Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans (original) (raw)
Abstract
Impaired immune functions leading to primary immunodeficiencies often correlate with paradoxical autoimmune complications; patients with hyper-IgM syndromes who are deficient in activation-induced cytidine deaminase (AID), which is required for class-switch recombination and somatic hypermutation, are prone to develop autoimmune diseases. To investigate the impact of AID-deficiency on early B-cell tolerance checkpoints in humans, we tested by ELISA the reactivity of recombinant antibodies isolated from single B cells from AID-deficient patients. New emigrant/transitional and mature naive B cells from AID-deficient patients express an abnormal Ig repertoire and high frequencies of autoreactive antibodies, demonstrating that AID is required for the establishment of both central and peripheral B-cell tolerance. In addition, B-cell tolerance was further breached in AID-deficient patients as illustrated by the detection of anti-nuclear IgM antibodies in the serum of all patients. Thus, we identified a major and previously unsuspected role for AID in the removal of developing autoreactive B cells in humans.
Hyper IgM (HIGM) syndromes are primary immunodeficiencies characterized by defects in class switch recombination (CSR), resulting in severely decreased numbers of circulating isotype-switched memory B cells (1). The genetic basis of HIGM is diverse and is caused by defects in (i) the CD40L/CD40 pathway essential for B-cell activation, germinal center (GC) formation, and CSR induction, or (ii) enzymes such as activation-induced cytidine deaminase (AID) and uracil DNA glycosylase mediating CSR and somatic hypermutation (2–5). Aside from the susceptibility to bacterial infections, patients with HIGM syndromes are prone to develop autoimmune conditions, suggesting that B-cell tolerance is not properly established or maintained in the absence of functional CD40L or AID (6, 7). Although developing autoreactive B cells are properly counterselected in the bone marrow of CD40L-deficient patients, their mature naive B cells express a high proportion of autoreactive antibodies, including antinuclear antibodies (ANAs), suggesting that CD40/CD40L interactions are essential for peripheral but not central B-cell tolerance in humans (8). The defective peripheral B-cell tolerance checkpoint in CD40L-deficient patients correlated with decreased regulatory T cell (Treg) numbers and elevated serum B-cell activating factor (BAFF) concentration, corroborating data from transgenic mouse models (8–11). The importance of Treg cells in the establishment or the maintenance of peripheral tolerance is demonstrated in foxp3-deficient mice and humans, who experience a severe autoimmune syndrome associated with the secretion of autoreactive antibodies (12–14). In addition, increased BAFF concentration inhibits the counterselection of autoreactive new emigrant/transitional B cells that failed to be removed from the B-cell population (15, 16). Hence, the elevated serum BAFF concentration and decreased Treg cell numbers in CD40L-deficient patients are likely to contribute to the accumulation of autoreactive mature naive B cells in the blood of these patients (8).
In contrast to CD40L-deficient patients, it is not known why AID-deficient subjects as well as AID-KO mice often suffer from autoimmune conditions (6, 17, 18). We report herein that AID deficiency affects both central and peripheral B-cell tolerance checkpoints, resulting in the accumulation of large numbers of autoreactive B cells that secrete autoreactive IgM antibodies detected in the serum of all AID-deficient patients.
Results
Defective Central B-Cell Tolerance in AID-Deficient Patients.
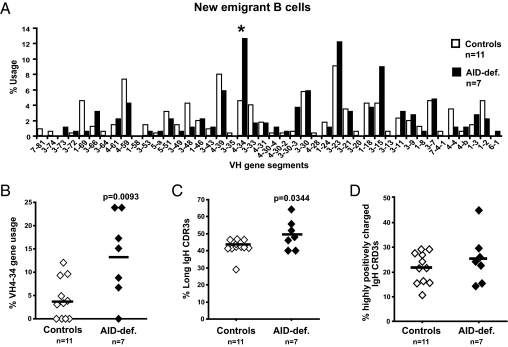
Central B-cell tolerance is achieved by the removal of most developing B cells that express highly polyreactive and ANAs in the bone marrow (19). To assess if this checkpoint is affected by AID deficiency, we cloned antibodies expressed by single CD10++CD21loIgMhiCD27− new emigrant/transitional B cells from AID-deficient patients (SI Appendix, Table S1) and tested their reactivity by ELISA (20). Patients were either homozygote or compound heterozygote for autosomal recessive AID mutations, preventing AID expression in most cases (21). The first evidence suggesting that central B-cell tolerance was not established properly in the absence of AID expression came from the analysis of the Ig repertoire of new emigrant/transitional B cells from seven AID-deficient patients (SI Appendix, Table S1). Pooled heavy-chain gene sequences from AID-deficient new emigrant/transitional B cells revealed a VH repertoire strongly enriched in the VH4-34 gene segment compared with that of healthy donors (Fig. 1_A_). The frequency of VH4-34, which is known to encode intrinsically self-reactive cold agglutinin antibodies that recognize carbohydrate antigens on erythrocytes (22, 23), exceeded 20% in some AID-deficient patients, but averaged only 4.2% in transitional B cells from healthy donors (P = 0.0093; Fig. 1_B_), suggesting an abnormal selection of developing B-cell precursors in the absence of AID. New emigrant/transitional B cells from AID-deficient patients also displayed a significantly higher frequency of long IgH complementarity determining regions 3 (CDR3s), a feature that favors antibody self-reactivity (Fig. 1_C_) (20, 24). However, the frequency of strongly positively charged IgH CDR3s, a feature also associated with autoreactive antibodies, was not significantly different between healthy donor and AID-deficient new emigrant/transitional B cells (Fig. 1_D_). Moreover, despite a similar D and JH gene segment repertoire in control and AID-deficient new emigrant/transitional B cells (SI Appendix, Fig. S1 and Tables S2–S25), we found that some commonly used D gene family members were used in a different reading frame in AID-deficient patients, which encoded hydrophobic residues known to favor self-reactivity (SI Appendix, Fig. S2) (25–27).
Fig. 1.
AID-deficient new emigrant/transitional B cells display an unusual IgH repertoire. (A) VH gene-usage frequencies in new emigrant/transitional B cells are represented for 11 healthy control subjects and seven AID-deficient patients. Sequences from 353 healthy control and 189 AID-deficient single transitional B cells were pooled. (B) The increased VH4-34 gene usage in AID-deficient new emigrant/transitional B cells is further analyzed. The frequencies of long IgH CDR3s (>14 aa) and IgH CDR3s containing two or more positively charged aa are represented in C and D, respectively. Each diamond represents an individual; the average is shown with a bar. Statistically significant differences are indicated.
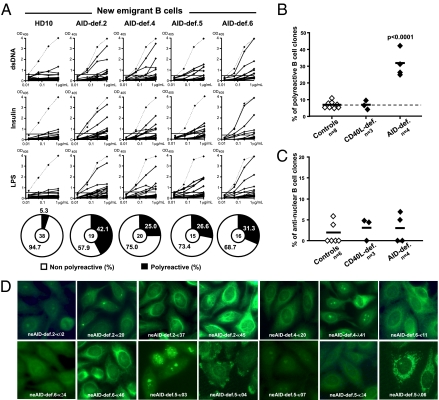
The reactivities of antibodies expressed by new emigrant/transitional B cells from four AID-deficient patients were then compared with their counterparts in healthy donor controls and CD40L-deficient patients with HIGM syndrome (Fig. 2 A_–_C) (8, 20, 27, 28). We found that polyreactive new emigrant/transitional B cells were significantly increased in AID-deficient patients (25.0–42.1% of the clones) compared with healthy controls (5.0–11.1%) or CD40L-deficient patients (4.3–9.5%; Fig. 2 A and B) (8, 20, 27, 28). Antibodies from AID-deficient new emigrant/transitional B cells predominantly recognized cytoplasmic structures, and only two of 70 clones, neAID-def.4 λ41 and neAID-def.5 κ03, bound nuclear antigens (Fig. 2 C and D), suggesting that the early counterselection of antinuclear expressing B cells, which requires IRAK-4 and MyD88 (29) may not be AID-dependent. Thus, the abnormal Ig repertoires and the increased frequency of polyreactive clones in new emigrant B cells from AID-deficient patients demonstrate that central B-cell tolerance is dependent on functional AID.
Fig. 2.
Defective central B-cell tolerance checkpoint in AID-deficient patients. (A) Antibodies from new emigrant/transitional B cells from a healthy donor and AID-deficient patients were tested by ELISA for reactivity against dsDNA, insulin, and lipopolysaccharide (LPS). Dotted lines show ED38-positive control and solid lines show binding for each cloned recombinant antibody (20, 26). Horizontal lines define cutoff OD405 for positive reactivity. The frequencies of polyreactive (B) and antinuclear (C) new emigrant/transitional B cells are compared between controls and CD40L- and AID-deficient patients, and statistically significant differences are indicated. (D) Autoreactive antibodies from AID-deficient new emigrant B cells show various patterns of HEp-2 staining.
AID Gene Transcription in Human Immature B Cells.
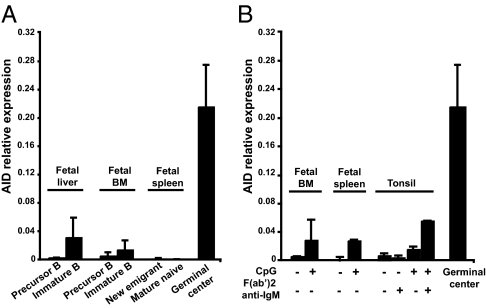
The analysis of patients with diverse primary immunodeficiencies revealed that central tolerance seems to be mostly controlled by B-cell–intrinsic factors regulating B-cell receptor or Toll-like receptor (TLR) signaling (27, 29). Although AID expression was previously believed to be restricted to activated B cells and GCs, it has now been observed in immature B cells from mice and humans, pointing to an earlier role for AID during bone marrow B-cell development (30–33). In agreement with these reports, we detected AID transcripts by quantitative PCR in CD19+CD10++IgM+ immature B cells from fetal liver and bone marrow (Fig. 3_A_) (30–33). The AID gene was three to five times more transcribed in immature B cells than CD19+CD10++IgM− early B-cell precursors, whereas AID transcripts were not amplified in more mature B-cell fractions isolated from fetal spleen or tonsils (Fig. 3 A and B). However, CD19+CD10++IgM+ immature B cells expressed approximately 20 to 25 times fewer AID transcripts than freshly isolated tonsillar GC B cells, bringing into question whether such low levels of AID transcription might be relevant to central tolerance (Fig. 3). Nonetheless, fetal bone marrow immature B cells were able to up-regulate in vitro AID transcription after TLR9 triggering by CpG for 2 d, similarly to mature naive B cells (Fig. 3_B_) (31). Hence, human developing immature B cells might up-regulate AID transcription after TLR9 triggering, potentially after binding DNA-containing self-antigens.
Fig. 3.
AID gene expression in human immature B cells. AID gene expression was assessed by quantitative PCR in unstimulated human precursors and immature B cells purified from fetal liver, bone marrow (BM), and spleen (A), or in immature B cells stimulated for 2 d with CpG and/or F(ab′)2 anti-IgM (B). Tonsillar GC B cells were used as positive control for AID gene expression. Error bars represent the mean ± SEM.
Defective Peripheral B-Cell Tolerance in AID-Deficient Patients.
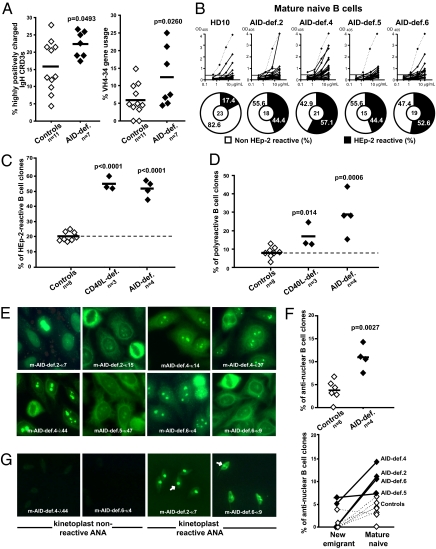
A second B-cell tolerance checkpoint eliminates autoreactive B cells in the periphery before they enter the CD19+CD10−CD21+IgM+CD27− mature naive B-cell compartment (20). As a consequence, the frequency of B cells expressing IgH CDR3s with two or more positively charged residues (a characteristic feature of autoreactive clones) decreases in healthy donors between the transitional (21.7% average) and mature naive (15.8% average) B-cell stages (Figs. 1_D_ and 4_A_). In contrast, we found that the proportion of B cells expressing highly positively charged CDR3s failed to significantly decrease between new emigrant/transitional and mature naive B-cell compartment of all AID-deficient patients (25.3% vs. 22.2%, respectively), suggesting B-cell selection defects (Figs. 1_D_ and 4_A_). In addition, VH4-34 gene segment usage continued to be significantly higher in mature naive B cells from AID-deficient patients than in their healthy donor counterparts (Fig. 4_B_ and SI Appendix, Fig. S3). Additional changes in the VH segment and D reading frame use of mature naive B cells from AID-deficient patients further suggest an alteration of the peripheral B-cell tolerance checkpoint in the absence of functional AID (SI Appendix, Figs. S3 and S4 and Tables S2–S25) (25–27). The full impact of AID-deficiency on the peripheral B-cell tolerance checkpoint was assessed by characterizing the reactivity of antibodies expressed by mature naive B cells from AID-deficient patients by using an ELISA to screen for binding to antigens expressed by the HEp-2 cell line (20). The frequency of HEp-2 reactive mature naive B cells was significantly increased (44.4–57.1%) in AID-deficient patients compared with healthy donors (16.7–23.3%) and was similar to that previously reported for CD40L-deficient patients (Fig. 4_C_) (8). AID-deficient mature naive B cells were also enriched in polyreactive clones compared with healthy controls (Fig. 4_D_). In agreement with the enrichment of clones expressing highly positively charged IgH CDR3s, we found an increased proportion of mature naive B cells expressing ANAs in all AID-deficient patients, further suggesting the existence of selection defects between transitional and mature naive B cells (Fig. 4 E and F). Indeed, whereas ANA-expressing B cells were almost absent in AID-deficient new emigrant B cells, their frequency significantly increased to 7.1–14.3% (total of eight of 73 clones) in the mature naive B-cell compartment (Fig. 4_F_). ANAs expressed by AID-deficient mature naive B cells displayed diverse nuclear staining patterns and the presence of chromatin-reactive antibodies was confirmed by the identification of clones recognizing the kinetoplast of Crithidia luciliae (Fig. 4 E and G). We conclude that the absence of functional AID alters the peripheral B-cell tolerance checkpoint and results in the accumulation in the mature naive B-cell compartment of large numbers of B cells expressing autoreactive antibodies including ANAs in AID-deficient patients.
Fig. 4.
Defective peripheral B-cell tolerance checkpoint in AID-deficient patients. The frequency of mature naive B-cell clones from healthy controls and AID-deficient patients expressing IgH CDR3s containing two or more positively charged aa (Left) or VH4-34 gene (Right) is represented in A, and the average is shown with a bar. (B) Antibodies from mature naive B cells from a healthy donor and AID-deficient patients were tested by ELISA for anti–HEp-2 cell reactivity. Dotted lines show ED38-positive control (20, 26). Horizontal lines define cutoff OD405 for positive reactivity. Frequencies of HEp-2–reactive (C) and polyreactive (D) clones in the mature naive fraction of CD40L and AID-deficient patients are higher than in controls. Mature naive B cells from AID-deficient patients contain ANA-expressing clones with diverse HEp-2 staining patterns (E). The frequency of ANA-expressing B cells in healthy controls and AID-deficient patients (Top) and its evolution between the new emigrant/transitional and mature naive B-cell compartments (Bottom) are represented in F. ANAs that bind the kinetoplast of C. luciliae (white arrows) are shown in G.
Decreased Treg Cell Frequencies and Increased Serum BAFF Concentrations in AID-Deficient Patients.
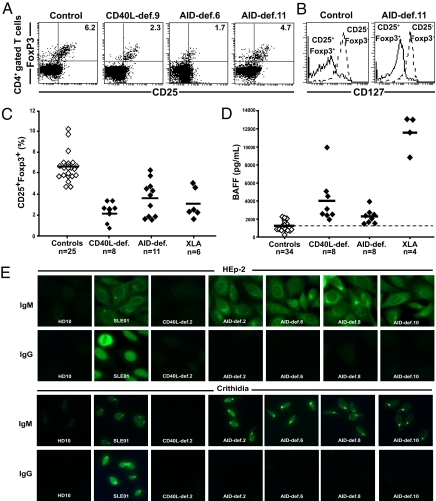
The emergence of ANA-expressing B cells was previously observed in CD40L-deficient patients and correlated with decreased Treg frequencies and increased concentrations of serum BAFF (8). We found that CD4+CD25hiFoxp3+ Treg cell frequency was significantly decreased in AID-deficient patients, who displayed, on average, approximately half the Treg cells as healthy controls (Fig. 5 A_–_C). The phenotype of the AID-deficient and control Treg cells was further confirmed by low levels of IL-7 receptor/CD127 expression on CD4+CD25hiFoxp3+ cells (Fig. 5_B_) (34, 35). This decreased Treg cell frequency in CD40L- and AID-deficient patients might reveal an involvement of isotype switched memory B cells missing in both types of HIGM patients in the generation or maintenance of some Treg cells in humans. The impact of B cells on Treg cells is further suggested in patients with X-linked agammaglobulinemia (XLA), who virtually lack B cells (36) and display decreased frequencies of Treg cells (Fig. 5_C_ and SI Appendix, Fig. S5). Serum BAFF concentrations were significantly (twofold) increased in AID-deficient patients, averaging 2,272 pg/mL compared with 1,226 pg/mL in healthy donors (P < 0.0001) and 4,009 pg/mL and 11,739 pg/mL in CD40L-deficient patients and those with XLA, respectively (Fig. 5_D_). The increased concentrations of BAFF in patients with XLA likely result from the virtual absence of B cells in these patients. However, it is unclear how AID and CD40L deficiencies impact serum BAFF concentrations because AID- and CD40L-deficient patients display normal numbers of B cells in their blood. Nevertheless, increased BAFF concentrations in AID-deficient patients are likely to interfere with the counterselection of autoreactive transitional B cells, as evidenced in mouse models (15, 16). Hence, decreased Treg cell frequencies and increased serum BAFF concentrations correlate with an altered peripheral B-cell tolerance checkpoint in both AID- and CD40L-deficient patients.
Fig. 5.
Low Treg cell frequency and increased serum BAFF concentrations and autoantibodies in AID-deficient patients. Treg cell frequencies among peripheral CD4+ T cells were assessed by analyzing the proportion of CD25+Foxp3+ cells. (A) Dot plots representative of a healthy control, a CD40L-deficient patient, and two AID-deficient patients. (B) CD127 expression was further analyzed by gating as indicated. (C) Treg cell frequencies from all patients were significantly lower than those in healthy controls (P < 0.0001 for CD40L-, AID-deficient, and XLA patients). (D) Significantly elevated serum BAFF concentrations (in pg/mL) in CD40L-, AID-deficient, and XLA patients were measured by ELISA (P < 0.0001 for each group of patients). (E) AID-deficient patients display secreted autoreactive IgM antibodies in their serum. Sera from six healthy donors, a patient with systemic Lupus erythematosus, four CD40L-deficient patients, and eight AID-deficient patients were tested for HEp-2–reactive (Top) and _C. luciliae_-reactive (Bottom) IgM and IgG antibodies.
AID-Deficient Patients Display Serum IgM ANAs.
We assessed whether tolerance was further breached in the periphery of AID-deficient patients by analyzing their sera for autoreactive antibodies. We found that eight of eight AID-deficient patients displayed autoreactive antibodies in their serum. These antibodies recognized many HEp-2 cell structures and the kinetoplast of C. luciliae, indicating the presence of anti-dsDNA autoantibodies in AID-deficient patients (Fig. 5_E_). Staining specificity was restricted to IgM antibodies and no IgG autoreactive antibodies were identified as expected for AID-deficient patients (Fig. 5_E_). A similar HEp-2 staining pattern was observed for IgM autoantibodies in the serum of a patient with systemic lupus erythematosus, whereas the IgG staining pattern was clearly nuclear. In contrast, none of five CD40L-deficient patients and six healthy donors we tested displayed any antibodies that recognized HEp-2 cells or C. luciliae in their serum (Fig. 5_E_). We conclude that B-cell tolerance is further breached in AID-deficient patients compared with CD40L-deficient patients, leading to the secretion of autoreactive antibodies including anti-dsDNA in the serum of all AID-deficient patients.
Discussion
The identification of defective central and peripheral B-cell tolerance checkpoints in AID-deficient patients demonstrates an important role for AID expression in the removal of developing autoreactive B cells. The mechanisms by which AID affects central B-cell tolerance are currently unknown, but because the mechanisms that ensure human central B-cell tolerance seem to be mostly controlled by intrinsic B-cell factors, AID expression in immature B cells might be relevant to tolerance induction (19, 37, 38). For instance, AID might induce DNA lesions that eventually lead to cell death and the elimination of autoreactive clones; AID-deficient B cells may therefore be less sensitive to apoptosis, a mechanism involved in central B-cell tolerance, as recently reported in mice (39). AID deamination of methylated cytidines might also induce DNA demethylation potentially required for the epigenetic regulation of gene expression [and perhaps V(D)J recombination and receptor editing (40)]. AID expression was previously believed to be restricted to GCs, but it has now been reported in ES cells (41, 42) as well as in immature B cells from mice and humans (30–33), suggesting an earlier role for AID during bone marrow B-cell development. We also detected AID transcripts in human immature B cells, but 20 to 25 times lower than in GC B cells. Although AID transcription may be up-regulated in immature B cells after TLR9 triggering, these low AID transcript levels may not be relevant to immature B-cell physiology and the removal of developing autoreactive B cells. Alternatively, early B-cell tolerance alteration observed in AID-deficient patients may not result from intrinsic B-cell defects but perhaps from a failure to control intestinal microflora (43). Indeed, intestinal inflammation and microflora changes may result in the alteration of Treg cell frequencies and serum BAFF concentrations in AID-deficient patients, thereby inducing peripheral B-cell tolerance defects similar to those reported in CD40L-deficient patients (8, 43, 44). Moreover, the lack of isotype-switched memory B cells in patients with both types of HIGM and XLA might also contribute to Treg cell alterations because B cells have been reported to be involved in T-cell activation and expansion (45–47). Treg cell frequencies were further decreased in CD40L-deficient patients compared with AID-deficient patients, probably because disruption of CD40/CD40L interactions between Treg cells and APCs such as dendritic cells, which are known to control Treg cell development (48), contributes to an even more pronounced defect in the induction or maintenance of Treg cells. Although both CD40L- and AID-deficient patients display high frequencies of autoreactive mature naive B cells, including ANA-expressing clones in their blood, an additional breach in B-cell tolerance is revealed in AID-deficient patients by the detection of ANAs and autoreactive IgM antibodies in their serum, which may favor the development of autoimmune disorders in these patients. It also demonstrates that ANA clone production and secretion can occur in the absence of somatic hypermutation despite its documented importance in the production of ANAs (49, 50). Interestingly, AID-deficient animals display B-cell tolerance defects similar to those reported herein, further attesting a conserved role for AID during early B-cell development in mice and humans (51). In conclusion, AID plays a major and previously unsuspected role in the establishment of both central and peripheral B-cell tolerance.
Materials and Methods
Patients and Donor Controls.
AID-deficient patients’ information is included in SI Appendix, Table S1. CD40L-deficient and XLA patients, as well as age-matched healthy donors, were previously reported besides HD12 (29-y-old white female), HD13 (31-y-old white male), HD14 (59-y-old white male), HD15 (53-y-old Hispanic female), and CD40L-deficient patients 8 and 9, who are two Hispanic brothers 11 and 14 y of age, respectively, who have an entire deletion of their CD40L gene (8, 20, 27–29, 52). Organs from two fetuses (109 and 120 d old, respectively) were obtained from the tissue collection and distribution program from the Laboratory of Developmental Biology at the University of Washington. Tonsil samples were obtained from the Yale/New Haven Hospital. All samples were collected in accordance with institutional review board-reviewed protocols.
Cell Staining and Sorting, cDNA, RT-PCR, Antibody Production, ELISAs, and Indirect Fluorescence Assays.
Peripheral B cells were purified from the blood of patients and control donors by positive selection by using CD20-magnetic beads (Miltenyi). Single CD21loCD10++IgMhiCD27− new emigrant/transitional and CD21+CD10−IgM+CD27− peripheral mature naive B cells from patients and control donors were sorted on a FACSVantage device (Becton Dickinson) into 96-well PCR plates, and antibody reactivities were tested as previously described (8, 20, 27–29, 52). For Treg cell analyses, peripheral mononuclear blood cells were stained with FITC anti-CD4, PECy7 anti-CD25, and APC-anti-CD127 (Biologend), and intracellular Foxp3 stainings were performed according to the manufacturer's instructions (eBioscience). Serum BAFF concentrations were determined by ELISA according to the manufacturer's instruction (R&D Systems).
Real-Time Quantitative RT-PCR.
Mature naive B cells were enriched from the blood or tonsils of donors by negative selection by using the Naive B Cell Isolation Kit II (Miltenyi). CD38+IgD− GC B cells were enriched from negatively selected tonsillar B cells by using total B Cell Isolation Kit (Miltenyi) and depletion of IgD+ B cells after phycoerythrin anti-IgD staining and antiphycoerythrin magnetic bead treatment (Miltenyi), followed by positive selection by using FITC-anti-CD38 and anti-FITC magnetic bead isolation (Miltenyi). CD19+CD10++IgM−CD27− early B-cell precursors and CD19+CD10++IgM+CD27− immature B cells from the liver and bone marrow of 109- and 120-d-old fetuses were sorted by flow cytometry after enrichment for B cells by using CD20 magnetic beads (Miltenyi). CD19+CD10++IgM+CD27− new emigrant and CD19+CD10−IgM+CD27− mature naive B cells were also isolated from the spleen of the same fetuses by using a similar approach. Total RNA was extracted from B cells using the Absolutely RNA MicroPrep kit (Stratagene), followed by cDNA synthesis with SuperScript II RT (Gibco BRL). Real-time quantification was performed with an iCycler IQ5 thermal cycler (BioRad) by using Evagreen (BioRad) and the following primers: sense CD79B, ccaggctggcgttgtctcctg; antisense CD79B, aggcgctgttcatgtagcagtg; sense AID, agacactctggacaccactatg; and antisense AID, ggaggaagagcaattccacgtg. Quantification of the gene of interest was analyzed by ΔCt method with CD79B used as the reference gene. Relative expression equals 2-(CTgene-CTactin).
Supplementary Material
Supporting Information
Acknowledgments
We thank Dr. S. Rudchenko and S. Semova for cell sorting, Dr. C. Price for providing CD40L-deficient patient samples, Mrs. M. C. Stolzenberg and M. Forveille for excellent technical assistance, Dr. J. Craft for providing tonsil samples, and Dr. D. Schatz for advice and discussions. We also thank all the members of the Immunodeficiency Clinic of the Centre Hospitalier de l'Université Laval. This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases Grants AI061093, AI071087, and AI082713 (to E.M.), Institut National de la Santé et de la Recherche Médicale, CEE European Primary Antibody Deficiencies Contract Seventh Framework Program (201549), and Association Contre le Cancer (F.R.-L. and A.D.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Gulino AV, Notarangelo LD. Hyper IgM syndromes. Curr Opin Rheumatol. 2003;15:422–429. doi: 10.1097/00002281-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Lee WI, et al. Molecular analysis of a large cohort of patients with the hyper immunoglobulin M (IgM) syndrome. Blood. 2005;105:1881–1890. doi: 10.1182/blood-2003-12-4420. [DOI] [PubMed] [Google Scholar]
- 3.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Revy P, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 5.Imai K, et al. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol. 2003;4:1023–1028. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- 6.Quartier P, et al. Clinical, immunologic and genetic analysis of 29 patients with autosomal recessive hyper-IgM syndrome due to activation-induced cytidine deaminase deficiency. Clin Immunol. 2004;110:22–29. doi: 10.1016/j.clim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Durandy A, Peron S, Fischer A. Hyper-IgM syndromes. Curr Opin Rheumatol. 2006;18:369–376. doi: 10.1097/01.bor.0000231905.12172.b5. [DOI] [PubMed] [Google Scholar]
- 8.Hervé M, et al. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. J Exp Med. 2007;204:1583–1593. doi: 10.1084/jem.20062287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rathmell JC, et al. CD95 (Fas)-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- 10.Schiemann B, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 11.Mackay F, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 13.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 14.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 15.Lesley R, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 16.Thien M, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Hase K, et al. Activation-induced cytidine deaminase deficiency causes organ-specific autoimmune disease. PLoS ONE. 2008;3:e3033. doi: 10.1371/journal.pone.0003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Guo L, Tian J, Zheng B, Han S. Deficiency in activation-induced cytidine deaminase promotes systemic autoimmunity in lpr mice on a C57BL/6 background. Clin Exp Immunol. 2010;159:169–175. doi: 10.1111/j.1365-2249.2009.04058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 21.Ta VT, et al. AID mutant analyses indicate requirement for class-switch-specific cofactors. Nat Immunol. 2003;4:843–848. doi: 10.1038/ni964. [DOI] [PubMed] [Google Scholar]
- 22.Silberstein LE, et al. Variable region gene analysis of pathologic human autoantibodies to the related i and I red blood cell antigens. Blood. 1991;78:2372–2386. [PubMed] [Google Scholar]
- 23.Pascual V, et al. Nucleotide sequence analysis of the V regions of two IgM cold agglutinins. Evidence that the VH4-21 gene segment is responsible for the major cross-reactive idiotype. J Immunol. 1991;146:4385–4391. [PubMed] [Google Scholar]
- 24.Klonowski KD, Primiano LL, Monestier M. Atypical VH-D-JH rearrangements in newborn autoimmune MRL mice. J Immunol. 1999;162:1566–1572. [PubMed] [Google Scholar]
- 25.Corbett SJ, Tomlinson IM, Sonnhammer ELL, Buck D, Winter G. Sequence of the human immunoglobulin diversity (D) segment locus: a systematic analysis provides no evidence for the use of DIR segments, inverted D segments, “minor” D segments or D-D recombination. J Mol Biol. 1997;270:587–597. doi: 10.1006/jmbi.1997.1141. [DOI] [PubMed] [Google Scholar]
- 26.Meffre E, et al. Surrogate light chain expressing human peripheral B cells produce self-reactive antibodies. J Exp Med. 2004;199:145–150. doi: 10.1084/jem.20031550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng Y-S, Wardemann H, Chelnis J, Cunningham-Rundles C, Meffre E. Bruton's tyrosine kinase is essential for human B cell tolerance. J Exp Med. 2004;200:927–934. doi: 10.1084/jem.20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuiji M, et al. A checkpoint for autoreactivity in human IgM+ memory B cell development. J Exp Med. 2006;203:393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isnardi I, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao C, et al. T cell-independent somatic hypermutation in murine B cells with an immature phenotype. Immunity. 2004;20:133–144. doi: 10.1016/s1074-7613(04)00019-6. [DOI] [PubMed] [Google Scholar]
- 31.Han JH, et al. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and Toll-like receptors. Immunity. 2007;27:64–75. doi: 10.1016/j.immuni.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueda Y, Liao D, Yang K, Patel A, Kelsoe G. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J Immunol. 2007;178:3593–3601. doi: 10.4049/jimmunol.178.6.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuraoka M, et al. Activation-induced cytidine deaminase expression and activity in the absence of germinal centers: Insights into hyper-IgM syndrome. J Immunol. 2009;183:3237–3248. doi: 10.4049/jimmunol.0901548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seddiki N, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conley ME. B cells in patients with X-linked agammaglobulinemia. J Immunol. 1985;134:3070–3074. [PubMed] [Google Scholar]
- 37.Goodnow CC. Balancing immunity and tolerance: Deleting and tuning lymphocyte repertoires. Proc Natl Acad Sci USA. 1996;93:2264–2271. doi: 10.1073/pnas.93.6.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nemazee D, et al. B-cell-receptor-dependent positive and negative selection in immature B cells. Curr Top Microbiol Immunol. 2000;245:57–71. doi: 10.1007/978-3-642-59641-4_3. [DOI] [PubMed] [Google Scholar]
- 39.Zaheen A, et al. AID constrains germinal center size by rendering B cells susceptible to apoptosis. Blood. 2009;114:547–554. doi: 10.1182/blood-2009-03-211763. [DOI] [PubMed] [Google Scholar]
- 40.Goodhardt M, et al. Methylation status of immunoglobulin kappa gene segments correlates with their recombination potential. Eur J Immunol. 1993;23:1789–1795. doi: 10.1002/eji.1830230809. [DOI] [PubMed] [Google Scholar]
- 41.Bhutani N, et al. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature. 2010;463:1042–1047. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popp C, et al. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature. 2010;463:1101–1105. doi: 10.1038/nature08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fagarasan S, et al. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 44.Hall JA, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diaz-de-Durana Y, Mantchev GT, Bram RJ, Franco A. TACI-BLyS signaling via B-cell-dendritic cell cooperation is required for naive CD8+ T-cell priming in vivo. Blood. 2006;107:594–601. doi: 10.1182/blood-2004-12-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouaziz JD, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci USA. 2007;104:20878–20883. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watanabe N, et al. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 49.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci USA. 1987;84:9150–9154. doi: 10.1073/pnas.84.24.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo W, et al. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. J Exp Med. 2010;207:2225–2237. doi: 10.1084/jem.20092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuraoka M, et al. Activation-induced cytidine deaminase mediates central tolerance in B cells. Proc Natl Acad Sci USA. 2011;108:11560–11565. doi: 10.1073/pnas.1102571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isnardi I, et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115:5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information