1530–1532. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y-L, Hernandez-Ono A, Ko C, Yasunaga K, Huang L-S, Ginsberg HN. Regulation of hepatic apolipoprotein B-lipoprotein assembly and secretion by the availability of fatty acids 1: Differential effects of delivering fatty acids via albumin or remnant-like emulsion particles. J Biol Chem. 2004;279:19362–19374. doi: 10.1074/jbc.M400220200. [DOI] [PubMed] [Google Scholar]
- 21.Castro Cabezas M, de Bruin TW, de Valk HW, Shoulders CC, Jansen H, Willem Erkelens D. Impaired fatty acid metabolism in familial combined hyperlipidemia. A mechanism associating hepatic apolipoprotein B overproduction and insulin resistance. J Clin Invest. 1993;92:160–168. doi: 10.1172/JCI116544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hajri T, Abumrad NA. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu Rev Nutr. 2002;22:383–415. doi: 10.1146/annurev.nutr.22.020402.130846. [DOI] [PubMed] [Google Scholar]
- 23.Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20:72–77. doi: 10.1016/j.tem.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simard JR, Pillai BK, Hamilton JA. Fatty acid flip-flop in a model membrane is faster than desorption into the aqueous phase. Biochemistry. 2008;47:9081–9089. doi: 10.1021/bi800697q. [DOI] [PubMed] [Google Scholar]
- 25.Miquilena-Colina ME, Lima-Cabello E, Sanchez-Campos S, Garcia-Mediavilla MV, Fernandez-Bermejo M, Lozano-Rodriguez T, Vargas-Castrillon J, Buque X, Ochoa B, Aspichueta P, et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in nonalcoholic steatohepatitis and chronic hepatitis C. Gut. 2011;26 doi: 10.1136/gut.2010.222844. [DOI] [PubMed] [Google Scholar]
- 26.Zhu L, Baker SS, Liu W, Tao MH, Patel R, Nowak NJ, Baker RD. Lipid in the livers of adolescents with nonalcoholic steatohepatitis: combined effects of pathways on steatosis. Metabolism. 2010;12 doi: 10.1016/j.metabol.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Ginsberg HN, Zhang Y-L, Hernandez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res. 2005;36:232–240. doi: 10.1016/j.arcmed.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Brunzell JD, Hazzard WR, Porte DJ, Bierman EL. Evidence for a common saturable triglyceride removal mechanism for chlomicrons and very low density lipoprotein in man. J Clin Invest. 1973;52:1578. doi: 10.1172/JCI107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Q, Avramoglu RK, Adeli K. Intestinal assembly and secretion of highly dense/lipid-poor apolipoprotein B48-containing lipoprotein particles in the fasting state: evidence for induction by insulin resistance and exogenous fatty acids. Metabolism. 2005;54:689–697. doi: 10.1016/j.metabol.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Duez H, Lamarche B, Uffelman KD, Valero R, Cohn JS, Lewis GF. Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein B48 containing lipoproteins in humans. Arteriosclerosis, Thromb. & Vasc. Biol. 2006;26:1357–1363. doi: 10.1161/01.ATV.0000222015.76038.14. [DOI] [PubMed] [Google Scholar]
- 31.Duez H, Lamarche B, Valero R, Pavlic M, Proctor S, Xiao C, Szeto L, Patterson BW, Lewis GF. Both intestinal and hepatic lipoprotein production are stimulated by an acute elevation of plasma free fatty acids in humans. Circulation. 2008;117:2369–2378. doi: 10.1161/CIRCULATIONAHA.107.739888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adeli K, Lewis GF. Intestinal lipoprotein overproduction in insulin-resistant states. Curr Opin Lipidol. 2008;19:221–228. doi: 10.1097/MOL.0b013e3282ffaf82. [DOI] [PubMed] [Google Scholar]
- 33.Cohn JS, Patterson BW, Uffelman KD, Davignon J, Steiner G. Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C-III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:3949–3955. doi: 10.1210/jc.2003-032056. [DOI] [PubMed] [Google Scholar]
- 34.Cooper AD. Hepatic uptake of chylomicron remnants. J Lipid Res. 1997;38:2173–2192. [PubMed] [Google Scholar]
- 35.Barrows BR, Timlin MT, Parks EJ. Spillover of dietary fatty acids and use of serum nonesterified fatty acids for the synthesis of VLDL-triacylblycerol under two different feeding regimens. Diabetes. 2005;54:2668–2673. doi: 10.2337/diabetes.54.9.2668. [DOI] [PubMed] [Google Scholar]
- 36.Miles JM, Nelson RH. Contribution of triglyceride-rich lipoproteins to plasma free fatty acids. Horm Metab Res. 2007;39:726–729. doi: 10.1055/s-2007-990273. [DOI] [PubMed] [Google Scholar]
- 37.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during nonalcoholic fatty liver disease. Diabetes Metab. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 38.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoprotein in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115 doi: 10.1172/JCI23621. 1343-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acidsynthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 2008;118:829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joseph SB, Laffitte BA, Patel PH, Watson MA, Matsukuma KE, Walczak R, Collins JL, Osborne TFTP. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J Biol Chem. 2002;277:11019–11025. doi: 10.1074/jbc.M111041200. [DOI] [PubMed] [Google Scholar]
- 43.Cha JY, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. the carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem. 2007;282:743–751. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- 44.Uyeda K, Repa JJ. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. PNAS. 2004;101:11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hegarty BD, Bobard A, Hainault I, Ferre P, Bossard P, Foufelle F. Distinct roles of insulin and liver X receptor in the induction and cleavage of sterol regulatory element-binding protein-1c. Proc. Natl. Acad. Sci. USA. 2005;102:791–796. doi: 10.1073/pnas.0405067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y, Shionoiri F, Iizuka Y, Ohashi K, Harada K, et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem. 1999;274:35832–35839. doi: 10.1074/jbc.274.50.35832. [DOI] [PubMed] [Google Scholar]
- 48.Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277:9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
- 49.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto M, Ogawa W, Teshigawara K, Inoue H, Miyake K, Sakaue H, Kasuga M. Role of the insulin receptor substrate 1 and phosphatidylinositol 3-kinase signalling pathway in insulin-induced expression of sterol regulatory element binding protein 1c and glucokinase genes in rat hepatocytes. Diabetes. 2002;51:1672–1680. doi: 10.2337/diabetes.51.6.1672. [DOI] [PubMed] [Google Scholar]
- 51.Dong X, Park S, Lin X, Copps K, Yi X, White MF. Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. J Clin Invest. 2006;116:101–114. doi: 10.1172/JCI25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo S, Copps KD, Dong X, Park S, Cheng Z, Pocai A, Rossetti L, Sajan M, Farese RV, White MF. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol. 2009;29:5070–5083. doi: 10.1128/MCB.00138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, Farese R, Cantley LC, Kahn R. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKCλ/ζ. Cell Metabolism. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Matsumoto M, Ogawa W, Akimoto K, Inoue H, Miyake K, Furukawa K, Hayashi Y, Iguchi H, Matsuki Y, Hiramatsu R, et al. PKClambda in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest. 2003;112:935–944. doi: 10.1172/JCI18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sajan MP, Standaert ML, Nimal S, Varanasi U, Pastoor T, Mastorides S, Braun U, Leitges M, Farese RV. The critical role of atypical protein kinase C in activating hepatic SREBP-1c and NF(kappa)B in obesity. J Lipid Res. 2009;50:1133–1145. doi: 10.1194/jlr.M800520-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ono H, Shimano H, Katagiri H, Yahagi N, Sakoda H, Onishi Y, Anai M, Ogihara T, Fujishiro M, Viana A, et al. Hepatic Akt activation induces marked hypoglycemia, hepatomegaly, and hypertriglyceridemia with sterol regulatory element binding protein involvement. Diabetes. 2003;52:2905–2913. doi: 10.2337/diabetes.52.12.2905. [DOI] [PubMed] [Google Scholar]
- 57.Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamagate A, Qu S, Perdomo G, Kim DH, Slusher S, Meseck M, Dong HH. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008;118:2347–2364. doi: 10.1172/JCI32914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. The Journal of Clinical Investigation. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, et al. FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]
- 61.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laplante M, Sabatini DM. mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3281–3282. doi: 10.1073/pnas.1000323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore KJ, Rayner KJ, Suarez Y, Fernandez-Hernando C. MicroRNAs and cholesterol metabolism. Trends Endocrinol Metab. 2010;21:699–706. doi: 10.1016/j.tem.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lliopoulos D, Drosatos K, Hiyama Y, Goldberg IJ, Zannis VI. MicroRNA-370 controls the expression of microRNA-122 and Cpt1alpha and affects lipid metabolsim. J Lipid Res. 2010;51:1513–1523. doi: 10.1194/jlr.M004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Gerin i, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, Leclercq IA, MacDougald OA, Bommer GT. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem. 2010;285:33652–33661. doi: 10.1074/jbc.M110.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burkhardt R, Toh SA, Lagor WR, Birkeland A, Levin M, Li X, Robblee M, Fedorov VD, Yamamoto M, Satoh T, et al. Trib1 is a lipid-and myocardial infarction-associated gene that regulates hepatic lipogenesis and VLDL production in mice. J Clin Invest. 2010;120:4410–4414. doi: 10.1172/JCI44213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dentin R. Liver-specific inhibition of ChREBP improves plasma glucose control in leptin-deficient (ob/ob) mice. J Biol Chem. 2006;270:1416–1421. [Google Scholar]
- 69.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. PNAS. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matsusue K, Haluzik M, Lambert G, Yim S-H, Gavrilova O, Ward JM, Brewer B, Jr, Reitman ML, Gonzalez FJ. Liver-specific disruption of PPARγ in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111:737–747. doi: 10.1172/JCI17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, Rao MS, Gonzalez FJ, Reddy JK. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma) overexpression. J Biol Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 72.Schadinger SE, Bucher NL, Schreiber BM, Farmer SR. PPAR(gamma) 2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab. 2005 doi: 10.1152/ajpendo.00513.2004. In Print. [DOI] [PubMed] [Google Scholar]
- 73.Siri P, Candela N, Ko C, Zhang Y, Eusufzai S, Ginsberg HN, Huang LS. Post-transcriptional stimulation of the assembly and secretion of triglyceride-rich apolipoprotein B-lipoproteins in a mouse with selective deficiency of brown adipose tissue, obesity and insulin resistance. J Biol Chem. 2001;276:46064–46072. doi: 10.1074/jbc.M108909200. [DOI] [PubMed] [Google Scholar]
- 74.Edvardsson U, Ljungberg A, Oscarsson J. Insulin and oleic acid increase PPAR 2 expression in cultured mouse hepatocytes. Biochem & Biophys Res Com. 2006;340:111–117. doi: 10.1016/j.bbrc.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 75.Iozzo P, Bucci M, Roivainen A, Nagren K, Jarvisalo MJ, Kiss J, Guiducci L, Fielding B, Naum AG, Borra R, et al. Fatty acid metabolism in the liver, measured by position emission tomography, is increased in obese individuals. Gastroenterology. 2010;139:846–856. doi: 10.1053/j.gastro.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 76.Kotronen A, Seppala-Lindroos A, Vehkavaara S, Bergholm R, Frayn KN, Fielding BA, Yki-Jarvinen H. Liver fat and lipid oxidation in humans. Liver Int. 2009;29:1439–1446. doi: 10.1111/j.1478-3231.2009.02076.x. [DOI] [PubMed] [Google Scholar]
- 77.Walther TC, Farese RV., Jr The life of lipid droplets. Biochim Biophys Acta. 2009;1791:459–466. doi: 10.1016/j.bbalip.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olofsson SO, Bostrom P, Andersson L, Rutberg M, Perman J, Boren J. Lipid droplets as dynmaic organelles connecting storage and efflux of lipids. Biochim Biophys Acta. 2009;1791:448–458. doi: 10.1016/j.bbalip.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 79.Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mamalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res. 2010;51:468–471. doi: 10.1194/jlr.R000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gong J, Sun Z, Li P. CIDE proteins and metabolic disorders. Curr Opin Lipidol. 2009;20:121–126. doi: 10.1097/MOL.0b013e328328d0bb. [DOI] [PubMed] [Google Scholar]
- 81.Kadereit B, Kumar P, Wang WJ, Miranda D, Snapp EL, Severina N, Torregroza I, Evans T, Silver DL. Evolutionarily conserved gene family important for fat storage. Proc Natl Acad Sci U S A. 2008;105:94–99. doi: 10.1073/pnas.0708579105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang BH, Li L, Saha P, Chan L. Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis, and improves whole body insulin resistance in leptin-deficient mice. J Lipid Res. 2010;51:2132–2142. doi: 10.1194/jlr.M004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Magnusson B, Asp L, Bostrom P, Ruiz M, Stillemark-Billton P, Linden D, Boren J, Olofsson SO. Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler Thromb Vasc Biol. 2006 doi: 10.1161/01.ATV.0000223345.11820.da. [DOI] [PubMed] [Google Scholar]
- 84.Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Executive summary of the third report of the NCEP expert panel on detection, evaluation, and treatment of high cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 85.Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reid BN, Ables GP, Otivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua SC, Jr, Huang L-S. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem. 2008;283:13087–13099. doi: 10.1074/jbc.M800533200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Farrell GC. PNPLeAse get the fats right: does lipogenesis or lipolysis cause NASH? Hepatology. 2010;52:818–821. doi: 10.1002/hep.23867. [DOI] [PubMed] [Google Scholar]
- 89.Dubuquoy C, Robichon C, Lasnier F, Langlois C, Dugail I, Foufelle F, Girard J, Burnol AF, Postic C, Moldes M. Distinct regulation of adiponutrin/PNPLA3 gene expression by the transcription factors ChREBP and SREBP1c in mouse and human hepatocytes. J Hepatol. 2010 doi: 10.1016/j.jhep.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 90.Speliotes EK, Butler JL, Palmer CD, Voight BF, Hirschhorn JN GIANT Consortium, MIGen Consortium, NASH CRN. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 2010;52:904–912. doi: 10.1002/hep.23768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, Hobbs HH. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stickel F, Buch S, Lau K, Schwabedissen HM, Berg T, Ridinger M, Rietschel M, Schafmayer C, Braun F, Hinrichsen H, et al. Generic variation in the PNPLA3 gene is associated with alcoholic liver injury in caucasians. Hepatology. 2011;53:86–95. doi: 10.1002/hep.24017. [DOI] [PubMed] [Google Scholar]
- 93.Chen W, Chang B, Li L, Chan L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology. 2010;52:1134–1142. doi: 10.1002/hep.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rutledge AC, Su Q, Adeli K. Apolipoprotein B100 biogenesis: a complex array of intracellular mechanisms regulating folding, stability, and lipoprotein assembly. Biochem Cell Biol. 2010;88:251–267. doi: 10.1139/o09-168. [DOI] [PubMed] [Google Scholar]
- 95.Zhou M, Fisher EA, Ginsberg HN. Regulated co-translational ubiquitination of apoliprotein B100. A new paradigm for proteasomal degradation of a secretory protein. J Biol Chem. 1998;273:24649–24653. doi: 10.1074/jbc.273.38.24649. [DOI] [PubMed] [Google Scholar]
- 96.Fisher EA, Zhou M, Mitchell DM, Wu X, Omura S, Wang H, Goldberg AL, Ginsberg HN. The degradation of apolipoprotein B100 is mediated by the ubiquitin-proteasome pathway and involves heat shock protein 70. J Biol Chem. 1997;272:20427–20434. doi: 10.1074/jbc.272.33.20427. [DOI] [PubMed] [Google Scholar]
- 97.Raabe M, Veniant MM, Sullivan MA, Zlot CH, Jorkegren J, Nielsen LB, Wong JS, Hamilton RL, Young SG. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest. 1999;103:1287–1298. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alexander CA, Hamilton RL, Havel RJ. Subcellular localization of B apoprotein of plasma lipoproteins in rat liver. J Cell Biol. 1976;69:241–263. doi: 10.1083/jcb.69.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang H, Gilham D, Lehner R. Proteomic and lipid characterization of apolipoprotein B-free luminal lipid droplets from mouse liver microsomes: implications for very-low density lipoprotein assembly. J Biol Chem. 2007 doi: 10.1074/jbc.M706841200. [DOI] [PubMed] [Google Scholar]
- 100.Pan M, Liang J-S, Fisher EA, Ginsberg HN. The late addition of core lipids to nascent apolipoprotein B100, resulting in the assembly and secretion of triglyceride-rich lipoproteins, is independent of both microsomal triglyceride transfer protein activity and new triglyceride synthesis. Journal of Biological Chemistry. 2002;277:4413–4421. doi: 10.1074/jbc.M107460200. [DOI] [PubMed] [Google Scholar]
- 101.Gilham D, Lehner R. The physiological role of triacylglycerol hydrolase in lipid metabolism. Rev Endocr Metab Disord. 2004;5:303–309. doi: 10.1023/B:REMD.0000045101.42431.c7. [DOI] [PubMed] [Google Scholar]
- 102.Wei E, Alam M, Sun F, Agellon LB, Vance DE, Lehner R. Apolipoprotein B and triacylglycerol secretion in human triacylglycerol hydrolase transgenic mice. J Lipid Res. 2007 doi: 10.1194/jlr.M700320-JLR200. [DOI] [PubMed] [Google Scholar]
- 103.Yamaguchi J, Gamble MV, Conlon D, Liang J-S, Ginsberg HN. The conversion of apoB100 low density lipoprotein/high density lipoprotein particles to apoB100 very low density lipoproteins in response to oleic acid occurs in the endoplasmic reticulum and not in the Golgi in McARH77 cells. J Biol Chem. 2003;278:42643–42651. doi: 10.1074/jbc.M306920200. [DOI] [PubMed] [Google Scholar]
- 104.Brodsky JL, Gusarova V, Fisher EA. Vesicular trafficking of hepatic apolipoprotein B100 and its maturation to very low-density lipoprotein particles. Trends Cardiovasc. Med. 2004;14:127–132. doi: 10.1016/j.tcm.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 105.Pan M, Maitin V, Parathath S, Andreo U, Lin SX, St Germain C, Yao Z, Maxfield FR, Williams KJ, Fisher EA. Presecretory oxidation, aggregation, and autophagic destruction of apoprotein-B: a pathway for late-stage quality control. Proc Natl Acad Sci USA. 2008;105:5862–5867. doi: 10.1073/pnas.0707460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brodsky JL, Fisher EA. The many intersecting pathways underlying apolipoprotein B secretion and degradation. Trends Endocrinol Metab. 2008;19:254–259. doi: 10.1016/j.tem.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qiu W, Zhang J, Dekker MJ, Wang H, Huang J, Brumell JH, Adeli K. Hepatic autophagy medites ER stress-induced degradation of misfolded apolipoprotein B. Hepatology. 2011 doi: 10.1002/hep.24269. [DOI] [PubMed] [Google Scholar]
- 108.Melish J, Le N-A, Ginsberg H, Steinberg D, Brown WV. Dissociation of triglyceride and apoprotein-B production in very low density lipoproteins. Am J Physiol. 1980;239:E354–E362. doi: 10.1152/ajpendo.1980.239.5.E354. [DOI] [PubMed] [Google Scholar]
- 109.Horton JD, Shimano H, Hamilton RL, Brown MS, Goldstein JL. Disruption of LDL receptor gene in transgenic SREBP-1a mice unmasks hyperlipidemia resulting from production of lipid-rich VLDL. J Clin Invest. 1999;103:1067–1076. doi: 10.1172/JCI6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ, et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J. Biol. Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 111.Sundaram M, Zhong S, Bou Khalil M, Links PH, Zhao Y, Iqbal J, Hussain MM, Parks RJ, Wang Y, Yao Z. Expression of apolipoprotein C-III in McA-RH7777 cells enhances VLDL assembly and secretion under lipid-rich conditions. J Lipid Res. 2010;51:150–161. doi: 10.1194/jlr.M900346-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nagashima K, Lopez C, Donovan D, Ngai C, Fontanez N, Bensadoun A, Fruchart-Najib J, Holleran S, Cohn JS, Ramakrishnan R, et al. Effects of the PPAR agonist pioglitazone on lipoprotein metabolism in patients with type 2 diabetes mellitus. JCI. 2005:1323–1332. doi: 10.1172/JCI23219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hussain MMe. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 114.Lin MC, Gordon D, Wetterau JR. Microsomal triglyceride transfer protein (MTP) regulation in HepG2 cells: insulin negatively regulates MTP gene expression. J Lipid Res. 1995;36:1073–1081. [PubMed] [Google Scholar]
- 115.Kamagate A, Dong HH. Fox01 integrates insulin signaling to VLDL production. Cell Cycle. 2008;7:3162–3170. doi: 10.4161/cc.7.20.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gutierrez-Juarez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006;116:1686–1695. doi: 10.1172/JCI26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Allister EM, Borradaile NM, Edwards JY, Huff MW. Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes. 2005;54:1676–1683. doi: 10.2337/diabetes.54.6.1676. [DOI] [PubMed] [Google Scholar]
- 118.Wolfrum C, Stoffel M. Coactivation of Fox2 through PGC-1 beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab. 2006;3:99–110. doi: 10.1016/j.cmet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 119.Sheena V, Hertz R, Nousbeck Jeal. Transcriptional regulation of human microsomal triglyceride transfer protein by hepatocyte nuclear factor-4 alpha. J Lipid Res. 2005;46:328–341. doi: 10.1194/jlr.M400371-JLR200. [DOI] [PubMed] [Google Scholar]
- 120.Koo SH, Montminy M. Fatty acids and insulin resistance: a perfect storm. Molecular Cell. 2006;21:449–450. doi: 10.1016/j.molcel.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 121.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bartels ED, Lauritsen M, Nielsen LB. Hepatic expression of microsomal triglyceride transfer protein and in vivo secretion of triglyceride-rich lipoproteins are increased in obese diabetic mice. Diabetes. 2002;51:1233–1239. doi: 10.2337/diabetes.51.4.1233. [DOI] [PubMed] [Google Scholar]
- 123.Sparks JD, Sparks CE. Insulin regulation of triacylglycerol-rich lipoprotein synthesis and secretion. Biochim Biophys Acta. 1994;1215:9–32. doi: 10.1016/0005-2760(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 124.Dashti N, Williams DL, Alaupovic P. Effects of oleate and insulin on the production rates and cellular mRNA concentrations of apolipoproteins in HepG2 cells. J Lipid Res. 1989;30:1365–1373. [PubMed] [Google Scholar]
- 125.Phung TL, Roncone A, Jensen KL, Sparks CE, Sparks JD. Phosphoinositide 3-kinase activity is necessary for insulin-dependent inhibition of apolipoprotein B secretion by rat hepatocytes and localizes to the endoplasmic reticulum. J Biol Chem. 1997;272:30693–30702. doi: 10.1074/jbc.272.49.30693. [DOI] [PubMed] [Google Scholar]
- 126.Sparks JD, Phung TL, Bolognino M, Sparks CE. Insulin-mediated inhibition of apolipoprotein B secretion requires an intracellular trafficking event and phosphatidylinositol 3-kinase activation: studies with brefeldin A and wortmannin in primary cultures of rat hepatocytes. Biochem. 1996;313:567–574. doi: 10.1042/bj3130567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brown AM, Gibbons GF. Insulin inhibits the maturation phase of VLDL assembly via a phosphoinositide 3-kinase-mediated event. Arterioscler Thromb Vasc Biol. 2001;21:1656–1661. doi: 10.1161/hq1001.096640. [DOI] [PubMed] [Google Scholar]
- 128.Au CS, Wagner A, Chong T, Qiu W, Sparks JD, Adeli K. Insulin regulates hepatic apolipoprotein B production independent of the mass or activity of Akt1/PKBalpha. Metabolism. 2004;53:228–235. doi: 10.1016/j.metabol.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 129.Zhong S, Magnolo AL, Sundaram M, Zhou H, Yao EF, Di Leo E, Loria P, Wang S, Bamji-MIrza M, Wang L, et al. Nonsynonymous mutations within APOB in human familial hypobetalipoproteinemia: evidence for feedback inhibition of lipogenesis and postendoplasmic reticulum degradation of apolipoprotein B. J Biol Chem. 2010;285:6453–6464. doi: 10.1074/jbc.M109.060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chirieac DV, Chirieac LR, Corsetti JP, Cianci J, Sparks CE, Sparks JD. Glucose-stimulated insulin secretion suppresses hepatic triglyceride-rich lipoprotein and apoB production. Am J Physiol Endocrinol Metab. 2000;279:E1003–E1011. doi: 10.1152/ajpendo.2000.279.5.E1003. [DOI] [PubMed] [Google Scholar]
- 131.Malmstrom R, Packard CJ, Caslake M, Bedford D, Stewart P, Yki-Jarvinen H, Shepherd J, Taskinen MR. Effects of insulin and acipimox on VLDL1 and VLDL2 apolipoprotein B production in normal subjects. Diabetes. 1998;47:779–787. doi: 10.2337/diabetes.47.5.779. [DOI] [PubMed] [Google Scholar]
- 132.Lewis GF, Uffelman KD, Szeto LW, Steiner G. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes. 1993;42:833–842. doi: 10.2337/diab.42.6.833. [DOI] [PubMed] [Google Scholar]
- 133.Pavlic M, Xiao C, Szeto L, Patterson BW, Lewis GF. Insulin acutely inhibits intestinal lipoprotein secretion in humans in part by suppressing plasma free fatty acids. Diabetes. 2010;59:580–587. doi: 10.2337/db09-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndroms. Artheroscler Thromb Vasc Biol. 2008;28:1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 135.Bartlett SM, Gibbons GF. Short- and longer-term regulation of very-low-density lipoprotein secretion by insulin, dexamethasone and lipogenic substrates in cultured hepatocytes. A biphasic effect of insulin. Biochem J. 1988;249:37–43. doi: 10.1042/bj2490037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bjornsson OG, Duerden JM, Bartlett SM, Sparks JD, Sparks CE, Gibbons GF. The role of pancreatic hormones in the regulation of lipid storage, oxidation and secretion in primary cultures of rat hepatocytes. Short- and long-term effects. Biochem J. 1992;281:381–386. doi: 10.1042/bj2810381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Malmstrom R, Packard CJ, Caslake M, Bedford D, Stewart P, Jarvinen H, Shepherd J, Taskinen MR. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia. 1997;40:454–462. doi: 10.1007/s001250050700. [DOI] [PubMed] [Google Scholar]
- 138.Stiles B, Wang Y, Stahl A, Bassilian S, Lee WP, Kim Y-J, Sherwin R, Devaskar S, Lesche R, Magnuson MA, et al. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity. Proc. Natl. Acad. Sci. USA. 2004;101:2082–2087. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, Vehkavaara S, Hakkinen A, Olofsson SO, Yki-Jarvinen H, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–765. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 140.Grefhorst A, Parks EJ. Reduced insulin-mediated inhibition of VLDL secretion upon pharmacological activation of the liver X receptor in mice. J Lipid Res. 2009;16 doi: 10.1194/jlr.M800505-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ginsberg H, Le N-A, Mays C, Gibson J, Brown WV. Lipoprotein metabolism in non-responders to increased dietary cholesterol. Arteriosclerosis. 1981;1:463–470. doi: 10.1161/01.atv.1.6.463. [DOI] [PubMed] [Google Scholar]
- 142.Ginsberg HN, Le N-A, Gibson JC. Regulation of the production and catabolism of plasma low density lipoproteins in hypertriglyceridemic subjects. Effect of weight loss. J Clin Invest. 1985;75:614–623. doi: 10.1172/JCI111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ron D, Walter P. Signal integrationin the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 144.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutation Research. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 145.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schroder M, Sutcliffe L. Consequences of stress in the secretory pathway: The ER stress response and its role int he metabolic syndrome. Methods Mol Biol. 2010;648:43–62. doi: 10.1007/978-1-60761-756-3_3. [DOI] [PubMed] [Google Scholar]
- 147.Gentile C, Frye M, Pagliassotti M. Endoplasmic reticulum stress and the unfolded protein response in nonalcoholic fatty liver disease. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rutkowski DT, Wu J, Back SH, Callaghan MU, Ferris SP, Iqbal J, Clark R, Miao H, Hassler JR, Fornek J, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Developmental Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J. Clin. Invest. 2008;118:316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qiu W, Avramoglu RK, Rutledge AC, Tsai J, Adeli K. Mechanisms of glucosamine-induced suppression of the hepatic assembly and secretion of apolipoprotein B-100-containing lipoproteins. J Lipid Res. 2006;47:1749–1761. doi: 10.1194/jlr.M500363-JLR200. [DOI] [PubMed] [Google Scholar]
- 151.Qiu W, Su Q, Rutledge AC, Zhang J, Adeli K. Glucosamine-induced endoplasmic reticulum stress attenuates apolipoprotein B100 synthesis via PERK signaling. J Lipid Res. 2009;50:1814–1823. doi: 10.1194/jlr.M800343-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Su Q, Tsai J, Xu E, Qiu W, Bereczki E, Santha M, Adeli K. Apolipoprotein B100 acts as a molecular link between lipid-induced endoplasmic reticulum stress and hepatic insulin resistance. Hepatology. 2009;50:77–84. doi: 10.1002/hep.22960. [DOI] [PubMed] [Google Scholar]
- 153.Charlton M, Sreekumar R, Rasmussen D, Lindor K, Nair KS. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35:898–904. doi: 10.1053/jhep.2002.32527. [DOI] [PubMed] [Google Scholar]
- 154.Singh R, Kaushik S, Wang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Horton JD. Physiology. Unfolding lipid metabolism. Science. 2008;320:1433–1434. doi: 10.1126/science.1159651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Flamment M, Kammoun HL, Hainault I, Ferre P, Foufelle F. Endoplasmic reticulum stress: a new actor in the development of hepatic steatosis. Curr Opin Lipidol. 2010;21:239–246. doi: 10.1097/MOL.0b013e3283395e5c. [DOI] [PubMed] [Google Scholar]
- 157.Sriburi R, Bommiasamy H, Buldak GL, Robbins GR, Frank M, Jackowski S, Brewer JW. Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP-1(S)-induced endoplasmic reticulum biogenesis. J Biol Chem. 2007;282:7024–7034. doi: 10.1074/jbc.M609490200. [DOI] [PubMed] [Google Scholar]
- 158.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translational initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Werstuck GH, Lentz SR, Dayal S, Hossain GS, Sood SK, Shi YY, Zhou J, Maeda N, Krisans SK, Malinow MR, et al. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest. 2001;107:1263–1273. doi: 10.1172/JCI11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 162.Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O'Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347–357. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mollica MP, Lionetti L, Putti R, Cavaliere G, Gaita M, Barletta A. From chronic overfeeding to hepatic injury: Role of endoplasmic reticulum stress and inflammation. Nutr Metab Cardiovasc Dis. 2011 doi: 10.1016/j.numecd.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 164.Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Ables EV, Ferrante AW., Jr C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes. 2010;59:916–925. doi: 10.2337/db09-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]